Abstract
Objective:
CD73 is an ectonucleotidase which catalyzes the conversion of AMP to adenosine. Adenosine has been shown to be anti-inflammatory and vasorelaxant. The impact of ectonucleotidases on age-dependent atherosclerosis remains unclear. Our aim was to investigate the role of CD73 in age-dependent accumulation of atherosclerosis.
Approach and results:
Mice doubly deficient in CD73 and apolipoprotein E (cd73−/−/apoE−/−) were generated, and the extent of aortic atherosclerotic plaque was compared to apoE−/− controls at 12, 20, 32 and 52 weeks. By 12 weeks of age, cd73−/−/apoE−/− mice exhibited a significant increase in plaque (1.4% ± 0.5% of the total vessel surface vs. 0.4% ± 0.1% in apoE−/−controls, p<0.005). By 20 weeks of age, this difference disappeared (2.9% ± 0.4% vs 3.3% ± 0.7%). A significant reversal in phenotype emerged at 32 weeks (9.8% ± 1.2% vs. 18.3% ± 1.4%, p<0.0001) and persisted at the 52 week timepoint (22.4% ± 2.1% vs 37.0% ± 2.1%, p<0.0001). The inflammatory response to aging was found to be comparable between cd73−/−/apoE−/− mice and apoE−/− controls. A reduction in lipolysis in CD73 competent mice was observed, even with similar plasma lipid levels (cd73−/−/apoE−/− vs. apoE−/− at 12 weeks (16.2 ± 0.7 vs. 9.5 ± 1.4 nmol glycerol/well) 32 weeks (24.1 ± 1.5 vs. 7.4 ± 0.4 nmol/well), and 52 weeks (13.8 ± 0.62 vs. 12.7 ± 2.0 nmol/well), p < 0.001).
Conclusions:
At early time points, CD73 exerts a subtle anti-atherosclerotic influence, but with age, the pattern reverses, and the presence of CD73 promoted suppression of lipid catabolism.
Keywords: ectonucleotidase, vascular aging, atherosclerosis, CD73, adenosine
Subject codes: Basic Science Research, Lipids and Cholesterol, Vascular Biology, Aging, Cardiovascular Disease, Vascular Disease
Category: Basic study
Subcategory: Thrombosis
Visual abstract
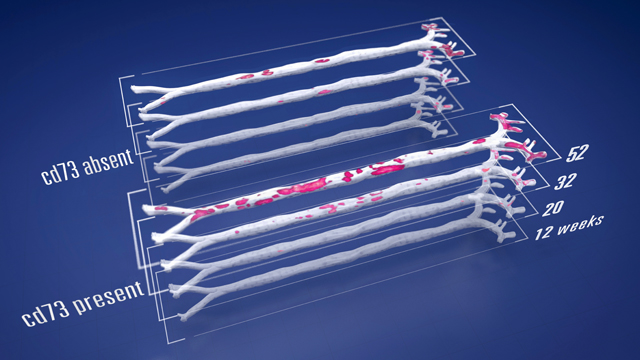
Visual depiction of study findings: In a murine model of atherosclerosis, at early ages (12 weeks), CD73 exerts a subtle anti-atherosclerotic influence, but with age (32 and 52 weeks), the pattern reverses. In aged mice, the presence of CD73 promotes aortic atherosclerosis. Red plaques represent areas of atherosclerosis.
Introduction
Age-dependent accrual of atherosclerotic plaque is a complex process which is marked by excess vascular lipid accumulation, inflammation, and apoptosis. While much has been discovered about the development of atherosclerotic plaque over the past several decades, the question of why atherosclerosis accelerates with age in some individuals remains poorly understood. Ectonucleotidases are cell surface enzymes responsible for balancing extracellular modulators of inflammation and thrombosis: ATP, ADP, AMP, and adenosine.1–3
CD73 is an ectonucleotidase which catalyzes the terminal step in extracellular adenine nucleotide breakdown: the conversion of AMP to adenosine. Adenosine, which binds to a discrete family of cell surface receptors to initiate intracellular signaling cascades, has been shown to be anti-inflammatory and vasorelaxant. Studies with CD73 knockout mice have shown increased inflammation, transplant rejection, and hypoxia-induced edema.4, 5, 6, 7
CD73 is expressed on circulating leukocytes as well as the vascular endothelium, where it acts as an anti-inflammatory and antithrombotic molecule. The proximity of endogenous CD73 to sites of plaque formation led us to hypothesize that CD73 could play an important role modulating the pace of atherosclerotic plaque accumulation. We hypothesized that CD73 would have anti-atherogenic properties with age. To test this hypothesis, we bred cd73−/− mice on a hyperlipidemic background using apolipoprotein E (apoE−/−) mice to generate cd73−/−/apoE−/− double knockout mice. This study examines how CD73 modulates the accrual of atherosclerotic plaque over time.
Materials & Methods
The authors declare that all supporting data are available within the article; further questions regarding the materials and methods used to support the findings of this study are available from the corresponding author upon reasonable request.
Mice
All animal experiments were approved by the University of Michigan Institutional Animal Care and Use Committee. CD73 knockout mice6 were bred to Apolipoprotein E (apoE−/−) knockout mice (The Jackson Laboratory, Bar Harbor, Maine) to generate mice doubly deficient in CD73 and ApoE. These cd73−/−/apoE−/− mice were phenotypically normal and fertile. Mice used in experiments were at least third-generation male homozygous double knockouts. Both cd73−/− and apoE−/− parental strains were crossed (> 6 generations) into a C57BL/6J background. Approximately 325 genetically engineered male mice were used for the data presented. Mice were kept on a normal laboratory diet (LabDiet 5001 containing 4.5% fat). Our studies comply with the recommendations of the Arteriosclerosis, Thrombosis, and Vascular Biology (ATVB) Council on considering sex in arterial pathology and on experimental atherosclerosis studies.8, 9 While this study did not focus on the effect of sex on aortic plaque burden, prior studies have suggested the possibility of increased atherosclerosis in young female apoE−/− mice compared with males, and others (more relevant to this study on aging) suggesting no difference (n = 5 per group) or greater aortic lipid accumulation (n not specified) in aged apoE−/− male mice compared to female mice.10–12
High pressure liquid chromatography
To determine the concentrations of AMP and adenosine in vivo, 100 μl of blood was drawn into a syringe containing 100 μl of an inhibitor solution to inactivate nucleotide breakdown: 40 mM Tricine buffer with 5 mM KCl, 118 mM NaCl, 4.15 mM ethylene diamine tetraacetate (EDTA), 100 μM dipyridamole, 10 μM erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA), 79 μM a,b-methylene adenosine diphosphate (AOPCP), 1 μM dihydrochloride hydrate, 5nM S-(4-Nitrobenzyl)-6-thioinosine (NBTI), 10 μM Forskolin and 100 μM isobutylmethylxanthine (IBMX).13 The blood was mixed and immediately centrifuged at maximum speed for 2 min at 4ºC. The supernatant was centrifuged and samples were kept at 4ºC until high pressure liquid chromatography was performed.
Flow cytometry
To determine which cell types expressed CD73 in apoE−/− mice, the following protocol was followed. After anesthetizing mice, the ascending and descending aorta was harvested. Tissue was minced and digested with 0.1% collagenase B (Roche). After incubating at 37° C for 25 minutes, tissue was homogenized, strained, and resuspended in Dulbecco’s Modified Eagle Medium (Gibco) with 10% fetal bovine serum. After lysis of red blood cells, resuspending cells in flow cytometry buffer, fixing and permeabilizing, and blocking nonspecific binding (Fc Block BD 553142), cells were stained for CD73 (BD 561545), endothelial cells/CD31 (BD 550765), leukocytes/CD45 (Biolegend 103123), and vascular smooth muscle cells/alpha smooth muscle actin, or Sm-22α (Biotium BNCR0247). Isotype controls were used (BD557676, BD550765, Biolegend 400626). Flow cytometry capturing 10,000 events per sample was performed on a FACSAria III and was analyzed using BD FACSDiva (8.0.1) software.
Blood pressure
Blood pressure measurements were performed as previously described14 with the following modifications: mice were anesthetized with Isofluorane (4% in room air) and kept at 31–33°C. An integrated sensor-cuff occluder (model B60–1/4, IITC Life Science) and return of tail pulsations-computerized blood pressure monitor (model 6M 229 6 channel mouse system, IITC Life Science) were used with a maximum inflation pressure of 200 mm Hg. Four to eight individual measurements per mouse were averaged.
