Abstract
Alzheimer’s disease (AD), the most common cause of dementia, is a progressive neurodegenerative disease. The number of AD cases has been rapidly growing worldwide. Several the related etiological hypotheses include atypical amyloid β (Aβ) deposition, neurofibrillary tangles of tau proteins inside neurons, disturbed neurotransmission, inflammation, and oxidative stress. During AD progression, aberrations in neurotransmission cause cognitive decline—the main symptom of AD. Here, we review the aberrant neurotransmission systems, including cholinergic, adrenergic, and glutamatergic network, and the interactions among these systems as they pertain to AD. We also discuss the key role of N-methyl-d-aspartate receptor (NMDAR) dysfunction in AD-associated cognitive impairment. Furthermore, we summarize the results of recent studies indicating that increasing glutamatergic neurotransmission through the alteration of NMDARs shows potential for treating cognitive decline in mild cognitive impairment or early stage AD. Future studies on the long-term efficiency of NMDA-enhancing strategies in the treatment of AD are warranted.
Keywords: Alzheimer’s disease, cholinergic, adrenergic, glutamatergic, NMDAR
1. Introduction
Patients with dementia experience declines in at least two areas of cognition: memory and thinking. Alzheimer’s disease (AD), the most common cause of dementia, is a progressive neurodegenerative disease that causes memory and cognitive impairments as well as behavioral and psychiatric abnormalities [1,2]. AD has gained increasing attention because of the growing burden it places on the global health care system [3]. The development of drugs to treat AD is difficult because the causes of AD remain poorly understood. No new drug or modifying treatment for AD has been approved since 2013 [4].
Patients with AD undergo two main histopathological changes: (i) the extracellular deposition of amyloid plaques, consisting of amyloid β (Aβ), in the brain tissue and (ii) the formation of intraneuronal neurofibrillary tangles of the phosphorylated tau proteins. Although the mechanism through which Aβ and hyperphosphorylated tau damage synaptic dysfunction warrants further clarification, an increasing amount of evidence suggests that the main cause is alterations in the neurotransmitter systems. The cholinergic, adrenergic, and glutamatergic pathways are the key points of focus of AD treatment and prevention. In this review, we first present the evidence describing the role of the cholinergic, adrenergic, and glutamatergic systems in AD development. Then, we review the literature on the effects of drugs targeting the cholinergic, adrenergic, and glutamatergic systems in cell cultures, animal models, and human trials of AD. Finally, we detail on how these three systems influence each other.
2. Current Etiological Hypothesis of AD Involving the Neurotransmitter System
2.1. Cholinergic Hypothesis
The changes in neurotransmitters and their receptors in patients with AD have been well studied. A consistent loss of cholinergic neurons and a considerable decline in choline acetyltransferase activity are the most prominent phenotypes of AD progression. Central cholinergic neurons in the nucleus basalis of Meynert are the primary source of cholinergic innervation to the neocortex. The nucleus basalis of Meynert has been widely studied with respect to AD [5,6,7,8,9], with all pertinent evidence showing that cholinergic neuronal cell degeneration is related to the progressive worsening of memory and cognitive loss in patients with AD. A considerable (29%) overall loss in neuron number was observed in the nucleus basalis of Meynert as well as an even larger loss (61%) in large neurons and a concurrent increase (59%) in small neurons [5].
Both nicotinic acetylcholine receptors (nAChR) and muscarinic ACh receptors have been suggested as drug targets for AD treatment. In the human brain, the heteromeric α4β2 and homomeric α7-nAChR are the major nAChR subtypes [10]. Considerable decreases in α4β2 levels (of up to 50%) have been observed in the brains of patients with AD though an examination of postmortem human brain tissue [11]. Decreases in the α7-nAChR levels observed in the early AD stage are associated with the progression of cognitive deficits [12,13]. However, the results of postmortem protein and mRNA expression studies have led to discrepancies. Although a decrease in α7 protein levels was noted in the cortex and hippocampus [13,14], α7 mRNA levels have been found to be considerably higher in the hippocampus of patients with AD than in that of control patients [15].
The α7-nAChR exhibits high binding affinity to the 42-amino acid Aβ peptide (Aβ(1–42)) [16,17]. Aβ(1–42) can induce cell death in human neuroblastoma cells which overexpress α7-nAChR, and the pretreatment of the α7-nAChR agonists nicotine and epibatidine can protect from Aβ(1–42)-mediated cell toxicity [16]. In one study, chronic nicotine treatment reduced more than 80% of Aβ(1–42)-positive plaques in the brain of a mouse AD model (APPsw) [18]. In addition, α7-nAChR is highly colocalized with Aβ(1–42) within the neurons of AD brains [19]. The crossing of an α7-nAChR-null mutant (α7KO) mouse with a mouse model with AD PDAPP (J9) has been reported to protect from dysfunctions in synaptic integrity and memory behavior [20].
The loss of cholinergic neurons and AChRs in patients with AD makes acetylcholinesterase (AChE) a therapeutic target. Three AChE inhibitors (AChEIs), namely donepezil, galantamine, and rivastigmine, can decelerate acetylcholine breakdown by reducing acetylcholinesterase activity and increasing ACh concentrations at the synapses [21]. The administration of rivastigmine decelerates whole-brain atrophy, hippocampal atrophy, white matter loss, and cognitive decline [22]. Randomized controlled trials on donepezil in patients with AD have indicated that donepezil can also decrease the rate of atrophy in the cortex [23], hippocampus [24], and basal forebrain [25]. Moreover, AChEIs can reduce free radicals and amyloid toxicity as well as cytokine release [26,27,28]. These anti-inflammatory effects demonstrate the positive effects of AChEIs on AD alleviation [29].
2.2. Adrenergic Hypothesis
Several studies have shown that the adrenergic system is critical in the CNS. The locus coeruleus (LC), the predominant source of noradrenergic projection neurons, determines the global states of the brain, covering the whole spectrum of brain activation [30], including learning, memory, attention [31,32], sleep–wake cycle regulation [33], active wake and physiological stress [30], as well as aggression regulation. The stimulation of postsynaptic α1-adrenergic receptor (α2AR) with guanfacine can improve working memory performance [34]. Studies have found that LC noradrenergic neurons could be considerably degenerated in the brains of patients with advanced AD [35,36,37,38,39]. A 30% loss in LC neurons was observed during the transition from a state of no cognitive impairment to amnestic mild cognitive impairment (MIC), with an additional 25% loss in LC neurons during AD [40].
Although anatomical and neurotransmitter changes in the adrenergic system have been observed in patients with AD, the expression of adrenergic receptors in postmortem brain tissues reveals an inconsistency. Kalaria showed that α1AR density decreased in the prefrontal cortex [41] but not in the hippocampus, the putamen, or the cerebellum, whereas Szot et al. found that α1AR binding sites increased in layers 1 and 2 of the prefrontal cortex [42]. In the brains of patients with Alzheimer-type dementia, the amount of α1ARs considerably decreased in the hippocampus and the cerebellar hemisphere, whereas that of α2ARs considerably decreased in the nucleus basalis of Meynert [43]. However, in patients with AD, the amount of β1ARs decreased whereas that of β2ARs increased in the cortex, and the amounts of both β1ARs and β2ARs increased in the hippocampus [44].
The distribution of adrenergic receptors in the regions of the brain is correlated with AD pathogenesis. β2ARs are predominant in the human hippocampus, and β1ARs are predominant in the rat hippocampus [45,46]. In addition, βARs play a crucial role in cognitive function. The administration of both selective and nonselective βAR antagonists, including isoproterenol, propranolol, timolol, and sotalol, can impair memory function [47,48,49,50,51]. However, the stimulation of βARs with agonists promotes memory consolidation [50,52,53] and synaptic long-term potentiation (LTP) [54,55,56,57]. In support of the idea that adrenergic neurotransmission participates in memory and leaning, the expression of adrenergic receptors mainly distributes in the dendrites of granule cells and interneurons in dentate gyrus [58,59].
βARs represent a target that might be altered by Aβ oligomers. The direct application of human Aβ oligomers can induce the internalization of transfected human β2ARs in fibroblasts and endogenous β2ARs in rodent prefrontal cortical neurons [60]. Large soluble oligomers of Aβ proteins from human brains with AD are less bioactive than low molecular-weight (approximately 8–70 kDa) Aβ oligomers (which are dissociated from high molecular-weight Aβ oligomers); nevertheless, low molecular-weight Aβ oligomers can decrease the neuron levels of β2ARs, impair hippocampal LTP, and activate microglia in vivo [61]. Furthermore, the binding of soluble Aβ peptides to β2ARs activates G-protein-cAMP-protein kinase A (PKA) signaling and further induces α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) receptor hyperactivity [62].
Adrenergic receptor activation restores memory impairment in animal models with AD. In amyloid precursor protein/presenilin 1 (APP/PS1) mice, the β2 adrenergic agonist clenbuterol enhances hippocampal neurogenesis, improves memory deficits, and upregulates dendritic branching and the density of dendritic spines [63]. The administration of the β1AR agonist xamoterol has been found to reduce the social recognition deficit in APP/PS1 mice by increasing nuclear phospho-CREB [64]. These findings suggest that the activation of βAR reduces cognitive deficits in animal models with AD.
