Abstract
Background and Aims:
Helicobacter pylori eradication and endoscopic surveillance of gastric precancerous lesions are strategies to reduce gastric cancer (GC) risk. This study is the longest prospective cohort of an H. pylori eradication trial in a Hispanic population.
Methods:
800 adults with precancerous lesions were randomized to anti-H. pylori treatment or placebo. Gastric biopsies at baseline, 3, 6, 12, 16, and 20 years were assessed by our Correa histopathology score. A generalized linear mixed model with a subject level random intercept was used to estimate the effect of H. pylori status on the score over time. Logistic regression models were used to estimate progression by baseline diagnosis, and GC risk by intestinal metaplasia (IM) subtype and anatomic location. ‘
Results:
356 individuals completed 20 years of follow-up. Anti-H. pylori therapy (intention-to-treat) reduced progression of the Correa score (odds ratio OR, 0.59, 95% confidence interval, CI, 0.38–0.93). H. pylori-negative status had a beneficial effect on the score over time (P=0.036). Among individuals with IM (including indefinite for dysplasia) at baseline, incidence rates per 100 person-years were 1.09 (95% CI, 0.85–1.33) for low-grade/high-grade dysplasia and 0.14 (95% CI, 0.06–0.22) for GC. Incomplete-type (vs. complete-type) IM at baseline presented higher GC risk (OR, 13.4; 95% CI, 1.8–103.8). Individuals with corpus (vs. antrum-restricted) IM showed an OR of 2.1 (95% CI, 0.7–6.6) for GC.
Conclusions:
In a high GC risk Hispanic population, anti-H. pylori therapy had a long-term beneficial effect against histological progression. Incomplete IM is a strong predictor of GC risk.
Keywords: H. pylori, multifocal atrophic gastritis, intestinal metaplasia, dysplasia
Short summary
In a Hispanic population, H. pylori eradication reduced the progression of gastric precancerous lesions during a 20-year follow-up period. Incomplete-type intestinal metaplasia is a high-risk factor for gastric cancer development.
INTRODUCTION
Gastric cancer is the third leading cause of cancer-related mortality worldwide.1 The most common type is intestinal non-cardia adenocarcinoma (hereafter referred to as GC). Most GC cases develop following a sequence of histological lesions known as the Correa cascade: non-atrophic gastritis (NAG), multifocal atrophic gastritis without intestinal metaplasia (MAG), intestinal metaplasia (IM), and dysplasia.2,3 MAG, IM, and dysplasia are precancerous stages with progressively increasing risks of GC.4–6
Helicobacter pylori (H. pylori) infection is the main driver of the precancerous cascade and the most important known etiological factor for GC.7 Eradication therapy is indicated in symptomatic individuals,8–10 and increasing evidence supports its effect in reducing GC incidence and mortality.11 Furthermore, GC screening programs and surveillance of individuals with precancerous lesions represent excellent opportunities for decreasing GC burden.10,12 Five-year survival rates from GC remain ~30% in most Western countries,13 reflecting the typically advanced stage at diagnosis. Much better survival rates in Japan and South Korea13 are partially attributed to national screening programs with more frequent detection of early-stage GC.12
In the US, the prevalence of gastric precancerous lesions14,15 and the incidence rates of GC16,17 are higher in racial/ethnic minorities compared to non-Hispanic whites. The need for GC control and prevention strategies in high-risk US populations is a focus of increasing interest and controversy.12,18–20
In Colombia, GC ranks first in cancer mortality in males.1 In the 1990s, our team conducted a randomized chemoprevention trial in individuals with gastric precancerous lesions from a high GC risk region in Colombia. Results of the 6-year trial showed a beneficial effect of either H. pylori eradication or antioxidant supplementation on the histological progression of lesions.21 After 12 years of follow-up, either anti-H. pylori treatment allocation or H. pylori clearance were significantly associated with histological regression (measured by the Correa histopathology score).22 Our analysis at the 16-year follow-up demonstrated that cumulative exposure to H. pylori was directly associated with histological progression.23
Although GC incidence rates continue to decrease globally, the number of new cases is predicted to keep rising due to aging and growth of high-risk populations.24 Long-term cohorts of individuals with precancerous lesions are increasingly needed for the formulation of appropriate GC surveillance guidelines. In this report, we present the histological evolution of H. pylori-associated precancerous lesions over 20 years after our eradication trial.
MATERIALS AND METHODS
Study participants, intervention, and follow-up
Adult volunteers (n=1,219) from two Colombian towns in a high GC risk area underwent upper gastrointestinal endoscopy with biopsy mapping to determine eligibility. Individuals with precancerous lesions (MAG, IM, or dysplasia), were invited to participate in a double-blind trial aimed to evaluate the effects of anti-H. pylori therapy and antioxidants in preventing histological progression. Participants were randomized to receive 2 weeks of anti-H. pylori therapy with or without beta-carotene and/or ascorbic acid supplementation for 6 years, or corresponding placebos. At the end of the 6-year trial anti-H pylori therapy was offered to untreated individuals. No other intervention was offered. The cohort was then followed with endoscopic visits at 12, 16, and 20 years after enrollment. After each visit, participants were referred with the pathology reports to their primary care physicians. Additional anti-H. pylori treatments were not part of the study protocol and were not recorded. Results at 6, 12, and 16 years of follow-up are published.21–23 The current analysis comprises 800 individuals, including five additional individuals compared to the last 2 reports. Additional information and participant flow chart are in Supplementary Methods and Supplementary Figure 1. The Institutional Review Boards of Louisiana State University Health Sciences Center and Vanderbilt University, and the Committees on Ethics of Universidad del Valle and Hospital Departamental de Nariño in Colombia approved the study protocol. Written informed consent was obtained from all participants.
Histopathology and H. pylori infection assessment
Upper gastrointestinal tract endoscopies using conventional white light at all visits were performed by one of two experienced gastroenterologists. Participants were required to stop proton pump inhibitors and antimicrobials at least two weeks prior to each endoscopic procedure throughout the study. A biopsy protocol was applied at each endoscopy: 2 antral biopsies (lesser and greater curvature), 1 from the incisura, and 1 from the corpus (anterior wall). Additional biopsies were obtained from suspicious lesions, and all samples were incorporated into the histological analysis using H&E-stained sections. A modified Steiner technique was used to detect H. pylori in sections from all biopsies. IM was classified as complete (denoting only complete type) or incomplete (denoting incomplete or mixed) types (see Supplementary Methods). The anatomic location of IM was classified as antrum (denoting IM restricted to antrum and/or incisura) or corpus (denoting corpus IM regardless of antral IM). All histological preparations were independently assessed by 2 experienced GI pathologists (MBP and JCB) blinded to treatment assignment and prior histological diagnoses. Disagreement was solved between the 2 pathologists by reviewing the cases on a double-headed microscope. If the disagreement remained, the final decision was made by a third senior GI pathologist (PC or MKW).