Blood/organ/cell harvest
To harvest blood and organs, mice were anesthetized with Ketamine (100 mg/kg) /Xylazine (10ng/kg) intraperitoneally and immobilized. Blood was drawn by direct cardiac puncture, and mice were perfused with 10 ml of ice-cold phosphate-buffered saline (PBS) followed by perfusion with 10 ml of 4% para-formaldehyde in PBS at pH 7.5. Carcasses were kept in para-formaldehyde at room temperature until dissection of the aorta.
En face aortic plaque quantification
After dissection from the aortic root to the iliacs, the aorta was Oil Red O stained, micro-dissected, and pinned en face. Digital images taken of each pinned aorta were given arbitrary numbers by a third party in order to blind analysis. Adobe Photo-Shop 7.0.1 (Adobe Systems Inc. San Jose, CA) was used to distinguish areas of plaque (red) from vessel (white) at a tolerance setting of 70. ImagePro 4.5.1.29 (Media Cybernetics Inc. Bethesda, MD) was used to quantify areas of plaque as a percentage of total aortic vessel surface.
Immunohistochemistry
Mouse hearts were incubated with 4% paraformaldehyde in PBS at pH 7.5 at 4º C for 3 hours followed by three washes with PBS, overnight incubation in 30% sucrose in PBS, and embedded in Tissue-Tek Optimal Cutting Temperature compound (Sakura, Japan). Cryosections of 6μm were taken of the aortic root and kept frozen until staining. We used a similar cut for each aortic root lesion analysis per animal, for each stain or plaque quantification.
Oil red O (Sigma) stains, counterstained with Harris Hematoxylin, were performed to identify and measure the extent of plaque. Absolute plaque area is reported. Stains were performed in adjacent serial sections. Trichrome staining was performed on frozen sections (Sigma Aldrich HT15). For calcium staining, 2% Alizarin red was used to identify calcium precipitates (Thermo Fisher AC400480250). The pH was adjusted to 4.1– 4.5 with 0.5% ammonium hydroxide. After staining, excess stain was blotted off, and sections were dehydrated in acetone for 10 seconds, followed by 10 seconds in 1:1 acetone:xylene 50:50. For apoptotic terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stain, sections were dehydrated in ice-cold acetone and dried for 30 minutes followed by use of the CardioTACS kit (Trevigen, Gaithersburg, MD) according to the manufacturer’s protocol, using mouse spleen cryosections as positive controls. Slides were counterstained with nuclear fast red. For macrophage stains, a rat anti-mouse CD68 was used (Biorad FA-11) with a secondary antibody (Invitrogen A11006) and corresponding isotype control (Thermo Fisher Rat IgG2a isotype 02–9688). For T cell staining, a rat anti-mouse CD3ε was used (BD 17A2). The corresponding isotype controls was used (BD rat IgG2b A95–1). We did not report macrophage and T cell content in plaques at 12 weeks given the small size of the plaque and sparsity of cells at that age in mice fed a normal laboratory diet. For adenosine deaminase staining, the following antibodies were used: ADA polyclonal antibody (Invitrogen PA5–51572) and rabbit IgG isotype control (Sigma-Aldrich NI01).
Photomicrographs were taken at 30x using a Nikon TE microscope or at 10x using an Olympic upright microscope. High-resolution pictures of the entire aortic root were analyzed using the Metamorph software (Molecular devices, Sunnyvale, CA) or using Nikon NIS-Elements AR (version 5.02.00). Plaque burden was measured at a comparable level of the aorta, at the level of the aortic valve leaflets. Stained cells and plaque area were quantified in the aortic root by a blinded investigator. Stained cells were expressed as total cells (TUNEL) or stained cells per area of plaque (CD68 and CD3 stained cells).
Lipid levels
Blood was drawn after fasting and was centrifuged twice at maximum speed at 4º C for 2 minutes. The serum was stored at −80º C until analysis. Total cholesterol was determined using the Amplex Red Cholesterol assay kit (Thermo Fisher) for 32 week old mice and for the 12 and 52 week old mice, total cholesterol was determined using a Randox RX Series Daytona analyzer (Randox CH 3810). Triglycerides, LDL, and HDL were determined for 12 and 52 week old mice, using a Randox RX Series Daytona analyzer (Randox TR 3823, CH3841, and CH3811, respectively). Free fatty acids were determined for 32 and 52 week old mice using a Randox RX Series Daytona analyzer (Randox FA 115).
Inflammatory markers
Plasma from 32 week and 52 week old mice was obtained as described, and concentrations of IFNγ, TNF-α, IL-1β were determined using a Luminex multiplexing assay (Millipore Milliplex MTH17MAG-47K).
Lipolysis assay
Plasma from sacrificed animals was snap frozen at −80° C. Analysis had previously shown non-significant differences in plasma lipids between groups. Using 3T3-L1 cells to which 10 μl of murine plasma had been added (kindly supplied by Dr. Eugene Chen, University of Michigan, Ann Arbor, MI), glycerol production was determined using a Lipolysis Assay Kit (Abcam 185433).
Statistical analysis
Student t-tests for 2 sample comparisons (assuming equal variances) or ANOVA with least significant difference post-hoc analysis for multiple group comparisons was performed using IBM SPSS Statistics 24. Data was also analyzed assuming non-normal distribution using the Mann Whitney test for 2 group comparisons and the Kruskal-Wallis test for multiple group comparisons. The statistical results remained unchanged. Therefore, we have reported the data (assuming normal distribution) with the associated t-test or ANOVA statistical significance with standard error.
Results
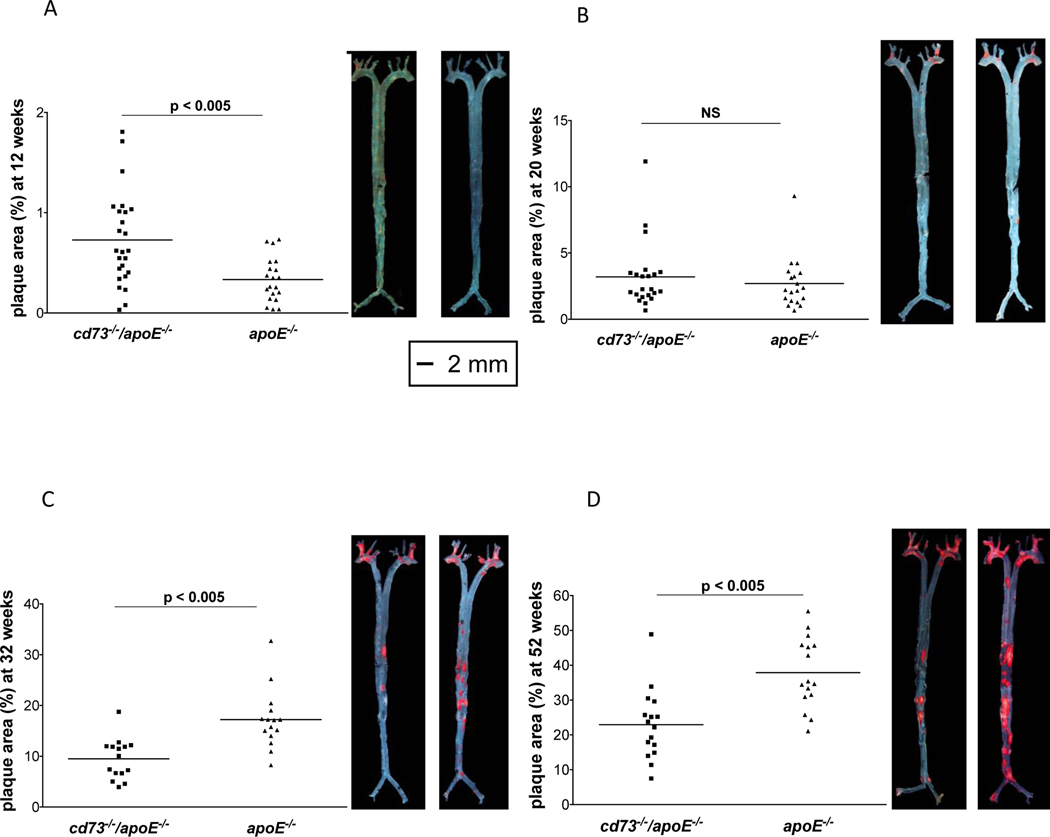
On a normal laboratory diet (4.5% fat), young mice show a very limited extent of atherosclerotic plaque. In 12 week old cd73/-/apoE−/− mice, plaque covered 0.7% ± 0.1% of the total vessel surface (n=25), while the apoE−/− controls had 0.3% ± 0.05% plaque (n=20), p<0.005, (Figure 1A). This difference disappeared in 20 week old mice, with 3.2% ± 0.5% plaque in cd73−/−/apoE−/− mice (n=22), and 2.6% ± 0.4% plaque in apoE−/− controls (n=19), p=NS, (Figure 1B). After 32 weeks, the phenotypic pattern of plaque accumulation reversed. A greater burden of atherosclerosis was seen in apoE−/− controls compared with cd73−/−/apoE−/− mice (9.5% ± 1.0% plaque in cd73−/−/apoE−/− mice (n=15) vs. 17.2% ± 1.5% plaque in apoE−/− controls (n=15), p<0.001 (Figure 1C). This pattern was maintained at 52 weeks with 22.9% ± 2.5% plaque in cd73−/−/apoE−/− mice (n=16) and 37.9% ± 2.5% plaque in apoE−/− control mice (n=16), p<0.001 (Figure 1D). Taken together, apoE−/− control mice had significantly less aortic surface plaque area than cd73−/−/apoE−/− mice at 12 weeks of age. This phenotype reversed by 32 weeks of age, with the apoE−/− control mice accumulating more plaque than in cd73−/−/apoE−/− mice (summarized in Figure 1E).