2.3. Glutamatergic Hypothesis
The glutamatergic pathway, particularly involving N-methyl d-aspartate receptor (NMDAR) activation, is the main mediator of synaptic strength and structure, which are related to long-term synaptic plasticity [65]. Ca2+ entry via NMDARs and l-type Ca2+ channels activate CREB signals in hippocampal pyramidal neurons [66,67]. Because vesicular glutamate transporters (VGLUT1–3) mediate glutamate packing into synaptic vesicles, scientists have used VGLUTs to detect glutamatergic neurons. VGLUT1 mRNA expression is mainly detected in the telencephalic regions, including the cerebral cortex, hippocampus and cerebellum [68,69], whereas VGLUT2 mRNA and proteins are mainly found in the thalamus and lower brainstem regions [69,70]. VGLUT1 and VGLUT2 detection through Western blotting has revealed that VGLUT1 and VGLUT2 are lower in the prefrontal dorsolateral cortices of patients with AD [71]. Many researchers have demonstrated that glutamatergic network dysfunction is associated with AD pathogenesis, which involves a decrease in glutamic acid content and receptor binding in the brain [72,73]. In addition, lowered glutamate uptake levels have been found in the cortex and the hippocampus, indicating glutamatergic synapse loss [74].
Soluble Aβ oligomers stimulate tau phosphorylation [75] and disturb the glutamatergic networks. Aβ42 binds to forebrain synaptosomes, which are associated with postsynaptic density complexes of the NMDA subunits NR1 and NR2B [76]. In cultured rat cortical neurons and entorhinal–hippocampal organotypic slices, soluble Aβ oligomers markedly induced NMDA-dependent inward Ca2+ currents and cell apoptosis through the NMDA and AMPA receptors [77]. NMDA and AMPA receptor overactivation leads to mitochondrial dysfunction, including excessive mitochondrial Ca2+ and mitochondrial damage. Aβ oligomers can inhibit LTP in hippocampal brain slices, cultured cells, and the cortices of brains with AD [78,79]; this process can be blocked by selective NR2B inhibitors. This observation indicates that soluble Aβ oligomers reduce LTP through the excessive activation of extrasynaptic NMDA receptors containing NR2B.
The overactivation of extrasynaptic NMDARs is linked to neurodegeneration. Memantine, which reduces extrasynaptic NMDAR overactivation [80], has therapeutic effects on moderate to severe AD because it reduces glutamate excitotoxicity [81,82,83]. Compared with other NMDAR antagonists, such as MK-801, the binding affinity of memantine is relatively low. Because of this low binding affinity, memantine strikes a balance between physiological synaptic activity and excessive extrasynaptic activity [84]. Whether NMDAR activation leads to cell survival or cell death depends on the location and strength of NMDAR stimulation. A low amount of NMDAR stimulation activates the synaptic NMDARs, which causes pro-survival signaling, whereas a considerable amount of simulation gradually leads to the stimulation of not only extrasynaptic but also synaptic NMDARs, thus triggering cell death [85]. Therefore, finding a mild NMDAR antagonist has become a research focus in neurodegenerative disease treatment.
NMDAR subunit composition depends on the location. In a mature hippocampus, synaptic NMDARs mainly contain NR2A, whereas extrasynaptic NMDA receptors mainly contain NR2B [86]. The administration of Aβ(1–42) oligomers can induce the nuclear accumulation of Jacob, which is caused by the activation of extrasynaptic NMDARs and correlated with pathological changes in the dendric spines; these effects can be blocked by the NR2B antagonist ifenprodil [87]. NMDARs containing NR2B have high levels of pathological expression with apoptosis at the hippocampus in rat models with AD, indicating the nature of the NR2B–AD relationship [88]. The application of traxoprodil and arcaine, both of which are NR2B antagonists, reverses the Aβ(25–35)-induced reduction in dendritic spine density and morphological changes to the spine [89]. By using an organotypic hippocampal slice from arcAβ transgenic mice combined with overexpressed human tau protein, Tackenberg et al. demonstrated that the blocking of NR2B-containing NMDARs inhibited Aβ-induced tau phosphorylation and cell toxicity because GSK-3β activation was lowered [90]. Although the reduced NR2B activity caused by the receptor antagonists was demonstrated to afford neuroprotection and neuropathic pain alleviation in animal models with Parkinson’s disease [91,92], a clinical trial reported that the effects of traxoprodil, a NR2B antagonist, on traumatic brain injury was nonsignificant [93]. Thus, additional studies investigating NR2B-targeting interventions on dementia are still warranted.
Enhancing NMDAR function through the NMDAR co-agonists d-serine and glycine can offer therapeutic potential. The binding of co-agonists and glutamate are essential for NMDAR activation [94,95,96]. The availability of NMDAR co-agonists varies according to locations. Although both d-serine and glycine originate from glial cells, d-serine is available for synaptic NMDARs and glycine is available for extrasynaptic NMDARs [97]. As stated in Section 2.2, the activation of the synaptic NMDARs plays a key role in neuroprotection, and d-serine is the main co-agonist for NMDARs in the forebrain and the hippocampus [98,99,100]. However, patients with dementia exhibit distinct changes in d-serine levels. Madeira et al. reported that d-serine levels were considerably high in the hippocampus and the parietal cortex in postmortem brains with AD as well as in the cerebrospinal fluid (CSF) of patients with AD [101]. In contrast to the study of Madeira et al., d-serine levels in the CSF of patients with AD did not differ from those in the CSF of the controls [102]. Nagata et al. analyzed the levels of free l-serine and d-serine in the frontal cortex of human brains with and without AD and found no notable difference between the two groups [103]. In a study using blood samples from patients with AD or MIC, patients with poor cognitive function demonstrated high d-serine concentrations [104]. High d-amino acid oxidase (DAAO) levels were also found [105]. The increase in DAAO concentration might have been a secondary response to the high concentration of d-serine, which contributed to late-phase AD because of neurotoxicity.
3. Interaction Among Adrenergic, Cholinergic, and Glutamatergic Systems
3.1. Link with Adrenergic and Glutamatergic Systems
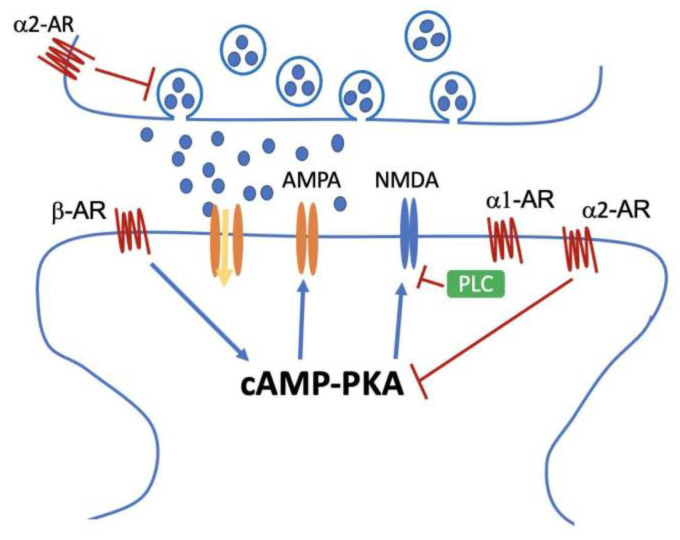
The glutamatergic system plays a key role in controlling cognitive function and working memory in the prefrontal cortex (PFC), but it does not function alone and its connection to other neurotransmitter systems warrants discussion. Because the adrenergic and cholinergic systems are both located on dendritic spines, they are thought to regulate the glutamatergic system and its associated activities [106]. The crossing of Ear2−/− mice that had severe LC neuron loss with APP/PS1 mice revealed a decrease in NR2A subunit levels but an increase in NR2B levels [107]. This deficiency in LC neurons causes early memory and learning impairment, suggesting that a reduction in adrenergic stimulation may cause cognitive decline through NMDAR dysfunction. Acute stress activates noradrenaline release in the brain [108]. The binding of noradrenaline to βARs activates PKA and CaMKII, which further phosphorylate GluR1 at S845 and S831. After the systemic administration of epinephrine to increase norepinephrine (NE) levels in the CNS, phosphorylation at Ser845 of GluR1 increases [109]. This phosphorylation can lower the threshold for the incorporation of AMPA receptors containing GluR1 into the synapses during LTP [109], and this synaptic delivery of AMPA receptors is critical to synaptic plasticity [109,110,111,112]. In older rats, impaired LTP is correlated with the misregulation of AMPA receptor trafficking under fear conditioning, and NE administration can reverse LTP impairment by enhancing the GluR1-transporting cell surface [113]. Kobayashi et al. reported that α1AR activation decreased eEPSPs through protein kinase C, whereas the activation of βARs evoked excitatory signals through cAMP-PKA in layer 5 pyramidal neurons [114]. Phenylephrine, the α1AR agonist, decreases miniature EPSC (mEPSC) amplitude. However, the βAR agonist isoproterenol greatly increases the eEPSC amplitude [115]. In addition to AMPA receptors, the application of NE and NE transporter inhibitors reduces the amplitude of EPSC mediated by NMDA receptors in the PFC pyramidal neurons [116]. A possible mechanism is that G-protein-coupled α1AR decreases NMDAR currents via the PLC-IP3 pathway, which is regulated by regulators of G-protein signaling 2. Activation of α2AR reduces PKA–ERK signaling and furthers NMDAR transports, leading to the downregulation of NMDAR currents [116]. Moreover, presynaptic α2AR inhibits glutamate transmission in the ventral tegmental area [117]. Because the activation of α1, α2, and βARs results in various effects for AMPA and NMDA receptors, it reveals that the effects of NE on glutamatergic receptors depend on each individual cell and receptor [118,119,120]. Figure 1 illustrates the mechanism of α1, α2, and βAR regulating NMDAR function.
