Abstract
Triclosan (TCS) is widely used in personal hygiene products, such as mouthwash and toothpaste, and is found in human tissues. Interleukin (IL)-1 beta (IL-1β), IL-6, tumor necrosis factor alpha (TNFα), and interferon gamma (IFNγ) are pro-inflammatory cytokines and inappropriately elevated levels of each have been associated with pathologies including rheumatoid arthritis and certain cancers. Here we examine effects of TCS on the secretion of the pro-inflammatory cytokines from human immune cell preparations. TCS at concentrations between 0.05–5 μM consistently increased the secretion of IL-1β, IL-6, and TNFα within 24 h of exposure and the increases often maintained out to 6 days of exposure. TCS also induced increases in IFNγ secretion, however the increases were most consistent after 48 h of exposure rather than within 24 h. Additionally, a role for both p44/42 and p38 MAPK in TCS-stimulated increases in IL-1β was seen in cells from some donors.
Keywords: NK cells, PBMCs, MD-PBMCs, Triclosan, pro-inflammatory cytokines
INTRODUCTION
Triclosan (TCS) is an organochlorine compound, containing both phenol and ether functional groups. It is used in personal hygiene products, mouthwash, toothpaste, beauty products, cleaning supplies and pesticides (Morrall et al., 2004; Thompson et al., 2005; Weatherly and Gosse, 2017). TCS can be ingested and absorbed through the skin and has been found in human blood, breast milk, and urine (Allmyr et al. 2006; Calafat et al., 2008; Yin et al., 2016). Ingestion of 13 mL (approximately 1 tablespoon) of mouthwash (0.03% TCS) resulted in a plasma concentration of as high as 1 μM within 1–3 h (Sandborgh-England et al. 2006). Exposure to TCS from toothpaste also resulted in a plasma level as high as 1 μM after 14 days (Allmyr et al. 2009). The levels of TCS that may be present in tissues just beneath the skin following exposure to products that contain as much as 0.3% TCS could be much higher (greater than 10 μM), based on findings on the absorption of TCS in human skin samples (Moss et al., 2000). Although triclosan was recently banned from over-the-counter soaps by the Food and Drug Administration, it is still widely used in oral hygiene products, as well as other beauty and personal hygiene merchandise (Kux, 2016) and is still a major safety concern and an emerging environmental pollutant (Kemsley, 2014; Weatherly and Gosse, 2017).
Interleukin (IL) 1 beta (IL-1β), IL-6, tumor necrosis factor alpha (TNFα), and interferon gamma (IFNγ) are several of the most potent pro-inflammatory cytokines. These cytokines have the capacity to regulate the innate and adaptive immune responses as well as to promotes cellular growth, and tissue repair (Dinarello, 1996; 2005; 2009; Tanaka and Kishimoto, 2014; Jorcyk et al., 2011; Fernando et al., 2014; Goetz et al., 2004; Billiau and Matthys, 2009).
IL-1β is produced by a wide range of cells including monocytes, macrophages, T cells, natural killer cells, neutrophils, keratinocytes, and fibroblasts (Dinarello, 2005; Apte et al., 2006; Voronov et al., 2002), while IL-6 is produced by monocytes, lymphocytes, macrophages, and muscle cells (Tanaka and Kishimoto, 2014; Jorcyk et al., 2011; Fernando et al., 2014). Elevation of either IL-1β or IL-6 in the absence of injury or infection can significantly contribute to a number of pathologies including rheumatoid arthritis, Crohn’s disease, bone loss, multiple sclerosis, cardiovascular disease, and cancer (Landskron et al., 2014; Choy and Panayi, 2001; Dinarello, 2011; Rubin et al., 2012; Elaraj et al., 2006; Jin et al., 1997; Lewis and Varghese, 2006; Voronov et al., 2002; Straub et al., 2015; Gabay, 2006; Tanaka and Kishimoto, 2014).
Both TNFα and IFNγ are produced by T cells, NK cells, monocytes, and macrophages (Billiau and Matthys, 2009; Darwich et al., 2009; Girart et al., 2007). Additional cell types are capable of producing TNFα such as smooth muscle cells, fibroblasts, endothelial cells, epithelial cells, and adipocytes (Locksley et al., 2001). As with IL-1β and IL-6, unwarranted elevations of IFNγ and TNFα cause chronic inflammation and its associated pathologies (Macarthur et al., 2004; Balkwill and Mantovani, 2001). Development of some gastrointestinal cancers is increased by IFN (Macarthur et al., 2004). TNFα can act as a stimulus for growth, angiogenesis, and epithelial-mesenchymal transition thus promoting tumor cell proliferation and invasiveness (Vajdic and van Leeuwen, 2009). Mitogen activated protein kinases (MAPKs) and nuclear factor kappa B (NFκB) regulate the secretion and synthesis of many pro-inflammatory cytokines from immune cells (Gaestel et al., 2009).
Previous studies in our lab have shown that the organochlorine contaminants pentachlorophenol (PCP) and dichlorodiphenyltrichloroethane (DDT) are able to increase the secretion of pro-inflammatory cytokines at certain exposures (Martin and Whalen., 2017; Martin et al., 2019; Massawe et al., 2017). The current study addresses whether TCS in the concentration range seen in human tissues (Thompson et al., 2005; Weatherly and Gosse, 2017; Allmyr et al. 2006; Calafat et al., 2008), is able to increase immune cell secretion of 4 potent pro-inflammatory cytokines. Any elevation of their secretion stimulated by TCS could have implications for a role for TCS in producing sterile (absence of injury or infection) chronic inflammation (Chen and Nunez, 2010) and its accompanying pathologies.
MATERIALS AND METHODS
Preparation of Peripheral blood mononuclear cells (PBMCs) and monocyte-depleted(MD- PBMCS
PBMCs were isolated from leukocyte filters (PALL- RCPL or RC2D) obtained from the Red Cross Blood Bank Facility (Nashville, TN) as described in Meyer et al., 2005. Leukocytes were retrieved from the filters by back-flushing them with an elution medium (sterile PBS containing 5 mM disodium EDTA and 2.5% [w/v] sucrose) and collecting the eluent. The eluent was layered onto lymphosep cell separation media (1.077g/mL) ((MP Biomedicals, Irvine, CA) and centrifuged at 1200g for 30 min. Mononuclear cells were collected and washed with phosphate buffered saline (PBS) pH 7.4 (500g, 10min). Following washing, the cells were layered on bovine calf serum for platelet removal. The cells were then suspended in RPMI-1640 (Mediatech, Inc, Manassas, VA) complete medium which consisted of RPMI-1640 supplemented with 10% heat-inactivated BCS, 2 mM L-glutamine and 50 U penicillin G with 50 μg streptomycin/mL. This preparation constituted PBMCs. Monocyte-depleted PBMCs (10–20% CD16+, 10–20 % CD56+, 70–80% CD3+, 3–5% CD19+, 2–20% CD14+) were prepared by incubating the cells in glass Petri dishes (150 X 15 mm) at 37 °C and air/CO2, 19:1 for 1 h. This cell preparation is referred to as MD-PBMCS cells. All cell isolation procedures were carried out under sterile conditions.
Preparation of NK cells
To prepare NK cells, leukocyte filters were back-flushed with elution media and the eluent was treated with RosetteSep human NK cell enrichment antibody cocktail (StemCell Technologies, Vancouver, British Columbia, Canada) at 0.6–0.8 mL per 50 mL of eluent. The treated eluent was incubated at room temperature (~ 25° C) for 20 min, then layered onto lymphosep cell separation media (1.077 g/mL) and centrifuged at 1200 g for 50 min. NK cells were collected and washed twice with phosphate buffered saline (PBS) pH 7.4 and stored in complete media at 37 °C and air/CO2, 19:1.
Chemical Preparation
TCS was purchased from Sigma-Aldrich (St. Louis, MO). A 100 mM stock solution was prepared using dimethyl sulfoxide (DMSO) as the solvent. Desired concentrations for cell treatments were made by diluting the stock into cell culture media. Enzyme inhibitors (MEK 1/2 pathway inhibitor (PD98059), p38 inhibitor (SB202190)) were purchased from Fischer Scientific (Pittsburgh, PA). 50 mM stock solutions of the inhibitors were prepared using DMSO, which were diluted for a final concentrations of 50 μM PD98059 and 25 μM SB202190. Appropriate DMSO controls were used in all experiments with the final DMSO concentration for experiments without inhibitors being less than 0.005% and for experiments using pathway inhibitors less than 0.1%.
Cell Treatments
PBMCs, Monocyte-depleted PBMCS and NK cells (at a concentration of 1.5 million cells/ mL) were treated with TCS at concentrations of 0.05–5.0 μM for 24h, 48h, or 6 days. Following the incubations, the cells were pelleted and supernatants were collected and stored at −70 C until assaying for IL-6. In experiments examining the role of p44/42 and p38 MAPKs, cells were pretreated with the inhibitor and appropriate controls for 1 h prior to a 24 h incubation with 5, 2.5, and 1 μM TCS.