Global histological diagnosis
Following international guidelines,25,26 global diagnoses were assigned numerical values along the carcinogenesis cascade: 1=normal, 2=NAG, 3=MAG, 4=IM, 5=dysplasia, and 6=GC. The diagnosis of MAG included the loss/shrinkage of glands and the presence of pseudopyloric metaplasia. The diagnosis of dysplasia comprised the categories indefinite for dysplasia (ID), low-grade (LGD), and high-grade (HGD). The most advanced lesion observed in the set of biopsies of an individual at each endoscopy was used as the global diagnosis. Because ID is not considered definite dysplasia in clinical settings, ID cases were grouped with IM in some of our analyses to make our results comparable with those of other studies.
Correa Histopathology Scoring System
To better discriminate the degree of severity of MAG, IM, and dysplasia, we developed and used this scoring system in previous reports.22,23 Referred to hereafter as the Correa score (range 1–6), values 1, 2, and 6 correspond to the global diagnoses of normal, NAG, and GC, respectively. Values for MAG (range 3.25–4.00) reflect the extent of atrophy without IM; values for IM (range 4.30–5.00) combine IM extent and type. Values for dysplasia were ID=5.25, LGD=5.50, and HGD=5.75 (see further details in Supplementary Methods and Supplementary Table 1).
Operative Link for Gastritis Assessment (OLGA) and Operative Link for Gastric IM (OLGIM) systems
Baseline and 20-year follow-up biopsy sets from individuals with 16 and 20-year visits were classified by a single pathologist (MBP) according to the OLGA and OLGIM staging systems,27,28 whenever possible. Semiquantitative scoring of overall gastric atrophy (combining all variants of atrophy, i.e., non-metaplastic atrophy, IM, and pseudopyloric metaplasia, for OLGA) and scoring of IM alone (for OLGIM) were performed in all antrum, incisura, and corpus biopsies. Antrum and incisura scores were combined, representing the antral compartment. Stages were obtained by combining antrum and corpus scores.27,28
Statistical analysis
Chi-square test was used to determine differences of IM subtype or anatomic location and histological outcomes. Multivariable logistic regression models were used to estimate progression (vs. regression/no change) of the Correa score between baseline and 20 years of follow-up by diagnosis at baseline. For this analysis, progression was defined as an increment of at least 0.1 units in the Correa score between baseline and 20 years. A generalized linear mixed model with a subject random intercept and a logistic link function was used to estimate the effect of the H. pylori status on the Correa score over time. In this model, we tested a multiplicative interaction between H. pylori status and time. Incidence rates of IM, LGD/HGD, and GC by person-years (PY) of follow-up were calculated; individuals with different outcomes at different time points were included in more than one analysis. For each analysis, individuals were censored at the time of outcome or last endoscopy. A Poisson mixed model with a subject level random intercept was used to estimate progression to GC by OLGA and OLGIM stages (high-risk III/IV vs. others) at baseline. These analyses were adjusted for time and change of H. pylori status. Multivariable logistic regression models were used to estimate GC risk by IM subtype (incomplete vs. complete) and IM anatomic location (corpus vs. antrum-restricted) at baseline. Analyses including anti-H. pylori therapy were by intention-to-treat (ITT). All P-values are two-sided and considered statistically significant if P<0.05. All statistical analyses were performed using STATA 16 software (StataCorp, College Station, TX, USA).
RESULTS
Out of the 800 randomized individuals included in this analysis, 356 had 20-year follow-up (participant flow chart in Supplementary Figure 1). Characteristics of the participants at each visit are presented in Table 1.
Table 1.
Demographic characteristics and H. pylori status of participants at each visit, and anti-H. pylori therapy
| Characteristics | Baseline n=800 | 6 years n=630 | 12 years n=612 | 16 years n=456 | 20 years n=356 |
|---|---|---|---|---|---|
| Age in years, mean ± SD | 51 ± 9 | 57 ± 8 | 62 ± 8 | 67 ± 8 | 69 ± 8 |
| Males, n (%) | 364 (46) | 284 (45) | 274 (45) | 205 (45) | 160 (45) |
| H. pylori positive, n (%) | 776 (97) | 345 (55) | 322 (53) | 201 (44) | 146 (41) |
| Anti-H. pylori therapy†, n (%) | 396 (50) | 198 (31) | n/a | n/a | n/a |
50% of individuals were randomized to receive eradication therapy at baseline and the other 50% were offered it at the end of the 6-year trial.
SD, standard deviation; n/a, not applicable.
Global histological diagnosis at baseline and 20 years
Diagnoses at 20-year biopsies (or GC outcome at any time during follow-up) vs. baseline are shown in Table 2. Individuals with baseline MAG had a higher rate of progression (39%) to a more advanced diagnosis than IM (25%) or dysplasia (13%). Most individuals with IM remained with the same diagnosis (55%), and individuals with dysplasia regressed the most (53%).
Table 2.
Baseline diagnosis and outcome of individuals who attended the 20-year endoscopy or developed GC at any time
| Diagnosis at 20 years |
GC at earlier time points | Total | Regression n (%) | No change n (%) | Progression n (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | NAG | MAG | IM | Dysplasia | GC | |||||
| MAG | 20 | 47 | 32 | 10 | 0 | 0 | 109 | 20 (18) | 47 (43) | 42 (39) |
| IM | 11 | 34 | 125 | 50 | 0 | 8 | 228 | 45 (20) | 125 (55) | 58 (25) |
| Dysplasia | 0 | 2 | 14 | 10 | 1 | 3 | 30 | 16 (53) | 10 (33) | 4 (13) |
| Total | 31 | 83 | 171 | 70 | 1 | 11 | 367 | 81 (22) | 182 (50) | 104 (28) |
NAG, non-atrophic gastritis; MAG, multifocal atrophic gastritis without intestinal metaplasia; IM, intestinal metaplasia.
Dysplasia includes indefinite for dysplasia, low-grade, and high-grade. Gray cells denote the numbers of individuals who had the same histological diagnosis at baseline and at 20 years of follow-up. On the left side of the diagonal are individuals who regressed; on the right, those who progressed.
With the exception of GC, all histological diagnoses from endoscopies at intermediate time points were excluded from this table.
IM subtype by anatomic location at baseline
A total of 345 baseline biopsy sets with at least a global diagnosis of IM (IM, ID, or LGD) were classified by IM subtype and location. Out of 256 (74%) individuals with antrum-restricted IM at baseline, 113 (44%) had incomplete IM. Out of 89 individuals with corpus IM, 71 (80%) had IM in both antrum and corpus. Of these 71 individuals, incomplete type was observed in the antrum of 54% of individuals and in the corpus of 27%.