Figure 1.
Proposed model of the mechanism of α1, α2, and βAR regulating NMDAR function. βAR enhances NMDAR currents through cAMP-PKA pathways, whereas α2AR reduces NMDAR activity by inhibiting the same pathway or presynaptic glutamate secretion. α1AR, which is a protein-coupled receptor, decreases NMDAR activity through PLC-IP3 signals.
3.2. Link to Cholinergic and Glutamatergic Systems
The most frequently discussed topic in studies on connections with the cholinergic and glutamatergic systems is that of the dorsolateral PFC (dlPFC). Nicotinic α7-nAChR is enriched in the glutamate network synapses in the dlPFC and is required for NMDA action, indicating that α7-nAChR and NMDARs work together in dlPFC circuits [121]. However, the action of acetylcholine on NMDA may be region dependent. In CA1 neurons of the hippocampus, ACh potentiates NMDA receptors through muscarinic receptors [122] and possibly through the inositol 1,4,5-trisphosphate pathway in the hippocampus [123]. Exposure of the cholinergic receptor agonist carbachol in the hippocampus can induce LTP, which is dependent on NMDA receptor activation [124]. By contrast, NMDAR-mediated currents can be directly inhibited through the application of acetylcholine in the cortical brain slice, and this inhibition is independent of G-protein and voltage [125]. The administration of the AChE inhibitor donepezil can significantly reduce the surface expression of NR1, the core subunit of NMDAR, and glutamate-induced toxicity. α7-nAChR blockage by methyllycaconitine reduces the donepezil-induced attenuation of glutamate-mediated Ca2+ entry [126]. Physostigmine, another AChE inhibitor, reduces NMDAR-mediated excitatory postsynaptic currents through Ca2+ and the ERK pathway [127]. nAChR activation can cause increases in neurotransmission and selective increases in the amplitudes of AMPA receptor (AMPAR)-mediated currents in layer 1 neurons in the PFC [128]. Nicotine increases intracellular calcium levels through α7-nAChR and modulates the phosphorylation of the GluR1 AMPAR subunit, which leads to an increase in AMPAR current. Nicotine-treated astrocytes exhibits high AMPAR levels in the hippocampal slices. This activation of α7-nAChR on astrocytes lead to AMPAR recruitment to the postsynaptic sites on the neuron surface [129].
The cholinergic and glutamatergic systems may be connected by d-serine. The binding of d-serine or glycine at the glycine modulatory site is essential to activate NMDARs [96]. Astrocytes produce l-serine from glucose, and l-serine is then transported to neurons and converted to d-serine by serine racemase [130,131]. Thus, serine racemase modulation plays a key role in NMDAR activity. In PC-12 phaeochromocytoma cells and 1321N1 astrocytoma cells, incubation with α7-nAChR antagonists decreases the expression of serine racemase [132]. The deletion of the α7-nAChR gene in mice can cause the loss of d-serine and NMDARs, which leads to glutamatergic synaptic deficiency in the cortex [133]. The stimulation of the nucleus basalis of Meynert, the major source of cholinergic innervation to the cortex, considerably increases d-serine levels in wild type mice compared with those in inositol-1,4,5-trisphosphate receptor type 2 knockout mice [134]. Moreover, this elevation in d-serine levels is involved in ACh-modulated and NMDAR-dependent synaptic plasticity.
4. Potential Therapeutic Targets of NMDAR Enhancers
Because the adrenergic, cholinergic, and glutamatergic systems influence each other and because synapses containing NMDARs are the main target of cognition dysfunction, most of the therapeutic strategies have focused on modulating glutamate neurotransmission [135]. Although glutamate excitotoxicity through NMDARs is the primary cause of acute neuronal injuries, the direct administration of NMDAR antagonists, such as ketamine and MK-801, may increase dopamine release and schizophrenia-like behavior [136,137,138,139,140,141,142]. Thus, the use of NMDAR co-agonists to alter NMDA neurotransmission and improve cognition has become an alternative method for modulating NMDARs. Several types of NMDA-enhancing agents targeting co-agonist sites have been identified. Glycine and d-serine are two co-agonists that bind to segments S1 and S2 on the GluN1 and GluN3 subunits of NDMARs [143]. The co-agonist site can be activated by full agonists, including glycine and d-serine. The administration of d-serine as a supplement in water can prevent immune-induced cognitive deficits in adult offspring after maternal exposure to poly(I:C) [144]. Acute oral administration of d-serine in older adults can improve spatial learning and problem-solving abilities [145]. Patients with schizophrenia who received d-serine treatment to enhance NMDARs were noted to exhibit notable improvements to cognitive impairment as well as to positive and negative symptoms [146,147]. However, a multicenter add-on randomized controlled trial of low-dose d-serine treatment in patients with schizophrenia did not reveal any notable differences between the d-serine treatment group and the placebo group [148]. Therefore, longer-term, higher-dose d-serine treatment regimens in patients with cognitive impairments should be investigated in future studies.
The second method for enhancing co-agonist sites is through partial agonists, such as d-cycloserine. d-cycloserine can facilitate NMDAR–ionophore complex activation and enhance cognition in patients with AD [149]. d-cycloserine can reverse scopolamine-induced acquisition performance impairment in rats [150]. In a study on the effects in humans, Jones et al. pretreated healthy patients with the anticholinergic drug scopolamine as a model for memory impairment associated with AD. The authors found that low doses of d-cycloserine had a positive effect on memory [151]. The short-term treatment with 100 mg/day d-cycloserine is associated with notable improvements in cognitive function [152,153]. However, several studies have indicated that d-cycloserine does not benefit patients with AD [154,155,156]. The results of two large and two small randomized controlled trials suggested that d-cycloserine does not improve cognitive impairments in patients with AD [157]. Another reason for the limited use of d-cycloserine is the side effects, include hyperexcitability, dizziness, depression, anxiety, confusion, memory loss, and lethargy, which were found in using high dose of d-cycloserine [158].
Another method is to alter d-serine and glycine levels by modulating their generators and metabolizers. Increasing synaptic glycine by using glycine transporter-1 inhibitors ASP2535 or TASP0315003 can reduce cognitive deficits in rodent models with schizophrenia and AD [159,160]. A recent study evaluated the efficacy and safety of orally administered BI 425809, a selective GlyT1 inhibitor, for patients with AD exhibiting cognitive deficits. However, no notable changes were observed after 12 weeks of treatment [161]. Another method for enhancing NMDAR levels through increasing d-serine levels is to reduce d-serine degradation through inhibiting DAAO, a primary mediator of d-serine metabolism in the brain [162,163]. The d-serine levels in mice increased after administration of sodium benzoate and PGM030756, both of which are DAAO inhibitors [164]. Several studies have evaluated the clinical effects of DAAO inhibitors on patients with schizophrenia [162,165,166] and dementia [167,168,169], and their results have suggested that sodium benzoate has potential for delaying AD progression. Sodium benzoate can reduce the activation of p21rac, oxidative stress, and neuronal apoptosis in the hippocampus and can protect against the degeneration of spatial learning and memory capabilities in 5XFAD Tg mice [170]. A randomized, double-blind, placebo-controlled trial on the use of sodium benzoate demonstrated that sodium benzoate can improve cognitive and overall function in patients with MIC or mild AD [167]. A 6-week sodium benzoate treatment in patients with behavioral and psychological symptoms of dementia did not yield any notable improvements [168]. Combined with a precision medicine approach, a 6-week treatment of sodium benzoate demonstrated potential to improve cognitive function in some patients with behavioral and psychological symptoms of dementia [169]. Through measures of working memory, verbal learning, and regional homogeneity maps, sodium benzoate has been demonstrated to modify brain activity and cognition in patients with MCI [171]. Among these clinical studies, no adverse effects were observed in line with small doses of sodium benzoate have little or no side effect on animals [172]. Taken together, sodium benzoate shows greater therapeutic potential in the clinical treatment of AD than other NMDAR enhancers.
5. Conclusions
The cholinergic, norepinephrine, and glutamatergic networks and their interactions are involved in cognitive dysfunction associated with AD, with the glutamatergic system playing an essential role in the regulation of synaptic plasticity and cognition. The modulation of the glutamatergic system and NMDAR-based enhancement therapy has been effective in some clinical trials. However, clinical trials have not yet determined potentiators for NMDAR activation in AD cases. The modulation of NMDAR co-agonist levels also benefits patients with other cognitive disorders, including schizophrenia. However, further research on the efficacy and safety of this treatment in both early and late AD stages is warranted.
