Abstract
Simple Summary
The tumor microenvironment (TME) is increasingly believed to be involved in therapy resistance and disease progression of different cancer types. Cancer-associated fibroblasts (CAFs) are prominent components and a central player in creating this TME. In particular, lung and pancreatic cancer, two solid tumor types associated with therapy resistance and poor long-term prognosis have clear evidence of CAFs. In order to provide a more holistic approach of therapy resistance, we highlight different mechanisms of CAF contribution to tumor progression and provide a comprehensive review on how CAFs affect their response to clinical therapy and prognosis, and present an overview of novel therapies and future perspectives involving CAF-targeting agents for both cancer types.
Abstract
Cancer arises from mutations accruing within cancer cells, but the tumor microenvironment (TME) is believed to be a major, often neglected, factor involved in therapy resistance and disease progression. Cancer-associated fibroblasts (CAFs) are prominent and key components of the TME in most types of solid tumors. Extensive research over the past decade revealed their ability to modulate cancer metastasis, angiogenesis, tumor mechanics, immunosuppression, and drug access through synthesis and remodeling of the extracellular matrix and production of growth factors. Thus, they are considered to impede the response to current clinical cancer therapies. Therefore, targeting CAFs to counteract these protumorigenic effects, and overcome the resistance to current therapeutic options, is an appealing and emerging strategy. In this review, we discuss how CAFs affect prognosis and response to clinical therapy and provide an overview of novel therapies involving CAF-targeting agents in lung and pancreatic cancer.
Keywords: cancer associated fibroblasts, therapy resistance, lung cancer, pancreatic cancer
1. Introduction
As our knowledge on cancer biology continuously evolves, the tumor microenvironment (TME) has gained interest over the past decade. Before, cancer research has been focusing primarily on the malignant cell itself [1]. However, cancers are not simply autonomous neoplastic cells [1] but interact with a complex surrounding ecosystem of stromal cells, known as the TME [2]. The TME consists of cancer-associated fibroblasts (CAFs), extracellular matrix (ECM), endothelial cells, and infiltrating innate and adaptive immune cells (e.g., natural killer (NK) cells and T cells, respectively) [3]. CAFs, the most prominent [4] and key component [5] of the TME, can either originate from normal fibroblasts, which are non-epithelial, non-endothelial, non-immune resting mesenchymal cells in diverse connective tissue components [6], or other precursor cells such as bone marrow-derived mesenchymal stem cells, epithelial cells, carcinoma cells, endothelial cells, pericytes, smooth muscle cells, adipocytes, fibrocytes, or specialized cells such as pancreatic stellate cells (PSCs) [3]. However, as there are no unique markers for fibroblasts that are not expressed by other cell types, it is hard to define a CAF [3,7]. Therefore, all fibroblasts associated with tumors [6] that are negative for epithelial, endothelial, and leukocyte markers with an elongated morphology and lacking the mutations found within cancer cells might be considered CAFs [7].
Fibroblasts reside in the ECM of healthy tissue to sustain normal tissue homeostasis and are found in an inactive quiescent state under normal physiologic conditions [8]. As a consequence of a disturbance of tissue integrity, such as tissue injury, they become activated and capable of producing transforming growth factor-β (TGF-β) and vascular endothelial growth factor A (VEGF-A) to stimulate wound healing and angiogenesis, respectively [8,9]. Besides, activated fibroblasts are also modulators of the immune response via the secretion of cytokines (e.g., TGF-β and interleukin (IL)-6 [6]) and chemokines (e.g., C-C motif chemokine ligand (CCL) 5, C-X-C motif chemokine ligand (CXCL) 10 [6]), and additionally promote immune surveillance [1,7]. When the process is complete and the activating stimulus is attenuated, the fibroblast activation is reversed to the inactive quiescent state by reprogramming or apoptosis [6]. However, diverse stimuli (reviewed in detail [7]) can cause chronic and irreversible fibroblast activation. This includes inflammatory signals (e.g., IL-1, IL-6, and tumor necrosis factor (TNF)), TGF-β, physical changes in the ECM, contact signals with cancer cells, and DNA damage [7], resulting in a gain of proliferation, secretory phenotype, migration, and ECM production and remodeling [6]. In case of (pre)malignant lesions, persistent accumulation of cancer cells represents an ongoing tissue injury. This initiates a chronic and irreversible hyperactivated fibroblast response towards the cancer cells and eventually the rise of CAFs, with enhanced proliferative properties and self-sustained activation. Additional fibroblast recruitment and proliferation is governed by the release of growth factors (e.g., TGF-β, PDGF, and fibroblast growth factor (FGF) 2) by the cancer cells and infiltrating immune cells [6]. Once CAFs are activated, they can contribute to tumor progression by a broad range of diverse functions.
2. CAFs Contribution to Tumor Progression
2.1. Desmoplasia
The chronic tissue repair response results in the excessive growth of fibrous or connective tissue around an invasive tumor [10]. This process of desmoplasia is achieved through enhanced ECM production and acquired remodeling ability of CAFs [6,10]. As such, desmoplasia causes the tumor to be hard and stiff, leading to a compromised tumor vasculature with blood vessel collapse and hypoxia, thereby promoting formation aggressive tumor clones and inhibition of drug penetration and uptake [7,11]. Furthermore, CAFs and their generated ECM serve as a physical barrier to tumor infiltration by immune cells, and the stiffness of the ECM has shown to enhance cancer cell invasion [6].
2.2. Secretion of Pro-Tumorigenic Modulators
The secretome of CAFs consists of several growth factors and cytokines [8], including connective tissue growth factor (CTGF), epidermal growth factor (EGF), insulin-like growth factor (IGF), hepatocyte growth factor (HGF), basic FGF (bFGF), nerve growth factor (NGF), and IL-6, which promote cancer cell survival as well as its proliferative and invasive behavior. Matrix metalloproteinases (MMPs) released by CAFs remodel the ECM, thus generating permissive tracks to facilitate motility and enhanced invasiveness of cancer cells, eventually boosting metastasis [6,7]. Through the secretion of stromal cell-derived factor 1 (SDF1), next to VEGF-A, CAFs are also able to recruit endothelial progenitor cells into the tumor, thereby stimulating tumor angiogenesis [1,12].
2.3. Generation of a Protumorigenic and Immunosuppressive TME
CAFs are generally considered to promote a protumorigenic and immunosuppressive TME [4,6,7,8,13] directly by releasing a plethora of immunosuppressive as well as immunomodulating and stimulating cytokines (e.g., IL-6, IL-4, IL-8, IL-10, IL-11, IL-17A, TNF, TGF-β, and HGF) and chemokines (e.g., CCL2, CCL5, CCL7, CXCL7, CXCL9, CXCL10, CXCL12, and SDF1), inflammatory factors (e.g., prostaglandin E2 (PGE2)) and immune-modulatory molecules (e.g., human leukocyte antigen (HLA)-G) that can retain suppressive immune subsets and impede proper function of cytotoxic lymphocytes, or indirectly via the remodeling of the ECM, forming a physical barrier for immune cell entry [4,13]. In addition to CAF-derived soluble factors CXCL12, IL-6, and TGF-β, CAFs also inhibit antitumor cytotoxicity of CD8+ T cells through the acquisition of immune checkpoint molecules, such as programmed cell death ligand 1 (PD-L1), PD-L2, and Fas ligand in several tumor types [13,14]. NK cell cytotoxic activity is mainly counteracted by PGE2 and indoleamine 2,3-dioxygenase (IDO) by reducing cytotoxic molecules (granzyme B and perforin) and cytokine release [13]. In summary, CAFs possess the ability to manipulate the immune system, both by excluding and opposing T and NK cell functions via tumor cell downregulation of antigen presentation, elevated expression of surface inhibitory molecules, and secretion of immunosuppressive factors, as well as by maintaining an aberrant inflammatory protumorigenic environment [8,13]. However, CAFs represent a heterogenous population within the TME, with different CAF subsets exerting distinct functions in tumors [4]. This heterogeneity is illustrated by the presence of both myofibroblastic (i.e., myCAF) and non-myofibroblastic (i.e., iCAF) CAF subpopulations, with associated ECM signature and inflammatory phenotype, respectively, in different cancer types (reviewed in detail) [15].
2.4. Mediation of Drug Resistance
CAFs have emerged as key players in mediating drug resistance, and the potential mechanisms to do this are diverse [6,8]. First, CAFs and their generated ECM can function as a physical barrier and thus prevent efficient drug delivery [8]. Moreover, as drug resistance develops over a long period of time, the physical barrier and interaction with the ECM may protect cancer cells from apoptosis while they acquire the mutations needed for cell-intrinsic resistance to therapy [8,16]. Furthermore, CAFs may inhibit the uptake of anticancer drugs as a result of increased intratumoral interstitial fluid pressure, generated by ECM components [6]. Second, the TME might aid cancer cell survival and induce epithelial–mesenchymal transition (EMT) through the enhanced adhesion of cancer cells to the ECM, leading to therapeutic resistance, often described as cell adhesion-mediated drug resistance (CAM-DR) [6,8,17]. As they have been identified as potential drug resistance mediators, CAF-secreted soluble factors TGF-β, IL-6, and HGF may also contribute to therapeutic resistance by binding to cancer cell receptors causing transcriptional changes and by activating non-transcriptional mechanisms including degradation or redistribution of activators of apoptosis (e.g., proapoptotic Bcl-2 member Bim [18] and c-Fas-associated death domain-like IL-1-converting enzyme-like inhibitory protein-long (c-FLIPL)) [19], and increased stability of suppressors of apoptosis and cell cycle regulators (e.g., p27Kip1-associated cell cycle arrest [20]) [6,8]. In addition, upon exposure to conventional chemotherapies, radiotherapy, and targeted agents, CAFs can gain new features with altered functionality and secretion of proteins (e.g., WNT16B [21]) and cytokines (e.g., IL-17A [22]) that drive tumorigenesis, which can ultimately facilitate the development of therapy resistance and contribute to a more aggressive cancer phenotype [7,8].
As CAFs aid tumor development and contribute to therapy resistance [3], CAFs and cancer cells are increasingly viewed as partners in crime. However, the contribution of CAFs is complex and dynamic, with the involvement of various other players of the TME. Given the profound evidence of a generally protumorigenic function of CAFs, there is a considerable heterogeneity and plasticity between different tumor types and divergent context-dependent CAF phenotypes within distinct tumors [6,7,8,23]. Therefore, in this review, we discuss two solid tumor types, i.e., lung cancer and pancreatic cancer, with clear evidence of CAFs contributing to therapy resistance and marked by poor long-term prognosis. We discuss how CAFs contribute to tumor progression, affect their response to clinical therapy and prognosis, and we provide an overview of novel therapies involving CAF-targeting agents for both cancer types.
3. Lung Cancer
Lung cancer remains a devastating disease and the leading cause of cancer-related death worldwide [24], independent of sex [25]. Approximately 84.3% of all lung malignancies are classified as non-small cell lung cancer (NSCLC), for which the predicted 5-year relative survival rate for newly diagnosed cases is 21.0% [25] compared to only 7.0% for small cell lung cancer (SCLC) [26].
Important advancements in the treatment of NSCLC have been achieved over the past two decades due to the introduction of small molecule tyrosine kinase inhibitors and immunotherapy [27]. This is in contrast to SCLC, where immunotherapy shows only minor benefit [28]. However, oncogenic driver mutations are only present in 15–20% of NSCLC patients and PD-L1 tumor proportion score (TPS) ≥ 50% in 28% of advanced NSCLC, thereby limiting the use of these novel treatment strategies [29,30]. As such, surgery, cytotoxic chemotherapy, and radiotherapy remain the backbone for the treatment of both NSCLC [24,31] and SCLC [32]. Nonetheless, the overall cure for NSCLC and SCLC remains low, particularly in metastatic disease [27,28], as the majority of patients have already progressed to a more advanced stage at diagnosis, and due to the occurrence of therapeutic resistance, especially to chemotherapy and targeted therapies [33]. Therefore, further molecular characterization of the tumor landscape is needed, in order to investigate resistance mechanisms and develop novel therapeutic strategies [10].