IM subtype and anatomic location in individuals with baseline IM or ID (IM/ID) and 20-year outcome
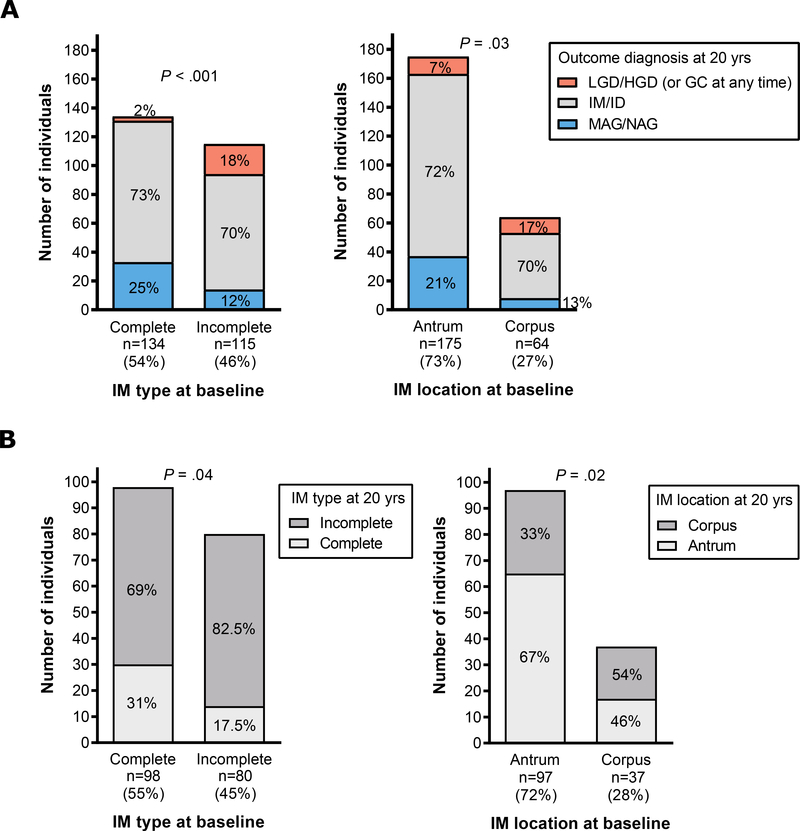
Outcome diagnosis by baseline IM subtype and location is shown in Figure 1A. A higher proportion of individuals with incomplete-type IM progressed (to LGD/HGD at 20 years or to GC at any time) compared to those with complete-type (18% vs. 2%, P<0.001; left panel). A higher proportion of individuals with corpus IM progressed compared to those with antrum-restricted IM (17% vs. 7%, P=0.03; right panel). Figure 1B shows changes in IM type and location in individuals with IM/ID at both baseline and 20 years. Sixty-nine percent of individuals with complete IM changed to incomplete-type at 20 years, while only 17.5% of those with incomplete-type changed to complete-type (P=0.04; left panel). Thirty-three percent of individuals with antrum-restricted IM at baseline had corpus IM at 20 years, while 46% of those with corpus IM at baseline had antrum-restricted IM at 20 years (P=0.02; right panel).
Figure 1.
Outcomes at 20 years in individuals with IM or ID (IM/ID) at baseline. A) Diagnosis at 20 years (or GC development at any time) by IM type and anatomic location at baseline. On the left, a higher proportion of individuals with incomplete-type IM at baseline progressed to a more advanced diagnosis compared to those with complete-type at baseline (18% vs. 2%; P<0.001). On the right, a higher proportion of individuals with corpus IM at baseline progressed to a more advanced diagnosis compared to those with antrum-restricted IM (17% vs. 7%, P=0.03). B) Individuals with a diagnosis of IM/ID at both baseline and 20-year biopsies. The left panel shows that 69% of individuals with complete IM at baseline changed to incomplete-type at 20 years, while only 17.5% of those with incomplete-type changed to complete-type (P=0.04). On the right, 33% of individuals with antrum-restricted IM at baseline had corpus IM at 20 years, while 46% of those with corpus IM at baseline had antrum-restricted IM at 20 years (P=0.02). Lower counts in right panels are due to the lack of adequate corpus representation in some biopsy sets.
OLGA and OLGIM stages at baseline and 20-year outcome
After excluding individuals with LGD/HGD at baseline, OLGA and OLGIM stages at baseline and at 20 years (or GC diagnosis at any time) could be assessed for 260 and 284 individuals, respectively (Tables 3 and 4). Overall, using OLGA, 54% of individuals progressed and 27% remained stable. Using OLGIM, 37% of individuals progressed and 45% remained stable. The greater progression observed using OLGA reflects in part that this system includes all histological variants of atrophy (non-metaplastic atrophy, IM, and pseudopyloric metaplasia), which may expand simultaneously, while OLGIM only evaluates IM.
Table 3.
OLGA stage at baseline and outcome of individuals who attended the 20-year endoscopy or developed GC at any time
| Stage or LGD/HGD diagnosis at 20-year of follow-up | GC at any time during follow-up | Total | Regression n (%) | No change n (%) | Progression n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline stage | 0 | I | II | III | IV | LGD | HGD | |||||
| I | 20 | 39 | 30 | 20 | 18 | 1 | 0 | 0 | 128 | 20 (16) | 39 (30) | 69 (54) |
| II | 5 | 8 | 10 | 22 | 15 | 2 | 0 | 1 | 63 | 13 (21) | 10 (16) | 40 (63) |
| III | 0 | 4 | 4 | 6 | 13 | 4 | 1 | 1 | 33 | 8 (24) | 6 (18) | 19 (58) |
| IV | 0 | 2 | 2 | 5 | 14 | 4 | 0 | 9 | 36 | 9 (25) | 14 (39) | 13 (36) |
| Total | 25 | 53 | 46 | 53 | 60 | 11 | 1 | 11* | 260 | 50 (19) | 69 (27) | 141 (54) |
Although 12 individuals developed GC, one of them had LGD at baseline, and is not included in this table. The OLGA system is not intended for staging individuals with LGD or HGD; however, as an observation of interest, the baseline OLGA stage in this patient was III.
Gray cells denote numbers of individuals with the same OLGA stage at baseline and 20 years of follow-up. On the left side of the diagonal are individuals who regressed; on the right, those who progressed.
Table 4.