Cell Viability
Cell viability was assessed at the beginning and end of each exposure period. Viability was determined using the trypan blue exclusion method. Briefly, cells were mixed with trypan blue and counted using a hemocytometer. The total number of cells and the total number of live cells were determined for both control and treated cells to determine the percent viable cells. Cell viability was not affected by any of the cell treatments (p>0.05). Viability for PBMCs treated with TCS for 24 h at 0 (control), 0.05, .01, 0.25, 0.5, 1.0, 2.5, and 5 μM were 99.6±0.6%, 99.7±0.8%, 99.2±1.1%, 99.6±0.5%, 99.4±1.0%, 99.5±0.7%, 99.3±1.1% and 97.9±2.0, respectively. After 48 h and 6 d of incubation with TCS viability of treated cells remained equivalent to that seen with control cells. This same result was seen with MD-PBMCs and enriched NK cells.
Measurement of cytokine secretion
IL-1 β levels were measured using the BD OptEIA™ Human IL-1 β enzyme linked immunosorbent assay (ELISA) kit (BD-Pharmingen, San Diego, CA). Briefly, a 96-well micro well plate, designed for ELISA (Fisher, Pittsburgh, PA), was coated with a capture antibody for IL-1β diluted in coating buffer. The plate was incubated with the capture antibody overnight at 4 ˚C. After incubation, the capture antibody was removed by washing the plate three times with wash buffer (PBS and 0.05% Tween-20). PBS containing 1% bovine calf serum (blocking solution) was added to each well and incubated at room temperature for 1 h. Blocking solution was removed by washing the plate three times, and the cell supernatants and IL-1β standards were added and incubated for 2 h at room temperature. Following this incubation, the plate was washed five times followed by incubation with biotinylated detection antibody for 1 h. Excess biotinylated detection antibody was removed by washing the plate five times. Streptavidin-linked horseradish peroxidase (HRP) was then added for 30 min during which it conjugated to the biotinylated detection antibody. Excess HRP was removed (seven washes) and a substrate solution was added for 30 min at room temperature. Incubation with substrate was ended by addition of acid and the absorbance was measured at 450 nm on a Thermo Labsystems Multiskan MCC/340 plate reader (Fisher Scientific).
IL-6, IFNγ, and TNFα levels were also measured using the BD OptEIA™ Human IL-6 ELISA kit, BD OptEIA™, Human IFNγ ELISA kit, and the BD OptEIA™ Human TNFα ELISA kit (BD-Pharmingen, San Diego, CA). The procedure was the same as that outlined for IL-1β, with the exception that detection antibodies for IL-6, IFNγ, and TNFα were conjugated with HRP prior to being applied to the microwell plate. Thus, there was not need for a separate incubation with detection antibody and then with HRP.
Statistical Analysis
Statistical analysis of the data was performed by using ANOVA and Student’s t test. Data were initially compared within a given experimental setup by ANOVA. A significant ANOVA was followed by pair wise analysis of control versus exposed data using Student’s t test, a p value of less than 0.05 was considered significant.
RESULTS
Effects of TCS Exposures on Secretion of IL-1β by NK cells, MD-PBMCs, and PBMCs
Table 1 shows the effects of exposing NK cells from 3 separate donors to 0, 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5 μM TCS for 24 h, 48 h and 6 days on IL-1β secretion. Within 24 h of exposure to TCS, cells from all 3 donors showed an increase in IL-1β secretion at one or more concentrations of TCS. Increases were seen at all TCS concentrations in cells from donor F467 while cells from donor F461 showed an increase at only at the 2.5 μM concentration. Cells from different donors varied in the concentration of TCS and length of exposure that caused the largest fold increase in IL-1β secretion. Cells from donor F461 showed the greatest TCS-induced increase (3.3 fold) in IL-1β after a 6 day exposure to 5 μM TCS while those from donor F467 had a maximal increase of 5.1 fold in IL-1β secretion after 24 h with 5 μM TCS. Cells from donor F475 showed a maximal increase (16 fold) at 0.05 μM TCS at 6 days. Cells from all donors showed TCS-induced decreases in IL-1β at 48 h at the highest concentration.
Table 1.
Effects of 24 h, 48 h, and 6 days exposures to TCS on IL-1β secretion from NK cells.
| 24 h | IL-1β secretion in pg/mL (mean±S.D.) | ||
|---|---|---|---|
| [TCS] μM | F461 | F467 | F475 |
| 0 | 1757±7 | 226±3 | 232±9 |
| 0.05 | 1534±30* | 935±9+ | 539±29+ |
| 0.1 | 1007±25* | 1288±9+ | 549±68+ |
| 0.25 | 1472±9* | 848±42+ | 354±34+ |
| 0.5 | 1275±19* | 946±11+ | 345±26+ |
| 1 | 1183±55* | 987±16+ | 425±17+ |
| 2.5 | 2164±72+ | 734±9+ | 289±25 |
| 5 | 1464±72* | 1136±29+ | 85±8* |
| 48 h | IL-1β secretion in pg/mL (mean±S.D.) | ||
| [TCS] μM | F461 | F467 | F475 |
| 0 | 4506±66 | 953±39 | 112±13 |
| 0.05 | 2493±86* | 1447±114+ | 1082±53+ |
| 0.1 | 2877±131* | 2137±27+ | 853±64+ |
| 0.25 | 2246±82* | 1209±47+ | 715±117+ |
| 0.5 | 3301±161* | 1321±75+ | 430±23+ |
| 1 | 2573±78* | 925±93 | 446±69+ |
| 2.5 | 2956±128* | 1063±35+ | 255±30+ |
| 5 | 2446±72* | 205±24* | 22±9* |
| 6 d | IL-1β secretion in pg/mL (mean±S.D.) | ||
| [TCS] μM | F461 | F467 | F475 |
| 0 | 345±8 | 1079±285 | 19±9 |
| 0.05 | 647±58+ | 1698±29 | 311±10+ |
| 0.1 | 669±3+ | 1148±32 | 303±22+ |
| 0.25 | 857±6+ | 1051±111 | 217±10+ |
| 0.5 | 833±8+ | 1169±106 | 185±23+ |
| 1 | 838±8+ | 1110±399 | 153±8+ |
| 2.5 | 904±7+ | 814±175 | 84±8+ |
| 5 | 1133±4+ | 62±46* | 31±4 |
Values are mean±S.D. of triplicate determinations.
indicates a significant increase and
indicates a significant decreases in secretion compared to control cells, p<0.05
The effects of exposing MD-PBMCs from 4 individual donors to TCS for 24 h, 48 h and 6 days on the secretion of IL-1β are shown in Table 2. Significant increases in IL-1β secretion were induced by TCS for all donors at the 5 μM concentrations after 24 h of exposure, with fold increases of 1.6, 5.4, 2.7, and 1.5 fold for cells from donors F390, F392, F406, and F451, respectively. TCS-stimulated increases in IL-1β maintained out to 6 days in cells from 3 of the 4 donors. MD-PBMCs did not show any consistent decreases in IL-1β secretion at any concentration of TCS or length of incubation.
Table 2.
Effects of 24 h, 48 h, and 6 days exposures to TCS on IL-1β secretion from monocyte-depleted PBMCs.
| 24 h | IL-1β secretion in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [TCS]μM | F390 | F392 | F406 | F451 |
| 0 | 1254±93 | 923±126 | 2107±181 | 4967±136 |
| 0.05 | 1400±43+ | 1651±130+ | 2293±39+ | 3728±323* |
| 0.1 | 1305±36 | 1638±282+ | 2844±111+ | 2425±578* |
| 0.25 | 1288±113 | 1561±146+ | 2537±23 | 3828±1323 |
| 0.5 | 1325±54 | 1704±200+ | 2778±144+ | 4587±748 |
| 1 | 1393±77 | 1535±86+ | 2789±128+ | 4121±971 |
| 2.5 | 1480±65+ | 2120±149+ | 3274±90+ | 4937±473 |
| 5 | 2040±164+ | 4944±488+ | 5630±145+ | 7604±256+ |
| 48 h | IL-1β secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F390 | F392 | F406 | F451 |
| 0 | 1357±55 | 778±138 | 1931±255 | 2761±218 |
| 0.05 | 1251±55 | 1126±34+ | 2303±232 | 3657±168+ |
| 0.1 | 1500±94 | 935±68 | 1903±80 | 3209±461 |
| 0.25 | 1563±94+ | 974±24 | 2531±58+ | 3389±340 |
| 0.5 | 1529±14+ | 1206±75+ | 3134±354 | 3185±220 |
| 1 | 1307±78 | 1187±32+ | 2638±435 | 3428±1006 |
| 2.5 | 1687±180 | 1762±170+ | 3415±39+ | 3551±775 |
| 5 | 3183±246+ | 3971±420+ | 5520±200+ | 4226±224+ |
| 6 d | IL-1β secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F390 | F392 | F406 | F451 |
| 0 | 464±59 | 458±9 | 1746±64 | 67±12 |
| 0.05 | 428±58 | 637±14+ | 2122±110+ | 42±20 |
| 0.1 | 457±13 | 647±4+ | 2279±76+ | 30±17* |
| 0.25 | 471±7 | 649±6+ | 2025±73+ | 46±11 |
| 0.5 | 494±21 | 646±6+ | 2169±52+ | 38±10* |
| 1 | 525±15 | 731±6+ | 2443±100+ | 41±9* |
| 2.5 | 564±46 | 980±49+ | 2704±224+ | 31±7* |
| 5 | 833±112+ | 1272±17+ | 4791±378+ | 41±1 |
Values are mean±S.D. of triplicate determinations.