Figure 1.
Reversal of atherosclerotic phenotype over time. All experiments were performed in mice of an Apolipoprotein E-deficient, hyperlipidemic background. All mice were a fed normal laboratory diet containing 4.5% fat. Mouse aortas were isolated, oil red O stained and pinned en face to quantify the percent of the total vessel area covered by plaque. Each point represents the percent of plaque coverage of the entire aorta for one mouse with representative photographs for each group shown on the right. Note the different scales of the graphs. (A) 12 weeks, (B) 20 weeks, (C) 32 weeks, and (D) 52 weeks. (E) synopsis of graphs a-d showing plaque growth over time and the reversal of phenotype around 20 weeks of age, leading to less plaque in cd73−/−/apoE−/− mice than in apoE−/− at 32 and 52 weeks of age.
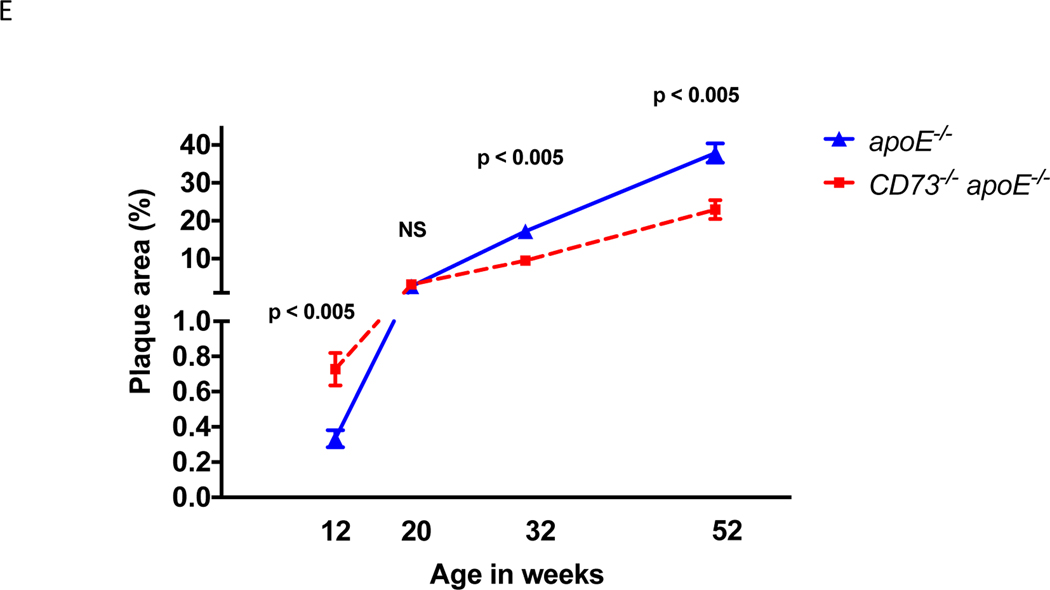
In addition to en face plaque area quantification, we also quantified plaque burden by the cross-sectional burden of plaque in the aortic wall. This is reported as the area of the aortic root covered by plaque. While no difference in plaque was detected in the aortic root at 12 weeks of age, by 32 weeks, cd73−/−/apoE−/− mice had significantly less plaque than apoE−/− controls (Figure 2).
Figure 2.
Plaque extent in the aortic root. While en face preparation in Figure 1 indicates the total vessel surface area of plaque, cross-sections of the aortic root capture plaque thickness. Plaque thickness is represented here by the aortic root atherosclerotic plaque area. (A) and (B) are examples of 6 μm cryosections of the aortic root which were oil red O stained. Shown in (A) is a representative 32 week old cd73−/−/apoE−/− mouse aortic root and in (B) is a representative 32 week old apoE-/−. (C) Plaque area of the aortic root at 12 and 32 weeks in cd73−/−/apoE−/− and apoE−/− mice. While there was no difference in plaque area at 12 weeks between genotypes, at 32 weeks, apoE−/− mice had significantly more cross-sectional plaque area at the aortic root compared with cd73−/−/apoE−/− mice. p < 0.001 using ANOVA.
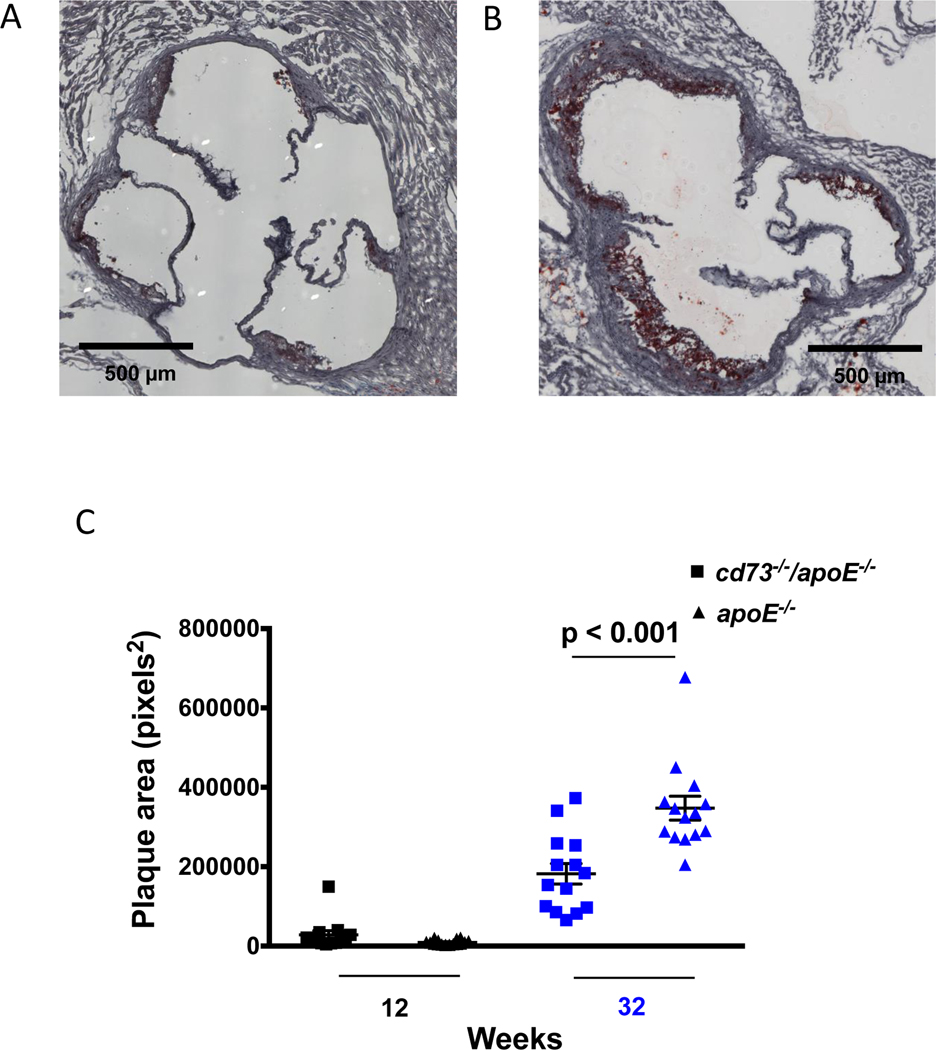
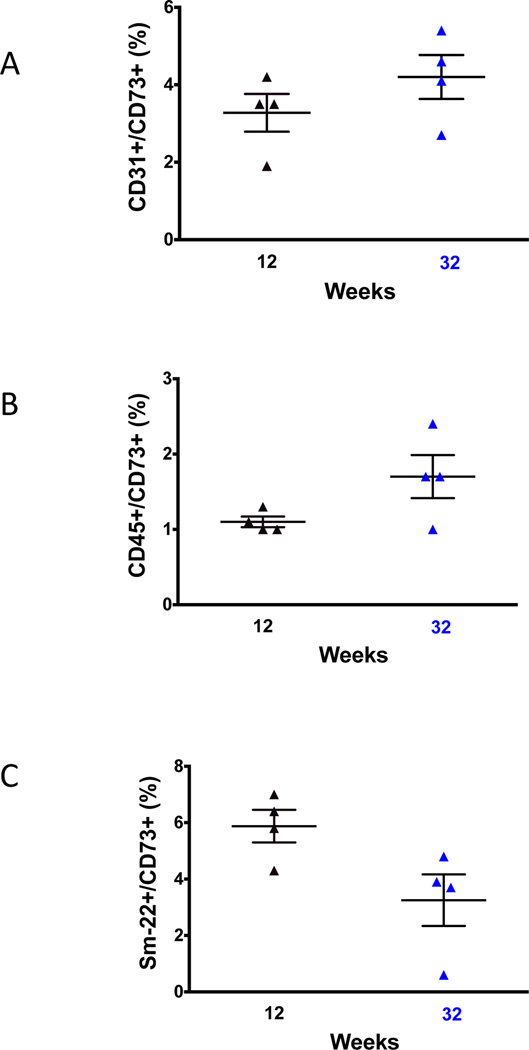
Since CD73 metabolizes AMP to adenosine, we measured the plasma concentration of AMP and adenosine in cd73−/−/apoE−/− and apoE−/− mice. At 12 and 32 weeks of age, and compared with apoE−/− mice, cd73−/−/apoE−/− demonstrated the expected impaired capacity to metabolize AMP to adenosine, represented by a higher ratio of AMP to adenosine (Figure 3). In order to understand which CD73-expressing cells could be contributing to the observed increase in plaque in aged apoE−/− mice, flow cytometry was performed on cells obtained from aortic homogenates (Figure 4 and Supplemental Figure I). At both the 12 and 32 week time points, in apoE−/− mice, CD73 