Abstract
Atopic dermatitis (AD) is a chronic, pruritic, inflammatory skin disease that predominantly affects children. However, it can persist in adulthood and/or start at older ages. Both dysfunction of the epidermal barrier and immune dysregulation are known to play a role in the pathogenesis of AD. In the last years, numerous studies showed that Janus kinase (JAK) enzymes have a key role in AD pathogenesis. Therefore, oral and topical JAK inhibitors are new emerging treatments for AD. We report the data relating to abrocitinib, an oral JAK1 inhibitor. For this purpose, we examined articles already published concerning, in particular, concluded clinical trials. Furthermore, we also report the design of current ongoing clinical trials. The search was carried out considering the main search engines relating to medical literature and clinical trials. From all the data we collected, abrocitinib proved to be an effective drug in significantly reducing the severity of moderate-to-severe AD when compared to placebo. Furthermore, the efficacy was similar to other well-established treatment for AD, such as dupilumab. Adverse events were generally mild; indeed, the drug was definitively suspended only in few patients.
Keywords: atopic dermatitis, abrocitinib, JAK inhibitors, JAK-STAT
Introduction
Atopic dermatitis (AD) is a chronic skin condition that has recently been the subject of volumes of basic science, clinical, and epidemiologic research, being considered a global health issue due to the increased prevalence in last decades.1,2 Currently, AD impacts approximately 15% to 20% of children and 10% of adults in industrialized countries, although there is a considerable countrywide variation of AD prevalence and severity.3
AD has a complex and multifactorial pathophysiology, consisting of multiple interactions between skin barrier defects and immunological disturbances.4 The most important pathway involved in AD pathogenesis is based on the T-helper (Th)2 lymphocyte axis. However, Th17, Th22, and Th1 cytokines have been shown to play important different roles in AD.4–8
The dysfunction of the skin barrier has been shown to be a fundamental alteration in the development of AD. Indeed, many alterations were related to skin barrier defects, such as immune dysregulation, filaggrin mutations, and skin dysbiosis.6 These factors interact with each other and may represent a trigger for immune activation, leading to an increase of interleukin (IL)-25, IL-33 as well as an increased activation of Th-2 pathways.9 These results in a downregulation of envelope proteins (filaggrin, loricrin, and claudins) and of defensive epithelial barrier proteins.4,9 The activation of Th2 pathways is also related to increased levels of IL-4, IL-13, and IL-31, key cytokines associated with type-2 inflammation. Indeed, their levels correlate with AD disease activity.10 Recently, some studies emphasized the role of innate lymphoid cells in expanding Th2 immunity through the release of IL-5 and IL-13, which in turn increase and support Th2 response.11 Th2 polarization facilitates Staphylococcus aureus binding and colonization, and IL-4 and IL-13 inhibit skin production of antimicrobial peptides, predisposing AD skin to bacterial infections.12 Furthermore, S. Aureus may induce modifications in skin barrier, inducing inflammatory processes via super-antigens and toxic shock syndrome toxin-1, which trigger cytokine release.4
