Abstract
Schizophrenia is a chronic mental devastating disease. Current therapy suffers from various limitations including low efficacy and serious side effects. Thus, there is an urgent necessity to develop new antipsychotics with higher efficacy and safety. The dried stigma of the plant Crocus sativus L., (CS) commonly known as saffron, are used in traditional medicine for various purposes. It has been demonstrated that saffron and its bioactive components crocins and safranal exert a beneficial action in different pathologies of the central nervous system such as anxiety, depression, epilepsy and memory problems. Recently, their role as potential antipsychotic agents is under investigation. In the present review, I intended to critically assess advances in research of these molecules for the treatment of schizophrenia, comment on their advantages over currently used neuroleptics as well-remaining challenges. Up to our days, few preclinical studies have been conducted to this end. In spite of it, results are encouraging and strongly corroborate that additional research is mandatory aiming to definitively establish a role for saffron and its bioactive components for the treatment of schizophrenia.
Keywords: Crocus sativus L., crocins, schizophrenia
1. Schizophrenia
Schizophrenia is a serious chronic mental disease that affects up to 1% of the world population. It is a complex heterogeneous psychiatric disorder that impairs social, occupational and individual functioning and causes an adjective decrease in the quality of life of patients. This disease usually is manifested in late adolescence or early adulthood. Schizophrenics display serious psychotic symptoms, which can be classified into three major categories: positive symptoms (e.g., hallucinations, delusions, disordered thinking, catatonic behavior), negative symptoms (e.g., social withdrawal, anhedonia, avolition, neglect of hygiene) and cognitive disturbances (e.g., in attention, executive functioning and memory) [1].
Schizophrenia’s causes and pathophysiology are not yet elucidated. Nevertheless, it is widely acknowledged as a composite neurodevelopmental disease affected by genetic and environmental factors [2,3]. Specifically, it has been revealed that monozygotic siblings of schizophrenics have a 50–80% risk of developing the disease. Further, incomplete maturation of the brain and abnormal synaptic connections between different brain areas are also evidenced [4] Interestingly, an increasing number of reports propose the implication of oxidative stress in the pathophysiology of schizophrenia [5].
Additionally, several lines of evidence suggest that malfunctioning of different neurotransmitter systems, as are dopamine (DA), glutamate, cholinergic, serotonergic and the GABAergic systems is associated with the appearance of this disease [6]. In particular, positive symptoms of schizophrenia are associated with overactivation of dopaminergic neurotransmission in the striatum, while negative symptoms and cognitive impairments appear to be dependent on dopaminergic hypofunction in the prefrontal cortex [7].
Glutamate hypofunction seems also to be involved in the pathophysiology of schizophrenia. Abnormal glutamatergic transmission is related to secondary dopaminergic dysfunction in the striatum and prefrontal cortex. In this context, it has been shown that pharmacological blockade of NMDA receptor induces negative symptoms and cognitive deficits that were not alleviated by neuroleptics [7]. Moreover, the functionality of the GABAergic system, the major inhibitory neurotransmitter in the brain, is compromised in schizophrenia [8]. Since GABAergic firing modulates dopaminergic transmission in the prefrontal cortex, the malfunctioning of GABA interneurons seems to play a role in the appearance of some of the clinical symptoms of schizophrenia [9].
Clinical findings indicate that conventional antipsychotics (either those of the first generation, or atypical) display a certain efficacy in the alleviation of positive symptoms but are inefficacious in relieving negative symptoms and cognitive impairments of schizophrenia patients. These medications, however, are associated with important side effects which compromise their benefit. Specifically, motor side effects (Parkinsonism) are related with the administration of traditional neuroleptics (e.g., chlorpromazine, haloperidol). Conversely, the administration of atypical antipsychotics (e.g., clozapine, olanzapine, risperidone) does not produce Parkinsonism but causes weight gain. In addition, 30% of patients are resistant to the above-described treatments. Collectively, these results suggest that there is a pressing necessity to find novel compounds which could provide alleviation of negative symptoms and cognitive deficits typical features of schizophrenia patients [10,11].
Among the different alternative approaches for the therapy of schizophrenia, the involvement of the plant saffron and its bioactive components as potential anti-schizophrenia agents has lately been suggested. In the current analysis, I intend to assess with critical feeling the potential beneficial action of saffron and its components for the treatment of schizophrenia.
2. Crocus sativus L. (Saffron)
Crocus sativus L. (CS), is a perennial herb and a member of the Iridaceae family, of genus Crocus, the line of Liliaceae. This plant is cultivated in a number of countries such as Azerbaijan, China, France, Greece, Egypt, India, Iran, Israel, Italy, Mexico, Morocco, Spain and Turkey. The spice saffron is the end product of this plant. Saffron, in filaments, is the dried dark-red stigmas of CS flower. The weight of a single stigma is circa 2 mg and each flower has three stigmata; 150.000 flowers must be thoroughly selected separately to gain 1 kg of spice. Saffron has a characteristic color, taste and smell. From ancient to modern times the history of saffron is full of applications. It is widely utilized as a perfume, as a spice for flavoring and staining food and drink preparations. The most common way to consume saffron is still to mix it with food or to add it to any hot or warm drink [12,13].
Additionally, saffron is commonly utilized in traditional medicine, as a beneficial agent for the therapy of various pathologies of the cardiovascular, respiratory, gastrointestinal and nervous system For review see [14].