indicates a significant increases and
indicates a significant decrease in secretion compared to control cells, p<0.05
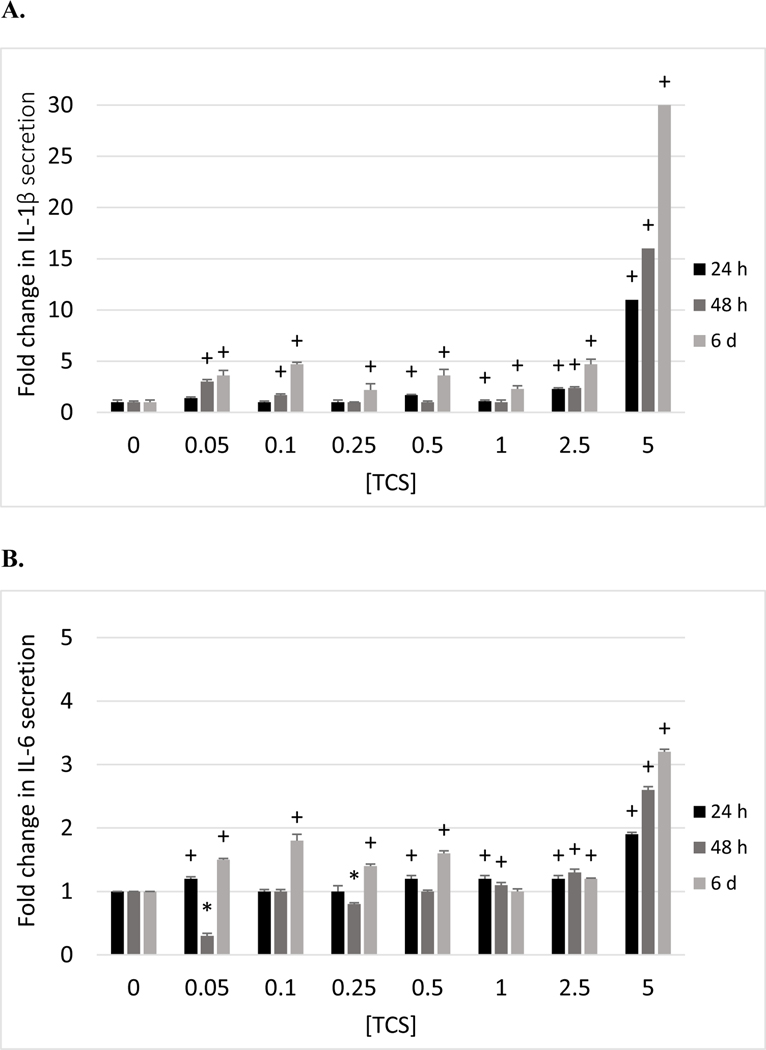
PBMCs showed TCS-induced increases in IL-1β secretion at multiple exposure concentrations after 24 h in cells from each of the 4 donors tested (Table 3). The maximal increase after 24 h was seen at the 5 μM exposure in cells from 3 of the 4 donors with 11 fold (F214), 5 fold (F224) and 25 fold (F238) increases. Cells from filter F217 showed the greatest increase (3.7 fold) at 0.1 μM TCS after 24 h. These increases continued out to 6 days of exposure (Table 3). The fold increases stimulated by TCS exposures at each time point were greatest in PBMCs as compared to MD-PBMCs and NK cells. This suggests that monocytes (which are major producers of IL-1β) are being stimulated by TCS to produce this cytokine within 24 h of exposure. As was seen with MD-PBMCs, TCS did not cause any consistent decreases in IL-secretion at any exposure condition. Figure 1A shows the fold changes in IL-1β secretion as compared to control of PBMCs from donor F214 exposed too TCS for 24h, 48 h, and 6 days.
Table 3.
Effects of 24 h, 48 h, and 6 days exposures to TCS on IL-1β secretion from PBMCs.
| 24 h | IL-1β secretion in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [TCS]μM | F214 | F217 | F224 | F238 |
| 0 | 545±35 | 5347±218 | 1906±143 | 287±7 |
| 0.05 | 781±100 | 7278±397+ | 1215±21* | 405±22+ |
| 0.1 | 519±43 | 19828±817+ | 1642±55 | 392±25+ |
| 0.25 | 581±31 | 6964±204+ | 1676±13 | 312±39 |
| 0.5 | 905±89+ | 6364±446+ | 1734±135 | 460±32+ |
| 1 | 623±27+ | 6240±94+ | 2043±290 | 409±44 |
| 2.5 | 1238±49+ | 2146±148* | 2306±192+ | 650±57+ |
| 5 | 5999±46+ | 7703±1341 | 9492±262+ | 7202±822+ |
| 48 h | IL-1β secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F214 | F217 | F224 | F238 |
| 0 | 349±41 | 6992±466 | 1652±62 | 179±49 |
| 0.05 | 1086±32+ | 10864±394+ | 2099±57+ | 201±65 |
| 0.1 | 596±69+ | 7174±347 | 2041±79+ | 118±19 |
| 0.25 | 387±30 | 8250±559+ | 2562±73+ | 190±116 |
| 0.5 | 314±8 | 8826±164+ | 2354±21+ | 170±7 |
| 1 | 362±28 | 8159±186+ | 2000±32+ | 285±20+ |
| 2.5 | 840±85+ | 8311±447+ | 3347±35+ | 321±64+ |
| 5 | 5773±281+ | 22401±1589+ | 4531±76+ | 6005±144+ |
| 6 d | IL-1β secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F214 | F217 | F224 | F238 |
| 0 | 261±36 | 7387±278 | 1679±10 | 353±134 |
| 0.05 | 949±60+ | 5286±55* | 1614±41 | 281±46 |
| 0.1 | 1228±143+ | 6503±63* | 1758±14+ | 258±6 |
| 0.25 | 571±63+ | 6684±174* | 2129±52+ | 463±29 |
| 0.5 | 975±154+ | 7510±616 | 1584±11+ | 208±28 |
| 1 | 601±146+ | 6445±906 | 2364±14+ | 216±20 |
| 2.5 | 1228±71+ | 8401±618 | 2638±80+ | 447±43 |
| 5 | 7881±138+ | 9735±961+ | 4771±24+ | 4056±134+ |
Values are mean±S.D. of triplicate determinations.
indicates a significant increase and
indicates a significant decrease in secretion compared to control cells, p<0.05
Figure 1.
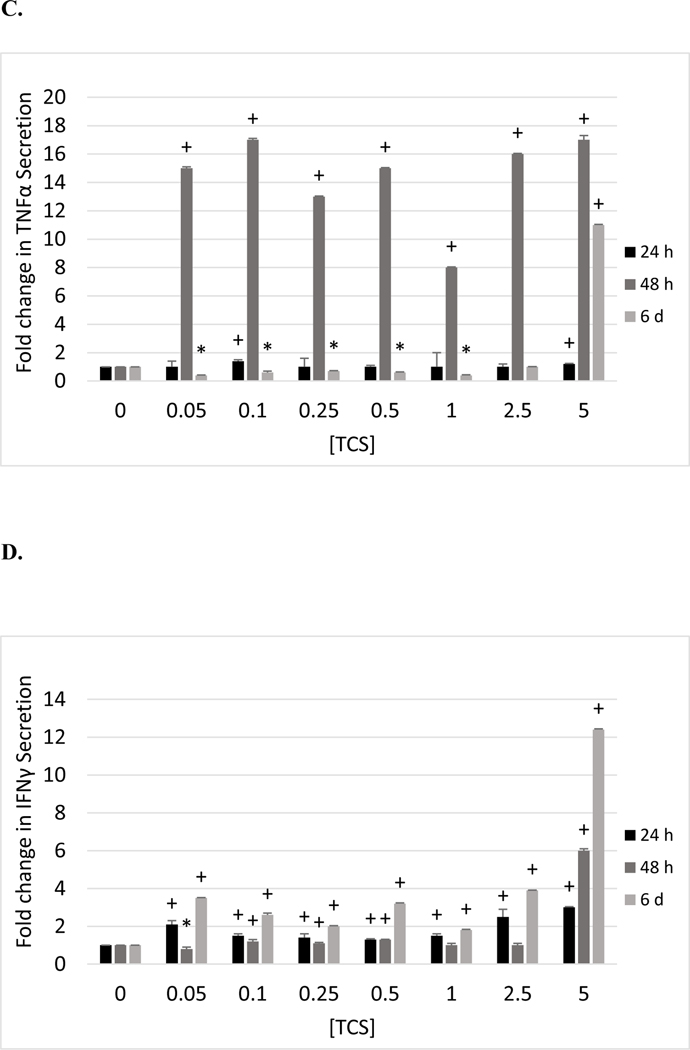
Effects of 24 h, 48 h and 6 days exposures to TCS on IL-1β, IL-6, TNFα, and IFNγ secretion from PBMCs. A) Fold changes (compared to control (0 μMTCS)) in IL-1β secretion in PBMCs exposed to 0–5 μM TCS (donor F214). B) Fold changes in IL-6 secretion in PBMCs exposed to 0–5 μM TCS (donor F214). C) Fold changes in TNFα secretion in PBMCs exposed to 0–5 μM TCS (donor F214). D) Fold changes in IFNγ secretion in PBMCs exposed to 0–5 μM TCS (donor F511). (+) indicates a significant increase (p<0.05) compared to control and (*) indicates a significant decrease compared to control.