was detected on vascular smooth muscle cells, endothelial cells, and leukocytes. The relative contributions (mean percent ± SEM) of CD73 at 12 and 32 weeks from vascular smooth muscle cells (5.9 ± 0.6 vs. 3.3 ± 0.9) and endothelial cells (3.3 ± 0.5 vs. 4.2 ± 0.6) did not differ, and there were fewer aortic leukocytes (1.1 ± 0.1 vs. 1.7 ± 0.3) expressing CD73 at both time points. This suggested that multiple cell types contribute to CD73-dependent accretion of atherosclerosis. As expected, cd73−/−/apoE−/− mice did not demonstrate any CD73 staining on endothelial cells, leukocytes, or vascular smooth muscle cells (Supplemental Figure I).
Figure 3.
Plasma CD73 activity. CD73 metabolizes AMP to adenosine, and the ratio of AMP to adenosine concentration is an indicator of CD73 enzymatic activity. Plasma was obtained from cd73−/−/apoE−/− and apoE−/− mice at 12 and 32 weeks. ApoE−/− mice were found to have reduced AMP/adenosine concentration ratios, as measured by HPLC, when compared with cd73−/−/apoE−/− mice. p < 0.001 using ANOVA.
Figure 4.
CD73 expression by cell type and age. Flow cytometry revealed that aortic tissue obtained from apoE−/− mice at 12 and 32 weeks expressed CD73 on multiple cell types: (A) endothelial cells (CD31+), (B) vessel wall leukocytes (CD45+), and (C) vascular smooth muscle cells (Sm-22). Counts are expressed as a percent of total gated cells per sample. No significant differences were present in CD73 expression with aging (t-test). Cd73−/−/apoE−/− mice did not express CD73 (Supplemental Figure I).
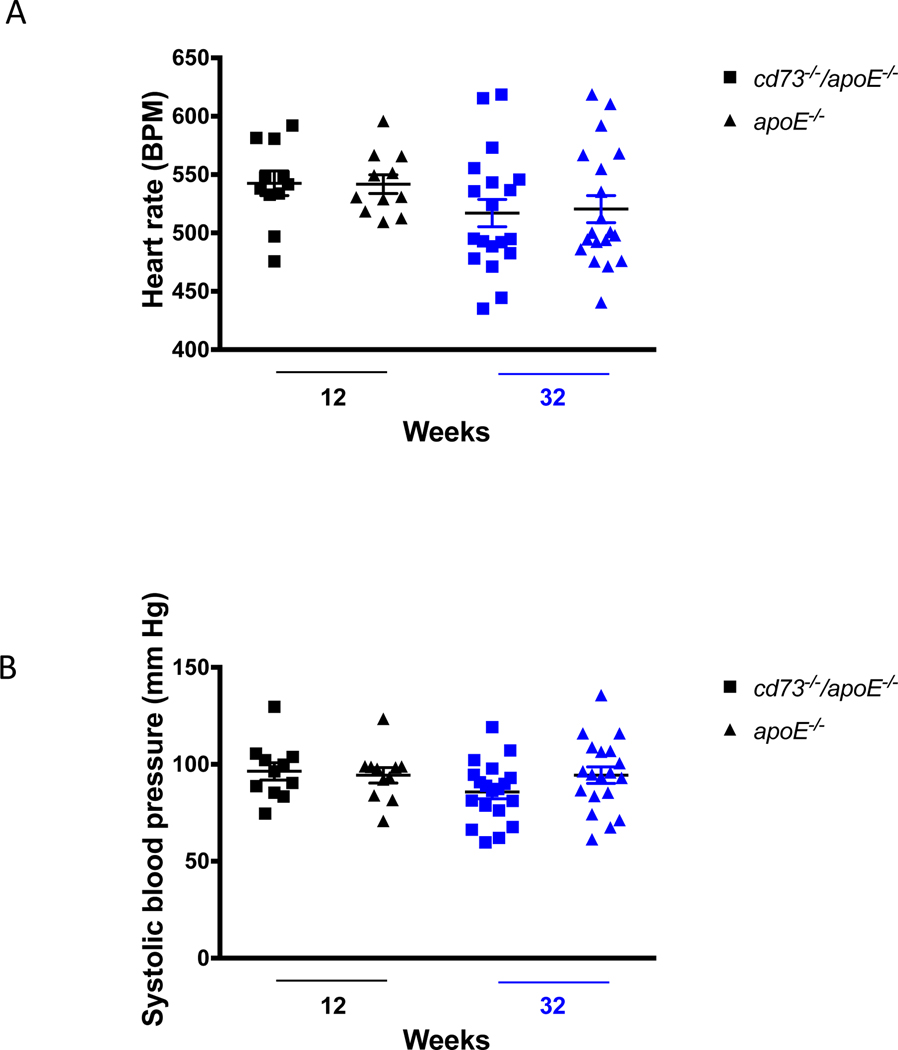
Blood pressure and heart rate were measured at 12 weeks (n = 11 for cd73−/−/apoE−/− mice, and n = 10 for apoE−/− controls) and 32 weeks (n = 14 for cd73−/−/apoE−/− mice, and n = 14 for apoE−/− controls). No differences were found between genotypes (Figure 5A, B).
Figure 5.
Hemodynamic findings. Heart rate and blood pressure were not altered by the presence of CD73 in apoE−/− male young or aged mice. (A) and (B) show averages of 4–8 individual measurements of blood pressure and heart rate per mouse.
Serum lipid analysis revealed that, in aggregate (mice 12–52 weeks), there was no difference in total cholesterol (403 ± 43 vs. 453 ± 39 mg/dL), LDL (78.3 ± 4.4 mg/dL vs. 85.6 ± 2.9 mg/dL), triglycerides (107 ± 13 mg/dL vs. 90 ±10 mg/dL), or HDL (18.4 ± 3.1 mg/dL vs. 11.2 ± 1.5 mg/dL) between cd73−/−/apoE−/− and apoE−/− mice, respectively. Specific lipid levels at aging time points are shown in Table 1 for cd73−/−/apoE−/− and apoE−/− mice. No difference in plasma free fatty acid was noted between cd73−/−/apoE−/− and apoE−/− mice (Supplemental Figure II). No differences in inflammatory markers (IFNγ, TNF-α, IL-1β) were present in aged cd73−/−/apoE−/− and apoE−/− mice (Supplemental Figure III).
Table 1.