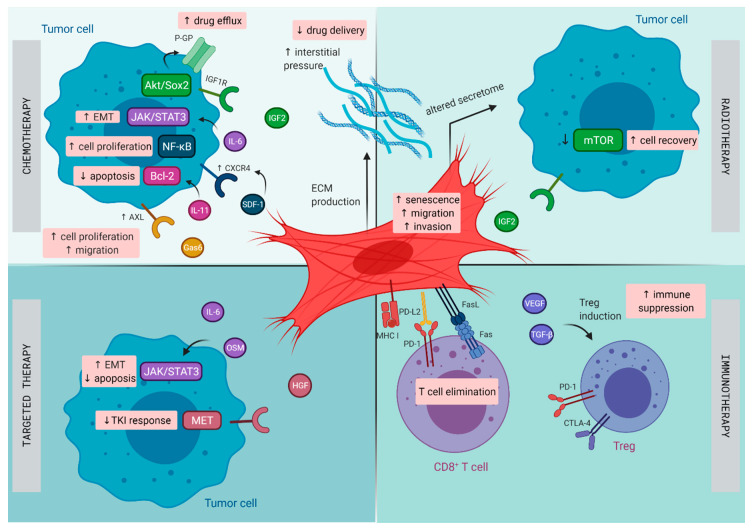
The TME in lung cancer has been recognized to play a central role in the initiation and progression of primary de novo lung cancer [10], as well as in contributing to therapeutic resistance [34]. Lung tumors harbor distinct subsets of CAFs, each expressing a unique repertoire of collagens and other ECM molecules, demonstrating phenotypic diversity in activated pathways, such as EMT and angiogenesis, that may promote invasiveness and metastasis [35]. Next to resistance resulting directly from CAFs, CAFs are able to promote resistance indirectly by modulating the response to chemotherapy, radiotherapy, targeted therapies, and immunotherapy through paracrine signaling to cancer and immune cells and mutual metabolic reprogramming (illustrated in Figure 1) [36].
Figure 1.
Mechanisms of therapy resistance in lung cancer orchestrated by cancer -associated fibroblasts (CAFs): Schematic illustration on how CAFs diminish the effect of chemotherapy, radiotherapy, targeted therapy, and immunotherapy in lung cancer. CTLA-4, cytotoxic T lymphocyte-associated protein 4; CXCR4, C-X-C chemokine receptor type 4; ECM, extracellular matrix; EMT, epithelial–mesenchymal transition; FasL, Fas ligand; Gas6, growth arrest specific-6 protein; HGF, hepatocyte growth factor; IGF1R, insulin-like growth factor 1 receptor; IGF2, insulin-like growth factor 2; IL, interleukin; MHC I, major histocompatibility complex I; OSM, oncostatin-M; PD-1, programmed cell death 1; PD-L2, programmed cell death ligand 2; P-GP, P-glycoprotein; SDF, stromal cell-derived factor; TGF-β, transforming growth factor-β; TKI, tyrosine kinase inhibitor; Treg, regulatory T cell; VEGF, vascular endothelial growth factor.
3.1. Resistance to Chemotherapy
Despite the introduction of numerous new treatment modalities, conventional platinum-based chemotherapy remains a pivotal pillar in the treatment of NSCLC, particularly in advanced stages [24,31]. CAFs are able to influence the response of lung cancer cells to chemotherapy through several signaling pathways and through their phenotypic diversity.
CAFs can modulate the response to cisplatin-based chemotherapy through the activation of the Gas6/AXL, IL-11/IL-11R/STAT3, SDF-1/CXCR4/NF-κB/Bcl-xL and SRGN/CD44 signaling pathways. CAF-derived growth arrest specific 6 (Gas6) protein, which is a natural ligand of tumor-associated macrophage (TAM) receptors and which has high affinity to the receptor tyrosine kinase AXL, increases during cisplatin-based chemotherapy. Tumoral AXL activation, which is linked to EMT and promoting cell survival, resistance, invasion, and metastasis in several cancers [37], results in proliferation and migration of lung cancer cells. Moreover, stromal Gas6 and tumoral AXL expression in NSCLC patient samples was associated with a worse 5-year disease-free survival rate [38]. CAFs treated with cisplatin also facilitate chemoresistance of lung adenocarcinoma by releasing IL-11, which results in the upregulation of antiapoptotic protein Bcl-2, thereby decreasing cisplatin-induced apoptosis [39]. By the release of SDF-1, CAFs are capable of promoting cell proliferation and drug resistance of lung cancer cells to cisplatin through upregulation of CXCR4, NF-κB, and Bcl-xL [40]. Furthermore, CAF-derived chondroitin sulfate proteoglycan serglycin (SRGN), a CD44 tumor cell-interacting factor, induces cancer cell stemness via Nanog induction accompanied with increased chemoresistance to cisplatin. In addition, increased expression of SRGN predicted poor prognosis NSCLC patients [41].
Other pathways that are involved in CAF-induced resistance of lung cancer cells to chemotherapy include the ANXA3/JNK, IGF2/AKT/Sox2/PG-P, and PI3K/Akt/GRP78 signaling pathways. CAFs increase Annexin A3 (ANXA3) expression in cancer cells, a Ca2+-dependent phospholipid-binding protein with an important role in tumor growth and progression [42]. This leads to the activation of c-jun N-terminal kinase (JNK) and survival, which inhibits cisplatin-induced apoptosis [42]. CAFs also produce IGF2 that binds to the IGF1 receptor in lung cancer cells and activate the AKT/Sox2 pathway, leading to enhanced expression of P glycoprotein (PG-P), a member of the ATP-binding cassette transporters, that is capable of pumping out the chemotherapeutic drug [43]. CAF-derived HGF inhibits paclitaxel-induced apoptosis of lung cancer cells by upregulating the PI3K/Akt pathway and glucose-regulated protein 78 (GRP78), a protective chaperone protein that supports cell survival and is associated with the development of chemoresistance [44].
Regarding the phenotypic diversity of CAFs, a subset with high CD10 and GPR77 expression correlated with chemoresistance and poor survival in breast and lung cancer patients [45]. Consistent with these findings, co-culture of CD10+GPR77+ CAFs with cancer cells dramatically enhanced survival upon treatment with cisplatin or docetaxel, by providing a constant source of paracrine IL-6 and IL-8 that stimulates the survival of cancer stem cells in the TME, ultimately inducing chemoresistance. Moreover, CD10+GPR77+ CAFs themselves were resistant to cisplatin and docetaxel by expressing numerous ATP binding cassette transporters [45].
Suppressing alpha-smooth muscle actin (α-SMA) myofibroblastic CAFs—which share the phenotypic traits of fibroblasts and smooth muscle cells, secrete ECM, and generate mechanical tension within tissue through cell contraction [46]—through the inhibition of plasminogen activator inhibitor-1 (PAI-1) limits resistance to cisplatin in lung cancer [47]. CAFs in tissue sections from lung carcinomas incubated with or without cisplatin turned out to be much less sensitive to cisplatin when compared to isolated CAFs, indicating that the TME also attenuates the sensitivity of lung cancer CAFs to cisplatin [48].
CAF-derived IL-6 is also found to be involved in maintenance of a paracrine loop in the interaction between fibroblasts and NSCLC cells. Treatment with cisplatin increases TGF-β production by NSCLC cells, which in turn activates CAFs, resulting in an increase of IL-6 and ultimately contributes to enhancing TGF-β-induced EMT in NSCLC. Furthermore, in human samples, stromal IL-6 expression was significantly correlated with the EMT status of cancer cells and with prognosis in patients, thus indicating that stromal IL-6 secreted from CAFs enhanced EMT signaling and induced resistance against chemotherapy or chemoradiotherapy [49]. In addition, CAF-derived IL-6 enhances the metastatic potential of lung cancer cells by activation of the JAK2/STAT3 signaling pathway [50,51], which in turn also mediates tumor angiogenesis through the upregulation of VEGF and bFGF [52].
In SCLC, the ECM enhances tumorigenicity and confers resistance to chemotherapeutic agents, such as doxorubicin, cisplatin, etoposide, and cyclophosphamide, as a result of β1 integrin-stimulated tyrosine phosphatidyl inositol 3-kinase (PI3-kinase) activation, overriding cell cycle arrest and chemotherapy-induced apoptosis, and allowing SCLC cells to survive with persistent DNA damage [53,54].
3.2. Resistance to Radiotherapy
For patients with comorbidities or those that are inoperable and present peripherally located stage I NSCLC, radiotherapy is the preferred treatment. Furthermore, for unresectable locally advanced stage IIIA and IIIB and stage IV NSCLC, radiotherapy is indicated with or without systemic chemotherapy or targeted treatment [24,31].
In general, CAFs are naturally resistant to fractionated radiotherapy, as radiation only causes cytotoxic but not cytolytic effects on CAFs [55]. Irradiated CAFs prior to implantation in mice lose their protumorigenic potential in vivo, as the enhanced tumorigenic effect observed in tumors co-implanted with control CAFs was abrogated. However, CAFs survive single high dose and fractionated radiation doses [56] and promote irradiated lung cancer cell recovery and tumor relapse after radiotherapy by CAF-derived IGF2-induced autophagy via mTOR suppression [57]. Consistent with these findings, CAFs isolated from freshly resected human lung tumors exposed to ablative doses of ionizing radiation show premature cellular senescence and reduced proliferative, migrative, and invasive capacity [58]. In addition, their secretory profile was transformed: the angiogenic factors SDF-1, angiopoietin, and thrombospondin-2 were downregulated in contrast to bFGF that was upregulated. Expression levels of HGF, IL-6, IL-8, IL-1β, and TNF-α remained unchanged. Even though CAFs had a transformed secretory profile, this did not affect the proliferative or migratory capacity of human lung tumor cells [59]. Nonetheless, prematurely induced senescence of human lung fibroblasts after exposure to ionizing radiation enhanced the growth of malignant lung epithelial cells in vitro and in vivo and may contribute to radiotherapy resistance and subsequent lung cancer progression [60]. Interestingly, soluble factors such as TGF-β produced by irradiated fibroblasts promoted murine lung adenocarcinoma cell migration and potential metastatic escape. However, in case of simultaneous irradiation of fibroblasts and carcinoma cells, carcinoma cell migration was repressed [61].
3.3. Resistance to Targeted Therapy
Targeted therapies against driver mutations and gene fusions provided significant therapeutic advances for a subset of NSCLC patients [62]. However, the clinical efficacy of such anticancer targeted therapies is strongly limited by the rapid development of acquired drug resistance in the majority of patients [62,63]. The TME has recently emerged as an important player in sustaining resistance to targeted therapies by activation of signals in a paracrine manner, able to compensate the drug-inhibited pathways in tumor cells [63].
Crosstalk between NSCLC cells and CAFs reduces sensitivity to targeted therapies, as CAFs show high expression levels of IL-6 and oncostatin-M (OSM), leading to paracrine JAK1/STAT3 activation in NSCLC cells, thus resulting in a switch to the EMT phenotype and protection from targeted drug-induced apoptosis. Moreover, OSM-receptor (OSMR) gene expression in surgically resected samples appeared to predict worse prognosis for patients with NSCLC, and selective inhibition of JAK1/STAT3 activation and OSMR expression with JAK1 inhibitor filgotinib reversed resistance to targeted drugs [62]. Previous findings also reported the role of CAF-mediated resistance of lung cancer cells to EGFR tyrosine kinase inhibitors (TKI) erlotinib [64] and gefitinib [65] through the induction of an EMT phenotype, suggesting that a specific phenotype of podoplanin- [66] and CD200-expressing [67] CAFs is responsible for the constituting resistance to EGFR TKIs. Despite the presence of podoplanin-expressing CAFs and a relatively poor response to EGFR TKIs among patients with lung adenocarcinoma harboring EGFR-activating mutations, the molecular mechanism remains unclear [66].
