Abstract
Exposure to PM2.5 has been associated with the prevalence of obesity. In the Greater Mexico City Area (GMCA), both are ranked among the highest in the world. Our aim was to analyze this association in children, adolescents, and adults in the GMCA. We used data from the 2006 and 2012 Mexican National Surveys of Health and Nutrition (ENSANUT). Participants’ past-year exposure to ambient PM2.5 was assessed using land use terms and satellite-derived aerosol optical depth estimates; weight and height were measured. We used survey-adjusted logistic regression models to estimate the odds ratios (ORs) of obesity (vs. normal-overweight) for every 10 µg/m3 increase in annual PM2.5 exposure for children, adolescents, and adults. Using a meta-analysis approach, we estimated the overall odds of obesity. We analyzed data representing 19.3 million and 20.9 million GMCA individuals from ENSANUT 2006 and 2012, respectively. The overall pooled estimate between PM2.5 exposure and obesity was OR = 1.96 (95% CI: 1.21, 3.18). For adolescents, a 10 µg/m3 increase in PM2.5 was associated with an OR of 3.53 (95% CI: 1.45, 8.58) and 3.79 (95% CI: 1.40, 10.24) in 2006 and 2012, respectively. More studies such as this are recommended in Latin American cities with similar air pollution and obesity conditions.
Keywords: PM2.5 exposure, obesity, Mexico, Latin America
1. Introduction
Expanding the epidemiologic evidence linking PM2.5 exposure to adverse health outcomes is key for advancing public health policies and regulations to improve air quality and preventing disease worldwide, such as the obesity epidemic. Between 1975 and 2014, the prevalence of obesity in adults worldwide increased from 3.2% (2.4–4.1) to 10.8% (9.7–12.0) for men, and from 6.4% (5.1–7.8) to 14.9% (13.6–16.1) in women [1]. Once considered a high-income country problem, we now know that obesity affects low- and middle-income countries equally [2], and it is becoming more prevalent at younger ages. According to the Mexican National Survey of Health and Nutrition (ENSANUT), in 2018, the combined prevalence of overweight and obesity in children (5 to 11 years-old), adolescents (12 to 19 years-old), and adults (≥20 years-old) were 35.6%, 38.4%, and 75.2%, respectively [3].
The Greater Mexico City Area (GMCA), one of the largest and most populated cities in Latin America, is home to over 21 million people, where PM2.5 concentrations frequently exceed the WHO air quality guidelines for short-term (24-h standard average, GMCA = 96 µg/m3 vs. WHO < 25 µg/m3) and chronic (annual average GMCA = 21 µg/m3 vs. WHO =10 µg/m3) exposure [4], and where the combined prevalence for overweight and obesity mimics the national prevalence for children, adolescents, and adults [5]. Existing studies on air pollution (not specifically for PM2.5) have diverse findings, suggesting further research in developing countries and heterogenous populations [6].
One of the proposed mechanisms linking PM2.5 exposure to obesity involves Toll-like receptors in the lung, which through inflammation and lipid oxidation can lead to metabolic dysfunction and weight gain [7]. A recent study in mice has shown that chronic hypothalamic inflammation, regulated by signaling of these Toll-like receptors, can lead to leptin resistance, hyperphagia, and decreased energy expenditure [8]. This is line with results from other animal studies that have shown that early life exposure to PM2.5 leads to the development of insulin resistance, adiposity, and inflammation [9]. Prenatal exposure to PM2.5 has also been linked to increased insulin levels and glucose intolerance [10]. Moreover, effects can be sex-specific [11]. Exposure during animal adulthood has been found to induce obesity, in part because of less energy expenditure (due to hypothalamic leptin resistance) [10]. Nevertheless, results from human studies on the association between PM2.5 exposure and obesity remain mixed [12]. Some have found null associations [13,14], while several others point to an increased risk of obesity from PM2.5 exposure at different life stages [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Therefore, the aim of this study was to analyze the association between past-year exposure to PM2.5 with the prevalence of obesity in two representative samples (ENSANUT 2006 and 2012) of children, adolescents, and adults from the GMCA.
2. Materials and Methods
2.1. Study Population
We used data from the 2006 and 2012 ENSANUT surveys that use a multi-stage and stratified clustered sampling procedure at the household level and are designed to be nationally and state-representative [32]. Both 2006 and 2012 surveys included overweight and obesity data for children (2–9 years-old), adolescents (10–19 years-old), and adults (≥20 years-old).
Our study used sample weights to ensure the sample representativeness for the 16 municipalities of Mexico City and 58 municipalities from the State of Mexico that comprise the GMCA. The Ethics Committee of the Mexican National Institute of Public Health (INSP) reviewed and approved the written consent forms employed in ENSANUT 2006 and 2012, as well as our research protocol.
2.2. PM2.5 Exposure Assessment
Geolocation of households was estimated at a block level; no further detail was pursued in order to maintain participant’s confidentiality. We used daily PM2.5 estimates from a hybrid spatio-temporal model developed by colleagues in our research team that has been previously described in more detail [33]. Briefly, the model estimates daily PM2.5 concentrations with a spatial resolution of 1 × 1 km2 for the entire GMCA by calibrating the association between PM2.5 measurements from ground monitors to satellite-derived aerosol optical depth measurements from the United States’ National Aeronautics and Space Administration (NASA), including land use terms and meteorological features specific to the GMCA. Daily exposure for each household was calculated from the 1 × 1 km2 PM2.5 cells, matching each household’s block area. We calculated the average past-year PM2.5 exposure according to the date the survey was administered.