2.1. Chemistry of CS
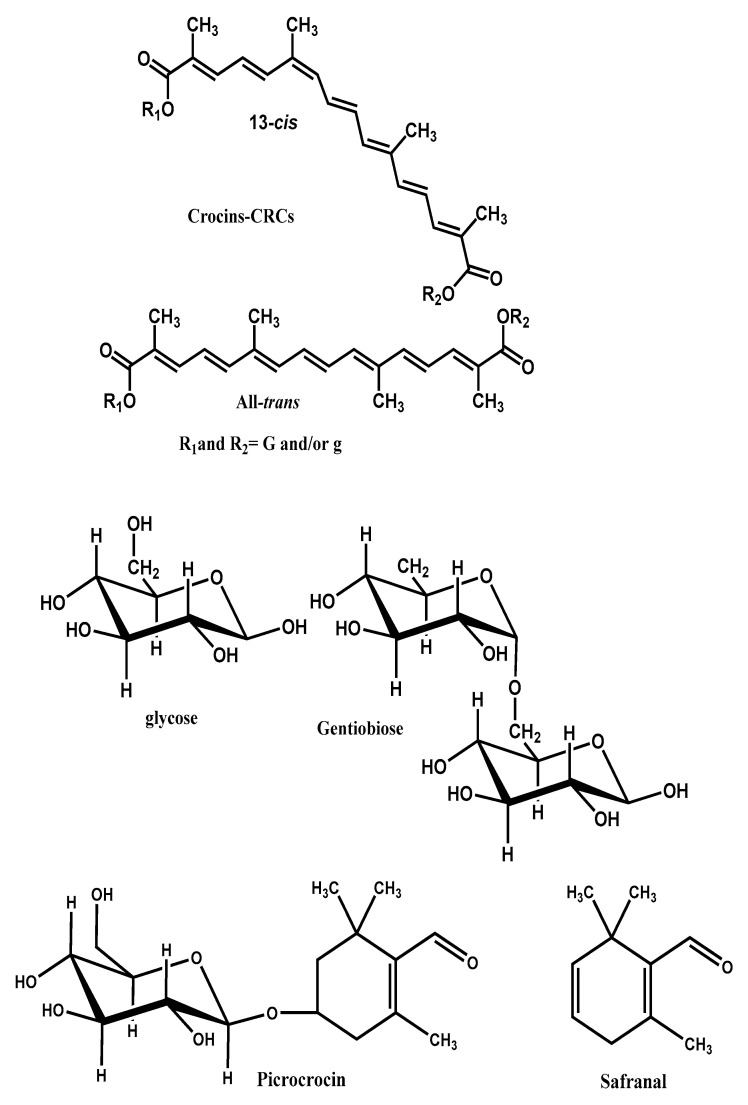
The prevailing non-volatile components of the saffron are crocins, crocetin, safranal picrocrocin and flavonoids (querectin and kaempferol) [12]. The coloring components of saffron are crocins (C44H64O24), which are unusual water-soluble carotenoids (glycosyl esters of crocetin). The major component is a digentiobiosyl ester of crocetin (C44H64O24, 8,8’-diapo-Ψ,Ψ’-carotenedioic acid bis (6-0-β-d-glucopyranosyl-β-d-glucopyranosyl) ester). Safranal (C10H14O, 2,6,6-trimethyl-1,3-cyclohexadiene-1- carboxaldehyde), which is responsible for the characteristic aroma of saffron is a monoterpene aldehyde. The principal bitter-tasting substance is picrocrocin a glycoside of safranal (C16H26O7, 4-(β-d-glucopyranosyloxy)-2,6,6-trimethyl-1-cyclohexene-1- carboxaldehyde) [15,16,17].
In Figure 1 the molecular structures of CS and its constituent crocins, picrocrocin and safranal are illustrated.
Figure 1.
Chemical structures of saffron bioactive components. Crocins (CRCs): Glucosyl ester of Crocetin, R1 = â-d-Geotiobiosyl, R2 = â-d-Geotiobiosyl, R1 = â-d-Gentiobiosyl, R2 = â-d-glucosyl., R1 = â-d-Gentiobiosyl, R2 = H, R1 = â-d-glucosyl, R2 = â-d-glucosyl., R1 = â-d-glucosyl, R2 = H, Crocetin R1 = H, R2 = H., B. Picrocrocin., C. Safranal.
2.2. Pharmacology of CS and Its Bioactive Components
Based on a conspicuous number of preclinical and clinical data an exciting pharmacological profile of saffron and its bioactive ingredients is turning up.
2.2.1. Effects of CS and Its Constituents on Non-Neurological/Neuropsychiatric Pathologies
In a series of preclinical in vitro and in vivo studies the anti-cancer, anti-nociceptive and anti-inflammatory properties of saffron have been revealed. Additionally, it has been reported that CS and its bioactive ingredients reduced atherosclerosis and hepatotoxicity, diminish hyperlipidemia, display a protective action on myocardial injury and consistently reduce blood pressure [14,18,19].
As a whole, what emerges from these preclinical findings is that CS and its active components appear to express a beneficial action in preclinical models of different non-neurological/neuropsychiatric pathologies. Until now, however, there is a lack of clinical results validating the therapeutic efficiency of saffron observed in the above-mentioned preclinical pathological models. Thus, clinical studies should be designed and conducted in order to properly address this important issue.