Author Contributions
Conceptualization: C.-H.L. and H.-Y.L.; writing, Y.-J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan (MOST 107-2628-B-182A-002; 108-2628-B-182A-002; 109-2628-B-182A-002; 109-2314-B-039-039-MY3), the National Health Research Institutes (NHRI-EX110-10816NC), Kaohsiung Chang Gung Memorial Hospital (CMRPG8G1391), and China Medical University (CMU 109-MOST-03).
Conflicts of Interest
The authors declare no conflict of interest. The sponsors were not involved in the design of the study; the collection, analysis, and interpretation of the data; the writing of the report; and the decision to submit the article for publication.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Epperly T., Dunay M.A., Boice J.L. Alzheimer Disease: Pharmacologic and Nonpharmacologic Therapies for Cognitive and Functional Symptoms. Am. Fam. Physician. 2017;95:771–778. [PubMed] [Google Scholar]
- 2.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Alzheimer’s Association 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020 doi: 10.1002/alz.12068. [DOI] [Google Scholar]
- 4.Yiannopoulou K.G., Anastasiou A.I., Zachariou V., Pelidou S.H. Reasons for Failed Trials of Disease-Modifying Treatments for Alzheimer Disease and Their Contribution in Recent Research. Biomedicines. 2019;7 doi: 10.3390/biomedicines7040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen S.J., Dawbarn D., Wilcock G.K. Morphometric immunochemical analysis of neurons in the nucleus basalis of Meynert in Alzheimer’s disease. Brain Res. 1988;454:275–281. doi: 10.1016/0006-8993(88)90827-X. [DOI] [PubMed] [Google Scholar]
- 6.Arendt T., Bigl V., Arendt A., Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer’s disease, paralysis agitans and Korsakoff’s Disease. Acta Neuropathol. 1983;61:101–108. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- 7.Candy J.M., Perry R.H., Perry E.K., Irving D., Blessed G., Fairbairn A.F., Tomlinson B.E. Pathological changes in the nucleus of Meynert in Alzheimer’s and Parkinson’s diseases. J. Neurol. Sci. 1983;59:277–289. doi: 10.1016/0022-510X(83)90045-X. [DOI] [PubMed] [Google Scholar]
- 8.Doucette R., Fisman M., Hachinski V.C., Mersky H. Cell loss from the nucleus basalis of Meynert in Alzheimer’s disease. Can. J. Neurol. Sci. 1986;13:435–440. doi: 10.1017/S0317167100037070. [DOI] [PubMed] [Google Scholar]
- 9.Etienne P., Robitaille Y., Wood P., Gauthier S., Nair N.P., Quirion R. Nucleus basalis neuronal loss, neuritic plaques and choline acetyltransferase activity in advanced Alzheimer’s disease. Neuroscience. 1986;19:1279–1291. doi: 10.1016/0306-4522(86)90142-9. [DOI] [PubMed] [Google Scholar]
- 10.Paterson D., Nordberg A. Neuronal nicotinic receptors in the human brain. Prog. Neurobiol. 2000;61:75–111. doi: 10.1016/S0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 11.Warpman U., Nordberg A. Epibatidine and ABT 418 reveal selective losses of alpha 4 beta 2 nicotinic receptors in Alzheimer brains. Neuroreport. 1995;6:2419–2423. doi: 10.1097/00001756-199511270-00033. [DOI] [PubMed] [Google Scholar]
- 12.Nordberg A. Nicotinic receptor abnormalities of Alzheimer’s disease: Therapeutic implications. Biol. Psychiatry. 2001;49:200–210. doi: 10.1016/S0006-3223(00)01125-2. [DOI] [PubMed] [Google Scholar]
- 13.Burghaus L., Schutz U., Krempel U., de Vos R.A., Jansen Steur E.N., Wevers A., Lindstrom J., Schroder H. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res. Mol. Brain Res. 2000;76:385–388. doi: 10.1016/S0169-328X(00)00031-0. [DOI] [PubMed] [Google Scholar]
- 14.Guan Z.Z., Zhang X., Ravid R., Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer’s disease. J. Neurochem. 2000;74:237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- 15.Hellstrom-Lindahl E., Mousavi M., Zhang X., Ravid R., Nordberg A. Regional distribution of nicotinic receptor subunit mRNAs in human brain: Comparison between Alzheimer and normal brain. Brain Res. Mol. Brain Res. 1999;66:94–103. doi: 10.1016/S0169-328X(99)00030-3. [DOI] [PubMed] [Google Scholar]
- 16.Wang H.Y., Lee D.H., D’Andrea M.R., Peterson P.A., Shank R.P., Reitz A.B. beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J. Biol. Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 17.Wang H.Y., Lee D.H., Davis C.B., Shank R.P. Amyloid peptide Abeta(1–42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J. Neurochem. 2000;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- 18.Nordberg A., Hellstrom-Lindahl E., Lee M., Johnson M., Mousavi M., Hall R., Perry E., Bednar I., Court J. Chronic nicotine treatment reduces beta-amyloidosis in the brain of a mouse model of Alzheimer’s disease (APPsw) J. Neurochem. 2002;81:655–658. doi: 10.1046/j.1471-4159.2002.00874.x. [DOI] [PubMed] [Google Scholar]
- 19.Nagele R.G., D’Andrea M.R., Anderson W.J., Wang H.Y. Intracellular accumulation of beta-amyloid(1–42) in neurons is facilitated by the alpha 7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience. 2002;110:199–211. doi: 10.1016/S0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- 20.Dziewczapolski G., Glogowski C.M., Masliah E., Heinemann S.F. Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer’s disease. J. Neurosci. 2009;29:8805–8815. doi: 10.1523/JNEUROSCI.6159-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rees T.M., Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today. 2003;39:75–83. doi: 10.1358/dot.2003.39.1.740206. [DOI] [PubMed] [Google Scholar]
- 22.Ferris S., Nordberg A., Soininen H., Darreh-Shori T., Lane R. Progression from mild cognitive impairment to Alzheimer’s disease: Effects of sex, butyrylcholinesterase genotype, and rivastigmine treatment. Pharmacogenet. Genom. 2009;19:635–646. doi: 10.1097/FPC.0b013e32832f8c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavedo E., Dubois B., Colliot O., Lista S., Croisile B., Tisserand G.L., Touchon J., Bonafe A., Ousset P.J., Rouaud O., et al. Reduced Regional Cortical Thickness Rate of Change in Donepezil-Treated Subjects With Suspected Prodromal Alzheimer’s Disease. J. Clin. Psychiatry. 2016;77:e1631–e1638. doi: 10.4088/JCP.15m10413. [DOI] [PubMed] [Google Scholar]
- 24.Dubois B., Chupin M., Hampel H., Lista S., Cavedo E., Croisile B., Louis Tisserand G., Touchon J., Bonafe A., Ousset P.J., et al. Donepezil decreases annual rate of hippocampal atrophy in suspected prodromal Alzheimer’s disease. Alzheimers Dement. 2015;11:1041–1049. doi: 10.1016/j.jalz.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Cavedo E., Grothe M.J., Colliot O., Lista S., Chupin M., Dormont D., Houot M., Lehericy S., Teipel S., Dubois B., et al. Reduced basal forebrain atrophy progression in a randomized Donepezil trial in prodromal Alzheimer’s disease. Sci. Rep. 2017;7:11706. doi: 10.1038/s41598-017-09780-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H.Y., Tang X.C. Huperzine B, a novel acetylcholinesterase inhibitor, attenuates hydrogen peroxide induced injury in PC12 cells. Neurosci. Lett. 2000;292:41–44. doi: 10.1016/S0304-3940(00)01433-6. [DOI] [PubMed] [Google Scholar]
- 27.Xiao X.Q., Wang R., Tang X.C. Huperzine A and tacrine attenuate beta-amyloid peptide-induced oxidative injury. J. Neurosci. Res. 2000;61:564–569. doi: 10.1002/1097-4547(20000901)61:5<564::AID-JNR11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 28.Kimura M., Akasofu S., Ogura H., Sawada K. Protective effect of donepezil against Abeta(1–40) neurotoxicity in rat septal neurons. Brain Res. 2005;1047:72–84. doi: 10.1016/j.brainres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Tabet N. Acetylcholinesterase inhibitors for Alzheimer’s disease: Anti-inflammatories in acetylcholine clothing! Age Ageing. 2006;35:336–338. doi: 10.1093/ageing/afl027. [DOI] [PubMed] [Google Scholar]
- 30.Atzori M., Cuevas-Olguin R., Esquivel-Rendon E., Garcia-Oscos F., Salgado-Delgado R.C., Saderi N., Miranda-Morales M., Trevino M., Pineda J.C., Salgado H. Locus Ceruleus Norepinephrine Release: A Central Regulator of CNS Spatio-Temporal Activation? Front. Synaptic Neurosci. 2016;8:25. doi: 10.3389/fnsyn.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berridge C.W., Waterhouse B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 32.Sara S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell H.A., Weinshenker D. Good night and good luck: Norepinephrine in sleep pharmacology. Biochem. Pharmacol. 2010;79:801–809. doi: 10.1016/j.bcp.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M., Ramos B.P., Paspalas C.D., Shu Y., Simen A., Duque A., Vijayraghavan S., Brennan A., Dudley A., Nou E., et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Ishino H., Otsuki S. Frequency of Alzheimer’s neurofibrillary tangles in the basal ganglia and brain-stem in Alzheimer’s disease, senile dementia and the aged. Folia Psychiatr. Neurol. Jpn. 1975;29:279–287. doi: 10.1111/j.1440-1819.1975.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 36.Bondareff W., Mountjoy C.Q., Roth M. Selective loss of neurones of origin of adrenergic projection to cerebral cortex (nucleus locus coeruleus) in senile dementia. Lancet. 1981;1:783–784. doi: 10.1016/S0140-6736(81)92657-X. [DOI] [PubMed] [Google Scholar]