Effects of TCS Exposure on Secretion of IL-6 by NK cells, MD-PBMCs, and PBMCs.
Table 4 shows the effects of exposing NK cells from 3 separate donors to TCS for 24 h, 48 h and 6 days on IL-6 secretion. Cells from all donors showed a significant increases in IL-6 secretion at one or more concentration after 24 h of exposure. Cells from donors F467 and F475 showed the largest fold increases at 24 h of exposure, 1.4 fold (0.05 μM) and 1.7 fold (0.1 μM), respectively. Cells from donor F461 showed maximal fold increase at 48 h of 2.2 fold (2.5 μM). Additionally, all donors demonstrated decreased IL-6 secretion in response to 5 μM TCS at each length of incubation. TCS-induced increases in NK cell IL-6 secretion were generally smaller than those seen for IL-1β, under the same conditions. For instance, cells from donor F475 showed a TCS-induced increase in IL-1β of 9.7 fold after 48 h of exposure at the 0.05 μM exposure (Table 1), while the TCS-induced increase in IL-6 was only 1.4 fold (Table 4). There were decreases in IL-6 secretion seen in cells from all donors at the 2.5 and 5 μM exposures at 24 h and 6 days and at 5 μM at 48 h.
Table 4.
Effects of 24 h, 48 h, and 6 days exposures to TCS on IL-6 secretion from NK cells.
| 24 h | IL-6 secretion in pg/mL (mean±S.D.) | ||
|---|---|---|---|
| [TCS] μM | F461 | F467 | F475 |
| 0 | 45142±470 | 22028±246 | 6449±153 |
| 0.05 | 24767±331* | 32043±605+ | 8652±372+ |
| 0.1 | 16906±419* | 25966±1263+ | 11145±262+ |
| 0.25 | 13156±146* | 24198±512+ | 8260±304+ |
| 0.5 | 22128±359* | 23609±372+ | 6623±240 |
| 1 | 33961±296* | 22710±619 | 6087±272 |
| 2.5 | 38419±569* | 13625±775* | 4957±115* |
| 5 | 37767±150* | 2679±827* | 435±314* |
| 48 h | IL-6 secretion in pg/mL (mean±S.D.) | ||
| [TCS] μM | F461 | F467 | F475 |
| 0 | 18325±260 | 27302±55 | 14486±265 |
| 0.05 | 25728±182+ | 27206±620 | 20787±324+ |
| 0.1 | 18381±188 | 28095±579 | 19835±442+ |
| 0.25 | 35144±237+ | 32063±715+ | 16803±1334 |
| 0.5 | 21992±182+ | 28476±719 | 21533±333+ |
| 1 | 21881±307+ | 25206±721* | 15343±126+ |
| 2.5 | 40228±251+ | 24984±810* | 7232±396* |
| 5 | 4630.6±87* | 2000±330* | 2882.5±306* |
| 6 d | IL-6 secretion in pg/mL (mean±S.D.) | ||
| [TCS] μM | F461 | F467 | F475 |
| 0 | 49128±105 | 44379±1221 | 24840±180 |
| 0.05 | 55489±120+ | 39621±784* | 29062±238+ |
| 0.1 | 48072±120* | 43258±1172 | 28744±552+ |
| 0.25 | 49906±501 | 47288±367+ | 26998±810+ |
| 0.5 | 44614±296* | 41894±548 | 27649±385+ |
| 1 | 47961±457* | 44712±1617 | 24538±378 |
| 2.5 | 44919±158* | 37561±1667* | 15094±120* |
| 5 | 44961±105* | 8651.5±533* | 12157±390* |
Values are mean±S.D. of triplicate determinations.
indicates a significant increase and
indicates a significant decreases in secretion compared to control cells, p<0.05
The effects of exposing MD-PBMCs from 4 individual donors to 0 to 5 μM TCS for 24 h, 48 h and 6 days on the secretion of IL-6 are shown in Table 5. Cells from all donors showed TCS-induced increases in secretion of IL-6 at two or more concentrations at 24 h. Maximal fold increases at 24 h were seen at different TCS concentrations among the different donors. In cells from donors F390 and F392, the largest increases in IL-6 secretion (3.1 and 3.0 fold, respectively) after 24 h were seen at 0.05 μM TCS. Cells from donors F401 and F451 showed the greatest increases of 2.0 and 4.1 fold after exposure to 5 μM TCS for 24 h. Increase in IL-6 secretion were stimulated at one or more TCS concentration after 48 h and 6 days. In contrast to NK cells, TCS-induced of IL-6 secretion was increased to a greater extent under a given condition in MD-PBMCs. For instance, 5 μM TCS at 24 h decreased IL-6 secretion in NK cells (Table 4) from all donors while the same treatment increased secretion from MD-PBMCS (Table 5).
Table 5.
Effects of 24 h, 48 h, and 6 days exposures to TCS on IL-6 secretion from monocyte-depleted PBMCs.
| 24 h | IL-6 secretion in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [TCS]μM | F390 | F392 | F401 | F451 |
| 0 | 12559±292 | 13847±314 | 21603±462 | 21712±367 |
| 0.05 | 38571±478+ | 41092±340+ | 31576±349+ | 63152±2566+ |
| 0.1 | 8044±81* | 8966±75* | 30536±487+ | 39525±927+ |
| 0.25 | 14337±491* | 16010±115+ | 31363±600+ | 61312±1250+ |
| 0.5 | 13074±427 | 14400±459 | 27176±560+ | 57312±625+ |
| 1 | 12044±214 | 13293±231 | 29256±788+ | 48005±244+ |
| 2.5 | 13378±405 | 144755±0 | 24296±288+ | 46859±1398+ |
| 5 | 14033±872 | 14928±75+ | 43576±1195+ | 89125±1281+ |
| 48 h | IL-6 secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F390 | F392 | F401 | F451 |
| 0 | 16128±362 | 15869±362 | 25600±1162 | 64077±2151 |
| 0.05 | 13226±2287 | 11660±45* | 29363±2134 | 63810±979 |
| 0.1 | 19736±1472+ | 19477±1472+ | 25481±1111 | 29914±975* |
| 0.25 | 18716±567+ | 18458±567+ | 26252±411 | 40610±724* |
| 0.5 | 17592±415+ | 17229±240+ | 24800±267 | 48729±948* |
| 1 | 17069±667 | 16810±667 | 26637±713 | 60551±1067 |
| 2.5 | 16024±1296 | 14458±770 | 22874±103 | 18092±51* |
| 5 | 17252±473+ | 16732±197+ | 35230±1030+ | 93173±2390+ |
| 6 d | IL-6 secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F390 | F392 | F401 | F451 |
| 0 | 32234±330 | 31418±1497 | 758±91 | 82949±311 |
| 0.05 | 36397±377+ | 36642±403+ | 3791±745+ | 69897±1761* |
| 0.1 | 38329±309+ | 38329±309+ | 3268±362+ | 76385±3867 |
| 0.25 | 35907±805+ | 35907±805+ | 3007±91+ | 79051±1351* |
| 0.5 | 32561±1034 | 32561±1034 | 2484±226+ | 67564±931* |
| 1 | 34737±1034+ | 34193±1506 | 2301±240+ | 88846±353+ |
| 2.5 | 32724±1644 | 32721±1640 | 2092±297+ | 70641±1974* |
| 5 | 27826±403* | 27820±402* | 1882±235+ | 95615±1533+ |
Values are mean±S.D. of triplicate determinations.
indicates a significant increases and
indicates a significant decrease in secretion compared to control cells, p<0.05
The effects of exposures to TCS on IL-6 secretion from PBMCs are shown in Table 6 and are very similar to those seen with MD-PBMCs. Cells from all donors showed increased IL-6 secretion in response to TCS after the 24 h, 48 h, or 6 day exposures. As with MD-PBMCs, the magnitude of the increases seen at a given TCS concentration varied from one donor to the next. The fold changes in IL-6 secretion stimulated by exposures to TCS in cells from donor F214 are shown in Figure 1B.
Table 6.