Moreover, treatment of lung cancer cells with TKIs targeting MET (proto-oncogene) or EGFR gene alterations can instruct the TME to sustain an adaptive resistance to targeted therapies by increasing lactate release. This leads to an increased HGF production by CAFs, which in turn activates MET in cancer cells, hence the diminished inhibitory effect of TKIs. Consistently, stromal HGF and tumor cell lactate transporter MCT4 were increased in NSCLC patients who progressed upon EGFR TKI therapy with erlotinib or gefitinib [63]. These results confirmed previous findings that c-MET-amplified tumor cells become dependent on HGF for survival upon pharmacologic MET inhibition [68], as well as for lung cancer cells harboring EGFR mutations upon treatment with EGFR TKI gefitinib [65,69].
3.4. Resistance to Immunotherapy
Immune-checkpoint inhibitor treatment, either as monotherapy or combination therapy depending on PD-L1 expression, has been established as the standard of care for patients with locally advanced/metastatic NSCLC without actionable oncogenic driver [24].
CAFs are able to modulate immune responses in the TME of lung cancer, regardless of immunotherapy [10]. CAFs derived from human NSCLC were found functionally and phenotypically heterogeneous and showed a constitutive upregulation of PD-L1 and PD-L2 resulting from autocrine interferon gamma (IFNγ), potentially enhancing or suppressing the activation of T cells. Furthermore, production of several cytokines and chemokines, such as IFNγ and TGF-β1, was demonstrated in these CAFs [70]. A more recent study revealed that CAFs directly contribute to the suppression of antitumor T cell responses by cross-presenting antigens complexed with major histocompatibility complex (MHC) I to antigen-specific CD8+ T cells, leading to antigen-specific upregulation of Fas/FasL and PD-1/PD-L2 on T cells and CAFs, respectively, which ultimately results in elimination of tumor-specific T cells and enhanced tumor viability [71].
Not surprisingly, recent studies have highlighted a major role for CAFs in promoting immunotherapy resistance by, at least in part, excluding T cells from tumor mass, which then accumulate at the tumor margin. As such, CAF-rich tumors are clinically aggressive and respond poorly to immunotherapy, as the success of most immunotherapies is dependent on CD8+ T cell-infiltrating tumors [72]. Consistently, in samples from patients with NSCLC who did not respond to immunotherapies, two subsets of immunosuppressive CAFs were enriched at time of diagnosis, of which one was able to increase the expression of PD-1 and CTLA-4 at the surface of FOXP3+ regulatory T cells (Tregs), which are critical in maintaining immune tolerance and homeostasis of the immune system [73]. Indeed, Tregs coexisting with CAFs are correlated with a poor outcome in NSCLC [74]. As such, the increased CTLA-4 expression by Tregs could explain the additive effect of antibodies blocking CTLA-4 in combination with anti-PD-1 checkpoint inhibitors [73], as this combination has recently been shown to improve overall survival, as compared with chemotherapy, in the first-line setting of patients with metastatic NSCLC [75].
4. Pancreatic Cancer
Pancreatic ductal adenocarcinoma (PDAC) is among the deadliest human malignancies. Despite the efforts that have been made, the overall prognosis remains extremely poor, with a 5-year overall survival that still stands below the bar of 10% [76]. Unfortunately, pancreatic cancer is even expected to become the second leading cause of cancer related deaths of the western world within the next decade, due to increasing age and risk factors (e.g., smoking, obesity, and diabetes) [77,78]. These devastating numbers reflect the resistance that is created by the tumor and its environment. Therefore, medical treatment of PDAC remains an ongoing challenge.
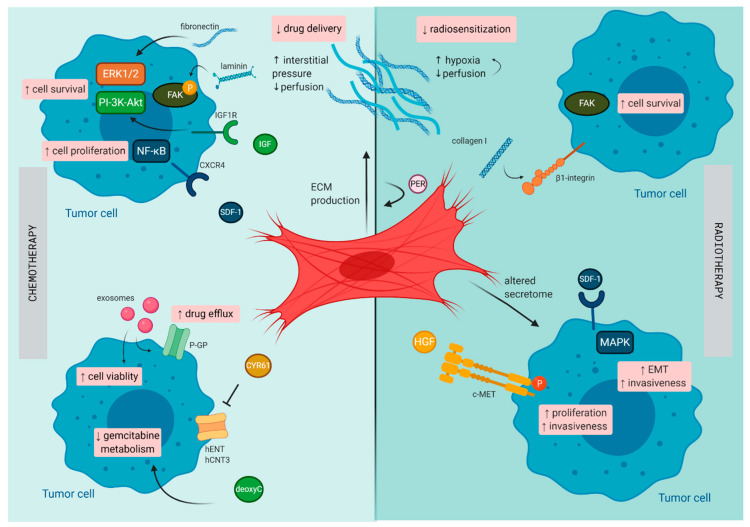
PDAC has a unique TME characterized by a dense stromal compartment that can even make up 90% of the whole tumor bulk [79]. This stromal shield is composed of a variety of cell types that are embedded in a dense ECM. CAFs play a central role in the formation of this fibrotic shield and mostly originate from PSCs, a resident stromal cell population of the pancreas [80]. By synthesis and secretion of various ECM components (e.g., collagen, laminin, and fibronectin), CAFs orchestrate the formation of the fibrotic tissue. Due to their presence within the PDAC tumor, along with their secreted factors, they are believed to be the major confounding factor involved in mediating therapeutic resistance (illustrated in Figure 2).
Figure 2.
Mechanisms of therapy resistance in pancreatic cancer orchestrated by cancer -associated fibroblasts (CAFs): Schematic illustration on how CAFs diminish the effect of chemotherapy and radiotherapy in pancreatic cancer. c-MET, tyrosine-protein kinase Met CXCR4, C-X-C chemokine receptor type 4; CYR61, cysteine-rich angiogenic inducer 61; deoxyC, deoxycytidine; EMT, epithelial–mesenchymal transition; FAK, focal adhesion kinase; hCNT3, human concentrative nucleoside transporter-3; hENT1, human equilibrative nucleoside transporter-1; HGF, hepatocyte growth factor; IGF, insulin-like growth factor; P, phosphorylated; PER, periostin; P-GP, P-glycoprotein; SDF-1, stromal cell-derived factor-1.
Currently, surgery is the sole curative treatment option for PDAC patients. Unfortunately, most patients present with locally advanced disease or distant metastasis at the time of diagnosis, precluding complete surgical resection of the tumor. Conventional chemotherapy treatment constitutes the standard of care for advanced or metastatic disease. Gemcitabine became the reference chemotherapeutic regimen for advanced PDAC and results in a median overall survival of 6.8 months, or 8.5 months when combined with nab-paclitaxel [81]. Recently, the use of FOLFIRINOX, a combination chemotherapy (oxaliplatin, irinotecan, fluorouracil, and leucovorin) increased the overall survival in these patients to a modest 11.1 months [82]. However, this intensified chemotherapeutic combination is only suitable for patients with a good performance status. Unfortunately, the failure of translating clinical response to chemotherapy into significant survival benefit could be attributed to poor penetration of the administered drug into the tumor and subsequent development of chemoresistance, in which CAFs play a pivotal role [83].
4.1. Resistance to Chemotherapy
4.1.1. Extrinsic Resistance
Various mechanisms have been unraveled by which CAFs can mediate chemoresistance in pancreatic cancer. At first, the physical barrier that is established by the stromal shield limits the delivery of administered drugs. Indeed, the dense stromal compartment creates high interstitial pressure primarily generated by hyaluronan [84], a polysaccharide overexpressed by activated CAFs in PDAC [85]. This causes vascular collapse and hypoperfusion, which alters the intratumoral physiology, hence limiting drug delivery. Moreover, increased hyaluronan expression has been correlated with poor prognosis in resected PDAC patients [86]. Therefore, enzymatic degradation of hyaluronan is an emerging strategy for the treatment of PDAC, which is discussed in the section CAF targeted treatment.
A second mechanism of chemoresistance, regulated by CAFs in PDAC, is their ability to alter the chemotherapeutic activity and efficacy by changing gemcitabine metabolism. To exert its antitumor effects, gemcitabine must first be metabolized within the tumor cell itself. Therefore, this hydrophilic molecule needs to be transported through the hydrophobic cell membrane via transmembrane nucleoside transporters, i.e., human equilibrative nucleoside transporter-1 (hENT1) and human concentrative nucleoside transporter-3 (hCNT3). Upon cytoplasmic entry, gemcitabine is phosphorylated by intracellular deoxycytidine kinase to perform its role as a deoxycytidine nucleoside analogue. This nucleoside analogue will then be incorporated into the replicated DNA strand, leading to chain termination, DNA damage, and apoptosis of the cancer cell [83]. However, CAFs are able to alter this crucial gemcitabine metabolism via expression of an arsenal of molecules. First, cysteine-rich angiogenic inducer 61 (CYR61) is a negative regulator of two important nucleoside transporters, i.e., hENT1 and hCNT3. It has been demonstrated that activated CAFs in the pancreatic tumor are a source of CYR61 [87]. Second, activated CAFs are also known to overexpress TGF-β. It has been demonstrated that TGF-β induces CYR61 expression, resulting in inhibition of hENT1 and hCNT3, hence impairing cellular uptake of gemcitabine by the tumor cells [87]. This highlights the potential of targeting CYR61 or TGF-β, to reverse the inhibited cellular uptake of gemcitabine, in order to improve its efficacy in PDAC. Last, CAFs are also able to release deoxycytidine into the TME. Deoxycytidine does not hinder the cellular uptake of gemcitabine, but rather competes downstream for the deoxycytidine kinase within the tumor cell. This results in a limited conversion to the active gemcitabine-triphosphate, thus diminishing its antitumor effect [88]. However, the lack of deoxycytidine secretion is not a property of all fibroblasts, highlighting the heterogeneity of fibroblast subsets, in which deoxycytidine expression might be a phenotypic subtype.
4.1.2. Intrinsic Resistance
The mechanisms described above involve extrinsic mechanisms of CAFs on chemoresistance in PDAC. However, intrinsic mechanisms of chemoresistance of tumor cells itself can also be altered directly or indirectly by CAFs, again helping cancer cells to survive under the influence of chemotherapy. Intrinsic mechanisms such as resistance to apoptosis and enhanced survival capacity of the cancer cells are mediated through various signaling pathways including RAS-RAF-MEK-ERK and PI3K-AKT. This crosstalk between cancer cells and CAFs is established via the expression of a broad range of molecules (e.g., laminin, fibronectin, IGF, SDF-1α, and perlecan), activating various subsequent signaling pathways that promote cancer cell survival [89,90,91,92,93,94]. Different ECM proteins, including laminin and fibronectin, are associated with CAM-DR in different cancer types [95], including pancreatic cancer [96]. Laminin, overexpressed by CAFs, induces phosphorylation of the non-receptor tyrosine kinase known as focal adhesion kinase (FAK), leading to cancer cell survival via the PI-3K-Akt pathway [90]. On the other hand, CAF-secreted fibronectin promotes high ERK1/2 activity in tumor cells, thereby protecting them from gemcitabine-induced cytotoxicity [90]. These findings imply combined use of gemcitabine and laminin or fibronectin inhibitors to counteract chemoresistance in PDAC. Furthermore, stromal-derived IGFs blunt the response to chemotherapy via activation of the insulin/IGF1R survival pathway within the cancer cells [91]. SDF-1, also named CXCL12, is a mediator of tumor–stromal interactions in different cancer types, including lung and pancreatic cancer, via the CXCR4/CXCL12 axis [97]. In PDAC, SDF-1α secretion by surrounding CAFs promotes chemoresistance mediated through paracrine SDF-1α/CXCR4 signaling and subsequently the IL-6 autocrine loop in pancreatic cancer cells. Upregulation of IL-6 in tumor cells, induced by SDF-1α, is due to downstream activation of FAK-AKT and ERK1/2 signaling pathways, thereby supporting cell survival and chemoresistance [92]. Lastl it has been demonstrated that stromal derived perlecan (or heparan sulfate proteoglycan 2, HSPG2) impairs the chemotherapy response in PDAC. In vivo experiments showed that perlecan depletion combined with gemcitabine prolonged mouse survival [94], supporting the potential of anti-stromal treatment for pancreatic cancer to enhance chemotherapy treatment.