The chronic stage of AD is characterized by intensified Th2 and Th22 axes, and activation of both Th1 and Th17 cells.10 Th1 pathway activation induces an upregulation of interferon gamma and IL-12, leading to the promotion of chronic inflammation and keratinocytes apoptosis.4 Th17 cytokines IL-17 and IL-22 contribute to immune dysregulation in AD, barrier abnormalities by down-regulating filaggrin, and down regulation of expression of keratinocyte genes associated with cellular adhesion.12,13
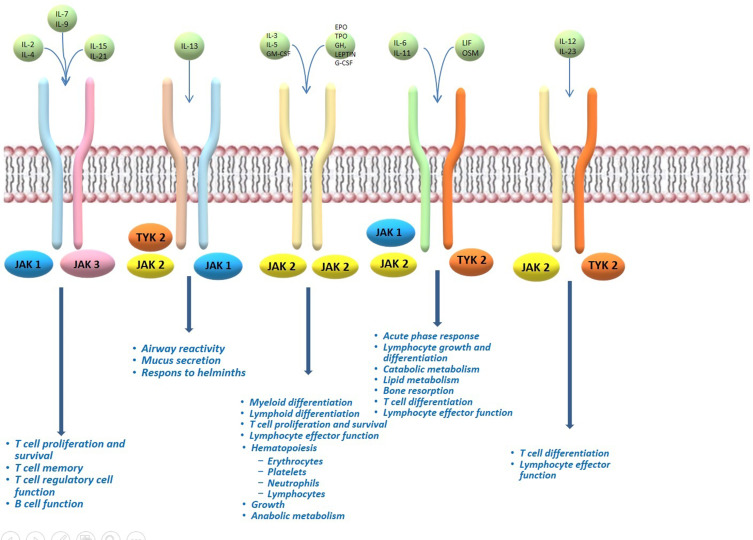
The Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signalling has been shown to play a central role in AD pathogenesis.14 The JAK kinase family is composed of JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2).15 While JAK1, JAK2, and TYK2 are expressed in all cells, JAK3 expression occurs primarily in hematopoietic cells.15 They operate intracellularly as signal transducers after that different cytokine bind their receptor chains associated with a JAK enzyme (Figure 1).16 The activation and dimerization of receptor activates JAK phosphorylation, leading to the binding with STAT, and subsequently STAT phosphorylation.17 The resulting STAT dimer translocates to the nucleus and modulates the upregulation of the proinflammatory cytokines and growth factors genes transcription.17 Each JAK dimer can be triggered by various cytokines and, sequentially, activate different STAT proteins.16 JAK1 can dimerize with JAK2, JAK3, and TYK2; JAK2 with itself or TYK2; JAK3 only with JAK1; and TYK2 with JAK1 and JAK2.16 Numerous studies showed that in AD pathogenesis there is an upregulation of epidermal chemokines, pro-inflammatory cytokines, and pro-angiogenic factors, as well as downregulation antimicrobial peptides and factors responsible for skin barrier function, all induced by the JAK-STAT pathway activation.18 In particular, Th2 cytokines are mostly responsible for JAK1 and JAK3 activation, and the next subsequent phosphorylation of STAT6.4
Figure 1.
Overview of JAK signalling in the different features of immune-cell development and function.
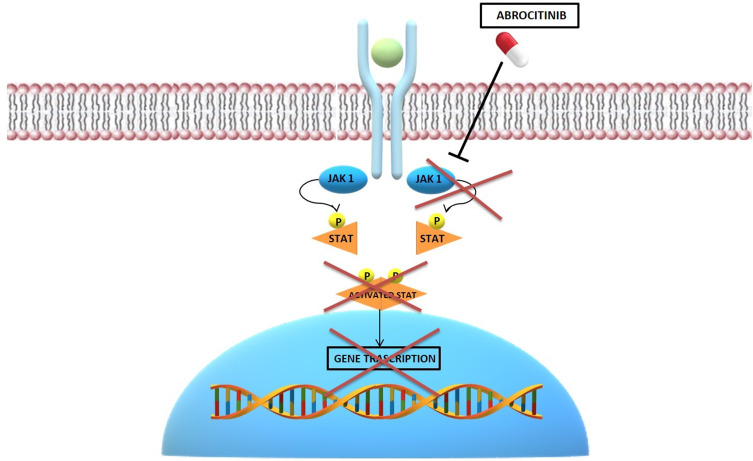
Oral and topical JAK inhibitors are new emerging treatments for AD.19 The inhibition of JAK prevents their phosphorylation, stopping the transduction of intracellular signaling.20 First-generation JAK inhibitors block multiple JAKs.21 Among these, baricitinib, an oral JAK1 and JAK2 inhibitor, was recently approved for the treatment of moderate-to-severe AD in adult patients who are candidates for systemic therapy.22 Second-generation JAK inhibitors target only one JAK isoform.20 Particularly, the selective JAK1-inhibition is important to modulate different cytokines involved in the AD, avoiding undesirable effects related to the inhibition of JAK2 (neutropenia and anaemia).23 Abrocitinib (PF-04965842) is a recently introduced oral JAK1 selective inhibitor that influences different important cytokines signalling pathways involved in the AD, like IL-4, IL-13, and IL-31 (Figure 2).23
Figure 2.
Schematic representation of the abrocitinib mechanism of action.
We herein present a review, which summarizes the data on efficacy and safety of abrocitinib in the treatment of moderate to severe AD.