2.2.2. Effects of CS and Its Constituents on Pathologies of The Central Nervous System
In a series of preclinical studies, the anticonvulsant properties of the aqueous and ethanolic extracts of CS and safranal have been observed and. Specifically, it has been demonstrated that CS extracts and safranal counteracted pentylenetetrazol-induced seizures in mice and rats and these effects seem to be mediated by their interaction with the GABAergic and opioids systems [20,21]. Further, both saffron and its active components were found to be protective in preclinical models of Parkinson’s disease (PD) and cerebral ischemia [22,23,24,25].
The efficacy of saffron and crocins to attenuate memory impairments in preclinical models associated with Alzheimer’s disease (AD), cerebral injuries, or schizophrenia is well documented For a review, see [26]. The outcome of clinical trials designed to examine the efficiency of saffron in alleviating memory problems, a common feature of AD, proposes that the effects exerted by CS on cognition, although modest, were similar to those displayed by the reference molecules donepezil and memantine. Importantly, in all human studies conducted, treatment with saffron, in contrast with donepezil and memantine, did not induce noticeable undesired effects [27].
Intensive preclinical research revealed a consistent antidepressant-like effect of saffron and its major constituents crocins and safranal [28]. This antidepressant-like effect of saffron observed in rodents was corroborated by clinical findings. Studies carried out in humans evidenced the efficacy of saffron in the therapy of mild-to-moderate depression [29,30]. In this context, it has been reported that saffron attenuated sexual malfunction in both males and females which was caused by the challenge with the selective serotonin re-uptake inhibitor (SSRI) antidepressant agent fluoxetine [31,32].
Up to now, few studies have been conducted aiming to investigate the potential anti-anxiety effect of CS and its components. In spite of the scant number of studies (preclinical and clinical), the results reported appear promising. Further research is mandatory in order to fully elucidate and establish the anxiolytic profile of saffron and its bioactive components. For review, please see [33].
2.2.3. Pharmacokinetic and Safety Studies
Pharmacokinetic studies revealed that crocins, following oral administration, are not absorbed in the gastrointestinal tract (GIT) but are hydrolyzed to crocetin and in this form are absorbed in the GIT [34,35]. Crocetin is the active metabolite among which crocins exert their beneficial actions. Crocetin reaches the blood circulation and is found to be relatively quickly distributed in all tissues of the human body [36] can be partially conjugated with mono and diglucuronides in the GIT and in the liver [37]. A recent report demonstrated, however, that after oral application also crocins can be absorbed through GIT with poorer bioavailability compared to crocetin [38]. As a whole, either crocins or crocetin when applied orally, display low stability, poor absorption and low bioavailability [39]. Reportedly in this context, it has been evidenced that intraperitoneal or intravenous rather oral administration of saffron and its active components might provide higher levels of absorption and bioavailability [38,40]. In particular, Zhang and colleagues have indicated that intravenous application of crocins in rats did not reveal the presence of crocetin in plasma but solely crocins were detected, eliciting thus that hepatic metabolism of crocins would be insignificant [38]. Further studies are required, however, aiming to clarify this important issue.
Interestingly, it has been shown that crocins despite their high hydrophilic profile, similarly to crocetine [41,42], can cross the blood–brain barrier (BBB) and reach the central nervous system [40]. Concerning safranal, it can be hypothesized that might be able to penetrate the BBB since in a series of studies its anticonvulsant and antidepressant properties have been revealed [20,21,28].
Toxicological investigations performed in rodents that have received saffron extracts shown that the hematological and the biochemical parameters of the animals were not altered by treatment with saffron and remained at physiological levels [43]. In this context, it has been reported that the oral LD50 of CS was 20.7 g/kg when was delivered as a decoction in mice [44]. Further research confirmed the good safety profile of CS and its ingredients. Specifically, it has been observed that acute treatment of mice with saffron (up to 3 g, either orally (p.o.) or intraperitoneally (i.p.)) and repeated with crocin (15–180 mg/kg, i.p.) did not affect a series of biochemical, hematological and pathological markers recorded [45].
The outline of human studies confirmed the safe profile of CS extracts and crocin observed in preclinical experiments. In a double-blind, placebo-controlled study, carried out on healthy volunteers, repeated challenge with saffron (200–400 mg/day, for seven consecutive days) did not induce appreciable abnormalities. It caused only some minor consistency clinical and laboratory parameter changes such as hypotension, reduced platelets and bleeding time and increased creatinine and blood urea nitrogen levels [46].
In agreement with the above, are the findings of another clinical trial conducted on healthy participants who received 20 mg/day of crocin for 30 consecutive days. Treatment with crocin did not produce any alteration of various hematological, biochemical, hormonal and urinary parameters recorded [47]. Finally, the challenge with very high doses of saffron (1.2–2 g) in healthy volunteers caused nausea, diarrhea, vomiting, and bleeding [48]. As a whole, saffron and its main bioactive components can be considered as safe natural products displaying very low toxicity.