2.3. Anthropometric Assessment
All participants were weighed twice (and averaged) using calibrated portable electronic scales (Tanita, Model 1583, Tokyo, Japan) recording the measurement to the nearest 10 g. Height was measured twice (and averaged) in orthostatic position using a stadiometer (Dynatop E1, Mexico City, Mexico) with a precision of 1 mm. Both anthropometric measures were performed by trained staff using standardized methods. We used these measurements to calculate body mass index (BMI) as the ratio of weight to height squared (Kg/m2). To determine participants’ overweight (25 ≤ BMI < 30) and obesity (BMI ≥ 30) status in adults, we followed the cut-off points in the WHO guidelines, and for children and adolescents, we followed age-specific guidelines from the Mexican Social Security Institute (IMSS) that follow age-specific WHO guidelines.
2.4. Covariates
Information on sex, age, smoking status (adolescents and adults), second-hand smoke (children), and socioeconomic status (SES) was collected by questionnaire during the survey home visit. SES was calculated using information on income levels and demographic and socioeconomic characteristics of Mexican households on the basis of the National Survey of Household Income and Expenditure [34]. For the adolescent and adult surveys, there was no information on second-hand smoking available. We considered non-smoking for individuals reporting never smoking vs. individuals that reported any smoking. For children, spending money on tobacco was used as a proxy for second-hand smoke. The same questions were asked in the 2006 and 2012 surveys. The field staff was trained and standardized previous to field work by a team of researchers from the National Institute of Public Health (INSP).
2.5. Statistical Analysis
We used Stata V.14 “svy” package for survey analysis (Stata Corp, College Station, TX, USA) to fit logistic regression models incorporating the sample design in the variance estimates. We estimated the association between the average past-year PM2.5 exposure and obesity prevalence and calculated Taylor linearized standard errors. Models were adjusted by sex, age in quintiles, SES, and smoking status. For this first analysis, we collapsed normal and overweight into one category and used it as the reference to compare with obesity. For each age group (i.e., children, adolescents, and adults), we performed separate models for each survey year (i.e., 2006, 2012). PM2.5 exposure estimates were included as continuous variables in our main analysis. In separate models, we included an interaction term with sex and then stratified by sex. We present adjusted odds ratios (ORs) for an increase of 10 μg/m3 in PM2.5. We also generated a multinomial regression model to evaluate overweight and obesity simultaneously compared to normal weight subjects and obtained relative risk ratios (RRR). To assess the non-linearity of the associations, we used quartiles of PM2.5 with a logistic model comparing normal and overweight to obesity. Lastly, we used a meta-analysis, random effect approach to estimate the overall association between PM2.5, and obesity (i.e., obesity vs. normal/overweight) using the combined results from all age groups from the 2006 and 2012 analyses and by age group.
Additionally, in order to exclude the possible measurement error due to smoking or second-hand smoke, we conducted the main analysis considering non-smoker adolescents and adults and children with no house-hold tobacco spending.
3. Results
A total of 4368 and 4521 persons participated in the ENSANUT 2006 and 2012 surveys representing 19.4 and 21 million inhabitants of the GMCA, respectively. Sex ratios were approximately 1:1. The mean age (±SD) of children, adolescents, and adults were similar in both survey years. While the prevalence of normal weight was similar between surveys, we saw a decrease in overweight and an increase in obesity prevalence for all age groups in the 2012 sample. The average past-year PM2.5 concentrations showed a slight decrease from 2006 to 2012 (Table 1).
Table 1.
Descriptive statistics in population samples for Mexican National Survey of Health and Nutrition (ENSANUT) 2006 and 2012 from the Greater Mexico City Area.
| Ensanut 2006 | Ensanut 2012 | |||||
|---|---|---|---|---|---|---|
| Variable | Children (0–9 Years) |
Adolescents (10–19 Years) |
Adults (≥20 Years) |
Children (0–9 Years) |
Adolescents (10–19 Years) |
Adults (≥20 Years) |
| Sample size (n) | 1005 | 1082 | 2281 | 1233 | 986 | 2302 |
| Sample representativeness (N) | 31,00,923 | 3,565,877 | 12,695,627 | 3,552,214 | 3,636,732 | 13,760,947 |
| Sex | ||||||
| Male (%) | 50.3 | 55.3 | 44.9 | 51 | 49.1 | 45.9 |
| Female (%) | 49.7 | 44.7 | 55.1 | 49 | 50.9 | 54.1 |
| Age mean (years) a | 4.7 ± 2.9 | 14.4 ± 2.9 | 42.9 ± 16.4 | 4.6 ± 2.7 | 14.3 ± 2.9 | 44.9 ± 16.3 |
| SES b | −0.16 ± 0.05 | −0.03 ± 0.06 | −0.16 ± 0.06 | 0.05 ± 0.10 | 0.03 ± 0.082 | −0.12 ± 0.05 |
| BMI | ||||||
| Normal (%) | 58.45% | 58.01% | 27.81% | 59.02% | 56.48% | 26.12% |
| Overweight (%) | 20.05% | 25.18% | 41.45% | 17.42% | 22.98% | 37.34% |
| Obesity (%) | 21.49% | 16.81% | 30.73% | 23.55% | 20.53% | 35.94% |
| Average past year PM2.5 (µg/m3) | 25.9 (2.4) | 24.8 (1.5) | ||||
a Age in years: mean ± SD. b SES: zero-centered mean ± SD.
Overall, exposure to PM2.5 was associated with higher odds of obesity in each survey and all age groups (Table 2), with stronger results for adolescents. For every 10 μg/m3 increase in PM2.5, the odds of obesity were 3.53 (95% CI: 1.45, 8.58) in 2006 and 3.79 (95% CI: 1.40, 10.24) in 2012. The results for the 2006 survey for children and adults also showed increased odds for obesity OR = 1.19 (95% CI: 0.47, 3.08) and OR = 1.01 (95% CI: 0.59, 1.73), respectively, and the results for children and adults in the 2012 survey showed increased odds of 1.98 (95% CI: 0.92, 4.22) and 2.73 (95% CI: 0.97, 7.71), respectively.