Serum lipid analysis from cd73−/−/apoE−/− and apoE−/− mice. NS = not significant. n = 3 per group for 12 weeks, 8–9 per group for 32 weeks, and n = 3 per group for 52 weeks. Significance determined by ANOVA.
| 12 weeks | 32 weeks | 52 weeks | |||||
|---|---|---|---|---|---|---|---|
| Mean (Std. Error) | cd73−/−/apoE−/− | apoE−/− | cd73−/−/apoE−/− | apoE−/− | cd73−/−/apoE−/− | apoE−/− | Significance |
| Total cholesterol (mg/dL) | 331 (75.8) | 439 (39.6) | 503 (38.0) | 475 (67.4) | 208 (62.6) | 412 (29.6) | NS |
| Triglycerides (mg/dL) | 137 (29.2) | 112 (14.2) | 103 (18.1) | 71.7 (12.4) | 81.0 (8.5) | 86.7 (18.3) | NS |
| HDL (mg/dL) | 18.7 (3.8) | 15.0 (1.5) | 7.0 (1.7) | 27.3 (3.7) | 9.3 (2.9) | 11.7 (2.6) | p < 0.01 |
| LDL (mg/dL) | 94.3 (8.4) | 82.3 (7.3) | 81.2 (2.6) | 89.5 (0.4) | 53.7 (9.0) | 78.7 (12.3) | NS |
Plaque characteristics were determined in cd73−/−/apoE−/− and apoE−/− mice by evaluating for differences in collagen, calcification, apoptosis, inflammatory cell infiltration, and adenosine deaminase expression.
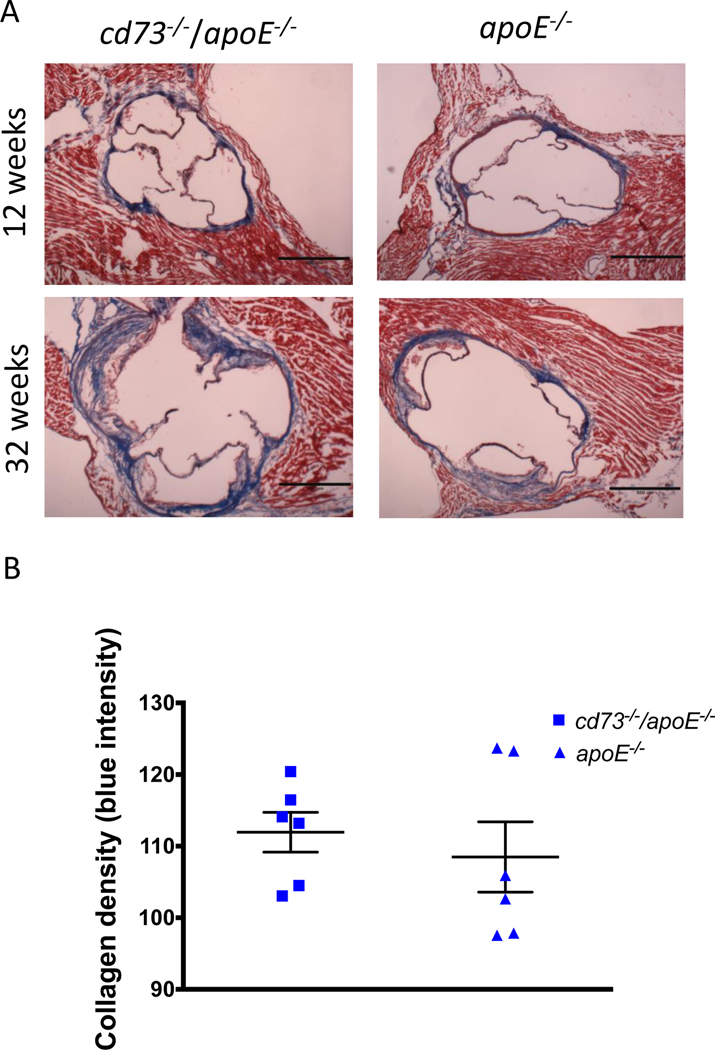
Qualitatively, patchy collagen was present in all plaques at 32 weeks and was present proportionate to the size of the plaque, as evaluated by Trichrome staining of aortic roots (Figure 6). The intensity of plaque connective tissue staining was used as a reflection of collagen density, and density was similar in cd73−/−/apoE−/− (which had smaller plaques) compared with apoE−/− mice (112 ± 2.8 vs. 108 ± 4.9). Calcium deposits were present in half of the apoE−/− aortic roots at 32 weeks, and none of the cd73−/−/apoE−/− mice were found to have plaque calcification (n = 6 per group)(Supplemental Figure IV).
Figure 6.
Aortic root collagen content. (A) Representative trichrome staining of aortic roots of young and aged cd73−/−/apoE−/− and apoE−/− mice. (B) Average intensity of collagen staining in regions of plaque was used as a reflection of collagen density, and 32 week old cd73−/−/apoE−/− mice were found to have similar collagen density in comparison with apoE−/− mice. 12 week old mice had scant plaque and therefore scant plaque collagen. For this reason, collagen density was not quantified in the young mice.
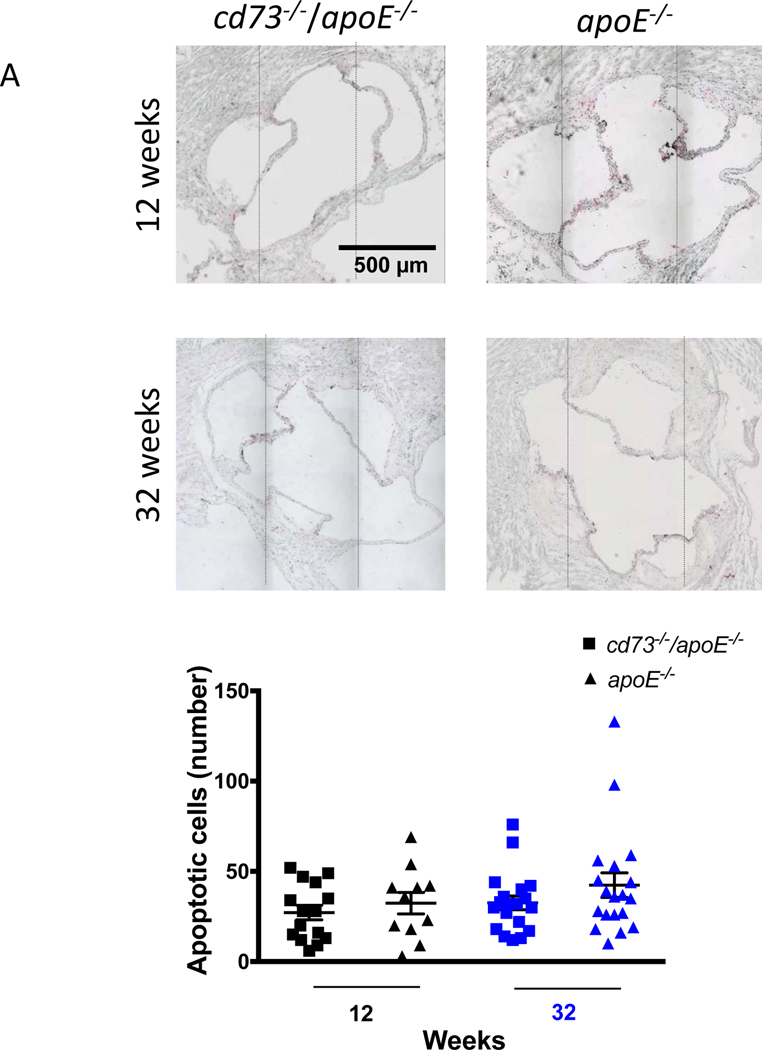
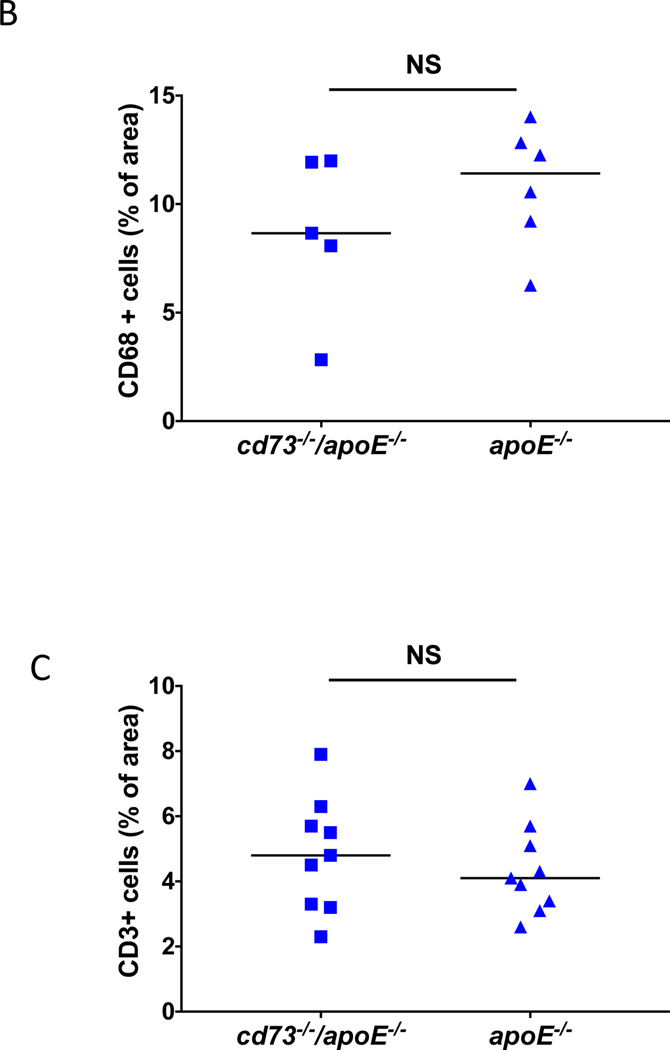
Differences in plaque burden were not due to a different rate of apoptosis, as TUNEL stains revealed no difference in apoptotic cell counts between genotypes at 12 or 32 weeks. (Figure 7A). To define whether differences in inflammatory cell infiltrate contributed to the differences in atherosclerotic plaque accumulation between genotypes, macrophages and T cells were quantified in the plaque of the aortic root at 32 weeks (Figure 7B and 7C and Supplemental Figure V) but did not differ between cd73−/−/apoE−/− mice and apoE−/− controls. Adenosine deaminase expression was found within the aortic root plaque as well as in the surrounding tissues in both cohorts at 12 and 32 weeks (Supplemental Figure VI).