3. Effects of CS and Its Constituents in Schizophrenia
3.1. Preclinical Studies
Table 1 summarizes the existing literature regarding the effects of crocins on animal models of schizophrenia. Crocins (15–30 mg/kg, acutely) counteracted disruption of non-spatial recognition memory caused by acute administration of the NMDA receptor antagonist ketamine (3 mg/kg, acutely) in rats. This finding strongly proposes the involvement of this bioactive ingredient of CS in schizophrenia-related cognitive impairments. Additionally, crocins (50 mg/kg, acutely) attenuated ketamine (25 mg/kg, acutely)—induced psychotomimetic effects (hypermotility, stereotypies and ataxia) in the rat. Further, in a behavioural procedure mimicking the negative symptoms of schizophrenia (social interaction test), these active components of saffron (50 mg/kg, acutely) were found able to reduce the social isolation-induced by treatment with ketamine (8 mg/kg, sub-chronically) in rats [49].
Table 1.
Effects of crocins on preclinical models of schizophrenia.
| Scheme | Agent | Dose Range | Route | Behavioral Test | Effect | Reference |
|---|---|---|---|---|---|---|
| Rat | Crocins | 15, 30, 50 mg/kg | i.p. acute | ORT | Crocins (15, 30 mg/kg) counteracted ketamine-induced non-spatial recognition memory deficits. | [49] |
| Ketamine | 3 mg/kg (NORT) | i.p. acute | ||||
| Ketamine | 8 mg/kg (SI) | i.p. sub-chronic | SI | Crocins (50 mg/kg) attenuated ketamine-induced social isolation. | [49] | |
| Ketamine | 25 mg/kg (motor activity) | i.p. acute | Motor activity, stereotypies, ataxia | Crocins (50 mg/kg) attenuated ketamine-induced hypermotility, stereotypies and ataxia. | [49] | |
| Rat | Crocins | 15, 30, 50 mg/kg | i.p. acute | ORT | Crocins (15, 30 mg/kg) counteracted ketamine-induced non-spatial recognition memory deficits. | [50] |
| Apomorphine | 1 mg/kg | i.p. acute | ||||
| Rat | Crocins | 15, 30, 50 mg/kg | i.p. acute | OLT | No effect | [50] |
| Apomorphine | 1 mg/kg | i.p. acute | ||||
| Rat | Crocins | 25, 50 mg/kg | i.p. acute | RR, OFT | Crocin (25, 50 mg/kg) counteracted MK-801-induced motor activity deficits. | [51] |
| MK-801 | 1 mg/kg | i.p. acute | ||||
| Crocins | 25, 50 mg/kg | i.p. acute | MWM | Crocin (25, 50 mg/kg) counteracted MK-801-induced spatial memory deficits. | [51] | |
| MK-801 | 1 mg/kg | i.p. acute |
Abbreviations:i.p, intraperitoneally; MWM, Morris water maze; OFT, open field test; OLT, object location task; ORT, object recognition task; RR, rotarod; SI, social interaction.
In agreement with the above, crocins (30 mg/kg, acutely) antagonized disruption of non-spatial recognition memory caused by a single injection of the mixed DA D1/D2 receptor agonist apomorphine (1 mg/kg). By contrast, crocins failed to counteracted spatial recognition memory induced by apomorphine (1 mg/kg, acutely). It has been suggested that this dual action of crocins on recognition memory deficits observed in a dopaminergic model of amnesia might depend to differences in stimuli intensity (higher in non-spatial tasks as compared to spatial tasks) [50].
It has recently been reported that crocin attenuated schizophrenia-like symptoms in a glutamatergic model of this psychiatric disease. In particular, crocin (25 and 50 mg/kg, acutely) attenuated motor disturbances and spatial navigation impairments induced by acute administration of the NMDA receptor antagonist MK-801 (1 mg/kg) in rats [51].
It is important to emphasize that the beneficial effects of crocins, summarized in Table 1, were observed following intraperitoneal application of them in rodents. Intraperitoneal compared to oral route of administration might be of higher utility since it can be avoided the first-pass metabolism and/or gastric hydrolysis and obtain consistent bioavailability profile of the compound (elimination of liver-induced metabolism as well exposure of crocins in a low pH of the stomach [52].
3.2. Clinical Studies
Up to our days, clinical information dealing with a potential anti-schizophrenia efficacy of CS and its bioactive constituents is inconsistent. Only one clinical study was conducted aiming to evaluate the safety and the tolerability of treatment with saffron and crocin in schizophrenia patients. This was a double-blind, placebo-controlled trial and participated 61 schizophrenics. Patients were treated twice daily with saffron or crocin (15 mg) or placebo for 12 consecutive weeks. In agreement with prior reports [27,29,53], the results of this study showed that saffron extracts, safranal and crocin were well-tolerated in schizophrenics [54]. In this context, is important to emphasize that challenge with a saffron aqueous extract (30 mg/day, for 12 weeks) administered in schizophrenics on treatment with olanzapine prevented the metabolic syndrome, a well-known side effect of this atypical neuroleptic [55].