4.1.3. Acquired Resistance
It has been demonstrated that chemotherapy is able to induce stromal responses that facilitate tumor progression. This mechanism of acquired resistance, mediated via different pathways, provides a rationale for the failure of chemotherapy following long-term treatment in clinical practice. In vitro experiments unraveled the molecular changes as well as the functional consequences associated with gemcitabine treatment of PDAC CAFs and confirmed that chemotherapy-treated CAFs are more tumor-supportive compared with their untreated counterpart [98]. This was mediated through upregulation of various inflammatory mediators, similar to the senescence-associated secretory phenotype (SASP). Induction of SASP increased the tumor cell viability, migration, and invasion, thus opposing the effects of chemotherapy treatment. Additionally, in vivo experiments showed that inhibition of upstream MAPK in CAFs resulted in attenuation of their tumor-supportive role [98]. This raises the possibility of SASP induction, via its upstream MAPK signaling pathway, as a potential therapeutic target to improve the efficacy of chemotherapy treatment.
Furthermore, gemcitabine treatment can also induce CAFs to secrete exosomes containing different tumor promoting molecules [99]. Studies have shown the production and release of exosomal miRNA-106b and Snai1, which lead to increased survival and enhanced proliferation in recipient cancer cells [99,100]. Additionally, miRNA-451a is highly present in the CAF exosome profile in PDAC [101]. This miRNA has been described to regulate the drug transporter protein P-glycoprotein [102], potentially promoting resistance to chemotherapy that utilize this transporter. However, this has not yet been confirmed in PDAC. Additionally, miR-451 contributes to promoted cell viability in vivo and in vitro and its elevated expression correlated with poor prognosis, including increased tumor invasion and metastasis in PDAC patients [103]. To conclude, the release of exosomes by CAFs offers an additional target to reduce resistance to chemotherapeutics.
4.2. Resistance to Radiotherapy
Patients with locally advanced PDAC are often treated with additional radiotherapy. Similar to their role in chemoresistance, CAFs have demonstrated their capacity to impede radiation response by direct or paracrine interaction. Remarkably, radiotherapy enhances the desmoplastic reaction in PDAC via an autocrine periostin loop [104]. Next to its chemoprotective characteristics, desmoplasia is also professed as a mediator of radioprotection via β1-integrin signaling and downstream FAK and MAPK-AKT signaling within tumor cells [105,106]. Furthermore, the desmoplastic reaction creates a hypoxic TME. As minimal levels of oxygen are required to sensitize tumor cells for radiation treatment, the present hypoxia leads to radiotherapy resistance [107,108]. Therefore, hypoxic radiosensitizers might be a rational-based combination strategy to surmount radiotherapy resistance.
Furthermore, in vitro experiments showed that irradiation of CAFs leads to an altered secretome, resulting in radioprotection. Indeed, culturing pancreatic cancer cells with conditioned medium from radiotherapy treated CAFs led to an elevated phosphorylation of c-Met, the HGF receptor. HGF, secreted by CAFs, plays a crucial role in invasion, proliferation and metastasis of cancer cells. Therefore, the overactive HGF-c-Met axis, induced by CAFs upon radiotherapy treatment, supports tumor progression [109]. Radiation of CAFs also promotes the secretion of high concentrations of CXCL12 both in vitro and in vivo via the P38 pathway, as such it promotes pancreatic cancer cell migration, invasion, and EMT [110]. Additionally, both EMT as well as cancer stem cells, modulated by stromal TGF-β secretion, are associated with resistance to radiotherapy [111]. This further supports the future for TGF-β as a therapeutic target for PDAC treatment.
5. CAF-Targeted Treatment
As CAFs display a broad range of mechanisms to stimulate tumorigenesis and drug resistance [8], combined with their genetic stability and relative abundance among stromal cells [36], targeting these cells is becoming an appealing and emerging therapeutic strategy. Several different approaches have been suggested for CAF-targeted anticancer treatment: (1) targeting the biophysical stromal barrier to increase drug delivery, (2) inhibiting CAF-secreted factors that stimulate tumorigenesis and drug resistance, (3) depleting or blocking the ECM components to induce stromal depletion and reduce adhesion-mediated signaling, (4) targeting the CAFs themselves to disable their downstream effects, (5) forcing CAFs to adopt an inactive quiescent phenotype (through the use of molecules such as all-trans retinoic acid (ATRA) or calcipotriol), and (6) modifying and using CAFs as in situ durable reservoir to deliver anticancer drugs (such as oncolytic adenoviruses, TNF-related apoptosis-inducing ligand (TRAIL), or IFN) [8,112]. Here, we will highlight the results of CAF-directed anticancer therapies for lung and pancreatic cancer that have been investigated in clinical studies and those under current clinical investigation (Table 1).
Table 1.
Active clinical trials targeting CAF in lung cancer and PDAC.
| Lung Cancer | ||||||
| Goal | Compound | Class | ID | Title | Phase | Status |
| Reduce ECM and IL-1β | Canakinumab | mAB targeting IL-1β | NCT03447769 | Canakinumab as Adjuvant Therapy in Adult Subjects with Stages AJCC/UICC v. 8 II-IIIA and IIIB (T > 5 cm N2) Completely Resected NSCLC | Phase III | Recruiting |
| Prevent activation of CAFs and block CAF secretome | Erdafitinib | TKI of FGF receptor 1-4 | NCT02699606 | Erdafitinib, A Pan- FGFR Tyrosine Kinase Inhibitor, In Asian Participants with Advanced NSCLC, Urothelial Cancer, Esophageal Cancer Or Cholangiocarcinoma | Phase II | Active, not recruiting |
| Prevent generation and activation of CAFs | Entinostat | HDAC inhibitor | NCT02437136 | Entinostat with Pembrolizumab in NSCLC with Expansion Cohorts in NSCLC, Melanoma, and Colorectal Cancer | Phase II | Active recruiting |
| NCT01928576 | Epigenetic Therapy with Azacitidine and Entinostat with Concurrent Nivolumab in Subjects With Metastatic NSCLC | Phase II | Recruiting | |||
| Prevent generation and activation of CAFs | Vorinstat | HDAC inhibitor | NCT02638090 | Pembrolizumab and Vorinostat in Patients with Immune Therapy Naïve and Immune Therapy Pretreated Stage IV NSCLC | Phase I/II | Active recruiting |
| Prevent generation and activation of CAFs | Mocetinostat | HDAC inhibitor | NCT02805660 | Mocetinostat and Durvalumab in Patients with Advanced Solid Tumors and NSCLC | Phase II | Completed, no results yet |
| PDAC | ||||||
| Goal | Compound | Class | ID | Title | Phase | Status |
| Reduce ECM and IL-1β | Canakinumab | mAB targeting IL-1β | NCT04581343 | A Phase 1B Study of Canakinumab, Spartalizumab, Nab-paclitaxel, and Gemcitabine in Metastatic PC Patients (PanCAN-SR1) | Phase Ib | Recruiting |
| Reduce ECM and TGF-β | Losartan | Angiotensin II receptor antagonist | NCT03563248 | Losartan and Nivolumab in Combination with FOLFIRINOX and SBRT in Localized Pancreatic Cancer | Phase II | Active recruiting |
| NCT01821729 | Proton w/FOLFIRINOX-Losartan for Pancreatic Cancer | Phase II | Active not recruiting | |||
| Normalize CAFs | ATRA | Vitamin A derivative | NCT04241276 | Phase IIb Randomized Trial of ATRA in a Novel Drug Combination for Pancreatic Cancer (STARPAC2) | Phase IIb | Not yet recruiting |
| Normalize CAFs | Paricalcitol | Vitamin D analogue | NCT03520790 | Paricalcitol Plus Gemcitabine and Nab-paclitaxel in Metastatic Pancreatic Cancer | Phase I/II | Active, not recruiting |
| Reduce CAF secretome | Plerixafor | CXCR4 antagonist | NCT04177810 | Plerixafor and Cemiplimab in Metastatic Pancreatic Cancer | Phase II | Recruiting |
| Reduce CAF secretome | GSK2256098 | FAK inhibitor | NCT02428270 | A Study of GSK2256098 and Trametinib in Advanced Pancreatic Cancer | Phase II | Active, not recruiting |
| Target FAP+ CAFs | BXCL701 (talabostat) | Small molecule inhibitor of FAP and dipeptidyl peptidases | NCT04123574 | A Pilot Study of BXCL701 in Patients With Pancreatic Cancer | Early phase I | Recruiting |
| Target FAP+ CAFs | CAR-T targeting nectin4/FAP | CAR-T cell | NCT03932565 | Interventional Therapy Sequential With the Fourth-generation CAR-T Targeting Nectin4/FAP for Malignant Solid Tumors | Phase I | Recruiting |
5.1. CAF-Targeted Treatment in Lung Cancer
CAFs are cells with an extensive proteome and secretome [3]. Therefore, drugs that have been developed successfully the past two decades, such as bevacizumab and atezolizumab, target at the same time CAF-derived VEGF-A and PD-L1 expression by CAFs, respectively, in the TME next to tumoral VEGF-A secretion and PD-L1-expression [10]. Furthermore, compounds such as canakinumab—a human anti-IL-1β monoclonal antibody and currently approved to treat uncommon autoimmune disorders, such as systemic juvenile idiopathic arthritis and periodic fever syndromes [113]—can be repurposed to target the secretome of CAFs, and are currently under investigation in a phase III trial as adjuvant therapy for completely resected (R0) NSCLC stages II-IIIA and IIIB (T > 5 cm N2) (NCT03447769) (Table 1).
In addition, several TKIs that simultaneously inhibit VEGF receptor 1, 2, and/or 3, FGF receptor 1, 2 and/or 3 and/or PDGF receptor α and/or β have been developed (e.g., nintedanib, motesanib, and sorafenib) [10]. These agents block the FGF and PDGF receptors on CAFs preventing activation, and the downstream effects of CAF-derived VEGF and FGF. Nintedanib has currently been approved by the European Medicines Agency (EMA) for use in combination with docetaxel for the treatment of patients with advanced NSCLC with disease progression after frontline platinum-based chemotherapy [114]. On the other hand, motesanib [115] and sorafenib [116], next to other similar compounds (reviewed by Altorki et al. [10]) all failed to show any benefit in phase III trials.
Rogaratinib, a FGFR-selective small-molecule kinase inhibitor of FGF receptor 1–4 aiming to prevent CAF activation, demonstrated in a phase I study partial response (PR) in 1 patient, stable disease (SD) in 15 patients, and progressive disease (PD) in the remaining 4 patients of the NSCLC cohort [117]. Infigratinib, a FGF receptor 1–3 inhibitor, also showed promising results with disease control in 18 (50%) out of 36 patients with FGFR1-amplified squamous NSCLC; four patients achieved PR and 14 had SD [118]. Currently, a phase II trial is investigating erdafitinib, a TKI of FGF receptor 1–4, in advanced solid tumors including NSCLC (NCT02699606) (Table 1).
Other specific CAF-targeted treatments using different approaches have been explored.