Table 2.
Association between past year PM2.5 (10 μg/m3 increase) with obesity in children, adolescents, and adults from the Greater Mexico City Area using data from the 2006 National Nutrition and Health Survey, ENSANUT 2006.
| ENSANUT 2006 | Children | Adolescents | Adults | |||
|---|---|---|---|---|---|---|
| n = 618 | n = 801 | n = 1559 | ||||
| n = 2,529,289 | n = 3,548,352 | n = 12,541,729 | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Logistic model * | ||||||
| Obesity | 1.19 | (0.47, 3.08) | 3.53 | (1.45, 8.58) | 1.01 | (0.59, 1.73) |
| Logistic model stratified by sex ** | ||||||
| Male | 0.57 | (0.17, 1.95) | 4.19 | (1.24, 14.19) | 1.09 | (0.40, 2.91) |
| Female | 2.7 | (0.71, 10.21) | 2.64 | (0.74, 9.44) | 0.94 | (0.49, 1.79) |
| p interaction | 0.07 | 0.53 | 0.82 | |||
| Multinomial model (RRR) * | ||||||
| Overweight | 1.57 | (0.54, 4.56) | 1.36 | (0.68, 2.73) | 0.82 | (0.41, 1.62) |
| Obesity | 1.43 | (0.53, 3.84) | 3.89 | (1.51, 10.03) | 0.89 | (0.43, 1.85) |
* Adjusted for age in quintiles, sex, socioeconomic status (SES), and smoking status. ** Adjusted for age in quintiles, SES, and smoking status. Statistically significant results (p <0.05) are highlighted in bold letters.
Only the results for the 2006 survey in children showed an interaction term between PM2.5 and sex (p for interaction = 0.07). There was no consistency in the models stratified by sex—while in the 2006 results, the effect of PM2.5 on obesity was stronger in male adolescents than in females (ORmale = 4.19 (95% CI 1.24, 14.19)), it was stronger for females in 2012 (ORfemale = 7.83 (95% CI 0.96, 64.12)). We found similar inconsistencies by sex in children and adults between surveys.
The results for adolescents in the multinomial models were in line with the logistic models showing a RRRoverweight of 1.36 (95% CI 0.68, 2.73) and RRRobesity of 3.89 (95% CI 1.51, 10.03) compared to normal weight in 2006 and a RRRoverweight of 2.34 (95% CI 0.83, 6.65) and RRRobesity of 4.82 (95% CI 2.16, 10.76) compared to normal weight in 2012 (Table 2 and Table 3). For children, the RRR for obesity were also higher than for overweight compared to normal weight in both surveys. For adults, the 2012 results showed a higher RRR for obesity than for overweight compared to normal weight.
Table 3.
Association between past year PM2.5 (10 μg/m3 increase) with obesity in children, adolescents, and adults from the Greater Mexico City Area using data from the 2012 National Nutrition and Health Survey, ENSANUT-2012.
| ENSANUT 2012 | Children | Adolescents | Adults | |||
|---|---|---|---|---|---|---|
| n = 752 | n = 718 | n = 1538 | ||||
| n = 2,769,354 | n = 3,245,296 | n = 11,415,512 | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Logistic model * | ||||||
| Obesity | 1.98 | (0.92, 4.22) | 3.79 | (1.40, 10.24) | 2.73 | (0.97, 7.71) |
| Logistic models stratified by sex ** | ||||||
| Male | 2.29 | (0.69, 7.55) | 2.61 | (0.41, 16.73) | 2.56 | (0.59, 11.20) |
| Female | 1.29 | (0.79, 2.10) | 7.83 | (0.96, 64.12) | 2.88 | (0.83, 10.04) |
| p interaction | 0.44 | 0.44 | 0.85 | |||
| Multinomial model (RRR) * | ||||||
| Overweight | 0.84 | (0.02, 42.96) | 2.34 | (0.83, 6.65) | 0.39 | (0.13, 1.18) |
| Obesity | 1.69 | (0.63, 4.59) | 4.82 | (2.16, 10.76) | 1.54 | (0.46, 5.14) |
* Adjusted for age in quintiles, sex, SES, and smoking status. ** Adjusted for age in quintiles, SES, and smoking status. Statistically significant results (p <0.05) are highlighted in bold letters.
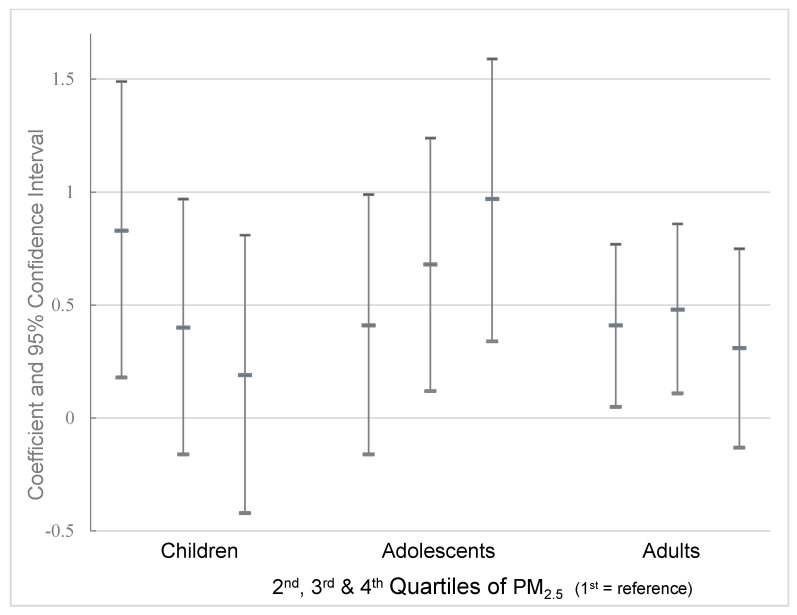
Associations from the models using PM2.5 in quartiles (with the first quartile of PM2.5 as a reference) are shown for the 2012 survey in Figure 1. Estimates of the model for adolescents showed an increasing dose–response relation as follows: second quartile ß = 0.41 (95% CI: −0.16, 0.99), third quartile ß = 0.68 (95% CI: 0.12, 1.24), and fourth quartile ß = 0.97 (95% CI: 0.34, 1.59). In children, an inverse relation was observed. Estimates were ß = 0.83 (95% CI: 0.18, 1.49) for the second quartile, ß = 0.40 (95% CI: −0.16, 0.97) for the third quartile, and ß = 0.19 (95% CI: −0.42, 0.81) for the fourth quartile (first quartile of PM2.5 as a reference). For adults, the estimates showed an increasing relation for the second and third quartiles at ß = 0.41 (95% CI: 0.05–0.77) and ß = 0.48 (95% CI: 0.11–0.86), respectively, but this did not hold for the fourth quartile at ß = 0.31 (95% CI: −0.13, 0.75) (first quartile of PM2.5 as reference).