3.3. Potential Mechanism of Action of CS and Its Constituents in Schizophrenia
The exact mechanism(s) through which crocins exert their effects on schizophrenia-like behavior caused by glutamatergic and dopaminergic dysfunction is (are) not yet elucidated. Research is needed aiming to clarify this important issue. That schizophrenia-like effects of NMDA receptor antagonists (e.g., ketamine, MK-801) are related to increased concentrations of glutamate, hypermotility, stereotypy and cognition deficits [56,57] is well-documented. In this context, it has been reported that acute systemic administration of safranal reduced kainic acid-induced increase of extracellular glutamate concentrations in the rat hippocampus [58]. Further, it has been observed that either saffron or crocetin but not crocins partly counteract the NMDA receptor by binding to the phencyclidine (PCP) binding site of it [59]. The apparent failure of crocins to bind at the NMDA receptor might depend on pharmacokinetic issues, and in particular, their poor intestinal absorption after oral administration in rats [59]. Moreover, it has been shown that CS extracts and crocetin normalized excessive glutamatergic synaptic transmission in rat cortical brain slices [60]. Finally, CS extracts and crocetin were found to display a strong affinity for the sigma (σ)1 receptor [59]. As a whole, these results propose that this decrease of glutamate concentrations by CS and its constituents might be crucial for the beneficial action exerted by crocins on NMDA receptor antagonists-induced psychotomimetic effects and cognitive deficits.
There is poor evidence concerning the mechanism(s) by which crocins could counteract the detrimental effects of apomorphine on non-spatial recognition memory. In this context, it has been reported that apomorphine prevented the induction of long-term potentiation (LTP) which is the electrophysiological correlate of cognition [61], while crocins promote it [62].
Although the pathogenesis of schizophrenia is not yet fully clarified, a possible association with oxidative stress [5,51], inflammation [51,63] and abnormally low concentrations of different neurotrophins as is the brain-derived neurotrophic factor (BDNF) [64] has been suggested. In line with the above, in a series of reports, the pro-oxidative and pro-inflammatory profile either of NMDA receptor antagonists [51,65] or apomorphine [61] has emerged. The potent antioxidant properties of crocins may offer an alternative explanation for the beneficial effects exerted by these bioactive components of saffron in preclinical models of schizophrenia [66,67,68,69]. In this context, it has recently been demonstrated that the neuroprotective action of crocins evidenced in a preclinical glutamatergic model of schizophrenia was related to their ability to restore the expression of BDNF and that of the silent information regulator-1 (SIRT-1), a modulator of oxidative stress and inflammation, thus eliciting alleviation of the oxidative stress [51].
4. Conclusions
There is poor available information (either preclinical or clinical) concerning a potential beneficial role for CS and its bioactive constituents in the therapy of schizophrenia. In spite of it, the few preclinical data produced do not lack consistency and are really promising. The latter elicits that future research is mandatory in order to definitively establish if these compounds are suitable candidates and provide a benefit in the therapy of schizophrenia. It is important to underline the clinical efficacy expressed by saffron and its constituents in depression [28,29,30] and anxiety [33] which are typical features of patients suffering from schizophrenia [70,71] and further emphasize their good safety profile expressed in human studies.
Future research should examine the efficacy of these natural products on preclinical models resembling attentional deficits and extensively evaluate their efficacy on animal models mimicking negative symptoms of this devastating psychiatric disorder. The utilization of other than pharmacological models (e.g., neurodevelopmental, genetic etc.) will be of high value. Finally, in the case of positive preclinical findings, human studies (double-blind, placebo-controlled studies) by recruiting an appropriate number of participants should be conducted in order to evaluate the efficacy of these compounds in schizophrenia. A summary of some future research activities (either preclinical or clinical) is provided in Table 2.
Table 2.
Summary of future studies designed to evaluate the role of Crocus sativus and its bioactive components in schizophrenia. Key plans.
| Preclinical research |
| Acute vs. repeated drug treatment Not pharmacological animal models of schizophrenia (genetic, neonatal ventral hippocampal lesions models etc) Evaluation of the effects of saffron and its constituents in animal models of attentional deficits Further evaluation of the effects of saffron and its constituents in animal models resembling cognitive impairments and negative symptoms of schizophrenia Investigation of potential mechanism(s) of action underlying the beneficial effects of saffron and its constituents observed in preclinical studies (molecular, biochemical, neurochemical, electrophysiological studies etc) |
| Clinical research |
| Multi-center, double-blind, placebo-controlled studies Studies of the effects of saffron alone in schizophrenia patients Studies of the effects of saffron in combination with atypical antipsychotics in schizophrenia patients Use of broad dose range of C. sativus Appropriate number of participants |