Sibrotuzumab, a 131I-radiolabeled anti-FAP monoclonal antibody on activated CAFs, was evaluated in a phase I study in patients with advanced or metastatic fibroblast activation protein (FAP) positive cancer, including NSCLC next to colorectal carcinoma. Despite sibrotuzumab was well tolerated and safe with repeat infusions, only two patients (one patient with NSCLC and one patient with colorectal carcinoma) had SD at 12 weeks, the remaining 21 patients showed PD at restaging [119]. In a phase II clinical trial for metastatic colorectal cancer, the anti-FAP monoclonal antibody failed as the minimal requirement for the continuation of the trial was not met [120]. Defactinib, an inhibitor of FAK expressed on CAFs and involved in inducing cell motility, extracellular matrix deposition, survival, and proliferation [121], demonstrated only modest clinical activity in heavily pretreated patients with KRAS mutant NSCLC with 15 (28%) patients that met the 12-week progression free-survival endpoint and with one patient achieving a PR [122].
Targeting the CAF secretome with S-3304, an MMP-inhibitor which most potently inhibits the activities of CAF-derived MMP-2 and MMP-9, in patients with advanced and refractory solid tumors, including NSCLC, showed no complete (CR) or PR, but only SD in seven out of 26 patients, of which none had NSCLC [123]. Furthermore, celecoxib, a selective COX-2 inhibitor and also part of the CAFs proteome [8], showed no significant improvement in PFS in combination with standard chemotherapy vs. placebo and chemotherapy in NSCLC patients with COX-2 overexpression [124]. A phase II study of belagenpumatucel-L, a TGF-β2 antisense gene-modified allogeneic tumor cell vaccine to inhibit cancer cell-derived (but not CAF-derived) TGF-β2, as maintenance therapy in NSCLC failed to meet its survival endpoint [125].
Currently, several histone deacetylase (HDAC) inhibitors, such as entinostat, vorinostat, and mocetinostat, in combination with PD-1 or PD-L1-inhibitors, are under clinical investigation in NSCLC. These agents can alter epigenetic regulation and intracellular signal transduction, including the JAK1/STAT3 pathway, not only in cancer cells, but also in CAFs or precursors of CAFs. As such, HDAC inhibitors potentially can reduce the generation and activation of CAFs and thus eliminate CAF infiltration in the TME [112].
5.2. CAF-Targeted Treatment in Pancreatic Cancer
Due to the central role of CAFs in impeding both chemo- and radiotherapy response in PDAC, stromal depletion swiftly emerged as a tempting therapeutic strategy. Preclinical models demonstrated the benefit of CAF ablation through blockade of the hedgehog signaling pathway, resulting in enhanced chemotherapeutic delivery [126]. Unfortunately, the use of different hedgehog inhibitors showed no benefit compared to gemcitabine or FOLFIRINOX monotherapy. Additionally, these clinical trials were halted due to a paradoxical accelerated disease progression as a result of more aggressive tumors [127,128,129,130], suggesting the presence of CAF subtypes carrying a tumor-restraining role, which has been confirmed in preclinical experiments [131]. This highlights the need for precise characterization of CAF heterogeneity in the context of developing effective therapies that target relevant CAF subtypes, while retaining the normal fibroblasts.
Additionally, an era of therapies emerged to target the ECM components with the purpose of reversing the desmoplastic reaction and thereby enhancing chemo- and radiotherapy [132]. High hopes were raised for PEGPH20 (pegylated hyaluronidase), as high hyaluronan is the main ECM component responsible for the elevated interstitial pressure [84] and correlated with poor prognosis in PDAC [86]. Unfortunately, the promising results obtained in a phase I/II clinical trial [133] were not translated in the subsequent phase III HALO 301 study [134]. On the other hand, losartan, an angiotensin receptor blocker, has been shown to reduce expression of TGF-β, HA synthases, and collagen I by CAFs [135] and is currently investigated in two clinical trials (Table 1). Prospective evaluation of losartan combined with FOLFIRINOX resulted in downstaging from locally advanced PDAC to a resectable tumor and prolonged survival [136].
As therapy resistance is also modulated via signals secreted by CAFs, blocking these signals might enhance current therapy options for PDAC patients. The central role of CAF-secreted CXCL12 in mediating both chemo- and radiotherapy resistance raised interest to target the CXCL12/CXCR4 axis (Table 1). Preclinical data on targeting this axis showed increased T cell infiltration, precluding its potential to be utilized in combination with immunotherapy [137]. Furthermore, TGF-β, a major player of the CAF secretome, is involved in therapy resistance via various mechanisms, described in previous sections. Therefore, therapies targeting this molecule were investigated in clinical trials [138]. However, addition of the TGF-β inhibitor galunisertib only led to a slight increase in overall survival versus gemcitabine treatment alone in unresectable pancreatic cancer patients [139].
Not only the broad range of CAF functions, but also additional subtype heterogeneity, introduces a challenge to the field of CAF targeting, which is demonstrated by the discouraging results in clinical trials. Therefore, clinical benefit might require targeting of CAF subtypes or reprogramming of CAFs to a normal fibroblast or an antitumorigenic phenotype. Indeed, more clinical trials targeting the specific FAP+ CAF subtype are emerging (Table 1), however results from these trials are not published yet. Moreover, CAF reprogramming is currently investigated with the use of paricalcitol and ATRA (Table 1). Although, it remains important to unravel if different states of CAF activation are represented by different subtypes, mediating various mechanisms of therapy resistance.
6. Conclusions and Future Perspectives
There is no doubt that CAFs mediate tumorigenesis and therapy resistance in lung and pancreatic cancer, and the mechanisms in doing so are diverse and ingenious, depending on the type of therapy. Therefore, targeting CAFs to counteract these protumorigenic effects and overcome the resistance to current therapeutic options is an appealing and emerging strategy. Moreover, their relative abundance in the TME and genetic stability [36] make them a valuable and sustainable target, as they are less prone to develop resistance phenotypes as a result of high mutation rates and clonal selection compared to cancer cells [8]. However, despite accumulating preclinical research exploring CAF-targeted cancer therapies [7,10,112], clinical trials with these agents have been disappointing so far (reviewed in detail [112]).
Substantial CAF heterogeneity is present in both lung [35] and PDAC [140,141,142] and is probably underestimated. Indeed, CAFs can originate from several different cell types and multiple mechanisms underlying CAF heterogeneity have been proposed (reviewed in detail) [142,143]. In combination with the lack and complex identification of specific CAF markers, CAF heterogeneity with distinct phenotypes and function provide a possible rationale for the current failure of clinical CAF-targeted treatments. Furthermore, differential effects between preclinical and clinical settings might be explained by the current challenge to construe preclinical models that precisely recapitulate this complex heterogeneity.
Therefore, preclinical in vitro 3D culture technologies, such as organoids, are opening avenues for the development of novel, more physiological human cancer models that incorporate CAFs [144] in contrast to conventional 2D homogenous cell culture experiments. Furthermore, a better understanding and identification of the CAF subpopulations that trigger resistance and the molecular mechanisms behind it, could tell us how CAFs can be effectively and specifically targeted to halt therapy resistance. Due to emerging molecular single cell analyzing strategies, more CAF subtypes will be defined. Digital deconvolution of bulk tumor expression profiling datasets demonstrated the presence of both activated and normal stromal signatures in PDAC [145]. Furthermore, distinct transcriptional profiles are displayed among subgroups of CAFs in lung [35] and PDAC [140,141], and epigenetic reprogramming (e.g., miRNA status and DNA methylation status) also contributes to the complexity of defining different CAF subtypes [142,143]. However, it is not merely about different CAF subtypes; mirroring their activation status is necessary to gain more insight in how resistance is governed by CAFs. Unravelling their molecular composition and epigenetic status might tell us how relevant CAF subtypes can be targeted specifically, while avoiding normal fibroblasts. We foresee that, in the future, therapies must be coupled with thorough molecular characterization of present CAFs in each individual to ensure optimal therapeutic efficacy and effectively overcome resistance.
Acknowledgments
Figures created with Biorender.com.
Author Contributions
Writing—original draft preparation, A.D., D.Q., S.Z. and H.P.; writing—review and editing, A.D., D.Q., S.Z., C.D., J.V.A., E.S., A.W., F.L., G.R., Y.V., A.J., T.V., P.v.D., M.P. and H.P.; supervision, C.D., J.V.A., E.S., A.W., F.L., G.R., A.J., T.V., P.v.D., M.P. and H.P. All authors have read and agreed to the published version of the manuscript.
