Abstract
Diabetic Retinopathy (DR) is a major cause of visual dysfunction, yet much remains unknown regarding the specific molecular events that contribute to diabetes-induced retinal pathophysiology. Herein, we review the impact of oxidative stress on DR, and explore evidence that supports a key role for the stress response protein regulated in development and DNA damage (REDD1) in the development of diabetes-induced oxidative stress and function defects in vision. It is well established that REDD1 mediates the cellular response to a number of diverse stressors through repression of the central metabolic regulator known as mechanistic target of rapamycin complex 1 (mTORC1). A growing body of evidence also supports that REDD1 acts independent of mTORC1 to promote oxidative stress by both enhancing the production of reactive oxygen species and suppressing the antioxidant response. Collectively, there is strong preclinical data to support a key role for REDD1 in the development and progression of retinal complications caused by diabetes. Furthermore, early proof-of-concept clinical trials have found a degree of success in combating ischemic retinal disease through intravitreal delivery of an siRNA targeting the REDD1 mRNA. Overall, REDD1-associated signaling represents an intriguing target for novel clinical therapies that go beyond addressing the symptoms of diabetes by targeting the underlying molecular mechanisms that contribute to DR.
Graphical Abstract
1. Introduction
Diabetic retinopathy (DR) is the leading cause of acquired blindness in working-age adults in western countries, with approximately 90% of cases presenting within 25 years of developing diabetes (1). DR is clinically defined by damage to the retinal microvasculature resulting in microaneurysms, hemorrhaging, angiogenesis and in the worst cases retinal detachment and blindness (2, 3). Clinical trials demonstrate that blocking vascular endothelial growth factor (VEGF) signaling can reduce edema and improve vision in patients with DR, but such interventions are effective in less than half of patients (4). One of the limitations of this approach is that it largely addresses the microvascular dysfunction and neovascularization that characterize later stages of disease progression. However, neuro-glial deficits can precede and often predict the development of microvascular disease in the retina of diabetic patients (5, 6). There is increasing consensus that a host of molecular events precede the clinically visible vascular pathologies of DR and cause significant metabolic and functional defects in retinal neurons and glia (7–11). Fluid accumulation into the neural retina, known as diabetic macular edema (DME), is most closely associated with an impairment in visual acuity; however, visual field loss and deficits contrast sensitivity can develop prior to overt microvascular dysfunction and cause significant visual difficulties, particularly in low light conditions. Despite extensive research, treatment options for DR that are preventative and/or provide interventions early in the preclinical and non-proliferative stages of DR are limited. Thus, there remains a need for therapeutics to address the initiating cause of retinal pathology. Developing an improved understanding of the molecular events that contribute to the development and progression of DR is critical for the design and implementation of improved therapeutic approaches for DR.
2. Diabetes-induced hyperglycemia as a driving factor in oxidative stress
A major causal factor in the development and progression of DR is hyperglycemia. The Diabetes Complications and Control Trial (DCCT) and the UK Prospective Diabetes Study (UKPDS) established that hyperglycemia is the leading contributor to the clinical manifestations of DR in both type 1 and type 2 diabetes (12, 13). The reports demonstrated that both the onset and progression of DR can be delayed with intensive glycemic control; though often difficult to achieve. Four principal pathways are believed to be responsible for hyperglycemia-induced tissue damage: the polyol pathway, advanced glycation end-products (AGE) pathway, the hexosamine biosynthetic pathway (HBP), and the protein kinase C (PKC) pathway (14–21). Each of these pathways has been well-studied and linked to DR [reviewed in (22)]. However, clinical trials evaluating inhibitors of these individual pathways have seen lackluster results in ameliorating DR progression (22).
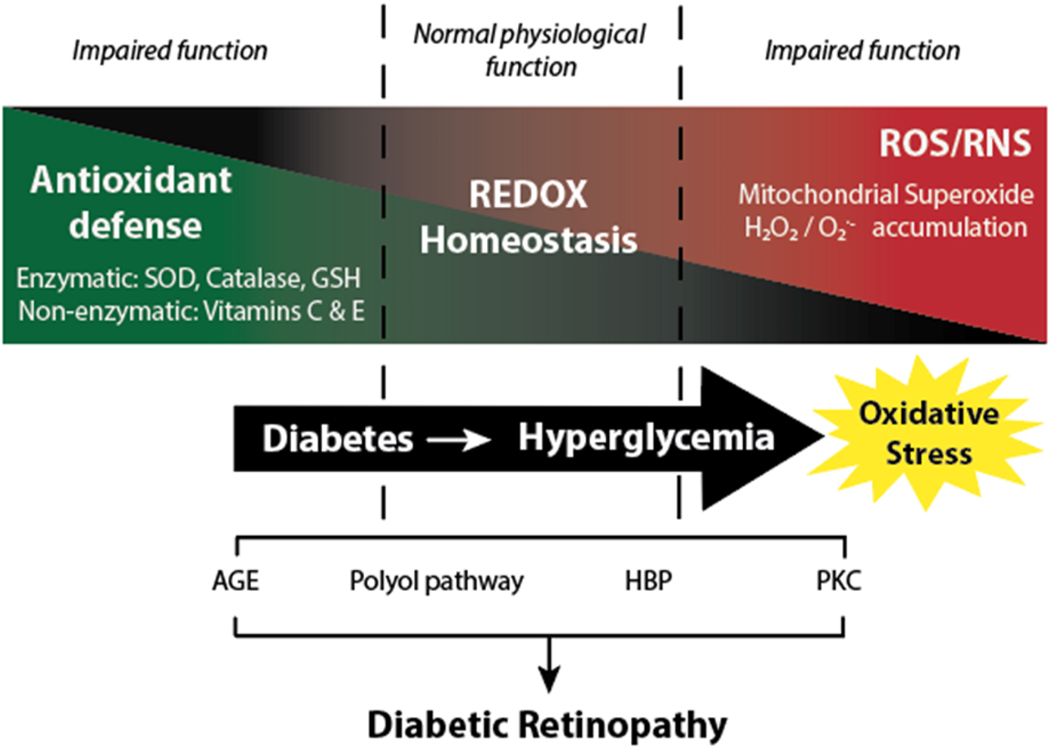
A unifying theory for the pathophysiology of diabetic complications suggests the four key pathways responsible for hyperglycemia-induced tissue damage are all linked to the excess production of reactive oxygen species (ROS) (22). Acute bursts of ROS play an important signaling role in proper physiological function (23). These effects are mediated, in large part, through the covalent modification of cysteine residues in reduction-oxidation (REDOX)-sensitive enzymes (24). However, prolonged exposure to excessive ROS levels damages all macromolecules, including proteins, nucleic acids, and lipids, leading to impaired physiological function. Thus, cells must maintain REDOX homeostasis by balancing the generation and elimination ROS (Fig. 1). A major source of ROS is the mitochondrial electron transport chain (ETC). In the retina, the outer segments of the photoreceptors and retinal pigment epithelium (RPE) contain particularly high mitochondrial density (25). Other sources of ROS include NAD(P)H oxidases, xanthine oxidase, and retinal excitation by light (26, 27). Focusing of light by the cornea and lens also results in an elevated blue light exposure that promotes formation of free radicals in the photoreceptors (25, 28).
Figure 1. Diabetes-induced hyperglycemia disrupts REDOX homeostasis in retina.
Under normal physiological conditions, there is a balance in the generation of free radicals and the antioxidant defense mechanisms. Diabetes-induced hyperglycemia promotes the production of reactive oxygen species (ROS)/reactive nitrogen species (RNS) and suppresses the retinal antioxidant response, resulting in a condition known as oxidative stress. The advanced glycation end-product (AGE) pathway, the polyol pathway, the hexosamine biosynthetic pathway (HBP), and the protein kinase C (PKC) pathway play an important role in hyperglycemia-induced tissue damage. All four pathways are associated REDOX imbalance and increased oxidative stress.
The ETC mediates the production of ATP via a voltage gradient across the inner mitochondrial membrane (ΔΨm) that drives ATP synthase (29, 30). Oxygen plays a critical role in the ETC, as the final electron acceptor; wherein the reduced oxygen picks up two protons to form water. Alternatively, 0.1%−5% of oxygen that enters the ETC is partially reduced to form mitochondrial superoxide (O2-). Evidence supports that hyperglycemic conditions increase ΔΨm in a manner that leads to excess production of superoxide (19, 31, 32). Enhanced glycolytic flux and the increased availability of substrates for the TCA cycle result in a surplus of electron donors entering the ETC via complexes I and II (32, 33). This increases ΔΨm until Complex III becomes overloaded and excess electrons are donated via Coenzyme Q to oxygen, resulting in production of superoxide (34). Evidence substantiates the hypothesis that hyperglycemic conditions activate a multi-component feedback loop that contributes to a high ΔΨm and the excess production of free radicals (35). The glycolytic enzyme mitochondrial hexokinase (HK) plays a particularly important role in regulating ΔΨm. Specifically, HK facilitates local ADP recycling through the VDAC (voltage-dependent anion channel)/ANT (adenine nucleotide translocator) complex to ATP synthase complex, which uses ΔΨm to generate ATP (36). Increased ROS levels in cells exposed to hyperglycemic conditions are prevented by inhibition of ETC flux, excess proton leak across the inner mitochondrial membrane, or increased expression of superoxide scavengers (31).
Often coincident with increased ROS levels is the production of reactive nitrogen species (RNS) including nitroxyl anion, nitrosonium cation, and peroxynitrite. Increased activity and expression of endothelial nitric oxide synthase (eNOS) is commonly seen in models of type 1 and type 2 diabetes (37–39). Elevated NOS levels can result in increased production of nitric oxide and consequently development of vascular and neurological complications (40). Though nitrous oxide plays an important role normal cellular function, its reaction with superoxide ion can manifest tissue damage through the production of peroxynitrite radical. Uncoupled nitric oxide synthase leads to increased peroxynitrite formation, which is believed to contribute to the cytotoxic effects of hyperglycemic conditions(41–43).