Funding
This research received no external fundings.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Freedman R. Schizophrenia. N. Engl. J. Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 2.Lewis D.A., Lieberman J.A. Catching up on schizophrenia: Natural history and neurobiology. Neuron. 2000;28:1738–1749. doi: 10.1016/S0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 3.Van Os J., Kenis G., Rutten B.P. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 4.Weinberger D.R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 5.Bitanihirwe B.K., Woo T.U. Oxidative stress in schizophrenia: An integrated approach. Neurosci. Biobehav. Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steeds H., Carhart-Harris R.L., Stone J.M. Drug models of schizophrenia. Ther. Adv. Psychopharmacol. 2015;5:43–58. doi: 10.1177/2045125314557797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javitt D.C. Glutamate and schizophrenia: Phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 8.Pratt J., Winchester C., Dawson N., Morris B. Advancing schizophrenia drug discovery: Optimizing rodent models to bridge the translational gap. Nat. Rev. Drug Discov. 2012;11:560–579. doi: 10.1038/nrd3649. [DOI] [PubMed] [Google Scholar]
- 9.Lewis D.A., Pierri J., Volk D., Melchitzky D., Woo T. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol. Psychiatry. 1999;46:616–626. doi: 10.1016/S0006-3223(99)00061-X. [DOI] [PubMed] [Google Scholar]
- 10.Field J.R., Walker A.G., Conn P.J. Targeting glutamate synapses in schizophrenia. Trends Mol. Med. 2011;17:689–698. doi: 10.1016/j.molmed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbott A. Schizophrenia: The drug deadlook. Nature. 2010;468:158–159. doi: 10.1038/468158a. [DOI] [PubMed] [Google Scholar]
- 12.Liakopoulou-Kyriakides M., Kyriakidis D. Crocus Sativus-biological active constituents. Stud. Nat. Prod. Chem. 2002;16:293–312. [Google Scholar]
- 13.Tarantilis P.A., Tsoupras G., Polissiou M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV/Visible photodiode-array detection-mass spectrometry. J. Chromatogr. A. 1995;699:107–118. doi: 10.1016/0021-9673(95)00044-N. [DOI] [PubMed] [Google Scholar]
- 14.Rios J.L., Recio M.C., Ginger R.M., Manz S. An update review of saffron and its active constituents. Phytother. Res. 1996;10:189–193. doi: 10.1002/(SICI)1099-1573(199605)10:3<189::AID-PTR754>3.0.CO;2-C. [DOI] [Google Scholar]
- 15.Tarantilis P.A., Polissiou M., Marnait M. Separation of picrocrocin, cis-trans-crocins and safranal of saffron using high-performance liquid chromatography with photodiode-array detection. J. Chromatogr. A. 1994;664:55–61. doi: 10.1016/0021-9673(94)80628-4. [DOI] [PubMed] [Google Scholar]
- 16.Kanakis C.D., Daferera D.J., Tarantilis P.A., Polissiou M.G. Qualitative determination of volatile compounds and quantitative evaluation of safranal and 4-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde. J. Agric. Food Chem. 2004;52:4515–4521. doi: 10.1021/jf049808j. [DOI] [PubMed] [Google Scholar]
- 17.Abdullaev F.I., Espinosa-Aguirre J.J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Bathaie S.Z., Mousavi S.Z. New applications and mechanisms of action of saffron and its important ingredients. Crit. Rev. Food Sci. Nutr. 2010;50:761–786. doi: 10.1080/10408390902773003. [DOI] [PubMed] [Google Scholar]
- 19.Alavizadeh S.H., Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinzadeh H., Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus Sativus, L., stigmas in mice. Arch. Iran Med. 2005;5:44–47. [Google Scholar]
- 21.Hosseinzadeh H., Sadeghnia H.R. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of the GABAergic and opioids systems. Phytomedicine. 2007;14:256–262. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad A.S., Ansari M.A., Ahmad M., Saleem S., Yousuf S., Hoda M.N., Islam F. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol. Biochem. Behav. 2005;81:805–813. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H., Sadeghnia H.R. Safranal a constituent of Crocus sativus (saffron) attenuated cerebral ischemia-induced oxidative damage in rat hippocampus. J. Pharm. Pharm. Sci. 2005;8:394–399. [PubMed] [Google Scholar]
- 24.Hosseinzadeh H., Sadeghnia H.R., Ghaeni F.A., Motamedshariaty V.S., Mohajeri S.A. Effects of saffron (Crocus sativus L.) and its active constituent crocin, on recognition and spatial memory after chronic cerebral hypofunction in rats. Phytother. Res. 2012;26:381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 25.Saleem S., Ahmad M., Ahmad A.S., Yousuf F., Ansari M.A., Khan M.B., Ishrat T., Islam F. Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J. Med. Food. 2006;9:246–253. doi: 10.1089/jmf.2006.9.246. [DOI] [PubMed] [Google Scholar]
- 26.Pitsikas N. The effects of Crocus sativus L. and its constituents on memory: Basic studies and clinical applications. Evid. Based Complement. Alternat. Med. 2015;2015:926284. doi: 10.1155/2015/926284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akhondzadeh S., Shafiee-Sabet M., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S., Hejazi S.S., Yousefi M.H., Alimardani R., Jasmshidi A., et al. A 22-week, multicentre randomized, double-blind controlled trial of Crocus sativus L., in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology. 2010;207:637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- 28.Hosseinzadeh H., Karimi G., Niapoor M. Antidepressant effects of crocus sativus stigma extracts and its constituents, crocins and safranal in mice. J. Med. Plants. 2004;3:48–58. [Google Scholar]