Figure 1.
Associations between quartiles of PM2.5 (annual mean) and obesity in three age groups, ENSANUT 2012.
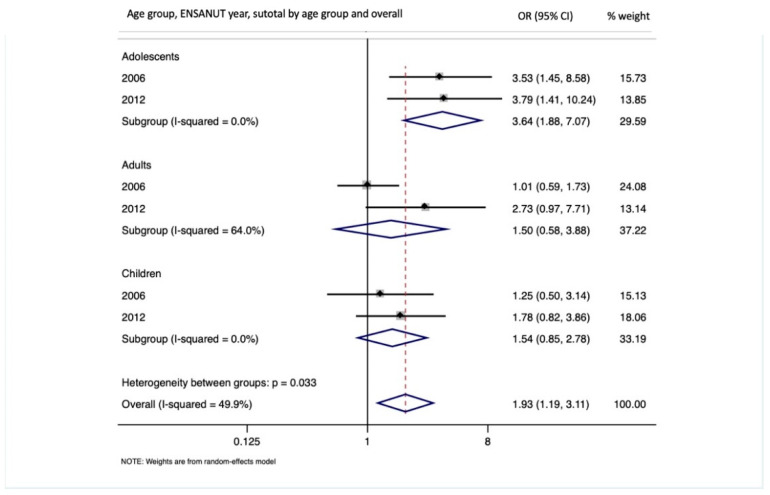
Figure 2 shows the combined results of the meta-analysis with an overall OR of 1.96 (95% CI: 1.21, 3.18) for the association between PM2.5 exposure and obesity. When analyzing by age group, we found that the combined OR for adolescents was 3.64 (95% CI: 1.88, 7.06), OR = 1.50 (95% CI: 0.58, 3.88) for adults, and OR = 1.62 (95% CI: 0.90, 2.93) for children. Most of the heterogeneity in the combined estimate was attributable to adults between the two surveys.
Figure 2.
Odds ratios for obesity and PM2.5 exposure (per 10 µg/m3 increase) using a meta-analysis approach, by age group and for the overall study.
In the analyses considering non-smokers (adults and adolescents) and no second-hand smoke (children), the odds for obesity in children were lower for both study years, whereas for adolescents and adults, the odds increased in comparison to the model with all participants. The meta-analysis showed an overall increase in the odds of the association, OR = 2.27 (95% CI: 1.08, 4.75) (data not shown).
4. Discussion
Our study analyzed the association between average past-year satellite-derived exposure to PM2.5 and the prevalence of obesity in of children, adolescents, and adults at two time points. This approach is different from other studies that have focused on a single age group or a specific population sample; furthermore, this is the first study with a representative population in one of the largest and most populated cities in Latin America. Overall, we observed an almost twofold increase in the odds of obesity (ORpooled = 1.96 (95% CI: 1.21, 3.18)) for each 10 µg/m3 of PM2.5. However, the association was strongest for adolescents, in line with other studies that have found, that exposure to high levels of traffic density was associated with higher attained BMI over an eight-year follow-up, and that traffic related air pollution had a stronger association compared to traffic density [25,28]. There is also evidence of obesity exacerbating the effects of air pollution on cardiometabolic disease markers [35]. A study in non-diabetic Indonesian adolescents found an association of long-term PM2.5 exposure and an increase in fasting plasma glucose levels [26], adding to the knowledge of possible mechanisms related to the association we observed in this age group. Although the results for children in the 2012 survey were marginally significant, those for 2006 were not. Nevertheless, both showed increased odds of obesity, similar to other cross-sectional studies in China and Spain that have found increased risk of obesity with PM10 and PM2.5, respectively [18,19]. Longitudinal studies in children that have assessed air pollution exposure since early stages (some including prenatal exposures) have mixed findings, with positive [23], null [13,36], and inverse associations [15]. Furthermore, the association for prenatal exposure to PM2.5 and childhood obesity seems to be stronger for those born to obese mothers [31]. Our study was unable to account for earlier life or prenatal PM2.5 exposure, but assuming there was no strong variability of PM2.5 between 2006 and 2012, our results suggest that there might be an increased risk for obesity as the population transitions from childhood to adolescence. Then, in the transition from adolescence to adulthood, unmeasured confounders (such as diet and physical activity) might be driving the apparent reduction in risk we observed.