In addition to promoting ROS/RNS production, diabetes-induced hyperglycemia is associated with a failure to sufficiently upregulate the cellular antioxidant response (44–46). In fact, clinical examinations have shown a significant decrease in antioxidant levels in patients diagnosed with type 1 and type 2 diabetes (47–50). The resulting imbalance between the production of ROS and the cellular antioxidant defense results in a condition known as oxidative stress. The cellular antioxidant defense system consists of both enzymatic and non-enzymatic antioxidants that are responsible for scavenging free radicals and non-radical oxidants (51). This includes enzymes such as superoxide dismutase, catalase, and those that are important for the synthesis of glutathione (GSH), one of the most abundant intracellular antioxidants (52–55). The transcription factor nuclear factor erythroid-related factor 2 (Nrf2) is a key regulatory element of the cellular antioxidant response (56, 57), and evidence supports a suppressive effect of hyperglycemic conditions on Nrf2 activation (58–60). Nrf2 promotes the transcription of antioxidant genes by binding to an enhancer region of the promoter sequence known as an antioxidant response element (ARE) (57, 61). The Nrf2 gene battery consists of more than 200 genes associated with not only REDOX balance, but also processes like inflammation and proteostasis. Nrf2-regulated genes include NAD(P)H quinone oxidoreductase (NQO1), Heme oxygenase 1 (HO-1) and subunits of Glutathione cysteine ligase (GCLC and GCLM) (62). Hyperglycemic conditions also negatively influence levels of non-enzymatic antioxidants such as β-carotene, vitamin C, and vitamin E (63).
3. Oxidative stress and diabetic retinopathy
The retina is composed of a variety of cells that have a high metabolic activity, and thus consume large amounts of oxygen and glucose (64). The retina also contains the highest concentration of polyunsaturated fatty acids (PUFAs) in the entire body, which are particularly vulnerable to lipid peroxidation (65). As a result, the retina is highly susceptible to the changes in REDOX homeostasis and the oxidative stress that result from diabetes-induced hyperglycemia. Clinical data from diabetic patients have correlated the formation of free radicals with the development of DR (66). A number of in vivo studies have also reported elevated ROS (26, 67–70) and RNS (71–74) in the retina of diabetic rodents. In DR, the accumulation of free radicals has been associated with activation of the four metabolic pathways responsible for hyperglycemia-induced tissue damage (22, 25, 75, 76). Activation of these metabolic pathways and others, culminate in hyperglycemia-induced tissue damage and apoptosis of retinal neurons and microvascular cells (77, 78). Exposure of endothelial cells, pericytes, and neurons to hyperglycemic conditions facilitates the production of free radicals coincident with cell death (52, 70, 79, 80). Retinal Müller cells, astrocytes, and photoreceptors are also impacted early in DR progression and their role in disease pathogenesis with regards to ROS/RNS production has been studied by a number of laboratories (81–84). In a rodent models of diabetes, the onset of hyperglycemia coincided with elevated nitric oxide and peroxynitrite formation in the retina (85). In the retina of diabetic mice, RNS production was significantly elevated shortly after diabetes onset, suggesting a potential role in the early tissue injury (86).
Diabetes-induced hyperglycemia also acts to suppress the retinal antioxidant defense (87). When compared to non-diabetics, pericytes isolated from post-mortem retinas of diabetic patients exhibited downregulation of mRNAs encoding the scavenging enzymes glutathione reductase and copper-zinc superoxide dismutase upon exposure to hyperglycemic culture conditions (88). In both preclinical models of diabetes and post-mortem samples from diabetic patients, retinal Nrf2 DNA-binding activity was found to be blunted (56, 89). In preclinical models, streptozotocin (STZ)-induced diabetes reduced retinal GSH levels and increased lipid peroxidation (90, 91). In vitro studies exposing bovine pericytes to AGEs have also documented a decrease in intracellular catalase and SOD activities (92).
A number of excellent studies support the potential benefits of antioxidant supplementation for combating DR pathology (93). In STZ-diabetic rats, administration of vitamins C and E reduced the appearance acellular capillaries and pericyte ghosts along with the development of DR in alloxan induced-diabetic rats (52). In vitro treatment with α-lipoic acid and N-acetyl cysteine (NAC) inhibited oxidative stress-induced damage in retinal endothelial cells, pericytes, and neuronal precursor cells (70, 94). NAC is a precursor of the amino acid cysteine, which is required for GSH synthesis. Dietary NAC supplementation attenuated the development of vascular pathology in the retina of STZ-diabetic rats (95), and our laboratory has shown that it prevents retinal cell death and contrast sensitivity deficits in STZ-diabetic mice (70). Administration of α-lipoic acid also prevented retinal capillary cell death and the development of microvascular defects in diabetic rats (94). However, epidemiological data from multiple clinical trials found no protective effect of β-carotene or vitamin C and E supplementation on DR (96–98). While clinical intervention with antioxidant administration should continue to be explored, efforts must also be targeted at understanding ways to restore and promote endogenous antioxidant production within the retina.
4. REDD1 as stress response factor associated with ROS
Regulated in Development and DNA Damage response 1 (REDD1, aka RTP801 or Dig2) is a 25 kDa protein encoded by the gene DNA Damage Inducible Transcript 4 (DDIT4). REDD1 is ubiquitously expressed at low levels in most adult human and mouse tissues, but is robustly upregulated in response to a variety of cell stresses. REDD1 was discovered and cloned in 2002 by two separate laboratories. Investigating hypoxia-regulated genes in rat glioma cells, Shoshani and colleagues identified REDD1 as a target gene for hypoxia-inducible factor 1 (HIF-1) that was involved in regulating ROS levels (99). REDD1 was concurrently identified by Ellisen and colleagues as a transcriptional target of p53 that was induced by DNA damage and directly correlated with intracellular ROS levels (100). While it had been previously suggested that ROS may be a means to promote p53-dependent apoptosis (101, 102), Ellisen et al. hypothesized that co-regulation of ROS by REDD1 and p63 was a mechanism for cellular differentiation and proliferation, wherein ROS act as secondary messengers to promote these processes (100).
Following its discovery, REDD1 expression was found to be influenced by a variety of stimuli including heat-shock, ionizing radiation, energy stress, glucocorticoids, and hyperglycemia; and could be chemically induced via dopaminergic toxins, ER stress inducers and DNA damaging agents (103–109). A common thread that connects each of these is the increased production of free radicals. In preclinical rodent models of type 1 and type 2 diabetes, retinal ROS levels were enhanced (67, 68, 70, 110, 111) and REDD1 protein content was increased (70, 83, 112, 113). However, in the retina of STZ-diabetic mice treated with NAC, retinal ROS levels were attenuated and REDD1 protein content was normalized (70). In R28 retinal cell cultures, exposure to hyperglycemic conditions promoted cellular ROS levels and enhanced REDD1 expression (70). Our laboratory also demonstrated that increased cellular ROS levels in cells exposed to H2O2 were sufficient to enhance REDD1 protein expression (102, 114). This suggests that ROS levels are an important regulator of REDD1 expression in the retina.
5. Models of REDD1 action
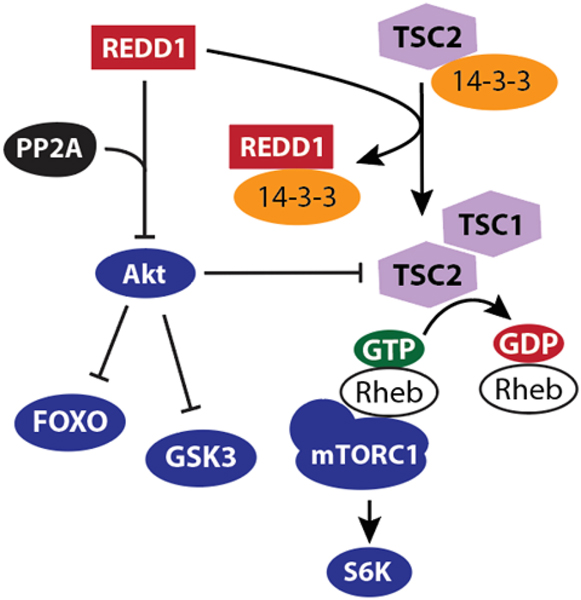
A landmark discovery in understanding REDD1 function was the observation that Scylla, the drosophila homolog of REDD1, repressed the phosphorylation of S6 Kinase (S6K), a substrate of the kinase target of rapamycin (TOR) (115). The results were later supported by the finding that REDD1 suppressed activation of mammalian target of rapamycin complex 1 (mTORC1) (116). Work extending from this finding (117) suggested that the inhibitory action of REDD1 on mTORC1 was due to its ability to activate the mTORC1 repressor tuberous sclerosis factor complex 2 (TSC2). TCS2 acts in a complex with TSC1 as a GTPase activating protein (GAP) for Ras homolog enriched in brain (Rheb). Rheb-GTP is an essential activator of mTORC1 phosphotransferase activity (118). Early studies led to development of a model (Fig. 2) wherein the competitive sequestration of 14-3-3 scaffolding proteins by REDD1 prevented formation of a TSC2/14-3-3 complex, thus freeing TSC2 to associate with its binding partner TSC1 and promote Rheb GTPase activity (119). However, structural analysis of the REDD1 protein suggested that the 14-3-3 binding motif of REDD1 was not conserved and immunoprecipitation studies failed to support a direct interaction between REDD1 and 14-3-3 proteins (120).
Figure 2. REDD1 acts via TSC2 to suppress mTORC1 by promoting Rheb GTPase activity.
Two working models have been proposed for the mechanism whereby REDD1 acts to suppress mTORC1. In both models, the suppressive effect of REDD1 on mTORC1 is mediated by a reduction in the proportion of GTP bound to Rheb. In the first model, REDD1 binds 14-3-3 protein to prevent TSC2 sequestration in an inactive complex. In the second, REDD1 promotes dephosphorylation of Akt at Thr308 by PP2A. Akt-dependent phosphorylation of TSC2 suppresses the GAP activity of TSC2 toward Rheb. In addition, REDD1-dependent dephosphorylation of Akt also influences other important targets downstream of Akt, including FOXO transcription factors and GSK3.