OLGIM stage at baseline and outcome of individuals who attended the 20-year endoscopy or developed GC at any time
| Stage or LGD/HGD diagnosis at 20-year of follow-up | GC at any time during follow-up | Total | Regression n (%) | No change n (%) | Progression n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline stage | 0 | I | II | III | IV | LGD | HGD | |||||
| 0 | 57 | 23 | 8 | 2 | 0 | 1 | 0 | 0 | 91 | - | 57 (63) | 34 (37) |
| I | 32 | 59 | 22 | 12 | 5 | 3 | 1 | 2 | 136 | 32 (24) | 59 (43) | 45 (33) |
| II | 4 | 9 | 7 | 3 | 3 | 3 | 0 | 2 | 31 | 13 (42) | 7 (23) | 11 (35) |
| III | 0 | 0 | 3 | 1 | 5 | 3 | 0 | 5 | 17 | 3 (18) | 1 (6) | 13 (76) |
| IV | 0 | 1 | 0 | 1 | 4 | 1 | 0 | 2 | 9 | 2 (22) | 4 (45) | 3 (33) |
| Total | 93 | 92 | 40 | 19 | 17 | 11 | 1 | 11* | 284 | 50 (18) | 128 (45) | 106 (37) |
Although 12 individuals developed GC, one of them had LGD at baseline, and is not included in this table. The OLGIM system is not intended for staging individuals with LGD or HGD; however, as an observation of interest, the baseline OLGIM stage in this patient was II.
Gray cells denote numbers of individuals with the same OLGIM stage at baseline and 20 years of follow-up. On the left side of the diagonal are individuals who regressed; on the right, those who progressed.
Progression of the Correa score at 20 years
Multivariable logistic regression models were used to estimate progression rates between baseline and 20 years by baseline global diagnosis, adjusting for age at enrollment, interventions, sex, and last H. pylori status (Table 5). Progression rates were similar for individuals with MAG or IM at baseline (59.5% and 61.3% respectively), and significantly lower (3.95%; P<0.001) for those with dysplasia. Anti-H. pylori treatment (ITT) had a significant effect in reducing the risk of progression by 41% (OR, 0.59; P=0.023) compared to placebo. Individuals who were H. pylori-positive (vs. H. pylori-negative) at the 20-year visit had a two-fold risk (OR, 1.92; P=0.007) of progression of the score. None of the other interventions or demographics had a significant effect on progression.
Table 5.
Estimated rates of progression (vs. regression/no change), adjusted odds ratios and 95% confidence intervals of the Correa score between baseline and 20 years by baseline diagnosis
| Characteristics | Progression rate % (95% CI) | Adjusted OR (95% CI) | P values (95%CI) |
|---|---|---|---|
| Baseline diagnosis | |||
| MAG | 59.5 (50.5 – 68.6) | 1.00 | - |
| IM | 61.3 (54.9 – 67.6) | 1.08 (0.66 – 1.74) | 0.77 |
| Dysplasia | 3.95 (0 – 11.6) | 0.02 (0.003 – 0.20) | <0.001 |
| Interventions (main effects) | |||
| None (placebos) | 63.1 (53.8 – 72.3) | 1.00 | - |
| H. pylori treatment | 51.1 (44.2 – 58.0) | 0.59 (0.38 – 0.93) | 0.023 |
| Beta-carotene | 55.3 (48.7 – 61.9) | 0.88 (0.56 – 1.38) | 0.57 |
| Ascorbic acid | 57.6 (50.8 – 64.5) | 1.09 (0.69 – 1.71) | 0.71 |
| Age at enrollment (years) | |||
| ≤45 | 55.1 (47.0 – 63.1) | 1.00 | |
| 46–55 | 58.1 (50.8 – 65.5) | 1.15 (0.69 – 1.92) | 0.58 |
| ≥56 | 56.3 (45.7 – 66.9) | 1.06 (0.57 – 1.96) | 0.85 |
| Sex | |||
| Female | 57.0 (50.6 – 63.4) | 1.00 | - |
| Male | 56.1 (48.8 – 63.5) | 0.97 (0.61 – 1.53) | 0.89 |
| H. pylori at 20 years | |||
| Negative | 50.8 (44.3 – 57.3) | 1.00 | - |
| Positive | 64.8 (57.5 – 72.0) | 1.92 (1.20 – 3.07) | 0.007 |
OR, odds ratio; CI, confidence interval; MAG, multifocal atrophic gastritis without intestinal metaplasia; IM, intestinal metaplasia. Dysplasia includes indefinite for dysplasia, low-grade, and high-grade.
Effect of H. pylori status on the Correa score over time (including all endoscopic visits)
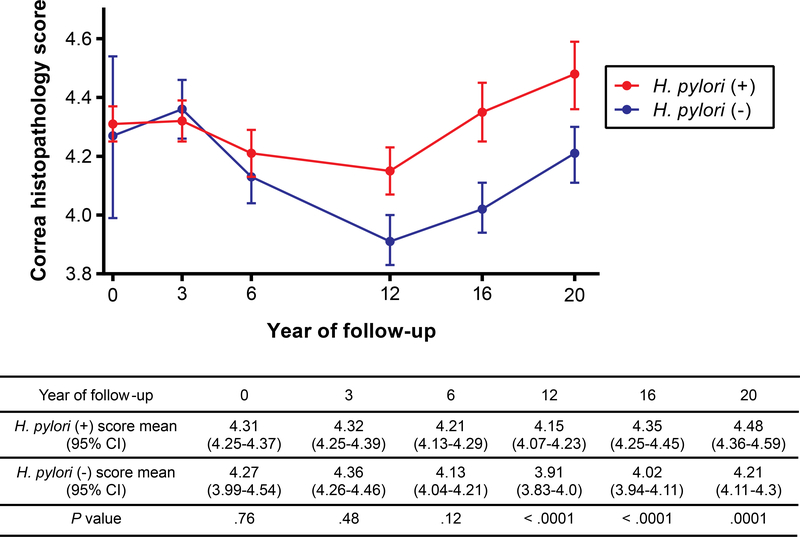
During 20 years, a total of 3,524 endoscopic procedures (mean 4.4 per individual) were performed, with a mean follow-up time of 12.6±4.4 years (median 15.1 years; interquartile range 5.9–15.8). As shown in Table 1, 97% of participants were H. pylori-positive at baseline, 55% at the end of the 6-year trial, and 41% at the 20-year visit. A generalized linear mixed effects model, including all individuals, and accounting for the loss of participants over time, showed a significant interaction between H. pylori status and time (P=0.036). For both H. pylori-positive and -negative individuals, the Correa score significantly changed over time (Figure 2). There was no significant effect of the H. pylori status on the score during the first 3 years of follow-up. Scores decreased from 3 to 12 years and increased afterwards. Notably, the scores between groups diverged after 6 years, remaining consistently lower in H. pylori-negative individuals. Age and sex did not modify the H. pylori effect on the score. At year 20, the highly significant H. pylori effect did not depend on clearing the bacterium at a particular age, as long as there was a minimum of 6 years free of infection.
Figure 2.