Our results for adults were inconsistent from 2006 to 2012, although evidence has also pointed at an increased risk of obesity in this age group [22]. Many adult studies [27,37] and reviews [38,39] have focused on the association of PM2.5 exposure and increased susceptibility to cardiovascular diseases and type 2 diabetes in adults with obesity. PM2.5 might be associated more directly with metabolic disorders in adults, rather than with obesity directly through oxidative stress and inflammation [37]. We found no consistent evidence of an interaction between PM2.5 and sex in any age group or across the time points, which is different to other studies that have found that women have a higher risk of obesity associated with exposure to intensive traffic [20].
We were able to include SES in our study, for which there is extensive research as an important predictor of both obesity [40,41] as well as of PM 2.5 exposure [42,43], therefore possibly confounding the association. In our study, adjusted models for each age group and survey year confirmed the association between lower SES and higher PM2.5 as well as increased SES and lower odds of obesity. The effect estimates of our models reflected an important change when adjusting for SES. For all age groups and years, increased SES was associated with reduced odds of obesity (reaching statistical significance only for children 2006 p < 0.05 and adolescents 2006 p = 0.05), except for adults in 2012 where increased SES was associated with and increased odds of obesity (OR 1.14; 95% CI: 0.99, 1.32).
Smoking status is an important factor to consider, since households with tobacco smoke exposure can be so overwhelming that it can mask the effects of outdoor exposure in its association with obesity [44], and although our main analyses were adjusted for smoking status and second-hand smoke for children, we investigated how our effect estimates would change by including only non-smokers and no second-hand smoke in children’s household. This strengthened the effect estimates for adolescents and adults as well as the overall association (data not shown). However, we could not account for second-hand smoke for adults or adolescents, which could be a different comparison than what we included in this analysis.
In terms of biological mechanisms, animal studies have shown associations between PM2.5 exposure and an increase in inflammation and adiposity [9,45,46], changes in energy metabolism [47], and alterations in food intake and dietary behaviors increasing the risk of obesity [11,48]. It has been also documented that that TLR2/4-dependent inflammatory activation and lipid oxidation in the lung triggered by PM2.5 exposure can spill over systemically, leading to metabolic dysfunction and weight gain [7], as well as a transgenerational transmission of obesity developmental programming [46]. Another recent animal study suggests suppressing oxidative stress and inflammatory response in order to prevent and treat air pollution-induced diseases such a non-alcoholic fatty liver disease [8]. A study in Mexico City demonstrated possible mechanisms in children associated with the development of obesity that included differences between exposed and non-exposed children in leptin, endothelin-1, glucagon-like peptide-1 (GLP 1), ghrelin, and glucagon [29]. We were unable to include biomarkers in our study and were limited in our exposure assessment since we considered exposure over the previous year to the ENSANUT survey, not capturing chronic nor cumulative exposure. Reconstructing history of exposure, for example, considering mobility, was impossible. Although a life-course assessment and biomarkers of PM2.5 exposure in the GMCA would be desirable, the limitations to carry out such a study in a representative sample of the population are considerable.
Several factors known to affect BMI, such as dietary habits, physical activity patterns, sedentary behavior, as well as history of respiratory illnesses (that could have restricted the performance of physical activity), were not available for this study. However, with the exception of physical activity, these are not confounders of the association. We were able to control for SES, which may be considered a surrogate for the omitted predictors, and this may have reduced unmeasured associations from omitted BMI predictors. As noted above, lower SES was associated with higher PM2.5 exposure; if SES can be a surrogate for diet quality, PM2.5 could be considered an obesogenic if lower SES was associated with lower diet quality; however, higher SES has been previously associated with a lower diet quality in this population [49]. Future studies should include diet quality to better investigate this complex relation. We were able to reproduce the prevalence of overweight and obesity found in other studies using ENSANUT data, showing a decrease in overweight and an increase in obesity between surveys, while normal weight remained similar, suggesting that obesity prevention interventions should particularly target individuals with overweight.
A strength of our research is the use of a state-of-the-art hybrid spatio-temporal model with satellite-derived aerosol optical depth measures to assess PM2.5 exposure with higher resolution than previous 10 × 10 Km2 estimates employed in similar studies [16,50]. This allowed us to assess exposure to PM2.5 with estimates from a novel model that predicts ground-level PM2.5 concentration with resolution of 1 × 1 km2 for the GMCA. The findings from our study add to the limited evidence on the advantages of using remote-sensing-derived estimates of PM2.5 in cities of the developing world that frequently have scarce coverage from ground-level monitoring networks. We used quartiles of PM2.5 to identify non-linearities in the exposure–response relationship; however, this approach ignores intra-category variation, which in turn could have reduced our study’s power to detect an association. Thus, future analyses should consider the use of non-linear terms (i.e., splines or fractional polynomials) to address this limitation [51].
Another strength of our study is the representativeness of our population sample; however, survey design sample weighted analysis methods trade estimation precision to obtain minimally biased coefficients that more accurately reflect the characteristics of the population from which the survey sample is drawn compared to coefficients derived from unweighted analysis of the same sample. The trade-off of precision for unbiasedness can be seen in the larger standard errors and confidence intervals of coefficients, due in part to the reduced degrees of freedom available in a design-based model (see supplementary Table S1 for complete models). Less biased coefficients resulting from survey design sample weighted analysis means we can more confidently generalize our results to the entire population.
Finally, regarding our meta-analysis approach, the term is used for two different purposes in epidemiology, the primary being a method for quantitatively summarizing previously published literature. However, when a single study has two groups or components, such as this one, it can be used to describe the method for deriving the overall effect estimate, since the statistical apparatus is identical. We used the inverse variance weighted averages to combine effect estimates between the two different groups. Regarding the different effect estimates in the two different years, random effect methods for combining effect estimates account for that difference. If the difference is greater than would have been expected given the confidence intervals in the two branches of the study, then a random variance component is greater than zero, and incorporating the variance component correctly accounts for the increased uncertainty in estimating the confidence intervals for the overall effect. We did not have a prior hypothesis as to why we would expect a different response in the two years of the study, and therefore we report the overall effect estimate, with correct confidence intervals that incorporate the differences between the two years.