Funding
A.D. is funded by the University Research Fund (BOF) of the University of Antwerp (FFB180188). D.Q. was funded by the University Research Fund (BOF) of the University of Antwerp (FFB190245) and is currently being funded by a fellowship from the Research Foundation Flanders (FWO-Vlaanderen; OZ8608). J.V.A., E.S., and M.P. also received funding from the University of Antwerp (FFI200135 and PP190006) and from the UZA Foundation. Y.V. received funding from the Emmanuel van der Schueren research grant (grant number CS 16628) of Kom op tegen Kanker (Stand up to Cancer), the Flemish cancer society. Y.V. is also a research fellow of the Research Foundation Flanders (FWO; fellowship number 1S69721N).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bremnes R.M., Dønnem T., Al-Saad S., Al-Shibli K., Andersen S., Sirera R., Camps C., Marinez I., Busund L.T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011;6:209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Coussens L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Santi A., Kugeratski F.G., Zanivan S. Cancer Associated Fibroblasts: The Architects of Stroma Remodeling. Proteomics. 2018;18:e1700167. doi: 10.1002/pmic.201700167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mhaidly R., Mechta-Grigoriou F. Fibroblast heterogeneity in tumor micro-environment: Role in immunosuppression and new therapies. Semin. Immunol. 2020;48:101417. doi: 10.1016/j.smim.2020.101417. [DOI] [PubMed] [Google Scholar]
- 5.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer. 2016;16:582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 7.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M., Fearon D., Greten F.R., Hingorani S.R., Hunter T., et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Öhlund D., Elyada E., Tuveson D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 2014;211:1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinz B., Phan S.H., Thannickal V.J., Galli A., Bochaton-Piallat M.L., Gabbiani G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altorki N.K., Markowitz G.J., Gao D., Port J.L., Saxena A., Stiles B., McGraw T., Mittal V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer. 2019;19:9–31. doi: 10.1038/s41568-018-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whatcott C.J., Diep C.H., Jiang P., Watanabe A., LoBello J., Sima C., Hostetter G., Shepard H.M., Von Hoff D.D., Han H. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin. Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V.J., Richardson A.L., Weinberg R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 13.Monteran L., Erez N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019;10:1835. doi: 10.3389/fimmu.2019.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Audenaerde J.R.M., De Waele J., Marcq E., Van Loenhout J., Lion E., Van den Bergh J.M.J., Jesenofsky R., Masamune A., Roeyen G., Pauwels P., et al. Interleukin-15 stimulates natural killer cell-mediated killing of both human pancreatic cancer and stellate cells. Oncotarget. 2017;8:56968. doi: 10.18632/oncotarget.18185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biffi G., Tuveson D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021;101:147–176. doi: 10.1152/physrev.00048.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meads M.B., Gatenby R.A., Dalton W.S. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat. Rev. Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 17.Schmidmaier R., Baumann P. ANTI-ADHESION evolves to a promising therapeutic concept in oncology. Curr. Med. Chem. 2008;15:978–990. doi: 10.2174/092986708784049667. [DOI] [PubMed] [Google Scholar]
- 18.Hazlehurst L.A., Argilagos R.F., Dalton W.S. Beta1 integrin mediated adhesion increases Bim protein degradation and contributes to drug resistance in leukaemia cells. Br. J. Haematol. 2007;136:269–275. doi: 10.1111/j.1365-2141.2006.06435.x. [DOI] [PubMed] [Google Scholar]
- 19.Shain K.H., Landowski T.H., Dalton W.S. Adhesion-Mediated Intracellular Redistribution of c-Fas-Associated Death Domain-Like IL-1-Converting Enzyme-Like Inhibitory Protein-Long Confers Resistance to CD95-Induced Apoptosis in Hematopoietic Cancer Cell Lines. J. Immunol. 2002;168:2544–2553. doi: 10.4049/jimmunol.168.5.2544. [DOI] [PubMed] [Google Scholar]
- 20.Lwin T., Hazlehurst L.A., Dessureault S., Lai R., Bai W., Sotomayor E., Moscinski L.C., Dalton W.S., Tao J. Cell adhesion induces p27Kip1-associated cell-cycle arrest through down-regulation of the SCFSkp2 ubiquitin ligase pathway in mantle-cell and other non-Hodgkin B-cell lymphomas. Blood. 2007;110:1631–1638. doi: 10.1182/blood-2006-11-060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y., Campisi J., Higano C., Beer T.M., Porter P., Coleman I., True L., Nelson P.S. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat. Med. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lotti F., Jarrar A.M., Pai R.K., Hitomi M., Lathia J., Mace A., Gantt G.A., Jr., Sukhdeo K., DeVecchio J., Vasanji A., et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med. 2013;210:2851–2872. doi: 10.1084/jem.20131195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeltz C., Primac I., Erusappan P., Alam J., Noel A., Gullberg D. Cancer-associated fibroblasts in desmoplastic tumors: Emerging role of integrins. Semin. Cancer Biol. 2020;62:166–181. doi: 10.1016/j.semcancer.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Planchard D., Popat S., Kerr K., Novello S., Smit E.F., Faivre-Finn C., Mok T.S., Reck M., Van Schil P.E., Hellmann M.D., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 25.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 26.Howlader N.N.A., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., Chen H.S., et al. SEER Cancer Statistics Review 1975–2017. [(accessed on 19 January 2021)]; Available online: https://seer.cancer.gov/csr/1975_2017/
- 27.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 28.Rudin C.M., Poirier J.T., Byers L.A., Dive C., Dowlati A., George J., Heymach J.V., Johnson J.E., Lehman J.M., MacPherson D., et al. Molecular subtypes of small cell lung cancer: A synthesis of human and mouse model data. Nat. Rev. Cancer. 2019;19:289–297. doi: 10.1038/s41568-019-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayekar M.K., Bivona T.G. Current Landscape of Targeted Therapy in Lung Cancer. Clin. Pharmacol. Ther. 2017;102:757–764. doi: 10.1002/cpt.810. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal C., Abreu D.R., Felip E., Carcereny E., Gottfried M., Wehler T., Ahn M.-J., Dolled-Filhart M., Zhang J., Shentu Y., et al. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024. Ann. Oncol. 2016;27:vi363. doi: 10.1093/annonc/mdw378.14. [DOI] [Google Scholar]
- 31.Postmus P.E., Kerr K.M., Oudkerk M., Senan S., Waller D.A., Vansteenkiste J., Escriu C., Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 32.Früh M., De Ruysscher D., Popat S., Crinò L., Peters S., Felip E. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24(Suppl. 6):vi99–vi105. doi: 10.1093/annonc/mdt178. [DOI] [PubMed] [Google Scholar]
- 33.Rotow J., Bivona T.G. Understanding and targeting resistance mechanisms in NSCLC. Nat. Rev. Cancer. 2017;17:637–658. doi: 10.1038/nrc.2017.84. [DOI] [PubMed] [Google Scholar]
- 34.Horvath L., Thienpont B., Zhao L., Wolf D., Pircher A. Overcoming immunotherapy resistance in non-small cell lung cancer (NSCLC)-novel approaches and future outlook. Mol. Cancer. 2020;19:141. doi: 10.1186/s12943-020-01260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambrechts D., Wauters E., Boeckx B., Aibar S., Nittner D., Burton O., Bassez A., Decaluwé H., Pircher A., Van den Eynde K., et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018;24:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 36.Fiori M.E., Di Franco S., Villanova L., Bianca P., Stassi G., De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol. Cancer. 2019;18:70. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antony J., Huang R.Y.-J. AXL-Driven EMT State as a Targetable Conduit in Cancer. Cancer Res. 2017;77:3725–3732. doi: 10.1158/0008-5472.CAN-17-0392. [DOI] [PubMed] [Google Scholar]
- 38.Kanzaki R., Naito H., Kise K., Takara K., Eino D., Minami M., Shintani Y., Funaki S., Kawamura T., Kimura T., et al. Gas6 derived from cancer-associated fibroblasts promotes migration of Axl-expressing lung cancer cells during chemotherapy. Sci. Rep. 2017;7:10613. doi: 10.1038/s41598-017-10873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao L., Huang G., Wang R., Pan Y., He Z., Chu X., Song H., Chen L. Cancer-associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL-11/IL-11R/STAT3 signaling pathway. Sci. Rep. 2016;6:38408. doi: 10.1038/srep38408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Guan J., Long X., Wang Y., Xiang X. mir-1-mediated paracrine effect of cancer-associated fibroblasts on lung cancer cell proliferation and chemoresistance. Oncol. Rep. 2016;35:3523–3531. doi: 10.3892/or.2016.4714. [DOI] [PubMed] [Google Scholar]
- 41.Guo J.Y., Hsu H.S., Tyan S.W., Li F.Y., Shew J.Y., Lee W.H., Chen J.Y. Serglycin in tumor microenvironment promotes non-small cell lung cancer aggressiveness in a CD44-dependent manner. Oncogene. 2017;36:2457–2471. doi: 10.1038/onc.2016.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L., Li X., Ren Y., Geng H., Zhang Q., Cao L., Meng Z., Wu X., Xu M., Xu K. Cancer-associated fibroblasts contribute to cisplatin resistance by modulating ANXA3 in lung cancer cells. Cancer Sci. 2019;110:1609–1620. doi: 10.1111/cas.13998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Q., Yang J., Bai J., Ren J. Reverse of non-small cell lung cancer drug resistance induced by cancer-associated fibroblasts via a paracrine pathway. Cancer Sci. 2018;109:944–955. doi: 10.1111/cas.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying L., Zhu Z., Xu Z., He T., Li E., Guo Z., Liu F., Jiang C., Wang Q. Cancer Associated Fibroblast-Derived Hepatocyte Growth Factor Inhibits the Paclitaxel-Induced Apoptosis of Lung Cancer A549 Cells by Up-Regulating the PI3K/Akt and GRP78 Signaling on a Microfluidic Platform. PLoS ONE. 2015;10:e0129593. doi: 10.1371/journal.pone.0129593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su S., Chen J., Yao H., Liu J., Yu S., Lao L., Wang M., Luo M., Xing Y., Chen F., et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell. 2018;172:841–856.e16. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Hanley C.J., Mellone M., Ford K., Thirdborough S.M., Mellows T., Frampton S.J., Smith D.M., Harden E., Szyndralewiez C., Bullock M., et al. Targeting the Myofibroblastic Cancer-Associated Fibroblast Phenotype Through Inhibition of NOX4. J. Natl. Cancer Inst. 2018;110:109–120. doi: 10.1093/jnci/djx121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masuda T., Nakashima T., Namba M., Yamaguchi K., Sakamoto S., Horimasu Y., Miyamoto S., Iwamoto H., Fujitaka K., Miyata Y., et al. Inhibition of PAI-1 limits chemotherapy resistance in lung cancer through suppressing myofibroblast characteristics of cancer-associated fibroblasts. J. Cell. Mol. Med. 2019;23:2984–2994. doi: 10.1111/jcmm.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonnenberg M., van der Kuip H., Haubeis S., Fritz P., Schroth W., Friedel G., Simon W., Mürdter T.E., Aulitzky W.E. Highly variable response to cytotoxic chemotherapy in carcinoma-associated fibroblasts (CAFs) from lung and breast. BMC Cancer. 2008;8:364. doi: 10.1186/1471-2407-8-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shintani Y., Fujiwara A., Kimura T., Kawamura T., Funaki S., Minami M., Okumura M. IL-6 Secreted from Cancer-Associated Fibroblasts Mediates Chemoresistance in NSCLC by Increasing Epithelial-Mesenchymal Transition Signaling. J. Thorac. Oncol. 2016;11:1482–1492. doi: 10.1016/j.jtho.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 50.Wang L., Cao L., Wang H., Liu B., Zhang Q., Meng Z., Wu X., Zhou Q., Xu K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017;8:76116. doi: 10.18632/oncotarget.18814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verhoeven Y., Tilborghs S., Jacobs J., De Waele J., Quatannens D., Deben C., Prenen H., Pauwels P., Trinh X.B., Wouters A., et al. The potential and controversy of targeting STAT family members in cancer. Semin. Cancer Biol. 2020;60:41–56. doi: 10.1016/j.semcancer.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Zhao M., Gao F.H., Wang J.Y., Liu F., Yuan H.H., Zhang W.Y., Jiang B. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung Cancer. 2011;73:366–374. doi: 10.1016/j.lungcan.