Effects of 24 h, 48 h, and 6 days exposures to TCS on IL-6 secretion from PBMCs.
| 24 h | IL-6 secretion in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [TCS]μM | F214 | F217 | F224 | F238 |
| 0 | 34790±355 | 126204±283 | 37857±1898 | 107320±551 |
| 0.05 | 42995±977+ | 134639±1158+ | 89639±3738+ | 106693±4563 |
| 0.1 | 34995±1081 | 4245±3558* | 96766±7446+ | 91268±5196* |
| 0.25 | 41764±3169 | 106830±1225* | 72815±6677+ | 96183±2396 |
| 0.5 | 42482±1616+ | 99646±1885* | 72233±5041+ | 100000±5979 |
| 1 | 42585±1891+ | 105306±1306* | 33542±2143 | 102771±5334 |
| 2.5 | 43405±1924+ | 117333±3885 | 64233±2052+ | 90013±181* |
| 5 | 67610±927+ | 114939±1496* | 157712±2925+ | 84502±91* |
| 48 h | IL-6 secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F214 | F217 | F224 | F238 |
| 0 | 55166±3291 | 52452±772 | 28827±118 | 186467±11449 |
| 0.05 | 17559±2366* | 50493±1401 | 66297±2171+ | 154217±1893* |
| 0.1 | 60636±1788 | 34982±618* | 58366±1708+ | 144633±3394* |
| 0.25 | 45456±1436* | 105132±1225+ | 16656±1788* | 188800±5629 |
| 0.5 | 52978±1202 | 45812±1177* | 16246±740* | 200000±1323 |
| 1 | 62072±2330+ | 41513±436* | 11186±1012* | 189717±5008 |
| 2.5 | 72875±3072+ | 53268±11018 | 20827±240* | 197300±3072 |
| 5 | 143713±2682+ | 72152±10235 | 9614±427* | 185383±4502 |
| 6 d | IL-6 secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F214 | F217 | F224 | F238 |
| 0 | 57240±3448 | 35223±137 | 10909±437 | 16440±489 |
| 0.05 | 86357±1144+ | 55762±4745+ | 10747±1307 | 20601±2322 |
| 0.1 | 101420±5923+ | 41634±2570+ | 34465±1009+ | 21773±902+ |
| 0.25 | 78789±4319+ | 46854±2492+ | 21172±426+ | 21040±863+ |
| 0.5 | 94357±2494+ | 38627±2409 | 23636±364+ | 18139±683+ |
| 1 | 60050±2208 | 43393±2578+ | 25495±280+ | 17641±396+ |
| 2.5 | 70645±499+ | 57691±260+ | 33172±1456+ | 24967±549+ |
| 5 | 182213±2219+ | 119762±10749+ | 57253±1330+ | 48791±4766+ |
Values are mean±S.D. of triplicate determinations.
indicates a significant increase and
indicates a significant decrease in secretion compared to control cells, p<0.05
Effects of TCS Exposure on Secretion of TNFα by PBMCs.
The effects of exposures to 0– 5 μM TSC for 24 h, 48 h, and 6 days on the secretion of TNFα from PBMCs are shown in Table 7. Cells from all donors showed statistically significant TCS-stimulated increases in TNFα secretion at the 5 μM exposure at 24 h. The fold increases stimulated by 5 μM TCS at 24 h were 1.2 (F214), 3.4 (F224), 6.4 (F509), and 1.6 (F510). The length of incubation that produced the largest TCS-induced fold increase in TNFα secretion varied among cells from different donors. Cells from donor F214 showed the greatest fold increase in TNFα secretion in response to TCS at 48 h, while those from F224, F509, and F510 were seen at 6 days, 24 h, and 6 days, respectively. There were TCS-stimulated increases in TNFα at one or more concentration of TCS at every length of incubation. This was similar to the effects of TSC on IL-1β from PBMCs, and unlike the effects on IL-6 secretion, where the length of incubation at which TCS-stimulated increases were seen varied among cells from different donors. Figure 1C illustrates the TCS- stimulated fold changes in TNFα secretion in PBMCs from donor F214.
Table 7.
Effects of 24 h, 48 h, and 6 days exposures to TCS on TNFα secretion from PBMCs.
| 24 h | TNFα secretion in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [TCS]μM | F214 | F224 | F509 | F510 |
| 0 | 669±29 | 290±7 | 670±108 | 595±10 |
| 0.05 | 1082±234 | 324±60 | 994±116+ | 519±47 |
| 0.1 | 917±80+ | 231±88 | 663±48 | 560±10* |
| 0.25 | 1327±425 | 351±27 | 885±66 | 621±4+ |
| 0.5 | 613±47 | 336±25 | 1041±59+ | 620±3+ |
| 1 | 1040±658 | 351±23+ | 1013±22+ | 622±8+ |
| 2.5 | 773±122 | 368±55 | 1387±81+ | 726±10+ |
| 5 | 800±24+ | 980±34+ | 4322±198+ | 941±13+ |
| 48 h | TNFα secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F214 | F224 | F509 | F510 |
| 0 | 40±7 | 153±7 | 218±2 | 243±18 |
| 0.05 | 593±69+ | 169±3+ | 450±46+ | 209±23 |
| 0.1 | 698±82+ | 115±12* | 287±8+ | 220±22 |
| 0.25 | 529±24+ | 140±4 | 419±24+ | 220±9 |
| 0.5 | 610±110+ | 157±22 | 281±3+ | 235±11 |
| 1 | 331±31+ | 138±5 | 345±15+ | 227±11 |
| 2.5 | 624±34+ | 173±10 | 243±12 | 238±30 |
| 5 | 681±41+ | 929±59+ | 890±8+ | 341±16+ |
| 6 d | TNFα secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F214 | F224 | F509 | F510 |
| 0 | 27±3 | 52±7 | 419±13 | 231±21 |
| 0.05 | 12±2* | 18±3* | 193±12* | 269±21 |
| 0.1 | 17±4* | 29±9* | 154±9* | 357±50+ |
| 0.25 | 18±1* | 32±2* | 305±23* | 248±33 |
| 0.5 | 15±1* | 26±3* | 344±8* | 510±29+ |
| 1 | 11±1* | 17±1* | 519±26+ | 522±40+ |
| 2.5 | 25±1 | 48±3 | 607±40+ | 514±6+ |
| 5 | 294±8+ | 651±18+ | 333±10* | 799±35+ |
Values are mean±S.D. of triplicate determinations.
indicates a significant increase and
indicates a significant decrease in secretion compared to control cells, p<0.05
Effects of TCS Exposure on Secretion of IFNγ by PBMCs.
Table 8 shows the effects of exposures to TSC for 24 h, 48 h, and 6 days on the secretion of IFNγ from PBMCs. TCS produced increases in IFNγ secretion in cells from all donors, at one or more concentration, at 48 h. Similar to what was seen with TCS stimulation of IL-6 secretion, the length of incubation at which TCS-induced increases in IFNγ secretion were seen varied among donor cells. For instance, cells from donor F217 and F511 showed TCS-stimulated increases in IFNγ secretion at each length of incubation, whereas those from F238 only demonstrated increased IFNγ secretion in response to TCS at 48 h and cells from F511 showed increased secretion at 24 h and 48 h. There was variation among donors as to the length of incubation and concentration of TSC at which the largest fold increase in TCS- stimulated IFNγ was seen. Maximum folds increases were: 26 fold at 0.1 μM TCS at 48 h for F217; 1.6 fold at 5 μM TCS at 48 h for F238; 1.7 fold at 0.1 μM TCS at 24 h for F509; and 12 fold at 5 μM at 6 days for F511. Alterations in secretion of IFNγ (fold change) caused by exposure of PBMCs from donor F511 to TCS are shown in Figure 1D.
Table 8.
Effects of 24 h, 48 h, and 6 days exposures to TCS on IFNγ secretion from PBMCs.
| 24 h | IFNγ secretion in pg/mL (mean±S.D.) | |||
|---|---|---|---|---|
| [TCS]μM | F217 | F238 | F509 | F511 |
| 0 | 103±3 | 122±7 | 74±3 | 26±1 |
| 0.05 | 141±21 | 124±9 | 84±9 | 55±6+ |
| 0.1 | 101±34 | 128±14 | 127±2+ | 38±2+ |
| 0.25 | 677±92+ | 111±15 | 81±8 | 37±4+ |
| 0.5 | 222±18+ | 84±6* | 108±2+ | 34±1+ |
| 1 | 120±14 | 104±4* | 93±9 | 39±3+ |
| 2.5 | 191±24+ | 75±6* | 99±8+ | 65±11+ |
| 5 | 67±5* | 86±1* | 36±3* | 78±1+ |
| 48 h | IFNγ secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F217 | F238 | F509 | F511 |
| 0 | 16±4 | 125±5 | 344±14 | 58±2 |
| 0.05 | 353±42+ | 140±8 | 273±6* | 46±3* |
| 0.1 | 418±50+ | 106±5* | 540±18+ | 72±4+ |
| 0.25 | 314±14+ | 99±6* | 481±19+ | 66±3+ |
| 0.5 | 364±67+ | 108±5* | 526±19+ | 77±1+ |
| 1 | 194±19+ | 122±6 | 383±21 | 57±5 |
| 2.5 | 372±21+ | 127±7 | 495±68 | 66±6 |
| 5 | 378±49+ | 206±3+ | 173±11* | 347±6+ |
| 6 d | IFNγ secretion in pg/mL (mean±S.D.) | |||
| [TCS]μM | F217 | F238 | F509 | F511 |
| 0 | 142±14 | 192±32 | 492±28 | 37±1 |
| 0.05 | 146±13 | 171±12 | 190±14* | 130±7+ |
| 0.1 | 186±4+ | 137±5 | 201±7* | 82±2+ |
| 0.25 | 185±8+ | 218±15 | 352±2* | 73±2+ |
| 0.5 | 173±14 | 210±26 | 295±12* | 120±11+ |
| 1 | 158±3 | 169±13 | 521±21 | 65±5+ |
| 2.5 | 184±3+ | 185±15 | 545±14 | 144±6+ |
| 5 | 202±2+ | 238±20 | 422±11* | 459±5+ |
Values are mean±S.D. of triplicate determinations.
indicates a significant increase and
indicates a significant decrease in secretion compared to control cells, p<0.05
p44/42 and p38 MAPK pathways in TCS-induced increases in IL-1β production.