5. Conclusions
We found evidence of an association between average past-year PM2.5 exposure and increased odds of obesity. Although we were unable to account for chronic PM2.5 exposure, mobility, or dietary information, important strengths of this study are the satellite-derived exposure assessment and its representativeness for the Greater Mexico City Area, among the largest cities in Latin America. More studies such as this are recommended in Latin American cities with similar air pollution and obesity conditions.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/18/5/2301/s1: Table S1. Results from crude and weighted models for the association between past year PM2.5 (10 μg/m3 increase) with obesity in children, adolescents and adults* from the Greater Mexico City Area using data from the National Nutrition and Health Surveys, ENSANUT-2006 and 2012.
Author Contributions
Conceptualization, M.T.-O., M.M.T.-R., A.C.J., L.F.B.-A., J.S., R.O.W., and H.R.-R.; data curation, S.J.R., I.K., J.L.T.-S., and M.R.-M.; formal analysis, M.T.-O., M.M.T.-R., S.J.R., I.G.-A., and J.S.; funding acquisition, M.T.-O., M.M.T.-R., L.F.B.-A., and R.O.W.; investigation, M.T.-O. and A.C.J.; methodology, M.M.T.-R., S.J.R., A.C.J., I.K., J.L.T.-S., M.R.-M., J.S., R.O.W., and H.R.-R.; project administration, M.M.T.-R. and L.F.B.-A.; software, I.K.; supervision, A.C.J.; validation, A.C.J.; writing—original draft, M.T.-O.; writing—review and editing, M.T.-O., M.M.T.-R., S.J.R., I.G.-A., A.C.J., I.K., J.L.T.-S., M.R.-M., L.F.B.-A., J.S., R.O.W., and H.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This study received funding from the Environmental Research Fund SEMARNAT-CONACyT (SEMARNAT-2014-1-249343), and NIEHS P30ES023515 and R00ES023450.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of The National Institute of Public Health, Mexico (protocol codes: CI:1033 and SEMARNAT-2014-1-249343, approved 21/07/2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Di Cesare M., Bentham J., Stevens G.A., Zhou B., Danaei G., Lu Y., Bixby H., Cowan M.J., Riley L.M., Hajifathalian K., et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ameye H., Swinnen J. Obesity, income and gender: The changing global relationship. Glob. Food Sec. 2019;23:267–281. doi: 10.1016/j.gfs.2019.09.003. [DOI] [Google Scholar]
- 3.Instituto Nacional de Salud Publica. Instituto Nacional de Estadistica y Geografia. Secreteria de Salud . Encuesta Nacional de Salud y Nutrición 2018 Presentación de Resultados. ENSANUT; Cuernavaca, Mexico: 2019. [Google Scholar]
- 4.Secretaria de Medio Ambiente CDMX . 5o Informe de Gobierno. Gobierno del Distrito Federal; Ciudad de México, México: 2017. [Google Scholar]
- 5.Shamah-Levi T., Cuevas-Nasu L., Dommarco-Rivera J., Hernandez-Avila M. Encuesta nacional de salud y nutrición de medio camino 2016 (ENSANUT MC 2016) Inst. Nac. Salud Pública. 2016;2016:151. doi: 10.21149/8593. [DOI] [Google Scholar]
- 6.An R., Ji M., Yan H., Guan C. Impact of ambient air pollution on obesity: A systematic review. Int. J. Obes. 2018;42:1112–1126. doi: 10.1038/s41366-018-0089-y. [DOI] [PubMed] [Google Scholar]
- 7.Gon Y. Toll-like receptors and airway inflammation. Allergol. Int. 2008;57:33–37. doi: 10.2332/allergolint.R-07-157. [DOI] [PubMed] [Google Scholar]
- 8.Xu M.X., Ge C.X., Qin Y.T., Gu T.T., Lou D.S., Li Q., Hu L.F., Feng J., Huang P., Tan J. Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic. Biol. Med. 2019;130:542–556. doi: 10.1016/j.freeradbiomed.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Xu X., Yavar Z., Verdin M., Ying Z., Mihai G., Kampfrath T., Wang A., Zhong M., Lippmann M., Chen L.-C., et al. Effect of early particulate air pollution exposure on obesity in mice: Role of p47phox. Arterioscler. Thromb. Vasc. Biol. 2010;30:2518–2527. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zordao O.P., Claro L.W., Saldiva P., Donato J., Veras M., Prada P.O. Air pollution exposure during pregnancy and lactation induces obesity and glucose intolerance in the offspring. Diabetes. 2018;67:223-OR. doi: 10.2337/db18-223-OR. [DOI] [Google Scholar]
- 11.Bolton J.L., Smith S.H., Huff N.C., Gilmour M.I., Foster W.M., Auten R.L., Bilbo S.D. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012;26:4743–4754. doi: 10.1096/fj.12-210989. [DOI] [PubMed] [Google Scholar]
- 12.Seo M.Y., Kim S.H., Park M.J. Air pollution and childhood obesity. Korean J. Pediatr. 2020;63:382–388. doi: 10.3345/cep.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fioravanti S., Cesaroni G., Badaloni C., Michelozzi P., Forastiere F., Porta D. Traffic-related air pollution and childhood obesity in an Italian birth cohort. Environ. Res. 2018;160:479–486. doi: 10.1016/j.envres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Fleisch A.F., Luttmann-Gibson H., Perng W., Rifas-Shiman S.L., Coull B.A., Kloog I., Koutrakis P., Schwartz J.D., Zanobetti A., Mantzoros C.S., et al. Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatr. Obes. 2017;12:48–57. doi: 10.1111/ijpo.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fossati S., Valvi D., Martinez D., Cirach M., Estarlich M., Fernández-Somoano A., Guxens M., Iñiguez C., Irizar A., Lertxundi A., et al. Prenatal air pollution exposure and growth and cardio-metabolic risk in preschoolers. Environ. Int. 2020;138:105619. doi: 10.1016/j.envint.2020.105619. [DOI] [PubMed] [Google Scholar]