- 29.Akhondzadeh S., Fallah-Pour H., Afkham K., Jamshidi A.H., Khalighi-Cigaroudi F. Comparison of Crocus sativus L., and imipramine in the treatment of mild to moderate depression: A pilot double-blind, randomized trial. BMC Complement. Altern. Med. 2004;4:12–16. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noorbala A.A., Akhondzadeh S., Tahmacebi-Pour N., Jamshidi A.H. Hydro-alcoholic extract of Crocus sativus L., versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized trial. J. Ethnopharmacol. 2005;97:281–284. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Kashani L., Raisi F., Saroukhani S., Sohrabi H., Moddabernia H., Nasehi A.A., Jamshidi A., Ashrafi M., Mansouri P., Ghaeli P., et al. Saffron for treatment of fluoxetine-induced sexual dysfunction in women: Randomized double-blind placebo-controlled study. Hum. Psychopharmacol. 2012;28:54–60. doi: 10.1002/hup.2282. [DOI] [PubMed] [Google Scholar]
- 32.Modabbernia A., Sohrabi H., Nasehi A.A., Raisi F., Saroukhani S., Jamshidi A., Tabrizi M., Ashrafi M., Akhonzadeh S. Effect of saffron on fluoxetine-induced sexual impairment in men: Randomized double-blind placebo-controlled trial. Psychopharmacology. 2013;223:381–388. doi: 10.1007/s00213-012-2729-6. [DOI] [PubMed] [Google Scholar]
- 33.Pitsikas N. Saffron the age-old panacea in a new light. Sarwat, M., Sumaiya, S., Eds. Academic Press; Cambridge, MA, USA: 2020. Assessment of Crocus sativus L., and its bioactive constituents as potential anti-anxiety compounds. Basic and clinical evidence; pp. 131–139. [Google Scholar]
- 34.Lautenschlager M., Sendker J., Huwel S., Galla H.J., Brandt S., Dufer M., Riehemann K., Hensel A. Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier. Phytomedicine. 2015;22:36–44. doi: 10.1016/j.phymed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Xi L., Quian Z., Xu G., Zheng S., Sun S., Wen N., Sheng L., Shi Y., Zhang Y. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J. Nutr. Biochem. 2007;18:64–72. doi: 10.1016/j.jnutbio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Kanakis C.D., Tarantilis P.A., Tahmir-Riahi H.A., Polissiou M.G. Crocetin, dimethylcrocetin and safranal, bind human serum albumin: Stability and antioxidative properties. J. Agric. Food Chem. 2007;55:970–977. doi: 10.1021/jf062638l. [DOI] [PubMed] [Google Scholar]
- 37.Asai A., Nakano T., Takahashi M., Nagao A. Orally administered crocetin and crocins are absorbed into blood plasma as crocetin and its glucuronide conjugates in mice. J. Agric. Food Chem. 2005;53:7302–7306. doi: 10.1021/jf0509355. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Fei F., Zhen L., Zhu X., Wang J., Li. S., Geng J., Sun R., Yu X., Chen T., et al. Sensitive analysis and simultaneous assessment of pharmacokinetic properties of crocin and crocetin after oral administration in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017;10:1–7. doi: 10.1016/j.jchromb.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Puglia C., Santonocito D., Musumeci T., Cardile V., Graziano A., Salerno L., Raciti G., Crasci’ L., Panico A., Puglisi G. Nanotechnological approach to increase the antioxidant and cytotoxic efficacy of crocin and crocetin. Planta Med. 2018;85:258–265. doi: 10.1055/a-0732-5757. [DOI] [PubMed] [Google Scholar]
- 40.Karkoula E., Lemonakis N., Kokras N., Dalla C., Gikas E., Skaltsounis A.L., Tsarbopoulos A. Trans-crocin 4 is not hydrolyzed to crocetin following i.p. administration in mice, while it shows penetration through the blood brain barrier. Fitoterapia. 2018;129:62–72. doi: 10.1016/j.fitote.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Linardaki Z.I., Orkoula M., Kokkosis A.G., Lamari F.N., Margarity M. Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem. Toxicol. 2013;52:163–170. doi: 10.1016/j.fct.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 42.Yoshino F., Yoshida A., Umigai N., Kubo K., Lee M.C.I. Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (SHRSPs) brain. J. Clin. Biochem. Nutr. 2011;49:182–187. doi: 10.3164/jcbn.11-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nair S.C., Panikkar B., Panikkar K.R. Antitumor activity of saffron. Cancer Lett. 1991;57:109–114. doi: 10.1016/0304-3835(91)90203-T. [DOI] [PubMed] [Google Scholar]
- 44.Abdullaev F.I. Cancer chemoprotective and tumoricidial properties of saffron (Crocus sativus L.) Exp. Biol. Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 45.Hosseinzadeh H., Motamedshariaty V.S., Sameni A.K., Vahabzadeh M. Acute and sub-acute toxicity of crocin, a constituent of Crocus sativus L., (saffron), in mice and rats. Pharmacologyonline. 2010;2:943–951. [Google Scholar]
- 46.Modaghegh M.H., Shahabian M., Esmaeli H.A., Rajbai O., Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Mohamadpour A.H., Ayati Z., Parizadeh M.R., Rajbai O., Hosseinzadeh H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran, J. Basic Med. Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt M., Betti G., Hensel A. Saffron in phytotherapy: Pharmacology and clinical uses. Wien Med. Wochenschr. 2007;157:315–319. doi: 10.1007/s10354-007-0428-4. [DOI] [PubMed] [Google Scholar]
- 49.Georgiadou G., Grivas V., Tarantilis P.A., Pitsikas N. Crocins the active constituents of Crocus Sativus, L., counteracted ketamine-induced behavioural deficits in rats. Psychopharmacology. 2014;231:717–726. doi: 10.1007/s00213-013-3293-4. [DOI] [PubMed] [Google Scholar]