Involvement of the p44/42 and p38 MAPK pathways in TCS-stimulated increases in IL-1β secretion were examined. TCS caused reproducible increases (in cells from all donors) in IL-1β secretion at the 1–5 μM concentrations after 24 h in PBMCs and thus, we went on to examine if MAPKs known to be involved in its (and other pro-inflammatory cytokines) production were needed for TCS to stimulate its secretion.
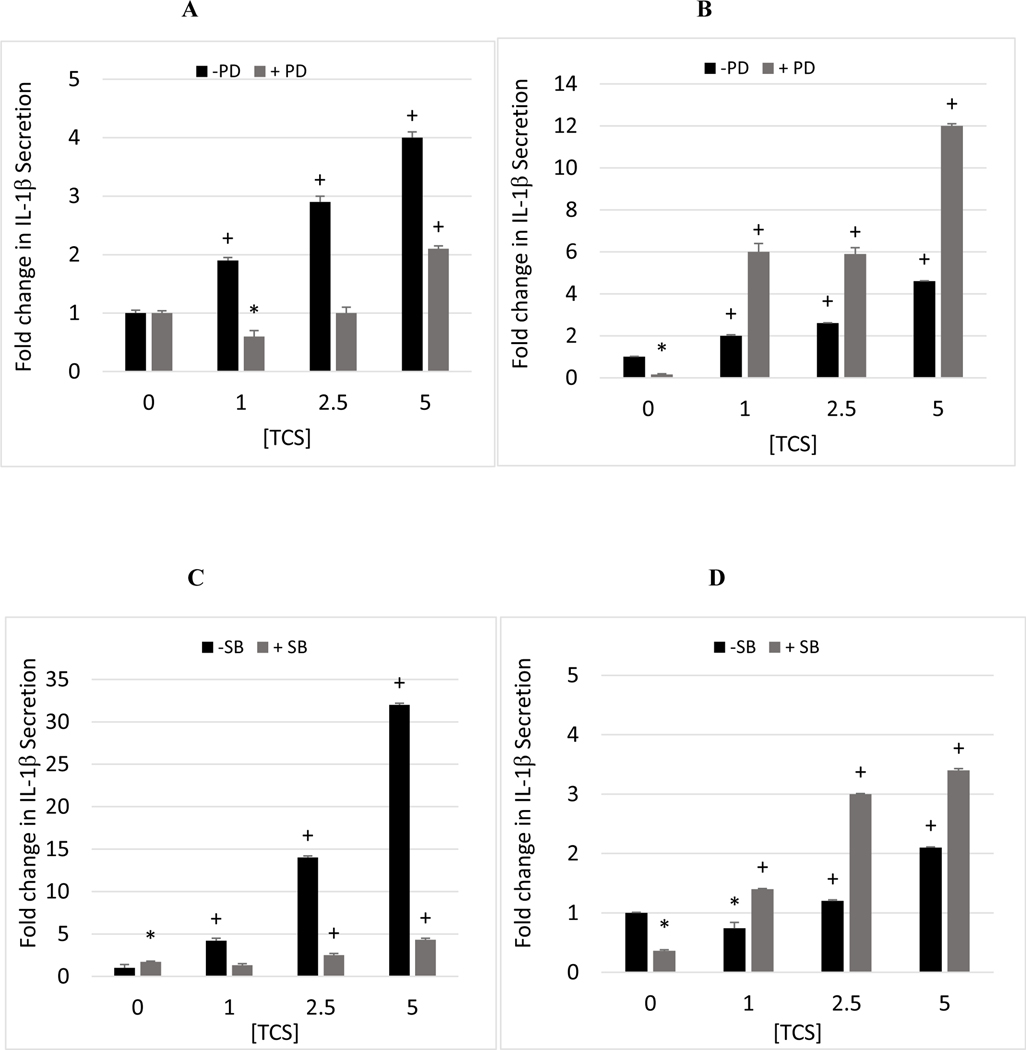
Cells from 4 of the 5 donors tested showed decreased TCS-stimulated secretion of IL-1β at 1 or more concentration of TCS when p44/42 was inhibited (Table 9). Cells from 3 of the 5 donors showed diminished TCS-induced IL-1β secretion at all concentrations tested when p44/42 was inhibited (F630, F631, F633). For cells from F630, IL-1β secretion was increased by 1.9, 2.9, and 4.0 fold at the 1, 2.5, and 5 μM exposures to TCS (Figure 2A). When p44/42 activity was inhibited prior to exposure to those same concentrations of TCS, there were no TCS-induced increases at 1 or 2.5 μM TCS and a 2.1 fold increase at 5 μM (Figure 2A). In contrast cells from donor F696 showed increased TCS stimulation of IL-1β secretion when p44/42 was blocked (Figure 2B). It is notable that there was a very large decrease in baseline IL-1β secretion in the cells from this donor, which was not as pronounced in the PBMCs from the other donors. These data indicate that p44/42 MAPK is utilized to a significant extent in TCS stimulation of IL-1β secretion, but this utilization is dependent on the baseline status of the donor cells.
Table 9.
Effect of MAPK pathway inhibitors on TCS-induced increases in IL-1β secretion from PBMCs.
| MEK1/2 Pathway Inhibitor (PD98059) |
| 24 h | TCS secreted in pg/mL (mean±S.D.) | ||||
|---|---|---|---|---|---|
| [TCS] μM | F630 | F631 | F633 | F696 | F697 |
| 0 | 350±19 | 2300±22 | 3470±42 | 1158±24 | 3032±14 |
| 0 + PD | 309±15 | 1163±25 | 2554±33 | 186±30 | 540±25 |
| 1 | 650±19+ | 3950±45+ | 3321±6+ | 2293±61+ | 2661±50 |
| 1 + PD | 199±27 | 804±31 | 1005±18+ | 1115±69+ | 682±31+ |
| 2.5 | 1012±30+ | 4346±64+ | 5526±99+ | 2960±18+ | 7473±64+ |
| 2.5 + PD | 306±40 | 1817±26+ | 1682±28+ | 1103±48+ | 907±7+ |
| 5 | 1390±19+ | 11642±26+ | 6696±24+ | 5341±18+ | 11019±26+ |
| 5 + PD | 640±15+ | 3550±22+ | 4429±122+ | 2254±20+ | 4057±19+ |
| p38 Inhibitor (SB202190) |
| 24 h | TCS secreted in pg/mL (mean±S.D.) | ||||
|---|---|---|---|---|---|
| [TCS] μM | F630 | F631 | F633 | F696 | F697 |
| 0 | 130±47 | 3119±46 | 1325±27 | 2734±34 | 4486±19 |
| 0 + SB | 216±14 | 952±23 | 593±30 | 1010±43 | 2244±14 |
| 1 | 554±36+ | 2352±68 | 2304±38+ | 2021±256 | 2994±112 |
| 1 + SB | 270±54 | 1093±8 | 1071±26+ | 1372±6+ | 2748±26+ |
| 2.5 | 1856±28+ | 4404±20+ | 6084±23+ | 3273±59+ | 5173±108+ |
| 2.5 + SB | 550±54+ | 1518±23+ | 2189±23+ | 2996±27+ | 4890±54+ |
| 5 | 4212±28+ | 10198±20+ | 8852±27+ | 5627±22+ | 8248±44+ |
| 5 + SB | 941±51+ | 3624±23+ | 3361±23+ | 3443±87+ | 6519±38+ |
Values are mean±S.D. of triplicate determinations.
indicates a significant increase in secretion compared to the appropriate control cells, p<0.05
Figure 2.
Effects of inhibiting p44/42 and p38 MAPKs on TCS-induced IL-1β secretion from PBMCs. A) Fold change in TCS-stimulated Il-1β secretion from PBMCs when p44/42 was inhibited by 50 μM PD98059 (donor F630). B) Fold change in TCS-stimulated Il-1β secretion from PBMCs when p44/42 was inhibited by 50 μM PD98059 (donor F696). C) Fold change in TCS-stimulated Il-1β secretion from PBMCs when p38 was inhibited by 25 μM SB202190 (donor F630). D) Fold change in TCS-stimulated Il-1β secretion from PBMCs when p38 was inhibited by 25 μM SB202190 (donor F696). (+) indicates a significant increase compared to control and (*) indicates a significant decrease compared to appropriate control (no TCS or no TCS+inhibitor).