Methods
A search in the PubMed, Embase, Cochrane Skin databases, and clinicaltrials.gov (until October 31, 2020) was performed using the search: “atopic dermatitis” or “atopic eczema” associated with “JAK1 inhibitors”, “abrocitinib”, or “PF-04965842”. Only the material in English language was selected. The resulting records were further redefined through full-text review. Each author independently reviewed all the articles and data. Subsequently, they compared their opinions on the content and shared the information to be reported in the review. Only the articles and data already published were included in the review. Moreover, the reference lists of those papers were examined to retrieve other studies that were considered relevant for our aim. Implementing these search criteria, we also evaluated the clinical trials ongoing, but with no results posted.
Results
A total of 15 records were identified for evaluation in this review. Among these, we selected 5 clinical trials of Phase I, II and III, reviewing data about effectiveness and safety of abrocitinib. We also describe 5 trials that are currently ongoing and whose results, therefore, are not yet available. The clinical trials considered in this review are summarized in Table 1. Furthermore, we selected 5 reviews, which could be useful for the consultation and interpretation of the data. Letters and comments, reviews of abrocitinib in other dermatological diseases, and papers in which, according to the opinion shared by all the authors, there was no relevant information were excluded.
Table 1.
Clinical Trials Evaluating Efficacy and Safety of Abrocitinib in Atopic Dermatitis
| Trial | Title | Phase | Enrollment | Arm | Primary Outcomes | Main Secondary Outcomes | Status |
|---|---|---|---|---|---|---|---|
| NCT01835197 | First-in-Human Study to Evaluate Safety and Tolerability of Single and Multiple Ascending Doses of Janus Kinase-1 Inhibitor PF-04965842 in Healthy Western and Japanese Subjects | 1 |
|
-Single doses of 3, 10, 30, 100, 200, 400, or 800 mg of abrocitinib in a dose escalation format; -single doses of abrocitinib matching placebo in a dose escalation format; -doses of 30, 100 or 200 mg QD for 10 days; Abrocitinib matching placebo QD for 10 days; -doses of 100 or 200 mg BID for 10 days; -abrocitinib matching placebo doses BID for 10 days; -200 mg dose BID for 10 days; -abrocitinib matching placebo dose BID for 10 days; -400 mg dose QD for 10 days. |
Changes from baseline to week 6 in vital signs; Changes from baseline to week in ECG parameters; Incidence of AE; Incidence of clinical laboratory abnormalities; 24-hour urine creatinine clearance; |
Complement Level (C3, C4, C3A); neutrophils count; reticulocytes count Apparent total body clearance; urinary pharmacokinetics; renal clearance; plasma decay half-life; maximum observed plasma concentration; apparent volume of distribution |
Completed |
| NCT02780167 | A Phase 2b Randomized, Double-Blind, Placebo-controlled, Parallel, Multicenter, Dose Ranging, Study to Evaluate the Efficacy and Safety Profile of PF-04965842 in Subjects with Moderate-to-Severe Atopic Dermatitis | 2 | 267 adult patients aged 18–75 years | -Abrocitinib 10 mg QD -Abrocitinib 30 mg QD; -abrocitinib 100 mg QD; -abrocitinib 200 mg QD; -placebo QD |
Proportion of patients achieving IGA score for clear or almost clear and [2-point improvement from baseline at week 12 | Variation rate from baseline to week 12 in EASI, BSA, SCORAD and P-NRS | Completed |
| NCT03349060 | Study to Evaluate Efficacy and Safety of PF-04965842 in Subjects Aged 12 Years And Older With Moderate to Severe Atopic Dermatitis (JADE Mono-1) | 3 | 387 patients aged ≥12 years | -Abrocitinib100 mg QD (135 patients); -abrocitinib 200 mg QD (137 patients); -placebo QD (61 patients) |
-Proportion of patients who had achieved an IGA score of 1 or 0 with a ≥2-grade improvement form baseline to week 12 -Proportion of patients achieving EASI75 at week 12 |
-Improvement in P-NRS response ≥4 point and in the SCORAD score. (≥75% or more); -change in DLQI from baseline to week 12; |
Completed |