An alternative model for TSC2-dependent suppression of mTORC1 by REDD1 was demonstrated by our laboratory in a series of studies that utilized human embryonic kidney (HEK293) cells with tetracycline-inducible REDD1 expression (121). In the absence of cellular stress, REDD1 facilitated the recruitment of protein phosphatase 2A (PP2A) to Akt, and site specific dephosphorylation of Akt at T308 (121). Phosphorylation of Akt at T308 and S473 is required for maximal kinase activity (122); however, phosphorylation of Akt substrates is differentially affected by variation in Akt multi-site phosphorylation (123). TSC2 is a direct target of Akt, and Akt-dependent phosphorylation of TSC2 promotes its GAP activity (124). Thus, REDD1 mediates recruitment of PP2A to dephosphorylate Akt, restrict Rheb-GTP loading, and suppress mTORC1 activation (121). Importantly, these two models of TSC2-dependent mTORC1 suppression are not necessarily mutually exclusive, and the specific signaling events whereby REDD1 acts may depend on cell type and stress condition.
Independent of an effect on mTORC1, the repressive effect of REDD1 on Akt phosphorylation has implications with regards to numerous downstream effectors that influence cell survival, proliferation, neuroprotection, and metabolism [e.g. the forkhead box O (FoxO) family of transcription factors and glycogen synthase kinase 3 (GSK3)]. In models of Parkinson’s disease, REDD1 reduced Akt phosphorylation resulting in increased neuronal cell death (106, 125, 126). Moreover, evidence supports a role for REDD1 in both glucocorticoid- and diabetes-induced muscle atrophy via suppression of Akt-dependent phosphorylation events (127, 128). Similarly, studies from our laboratory supported that exposure to hyperglycemic conditions suppressed cell survival by REDD1-dependent Akt repression in the retina of STZ-diabetic mice and R28 retinal cells (105).
6. REDD1 as a positive regulator of ROS production
A number of prior studies provide evidence that REDD1 is a positive regulator of cellular ROS (Table 1). Tissues of REDD1-deficient mice and REDD1 knockout cell lines exhibited reduced basal ROS levels (129). Notably, cells deficient for TSC2 exhibited an increase in ROS levels, as opposed to the decrease that was seen with REDD1 ablation, suggesting that an increase in mTORC1 activity was not sufficient to recapitulate the suppressive effect of REDD1 deletion on ROS levels (129). A number of studies have demonstrated that REDD1 is necessary for stress-induced ROS. For example, REDD1 knockdown prevented lipopolysaccharide-induced oxidative stress in endothelial cell cultures (130). Similarly, REDD1 knockdown suppressed mitochondrial ROS generation in bone marrow mesenchymal stromal cells exposed to ionizing radiation (131). With regards to DR, REDD1 deletion prevented the increase in retinal ROS levels in STZ-diabetic mice (70). This observation is supported by cell culture studies that we performed in both retinal R28 and MIO-M1 cell cultures, wherein an increase in cellular ROS levels in cells exposed to hyperglycemic conditions required REDD1 (70, 83).
Table 1.
REDD1 contributes to the development of oxidative stress
| Model | Cell type or Tissue | Key Findings | PMID |
|---|---|---|---|
| Diabetic retinopathy | Retinal cells and tissue | REDD1 deletion prevents neurodegeneration, ERG defects, and deficits in functional vision in STZ-mice | 29074598 |
| REDD1 deletion normalizes oxidative stress in retina of STZ-mice | 31141608 | ||
| REDD1 promotes Nrf2 degradation | 32295843 | ||
| Retinopathy of prematurity | Retina | REDD1 deletion prevents hypoxia-induced vaso-obliteration, neovascularization, and retinal cell apoptosis | 15452091 |
| Hypoxia | Glioma cells | REDD1 elevated under conditions that promote HIF-1 stabilization and ROS | 11884613 |
| Dry eye disease | Human corneal epithelial cells and whole cornea | REDD1 knockdown reduces ROS levels | 31266058 |
| DNA damage | Fibroblasts and osteosarcoma cells | REDD1 increases ROS levels in p63-null cells | 12453409 |
| Cancer | Prostate cancer cells and fibroblasts | REDD1 inhibits activation of PDH and promotes of HIF-1 accumulation | 24515947 |
| Fibroblasts, human breast carcinomas and mice | REDD1 expression associated with HIF-1 and ROS levels | 20176937 | |
| Stress-induced autophagy | Fibroblasts, osteosarcoma cells and splenocytes | REDD1 and TXNIP form a pro-oxidant complex to induce autophagy through induction of ROS | 25916556 |
| Vascular endothelial injury | Endothelial cells (HUVEC) | REDD1-TXNIP complex promotes inflammatory cytokine secretion, ROS and apoptosis | 30485138 |
| Cigarette smoke-induced lung damage | Lung | REDD1 amplifies ROS-associated pathology. REDD1 deletion prevents emphysema | 20473305 |
| Mouse Lung Fibroblasts | REDD1 promotes ROS by enhancing Nox4.
REDD1 necessary for downregulation of Sod2 and Gpx1 |
2556956 | |
| Ionizing radiation | Bone marrow mesenchymal stromal cells | REDD1 induction reduces mitochondrial ROS production, autophagy, and apoptosis | 30846680 |
| Ischemia reperfusion | Cardiomyocytes | REDD1 depletion ameliorates oxidative stress modulated by Akt/mTORC1/Nrf2 signaling | 31493869 |
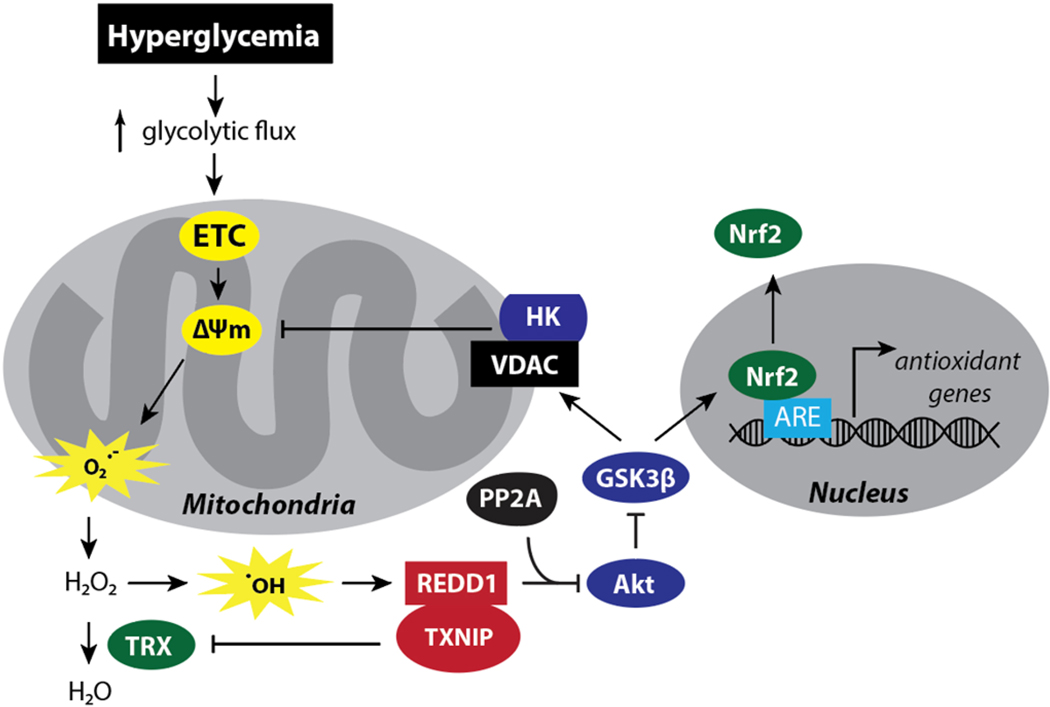
Our laboratory has also provided evidence that REDD1 is necessary for hyperglycemic conditions to enhance ΔΨm (Fig. 3) (70). Upon an increase in the glucose flux through the central metabolic pathways, Hexokinase II (HKII) translocates to the mitochondria to interact with voltage-dependent ion channel (VDAC), an event mediated by GSK3β-dependent phosphorylation of VDAC (132). Akt is an important regulator of this process, as the kinase directly phosphorylates GSK3β to block substrate recognition (133, 134). The selective dephosphorylation of Akt on T308 that occurs in response to REDD1 was associated with decreased phosphorylation of GSK3β (121). In turn, increased GSK3β activity is associated with transient hyperglycemia-induced ROS production and retinal cell death (35, 132). Akt kinase activity was attenuated in the retina of STZ-diabetic rodents (105, 135). However, REDD1 deletion was sufficient to prevent the diabetes-induced deficit in retinal Akt activity (105) and restore GSK3 phosphorylation in the retina of STZ-diabetic mice to levels observed in the retina of non-diabetic mice (83). In R28 cells exposed to hyperglycemic conditions, enhanced REDD1 expression was required to increase ΔΨm (70). However, expression of a dominant negative form of Akt or a constitutively active GSK3 was sufficient to enhance ΔΨm and promote ROS levels in REDD1-deficient R28 cells exposed to hyperglycemic conditions (70). Together these studies demonstrated a critical role for REDD1 in regulating mitochondrial ROS via inhibition of the Akt/GSK3β signaling axis.
Figure 3. REDD1 contributes to diabetes-induced oxidative stress by increasing mitochondrial ROS production and suppressing the antioxidant response.
REDD1 and TXNIP protein expression are enhanced in the retina by diabetes and hyperglycemic conditions. REDD1 and TXNIP form a pro-oxidant complex and both proteins are required for suppression of thioredoxin (TRX) antioxidant function. Evidence also supports that REDD1 contributes to enhanced mitochondrial ROS production in response to hyperglycemic conditions through activation of a multi-component feedback loop. Hyperglycemia promotes electron transport chain (ETC) flux, increasing the voltage gradient across the inner mitochondrial membrane (ΔΨm), thereby the production of mitochondrial superoxide (O2.-). REDD1 acts to maintain a high ΔΨm via suppression of Akt/GSK3β signaling. GSK3β phosphorylates the voltage-dependent anion channel (VDAC) to suppress mitochondrial localization of hexokinase (HK). HK uses ATP produced by the mitochondria to phosphorylate glucose, and thus provides local ADP recycling at the expense of ΔΨm. Thus, REDD1-dependent activation of GSK3β helps to maintain a high ΔΨm. GSK3β also directly phosphorylates the transcription factor Nrf2 to promote its nuclear exclusion and attenuated expression of antioxidant genes with Antioxidant Response Element (ARE) promoters.