- 16.Chiu Y.H., Hsu H.H., Wilson A., Coull B.A., Pendo M.P., Baccarelli A., Kloog I., Schwartz J., Wright R.O., Taveras E.M., et al. Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environ. Res. 2017;158:798–805. doi: 10.1016/j.envres.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei Y., Zhang J., Li Z., Gow A., Chung K.F., Hu M., Sun Z., Zeng L., Zhu T., Jia G., et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: Findings from a natural experiment in Beijing. FASEB J. 2016;30:2115–2122. doi: 10.1096/fj.201500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bont J., Casas M., Barrera-Gómez J., Cirach M., Rivas I., Valvi D., Álvarez M., Dadvand P., Sunyer J., Vrijheid M. Ambient air pollution and overweight and obesity in school-aged children in Barcelona, Spain. Environ. Int. 2019;125:58–64. doi: 10.1016/j.envint.2019.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong G.H., Qian Z., Liu M.M., Wang D., Ren W.H., Flick L.H., Fu J., Wang J., Chen W., Simckes M., et al. Ambient air pollution and the prevalence of obesity in Chinese children: The seven northeastern cities study. Obesity. 2014;22:795–800. doi: 10.1002/oby.20198. [DOI] [Google Scholar]
- 20.Zhang X., Zhao H., Chow W., Bixby M., Durand C., Markham C., Zhang K. Population-based study of traffic-related air pollution and obesity in Mexican Americans. Obesity. 2020;28:412–420. doi: 10.1002/oby.22697. [DOI] [PubMed] [Google Scholar]
- 21.Bloemsma L.D., Wijga A.H., Klompmaker J.O., Janssen N.A.H., Smit H.A., Koppelman G.H., Brunekreef B., Lebret E., Hoek G., Gehring U. The associations of air pollution, traffic noise and green space with overweight throughout childhood: The PIAMA birth cohort study. Environ. Res. 2019;169:348–356. doi: 10.1016/j.envres.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Zhang N., Wang L., Zhang M., Nazroo J. Air quality and obesity at older ages in China: The role of duration, severity and pollutants. PLoS ONE. 2019;14:e0226279. doi: 10.1371/journal.pone.0226279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrijheid M., Fossati S., Maitre L., Márquez S., Roumeliotaki T., Agier L., Andrusaityte S., Cadiou S., Casas M., de Castro M., et al. Early-life environmental exposures and childhood obesity: An exposome-wide approach. Environ. Health Perspect. 2020;128:1–14. doi: 10.1289/EHP5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z., Herting M.M., Chatzi L., Belcher B.R., Alderete T.L., McConnell R., Gilliland F.D. Regional and traffic-related air pollutants are associated with higher consumption of fast food and trans fat among adolescents. Am. J. Clin. Nutr. 2019;109:99–108. doi: 10.1093/ajcn/nqy232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jerrett M., McConnell R., Chang C.C.R., Wolch J., Reynolds K., Lurmann F., Gilliland F., Berhane K. Automobile traffic around the home and attained body mass index: A longitudinal cohort study of children aged 10–18 years. Prev. Med. (Baltim). 2010;50:S50–S58. doi: 10.1016/j.ypmed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu W., Sulistyoningrum D.C., Gasevic D., Xu R., Julia M., Murni I.K., Chen Z., Lu P., Guo Y., Li S. Long-term exposure to PM2.5 and fasting plasma glucose in non-diabetic adolescents in Yogyakarta, Indonesia. Environ. Pollut. 2020;257:113423. doi: 10.1016/j.envpol.2019.113423. [DOI] [PubMed] [Google Scholar]
- 27.Li W., Dorans K.S., Wilker E.H., Rice M.B., Schwartz J., Coull B.A., Koutrakis P., Gold D.R., Fox C.S., Mittleman M.A. Residential proximity to major roadways, fine particulate matter, and adiposity: The framingham heart study. Obesity. 2016;24:2593–2599. doi: 10.1002/oby.21630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerrett M., McConnell R., Wolch J., Chang R., Lam C., Dunton G., Gilliland F., Lurmann F., Islam T., Berhane K. Traffic-related air pollution and obesity formation in children: A longitudinal, multilevel analysis. Environ. Health. 2014;13:49. doi: 10.1186/1476-069X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calderón-Garcidueñas L., Franco-Lira M., D’Angiulli A., Rodríguez-Díaz J., Blaurock-Busch E., Busch Y., Chao C., Thompson C., Mukherjee P.S., Torres-Jardón R., et al. Mexico City normal weight children exposed to high concentrations of ambient PM2.5 show high blood leptin and endothelin-1, vitamin D deficiency, and food reward hormone dysregulation versus low pollution controls. Relevance for obesity and Alzheimer dise. Environ. Res. 2015;140:579–592. doi: 10.1016/j.envres.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 30.O’Neill M.S., Veves A., Sarnat J.A., Zanobetti A., Gold D.R., Economides P.A., Horton E.S., Schwartz J. Air pollution and inflammation in type 2 diabetes: A mechanism for susceptibility. Occup. Environ. Med. 2007;64:373–379. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao G., Nachman R.M., Sun Q., Zhang X., Koehler K., Chen Z., Hong X., Wang G., Caruso D., Zong G., et al. Individual and joint effects of early-life ambient PM2.5 exposure and maternal prepregnancy obesity on childhood overweight or obesity. Environ. Health Perspect. 2017;125:067005. doi: 10.1289/EHP261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barquera S., Campos-Nonato I., Hernández-Barrera L., Pedroza A., Rivera-Dommarco J.A. Prevalencia de obesidad en adultos mexicanos, 2000. Salud Publica Mex. 2013;55:S151–S160. doi: 10.21149/spm.v55s2.5111. [DOI] [PubMed] [Google Scholar]