Figure 7.
Plaque qualities by genotype. Differences in atherosclerotic plaque burden between cd73−/−/apoE−/− mice and apoE−/− controls were not accompanied by changes in the rate of cellular apoptosis or the degree of immune cell infiltration. Cross sections of the aortic root were stained for apoptotic cells (A) by TUNEL, for (B) macrophages, identified by CD68, and (C) T cells, identified by CD3. Immune cell infiltration was examined at the 32 week time point. Dotted lines represent patched photographs.
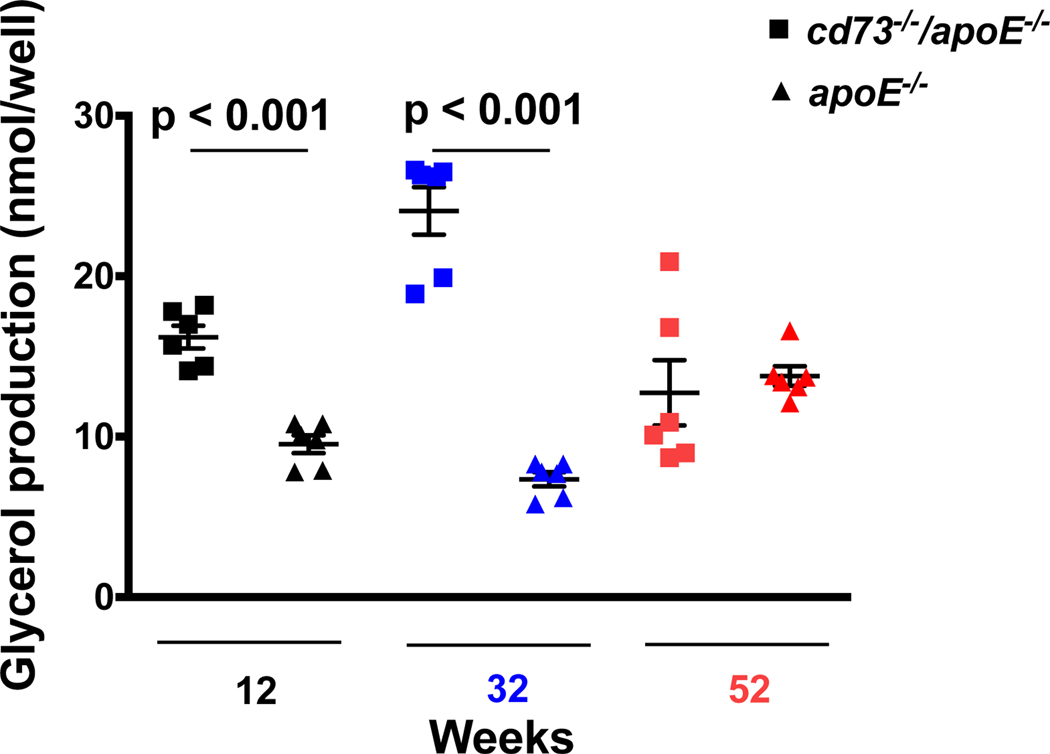
Given that CD73 promoted the accretion of atherosclerosis over time and that no differences in plaque inflammation or apoptosis were found, we explored whether differences in lipolysis could have contributed to plaque accumulation in apoE−/− controls compared with cd73−/−/apoE−/− mice. Adenosine, the product of CD73 activity, is known to suppress lipolysis.15–17 Plasma from apoE−/− mice with intact CD73-mediated adenosine production was found to reduce lipolysis in adipocytes (as determined by glycerol production) more than plasma from cd73−/−/apoE−/− mice. This reduction in lipolysis in CD73 competent mice was observed, even with similar plasma lipid levels, at early time points (cd73−/−/apoE−/− vs. apoE−/− at 12 weeks (16.2 ± 0.7 vs. 9.5 ± 1.4 nmol/well) 32 weeks (24.1 ± 1.5 vs. 7.4 ± 0.4 nmol/well), and 52 weeks (13.8 ± 0.62 vs. 12.7 ± 2.0 nmol/well), p < 0.001) (Figure 8). This provocative finding suggests that an intact CD73/adenosine axis can promote atherogenesis, particularly in older mice, through suppression of lipid catabolism.
Figure 8.
Distinct lipolysis activity by genotype. Adenosine suppresses lipolysis, and cd73−/−/apoE−/− mice, which demonstrated reduced AMP to adenosine ratios, were found to have increased plasma lipolysis in comparison to apoE−/− controls, suggesting that reduced lipolysis might account for plaque accumulation in mice with active CD73 over time. p < 0.001 by ANOVA.
Discussion
Endogenous ectonucleotidase activity mediates the intravascular balance of thrombosis and inflammation, by dissipating ATP, ADP, and AMP. In this work, we describe an age-dependent role for the ectonucleotidase CD73. Here we demonstrate for the first time that CD73 exerts disparate influences on the accumulation of atherosclerosis, depending upon the age of the animal. At younger ages, CD73 played a subtle protective role in preventing plaque accretion. Surprisingly, this phenotype reversed with aging, as the presence of CD73 on the vessel wall created an environment that permitted the accumulation of plaque in older mice.
The ectonucleotidase CD73 plays an important role in the terminal phosphohydrolysis of AMP, thereby generating adenosine. Adenosine in turn signals through a receptors situated in close proximity to CD73 on the cell surface.18 Each adenosine receptor has a unique tissue distribution, ligand affinity, and signal transduction pathway. The A2A and A2B receptors stimulate adenylyl cyclase, inducing cyclic adenosine monophosphate (cAMP) production, whereas A1A and A3 inhibit adenylyl cyclase.19 Because adenosine has predominantly anti-inflammatory properties through the adenosine A2A and A2B receptors,20, 21 we expected that CD73 would continue to play a protective role in aged mice in terms of preventing accumulation of atherosclerosis. However, our results were contrary to our expectations, and aged mice, particularly those in which CD73 was present on the vessel wall, demonstrated a greater burden of atherosclerosis in comparison to their aged counterparts lacking CD73 on the vessel wall.