7. REDD1 as a negative regulator of the antioxidant response
REDD1 also acts to promote ROS levels, at least in part, by suppressing the endogenous antioxidant response (Fig. 3). REDD1 forms a pro-oxidant complex with thioredoxin inhibiting protein (TXNIP) (129). TXNIP is well known for suppressing thioredoxin (TRX) antioxidant function, but also has a number of other important binding partners, including REDD1 (136). Notably, a potential role for TXNIP in DR pathology is supported by preclinical models [reviewed in (137)]. Cellular stress resulting from hypoxia or exercise upregulated the REDD1-TXNIP complex to promote ROS levels (129). Co-immunoprecipitation studies showed that REDD1-TXNIP association was enhanced by hypoxia or energy stress (129). Furthermore, it was determined that REDD1 potentiated TRX inhibition by TXNIP. Deletion of either REDD1 or TXNIP enhanced TRX activity and reduced ROS levels (129). Overexpression of TXNIP alone in REDD1-deficient cells did not increase ROS levels, but reintroduction of REDD1 was sufficient to do so.
Studies have also demonstrated that REDD1 acts to repress the cellular antioxidant response through negative regulation of Nrf2 (83, 138). In the absence of oxidative stress, Nrf2 is sequestered in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1) (139). Keap1 is a homodimer that binds to Nrf2 and presents it for ubiquitination by the E3 ligase Cullin 3, so that it may be subsequently targeted to the proteasome for degradation. Keap1 is redox-sensitive, as oxidation of multiple cysteine residues in Keap1 reduces binding affinity for Nrf2, and thus allows Nrf2 to translocate into the nucleus to promote ARE-dependent transcription (140). A range of therapeutics that attempt to augment Nrf2 activity by disrupting Keap1-mediated Nrf2 degradation have gained significant attention as options to combat oxidative stress in different disease states (141). Prior studies have consistently demonstrated that diabetes enhances oxidative stress in the retina (140, 142). However, prior studies have also demonstrated a failure to upregulate ARE-mediated transcription in DR models (56, 89, 143). In fact, Nrf2 nuclear localization was reduced in both retinal epithelial cells cultured under hyperglycemic conditions and in isolates obtained from STZ-induced diabetic rats (56, 89). This created a paradox, because the oxidative stress associated with the diabetic metabolic environment should result in modification of redox-sensitive Keap1 cysteine residues to enhance Nrf2 nuclear translocation.
In addition to Keap1-mediated regulation, Akt/GSK3 signaling is an important regulator of Nrf2 action (144) (145). Specifically, GSK3 phosphorylates Nrf2 to promote its nuclear export and degradation by a protein complex consisting of the adapter protein beta transducing repeat containing protein (β-TrCP) and the E3 ubiquitin ligase S-phase kinase-associated protein1-Cullin1–F-box protein (145). Thus, the diabetes-induced defect in retinal Nrf2 activation may be caused by the suppressive effect of REDD1 on retinal Akt/GSK3 signaling (135). As a result, diabetes-induced defects in Nrf2 activation may not be fully addressed by Keap1 targeting. Indeed, a recent study from our laboratory supported that the protective effects of decreasing REDD1 expression on oxidative stress were, in part, due to Keap1-independent Nrf2 upregulation (83, 138). Nrf2 activity in the retina of REDD1-deficient mice was significantly elevated and insensitive to the suppressive effect of diabetes (83). In fact, REDD1 promoted GSK3-dependent proteasomal degradation of Nrf2, even in the presence of pharmacological Keap1 inhibition or targeted mutations to Nrf2 that disrupt Keap1 binding (83). By contrast, pharmacological inhibition of GSK3 or alanine substitutions at the GSK3 phosphorylation sites in Nrf2 blocked the suppressive effects of REDD1 on Nrf2 expression. These data suggest that targeting REDD1-mediated GSK3 activation may be beneficial in augmenting the Nrf2 antioxidant response in diabetes. Indeed, despite its toxicity and remarkably narrow therapeutic window, the archetypal GSK3 inhibitor, lithium chloride, decreased TUNEL-positive nuclei in the retina of STZ-diabetic rats (146). Moreover, in the retina of STZ-diabetic mice, GSK3 suppression with VP3.15 enhanced Nrf2 activity and prevented diabetes-induced ROS (83).
8. Protective effects of REDD1 deletion on retinal pathology
A role for REDD1 in retinal pathology was first demonstrated in an experimental model of retinopathy of prematurity (ROP) (147). In this model, newborn pups are exposed to 75% oxygen and later transferred to normoxic conditions. As a result, the retina develops hypoxia, vasoproliferation, and neurodegeneration (148, 149), which are hallmarks of ischemic retinopathies, including DR. In the ROP model, mice deficient for REDD1 exhibited reduced retinal vaso-obliteration and a marked reduction in neovascularization as compared to wild-type (147). Moreover, the number of TUNEL-positive nuclei in the inner nuclear retinal layers of these mice was reduced by REDD1 deletion. A similar protective role for REDD1 knockout in retinal neurodegeneration was observed in STZ-diabetic mice (105). Defects in Akt signaling play a key role in the retinal neurodegeneration that is caused by diabetes (150). In fact, subconjunctival insulin administration was sufficient to prevent diabetes-induced attenuation of retinal Akt kinase activity and retinal cell death in STZ-diabetic rats (151). Similarly, the suppressive effects of diabetes on Akt kinase activity was not observed in the retina of mice deficient for REDD1, and diabetes-induced retinal cell death was absent (105).
REDD1 deletion also had protective effects in maintaining retinal function in STZ-diabetic mice (105). Deficiencies in the electrical response to the neural retina to flashes of light is one of the earliest signs of functional deficits in diabetic patients (152). Oscillatory potentials (OP) of the electroretinogram (ERG) are among the most commonly reported ERG abnormalities associated with DR (153), as they have been shown to predict the onset and progression of DR (154). These high frequency wavelets occur in the initial phase of the b-wave and are indicative of inner retinal function (155). Changes in OP and b-waves have been observed in rodent models of diabetes, almost immediately after the onset of hyperglycemia (152, 156). In wild-type mice, diabetes suppressed mean OP amplitude and extended OP latency; however, neither of these diabetes-induced defects were observed in the retina of REDD1-deficient mice (105). Diabetes has been found to decrease b-wave amplitudes in REDD1-deficient mice, however the magnitude of the effect was markedly reduced compared to that observed in wild-type mice.
Protective effects of REDD1 deletion on retinal function have also been observed with behavioral optometry (105). STZ-diabetic wild-type mice exhibited functional deficiencies in visual acuity (spatial frequency threshold) and contrast sensitivity shortly after the onset of hyperglycemia, whereas REDD1-deficient diabetic mice did not show the same deficits. An important caveat to this observation is that older REDD1-deficient mice exhibited a modest impairment in visual acuity, as compared to age-matched wild-type mice. Regardless, diabetes-induced attenuation in visual function was absent. Notably, the protective effects of REDD1 deletion on contrast sensitivity suggests that diabetes-induced REDD1 may disrupt information processing in the inner retina as opposed to the outer retina and photoreceptors (157). This is consistent with localization of the REDD1 mRNA to the inner retina (147).
9. Retinal REDD1 suppression in clinical trials
Due to the remarkable effects of REDD1 deletion in preclinical retinopathy models, REDD1 knockdown has been pursued as a therapeutic intervention. PF-04523655 is an 19 nucleotide O-methyl stabilized small interfering RNA (siRNA) that targets the REDD1 mRNA for cleavage by the RNA-induced silencing complex (158). When administered to STZ-diabetic rats via intravitreal injection, PF-04523655 attenuated REDD1 mRNA abundance, and the suppressive effect was maintained for up to 2-weeks (152). The DEGAS study was a prospective, randomized Phase 2 clinical trial to evaluate the safety and efficacy of PF-04523655 in patients with DME (158). The study was ultimately terminated early, due to interim analysis suggesting that significantly higher doses would be necessary to produce therapeutic effects superior to VEGF blockade. However, in patients treated with PF-04523655 there was a trend toward improvement in best corrected visual acuity (BCVA) when compared to focal/grid laser (+5.8 letters with 3 mg PF-04523655 versus +2.4 letters with laser, p = 0.08) (158). In a secondary analysis of patients that completed 12-month follow up, mean improvement in BCVA with 3 mg PF-04523655 was +9.1 letters, versus +3.2 with laser treatment (p<0.01). Patients treated with PF-04523655 showed no improvement in anatomical features or changes in fluorescein leakage, suggesting the benefits were independent of an effect on vascular permeability. In 2011, a Phase 2b study was initiated to explore intervention with higher doses of PF-04523655 with and without ranibizumab (MATISSE study, ClinicalTrials.gov Identifier: NCT01445899); but to date, there is no publicly available data comparing the effects of PF-04523655 to anti-VEGF antibodies in the treatment of DME.
A comparison between ranibizumab and PF-04523655 was carried out in the MONET Phase 2 clinical trial to assess their efficacy in age-related macular degeneration (159). In that trial, ranibizumab outperformed PF-04523655 as a monotherapy, but combining 1 mg PF-04523655 with ranibizumab increased the average gain in BCVA as compared to either monotherapy. Notably, up to half of patients with DME failed to fully respond to anti-VEGF therapies (4). Thus, it is possible that effectively preventing the increase in REDD1 protein expression in response to diabetes, or targeting the events downstream of REDD1 that lead to retinal pathology, could improve the current standard of care as a combination therapeutic with anti-VEGF.