- 37.Chan-Palay V., Asan E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J. Comp. Neurol. 1989;287:373–392. doi: 10.1002/cne.902870308. [DOI] [PubMed] [Google Scholar]
- 38.Davies P., Maloney A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/S0140-6736(76)91936-X. [DOI] [PubMed] [Google Scholar]
- 39.Whitehouse P.J., Price D.L., Clark A.W., Coyle J.T., DeLong M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 40.Kelly S.C., He B., Perez S.E., Ginsberg S.D., Mufson E.J., Counts S.E. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2017;5:8. doi: 10.1186/s40478-017-0411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalaria R.N. Characterization of [125I]HEAT binding to alpha 1-receptors in human brain: Assessment in aging and Alzheimer’s disease. Brain Res. 1989;501:287–294. doi: 10.1016/0006-8993(89)90645-8. [DOI] [PubMed] [Google Scholar]
- 42.Szot P., White S.S., Greenup J.L., Leverenz J.B., Peskind E.R., Raskind M.A. Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: Evidence of compensatory changes. Neuroscience. 2007;146:471–480. doi: 10.1016/j.neuroscience.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimohama S., Taniguchi T., Fujiwara M., Kameyama M. Biochemical characterization of alpha-adrenergic receptors in human brain and changes in Alzheimer-type dementia. J. Neurochem. 1986;47:1295–1301. [PubMed] [Google Scholar]
- 44.Kalaria R.N., Andorn A.C., Tabaton M., Whitehouse P.J., Harik S.I., Unnerstall J.R. Adrenergic receptors in aging and Alzheimer’s disease: Increased beta 2-receptors in prefrontal cortex and hippocampus. J. Neurochem. 1989;53:1772–1781. doi: 10.1111/j.1471-4159.1989.tb09242.x. [DOI] [PubMed] [Google Scholar]
- 45.Oleskevich S., Descarries L., Lacaille J.C. Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J. Neurosci. 1989;9:3803–3815. doi: 10.1523/JNEUROSCI.09-11-03803.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daly C.J., McGrath J.C. Previously unsuspected widespread cellular and tissue distribution of beta-adrenoceptors and its relevance to drug action. Trends Pharmacol. Sci. 2011;32:219–226. doi: 10.1016/j.tips.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan R.M., McGaugh J.L., Leon M. Norepinephrine-induced plasticity and one-trial olfactory learning in neonatal rats. Brain Res. Dev. Brain Res. 1991;60:219–228. doi: 10.1016/0165-3806(91)90050-S. [DOI] [PubMed] [Google Scholar]
- 48.Izquierdo I., Medina J.H., Izquierdo L.A., Barros D.M., de Souza M.M., Mello e Souza T. Short- and long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol. Learn. Mem. 1998;69:219–224. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- 49.Cahill L., Pham C.A., Setlow B. Impaired memory consolidation in rats produced with beta-adrenergic blockade. Neurobiol. Learn. Mem. 2000;74:259–266. doi: 10.1006/nlme.1999.3950. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan R.M., Stackenwalt G., Nasr F., Lemon C., Wilson D.A. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav. Neurosci. 2000;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephenson R.M., Andrew R.J. Amnesia due to beta-antagonists in a passive avoidance task in the chick. Pharmacol. Biochem. Behav. 1981;15:597–604. doi: 10.1016/0091-3057(81)90216-1. [DOI] [PubMed] [Google Scholar]
- 52.Ferry B., McGaugh J.L. Clenbuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiol. Learn. Mem. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- 53.Crowe S.F., Shaw S. Salbutamol overcomes the effect of the noradrenergic neurotoxin DSP-4 on memory function in the day-old chick. Behav. Pharmacol. 1997;8:216–222. [PubMed] [Google Scholar]
- 54.Connor S.A., Wang Y.T., Nguyen P.V. Activation of {beta}-adrenergic receptors facilitates heterosynaptic translation-dependent long-term potentiation. J. Physiol. 2011;589:4321–4340. doi: 10.1113/jphysiol.2011.209379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gelinas J.N., Nguyen P.V. Beta-adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J. Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin Y.W., Min M.Y., Chiu T.H., Yang H.W. Enhancement of associative long-term potentiation by activation of beta-adrenergic receptors at CA1 synapses in rat hippocampal slices. J. Neurosci. 2003;23:4173–4181. doi: 10.1523/JNEUROSCI.23-10-04173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian H., Matt L., Zhang M., Nguyen M., Patriarchi T., Koval O.M., Anderson M.E., He K., Lee H.K., Hell J.W. beta2-Adrenergic receptor supports prolonged theta tetanus-induced LTP. J. Neurophysiol. 2012;107:2703–2712. doi: 10.1152/jn.00374.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milner T.A., Shah P., Pierce J.P. beta-adrenergic receptors primarily are located on the dendrites of granule cells and interneurons but also are found on astrocytes and a few presynaptic profiles in the rat dentate gyrus. Synapse. 2000;36:178–193. doi: 10.1002/(SICI)1098-2396(20000601)36:3<178::AID-SYN3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 59.O’Dell T.J., Connor S.A., Guglietta R., Nguyen P.V. beta-Adrenergic receptor signaling and modulation of long-term potentiation in the mammalian hippocampus. Learn. Mem. 2015;22:461–471. doi: 10.1101/lm.031088.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang D., Yuen E.Y., Zhou Y., Yan Z., Xiang Y.K. Amyloid beta peptide-(1–42) induces internalization and degradation of beta2 adrenergic receptors in prefrontal cortical neurons. J. Biol. Chem. 2011;286:31852–31863. doi: 10.1074/jbc.M111.244335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang T., Li S., Xu H., Walsh D.M., Selkoe D.J. Large Soluble Oligomers of Amyloid beta-Protein from Alzheimer Brain Are Far Less Neuroactive Than the Smaller Oligomers to Which They Dissociate. J. Neurosci. 2017;37:152–163. doi: 10.1523/JNEUROSCI.1698-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D., Govindaiah G., Liu R., De Arcangelis V., Cox C.L., Xiang Y.K. Binding of amyloid beta peptide to beta2 adrenergic receptor induces PKA-dependent AMPA receptor hyperactivity. FASEB J. 2010;24:3511–3521. doi: 10.1096/fj.10-156661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chai G.S., Wang Y.Y., Yasheng A., Zhao P. Beta 2-adrenergic receptor activation enhances neurogenesis in Alzheimer’s disease mice. Neural Regen. Res. 2016;11:1617–1624. doi: 10.4103/1673-5374.193241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coutellier L., Ardestani P.M., Shamloo M. beta1-adrenergic receptor activation enhances memory in Alzheimer’s disease model. Ann. Clin. Transl. Neurol. 2014;1:348–360. doi: 10.1002/acn3.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gnegy M.E. Ca2+/calmodulin signaling in NMDA-induced synaptic plasticity. Crit. Rev. Neurobiol. 2000;14:91–129. doi: 10.1615/CritRevNeurobiol.v14.i2.10. [DOI] [PubMed] [Google Scholar]
- 66.Deisseroth K., Bito H., Tsien R.W. Signaling from synapse to nucleus: Postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/S0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 67.Deisseroth K., Heist E.K., Tsien R.W. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 68.Vigneault E., Poirel O., Riad M., Prud’homme J., Dumas S., Turecki G., Fasano C., Mechawar N., El Mestikawy S. Distribution of vesicular glutamate transporters in the human brain. Front. Neuroanat. 2015;9:23. doi: 10.3389/fnana.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaneko T., Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci. Res. 2002;42:243–250. doi: 10.1016/S0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 70.Balaram P., Takahata T., Kaas J.H. VGLUT2 mRNA and protein expression in the visual thalamus and midbrain of prosimian galagos (Otolemur garnetti) Eye Brain. 2011;2011:5–15. doi: 10.2147/EB.S16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kashani A., Lepicard E., Poirel O., Videau C., David J.P., Fallet-Bianco C., Simon A., Delacourte A., Giros B., Epelbaum J., et al. Loss of VGLUT1 and VGLUT2 in the prefrontal cortex is correlated with cognitive decline in Alzheimer disease. Neurobiol. Aging. 2008;29:1619–1630. doi: 10.1016/j.neurobiolaging.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 72.Procter A.W., Palmer A.M., Francis P.T., Lowe S.L., Neary D., Murphy E., Doshi R., Bowen D.M. Evidence of glutamatergic denervation and possible abnormal metabolism in Alzheimer’s disease. J. Neurochem. 1988;50:790–802. doi: 10.1111/j.1471-4159.1988.tb02983.x. [DOI] [PubMed] [Google Scholar]