| NCT03575871 | Study Evaluating Efficacy and Safety of PF-04965842 in Subjects Aged 12 Years And Older With Moderate to Severe Atopic Dermatitis (JADE Mono-2) | 3 | 391 patients aged ≥12 years | -Abrocitinib 100 mg QD; -abrocitinib 200 mg QD; -placebo QD |
-The proportion of subjects the at 12 weeks reaching IGA 0/1 or with an improvement of ≥2 grades from baseline; -The proportion of subjects reaching EASI-75 response |
-The proportion of subjects with an improvement of ≥4 points in P-NRS score from baseline to week 12 -The change from baseline to week 12 in AD-PSA; -change in BSA from baseline to week 12; -change in SCORAD from baseline to week 12; -change in DLQI from baseline to week 12; |
Completed |
| NCT03720470 | Study Evaluating Efficacy and Safety of PF-04965842 and Dupilumab in Adult Subjects With Moderate to Severe Atopic Dermatitis on Background Topical Therapy (JADE Compare) | 3 | 838 patients aged 18–75 years | -Abrocitinib 100 mg + placebo injection followed by abrocitinib 100 mg; -abrocitinib 200 mg + placebo injection followed by abrocitinib 200mg; -dupilumab injection + oral placebo followed by oral placebo; -oral placebo + placebo injection followed by 100 mg abrocitinib; -oral placebo + placebo injection followed by 200 mg abrocitinib; |
-The proportion of subjects the at 12 weeks reaching IGA 0/1 or with an improvement of ≥2 grades from baseline; -The proportion of subjects reaching EASI-75 response |
-The proportion of subjects with an improvement of ≥4 points in P-NRS score from baseline to week 12; -change in BSA from baseline to week 12; -change in SCORAD from baseline to week 12; -change in DLQI from baseline to week 12; |
Completed |
| NCT03627767 | Study to Investigate Efficacy and Safety of PF-04965842 in Subjects Aged 12 Years and Over With Moderate to Severe Atopic Dermatitis With the Option of Rescue Treatment in Flaring Subjects | 3 | 1234 subjects aged ≥12 years | -Abrocitinib 100 mg, administered as two tablets to be taken orally QD; -abrocitinib 200 mg, administered as two tablets to be taken orally QD; -placebo administered as two tablets to be taken orally QD; |
Percentage of patients with a loss of response to abrocitinib (defined as a loss of at least 50% of the EASI) at week 12 | - Change in IGA from baseline to week 12; -change in EASI score from baseline to week 12; -change in SCORAD score from baseline to week 12; -change in BSA score from baseline to week 12; -change in DLQI score from baseline to week 12; - the proportion of subjects with an improvement of ≥4 points in P-NRS score from baseline to week 12 |
Completed but result no posted |
| NCT03422822 | Study to Evaluate Efficacy and Safety of PF-04965842 With or Without Topical Medications in Subjects Aged 12 Years and Older With Moderate to Severe Atopic Dermatitis (JADE EXTEND) | 3 | 3000 subjects aged ≥12 years | -Abrocitinib 100 mg QD; -abrocitinib 200 mg QD |
-Incidence of AEs during the blinded phase of the study and until availability of commercial product; -Changes from baseline in clinical laboratory values; -Changes from baseline in electrocardiogram measurements; -Changes from baseline in vital signs. |
- Change in IGA from baseline to week 92; -change in EASI score from baseline to week 92; -change in SCORAD score from baseline to week 92; -change in BSA score from baseline to week 92; -change in DLQI score from baseline to week 92; - the proportion of subjects with an improvement of ≥4 points in P-NRS score from baseline to week 92 |
Enrolling by invitation |
| NCT04345367 | Study of Abrocitinib Compared With Dupilumab in Adults With Moderate to Severe Atopic Dermatitis on Background Topical Therapy | 3b | 600 patients aged ≥18 years | - Abrocitinib 200 mg plus placebo injection; - dupilumab 300 mg plus placebo tablets |
-Change percentage in P-NRS from baseline to week 4 (Response based on achieving at least a 4-point improvement); -change percentage in EASI values from baseline to week 4 |
-Percentage of patients achieving EASI90 (≥90% improvement from baseline) at week 16; -percentage of participants achieving EASI75 at week 16; -ercentage of patients achieving IGA 0/1 or with a reduction from baseline of ≥2 points at all scheduled time points; -percentage of subjects with at least a 4-point improvement in the severity of P-NRS; -percentage reduction of BSA |