10. Conclusions and future perspectives
The studies reviewed herein support that diabetes promotes REDD1 protein expression in the retina, and that REDD1 plays an important role in the visual dysfunction that is caused by diabetes. A causal role for diabetes-induced oxidative stress in retinal pathology is well accepted, and evidence supports that REDD1 is an important contributor to this effect. REDD1 not only promotes hyperglycemia-induced ROS production, but also acts to undermine the antioxidant response. Beneficial effects of REDD1 deletion on visual function have been observed in both preclinical rodent models and patients with DME. These proof-of-concept studies support the therapeutic benefits of targeting REDD1. While modest benefits in visual function were seen in patients with DME receiving an siRNA targeting the REDD1 mRNA, it remains to be seen if this is an effective therapeutic approach to prevent diabetes-induced REDD1 protein expression or avert the signaling events downstream of REDD1 that contribute to retinal pathology. Based on an improved understanding of the neurovascular complications that contribute to diabetes-induced visual deficits, it will be important for future studies consider the potential benefits of REDD1 targeted therapeutics beyond improvement in BCVA. In light of recent studies that more fully delineate REDD1 mechanistic action, phosphatase-specific inhibitors, Akt agonists, or GSK3 suppression should also be considered as therapeutic alternatives to address the molecular events that cause DR.
Highlights.
Diabetes-induced hyperglycemia causes oxidative stress in the retina.
Oxidative stress contributes to the development of diabetic retinopathy.
Diabetes promotes expression of the stress response protein REDD1 in retina.
REDD1 promotes ROS production and suppresses the retinal antioxidant response.
REDD1 contributes to diabetes-induced retinal pathology and deficits in vision.
Acknowledgements.
This research was supported by the American Diabetes Association Pathway to Stop Diabetes Grant 1-14-INI-04, R01 EY029702 (to MDD), and F31 EY031199 (to WPM). The authors thank Alistair Barber and Scot Kimball (Penn State College of Medicine) for critically evaluating the manuscript.
Footnotes
Duality of Interest. The authors declare that they have no conflicts of interest with the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheung N, Mitchell P, and Wong TY (2010) Diabetic retinopathy. Lancet. 376, 124–136 [DOI] [PubMed] [Google Scholar]
- 2.Frank RN (2004) Diabetic Retinopathy. N. Engl. J. Med 350, 48–58 [DOI] [PubMed] [Google Scholar]
- 3.Aylward GW (2005) Progressive changes in diabetics and their management. Eye. 19, 1115–1118 [DOI] [PubMed] [Google Scholar]
- 4.Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, Adamis AP, Ehrlich JS, and Hopkins JJ (2013) Long-term Outcomes of Ranibizumab Therapy for Diabetic Macular Edema: The 36-Month Results from Two Phase III Trials: RISE and RIDE. Ophthalmology. 120, 2013–2022 [DOI] [PubMed] [Google Scholar]
- 5.Adams AJ, and Bearse MA Jr (2012) Retinal neuropathy precedes vasculopathy in diabetes: a function-based opportunity for early treatment intervention? Clin. Exp. Optom. 95, 256–265 [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Bearse MA Jr, Schneck ME, Barez S, Jacobsen CH, and Adams AJ (2004) Multifocal Electroretinogram Delays Predict Sites of Subsequent Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci 45, 948–954 [DOI] [PubMed] [Google Scholar]
- 7.Safi SZ, Qvist R, Kumar S, Batumalaie K, and Ismail IS Bin (2014) Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res. Int 2014, 801269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payne AJ, Kaja S, Sabates NR, and Koulen P. (2013) A case for neuroprotection in ophthalmology: developments in translational research. Mo. Med 110, 429–436 [PMC free article] [PubMed] [Google Scholar]
- 9.Payne AJ, Kaja S, Naumchuk Y, Kunjukunju N, and Koulen P. (2014) Antioxidant drug therapy approaches for neuroprotection in chronic diseases of the retina. Int. J. Mol. Sci 15, 1865–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whiteside CI (2005) Cellular mechanisms and treatment of diabetes vascular complications converge on reactive oxygen species. Curr. Hypertens. Rep 7, 148–154 [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Tang L, and Chen B. (2014) Oxidative stress: implications for the development of diabetic retinopathy and antioxidant therapeutic perspectives. Oxid. Med. Cell. Longev 2014, 752387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus (1993) N. Engl. J. Med 329, 977–986 [DOI] [PubMed] [Google Scholar]
- 13.King P, Peacock I, and Donnelly R. (1999) The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol 48, 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kowluru RA (2005) Diabetic Retinopathy: Mitochondrial Dysfunction and Retinal Capillary Cell Death. Antioxid. Redox Signal 7, 1581. [DOI] [PubMed] [Google Scholar]
- 15.DeBosch BJ, Baur E, Deo BK, Hiraoka M, and Kumagai AK (2001) Effects of insulin-like growth factor-1 on retinal endothelial cell glucose transport and proliferation. J. Neurochem 77, 1157–1167 [DOI] [PubMed] [Google Scholar]
- 16.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AEB, Al-Shabrawey M, Platt DH, and Caldwell GIL and R. W. (2005) Vascular Endothelial Growth Factor and Diabetic Retinopathy: Role of Oxidative Stress. Curr. Drug Targets 6, 511–524 [DOI] [PubMed] [Google Scholar]
- 17.Koya D, and King GL (1998) Protein kinase C activation and the development of diabetic complications. Diabetes. 47, 859LP–866 [DOI] [PubMed] [Google Scholar]
- 18.Stäuble B, Boscoboinik D, Tasinato A, and Azzi A. (1994) Modulation of Activator Protein-1 (AP-1) Transcription Factor and Protein Kinase C by Hydrogen Peroxide and d-α-Tocopherol in Vascular Smooth Muscle Cells. Eur. J. Biochem 226, 393–402 [DOI] [PubMed] [Google Scholar]
- 19.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, and Brownlee M. (2000) Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. U. S. A 97, 12222–12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beisswenger PJ, Howell SK, Smith K, and Szwergold BS (2003) Glyceraldehyde-3-phosphate dehydrogenase activity as an independent modifier of methylglyoxal levels in diabetes. Biochim. Biophys. Acta - Mol. Basis Dis 1637, 98–106 [DOI] [PubMed] [Google Scholar]
- 21.Miwa K, Nakamura J, Hamada Y, Naruse K, Nakashima E, Kato K, Kasuya Y, Yasuda Y, Kamiya H, and Hotta N. (2003) The role of polyol pathway in glucose-induced apoptosis of cultured retinal pericytes. Diabetes Res. Clin. Pract 60, 1–9 [DOI] [PubMed] [Google Scholar]
- 22.Brownlee M. (2005) The Pathobiology of Diabetic Complications. Diabetes. 54, 1615LP–1625 [DOI] [PubMed] [Google Scholar]
- 23.Di Meo S, Reed TT, Venditti P, and Victor VM (2016) Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev 2016, 1245049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forman HJ, Maiorino M, and Ursini F. (2010) Signaling functions of reactive oxygen species. Biochemistry. 49, 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Medina J, Zanon-Moreno V, Pinazo-Duran M, Foulquie-Moreno E, Rubio-Velazquez E, Casaroli-Marano R, and Del-Rio-Vellosillo M. (2020) Oxidative stress in diabetic retinopathy. in Diabetes: Oxidative Stress and Dietary Antioxidants, 2nd Ed. (Preedy VR ed), p. 52, Academic Press (Elsevier Inc) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowluru RA (2003) Effect of Reinstitution of Good Glycemic Control on Retinal Oxidative Stress and Nitrative Stress in Diabetic Rats. Diabetes. 52, 818LP–823 [DOI] [PubMed] [Google Scholar]
- 27.Rohowetz LJ, Kraus JG, and Koulen P. (2018) Reactive Oxygen Species-Mediated Damage of Retinal Neurons: Drug Development Targets for Therapies of Chronic Neurodegeneration of the Retina. Int. J. Mol. Sci 19, 3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Field MG, Yang D, Bian Z-M, Petty HR, and Elner VM (2011) Retinal flavoprotein fluorescence correlates with mitochondrial stress, apoptosis, and chemokine expression. Exp. Eye Res 93, 548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace DC (1992) Diseases Of The Mitochondrial DNA. Annu. Rev. Biochem 61, 1175–1212 [DOI] [PubMed] [Google Scholar]
- 30.Trumpower BL (1990) The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J. Biol. Chem. 265, 11409–11412 [PubMed] [Google Scholar]
- 31.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes H-P, Giardino I, and Brownlee M. (2000) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 404, 787. [DOI] [PubMed] [Google Scholar]
- 32.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, and Brownlee M. (2001) Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J. Clin. Invest 108, 1341–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giugliano D, Ceriello A, and Paolisso G. (1996) Oxidative Stress and Diabetic Vascular Complications. Diabetes Care. 19, 257LP–267 [DOI] [PubMed] [Google Scholar]
- 34.Korshunov SS, Skulachev VP, and Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 416, 15–18 [DOI] [PubMed] [Google Scholar]