- 73.Cross A.J., Slater P., Simpson M., Royston C., Deakin J.F., Perry R.H., Perry E.K. Sodium dependent D-[3H]aspartate binding in cerebral cortex in patients with Alzheimer’s and Parkinson’s diseases. Neurosci. Lett. 1987;79:213–217. doi: 10.1016/0304-3940(87)90699-9. [DOI] [PubMed] [Google Scholar]
- 74.Cowburn R., Hardy J., Roberts P., Briggs R. Presynaptic and postsynaptic glutamatergic function in Alzheimer’s disease. Neurosci. Lett. 1988;86:109–113. doi: 10.1016/0304-3940(88)90192-9. [DOI] [PubMed] [Google Scholar]
- 75.De Felice F.G., Wu D., Lambert M.P., Fernandez S.J., Velasco P.T., Lacor P.N., Bigio E.H., Jerecic J., Acton P.J., Shughrue P.J., et al. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol. Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lacor P.N., Buniel M.C., Furlow P.W., Clemente A.S., Velasco P.T., Wood M., Viola K.L., Klein W.L. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alberdi E., Sanchez-Gomez M.V., Cavaliere F., Perez-Samartin A., Zugaza J.L., Trullas R., Domercq M., Matute C. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010;47:264–272. doi: 10.1016/j.ceca.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 78.Bieschke J., Herbst M., Wiglenda T., Friedrich R.P., Boeddrich A., Schiele F., Kleckers D., Lopez del Amo J.M., Gruning B.A., Wang Q., et al. Small-molecule conversion of toxic oligomers to nontoxic beta-sheet-rich amyloid fibrils. Nat. Chem. Biol. 2011;8:93–101. doi: 10.1038/nchembio.719. [DOI] [PubMed] [Google Scholar]
- 79.Li S., Jin M., Koeglsperger T., Shepardson N.E., Shankar G.M., Selkoe D.J. Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J. Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xia P., Chen H.S., Zhang D., Lipton S.A. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reisberg B., Doody R., Stoffler A., Schmitt F., Ferris S., Mobius H.J., Memantine Study G. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 82.Tariot P.N., Farlow M.R., Grossberg G.T., Graham S.M., McDonald S., Gergel I., Memantine Study G. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: A randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 83.Molinuevo J.L., Llado A., Rami L. Memantine: Targeting glutamate excitotoxicity in Alzheimer’s disease and other dementias. Am. J. Alzheimers Dis. Other Dement. 2005;20:77–85. doi: 10.1177/153331750502000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okamoto S., Pouladi M.A., Talantova M., Yao D., Xia P., Ehrnhoefer D.E., Zaidi R., Clemente A., Kaul M., Graham R.K., et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat. Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou X., Hollern D., Liao J., Andrechek E., Wang H. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis. 2013;4:e560. doi: 10.1038/cddis.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tovar K.R., Westbrook G.L. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ronicke R., Mikhaylova M., Ronicke S., Meinhardt J., Schroder U.H., Fandrich M., Reiser G., Kreutz M.R., Reymann K.G. Early neuronal dysfunction by amyloid beta oligomers depends on activation of NR2B-containing NMDA receptors. Neurobiol. Aging. 2011;32:2219–2228. doi: 10.1016/j.neurobiolaging.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z., Lv C., Zhao W., Song Y., Pei D., Xu T. NR2B-containing NMDA receptors expression and their relationship to apoptosis in hippocampus of Alzheimer’s disease-like rats. Neurochem. Res. 2012;37:1420–1427. doi: 10.1007/s11064-012-0726-0. [DOI] [PubMed] [Google Scholar]
- 89.Gomes G.M., Dalmolin G.D., Bar J., Karpova A., Mello C.F., Kreutz M.R., Rubin M.A. Inhibition of the polyamine system counteracts beta-amyloid peptide-induced memory impairment in mice: Involvement of extrasynaptic NMDA receptors. PLoS ONE. 2014;9:e99184. doi: 10.1371/journal.pone.0099184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tackenberg C., Grinschgl S., Trutzel A., Santuccione A.C., Frey M.C., Konietzko U., Grimm J., Brandt R., Nitsch R.M. NMDA receptor subunit composition determines beta-amyloid-induced neurodegeneration and synaptic loss. Cell Death Dis. 2013;4:e608. doi: 10.1038/cddis.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leaver K.R., Allbutt H.N., Creber N.J., Kassiou M., Henderson J.M. Neuroprotective effects of a selective N-methyl-D-aspartate NR2B receptor antagonist in the 6-hydroxydopamine rat model of Parkinson’s disease. Clin. Exp. Pharmacol. Physiol. 2008;35:1388–1394. doi: 10.1111/j.1440-1681.2008.05046.x. [DOI] [PubMed] [Google Scholar]
- 92.Huang L.E., Guo S.H., Thitiseranee L., Yang Y., Zhou Y.F., Yao Y.X. N-methyl D-aspartate receptor subtype 2B antagonist, Ro 25-6981, attenuates neuropathic pain by inhibiting postsynaptic density 95 expression. Sci. Rep. 2018;8:7848. doi: 10.1038/s41598-018-26209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yurkewicz L., Weaver J., Bullock M.R., Marshall L.F. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J. Neurotrauma. 2005;22:1428–1443. doi: 10.1089/neu.2005.22.1428. [DOI] [PubMed] [Google Scholar]
- 94.Johnson J.W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 95.Kleckner N.W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- 96.Mothet J.P., Parent A.T., Wolosker H., Brady R.O., Jr., Linden D.J., Ferris C.D., Rogawski M.A., Snyder S.H. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papouin T., Ladepeche L., Ruel J., Sacchi S., Labasque M., Hanini M., Groc L., Pollegioni L., Mothet J.P., Oliet S.H. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 98.Labrie V., Roder J.C. The involvement of the NMDA receptor D-serine/glycine site in the pathophysiology and treatment of schizophrenia. Neurosci. Biobehav. Rev. 2010;34:351–372. doi: 10.1016/j.neubiorev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Wolosker H., Radzishevsky I. The serine shuttle between glia and neurons: Implications for neurotransmission and neurodegeneration. Biochem. Soc. Trans. 2013;41:1546–1550. doi: 10.1042/BST20130220. [DOI] [PubMed] [Google Scholar]
- 100.Hashimoto A., Kumashiro S., Nishikawa T., Oka T., Takahashi K., Mito T., Takashima S., Doi N., Mizutani Y., Yamazaki T., et al. Embryonic development and postnatal changes in free D-aspartate and D-serine in the human prefrontal cortex. J. Neurochem. 1993;61:348–351. doi: 10.1111/j.1471-4159.1993.tb03575.x. [DOI] [PubMed] [Google Scholar]
- 101.Madeira C., Lourenco M.V., Vargas-Lopes C., Suemoto C.K., Brandao C.O., Reis T., Leite R.E., Laks J., Jacob-Filho W., Pasqualucci C.A., et al. d-serine levels in Alzheimer’s disease: Implications for novel biomarker development. Transl. Psychiatry. 2015;5:e561. doi: 10.1038/tp.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Biemans E.A., Verhoeven-Duif N.M., Gerrits J., Claassen J.A., Kuiperij H.B., Verbeek M.M. CSF d-serine concentrations are similar in Alzheimer’s disease, other dementias, and elderly controls. Neurobiol. Aging. 2016;42:213–216. doi: 10.1016/j.neurobiolaging.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 103.Nagata Y., Borghi M., Fisher G.H., D’Aniello A. Free D-serine concentration in normal and Alzheimer human brain. Brain Res. Bull. 1995;38:181–183. doi: 10.1016/0361-9230(95)00087-U. [DOI] [PubMed] [Google Scholar]
- 104.Lin C.H., Yang H.T., Lane H.Y. D-glutamate, D-serine, and D-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol. Biochem. Behav. 2019;185:172760. doi: 10.1016/j.pbb.2019.172760. [DOI] [PubMed] [Google Scholar]
- 105.Lin C.H., Yang H.T., Chiu C.C., Lane H.Y. Blood levels of D-amino acid oxidase vs. D-amino acids in reflecting cognitive aging. Sci. Rep. 2017;7:14849. doi: 10.1038/s41598-017-13951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arnsten A.F., Jin L.E. Molecular influences on working memory circuits in dorsolateral prefrontal cortex. Prog. Mol. Biol. Transl. Sci. 2014;122:211–231. doi: 10.1016/B978-0-12-420170-5.00008-8. [DOI] [PubMed] [Google Scholar]