Little is known about how ectonucleotidases influence the accumulation and composition of atherosclerotic plaque. Because plaque does not accrue linearly with age22, in order to fully understand whether ectonucleotidases have bearing on the development of atherosclerosis, it is critical to look at plaque accumulation at varying time points, particularly as vascular disease progresses with age. We believe that this is the first study to examine the role of CD73 in aging animals over a 12 month period. Previously, it was shown that in a hyperlipidemic environment, CD73 prevented plaque accumulation.23 We noted a dichotomous effect of CD73, in which it demonstrated a subtle early protective effect, but then promoted atherosclerosis beginning at 32 weeks, which continued at the latest timepoint studied, 52 weeks. Our position is that the findings, while at face value, appear discordant, upon closer evaluation may not be. The 26 week results from the prior report on atherosclerotic plaque burden in the cd73−/−/apoE−/− mice were very similar numerically to our 32 week results. The likely explanation for the seeming difference is an exponential rise in plaque burden beginning in the apoE−/− mice, which began between 20 and 32 weeks of age. It is also noted that the prior report included n = 8 mice per group in the 26 week cohort, while our study reports on n = 15–16 mice per group at 12, 20, 32, and 52 week time points.23 As a result of the lower n in the prior report, the confidence intervals (5.5 ± 1.6% for apoE−/− and 9.8 ± 4.5 for the cd73−/−/apoE−/− mice) were wide enough that, although the result was significant at p < 0.05, it is possible that the degree of difference from our findings was also overestimated. Rather than contradicting the prior findings, our findings highlight that age is a critical modifier of CD73 and the rate of accumulation and composition of plaque. Notably, ApoE−/− mice null for A2A receptor expression were shown to be protected from atheromatous disease.24 Differences in diet and younger age limit direct comparison of these data to our findings, and differences in findings emphasize the challenges in describing the ectonucleotidase CD73 in binary (protective or harmful) terms.
CD73 is known to have other effects on the vessel wall. A striking phenotype of predominantly lower extremity vascular calcification in humans lacking functional CD73 has been described.25 To date, this has not been phenocopied in murine models, highlighting the limitation of extrapolating findings from animal models to humans.26 Explanted atherosclerotic plaques have been shown to have low CD73 expression; the authors speculated that this could be due to shedding of CD73 from dysfunctional endothelium.27 Hydrogen peroxide, palmitate, and antidiabetic sulfonylurea drugs can relocate CD73 from lipid rafts in the cell membrane to lipid droplets released from the cell, as well as increase the phospholipase-cleavage of glycosylphosphatidylinositol (GPI)-anchors, releasing the enzymatic moiety from the cell surface.28 An alternative explanation is that dysfunctional endothelial cells lead to both atherosclerosis and that loss of CD73 is intrinsic to this process; the source of increased serum CD73 could be endothelial shedding, but leukocyte origin or active microparticle production from other cell sources are also possible.29
Closely related to CD73 is CD39, which operates upstream of CD73 by phosphohydrolyzing extracellular ATP and ADP to AMP. Prior work by our group demonstrated that CD39 has regional expression on blood vessels, tending to be expressed in areas of lower plaque burden and laminar, rather than turbulent blood flow.30 Mice haploinsufficient for CD39 accumulated more atherosclerosis in comparison to control mice with fully functional CD39, driven by increased platelet reactivity in mice haploinsufficient for CD39.30 Of relevance is an additional report suggesting that CD39 paradoxically protected against the development of atherosclerosis, driven by attenuated platelet activation, increased plasma HDL levels, and enhanced cholesterol efflux.31 While differences in findings may be due to differing knockout strategies or dietary or housing differences, both studies implicate the impact of CD39 on platelet reactivity in the development of atherosclerosis, highlighting the importance of the purinergic axis in modulating the accumulation of atherosclerosis.30, 31 As a corollary, little is known about the role of CD39 in human vascular disease; ADPase activity has been reported to be found at higher levels in humans with lower extremity peripheral vascular disease.32
Although CD73 has been shown in other models to reduce inflammation, in this study, we did not find that CD73 had an impact on plaque macrophage or T cell counts in our aged mice.4, 5, 7, 20, 33–35 This is of relevance since plaque inflammation is a primary driver of plaque destabilization and rupture, ultimately leading to stroke or myocardial infarction.36 Our findings are concordant with a prior report that in a hyperlipidemic environment, CD73 did not have any additional impact on plaque inflammation.23 Taken together, these reports suggest an interaction between CD73 and aging, such that CD73 allows for progressive accumulation of atherosclerotic plaque with age but may not have an additional impact on plaque inflammation beyond that stimulated by the hyperlipidemic environment. It is also possible that CD73 could be protective under provoked inflammatory vascular conditions, but exerts a disparate effect in the setting of chronic progressive atherosclerotic plaque accumulation, without an additional inciting inflammatory stimulus.37
Besides regulating inflammation, CD73 has also been shown to play a role in cellular apoptosis. The product of CD73 enzymatic activity, adenosine, induces smooth muscle cell apoptosis through adenosine receptor A2B receptor signaling.38 On the other hand, CD73 conferred resistance to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis independent of its enzymatic AMPase activity.39 As described in our study, TUNEL-stains for apoptotic cells within the plaque of the aortic root in this study showed no difference in apoptosis in the plaques of cd73−/−/apoE−/− mice and control apoE−/− mice.
To further elucidate the molecular underpinnings of CD73-driven plaque accumulation with age in this model of murine atherosclerosis, we examined the collagen content of the plaque and found that collagen was proportional to the size of the plaque. This observation suggested that there could be less lipid accumulation in the plaques of mice lacking CD73 and prompted us to investigate whether differences in lipid metabolism were present between the two genotypes. Consistent with this hypothesis, we found a striking difference in lipolysis between cd73−/−/apoE−/− and apoE−/− mice in spite of similar baseline plasma lipid levels. This strongly supports a role for CD73 in suppressing lipolysis, leading to plaque accumulation as animals aged. We propose that plaque accumulation occurred in apoE−/− mice in the presence of CD73 due to adenosine mediated suppression of lipolysis. In the micro-environment of the atherosclerotic plaque, we theorize that this led to plaque accumulation. In the absence of CD73, lipolysis readily occurred, leading to smaller plaques. In prior in vivo studies, it has been shown that A1 receptors are likely responsible for adenosine-mediated suppression of lipolysis, and variations in cholesterol metabolism can alter atherosclerotic plaque accumulation in apoE−/− mice.17, 40, 41
A limitation of this study is that we did not consider the effect of sex on aortic plaque burden. Prior studies have suggested the possibility of increased atherosclerosis in young female apoE−/− mice compared with males.11 Other studies more relevant to this study on aging suggested no difference or greater aortic lipid accumulation in aged apoE−/− male mice compared to female mice.10, 12 Taken together, we do not have reason to suspect a sex effect on our findings, but further investigation would be required to confirm this.
These data suggest that ectonucleotidases could play a role in age-determined atherosclerosis by nature of the interactions between lipids and purinergic metabolism. In conclusion, CD73 has previously unknown, potent pro-atherosclerotic effects that occur as a function of aging.
Supplementary Material
Highlights.
At early time points, CD73 is subtly protective against the accrual of aortic atherosclerosis. With aging, CD73 allows for accretion of atherosclerotic plaque.
In atherosclerotic plaques of aged mice, CD73, which is responsible for in situ adenosine production, did not temper the inflammatory response nor did it impact the quantity of apoptotic cells.
CD73 can promote atherogenesis, particularly in older mice, through suppression of lipid catabolism.
Acknowledgements
The authors would like to thank Linda F. Thompson, Ph.D. for providing the cd73−/− mouse strain; Yang Zhang, Tony Ren, Breanne Kassarjian, Shakeeta Nicholson, Jun Chen, Sharon Koonse, and Jessica Ray for technical assistance; and Stephen Alvey, for graphical design.
Sources of funding
This work was funded by grants from the NIH (R01 NS087147, R01 HL127151, R01 HL127687, P30DK020572, and T32 HL007853) and from the Taubman Medical Research Institute and the University of Michigan Biology of Cardiovascular Aging Program.
Abbreviations:
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- ApoE
apolipoprotein E
- cAMP
cyclic adenosine monophosphate
- HDL
high density lipoprotein
- PBS
phosphate-buffered saline
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- LDL
low density lipoprotein
Footnotes
Disclosures
None.