Inhibiting p38 MAPK with SB202190 diminished the ability of TCS to increase IL-1β secretion in cells from 2 of the 5 donors tested (Table 9). For instance in cells from donor F630 TCS stimulated secretion of IL-1β by 4.2, 14, and 32 fold at the 1, 2.5, and 5 μM concentrations, however in the presence of SB202120 these same concentrations of TCS caused 1.3, 2.5, and 4.3 fold increases (Figure 2C). When p38 was inhibited in cells from the other 3 donors, TCS was as effective (or more effective) at increasing IL-1β secretion as when no inhibitor was present (Figure 2D).
DISCUSSION
When secreted in the absence of injury or infection, pro-inflammatory cytokines such as IL-1β, IL-6, TNFα, and IFNγ can cause chronic inflammation leading to a number of pathologies such as rheumatoid arthritis, Crohn’s disease, diabetes type II, multiple sclerosis, systemic lupus erythematosus, and cancer (Lucas and Hohlfeld., 1995 Landskron et al., 2014; Choy and Panayi, 2001; Dinarello, 2011; Tsalamandris et al., 2019; Elaraj et al., 2006; Jin et al., 1997; Lewis and Varghese, 2006; Voronov et al., 2002; Rubin et al., 2012; Straub et al., 2015; Gabay, 2006; Tanaka and Kishimoto, 2014; Coussens and Werb, 2002). Pro-inflammatory cytokines such as TNFα and IL-1β can further stimulate the production of IL-6 (Luo et al., 2003; Ostrowski et al., 1998b; 1998a; Tilg et al., 1997). TCS is an organochlorine environmental contaminant due to its use as an anti-microbial in a wide range of products and has been found in human blood and other tissues (Thompson et al., 2005; Weatherly and Gosse, 2017; Allmyr et al. 2006; Calafat et al., 2008). Ingestion of products containing TSC can lead to plasma levels of 1 μM (Sandborgh-England et al. 2006) and baseline levels can be in the range of 0.05 μM in individuals with no known exposure (Allmyr et al. 2006; 2008). Triclosan has been shown to disrupt estrogen and thyroid hormone responses in humans and other mammals (Wang et al., 2015; Wang and Tian, 2015; Feng et al., 2016; Ha et al., 2019) Two other organochlorine contaminants PCP and DDT have been shown to increase the secretion of IL-1β, IL-6, TNFα, and IFNγ (Martin and Whalen, 2017; Martin et al., 2019; Massawe et al., 2017). Here we examined whether TCS in a concentration range that could be seen in human plasma (as well as other tissues) had the ability to stimulate secretion of pro-inflammatory cytokines and thus have the potential to produce chronic inflammation.
Cell preparations of varying complexity were examined for the effects of TCS exposures on both IL-1β and IL-6 secretion. A preparation that was highly enriched in NK lymphocytes showed TCS-induced increases in IL-1β and IL-6 secretion in all donors at 24 h. This cell preparation also showed TCS-induced decreases in secretion of IL-1β at the 5 μM exposure at 48 h and of IL-6 secretion at 5 μM at 24 h, 48 h, and 6 days. In contrast, MD-PBMCs (a preparation of NK and T lymphocytes) from all donors showed increased IL-1β and IL-6 secretion when exposed to 5 μM TCS (as well as several other concentrations) for 24 h and/or 48 h. Exposure of the most complex cell preparation, PBMCs (NK lymphocytes, T lymphocytes, and monocytes), to TCS under the same exposure conditions produced results very similar to those seen with MD-PBMCs. Thus, there appears to be a difference in effect of the higher concentrations of TCS on IL-1β and IL-6 secretion depending on the composition of the cell preparation, with 5 μM and often 2.5 μM TCS causing decreased secretion of both cytokines in NK cells but increased secretion in preparations that contained T cells (MD-PBMCs) and T cells+monocytes (PBMCs). The more complex preparations may be more reflective of the situation in vivo and thus, TCS-induced stimulation of IL-1β and IL-6 secretion might be more likely than the decreases seen at those exposures. This difference between NK cells and the more complex preparations was also seen when measuring the effects of the organochlorine contaminant PCP on IL-1β and IL-6 secretion (Martin and Whalen, 2017; Martin et al. 2019). This suggests that the regulation of secretion and/or production of IL-1β and IL-6 from NK cells may be different than that of T cells and/or monocytes (Gaestel et al., 2009).
The most consistent and greatest stimulations of IL-1β and IL-6 occurred in the most complex cell preparation (PBMCs). Thus, effects of TSC on secretion of two additional pro-inflammatory cytokines were studied in PBMCSs. TCS was able to induce increases in TNFα secretion at a number of exposure concentrations at 24 h, 48 h, and 6 days (in cells from all donors tested). TCS-stimulated increases in IFNγ secretion were seen at 48 h across donors. The organochlorine PCP was also able to elevate secretion of IFNγ under some conditions (Massawe et al., 2017).
With cells from donor F214, we had adequate sample to monitor the levels of 3 of the cytokines (IL-1β, IL-6, and TNFα) allowing comparison of the effects of TCS on each of these cytokines in cells from the same donor (Figure 1A, 1B, 1C). TCS stimulated a much greater fold increase in IL-1β at each effective concentration and at each length of incubation than was seen with IL-6 or TNFα (in most cases). IL-1β secretion was increased 11, 16, and 30 fold by 5 μM TCS at 24 h, 48 h, and 6 days, while IL-6 was increased by 1.9, 2.6, and 3.2 fold under the same conditions. TNFα was increased by1.2, 17, and 11 fold by 5 μM TCS at 24 h, 48 h, and 6 days. Clearly IL-1β, which is considered a master regulator of inflammation (Dinarello, 2009), is increased to the greatest extent by TCS exposure, showing increased secretion as the length of exposure increases. TNFα is also increased to a significant extent by TCS however, the greatest fold increase occurred at 48 h and began to diminish at 6 days. Thus, 2 of the most potent pro-inflammatory cytokines are increased to the greatest extent by TCS.
Based on the fact that IL-1β was the cytokine whose secretion was increased to the greatest extent by TCS exposures, we investigated the role of p44/42 and p38 MAPKs in the mechanism of TCS-induction of its secretion. Both of these MAPKs are key components of the pathways that regulate immune cell secretion of pro-inflammatory cytokines (Gaestel, 2009). It appears that TCS-induced elevation of IL-1β involves activation of p44/42 and p38 under some conditions.
As shown here, TCS has the capacity to elevate the secretion of each of four pro-inflammatory cytokines. These increases were most pronounced with IL-1β, but were also seen with IL-6, TNFα, and IFNγ. As mentioned earlier, stimulation of pro-inflammatory cytokine secretion in the absence of appropriate stimuli (infection and/or injury) can lead to chronic “sterile” inflammation (Chen and Nunez, 2010), which can then trigger/exacerbate a number of disease states. The crucial role of IL-1β, IL-6, and TNFα in certain pathologies, including diabetes type II, rheumatoid arthritis, Crohn’s disease and cancer, has been demonstrated by the therapeutic action of blocking these cytokines (Ruscitti et al. 2019; Dinarello, 2011; Guo et al., 2012; Adegbola et al., 2018).
In summary, the results of the current study indicate that TCS, at biologically relevant concentrations, was able to stimulate secretion of IL-1β and IL-6 from NK-cells, lymphocytes (MD-PBMCs), and lymphocytes+monocytes (PBMCs) at one or more concentration within 24 h of exposure. TCS was also able to stimulate TNFα secretion from PBMCs within 24 h of exposure and IFNγ secretion within 48 h. TCS appeared to utilize p44/42 and p38 MAPKs as part of its mechanism of stimulating IL-1β secretion in a donor dependent manner. These data suggest that TCS has the capacity to stimulate inflammatory cytokine secretion from a complex mixture of immune cells and thus may contribute to chronic inflammation.