Recruiting |
| NCT04564755 | Abrocitinib Expanded Access Protocol in Adolescents and Adults With Moderate to Severe Atopic Dermatitis | 3 | Approximately 500 patients aged ≥ 12 years | -Abrocitinib 100 mg QD; -abrocitinib 200 mg QD |
No posted | No posted | Available |
| NCT03915496 | Study Evaluating the Mechanism of Action of PF-04965842 Monotherapy for Moderate-to-severe Atopic Dermatitis (JADE MOA) | 2A | 51 adult patients aged ≥18 years | -Abrocitinib 100 mg QD; -abrocitinib 200 mg QD; -placebo QD |
Changes from baseline in atopic dermatitis biomarkers in lesional and non-lesional skin | -Changes in gene expression from baseline to week 12; -changes in T-cell and dendritic cell inflammation markers from baseline to week 12; -changes in in epidermal hyperplasia markers; -changes in T-cell lymphocyte subset populations from baseline to week 12; -changes from baseline in pruritus and its correlation to immunohistochemistry and genetic markers from baseline to week 12. |
Recruiting |
Abbreviations: QD, once daily; BID, twice daily; AEs, adverse event; IGA, Investigator’s Global Assessment; EASI, Eczema Area and Severity Index; BSA, body surface area; SCORAD, Scoring Atopic Dermatitis; P-NRS, Pruritus Numerical Rating Scale; PSA, Pruritus and Symptoms Assessment; DLQI, Dermatology Life Quality Index; Inj, injective.
Phase I
The Phase I study (NCT01835197) evaluated abrocitinib safety, tolerability, pharmacokinetics and pharmacodynamics in 79 healthy adults.24 This was a single-center, double-blind (subject and investigator), and sponsor open study.24 The patients received a single ascending dose phase (SAD) of placebo or 3, 10, 30, 100, 200, 400, or 800 mg of abrocitinib and placebo or 30 mg once daily, 100 mg once daily, 200 mg once daily, 400 mg once daily, 100 mg twice daily, or 200 mg twice daily of abrocitinib for 10 consecutive days [multiple ascending dose phase] (MAD).24 After abrocitinib intake, the peak plasma concentration has been observed within 1 hour (in both SAD and MAD phases) with a mean half-life of 2.8–5.4 hours.24 Urinary recuperation was less than 4.4% and kidney excretion about 0.6 L h−1.24 No serious adverse events (AEs) or death have been reported. Common AEs were headache (16.14%), nausea (13.9%), and diarrhoea (13.9%).24 Only 4 treated patients discontinued due to AEs. For 3 of them, the drug discontinuation was considered treatment-related by the investigators (2 patients referred vomiting and one patient developed a respiratory syncytial virus infection), while for one patient the appearance of a second‐degree atrioventricular block was not considered treatment‐related.24 The most common abnormal laboratory tests were a decrease in mean platelet volume and a positive urine blood test in the SAD phase, and a reduction in reticulocyte counts and in mean platelet volume, and an increased lymphocyte counts in the MAD phase.24
Phase II
Abrocitinib safety and efficacy were evaluated in a randomized, double-blinded, placebo controlled phase IIb trial (NCT02780167), including 267 adult patients aged 18–75 years with inadequate responses or contraindications to topical treatment.23 Patients were randomly treated with abrocitinib (200, 100, 30, or 10 mg) or placebo for 12 weeks.23 Patients achieving an Investigator’s Global Assessment (IGA) of clear (0) or almost clear (1) with an improvement from baseline of 2 grades or more at week 12 was the primary outcome, while secondary outcomes were the variation rate from baseline to week 12 in the Eczema Area and Severity Index (EASI), Body Surface Area (BSA), Scoring Atopic Dermatitis (SCORAD) and Pruritus Numerical Rating Scale (P-NRS).23