- 35.Giacco F, Du X, Carratú A, Gerfen GJ, D’Apolito M, Giardino I, Rasola A, Marin O, Divakaruni AS, Murphy AN, Shah MS, and Brownlee M. (2015) GLP-1 Cleavage Product Reverses Persistent ROS Generation After Transient Hyperglycemia by Disrupting an ROS-Generating Feedback Loop. Diabetes. 64, 3273LP–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da-Silva WS, Gómez-Puyou A, de Gómez-Puyou MT, Moreno-Sanchez R, De Felice FG, de Meis L, Oliveira MF, and Galina A. (2004) Mitochondrial Bound Hexokinase Activity as a Preventive Antioxidant Defense: Steady-State ADP Formation As A Regulatory Mechanism of Membrane Potential and Reactive Oxygen Species Generation In Mitochondria. J. Biol. Chem 279, 39846–39855 [DOI] [PubMed] [Google Scholar]
- 37.Takahashi T, and Harris RC (2014) Role of endothelial nitric oxide synthase in diabetic nephropathy: lessons from diabetic eNOS knockout mice. J. Diabetes Res 2014, 590541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felaco M, Grilli A, De Lutiis MA, Patruno A, Libertini N, Taccardi AA, Di Napoli P, Di Giulio C, Barbacane R, and Conti P. (2001) Endothelial Nitric Oxide Synthase (eNOS) Expression and Localization in Healthy and Diabetic Rat Hearts. Ann. Clin. Lab. Sci. 31, 179–186 [PubMed] [Google Scholar]
- 39.Carmo A, Cunha-Vaz JG, Carvalho AP, and Lopes MC (2000) Nitric Oxide Synthase Activity in Retinas from Non-Insulin-Dependent Diabetic Goto-Kakizaki Rats: Correlation with Blood–Retinal Barrier Permeability. Nitric Oxide. 4, 590–596 [DOI] [PubMed] [Google Scholar]
- 40.Martínez MC, and Andriantsitohaina R. (2008) Reactive Nitrogen Species: Molecular Mechanisms and Potential Significance in Health and Disease. Antioxid. Redox Signal 11, 669–702 [DOI] [PubMed] [Google Scholar]
- 41.Afanas’ev I. (2010) Signaling of reactive oxygen and nitrogen species in Diabetes mellitus. Oxid. Med. Cell. Longev 3, 361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du X, Stockklauser-Färber K, and Rösen P. (1999) Generation of reactive oxygen intermediates, activation of NF-κB, and induction of apoptosis in human endothelial cells by glucose: role of nitric oxide synthase? Free Radic. Biol. Med 27, 752–763 [DOI] [PubMed] [Google Scholar]
- 43.Gupta S, Chough E, Daley J, Oates P, Tornheim K, Ruderman NB, and Keaney JF (2002) Hyperglycemia increases endothelial superoxide that impairs smooth muscle cell Na+-K+-ATPase activity. Am. J. Physiol. Physiol 282, C560–C566 [DOI] [PubMed] [Google Scholar]
- 44.Maritim AC, Sanders RA, and Watkins JB III (2003) Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol 17, 24–38 [DOI] [PubMed] [Google Scholar]
- 45.Golbidi S, and Laher SAE and I. (2011) Antioxidants in the Treatment of Diabetes. Curr. Diabetes Rev 7, 106–125 [DOI] [PubMed] [Google Scholar]
- 46.Sheikh-Ali M, Chehade JM, and Mooradian AD (2011) The Antioxidant Paradox in Diabetes Mellitus. Am. J. Ther 18, 266–78 [DOI] [PubMed] [Google Scholar]
- 47.Marra G, Cotroneo P, Pitocco D, Manto A, Di Leo MAS, Ruotolo V, Caputo S, Giardina B, Ghirlanda G, and Santini SA (2002) Early Increase of Oxidative Stress and Reduced Antioxidant Defenses in Patients With Uncomplicated Type 1 Diabetes. Diabetes Care. 25, 370LP–375 [DOI] [PubMed] [Google Scholar]
- 48.Martín-Gallán P, Carrascosa A, Gussinye M, and Domínguez C. (2005) Estimation of lipoperoxidative damage and antioxidant status in diabetic children: Relationship with individual antioxidants. Free Radic. Res 39, 933–942 [DOI] [PubMed] [Google Scholar]
- 49.Bhatia S, Shukla R, Venkata Madhu S, Kaur Gambhir J, and Madhava Prabhu K. (2003) Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin. Biochem 36, 557–562 [DOI] [PubMed] [Google Scholar]
- 50.Vijayalingam S, Parthiban A, Shanmugasundaram KR, and Mohan V. (1996) Abnormal Antioxidant Status in Impaired Glucose Tolerance and Non-insulin-dependent Diabetes Mellitus. Diabet. Med 13, 715–719 [DOI] [PubMed] [Google Scholar]
- 51.Birben E, Sahiner UM, Sackesen C, Erzurum S, and Kalayci O. (2012) Oxidative stress and antioxidant defense. World Allergy Organ. J 5, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kowluru RA, Tang J, and Kern TS (2001) Abnormalities of Retinal Metabolism in Diabetes and Experimental Galactosemia. Diabetes. 50, 1938LP–1942 [DOI] [PubMed] [Google Scholar]
- 53.Wohaieb SA, and Godin DV (1987) Alterations in Free Radical Tissue-Defense Mechanisms in Streptozocin-Induced Diabetes in Rat: Effects of Insulin Treatment. Diabetes. 36, 1014LP–1018 [DOI] [PubMed] [Google Scholar]
- 54.Baynes JW, and Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 48, 1LP–9 [DOI] [PubMed] [Google Scholar]
- 55.Haskins K, Bradley B, Powers K, Fadok V, Flores S, Ling X, Pugazhenthi S, Reusch J, and Kench J. (2003) Oxidative Stress in Type 1 Diabetes. Ann. N. Y. Acad. Sci 1005, 43–54 [DOI] [PubMed] [Google Scholar]
- 56.Deliyanti D, Alrashdi SF, Tan SM, Meyer C, Ward KW, de Haan JB, and Wilkinson-Berka JL (2018) Nrf2 Activation Is a Potential Therapeutic Approach to Attenuate Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci 59, 815–825 [DOI] [PubMed] [Google Scholar]
- 57.Tonelli C, Chio IIC, and Tuveson DA (2018) Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 29, 1727–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Yu H, Pan H, Zhou X, Ruan Q, Kong D, Chu Z, Li H, Huang J, Huang X, Chau A, Xie W, Ding Y, and Yao P. (2019) Nrf2 Suppression Delays Diabetic Wound Healing Through Sustained Oxidative Stress and Inflammation. Front. Pharmacol 10, 1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen G, Chen X, Niu C, Huang X, An N, Sun J, Huang S, Ye W, Li S, Shen Y, Liang J, Cong W, and Jin L. Baicalin alleviates hyperglycemia-induced endothelial impairment via Nrf2. J. Endocrinol 240, 81–98 [DOI] [PubMed] [Google Scholar]
- 60.Shivarudrappa AH, and Ponesakki G. (2020) Lutein reverses hyperglycemia-mediated blockage of Nrf2 translocation by modulating the activation of intracellular protein kinases in retinal pigment epithelial (ARPE-19) cells. J. Cell Commun. Signal 14, 207–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen T, Sherratt PJ, and Pickett CB (2003) Regulatory Mechanisms Controlling Gene Expression Mediated by the Antioxidant Response Element. Annu. Rev. Pharmacol. Toxicol 43, 233–260 [DOI] [PubMed] [Google Scholar]
- 62.Hayes JD, and Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci 39, 199–218 [DOI] [PubMed] [Google Scholar]
- 63.Ford ES, Mokdad AH, Giles WH, and Brown DW (2003) The Metabolic Syndrome and Antioxidant Concentrations. Diabetes. 52, 2346LP–2352 [DOI] [PubMed] [Google Scholar]
- 64.Wong-Riley MTT (2010) Energy metabolism of the visual system. Eye Brain. 2, 99–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donato L, Scimone C, Rinaldi C, Aragona P, Briuglia S, D’Ascola A, D’Angelo R, and Sidoti A. (2018) Stargardt Phenotype Associated With Two ELOVL4 Promoter Variants and ELOVL4 Downregulation: New Possible Perspective to Etiopathogenesis? Invest. Ophthalmol. Vis. Sci 59, 843–857 [DOI] [PubMed] [Google Scholar]
- 66.Gürler B, Vural H, Yilmaz N, Oguz H, Satici A, and Aksoy N. (2000) The role of oxidative stress in diabetic retinopathy. Eye (Lond). 14 Pt 5, 730–735 [DOI] [PubMed] [Google Scholar]
- 67.Kowluru RA, and Abbas SN (2003) Diabetes-Induced Mitochondrial Dysfunction in the Retina. Invest. Ophthalmol. Vis. Sci 44, 5327–5334 [DOI] [PubMed] [Google Scholar]
- 68.Du Y, Miller CM, and Kern TS (2003) Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic. Biol. Med 35, 1491–1499 [DOI] [PubMed] [Google Scholar]
- 69.Ellis EA, Guberski DL, Somogyi-Mann M, and Grant MB (2000) Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/WOR diabetic rat. Free Radic. Biol. Med 28, 91–101 [DOI] [PubMed] [Google Scholar]
- 70.Miller WP, Toro AL, Barber AJ, and Dennis MD (2019) REDD1 Activates a ROS-Generating Feedback Loop in the Retina of Diabetic Mice REDD1 Promotes Oxidative Stress in Retina. Invest. Ophthalmol. Vis. Sci 60, 2369–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Kern TS, Ball S, and Berkowitz BA (2007) Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 50, 1987–1996 [DOI] [PubMed] [Google Scholar]
- 72.Du Y, Smith MA, Miller CM, and Kern TS (2002) Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J. Neurochem 80, 771–779 [DOI] [PubMed] [Google Scholar]
- 73.Kowluru RA, Kanwar M, and Kennedy A. (2007) Metabolic memory phenomenon and accumulation of peroxynitrite in retinal capillaries. Exp. Diabetes Res 2007, 21976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, and Caldwell RB (2003) Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am. J. Pathol 162, 1995–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dierschke SK, Miller WP, Favate JS, Shah P, Imamura Kawasawa Y, Salzberg AC, Kimball SR, Jefferson LS, and Dennis MD (2019) O-GlcNAcylation alters the selection of mRNAs for translation and promotes 4E-BP1-dependent mitochondrial dysfunction in retina. J. Biol. Chem 294, 5508–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dierschke SK, Toro AL, Barber AJ, Arnold AC, and Dennis MD (2020) Angiotensin-(1–7) Attenuates Protein O-GlcNAcylation in the Retina by EPAC/Rap1-Dependent Inhibition of O-GlcNAc Transferase. Invest. Ophthalmol. Vis. Sci 61, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakamura M, Barber AJ, Antonetti DA, LaNoue KF, Robinson KA, Buse MG, and Gardner TW (2001) Excessive Hexosamines Block the Neuroprotective Effect of Insulin and Induce Apoptosis in Retinal Neurons. J. Biol. Chem . 276, 43748–43755 [DOI] [PubMed] [Google Scholar]