- 107.Kummer M.P., Hammerschmidt T., Martinez A., Terwel D., Eichele G., Witten A., Figura S., Stoll M., Schwartz S., Pape H.C., et al. Ear2 deletion causes early memory and learning deficits in APP/PS1 mice. J. Neurosci. 2014;34:8845–8854. doi: 10.1523/JNEUROSCI.4027-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morilak D.A., Barrera G., Echevarria D.J., Garcia A.S., Hernandez A., Ma S., Petre C.O. Role of brain norepinephrine in the behavioral response to stress. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 109.Hu H., Real E., Takamiya K., Kang M.G., Ledoux J., Huganir R.L., Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 110.Thomas M.J., Moody T.D., Makhinson M., O’Dell T.J. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17:475–482. doi: 10.1016/S0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- 111.Tenorio G., Connor S.A., Guevremont D., Abraham W.C., Williams J., O’Dell T.J., Nguyen P.V. ‘Silent’ priming of translation-dependent LTP by ss-adrenergic receptors involves phosphorylation and recruitment of AMPA receptors. Learn. Mem. 2010;17:627–638. doi: 10.1101/lm.1974510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Joiner M.L., Lise M.F., Yuen E.Y., Kam A.Y., Zhang M., Hall D.D., Malik Z.A., Qian H., Chen Y., Ulrich J.D., et al. Assembly of a beta2-adrenergic receptor—GluR1 signalling complex for localized cAMP signalling. EMBO J. 2010;29:482–495. doi: 10.1038/emboj.2009.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Luo Y., Zhou J., Li M.X., Wu P.F., Hu Z.L., Ni L., Jin Y., Chen J.G., Wang F. Reversal of aging-related emotional memory deficits by norepinephrine via regulating the stability of surface AMPA receptors. Aging Cell. 2015;14:170–179. doi: 10.1111/acel.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kobayashi M. Differential regulation of synaptic transmission by adrenergic agonists via protein kinase A and protein kinase C in layer V pyramidal neurons of rat cerebral cortex. Neuroscience. 2007;146:1772–1784. doi: 10.1016/j.neuroscience.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 115.Kobayashi M., Kojima M., Koyanagi Y., Adachi K., Imamura K., Koshikawa N. Presynaptic and postsynaptic modulation of glutamatergic synaptic transmission by activation of alpha(1)- and beta-adrenoceptors in layer V pyramidal neurons of rat cerebral cortex. Synapse. 2009;63:269–281. doi: 10.1002/syn.20604. [DOI] [PubMed] [Google Scholar]
- 116.Liu W., Yuen E.Y., Allen P.B., Feng J., Greengard P., Yan Z. Adrenergic modulation of NMDA receptors in prefrontal cortex is differentially regulated by RGS proteins and spinophilin. Proc. Natl. Acad. Sci. USA. 2006;103:18338–18343. doi: 10.1073/pnas.0604560103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jimenez-Rivera C.A., Figueroa J., Vazquez-Torres R., Velez-Hernandez M.E., Schwarz D., Velasquez-Martinez M.C., Arencibia-Albite F. Presynaptic inhibition of glutamate transmission by alpha2 receptors in the VTA. Eur. J. Neurosci. 2012;35:1406–1415. doi: 10.1111/j.1460-9568.2012.08029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Velasquez-Martinez M.C., Vazquez-Torres R., Jimenez-Rivera C.A. Activation of alpha1-adrenoceptors enhances glutamate release onto ventral tegmental area dopamine cells. Neuroscience. 2012;216:18–30. doi: 10.1016/j.neuroscience.2012.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lur G., Higley M.J. Glutamate Receptor Modulation Is Restricted to Synaptic Microdomains. Cell Rep. 2015;12:326–334. doi: 10.1016/j.celrep.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patriarchi T., Buonarati O.R., Hell J.W. Postsynaptic localization and regulation of AMPA receptors and Cav1.2 by beta2 adrenergic receptor/PKA and Ca(2+)/CaMKII signaling. EMBO J. 2018;37 doi: 10.15252/embj.201899771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang Y., Paspalas C.D., Jin L.E., Picciotto M.R., Arnsten A.F., Wang M. Nicotinic alpha7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2013;110:12078–12083. doi: 10.1073/pnas.1307849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Markram H., Segal M. Acetylcholine potentiates responses to N-methyl-D-aspartate in the rat hippocampus. Neurosci. Lett. 1990;113:62–65. doi: 10.1016/0304-3940(90)90495-U. [DOI] [PubMed] [Google Scholar]
- 123.Markram H., Segal M. The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J. Physiol. 1992;447:513–533. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Navakkode S., Korte M. Cooperation between cholinergic and glutamatergic receptors are essential to induce BDNF-dependent long-lasting memory storage. Hippocampus. 2012;22:335–346. doi: 10.1002/hipo.20902. [DOI] [PubMed] [Google Scholar]
- 125.Flores-Hernandez J., Salgado H., De La Rosa V., Avila-Ruiz T., Torres-Ramirez O., Lopez-Lopez G., Atzori M. Cholinergic direct inhibition of N-methyl-D aspartate receptor-mediated currents in the rat neocortex. Synapse. 2009;63:308–318. doi: 10.1002/syn.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shen H., Kihara T., Hongo H., Wu X., Kem W.R., Shimohama S., Akaike A., Niidome T., Sugimoto H. Neuroprotection by donepezil against glutamate excitotoxicity involves stimulation of alpha7 nicotinic receptors and internalization of NMDA receptors. Br. J. Pharmacol. 2010;161:127–139. doi: 10.1111/j.1476-5381.2010.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen G., Chen P., Tan H., Ma D., Dou F., Feng J., Yan Z. Regulation of the NMDA receptor-mediated synaptic response by acetylcholinesterase inhibitors and its impairment in an animal model of Alzheimer’s disease. Neurobiol. Aging. 2008;29:1795–1804. doi: 10.1016/j.neurobiolaging.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tang B., Luo D., Yang J., Xu X.Y., Zhu B.L., Wang X.F., Yan Z., Chen G.J. Modulation of AMPA receptor mediated current by nicotinic acetylcholine receptor in layer I neurons of rat prefrontal cortex. Sci. Rep. 2015;5:14099. doi: 10.1038/srep14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X., Lippi G., Carlson D.M., Berg D.K. Activation of alpha7-containing nicotinic receptors on astrocytes triggers AMPA receptor recruitment to glutamatergic synapses. J. Neurochem. 2013;127:632–643. doi: 10.1111/jnc.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martineau M., Parpura V., Mothet J.P. Cell-type specific mechanisms of D-serine uptake and release in the brain. Front. Synaptic Neurosci. 2014;6:12. doi: 10.3389/fnsyn.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wolosker H., Blackshaw S., Snyder S.H. Serine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc. Natl. Acad. Sci. USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singh N.S., Paul R.K., Ramamoorthy A., Torjman M.C., Moaddel R., Bernier M., Wainer I.W. Nicotinic acetylcholine receptor antagonists alter the function and expression of serine racemase in PC-12 and 1321N1 cells. Cell. Signal. 2013;25:2634–2645. doi: 10.1016/j.cellsig.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin H., Hsu F.C., Baumann B.H., Coulter D.A., Lynch D.R. Cortical synaptic NMDA receptor deficits in alpha7 nicotinic acetylcholine receptor gene deletion models: Implications for neuropsychiatric diseases. Neurobiol. Dis. 2014;63:129–140. doi: 10.1016/j.nbd.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takata N., Mishima T., Hisatsune C., Nagai T., Ebisui E., Mikoshiba K., Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Billard J.M. D-Serine in the aging hippocampus. J. Pharm. Biomed. Anal. 2015;116:18–24. doi: 10.1016/j.jpba.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 136.Rao T.S., Kim H.S., Lehmann J., Martin L.L., Wood P.L. Differential effects of phencyclidine (PCP) and ketamine on mesocortical and mesostriatal dopamine release in vivo. Life Sci. 1989;45:1065–1072. doi: 10.1016/0024-3205(89)90163-X. [DOI] [PubMed] [Google Scholar]
- 137.Irifune M., Sato T., Kamata Y., Nishikawa T., Nomoto M., Fukuda T., Kawahara M. Inhibition by diazepam of ketamine-induced hyperlocomotion and dopamine turnover in mice. Can. J. Anaesth. 1998;45:471–478. doi: 10.1007/BF03012584. [DOI] [PubMed] [Google Scholar]
- 138.Wedzony K., Klimek V., Golembiowska K. MK-801 elevates the extracellular concentration of dopamine in the rat prefrontal cortex and increases the density of striatal dopamine D1 receptors. Brain Res. 1993;622:325–329. doi: 10.1016/0006-8993(93)90839-F. [DOI] [PubMed] [Google Scholar]
- 139.Corbett R., Camacho F., Woods A.T., Kerman L.L., Fishkin R.J., Brooks K., Dunn R.W. Antipsychotic agents antagonize non-competitive N-methyl-D-aspartate antagonist-induced behaviors. Psychopharmacology. 1995;120:67–74. doi: 10.1007/BF02246146. [DOI] [PubMed] [Google Scholar]