Correa histopathology score (mean and 95% CIs) by H. pylori status over time.
Effect of H. pylori status at the time of the 20-year biopsies on the Correa score
H. pylori status at the last endoscopy had a significant effect on the score. Individuals who were H. pylori-negative at 20 years had a net regression of −0.12 (95% CI, −0.01 to −0.23; P=0.03) units compared to baseline, while those who were still infected at year 20 had a net progression of 0.28 (95% CI, 0.41 to 0.14; P<0.001) score units.
Reversibility of MAG or IM after H. pylori eradication
Significant regression of MAG to NAG was achieved after 6 years free of infection. Among individuals with MAG at baseline, those who were H. pylori-negative (vs. H. pylori-positive) at both 6 and 12 years, showed a decline of a full unit (−1.01, 95% CI, −0.55 to −1.48) in the Correa score at 12 years when compared with H. pylori-positive individuals (at both time points). Similar declines were observed in individuals with MAG at baseline who were H. pylori-negative at 12 and 16 years (16-year score decline was −1.18, 95% CI, −0.76 to −1.60) or H. pylori-negative at 16 and 20 years (20-year score decline was −0.95, 95% CI, −0.52 to −1.37), when compared to individuals who were H. pylori-positive at both corresponding visits.
Overall, IM was not reversible to MAG. However, in individuals with baseline IM who were H. pylori-negative at both 16- and 20-year visits, we observed a maximum decline of −0.27 units (95% CI −0.05 to −0.50) in the Correa score at year 20 when compared to individuals who were H. pylori-positive at both visits. Thus, regression from IM to MAG was possible in a subset of individuals with the minimum score for IM (4.3 units).
Incidence rates of IM, ID, LGD/HGD, and GC
MAG at baseline.
222 individuals with MAG at baseline accumulated 3,440 years of follow-up (mean 15.5 years; median 16.6 years). Among them, 117 individuals progressed to IM, 16 progressed to ID, and 3 to LGD/HGD. Incidence rates were 4.70/100 PY (95% CI, 3.84 to 5.54) for IM, 0.47/100 PY (95% CI, 0.24 to 0.70) for ID, and 0.09/100 PY (95% CI, 0 to 0.18) for LGD/HGD. None of the individuals with MAG developed GC.
IM at baseline.
502 individuals with IM at baseline accumulated a total of 7,133 years of follow-up (mean 14.2 years; median 16.1 years). Among them, 166 individuals progressed to ID, 66 to LGD/HGD, and 8 to GC. Incidence rates were 2.43/100 PY (95% CI, 2.05 to 2.79) for ID, 0.98/100 PY (95% CI, 0.74 to 1.21) for LGD/HGD, and 0.11/100 PY (95% CI, 0.03 to 0.19) for GC.
IM/ID at baseline.
559 individuals with IM or ID at baseline, accumulated a total of 7,900 years of follow up (mean 14.1 years; median 16.1 years). Among them, 79 individuals progressed to LGD/HGD, and 11 to GC. Incidence rates were 1.09/100 PY (95% CI 0.85 to 1.33) for LGD/HGD, and 0.14/100 PY (95% CI 0.06 to 0.22) for GC.
Gastric cancer cases
During 20 years of follow-up, 12 individuals (8 males, 4 females, mean time to GC 9.5 years; median 10 years; interquartile range 3–12 years) developed GC, excluding cases identified at baseline or before 2 years of follow-up. Mean age at GC diagnosis was 64.9 years (SD, 11.1). Individuals with GC had IM (n=8; 7 incomplete, 1 complete), ID (n=3) or LGD (n=1) as the most advanced lesion at baseline. All dysplastic lesions were observed in a background of incomplete IM. Five of the 12 individuals who developed GC had received anti-H. pylori therapy at baseline.
Progression to GC by histological subtype or anatomic location of IM at baseline
Of a total of 361 baseline biopsy sets with at least a diagnosis of IM (IM, ID, or LGD), 197 (55%) showed complete-type IM, and the remaining had incomplete type. The rate of progression to GC among individuals with complete IM at baseline was 0.028/100 PY (95% CI, 0.026 to 0.082) and for incomplete IM was 0.37/100 PY (95% CI, 0.15 to 0.59). Multivariable analyses showed that individuals with incomplete-type IM were 13.4 times more likely to progress to GC than those with complete-type (95% CI, 1.8 to 103.8).
Among 345 baseline biopsy sets with at least a diagnosis of IM (IM, ID, or LGD), 256 (74%) showed antrum-restricted IM, and 89 (26%) had corpus IM. The rate of progression to GC among individuals with antrum-restricted IM was 0.15/100 PY (95% CI, 0.03 to 0.26) and for those with corpus IM was 0.32/100 PY (95% CI, 0.04 to 0.59). Multivariable analyses showed that individuals with corpus IM were twice more likely to progress to GC than those with antral-restricted IM (OR, 2.1; 95% CI 0.7 to 6.6).
Progression to GC by OLGA and OLGIM stages at baseline
After excluding individuals with baseline LGD or HGD, 484 and 499 sets of baseline biopsies were classified by OLGA and OLGIM, respectively. Stages were grouped as low- (0-II) and high-risk (III-IV) in both systems. By study design, all individuals had at least MAG at baseline; therefore, no individuals had OLGA stage 0. Using a Poisson mixed effects model, individuals with high-risk OLGA stages were 26.4 (95% CI 3.4 to 206.1) times more likely to develop GC than those with low-risk stages. Similarly, individuals with high-risk OLGIM stages were 19.4 (95% CI 5.7 to 66.5) times more likely to develop GC than those with low-risk stages. The proportion of individuals with incomplete-type IM increased significantly with baseline OLGA or OLGIM stages, being 11%, 48%, 56% and 85% for consecutive OLGA stages I to IV (P<0.001) and 28%, 75%, 96% and 83% for consecutive OLGIM stages I to IV (P<0.001).