- 78.Li L, and Renier G. (2006) Activation of nicotinamide adenine dinucleotide phosphate (reduced form) oxidase by advanced glycation end products links oxidative stress to altered retinal vascular endothelial growth factor expression. Metabolism. 55, 1516–1523 [DOI] [PubMed] [Google Scholar]
- 79.Kowluru RA, and Koppolu P. (2002) Diabetes-induced Activation of Caspase-3 in Retina: Effect of Antioxidant Therapy. Free Radic. Res 36, 993–999 [DOI] [PubMed] [Google Scholar]
- 80.Kowluru RA, Koppolu P, Chakrabarti S, and Chen S. (2003) Diabetes-induced Activation of Nuclear Transcriptional Factor in the Retina, and its Inhibition by Antioxidants. Free Radic. Res 37, 1169–1180 [DOI] [PubMed] [Google Scholar]
- 81.Mizutani M, Gerhardinger C, and Lorenzi M. (1998) Müller cell changes in human diabetic retinopathy. Diabetes. 47, 445LP–449 [DOI] [PubMed] [Google Scholar]
- 82.Phipps JA, Fletcher EL, and Vingrys AJ (2004) Paired-Flash Identification of Rod and Cone Dysfunction in the Diabetic Rat. Invest. Ophthalmol. Vis. Sci 45, 4592–4600 [DOI] [PubMed] [Google Scholar]
- 83.Miller WP, Sunilkumar S, Giordano JF, Toro AL, Barber AJ, and Dennis MD (2020) The stress response protein REDD1 promotes diabetes-induced oxidative stress in the retina by Keap1-independent Nrf2 degradation. J. Biol. Chem . 295, 7350–7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali TK, Al-Gayyar MMH, Matragoon S, Pillai BA, Abdelsaid MA, Nussbaum JJ, and El-Remessy AB (2011) Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 54, 657–668 [DOI] [PubMed] [Google Scholar]
- 85.Ellis EA, Guberski DL, Hutson B, and Grant MB (2002) Time Course of NADH Oxidase, Inducible Nitric Oxide Synthase and Peroxynitrite in Diabetic Retinopathy in the BBZ/WOR Rat. Nitric Oxide. 6, 295–304 [DOI] [PubMed] [Google Scholar]
- 86.Stadler K, Jenei V, von Bölcsházy G, Somogyi A, and Jakus J. (2004) Role of free radicals and reactive nitrogen species in the late complications of diabetes mellitus in rats. Orv. Hetil 145, 1135–1140 [PubMed] [Google Scholar]
- 87.Santos JM, Mohammad G, and Kowluru QZ and R. A. (2011) Diabetic Retinopathy, Superoxide Damage and Antioxidants. Curr. Pharm. Biotechnol 12, 352–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li W, Yanoff M, B J, and Z H. (1999) Altered mRNA Levels of Antioxidant Enzymes in Pre-Apoptotic Pericytes From Human Diabetic Retinas. Cell Mol Biol. 45, 59–66 [PubMed] [Google Scholar]
- 89.Zhong Q, Mishra M, and Kowluru RA (2013) Transcription Factor Nrf2-Mediated Antioxidant Defense System in the Development of Diabetic Retinopathy. Invest. Ophthalmol. Vis. Sci 54, 3941–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yue KKM, Chung W, Leung AWN, and Cheng CHK (2003) Redox changes precede the occurrence of oxidative stress in eyes and aorta, but not in kidneys of diabetic rats. Life Sci. 73, 2557–2570 [DOI] [PubMed] [Google Scholar]
- 91.Miranda M, Muriach M, Roma J, Bosch-Morell F, Genovés JM, Barcia J, Araiz J, Díaz-LLopis M, and Romero FJ (2006) Oxidative Stress In a Model of Experimental Diabetic Retinopathy: The Utility of Peroxynitrite Scavengers. Arch. Soc. Esp. Oftalmol 81, 27–32 [DOI] [PubMed] [Google Scholar]
- 92.Chen B, Jiang D, and Tang L. (2006) Advanced glycation end-products induce apoptosis involving the signaling pathways of oxidative stress in bovine retinal pericytes. Life Sci. 79, 1040–1048 [DOI] [PubMed] [Google Scholar]
- 93.Calderon GD, Juarez OH, Hernandez GE, Punzo SM, and De la Cruz ZD (2017) Oxidative stress and diabetic retinopathy: development and treatment. Eye (Lond). 31, 1122–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kowluru RA (2005) Effect of advanced glycation end products on accelerated apoptosis of retinal capillary cells under in vitro conditions. Life Sci. 76, 1051–1060 [DOI] [PubMed] [Google Scholar]
- 95.Zhu Y, Zhang X-L, Zhu B-F, and Ding Y-N (2012) Effect of antioxidant N-acetylcysteine on diabetic retinopathy and expression of VEGF and ICAM-1 from retinal blood vessels of diabetic rats. Mol. Biol. Rep 39, 3727–3735 [DOI] [PubMed] [Google Scholar]
- 96.Millen AE, Klein R, Folsom AR, Stevens J, Palta M, and Mares JA (2004) Relation between intake of vitamins C and E and risk of diabetic retinopathy in the Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr 79, 865–873 [DOI] [PubMed] [Google Scholar]
- 97.Mayer-Davis EJ, Bell RA, Reboussin BA, Rushing J, Marshall JA, and Hamman RF (1998) Antioxidant nutrient intake and diabetic retinopathy: The San Luis Valley Diabetes Study. Ophthalmology. 105, 2264–2270 [DOI] [PubMed] [Google Scholar]
- 98.Millen AE, Gruber M, Klein R, Klein BEK, Palta M, and Mares JA (2003) Relations of Serum Ascorbic Acid and α-Tocopherol to Diabetic Retinopathy in the Third National Health and Nutrition Examination Survey . Am. J. Epidemiol 158, 225–233 [DOI] [PubMed] [Google Scholar]
- 99.Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, Kalinski H, Kamer I, Rozen A, Mor O, Keshet E, Leshkowitz D, Einat P, Skaliter R, and Feinstein E. (2002) Identification of a Novel Hypoxia-Inducible Factor 1-Responsive Gene Involved in Apoptosis. Mol. Cell. Biol 22, 2283LP–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, Oliner JD, McKeon F, and Haber DA (2002) REDD1, a Developmentally Regulated Transcriptional Target of p63 and p53, Links p63 to Regulation of Reactive Oxygen Species. Mol. Cell 10, 995–1005 [DOI] [PubMed] [Google Scholar]
- 101.Polyak K, Xia Y, Zweier JL, Kinzler KW, and Vogelstein B. (1997) A model for p53-induced apoptosis. Nature. 389, 300–305 [DOI] [PubMed] [Google Scholar]
- 102.Li PF, Dietz R, and von Harsdorf R. (1999) p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J. 18, 6027–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin L, Stringfield TM, Shi X, and Chen Y. (2005) Arsenite induces a cell stress-response gene, RTP801, through reactive oxygen species and transcription factors Elk-1 and CCAAT/enhancer-binding protein. Biochem. J 392, 93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sofer A, Lei K, Johannessen CM, and Ellisen LW (2005) Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol. Cell. Biol 25, 5834–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miller WP, Yang C, Mihailescu ML, Moore JA, Dai W, Barber AJ, and Dennis MD (2018) Deletion of the Akt/mTORC1 Repressor REDD1 Prevents Visual Dysfunction in a Rodent Model of Type 1 Diabetes. Diabetes. 67, 110LP–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Malagelada C, Ryu EJ, Biswas SC, Jackson-Lewis V, and Greene LA (2006) RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. J. Neurosci 26, 9996–10005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim H-R, Lee G-H, Yi Cho E, Chae S-W, Ahn T, and Chae H-J (2009) Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J. Cell Sci 122, 1126LP–1133 [DOI] [PubMed] [Google Scholar]
- 108.McIntosh LJ, and Sapolsky RM (1996) Glucocorticoids Increase the Accumulation of Reactive Oxygen Species and Enhance Adriamycin-Induced Toxicity in Neuronal Culture. Exp. Neurol 141, 201–206 [DOI] [PubMed] [Google Scholar]
- 109.Leach JK, Van Tuyle G, Lin P-S, Schmidt-Ullrich R, and Mikkelsen RB (2001) Ionizing Radiation-induced, Mitochondria-dependent Generation of Reactive Oxygen/Nitrogen. Cancer Res. 61, 3894 LP – 3901 [PubMed] [Google Scholar]
- 110.He M, Pan H, Xiao C, and Pu M. (2013) Roles for Redox Signaling by NADPH Oxidase in Hyperglycemia-Induced Heme Oxygenase-1 Expression in the Diabetic Retina. Invest. Ophthalmol. Vis. Sci 54, 4092–4101 [DOI] [PubMed] [Google Scholar]
- 111.Ramos H, Bogdanov P, Sampedro J, Huerta J, Simó R, and Hernández C. (2020) Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress. Antioxidants. 9, 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dai W, Miller WP, Toro AL, Black AJ, Dierschke SK, Feehan RP, Kimball SR, and Dennis MD (2018) Deletion of the stress-response protein REDD1 promotes ceramide-induced retinal cell death and JNK activation. FASEB J. 10.1096/fj.201800413RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dennis MD, Kimball SR, Fort PE, and Jefferson LS (2015) Regulated in development and DNA damage 1 is necessary for hyperglycemia-induced vascular endothelial growth factor expression in the retina of diabetic rodents. J. Biol. Chem 290, 3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jin H-O, Seo S-K, Woo S-H, Kim E-S, Lee H-C, Yoo D-H, An S, Choe T-B, Lee S-J, Hong S-I, Rhee C-H, Kim J-I, and Park I-C (2009) Activating transcription factor 4 and CCAAT/enhancer-binding protein-β negatively regulate the mammalian target of rapamycin via Redd1 expression in response to oxidative and endoplasmic reticulum stress. Free Radic. Biol. Med 46, 1158–1167 [DOI] [PubMed] [Google Scholar]
- 115.Reiling JH, and Hafen E. (2004) The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 18, 2879–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Corradetti MN, Inoki K, and Guan K-L (2005) The Stress-inducted Proteins RTP801 and RTP801L Are Negative Regulators of the Mammalian Target of Rapamycin Pathway. J. Biol. Chem . 280, 9769–9772 [DOI] [PubMed] [Google Scholar]