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Sethi T., Rintoul R.C., Moore S.M., MacKinnon A.C., Salter D., Choo C., Chilvers E.R., Dransfield I., Donnelly S.C., Strieter R., et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 54.Hodkinson P.S., Elliott T., Wong W.S., Rintoul R.C., Mackinnon A.C., Haslett C., Sethi T. ECM overrides DNA damage-induced cell cycle arrest and apoptosis in small-cell lung cancer cells through β1 integrin-dependent activation of PI3-kinase. Cell Death Differ. 2006;13:1776–1788. doi: 10.1038/sj.cdd.4401849. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z., Tang Y., Tan Y., Wei Q., Yu W. Cancer-associated fibroblasts in radiotherapy: Challenges and new opportunities. Cell Commun. Signal. 2019;17:47. doi: 10.1186/s12964-019-0362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grinde M.T., Vik J., Camilio K.A., Martinez-Zubiaurre I., Hellevik T. Ionizing radiation abrogates the pro-tumorigenic capacity of cancer-associated fibroblasts co-implanted in xenografts. Sci. Rep. 2017;7:46714. doi: 10.1038/srep46714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y., Gan G., Wang B., Wu J., Cao Y., Zhu D., Xu Y., Wang X., Han H., Li X., et al. Cancer-associated Fibroblasts Promote Irradiated Cancer Cell Recovery Through Autophagy. EBioMedicine. 2017;17:45–56. doi: 10.1016/j.ebiom.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hellevik T., Pettersen I., Berg V., Winberg J.O., Moe B.T., Bartnes K., Paulssen R.H., Busund L.T., Bremnes R., Chalmers A., et al. Cancer-associated fibroblasts from human NSCLC survive ablative doses of radiation but their invasive capacity is reduced. Radiat. Oncol. 2012;7:59. doi: 10.1186/1748-717X-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hellevik T., Pettersen I., Berg V., Bruun J., Bartnes K., Busund L.T., Chalmers A., Bremnes R., Martinez-Zubiaurre I. Changes in the Secretory Profile of NSCLC-Associated Fibroblasts after Ablative Radiotherapy: Potential Impact on Angiogenesis and Tumor Growth. Transl. Oncol. 2013;6:66–74. doi: 10.1593/tlo.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Papadopoulou A., Kletsas D. Human lung fibroblasts prematurely senescent after exposure to ionizing radiation enhance the growth of malignant lung epithelial cells in vitro and in vivo. Int. J. Oncol. 2011;39:989–999. doi: 10.3892/ijo.2011.1132. [DOI] [PubMed] [Google Scholar]
- 61.Arshad A., Deutsch E., Vozenin M.C. Simultaneous irradiation of fibroblasts and carcinoma cells repress the secretion of soluble factors able to stimulate carcinoma cell migration. PLoS ONE. 2015;10:e0115447. doi: 10.1371/journal.pone.0115447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shien K., Papadimitrakopoulou V.A., Ruder D., Behrens C., Shen L., Kalhor N., Song J., Lee J.J., Wang J., Tang X., et al. JAK1/STAT3 Activation through a Proinflammatory Cytokine Pathway Leads to Resistance to Molecularly Targeted Therapy in Non–Small Cell Lung Cancer. Mol. Cancer Ther. 2017;16:2234–2245. doi: 10.1158/1535-7163.MCT-17-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Apicella M., Giannoni E., Fiore S., Ferrari K.J., Fernández-Pérez D., Isella C., Granchi C., Minutolo F., Sottile A., Comoglio P.M., et al. Increased Lactate Secretion by Cancer Cells Sustains Non-cell-autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab. 2018;28:848–865.e846. doi: 10.1016/j.cmet.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Choe C., Shin Y.S., Kim C., Choi S.J., Lee J., Kim S.Y., Cho Y.B., Kim J. Crosstalk with cancer-associated fibroblasts induces resistance of non-small cell lung cancer cells to epidermal growth factor receptor tyrosine kinase inhibition. Onco Targets Ther. 2015;8:3665–3678. doi: 10.2147/OTT.S89659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yi Y., Zeng S., Wang Z., Wu M., Ma Y., Ye X., Zhang B., Liu H. Cancer-associated fibroblasts promote epithelial-mesenchymal transition and EGFR-TKI resistance of non-small cell lung cancers via HGF/IGF-1/ANXA2 signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:793–803. doi: 10.1016/j.bbadis.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida T., Ishii G., Goto K., Neri S., Hashimoto H., Yoh K., Niho S., Umemura S., Matsumoto S., Ohmatsu H., et al. Podoplanin-Positive Cancer-Associated Fibroblasts in the Tumor Microenvironment Induce Primary Resistance to EGFR-TKIs in Lung Adenocarcinoma with EGFR Mutation. Clin. Cancer Res. 2015;21:642–651. doi: 10.1158/1078-0432.CCR-14-0846. [DOI] [PubMed] [Google Scholar]
- 67.Ishibashi M., Neri S., Hashimoto H., Miyashita T., Yoshida T., Nakamura Y., Udagawa H., Kirita K., Matsumoto S., Umemura S., et al. CD200-positive cancer associated fibroblasts augment the sensitivity of Epidermal Growth Factor Receptor mutation-positive lung adenocarcinomas to EGFR Tyrosine kinase inhibitors. Sci. Rep. 2017;7:46662. doi: 10.1038/srep46662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pennacchietti S., Cazzanti M., Bertotti A., Rideout W.M., Han M., Gyuris J., Perera T., Comoglio P.M., Trusolino L., Michieli P. Microenvironment-Derived HGF Overcomes Genetically Determined Sensitivity to Anti-MET Drugs. Cancer Res. 2014;74:6598–6609. doi: 10.1158/0008-5472.CAN-14-0761. [DOI] [PubMed] [Google Scholar]
- 69.Wang W., Li Q., Yamada T., Matsumoto K., Matsumoto I., Oda M., Watanabe G., Kayano Y., Nishioka Y., Sone S., et al. Crosstalk to Stromal Fibroblasts Induces Resistance of Lung Cancer to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Clin. Cancer Res. 2009;15:6630–6638. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]
- 70.Nazareth M.R., Broderick L., Simpson-Abelson M.R., Kelleher R.J., Yokota S.J., Bankert R.B. Characterization of Human Lung Tumor-Associated Fibroblasts and Their Ability to Modulate the Activation of Tumor-Associated T Cells. J. Immunol. 2007;178:5552–5562. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 71.Lakins M.A., Ghorani E., Munir H., Martins C.P., Shields J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8+T Cells to protect tumour cells. Nat. Commun. 2018;9:948. doi: 10.1038/s41467-018-03347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanley C.J., Thomas G.J. T-cell tumour exclusion and immunotherapy resistance: A role for CAF targeting. Br. J. Cancer. 2020;123:1353–1355. doi: 10.1038/s41416-020-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kieffer Y., Hocine H.R., Gentric G., Pelon F., Bernard C., Bourachot B., Lameiras S., Albergante L., Bonneau C., Guyard A., et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020;10:1330–1351. doi: 10.1158/2159-8290.CD-19-1384. [DOI] [PubMed] [Google Scholar]
- 74.Kinoshita T., Ishii G., Hiraoka N., Hirayama S., Yamauchi C., Aokage K., Hishida T., Yoshida J., Nagai K., Ochiai A. Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Sci. 2013;104:409–415. doi: 10.1111/cas.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hellmann M.D., Paz-Ares L., Bernabe Caro R., Zurawski B., Kim S.W., Carcereny Costa E., Park K., Alexandru A., Lupinacci L., de la Mora Jimenez E., et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 76.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 77.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 78.Quante A.S., Ming C., Rottmann M., Engel J., Boeck S., Heinemann V., Westphalen C.B., Strauch K. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. 2016;5:2649–2656. doi: 10.1002/cam4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neesse A., Michl P., Frese K.K., Feig C., Cook N., Jacobetz M.A., Lolkema M.P., Buchholz M., Olive K.P., Gress T.M., et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 80.Bynigeri R.R., Jakkampudi A., Jangala R., Subramanyam C., Sasikala M., Rao G.V., Reddy D.N., Talukdar R. Pancreatic stellate cell: Pandora’s box for pancreatic disease biology. World J. Gastroenterol. 2017;23:382–405. doi: 10.3748/wjg.v23.i3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L., Choné L., Francois E., Artru P., Biagi J.J., et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 83.Amrutkar M., Gladhaug I.P. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers. 2017;9:157. doi: 10.3390/cancers9110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DuFort C.C., DelGiorno K.E., Carlson M.A., Osgood R.J., Zhao C., Huang Z., Thompson C.B., Connor R.J., Thanos C.D., Scott Brockenbrough J., et al. Interstitial Pressure in Pancreatic Ductal Adenocarcinoma Is Dominated by a Gel-Fluid Phase. Biophys. J. 2016;110:2106–2119. doi: 10.1016/j.bpj.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu J., Wang L., Liang X., Liu X., Wu H., Liang Z. High-molecular-weight hyaluronan produced by activated pancreatic stellate cells promotes pancreatic cancer cell migration via paracrine signaling. Biochem. Biophys. Res. Commun. 2019;515:493–498. doi: 10.1016/j.bbrc.2019.05.167. [DOI] [PubMed] [Google Scholar]
- 86.Cheng X.B., Sato N., Kohi S., Yamaguchi K. Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS ONE. 2013;8:e80765. doi: 10.1371/journal.pone.0080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hesler R.A., Huang J.J., Starr M.D., Treboschi V.M., Bernanke A.G., Nixon A.B., McCall S.J., White R.R., Blobe G.C. TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in pancreatic ductal adenocarcinoma through downregulation of the nucleoside transporters hENT1 and hCNT3. Carcinogenesis. 2016;37:1041–1051. doi: 10.1093/carcin/bgw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dalin S., Sullivan M.R., Lau A.N., Grauman-Boss B., Mueller H.S., Kreidl E., Fenoglio S., Luengo A., Lees J.A., Vander Heiden M.G., et al. Deoxycytidine Release from Pancreatic Stellate Cells Promotes Gemcitabine Resistance. Cancer Res. 2019;79:5723–5733. doi: 10.1158/0008-5472.CAN-19-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu H., Liang Z., Shi X., Ren X., Wang K., Liu T. Intrinsic chemoresistance to gemcitabine is associated with constitutive and laminin-induced phosphorylation of FAK in pancreatic cancer cell lines. Mol. Cancer. 2009;8:125. doi: 10.1186/1476-4598-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amrutkar M., Aasrum M., Verbeke C.S., Gladhaug I.P. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer. 2019;19:596. doi: 10.1186/s12885-019-5803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ireland L., Santos A., Ahmed M.S., Rainer C., Nielsen S.R., Quaranta V., Weyer-Czernilofsky U., Engle D.D., Perez-Mancera P.A., Coupland S.E., et al. Chemoresistance in Pancreatic Cancer Is Driven by Stroma-Derived Insulin-Like Growth Factors. Cancer Res. 2016;76:6851–6863. doi: 10.1158/0008-5472.CAN-16-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H., Wu H., Guan J., Wang L., Ren X., Shi X., Liang Z., Liu T. Paracrine SDF-1α signaling mediates the effects of PSCs on GEM chemoresistance through an IL-6 autocrine loop in pancreatic cancer cells. Oncotarget. 2014;6:3085. doi: 10.18632/oncotarget.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh S., Srivastava S.K., Bhardwaj A., Owen L.B., Singh A.P. CXCL12–CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: A novel target for therapy. Br. J. Cancer. 2010;103:1671–1679. doi: 10.1038/sj.bjc.6605968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vennin C., Mélénec P., Rouet R., Nobis M., Cazet A.S., Murphy K.J., Herrmann D., Reed D.A., Lucas M.C., Warren S.C., et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 2019;10:3637. doi: 10.1038/s41467-019-10968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shain K.H., Dalton W.S. Cell adhesion is a key determinant in de novo multidrug resistance (MDR): New targets for the prevention of acquired MDR. Mol. Cancer Ther. 2001;1:69–78. [PubMed] [Google Scholar]
- 96.Miyamoto H., Murakami T., Tsuchida K., Sugino H., Miyake H., Tashiro S. Tumor-stroma interaction of human pancreatic cancer: Acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 97.Domanska U.M., Kruizinga R.C., Nagengast W.B., Timmer-Bosscha H., Huls G., de Vries E.G., Walenkamp A.M. A review on CXCR4/CXCL12 axis in oncology: No place to hide. Eur. J. Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 98.Toste P.A., Nguyen A.H., Kadera B.E., Duong M., Wu N., Gawlas I., Tran L.M., Bikhchandani M., Li L., Patel S.G., et al. Chemotherapy-Induced Inflammatory Gene Signature and Protumorigenic Phenotype in Pancreatic CAFs via Stress-Associated MAPK. Mol. Cancer Res. 2016;14:437–447. doi: 10.1158/1541-7786.MCR-15-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richards K.E., Zeleniak A.E., Fishel M.L., Wu J., Littlepage L.E., Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang Y., Zhou W., Rong Y., Kuang T., Xu X., Wu W., Wang D., Lou W. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Exp. Cell Res. 2019;383:111543. doi: 10.1016/j.yexcr.2019.111543. [DOI] [PubMed] [Google Scholar]