References
- 1.Baek AE, Sutton NR, Petrovic-Djergovic D, Liao H, Ray JJ, Park J, Kanthi Y and Pinsky DJ. Ischemic Cerebroprotection Conferred by Myeloid Lineage-Restricted or Global CD39 Transgene Expression. Circulation. 2017;135:2389–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutton NR, Hayasaki T, Hyman MC, Anyanwu AC, Liao H, Petrovic-Djergovic D, Badri L, Baek AE, Walker N, Fukase K, Kanthi Y, Visovatti SH, Horste EL, Ray JJ, Goonewardena SN and Pinsky DJ. Ectonucleotidase CD39-driven control of postinfarction myocardial repair and rupture. JCI Insight. 2017;2:e89504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanthi YM, Sutton NR and Pinsky DJ. CD39: Interface between vascular thrombosis and inflammation. Curr Atheroscler Rep. 2014;16:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohtsuka T, Changelian PS, Bouis D, Noon K, Harada H, Lama VN and Pinsky DJ. Ecto-5’-nucleotidase (CD73) attenuates allograft airway rejection through adenosine 2A receptor stimulation. J Immunol. 2010;185:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grunewald JK and Ridley AJ. CD73 represses pro-inflammatory responses in human endothelial cells. J Inflamm (Lond). 2010;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC and Colgan SP. Crucial role for ecto-5’-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrovic-Djergovic D, Hyman MC, Ray JJ, Bouis D, Visovatti SH, Hayasaki T and Pinsky DJ. Tissue-resident ecto-5’ nucleotidase (CD73) regulates leukocyte trafficking in the ischemic brain. J Immunol. 2012;188:2387–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS and Smith JD. Consideration of Sex Differences in Design and Reporting of Experimental Arterial Pathology Studies-Statement From ATVB Council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, Garcia-Cardena G, Lusis AJ, Owens AP 3rd, Rosenfeld ME, Virmani R, American Heart Association Council on Arteriosclerosis T, Vascular B and Council on Basic Cardiovascular S. Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association. Arterioscler Thromb Vasc Biol. 2017;37:e131–e157. [DOI] [PubMed] [Google Scholar]
- 10.Pereira TM, Nogueira BV, Lima LC, Porto ML, Arruda JA, Vasquez EC and Meyrelles SS. Cardiac and vascular changes in elderly atherosclerotic mice: the influence of gender. Lipids Health Dis. 2010;9:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda N, Johnson L, Kim S, Hagaman J, Friedman M and Reddick R. Anatomical differences and atherosclerosis in apolipoprotein E-deficient mice with 129/SvEv and C57BL/6 genetic backgrounds. Atherosclerosis. 2007;195:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caligiuri G, Nicoletti A, Zhou X, Tornberg I and Hansson GK. Effects of sex and age on atherosclerosis and autoimmunity in apoE-deficient mice. Atherosclerosis. 1999;145:301–308. [DOI] [PubMed] [Google Scholar]
- 13.Tune JD, Richmond KN, Gorman MW, Olsson RA and Feigl EO. Adenosine is not responsible for local metabolic control of coronary blood flow in dogs during exercise. Am J Physiol Heart Circ Physiol. 2000;278:H74–84. [DOI] [PubMed] [Google Scholar]
- 14.Whitesall SE, Hoff JB, Vollmer AP and D’Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol. 2004;286:H2408–415. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman BB, Chang H, Farahbakhsh Z and Reaven G. Inhibition of lipolysis by adenosine is potentiated with age. J Clin Invest. 1984;74:1750–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonnroth P, Jansson PA, Fredholm BB and Smith U. Microdialysis of intercellular adenosine concentration in subcutaneous tissue in humans. Am J Physiol. 1989;256:E250–255. [DOI] [PubMed] [Google Scholar]
- 17.Johansson SM, Lindgren E, Yang JN, Herling AW and Fredholm BB. Adenosine A1 receptors regulate lipolysis and lipogenesis in mouse adipose tissue-interactions with insulin. Eur J Pharmacol. 2008;597:92–101. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V and Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616:7–15. [DOI] [PubMed] [Google Scholar]
- 19.Ernst PB, Garrison JC and Thompson LF. Much Ado about Adenosine: Adenosine Synthesis and Function in Regulatory T Cell Biology. J Immunol. 2010;185:1993–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB and Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K and Eltzschig HK. Cardioprotection by ecto-5’-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. [DOI] [PubMed] [Google Scholar]
- 22.Pescatore LA, Gamarra LF and Liberman M. Multifaceted Mechanisms of Vascular Calcification in Aging. Arterioscler Thromb Vasc Biol. 2019;39:1307–1316. [DOI] [PubMed] [Google Scholar]
- 23.Buchheiser A, Ebner A, Burghoff S, Ding Z, Romio M, Viethen C, Lindecke A, Kohrer K, Fischer JW and Schrader J. Inactivation of CD73 promotes atherogenesis in apolipoprotein E-deficient mice. Cardiovasc Res. 2011;92:338–347. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Zhang W, Zhu C, Bucher C, Blazar BR, Zhang C, Chen JF, Linden J, Wu C and Huo Y. Inactivation of the adenosine A2A receptor protects apolipoprotein E-deficient mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA and Boehm M. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joolharzadeh P and St Hilaire C. CD73 (Cluster of Differentiation 73) and the Differences Between Mice and Humans. Arterioscler Thromb Vasc Biol. 2019;39:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalkanen J, Hollmen M, Jalkanen S and Hakovirta H. Regulation of CD73 in the development of lower limb atherosclerosis. Purinergic Signal. 2017;13:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller G, Wied S, Walz N and Jung C. Translocation of Glycosylphosphatidylinositol-Anchored Proteins from Plasma Membrane Microdomains to Lipid Droplets in Rat Adipocytes Is Induced by Palmitate, H2O2, and the Sulfonylurea Drug Glimepiride. Mol Pharmacol. 2008;73:1513–1529. [DOI] [PubMed] [Google Scholar]
- 29.Visovatti SH, Hyman MC, Bouis D, Neubig R, McLaughlin VV and Pinsky DJ. Increased CD39 nucleotidase activity on microparticles from patients with idiopathic pulmonary arterial hypertension. PloS one. 2012;7:e40829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanthi Y, Hyman MC, Liao H, Baek AE, Visovatti SH, Sutton NR, Goonewardena SN, Neral MK, Jo H and Pinsky DJ. Flow-dependent expression of ectonucleotide tri(di)phosphohydrolase-1 and suppression of atherosclerosis. J Clin Invest. 2015;125:3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Giorgi M, Enjyoji K, Jiang G, Csizmadia E, Mitsuhashi S, Gumina RJ, Smolenski RT and Robson SC. Complete deletion of Cd39 is atheroprotective in apolipoprotein E-deficient mice. J Lipid Res. 2017;58:1292–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jalkanen J, Yegutkin GG, Hollmen M, Aalto K, Kiviniemi T, Salomaa V, Jalkanen S and Hakovirta H. Aberrant circulating levels of purinergic signaling markers are associated with several key aspects of peripheral atherosclerosis and thrombosis. Circ Res. 2015;116:1206–1215. [DOI] [PubMed] [Google Scholar]
- 33.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC and Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa T, Bouis D, Liao H, Visovatti SH and Pinsky DJ. Ecto-5’ nucleotidase (CD73)-mediated adenosine generation and signaling in murine cardiac allograft vasculopathy. Circ Res. 2008;103:1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chadjichristos CE, Scheckenbach KE, van Veen TA, Richani Sarieddine MZ, de Wit C, Yang Z, Roth I, Bacchetta M, Viswambharan H, Foglia B, Dudez T, van Kempen MJ, Coenjaerts FE, Miquerol L, Deutsch U, Jongsma HJ, Chanson M and Kwak BR. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation. 2010;121:123–131. [DOI] [PubMed] [Google Scholar]
- 36.Lendon CL, Davies MJ, Born GV and Richardson PD. Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis. 1991;87:87–90. [DOI] [PubMed] [Google Scholar]
- 37.Zernecke A, Bidzhekov K, Ozuyaman B, Fraemohs L, Liehn EA, Luscher-Firzlaff JM, Luscher B, Schrader J and Weber C. CD73/ecto-5’-nucleotidase protects against vascular inflammation and neointima formation. Circulation. 2006;113:2120–2127. [DOI] [PubMed] [Google Scholar]
- 38.Peyot ML, Gadeau AP, Dandre F, Belloc I, Dupuch F and Desgranges C. Extracellular adenosine induces apoptosis of human arterial smooth muscle cells via A(2b)-purinoceptor. Circ Res. 2000;86:76–85. [DOI] [PubMed] [Google Scholar]
- 39.Mikhailov A, Sokolovskaya A, Yegutkin GG, Amdahl H, West A, Yagita H, Lahesmaa R, Thompson LF, Jalkanen S, Blokhin D and Eriksson JE. CD73 participates in cellular multiresistance program and protects against TRAIL-induced apoptosis. J Immunol. 2008;181:464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castan I, Valet P, Quideau N, Voisin T, Ambid L, Laburthe M, Lafontan M and Carpene C. Antilipolytic effects of alpha 2-adrenergic agonists, neuropeptide Y, adenosine, and PGE1 in mammal adipocytes. Am J Physiol. 1994;266:R1141–1147. [DOI] [PubMed] [Google Scholar]
- 41.Grosskopf I, Shaish A, Afek A, Shemesh S, Harats D and Kamari Y. Apolipoprotein A-V modulates multiple atherogenic mechanisms in a mouse model of disturbed clearance of triglyceride-rich lipoproteins. Atherosclerosis. 2012;224:75–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.