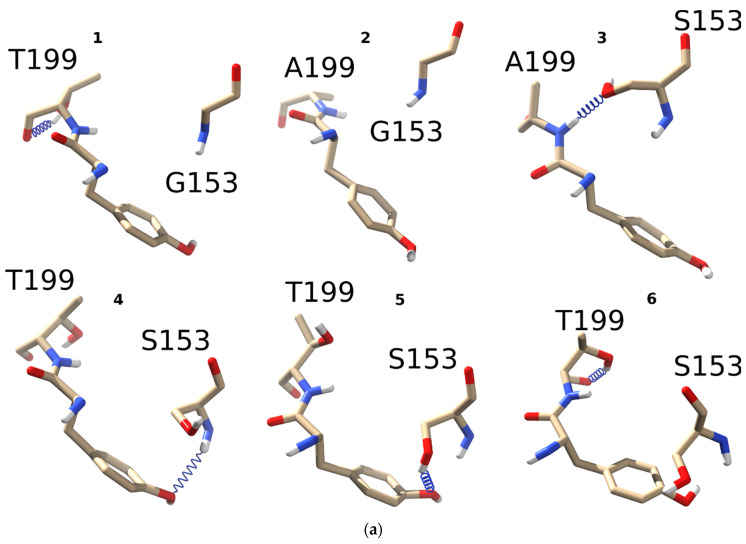
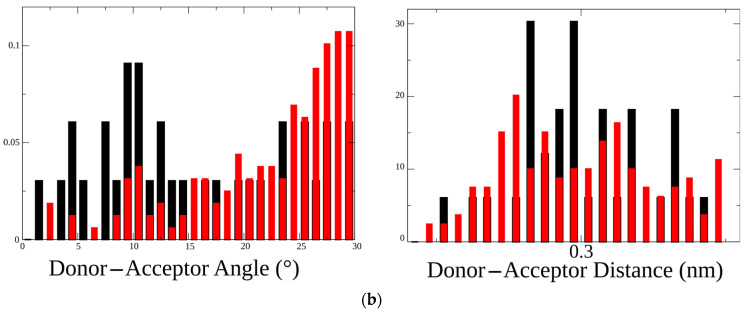
Figure 2.
Hydrogen bond patterns formed by threonine, alanine, or leucine in the 199 position of the C-loop with either glycine or serine in position 153: (a) four different α1 nAChR mutants were generated in silico, namely L199T (1), L199A (2), L199A/G153S (3), and L199T/G153S (4-6). All four mutants underwent 5 ns molecular dynamics to monitor hydrogen bond formation between residues 153 and 199. All possible combinations and observed hydrogen bonds (blue spring) are depicted in the figure. Among all mutants L199A did not show any hydrogen bonds between G153 and A199, while L199T/G153S showed three different hydrogen bonds; (b) comparison of hydrogen bonds distribution between residues 153 and 199 in double mutants L199A/G153S (black bars) and L199T/G153S (red bars). Distribution of H-bonds by the donor–acceptor angle shows the most prominent difference between these two mutants implying the possibility of different functional properties.