DISCUSSION
Our Colombian cohort represents the longest prospective study of Hispanic individuals after an H. pylori eradication trial. The current analysis shows that the protective effect of anti-H. pylori therapy (ITT) against progression of precancerous lesions remains after 20 years. Compared to participants who received placebo at baseline, those who were treated had a significant 41% reduction of the risk of progression as assessed by the comprehensive Correa score. Using the less sensitive global diagnoses, the ITT effect was not statistically significant. We found that MAG is reversible to a non-atrophic stage after a minimum of 6 years free of infection. In contrast, IM is virtually irreversible to a less advanced diagnosis, except when the lesion is minimal (e.g., focal and complete-type). These findings support the concept that the earlier the H. pylori clearance, the greater the benefits. In line with previous observations,29–32 our current results support the reversibility of MAG, and shed light on the controversial findings about the benefits of anti-H. pylori therapy on IM.29–31,33,34 Most of the evidence suggests that IM is largely irreversible by H. pylori eradication. Nevertheless, the lack of IM reversibility should not be interpreted as absence of benefit in preventing progression. Indeed, our observations indicate that clearance of the infection certainly reduces further epithelial damage, which agrees with eradication data on GC prevention, even reducing the risk for metachronous GC.35
Notably, ~40% of the individuals who attended the 20-year endoscopy were still infected. This high prevalence may likely represent the lack of a systematic intervention after the trial. The H. pylori-positive status at the most recent endoscopy was a factor that significantly contributed to a more advanced histopathology. Our analysis of the cumulative effect of H. pylori status from baseline to 20 years showed that bacterial clearance did not lead to visible histological changes during the first years of follow-up, but greater effects were observed subsequently, as years free of infection accumulated (Figure 2). A sustained H. pylori-negative status led to a fundamental change, dampening the natural progression along the Correa cascade, suggestive of a resetting of the epithelial damage to a less advanced point. Substantial benefit is certainly achieved when the continuous detrimental effects of the infection are removed, potentially by decreasing inflammation and the accompanying reactive oxygen species, but also by eliminating the direct effects of the bacterium on the host epithelial cells. A sustained lower Correa score over time was observed in individuals who remained free of infection. The maximum histological regression achieved by both H. pylori-positive and -negative individuals was observed 12 years after enrollment, probably due to the residual effect of interventions. Afterwards, a steady increase in the scores was observed at 16 and 20 years, roughly parallel in both groups, and significantly lower in the absence of H. pylori. This increase is in line with observations on the age-dependent occurrence of gastric histopathology,15,36 which may be due to H. pylori infection sequelae, but also a result of the long-term cumulative damage to the gastric mucosa by other noxious environmental factors, such as smoking and salt consumption, or a possible role of other members of the gastric microbiota.
Other randomized clinical trials have found that H. pylori treatment is effective in preventing GC. In the Shandong Trial, a protective effect of H pylori treatment on GC incidence and mortality remained 22 years post-intervention.37 A recent Cochrane review concluded that H. pylori eradication therapy is superior to placebo or no treatment (RR 0.54, 95% CI, 0.40 to 0.72) in preventing GC development.11 In our study, which was not statistically powered to address GC prevention and with only 12 incident cases, H. pylori therapy was not associated with a reduction in GC risk.
Our data showed that IM was the most prevalent precancerous lesion and the most stable diagnosis over time. Dysplasia was always observed in a background of IM, and while it may seem unexpected that individuals with dysplasia regressed the most, this is not an uncommon finding.4,38 Although no true regression should be assumed, reversibility of dysplasia based on repeated endoscopic procedures may be due to sampling error, to resolution of atypical epithelial changes (such as in resolution of inflammation), or to the possibility of being completely removed at the initial biopsy. In our cohort, most dysplastic lesions were focal and very mild, with hyperproliferative phenotypes and nuclear atypia that did not completely fit the patterns of dysplasia, falling into the category ID. This category may represent an advanced stage of IM.39 All individuals who developed GC had IM, ID, or LGD as the most advanced lesion at baseline. The overall incidence rate of GC in individuals with IM or ID was 0.14/100 PY, and for all individuals with incomplete IM at baseline (including those with any degree of dysplasia) the rate was 0.37/100 PY. A wide variation in the quantification of GC risk in individuals with IM has been reported, ranging from 0.04 to 0.4/100 PY.4–6,40,41 Causes of variation (besides actual differences), include heterogeneity in studied population (patients vs. healthy volunteers) and histological diagnostic criteria, and exclusion of GC cases detected early after an index endoscopy. Actual differences may result from the complex interaction of host genetics and environmental factors. To date, there is no evidence supporting that progression rates of IM vary by race/ethnicity.40 Thus, the clinical management of patients with IM should not differ based on population origin.15,18
OLGA and OLGIM systems are applied for GC risk stratification.27,42 Unlike the OLGIM system, which seems to be easily understood, considerable confusion exists in the interpretation of the OLGA system.20 While OLGA assesses the extent of total atrophy (combining IM, pseudopyloric metaplasia, and non-metaplastic atrophy), OLGIM exclusively evaluates IM. Therefore, the OLGA stage of an individual based on a given set of biopsies can be equal or higher than the OLGIM stage, but not lower.20 Consistent with the literature,27,43,44 including our previous report on this cohort,23 our current findings showed that individuals classified in high-risk OLGA or OLGIM stages at baseline were about 20-fold more likely to progress to GC than those in low-risk stages. Our results provide additional support to international guidelines recommending that individuals in high-risk stages should enter surveillance programs aimed to reduce GC mortality.10
Unlike MAG, the diagnosis of IM is highly reproducible among pathologists.4 IM is a marker of chronic injury to the gastric mucosa and is considered the “cancerization field” in which GC originates.45 However, most individuals with IM will not develop GC. Molecular studies of IM have characterized its heterogeneity and identified several features associated with its progression to neoplasia, including telomere shortening and somatic copy number alterations.46 Several histological classifications exist and cumulative evidence supports IM subtyping into complete and incomplete, as the incomplete-type confers a higher GC risk.40,41,47 Complete IM usually displays characteristics of a more stable lesion, with better organized epithelium, less architectural irregularity, and a lower proliferation rate than incomplete IM. Our study supports the concept that as complete IM progresses over time, the possibility of finding foci of incomplete IM increases. In our cohort, most individuals who remained with IM over 20 years presented a change from complete to incomplete (or mixed), supporting the incomplete type as a more advanced lesion along the Correa cascade. In agreement with our previous report,23 we now found that the risk of progression to GC was 13 times higher for individuals with incomplete-type IM compared to those with only complete-type. Although incomplete-type IM is recognized by recent guidelines10,19 as a risk factor to consider for endoscopic surveillance, routine IM subtyping is still not recommended for clinical practice. Unfortunately, IM subtyping is a resource that has remained largely underappreciated. Most cases of IM may be classified based on morphology on H&E-stained sections; for the rest, an inexpensive histochemical stain (Alcian blue, pH 2.5/periodic acid Schiff) is useful.48 Incomplete-type IM is an indicator of extensive IM, as our current analysis and previous reports show a strong association between incomplete-type IM and extent of atrophic/metaplastic changes.23,39,49 Therefore, in the absence of a recommended 5-biopsy protocol (updated Sydney system)25 to properly assess the extent of IM, the identification of incomplete-type IM is clinically useful.