- 33.Just A.C., Wright R.O., Schwartz J., Coull B.A., Baccarelli A.A., Tellez-Rojo M.M., Moody E., Wang Y., Lyapustin A., Kloog I. Using High-resolution satellite aerosol optical depth to estimate daily PM2.5 geographical distribution in Mexico City. Environ. Sci. Technol. 2015;49:8576–8584. doi: 10.1021/acs.est.5b00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutiérrez J.P. Household socioeconomic classification in the National Health and Nutrition Survey. Salud Publica Mex. 2013;55:341–346. doi: 10.21149/spm.v55s2.5133. [DOI] [PubMed] [Google Scholar]
- 35.Kim J.S., Chen Z., Alderete T.L., Toledo-Corral C., Lurmann F., Berhane K., Gilliland F.D. Associations of air pollution, obesity and cardiometabolic health in young adults: The Meta-AIR study. Environ. Int. 2019;133:105180. doi: 10.1016/j.envint.2019.105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleisch A.F., Rifas-Shiman S.L., Koutrakis P., Schwartz J.D., Kloog I., Melly S., Coull B.A., Zanobetti A., Gillman M.W., Gold D.R., et al. Prenatal exposure to traffic pollution: Associations with reduced fetal growth and rapid infant weight gain. Epidemiology. 2015;26:43–50. doi: 10.1097/EDE.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazidi M., Speakman J.R. Ambient particulate air pollution (PM2.5) is associated with the ratio of type 2 Diabetes to obesity. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-08287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weichenthal S., Hoppin J.A., Reeves F. Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity. 2014;22:1580–1589. doi: 10.1002/oby.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koman P.D., Mancuso P. Ozone exposure, cardiopulmonary health, and obesity: A substantive review. Chem. Res. Toxicol. 2017;30:1384–1395. doi: 10.1021/acs.chemrestox.7b00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim H.J., Xue H., Wang Y. Handbook of Eating and Drinking. Springer International Publishing; Berlin/Heidelberg, Germany: 2020. Global trends in obesity; pp. 1217–1235. [Google Scholar]
- 41.Vazquez C.E., Cubbin C. Socioeconomic status and childhood obesity: A review of literature from the past decade to inform intervention research. Curr. Obes. Rep. 2020;9:562–570. doi: 10.1007/s13679-020-00400-2. [DOI] [PubMed] [Google Scholar]
- 42.Han C., Xu R., Gao C.X., Yu W., Zhang Y., Han K., Yu P., Guo Y., Li S. Socioeconomic disparity in the association between long-term exposure to PM2.5 and mortality in 2640 Chinese counties. Environ. Int. 2021;146:106241. doi: 10.1016/j.envint.2020.106241. [DOI] [PubMed] [Google Scholar]
- 43.Hajat A., Diez-Roux A.V., Adar S.D., Auchincloss A.H., Lovasi G.S., O’Neill M.S., Sheppard L., Kaufman J.D. Air pollution and individual and neighborhood socioeconomic status: Evidence from the multi-ethnic study of atherosclerosis (MESA) Environ. Health Perspect. 2013;121:1325–1333. doi: 10.1289/ehp.1206337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villeneuve P.J., Goldberg M.S., Burnett R.T., Van Donkelaar A., Chen H., Martin R.V. Associations between cigarette smoking, obesity, sociodemographic characteristics and remote-sensing-derived estimates of ambient PM2.5: Results from a Canadian population-based survey. Occup. Environ. Med. 2011;68:920–927. doi: 10.1136/oem.2010.062521. [DOI] [PubMed] [Google Scholar]
- 45.Sun Q., Yue P., Deiuliis J.A., Lumeng C.N., Kampfrath T., Mikolaj M.B., Cai Y., Ostrowski M.C., Lu B., Parthasarathy S., et al. Ambient Air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Y., Wang W., Chen M., Zhou J., Huang X., Tao S., Pan B., Li Z., Xie X., Li W., et al. Developmental programming of obesity by maternal exposure to concentrated ambient PM2.5 is maternally transmitted into the third generation in a mouse model. Part. Fibre Toxicol. 2019;16:1–11. doi: 10.1186/s12989-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen M., Liang S., Zhou H., Xu Y., Qin X., Hu Z., Wang X., Qiu L., Wang W., Zhang Y., et al. Prenatal and postnatal mothering by diesel exhaust PM2.5-exposed dams differentially program mouse energy metabolism. Part. Fibre Toxicol. 2017;14:3. doi: 10.1186/s12989-017-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kenny P.J. Reward mechanisms in obesity: New insights and future directions. Neuron. 2011;69:664–679. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.López-Olmedo N., Popkin B.M., Taillie L.S. Association between socioeconomic status and diet quality in Mexican men and women: A cross-sectional study. PLoS ONE. 2019;14:e0224385. doi: 10.1371/journal.pone.0224385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kloog I., Koutrakis P., Coull B.A., Lee H.J., Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos. Environ. 2011;45:6267–6275. doi: 10.1016/j.atmosenv.2011.08.066. [DOI] [Google Scholar]
- 51.Bennette C., Vickers A. Against quantiles: Categorization of continuous variables in epidemiologic research, and its discontents. BMC Med. Res. Methodol. 2012;12:21. doi: 10.1186/1471-2288-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.