- 101.Takikawa T., Masamune A., Yoshida N., Hamada S., Kogure T., Shimosegawa T. Exosomes Derived From Pancreatic Stellate Cells: MicroRNA Signature and Effects on Pancreatic Cancer Cells. Pancreas. 2017;46:19–27. doi: 10.1097/MPA.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 102.van Jaarsveld M.T., Helleman J., Berns E.M., Wiemer E.A. MicroRNAs in ovarian cancer biology and therapy resistance. Int. J. Biochem. Cell Biol. 2010;42:1282–1290. doi: 10.1016/j.biocel.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 103.Guo R., Gu J., Zhang Z., Wang Y., Gu C. MiR-451 Promotes Cell Proliferation and Metastasis in Pancreatic Cancer through Targeting CAB39. Biomed Res. Int. 2017;2017:2381482. doi: 10.1155/2017/2381482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Erkan M., Kleeff J., Gorbachevski A., Reiser C., Mitkus T., Esposito I., Giese T., Büchler M.W., Giese N.A., Friess H. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–1464. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 105.Mantoni T.S., Lunardi S., Al-Assar O., Masamune A., Brunner T.B. Pancreatic Stellate Cells Radioprotect Pancreatic Cancer Cells through β1-Integrin Signaling. Cancer Res. 2011;71:3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hwang R.F., Moore T., Arumugam T., Ramachandran V., Amos K.D., Rivera A., Ji B., Evans D.B., Logsdon C.D. Cancer-Associated Stromal Fibroblasts Promote Pancreatic Tumor Progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Horsman M.R., Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J. Radiat. Res. 2016;57(Suppl. 1):i90–i98. doi: 10.1093/jrr/rrw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hill R.P., Bristow R.G., Fyles A., Koritzinsky M., Milosevic M., Wouters B.G. Hypoxia and Predicting Radiation Response. Semin. Radiat. Oncol. 2015;25:260–272. doi: 10.1016/j.semradonc.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 109.Ohuchida K., Mizumoto K., Murakami M., Qian L.-W., Sato N., Nagai E., Matsumoto K., Nakamura T., Tanaka M. Radiation to Stromal Fibroblasts Increases Invasiveness of Pancreatic Cancer Cells through Tumor-Stromal Interactions. Cancer Res. 2004;64:3215–3222. doi: 10.1158/0008-5472.CAN-03-2464. [DOI] [PubMed] [Google Scholar]
- 110.Li D., Qu C., Ning Z., Wang H., Zang K., Zhuang L., Chen L., Wang P., Meng Z. Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts. Am. J. Cancer Res. 2016;6:2192–2206. [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Assar O., Demiciorglu F., Lunardi S., Gaspar-Carvalho M.M., McKenna W.G., Muschel R.M., Brunner T.B. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother. Oncol. 2014;111:243–251. doi: 10.1016/j.radonc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 112.Chen X., Song E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 113.Sehested T.S.G., Bjerre J., Ku S., Chang A., Jahansouz A., Owens D.K., Hlatky M.A., Goldhaber-Fiebert J.D. Cost-effectiveness of Canakinumab for Prevention of Recurrent Cardiovascular Events. JAMA Cardiol. 2019;4:128–135. doi: 10.1001/jamacardio.2018.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reck M., Kaiser R., Mellemgaard A., Douillard J.Y., Orlov S., Krzakowski M., von Pawel J., Gottfried M., Bondarenko I., Liao M., et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 115.Scagliotti G.V., Vynnychenko I., Park K., Ichinose Y., Kubota K., Blackhall F., Pirker R., Galiulin R., Ciuleanu T.E., Sydorenko O., et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J. Clin. Oncol. 2012;30:2829–2836. doi: 10.1200/JCO.2011.41.4987. [DOI] [PubMed] [Google Scholar]
- 116.Paz-Ares L., Hirsh V., Zhang L., de Marinis F., Yang J.C., Wakelee H.A., Seto T., Wu Y.L., Novello S., Juhász E., et al. Monotherapy Administration of Sorafenib in Patients With Non-Small Cell Lung Cancer (MISSION) Trial: A Phase III, Multicenter, Placebo-Controlled Trial of Sorafenib in Patients with Relapsed or Refractory Predominantly Nonsquamous Non-Small-Cell Lung Cancer after 2 or 3 Previous Treatment Regimens. J. Thorac. Oncol. 2015;10:1745–1753. doi: 10.1097/jto.0000000000000693. [DOI] [PubMed] [Google Scholar]
- 117.Schuler M., Cho B.C., Sayehli C.M., Navarro A., Soo R.A., Richly H., Cassier P.A., Tai D., Penel N., Nogova L., et al. Rogaratinib in patients with advanced cancers selected by FGFR mRNA expression: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1454–1466. doi: 10.1016/S1470-2045(19)30412-7. [DOI] [PubMed] [Google Scholar]
- 118.Nogova L., Sequist L.V., Perez Garcia J.M., Andre F., Delord J.P., Hidalgo M., Schellens J.H., Cassier P.A., Camidge D.R., Schuler M., et al. Evaluation of BGJ398, a Fibroblast Growth Factor Receptor 1-3 Kinase Inhibitor, in Patients With Advanced Solid Tumors Harboring Genetic Alterations in Fibroblast Growth Factor Receptors: Results of a Global Phase I, Dose-Escalation and Dose-Expansion Study. J. Clin. Oncol. 2017;35:157–165. doi: 10.1200/jco.2016.67.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scott A.M., Wiseman G., Welt S., Adjei A., Lee F.-T., Hopkins W., Divgi C.R., Hanson L.H., Mitchell P., Gansen D.N., et al. A Phase I Dose-Escalation Study of Sibrotuzumab in Patients with Advanced or Metastatic Fibroblast Activation Protein-positive Cancer. Clin. Cancer Res. 2003;9:1639–1647. [PubMed] [Google Scholar]
- 120.Hofheinz R.D., al-Batran S.E., Hartmann F., Hartung G., Jäger D., Renner C., Tanswell P., Kunz U., Amelsberg A., Kuthan H., et al. Stromal antigen targeting by a humanised monoclonal antibody: An early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26:44–48. doi: 10.1159/000069863. [DOI] [PubMed] [Google Scholar]
- 121.Demircioglu F., Wang J., Candido J., Costa A.S.H., Casado P., de Luxan Delgado B., Reynolds L.E., Gomez-Escudero J., Newport E., Rajeeve V., et al. Cancer associated fibroblast FAK regulates malignant cell metabolism. Nat. Commun. 2020;11:1290. doi: 10.1038/s41467-020-15104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gerber D.E., Camidge D.R., Morgensztern D., Cetnar J., Kelly R.J., Ramalingam S.S., Spigel D.R., Jeong W., Scaglioni P.P., Zhang S., et al. Phase 2 study of the focal adhesion kinase inhibitor defactinib (VS-6063) in previously treated advanced KRAS mutant non-small cell lung cancer. Lung Cancer. 2020;139:60–67. doi: 10.1016/j.lungcan.2019.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chiappori A.A., Eckhardt S.G., Bukowski R., Sullivan D.M., Ikeda M., Yano Y., Yamada-Sawada T., Kambayashi Y., Tanaka K., Javle M.M., et al. A Phase I Pharmacokinetic and Pharmacodynamic Study of S-3304, a Novel Matrix Metalloproteinase Inhibitor, in Patients with Advanced and Refractory Solid Tumors. Clin. Cancer Res. 2007;13:2091–2099. doi: 10.1158/1078-0432.CCR-06-1586. [DOI] [PubMed] [Google Scholar]
- 124.Edelman M.J., Wang X., Hodgson L., Cheney R.T., Baggstrom M.Q., Thomas S.P., Gajra A., Bertino E., Reckamp K.L., Molina J., et al. Phase III Randomized, Placebo-Controlled, Double-Blind Trial of Celecoxib in Addition to Standard Chemotherapy for Advanced Non-Small-Cell Lung Cancer With Cyclooxygenase-2 Overexpression: CALGB 30801 (Alliance) J. Clin. Oncol. 2017;35:2184–2192. doi: 10.1200/JCO.2016.71.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Giaccone G., Bazhenova L.A., Nemunaitis J., Tan M., Juhász E., Ramlau R., van den Heuvel M.M., Lal R., Kloecker G.H., Eaton K.D., et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur. J. Cancer. 2015;51:2321–2329. doi: 10.1016/j.ejca.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 126.Olive K.P., Jacobetz M.A., Davidson C.J., Gopinathan A., McIntyre D., Honess D., Madhu B., Goldgraben M.A., Caldwell M.E., Allard D., et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim E.J., Sahai V., Abel E.V., Griffith K.A., Greenson J.K., Takebe N., Khan G.N., Blau J.L., Craig R., Balis U.G., et al. Pilot Clinical Trial of Hedgehog Pathway Inhibitor GDC-0449 (Vismodegib) in Combination with Gemcitabine in Patients with Metastatic Pancreatic Adenocarcinoma. Clin. Cancer Res. 2014;20:5937–5945. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ko A.H., LoConte N., Tempero M.A., Walker E.J., Kate Kelley R., Lewis S., Chang W.-C., Kantoff E., Vannier M.W., Catenacci D.V., et al. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas. 2016;45:370–375. doi: 10.1097/MPA.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Catenacci D.V.T., Junttila M.R., Karrison T., Bahary N., Horiba M.N., Nattam S.R., Marsh R., Wallace J., Kozloff M., Rajdev L., et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J. Clin. Oncol. 2015;33:4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Jesus-Acosta A., Sugar E.A., O’Dwyer P.J., Ramanathan R.K., Von Hoff D.D., Rasheed Z., Zheng L., Begum A., Anders R., Maitra A., et al. Phase 2 study of vismodegib, a hedgehog inhibitor, combined with gemcitabine and nab-paclitaxel in patients with untreated metastatic pancreatic adenocarcinoma. Br. J. Cancer. 2020;122:498–505. doi: 10.1038/s41416-019-0683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Özdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C.C., Simpson T.R., Laklai H., Sugimoto H., Kahlert C., Novitskiy S.V., et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weniger M., Honselmann K.C., Liss A.S. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers. 2018;10:316. doi: 10.3390/cancers10090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Infante J.R., Korn R.L., Rosen L.S., LoRusso P., Dychter S.S., Zhu J., Maneval D.C., Jiang P., Shepard H.M., Frost G., et al. Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br. J. Cancer. 2018;118:153–161. doi: 10.1038/bjc.2017.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hakim N., Patel R., Devoe C., Saif M.W. Why HALO 301 Failed and Implications for Treatment of Pancreatic Cancer. Pancreas (Fairfax) 2019;3:e1–e4. doi: 10.17140/POJ-3-e010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chauhan V.P., Martin J.D., Liu H., Lacorre D.A., Jain S.R., Kozin S.V., Stylianopoulos T., Mousa A.S., Han X., Adstamongkonkul P., et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Murphy J.E., Wo J.Y., Ryan D.P., Clark J.W., Jiang W., Yeap B.Y., Drapek L.C., Ly L., Baglini C.V., Blaszkowsky L.S., et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:1020–1027. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Feig C., Jones J.O., Kraman M., Wells R.J., Deonarine A., Chan D.S., Connell C.M., Roberts E.W., Zhao Q., Caballero O.L., et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ciardiello D., Elez E., Tabernero J., Seoane J. Clinical development of therapies targeting TGFβ: Current knowledge and future perspectives. Ann. Oncol. 2020;31:1336–1349. doi: 10.1016/j.annonc.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 139.Melisi D., Garcia-Carbonero R., Macarulla T., Pezet D., Deplanque G., Fuchs M., Trojan J., Oettle H., Kozloff M., Cleverly A., et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer. 2018;119:1208–1214. doi: 10.1038/s41416-018-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Helms E., Onate M.K., Sherman M.H. Fibroblast Heterogeneity in the Pancreatic Tumor Microenvironment. Cancer Discov. 2020;10:648–656. doi: 10.1158/2159-8290.CD-19-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dominguez C.X., Müller S., Keerthivasan S., Koeppen H., Hung J., Gierke S., Breart B., Foreman O., Bainbridge T.W., Castiglioni A., et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15+ Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2020;10:232–253. doi: 10.1158/2159-8290.CD-19-0644. [DOI] [PubMed] [Google Scholar]
- 142.Pereira B.A., Vennin C., Papanicolaou M., Chambers C.R., Herrmann D., Morton J.P., Cox T.R., Timpson P. CAF Subpopulations: A New Reservoir of Stromal Targets in Pancreatic Cancer. Trends Cancer. 2019;5:724–741. doi: 10.1016/j.trecan.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 143.Du H., Che G. Genetic alterations and epigenetic alterations of cancer-associated fibroblasts. Oncol. Lett. 2017;13:3–12. doi: 10.3892/ol.2016.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Drost J., Clevers H. Organoids in cancer research. Nat. Rev. Cancer. 2018;18:407–418. doi: 10.1038/s41568-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 145.Moffitt R.A., Marayati R., Flate E.L., Volmar K.E., Loeza S.G.H., Hoadley K.A., Rashid N.U., Williams L.A., Eaton S.C., Chung A.H., et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]