International guidelines consider individuals with atrophic/metaplastic lesions in the corpus at higher GC risk than those with only antral involvement.10 It is well-known that the H. pylori-associated inflammation and subsequent atrophic changes are initially observed in the antrum. As the process advances, the bacterium and the precancerous changes spread proximally. Our current analysis shows a non-significant two-fold risk of developing GC in individuals with corpus IM at baseline compared to those with antral-restricted IM. The limited data in the literature40 should not lead to the misconception that all individuals with antral-restricted IM are at low risk of progression. Indeed, 58% of individuals who developed GC in our cohort had IM restricted to the antrum at baseline. Despite the importance of identifying corpus atrophy (with or without IM) as a risk factor, in the routine assessment of gastritis, pathologists may not be able to recognize oxyntic atrophy when all biopsy specimens obtained at an endoscopic procedure are submitted in a single jar. In oxyntic atrophy, parietal and chief cells are lost and are often replaced by mucous-type cells resembling antropyloric mucosa (i.e., pseudopyloric metaplasia). Furthermore, in advanced stages of IM, the gastric phenotype of the mucosa may be completely absent. Therefore, biopsy samples should be submitted separately in at least two jars, labeled antrum (including incisura) or corpus. This recommendation is gaining importance considering the recently recognized increase of GC in young adults in the US,16 mainly located in the corpus.
The Shandong trial found that vitamin (C, E, and selenium) or garlic supplementation for 7 years significantly reduced GC mortality for more than 22 years.37 Vitamin supplementation also reduced GC incidence.37 In our trial, the loss of the beneficial effect of antioxidant supplementation remained at 20 years.
The strengths of our study include the long period of observation, a consistent team of skilled endoscopists, a standardized biopsy protocol, and the blinded assessment of histopathology by a consistent group of experienced GI pathologists. Unique features of our analysis are the use of a detailed histopathology score and a comprehensive assessment of the cumulative time of H. pylori infection. One limitation is the relatively high drop-out rate throughout the study. However, individuals at the last visit are a representative subset of the initial group, with similar distribution regarding sex, age, and baseline diagnoses. Another limitation is the possibility of diagnosis and/or H. pylori status misclassification due to biopsy sampling error. Although H. pylori was assessed in all biopsy samples by using a silver stain, false-negative results may be obtained, particularly in individuals with extensive IM. Other limitations are the lack of complete information on risk factors such as smoking (prevalence of smoking at baseline was 30% and most were light smokers, with a median of 3 cigarettes/day)50 or other environmental influences, and anti-H. pylori therapy after the 12-year follow-up.
Mass eradication of H. pylori infection in high GC risk populations has been proposed and implemented in some populations as a strategy to reduce GC rates.30,51 However, important concerns remain, such as the increase in antibiotic resistance and alterations of the intestinal microbiota with yet unknown consequences. Therefore, any screen-and-treat mass strategy should be tailored to local conditions, and measures should be taken to prevent widespread implementation before obtaining sufficient knowledge about its efficacy, safety, and economic impact.52
In conclusion, both anti-H. pylori therapy and clearance of the infection showed a long-term beneficial effect on the progression of precancerous lesions. Clearance of H. pylori led to regression of MAG and reduced the progression of IM. Our observations on high-risk features (incomplete-type IM or its extension to the corpus, and OLGA/OLGIM stages III/IV) are in line with those considered in international guidelines. We additionally recommend IM subtyping implementation in clinical practice, especially when assessing its extent is not possible. Organized multidisciplinary efforts are required for the control and prevention of GC globally. As a first step, the adoption of clear and strong guidelines on the management of individuals at high risk should be seriously considered, even in countries with overall low- and moderate-risk of GC. Additional strategies should include implementation of careful high-quality endoscopic examinations, optimal handling and processing of biopsy specimens, and adoption of pathology reports reflecting GC risk.
Supplementary Material
What You Need to Know.
BACKGROUND AND CONTEXT:
Anti-Helicobacter pylori treatment and endoscopic surveillance of gastric precancerous lesions reduce gastric cancer risk. European guidelines recommend surveillance in individuals with incomplete-type intestinal metaplasia, but subtyping is not recommended in clinical practice.
NEW FINDINGS:
In a Hispanic population, anti-H. pylori treatment or long-term clearance of the bacterium reduced histological progression during a 20-year period. Incomplete-type (vs. complete-type) intestinal metaplasia conferred a 13-fold higher gastric cancer risk.
LIMITATIONS:
Potential misclassification of histological diagnosis and/or H. pylori status due to random biopsy sampling error.
IMPACT:
This is the longest follow-up of a cohort of Hispanics after an eradication trial. Subtyping of intestinal metaplasia is an underutilized tool that should be used to identify individuals for gastric cancer surveillance.
Acknowledgements:
The authors thank the study participants and appreciate the invaluable efforts of the field research team in Colombia.
Grant support: U.S. National Institutes of Health grants P01CA028842 (KTW, PC, and DRM), R01 CA190612 (KTW and DRM), P01CA116087 (RMP and KTW), R21AI142042 (KTW), R01CA077955 and R01DK058587 (RMP), UL1RR024975 (Vanderbilt CTSA, Pilot Project to KTW), 5P30 DK058404 (Vanderbilt Digestive Disease Research Center; RMP and KTW), Merit Review Grants I01CX002171 and I01BX001453 from the Office of Medical Research, Department of Veterans Affairs (KTW), Department of Defense grant W81XWH-18-1-0301 (KTW). MCC is supported by funds from the intramural program of the U.S. National Institutes of Health, National Cancer Institute.
Abbreviations:
- CI
confidence interval
- GC
noncardia gastric cancer
- IM
intestinal metaplasia
- MAG
multifocal atrophic gastritis without intestinal metaplasia
- NAG
non-atrophic gastritis
- OLGA
Operative Link for Gastritis Assessment
- OLGIM
Operative Link on Gastric Intestinal Metaplasia Assessment
- OR
odds ratio
- PY
person-years
- SD
standard deviation
- SE
standard error
Footnotes
Disclosures (for all authors): Nothing to disclose.