ACKNOWLEDGEMENT
Grants 5T34GM007663 and U54CA163066 from the National Institutes of Health
REFERENCES
- Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. 2018. Anti-TNF Therapy in Crohn’s Disease. International journal of molecular sciences 19: 2244. 10.3390/ijms19082244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandbourgh-Englund G. 2006. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ 372: 87–93. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G. 2009. Human exposure to triclosan via toothpaste does not change CYP3A4 activity or plasma concentrations of thyroid hormones. Basic & clinical pharmacology & toxicology, 105: 339–344. [DOI] [PubMed] [Google Scholar]
- Allmyr M, Harden F, Toms L-ML, Mueller JF, McLachlan MS, Adolfsson-Erici M, Sandborgh-Englund G. 2008. The influence of age and gender on triclosan concentrations in Australian human blood serum. Sci Total Environ 393:162–167. [DOI] [PubMed] [Google Scholar]
- Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov, E. 2006. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer and Metastasis Reviews 25: 387–408. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. 2001. “Inflammation and cancer: back to Virchow?” The Lancet 357: 539–545. [DOI] [PubMed] [Google Scholar]
- Billiau A, Matthys P. 2009. Interferon-γ: A historical perspective. Cytokine & Growth Factor Reviews 20: 97–113. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. 2008. Urinary concentrations of triclosan in the U. S. population 2003–2004. Environ. Health Persp 116: 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Nunez G. 2010. Sterile inflammation: sensing and reacting to damage. Nature Reviews/Immunology 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E, Panayi G. 2001. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. New England Journal of Medicine 344: 907–916. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. 2002. Inflammation and cancer. Nature 420: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwich L, Coma G, Peña R, Bellido R, Blanco EJ, Este JA, Borras FE, Clotet B, Ruiz L, Rosell A, Andreo F, Parkhouse RM, Bofill M. 2009. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology126:386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. 1996. Biologic Basis for IL-1 in Disease. Blood 87: 2095–2147. [PubMed] [Google Scholar]
- Dinarello CA. 2005. Blocking IL-1 in systemic inflammation. The Journal of Experimental Medicine 201: 1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. 2009. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annual Review of Immunology 27: 519–550. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. 2011. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117: 3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, Feldman ED, Turner EM, Alexander HR. 2006. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clinical Cancer Research 12: 1088–1096. [DOI] [PubMed] [Google Scholar]
- Feng Y, Zhang P, Zhang Z, Shi J, Jiao Z, Shao B. 2016. Endocrine Disrupting Effects of Triclosan on the Placenta in Pregnant Rats. PloS one, 11(5), e0154758. 10.1371/journal.pone.0154758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando M, Reyes J, Iannuzzi J, Leung G, McKay D. 2014. The Pro-Inflammatory Cytokine, Interleukin-6, Enhances the Polarization of Alternatively Activated Macrophages. PLoS ONE 9: p. e94188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C 2006. Interleukin-6 and chronic inflammation. Arthritis Research and Therapy 8: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaestel M, Kotlyarov A, Kracht M. 2009. Targeting innate immunity protein kinase signaling in inflammation. Nature Rev. Drug Disc. 8: 480–481. [DOI] [PubMed] [Google Scholar]
- Girart MV, Fuertes MB, Domaica CI, Rossi LE, Zwirner NW. 2007. Engagement of TLR3, TLR7, and NKG2D regulate IFN-γ secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J. Immunol. 179:3472–3479. [DOI] [PubMed] [Google Scholar]
- Goetz FW, Planas JV, MacKenzie S. 2004. Tumor necrosis factors. Develop. Comp. Immunol. 28: 487–497. [DOI] [PubMed] [Google Scholar]
- Guo Y, Xu F, Lu T, Duan Z, Zhang Z. 2012. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treatment Reviews 38: 904–910. [DOI] [PubMed] [Google Scholar]
- Ha NY, Kim DH, Ryu JY. 2019. Relationship between triclosan exposure and thyroid hormones: the Second Korean National Environmental Health Survey (2012–2014). Annals of occupational and environmental medicine, 31, e22. 10.35371/aoem.2019.31.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guidae A, Hastings HM, Andres J, Turkel G, Polverini PJ, Goldberg ID, Rosen EM. 1997. Expression of interleukin-1β in human breast carcinoma. Cancer 80: 421–434. [DOI] [PubMed] [Google Scholar]
- Jorcyk C, Tawara K, Oxford J. 2011. Clinical significance of interleukin (IL)-6 in cancer metastasis to bone: potential of anti-IL-6 therapies. Cancer Management and Research 3:177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemsley J 2014. Triclosan under the microscope. Chemical and Engineering News 92:10–13. [Google Scholar]
- Kux L 2016. “Federal Register Vol. 81 No.126 https://www.gpo.gov/fdsys/pkg/FR-2016-06-30/pdf/2016-15410.pdf [Google Scholar]
- Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. 2014. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A, Varghese S. 2006. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. Journal of Translational Medicine 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley RM, Killeen N, Lenardo MJ. 2001. “The TNF and TNF receptor superfamilies: integrating mammalian biology”. Cell 104: 487–501. [DOI] [PubMed] [Google Scholar]
- Lucas K, Hohlfeld R. 1995. Different aspects of cytokines in the immunopathology of multiple sclerosis. Neurology 45: S4–S5. [DOI] [PubMed] [Google Scholar]
- Luo G, Hershko DD, Robb BW, Wray CJ, Hasselgren PO. 2003. IL-1β stimulates IL-6 production in cultured skeletal muscle cells through activation of MAP kinase signaling pathway and NF-κB. American Journal of Physiology-Regulatory, Integrative, and Comparative 284: 1249–1254. [DOI] [PubMed] [Google Scholar]
- Macarthur M, Hold GL, El-Omar EM. 2004. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal maliganancy. Am. J. Physiol. Gastrointest. Liver Physiol. 286: G515–G520. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Whalen MM. 2017.Exposures to the Environmental Toxicants Pentachlorophenol (PCP) and Dichlorodiphenyltrichloroethane (DDT) Modify Secretion of Interleukin 1-Βeta (IL-1β) from Human Immune Cells. Archives of Toxicology 91: 1795–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ, Gabure S, Maise J, Snipes S, Peete M, Whalen MM. 2019. The Organochlorine Pesticides Pentachlorophenol and Dichlorodiphenyltrichloroethane Increase Secretion and Production of Interleukin 6 by Human Immune cells. Environ. Toxicol. Pharm. 72: 103263 11pages [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massawe R, Drabo L, Whalen M. 2017. Effects of pentachlorophenol and dichlorodiphenyltrichloroethane on secretion of interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) from human immune cells. Toxicology Mechanisms and Methods 27: 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, Weinauer F, Schmitz J, Illert WE. 2005. Filter Buffy Coats (FBC): A source of peripheral blood leukocytes recovered from leukocyte depletion filters. Journal of Immunological Methods 307: 150–166. [DOI] [PubMed] [Google Scholar]
- Morrall D, McAvoy D, Schatowitz B, Inauen J, Jacob M, Hauk A, Eckhoff W. 2004. A field study of triclosan loss rates in river water (Cibolo Creek, TX). Chemosphere 54: 653–660. [DOI] [PubMed] [Google Scholar]
- Moss T, Howes D, Williams FM. 2000. Percutaneous Penetration and Dermal Metabolism of Triclosan (2,4,4’-Trichloro-2’- hydroxydiphenyl Ether. Food and Chemical Toxicology 38: 361–370 [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK. 1998a. A trauma-like elevation in plasma cytokines in humans in response to treadmill running. Journal of Physiology 513: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. 1998b. Evidence that IL-6 is produced in skeletal muscle during intense long-term muscle activity. Journal of Physiology. (London) 508: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, Shaker A, Levin MS. 2012. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Frontiers in immunology 3: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscitti P, Masedu F, Alvaro S, Airò P, Battafarano N, Cantarini L, Cantatore FP, Carlino G, D’Abrosca V, Frassi M, Frediani B, Iacono D, Liakouli V, Maggio R, Mulè R, Pantano I, Prevete I, Sinigaglia L, Valenti M, Viapiana O, Cipriani P, Giacomelli R. 2019. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial. PLoS Med. 16(9):e1002901. doi: 10.1371/journal.pmed.1002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. 2006. Pharmacokinetics of Triclosan Following Oral Ingestion in Humans. J. of Toxic. and Env. Health Part A, 69: 1861–1873 [DOI] [PubMed] [Google Scholar]
- Straub RH, Cutolo M, Pacifici R. 2015. Evolutionary medicine and bone loss in chronic inflammatory diseases--A theory of inflammation-related osteopenia. Seminars in arthritis and rheumatism 45: 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kishimoto T. 2014. The Biology and Medical Implications of Interleukin-6. Cancer Immunology Research 2:288–294. [DOI] [PubMed] [Google Scholar]
- Thompson A, Griffin P, Stuetz R, Cartmell E. 2005. The fate and removal of triclosan during wastewater treatment. Water Environ Res. 77:63–67. [DOI] [PubMed] [Google Scholar]
- Tilg H, Dinarello CA, Mier JW. 1997. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunology Today 18: 428–432. [DOI] [PubMed] [Google Scholar]
- Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D. 2019. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. European cardiology 14: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajdic CM, van Leeuwen MT. 2009. Cancer incidence and risk factors after solid organ transplantation. Int. J. Cancer 125: 1747–1754. [DOI] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. 2002. IL-1 is required for tumor invasiveness and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America 100: 2645–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen X, Feng X, Chang F, Chen M, Xia Y, Chen, L. 2015. Triclosan causes spontaneous abortion accompanied by decline of estrogen sulfotransferase activity in humans and mice. Scientific reports, 5, 18252. 10.1038/srep18252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CF, Tian Y. 2015. Reproductive endocrine-disrupting effects of triclosan: Population exposure, present evidence and potential mechanisms. Environ Pollut. 206:195–201. [DOI] [PubMed] [Google Scholar]
- Weatherly LM, Gosse JA. 2017. Triclosan exposure, transformation, and human health effects. Journal of toxicology and environmental health. Part B, Critical reviews, 20(8): 447–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Wei L, Shi Y, Zhang J, Wu Q, Shao B. 2016. Chinese population exposure to triclosan and triclocarban as measured via human urine and nails. Environ Geochem Health 38:1125–1135 [DOI] [PubMed] [Google Scholar]