- 117.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, and Kaelin WG Jr (2004) Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, and Avruch J. (2005) Rheb Binds and Regulates the mTOR Kinase. Curr. Biol 15, 702–713 [DOI] [PubMed] [Google Scholar]
- 119.DeYoung MP, Horak P, Sofer A, Sgroi D, and Ellisen LW (2008) Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 22, 239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vega-Rubin-de-Celis S, Abdallah Z, Kinch L, Grishin NV, Brugarolas J, and Zhang X. (2010) Structural analysis and functional implications of the negative mTORC1 regulator REDD1. Biochemistry. 49, 2491–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dennis MD, Coleman CS, Berg A, Jefferson LS, and Kimball SR (2014) REDD1 enhances protein phosphatase 2A-mediated dephosphorylation of Akt to repress mTORC1 signaling. Sci. Signal 7, ra68–ra68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, and Hemmings BA (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 123.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, and Su B. (2006) SIN1/MIP1 Maintains rictor-mTOR Complex Integrity and Regulates Akt Phosphorylation and Substrate Specificity. Cell. 127, 125–137 [DOI] [PubMed] [Google Scholar]
- 124.Inoki K, Li Y, Zhu T, Wu J, and Guan K-L (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol 4, 648–657 [DOI] [PubMed] [Google Scholar]
- 125.Malagelada C, Jin ZH, and Greene LA (2008) RTP801 is induced in Parkinson’s disease and mediates neuron death by inhibiting Akt phosphorylation/activation. J. Neurosci 28, 14363–14371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, and Greene LA (2010) Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J. Neurosci 30, 1166–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hulmi JJ, Silvennoinen M, Lehti M, Kivelä R, and Kainulainen H. (2011) Altered REDD1, myostatin, and Akt/mTOR/FoxO/MAPK signaling in streptozotocin-induced diabetic muscle atrophy. Am. J. Physiol. Metab 302, E307–E315 [DOI] [PubMed] [Google Scholar]
- 128.Britto FA, Cortade F, Belloum Y, Blaquière M, Gallot YS, Docquier A, Pagano AF, Jublanc E, Bendridi N, Koechlin-Ramonatxo C, Chabi B, Francaux M, Casas F, Freyssenet D, Rieusset J, Giorgetti-Peraldi S, Carnac G, Ollendorff V, and Favier FB (2018) Glucocorticoid-dependent REDD1 expression reduces muscle metabolism to enable adaptation under energetic stress. BMC Biol. 16, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiao S, Dennis M, Song X, Vadysirisack DD, Salunke D, Nash Z, Yang Z, Liesa M, Yoshioka J, Matsuzawa S-I, Shirihai OS, Lee RT, Reed JC, and Ellisen LW (2015) A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat. Commun 6, 7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hou X, Yang S, and Yin J. (2018) Blocking the REDD1/TXNIP axis ameliorates LPS-induced vascular endothelial cell injury through repressing oxidative stress and apoptosis. Am. J. Physiol. Physiol 316, C104–C110 [DOI] [PubMed] [Google Scholar]
- 131.Liu Z, Li T, Zhu F, Deng S, Li X, and He Y. (2019) Regulatory roles of miR-22/Redd1-mediated mitochondrial ROS and cellular autophagy in ionizing radiation-induced BMSC injury. Cell Death Dis. 10, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pastorino JG, Hoek JB, and Shulga N. (2005) Activation of Glycogen Synthase Kinase 3β Disrupts the Binding of Hexokinase II to Mitochondria by Phosphorylating Voltage-Dependent Anion Channel and Potentiates Chemotherapy-Induced Cytotoxicity. Cancer Res. 65, 10545 LP – 10554 [DOI] [PubMed] [Google Scholar]
- 133.Cross DAE, Alessi DR, Cohen P, Andjelkovich M, and Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 134.Frame S, Cohen P, and Biondi RM (2001) A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 7, 1321–1327 [DOI] [PubMed] [Google Scholar]
- 135.Reiter CEN, Wu X, Sandirasegarane L, Nakamura M, Gilbert KA, Singh RSJ, Fort PE, Antonetti DA, and Gardner TW (2006) Diabetes Reduces Basal Retinal Insulin Receptor Signaling. Diabetes. 55, 1148 LP – 1156 [DOI] [PubMed] [Google Scholar]
- 136.Forred BJ, Neuharth S, Kim DI, Amolins MW, Motamedchaboki K, Roux KJ, and Vitiello PF (2016) Identification of Redox and Glucose-Dependent Txnip Protein Interactions. Oxid. Med. Cell. Longev 2016, 5829063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Singh LP (2013) Thioredoxin Interacting Protein (TXNIP) and Pathogenesis of Diabetic Retinopathy. J. Clin. Exp. Ophthalmol 4, 2155–9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li P, Lin N, Guo M, Huang H, Yu T, and Zhang L. (2019) REDD1 knockdown protects H9c2 cells against myocardial ischemia/reperfusion injury through Akt/mTORC1/Nrf2 pathway-ameliorated oxidative stress: An in vitro study. Biochem. Biophys. Res. Commun 519, 179–185 [DOI] [PubMed] [Google Scholar]
- 139.Cullinan SB, Gordan JD, Jin J, Harper JW, and Diehl JA (2004) The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol 24, 8477–8486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, and Talalay P. (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. U. S. A 99, 11908–11913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Robledinos-Antón N, Fernández-Ginés R, Manda G, and Cuadrado A. (2019) Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev 2019, 9372182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, and Klein R. (2003) Diabetic Retinopathy. Diabetes Care. 26, 226LP–229 [DOI] [PubMed] [Google Scholar]
- 143.Mishra M, Zhong Q, and Kowluru RA (2014) Epigenetic modifications of Nrf2-mediated glutamate–cysteine ligase: implications for the development of diabetic retinopathy and the metabolic memory phenomenon associated with its continued progression. Free Radic. Biol. Med 75, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang L, Chen Y, Sternberg P, and Cai J. (2008) Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest. Ophthalmol. Vis. Sci 49, 1671–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, and Cuadrado A. (2011) SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol 31, 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Z, Ma L, Chen X, Li Y, Li S, Zhang J, and Lu L. (2014) Glycogen synthase kinase-3: A key kinase in retinal neuron apoptosis in early diabetic retinopathy. Chin. Med. J. (Engl). 127, 3464–3470 [PubMed] [Google Scholar]
- 147.Brafman A, Mett I, Shafir M, Gottlieb H, Damari G, Gozlan-Kelner S, Vishnevskia-Dai V, Skaliter R, Einat P, Faerman A, Feinstein E, and Shoshani T. (2004) Inhibition of Oxygen-Induced Retinopathy in RTP801-Deficient Mice. Invest. Ophthalmol. Vis. Sci 45, 3796–3805 [DOI] [PubMed] [Google Scholar]
- 148.Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, and Campochiaro PA (1999) Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest. Ophthalmol. Vis. Sci 40, 182–189 [PubMed] [Google Scholar]
- 149.Sennlaub F, Courtois Y, and Goureau O. (2002) Inducible nitric oxide synthase mediates retinal apoptosis in ischemic proliferative retinopathy. J. Neurosci 22, 3987–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, and Gardner TW (1998) Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Invest 102, 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fort PE, Losiewicz MK, Reiter CEN, Singh RSJ, Nakamura M, Abcouwer SF, Barber AJ, and Gardner TW (2011) Differential roles of hyperglycemia and hypoinsulinemia in diabetes induced retinal cell death: evidence for retinal insulin resistance. PLoS One. 6, e26498–e26498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tzekov R, and Arden GB (1999) The Electroretinogram in Diabetic Retinopathy. Surv. Ophthalmol 44, 53–60 [DOI] [PubMed] [Google Scholar]
- 153.Juen S, and Kieselbach GF (1990) Electrophysiological Changes in Juvenile Diabetics Without Retinopathy. Arch. Ophthalmol 108, 372–375 [DOI] [PubMed] [Google Scholar]
- 154.Bresnick GH, Korth K, Groo A, and Palta M. (1984) Electroretinographic Oscillatory Potentials Predict Progression of Diabetic Retinopathy: Preliminary Report. Arch. Ophthalmol 102, 1307–1311 [DOI] [PubMed] [Google Scholar]
- 155.Ogden TE (1973) The oscillatory waves of the primate electroretinogram. Vision Res. 13, 1059-IN4 [DOI] [PubMed] [Google Scholar]
- 156.Sakai H, Tani Y, Shirasawa E, Shirao Y, and Kawasaki K. (1995) Development of Electroretinographic Alterations in Streptozotocin-lnduced Diabetes in Rats. Ophthalmic Res. 27, 57–63 [DOI] [PubMed] [Google Scholar]
- 157.Puell MC, Palomo-Álvarez C, and Pérez-Carrasco MJ (2018) Macular Inner Retinal Layer Thickness in Relation to Photopic and Mesopic Contrast Sensitivity in Healthy Young and Older Subjects. Invest. Ophthalmol. Vis. Sci 59, 5487–5493 [DOI] [PubMed] [Google Scholar]
- 158.Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, Chi-Burris K, Yan E, Paggiarino DA, Rosenblatt I, Aitchison R, and Erlich SS (2012) Dose-Ranging Evaluation of Intravitreal siRNA PF-04523655 for Diabetic Macular Edema (the DEGAS Study). Invest. Ophthalmol. Vis. Sci 53, 7666–7674 [DOI] [PubMed] [Google Scholar]
- 159.Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Klamerus KJ, Chi-Burris K, Yan E, Paggiarino DA, Rosenblatt I, Aitchison R, and Erlich SS (2012) Evaluation of the siRNA PF-04523655 versus Ranibizumab for the Treatment of Neovascular Age-related Macular Degeneration (MONET Study). Ophthalmology. 119, 1867–1873 [DOI] [PubMed] [Google Scholar]