- 50.Pitsikas N., Tarantilis P.A. Crocins, the active constituents of Crocus sativus L., counteracted apomorphine-induced performance deficits in the novel object recognition task, but not novel location task, in rats. Neurosci. Lett. 2017;644:37–42. doi: 10.1016/j.neulet.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 51.Sun X.J., Zhao X., Xie J.N., Wan H. Crocin alleviates schizophrenia-like symptoms in rats by upregulating silent information regulator-1 and brain derived neurotrophic factor. Compr. Psychiatry. 2020;103:152209. doi: 10.1016/j.comppsych.2020.152209. [DOI] [PubMed] [Google Scholar]
- 52.Tumer P.V., Brabb T., Pekow C., Vasbinder M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 53.Farokhnia M., Shafiee-Sabet M., Iranpour N., Gougol A., Yekehtaz H., Alimardani R., Farsad F., Kamalipour M., Akhondzadeh S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Hum. Psychopharmacol. 2014;29:351–359. doi: 10.1002/hup.2412. [DOI] [PubMed] [Google Scholar]
- 54.Mousavi B., Bathaie S.Z., Fadai F., Ashtari Z., Ali Beigi N., Farhang S., Hashempour S., Shahhamzei N., Heidarzadeh H. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J. Phytomed. 2015;5:413–419. [PMC free article] [PubMed] [Google Scholar]
- 55.Fadai F., Moosavi B., Ashtari Z., Ali Beigi N., Farhang S., Hashempour S., Shahhamzei N., Zahra Bathaie S. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: A randomized triple blind placebo controlled study. Pharmacipsychiatry. 2014;47:156–161. doi: 10.1055/s-0034-1382001. [DOI] [PubMed] [Google Scholar]
- 56.Moghaddam B., Adams B., Verma A., Daly D. Activation of glutamatergic transmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tricklebank M.D., Singh L., Oles R.J., Preston C., Iversen S.D. The behavioral effects of Mk-801: A comparison with antagonists acting non-competitively and competitively at the NMDA receptor. Eur. J. Pharmacol. 1989;167:127–135. doi: 10.1016/0014-2999(89)90754-1. [DOI] [PubMed] [Google Scholar]
- 58.Hosseinzadeh H., Sadeghnia H.R., Rahimi A. Effects of safranal on extracellular hippocampal levels of glutamate and aspartate during kainic acid treatment in anesthetized rats. Planta Med. 2008;74:1441–1445. doi: 10.1055/s-2008-1081335. [DOI] [PubMed] [Google Scholar]
- 59.Lechtenberg M., Schepmann D., Niehues M., Hellenbrand M., Wunsch B., Hensel A. Quality and functionality of saffron: Quality control, species assortment and affinity of extract and isolated saffron compounds to NMDA and σ1 (Sigma-1) receptors. Planta Med. 2008;74:764–772. doi: 10.1055/s-2008-1074535. [DOI] [PubMed] [Google Scholar]
- 60.Berger F., Hensel A., Nieber K. Saffron extracts and trans-crocetin inhibit glutamatergic synaptic transmission in rat cortical brain slices. Neuroscience. 2011;180:238–247. doi: 10.1016/j.neuroscience.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 61.Arroyo-Garcia L.E., Rodriguez-Moreno A., Flores G. Apomorphine effects on hippocampus. Neural Regen. Res. 2018;13:2064–2066. doi: 10.4103/1673-5374.241443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sugiura M., Shoyama Y., Saito H., Abe K. Crocin (crocetin di-gentiobiose ester) prevents the inhibitory effect of ethanol on long-term potentiation in the dentate gyrus in vivo. J. Pharmacol. Exp. Ther. 1994;271:703–707. [PubMed] [Google Scholar]
- 63.Khandaker G.M., Cousins L., Deakin J., Lennox R.B., Yolken R., Jones P.B. Inflammation and immunity in schizophrenia: Implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2:258–270. doi: 10.1016/S2215-0366(14)00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez-Amorim D., Rivera-Baltands T., Bessa J., Sousa N., Vallejo-Curto M.C., Rodriguez-Jamardo C., de las Heras M.E., Diaz R., Agis-Balboa R.C., Olivares J.M., et al. The neurobiological hypothesis of neurotrophins in the pathophysiology of schizophrenia: a meta-analysis. J. Psychiatr. Res. 2018;106:43–53. doi: 10.1016/j.jpsychires.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 65.De Oliveira L., Spiazzi C.M., Bortolin T., Canever L., Petronilho F., Mina F.G., Dal-Pizzol F., Quevedoa J., Zugno A.I. Different sub-anesthetic doses of ketamine increase oxidative stress in brain of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1003–1008. doi: 10.1016/j.pnpbp.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 66.Naghizadeh B., Mansouri M.T., Ghorbanzadeh B., Farbood Y., Sarkaki A. Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine. 2013;20:537–543. doi: 10.1016/j.phymed.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 67.Ochiai T., Soeda S., Ohno S., Tanaka H., Shoyama Y., Shimeno H. Crocin prevent the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem. Int. 2004;44:321–330. doi: 10.1016/S0197-0186(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 68.Papandreou M.A., Tsachaki M., Efthimiopoulos S., Cordopatis P., Lamari F.N., Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav. Brain Res. 2011;219:197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Zheng Y.Q., Liu J.X., Wang J.N., Xu L. Effects of crocin on reperfusion induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 70.Upthegrove R., Marwaha S., Birchwood M. Depression and schizophrenia: Cause, consequence or trans-diagnostic tool? Schizophr. Bull. 2016;43:240–244. doi: 10.1093/schbul/sbw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Temmingh H., Stein D.J. Anxiety in patients with schizophrenia: Epidemiology and management. CNS Drugs. 2015;29:819–832. doi: 10.1007/s40263-015-0282-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.