- 140.Lapin I.P., Rogawski M.A. Effects of D1 and D2 dopamine receptor antagonists and catecholamine depleting agents on the locomotor stimulation induced by dizocilpine in mice. Behav. Brain Res. 1995;70:145–151. doi: 10.1016/0166-4328(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 141.Verma A., Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: Modulation by dopamine. J. Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xiu Y., Kong X.R., Zhang L., Qiu X., Chao F.L., Peng C., Gao Y., Huang C.X., Wang S.R., Tang Y. White matter injuries induced by MK-801 in a mouse model of schizophrenia based on NMDA antagonism. Anat. Record. 2014;297:1498–1507. doi: 10.1002/ar.22942. [DOI] [PubMed] [Google Scholar]
- 143.Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fujita Y., Ishima T., Hashimoto K. Supplementation with D-serine prevents the onset of cognitive deficits in adult offspring after maternal immune activation. Sci. Rep. 2016;6:37261. doi: 10.1038/srep37261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Avellar M., Scoriels L., Madeira C., Vargas-Lopes C., Marques P., Dantas C., Manhaes A.C., Leite H., Panizzutti R. The effect of D-serine administration on cognition and mood in older adults. Oncotarget. 2016;7:11881–11888. doi: 10.18632/oncotarget.7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tsai G., Yang P., Chung L.C., Lange N., Coyle J.T. D-serine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry. 1998;44:1081–1089. doi: 10.1016/S0006-3223(98)00279-0. [DOI] [PubMed] [Google Scholar]
- 147.Heresco-Levy U., Javitt D.C., Ebstein R., Vass A., Lichtenberg P., Bar G., Catinari S., Ermilov M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol. Psychiatry. 2005;57:577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 148.Weiser M., Heresco-Levy U., Davidson M., Javitt D.C., Werbeloff N., Gershon A.A., Abramovich Y., Amital D., Doron A., Konas S., et al. A multicenter, add-on randomized controlled trial of low-dose d-serine for negative and cognitive symptoms of schizophrenia. J. Clin. Psychiatry. 2012;73:e728–e734. doi: 10.4088/JCP.11m07031. [DOI] [PubMed] [Google Scholar]
- 149.Chessell I.P., Procter A.W., Francis P.T., Bowen D.M. D-cycloserine, a putative cognitive enhancer, facilitates activation of the N-methyl-D-aspartate receptor-ionophore complex in Alzheimer brain. Brain Res. 1991;565:345–348. doi: 10.1016/0006-8993(91)91668-Q. [DOI] [PubMed] [Google Scholar]
- 150.Pitkanen M., Sirvio J., MacDonald E., Ekonsalo T., Riekkinen P., Sr. The effects of d-cycloserine, a partial agonist at the glycine binding site, on spatial learning and working memory in scopolamine-treated rats. J. Neural. Transm. Parkinson’s Dis. Dement. Sect. 1995;9:133–144. doi: 10.1007/BF02259655. [DOI] [PubMed] [Google Scholar]
- 151.Jones R.W., Wesnes K.A., Kirby J. Effects of NMDA modulation in scopolamine dementia. Ann. N. Y. Acad. Sci. 1991;640:241–244. doi: 10.1111/j.1749-6632.1991.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 152.Tsai G.E., Falk W.E., Gunther J., Coyle J.T. Improved cognition in Alzheimer’s disease with short-term D-cycloserine treatment. Am. J. Psychiatry. 1999;156:467–469. doi: 10.1176/ajp.156.3.467. [DOI] [PubMed] [Google Scholar]
- 153.Schwartz B.L., Hashtroudi S., Herting R.L., Schwartz P., Deutsch S.I. d-Cycloserine enhances implicit memory in Alzheimer patients. Neurology. 1996;46:420–424. doi: 10.1212/WNL.46.2.420. [DOI] [PubMed] [Google Scholar]
- 154.Randolph C., Roberts J.W., Tierney M.C., Bravi D., Mouradian M.M., Chase T.N. D-cycloserine treatment of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1994;8:198–205. doi: 10.1097/00002093-199408030-00006. [DOI] [PubMed] [Google Scholar]
- 155.Tsai G.E., Falk W.E., Gunther J. A preliminary study of D-cycloserine treatment in Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci. 1998;10:224–226. doi: 10.1176/jnp.10.2.224. [DOI] [PubMed] [Google Scholar]
- 156.Fakouhi T.D., Jhee S.S., Sramek J.J., Benes C., Schwartz P., Hantsburger G., Herting R., Swabb E.A., Cutler N.R. Evaluation of cycloserine in the treatment of Alzheimer’s disease. J. Geriatr. Psychiatry Neurol. 1995;8:226–230. doi: 10.1177/089198879500800405. [DOI] [PubMed] [Google Scholar]
- 157.Laake K., Oeksengaard A.R. D-cycloserine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2002:CD003153. doi: 10.1002/14651858.CD003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schade S., Paulus W. D-Cycloserine in Neuropsychiatric Diseases: A Systematic Review. Int. J. Neuropsychopharmacol. 2016;19 doi: 10.1093/ijnp/pyv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Harada K., Nakato K., Yarimizu J., Yamazaki M., Morita M., Takahashi S., Aota M., Saita K., Doihara H., Sato Y., et al. A novel glycine transporter-1 (GlyT1) inhibitor, ASP2535 (4-[3-isopropyl-5-(6-phenyl-3-pyridyl)-4H-1,2,4-triazol-4-yl]-2,1,3-benzoxadiazol e), improves cognition in animal models of cognitive impairment in schizophrenia and Alzheimer’s disease. Eur. J. Pharmacol. 2012;685:59–69. doi: 10.1016/j.ejphar.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 160.Chaki S., Shimazaki T., Karasawa J., Aoki T., Kaku A., Iijima M., Kambe D., Yamamoto S., Kawakita Y., Shibata T., et al. Efficacy of a glycine transporter 1 inhibitor TASP0315003 in animal models of cognitive dysfunction and negative symptoms of schizophrenia. Psychopharmacology. 2015;232:2849–2861. doi: 10.1007/s00213-015-3920-3. [DOI] [PubMed] [Google Scholar]
- 161.Boehringer Ingelheim 2 June 2016–6 November 2020. BI 425809 in Patients with Cognitive Impairment Due to Alzheimer’s Disease. Identifier NCT02788513. [(accessed on 31 January 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02788513.
- 162.Adage T., Trillat A.C., Quattropani A., Perrin D., Cavarec L., Shaw J., Guerassimenko O., Giachetti C., Greco B., Chumakov I., et al. In vitro and in vivo pharmacological profile of AS057278, a selective d-amino acid oxidase inhibitor with potential anti-psychotic properties. Eur. Neuropsychopharmacol. 2008;18:200–214. doi: 10.1016/j.euroneuro.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 163.Nagata Y. Involvement of D-amino acid oxidase in elimination of D-serine in mouse brain. Experientia. 1992;48:753–755. doi: 10.1007/BF02124295. [DOI] [PubMed] [Google Scholar]
- 164.Howley E., Bestwick M., Fradley R., Harrison H., Leveridge M., Okada K., Fieldhouse C., Farnaby W., Canning H., Sykes A.P., et al. Assessment of the Target Engagement and D-Serine Biomarker Profiles of the D-Amino Acid Oxidase Inhibitors Sodium Benzoate and PGM030756. Neurochem. Res. 2017;42:3279–3288. doi: 10.1007/s11064-017-2367-9. [DOI] [PubMed] [Google Scholar]
- 165.Duplantier A.J., Becker S.L., Bohanon M.J., Borzilleri K.A., Chrunyk B.A., Downs J.T., Hu L.Y., El-Kattan A., James L.C., Liu S., et al. Discovery, SAR, and pharmacokinetics of a novel 3-hydroxyquinolin-2(1H)-one series of potent D-amino acid oxidase (DAAO) inhibitors. J. Med. Chem. 2009;52:3576–3585. doi: 10.1021/jm900128w. [DOI] [PubMed] [Google Scholar]
- 166.Ferraris D.V., Tsukamoto T. Recent advances in the discovery of D-amino acid oxidase inhibitors and their therapeutic utility in schizophrenia. Curr. Pharm. Des. 2011;17:103–111. doi: 10.2174/138161211795049633. [DOI] [PubMed] [Google Scholar]
- 167.Lin C.H., Chen P.K., Chang Y.C., Chuo L.J., Chen Y.S., Tsai G.E., Lane H.Y. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: A randomized, double-blind, placebo-controlled trial. Biol. Psychiatry. 2014;75:678–685. doi: 10.1016/j.biopsych.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 168.Lin C.H., Chen P.K., Wang S.H., Lane H.Y. Sodium benzoate for the treatment of behavioral and psychological symptoms of dementia (BPSD): A randomized, double-blind, placebo-controlled, 6-week trial. J. Psychopharmacol. 2019;33:1030–1033. doi: 10.1177/0269881119849815. [DOI] [PubMed] [Google Scholar]
- 169.Lin C.H., Yang H.T., Chen P.K., Wang S.H., Lane H.Y. Precision Medicine of Sodium Benzoate for the Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD) Neuropsychiatr. Dis. Treat. 2020;16:509–518. doi: 10.2147/NDT.S234371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Modi K.K., Roy A., Brahmachari S., Rangasamy S.B., Pahan K. Cinnamon and Its Metabolite Sodium Benzoate Attenuate the Activation of p21rac and Protect Memory and Learning in an Animal Model of Alzheimer’s Disease. PLoS ONE. 2015;10:e0130398. doi: 10.1371/journal.pone.0130398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lane H.Y., Tu C.H., Lin W.C., Lin C.H. Brain activity of benzoate, a D-amino acid oxidase inhibitor, in patients with mild cognitive impairment in a randomized, double-blind, placebo controlled clinical trial. Int. J. Neuropsychopharmacol. 2021 doi: 10.1093/ijnp/pyab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nair B. Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int. J. Toxicol. 2001;20(Suppl. S3):23–50. doi: 10.1080/10915810152630729. [DOI] [PubMed] [Google Scholar]