Reference #8 states: “all authors contributed equally to the creation of this guideline”:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Correa P Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735–40. [PubMed] [Google Scholar]
- 3.Correa P, Haenszel W, Cuello C, et al. A model for gastric cancer epidemiology. Lancet 1975;2:58–60. [DOI] [PubMed] [Google Scholar]
- 4.Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res 1990;50:4737–40. [PubMed] [Google Scholar]
- 5.de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 2008;134:945–52. [DOI] [PubMed] [Google Scholar]
- 6.Song H, Ekheden IG, Zheng Z, et al. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ 2015;351:h3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Martel C, Georges D, Bray F, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 2020;8:e180–e190. [DOI] [PubMed] [Google Scholar]
- 8.Chey WD, Leontiadis GI, Howden CW, et al. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017;112:212–239. [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017;66:6–30. [DOI] [PubMed] [Google Scholar]
- 10.Pimentel-Nunes P, Libanio D, Marcos-Pinto R, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019;51:365–388. [DOI] [PubMed] [Google Scholar]
- 11.Ford AC, Yuan Y, Forman D, et al. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst Rev 2020;7:CD005583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang RJ, Koh H, Hwang JH, et al. A Summary of the 2020 Gastric Cancer Summit at Stanford University. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altayar O, Davitkov P, Shah SC, et al. AGA Technical Review on Gastric Intestinal Metaplasia-Epidemiology and Risk Factors. Gastroenterology 2020;158:732–744 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang RJ, Ende AR, Singla A, et al. Prevalence, risk factors, and surveillance patterns for gastric intestinal metaplasia among patients undergoing upper endoscopy with biopsy. Gastrointest Endosc 2020;91:70–77 e1. [DOI] [PubMed] [Google Scholar]
- 16.Anderson WF, Rabkin CS, Turner N, et al. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J Natl Cancer Inst 2018;110:608–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S, Tao L, Murphy JD, et al. Race/Ethnicity-, Socioeconomic Status-, and Anatomic Subsite-Specific Risks for Gastric Cancer. Gastroenterology 2019;156:59–62 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinis-Ribeiro M, Kuipers EJ. How to Manage a Patient With Gastric Intestinal Metaplasia: An International Perspective. Gastroenterology 2020;158:1534–1537. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Li D, El Serag HB, et al. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology 2020;158:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matysiak-Budnik T, Camargo MC, Piazuelo MB, et al. Recent Guidelines on the Management of Patients with Gastric Atrophy: Common Points and Controversies. Dig Dis Sci 2020;65:1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst 2000;92:1881–8. [DOI] [PubMed] [Google Scholar]
- 22.Mera R, Fontham ET, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut 2005;54:1536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mera RM, Bravo LE, Camargo MC, et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 2018;67:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold M, Park JY, Camarg MC, et al. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020;69:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161–81. [DOI] [PubMed] [Google Scholar]
- 26.Rugge M, Correa P, Dixon MF, et al. Gastric dysplasia: the Padova international classification. Am J Surg Pathol 2000;24:167–76. [DOI] [PubMed] [Google Scholar]
- 27.Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc 2010;71:1150–8. [DOI] [PubMed] [Google Scholar]
- 28.Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis 2008;40:650–8. [DOI] [PubMed] [Google Scholar]
- 29.Hwang YJ, Kim N, Lee HS, et al. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication - a prospective study for up to 10 years. Aliment Pharmacol Ther 2018;47:380–390. [DOI] [PubMed] [Google Scholar]
- 30.Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rokkas T, Pistiolas D, Sechopoulos P, et al. The long-term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta-analysis. Helicobacter 2007;12 Suppl 2:32–8. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz B, Garay J, Correa P, et al. Morphometric evaluation of gastric antral atrophy: improvement after cure of Helicobacter pylori infection. Am J Gastroenterol 2001;96:3281–7. [DOI] [PubMed] [Google Scholar]
- 33.Leung WK, Lin SR, Ching JY, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 2004;53:1244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li WQ, Ma JL, Zhang L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi IJ, Kook MC, Kim YI, et al. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med 2018;378:1085–1095. [DOI] [PubMed] [Google Scholar]
- 36.Sonnenberg A, Genta RM. Changes in the Gastric Mucosa With Aging. Clin Gastroenterol Hepatol 2015;13:2276–81. [DOI] [PubMed] [Google Scholar]
- 37.Li WQ, Zhang JY, Ma JL, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ 2019;366:l5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.den Hollander WJ, Holster IL, den Hoed CM, et al. Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut 2019;68:585–593. [DOI] [PubMed] [Google Scholar]
- 39.Tava F, Luinetti O, Ghigna MR, et al. Type or extension of intestinal metaplasia and immature/atypical “indefinite-for-dysplasia” lesions as predictors of gastric neoplasia. Hum Pathol 2006;37:1489–97. [DOI] [PubMed] [Google Scholar]
- 40.Gawron AJ, Shah SC, Altayar O, et al. AGA Technical Review on Gastric Intestinal Metaplasia-Natural History and Clinical Outcomes. Gastroenterology 2020;158:705–731 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez CA, Sanz-Anquela JM, Companioni O, et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: results of the Spanish follow-up multicenter study. J Gastroenterol Hepatol 2016;31:953–8. [DOI] [PubMed] [Google Scholar]
- 42.Rugge M, Meggio A, Pennelli G, et al. Gastritis staging in clinical practice: the OLGA staging system. Gut 2007;56:631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rugge M, Genta RM, Fassan M, et al. OLGA Gastritis Staging for the Prediction of Gastric Cancer Risk: A Long-term Follow-up Study of 7436 Patients. Am J Gastroenterol 2018;113:1621–1628. [DOI] [PubMed] [Google Scholar]
- 44.Rugge M, Meggio A, Pravadelli C, et al. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: a 5-year prospective study of 1755 patients. Gut 2019;68:11–17. [DOI] [PubMed] [Google Scholar]
- 45.Curtius K, Wright NA, Graham TA. An evolutionary perspective on field cancerization. Nat Rev Cancer 2018;18:19–32. [DOI] [PubMed] [Google Scholar]
- 46.Huang KK, Ramnarayanan K, Zhu F, et al. Genomic and Epigenomic Profiling of High-Risk Intestinal Metaplasia Reveals Molecular Determinants of Progression to Gastric Cancer. Cancer Cell 2018;33:137–150 e5. [DOI] [PubMed] [Google Scholar]
- 47.Filipe MI, Munoz N, Matko I, et al. Intestinal metaplasia types and the risk of gastric cancer: a cohort study in Slovenia. Int J Cancer 1994;57:324–9. [DOI] [PubMed] [Google Scholar]
- 48.Shah SC, Gawron AJ, Mustafa RA, et al. Histologic Subtyping of Gastric Intestinal Metaplasia: Overview and Considerations for Clinical Practice. Gastroenterology 2020;158:745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rugge M, Fassan M, Pizzi M, et al. Operative Link for Gastritis Assessment gastritis staging incorporates intestinal metaplasia subtyping. Hum Pathol 2011;42:1539–44. [DOI] [PubMed] [Google Scholar]
- 50.Camargo MC, Piazuelo MB, Mera RM, et al. Effect of smoking on failure of H. pylori therapy and gastric histology in a high gastric cancer risk area of Colombia. Acta Gastroenterol Latinoam 2007;37:238–45. [PMC free article] [PubMed] [Google Scholar]
- 51.Leja M, Park JY, Murillo R, et al. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: the GISTAR study. BMJ Open 2017;7:e016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrero R, Park JY, Forman D. The fight against gastric cancer - the IARC Working Group report. Best Pract Res Clin Gastroenterol 2014;28:1107–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.