After 12 weeks, IGA0/1 was achieved by 43.8%, 29.6%, 8.9% and 10.9% of patients treated with abrocitinib 200 mg,100 mg, 30 mg, and placebo, respectively.23 Concerning the variation of EASI, in the group treated with abrocitinib 200 mg, there was a reduction of EASI of 82.6%, while the reduction was of 59.0% and 40.7% in the groups treated with abrocitinib 100 mg and 30 mg, respectively.23 A significant reduction of EASI was observed in both 200-mg and 100-mg groups compared with placebo, while these were not significant in the 30 mg and 10 mg groups.23 At week 12, a significant reduction in P-NRS was observed in the 200 mg and 100 mg groups as well. P-NRS decreased and plateau by week 2 and week 4 in the 200 mg and 100 mg group, respectively. Finally, from baseline to week 12 a reduction of BSA and SCORAD in all treatment groups was observed.23 AEs were reported by 184 (68.9%) patients. Of these 24.0% were considered related to treatments.23 The most frequent reported AEs were upper respiratory tract infection, headache, nausea, diarrhoea, and AD worsening.23 Among patients treated with abrocitinib 200 mg and abrocitinib 100 mg, a dose-dependent decrease in the platelet-count was found, which trended toward normalization.23 Two serious AEs were reported in the treatment groups: a case of pneumonia in the abrocitinib 200 mg group and one case of eczema herpeticum in the abrocitinib 100 mg group.23 Treatment discontinuation for AEs was reported in the 16.5% (n=44).23
Phase III
JADE MONO-1
In a multicentre, randomised Phase III trial (JADE MONO-1; NCT03349060), patients suffering from moderate-to-severe AD, were enrolled at 69 sites.25 Patients were randomly assigned (2:2:1) in one of the three study arms: abrocitinib 100 mg, abrocitinib 200 mg, and placebo QD for 12 weeks.25 Enrolled patients have been suffering from AD for at least 1 year, showing IGA score ≥3, EASI score ≥16, BSA ≥10% and P-NRS ≥4.25 The coprimary endpoints were the proportion of patients who had achieved an IGA score of 1 or 0 with a ≥2-grade improvement from baseline, and the proportion of patients achieving EASI75.25 The main secondary endpoints were the proportion of patients who from baseline to week 12 achieved a P-NRS response ≥4 point improvement and an improvement of ≥75% or more in the SCORAD score.
Three hundred and eighty-seven patients were enrolled, but 333 patients concluded the study: 135 patients in the abrocitinib 100 mg group, 137 in the abrocitinib 200 mg group, and 61 in the placebo group. After 12 weeks, IGA response was reached by 43.8% of patients of abrocitinib 200 mg group, 23.7%, of patients of 100 mg group, and 7.9% placebo group.25 As regards the different variations in IGA responses between placebo and treatment groups, it was 15.8% (p=0.0037) abrocitinib 100 mg group and 36.0% (p<0.0001) in the abrocitinib 200 mg group.25 Furthermore, EASI75 was achieved by 62.7% of patients receiving abrocitinib 200 mg, 39.7% of subjects receiving abrocitinib 100 mg, and 11.8% of placebo group.25 A statistically significant improvement in the P-NRS score was observed in all treatment groups (57.2% of patients receiving abrocitinib 200 mg and 37.7% of those receiving abrocitinib 100 mg), vs 15.3% of placebo groups.25 An effective response in P-NRS has been already observed at week 2.25 P-NRS response was noted after a median of 14 days in patients treated with abrocitinib 200 mg, after a median of 84 days in patients treated with abrocitinib 100 mg and a median of 92 days in patients treated with placebo.25 As regards SCORAD variations rates, these resulted considerably higher in abrocitinib treatment groups than placebo groups, reaching a SCORAD response of ≥75% in 30.8% and 12.4% of patients in the 200mg and 100mg arms, respectively, vs 4.1% of those in placebo group.25
In the JADE MONO-1 trial, AEs were described in 69% (n=108) of the abrocitinib 100 mg group, 78% (n=120) of the abrocitinib 200 mg group, and 57% (n=44) of the placebo group. The most frequent reported AEs were nasopharyngitis, headache, upper respiratory tract infection, nausea, and AD.25 Serious AEs were observed in 5/156 (3.2%) of patients treated with abrocitinib 200 mg (including: asthma, inflammatory bowel disease, dehydration, and peri-tonsillitis), in 5/154 (3.2%) of abrocitinib 100 mg group (appendicitis, seizures, dizziness and acute pancreatitis) and in 3/77 (3.9%) of placebo group (worsening of AD, appendicitis, and meniscal degeneration).25 Two serious AEs were considered treatment-related: one patient in the abrocitinib 200 mg group developed chronic inflammatory bowel disease and another patient in the abrocitinib 100 mg acute pancreatitis.25 Abrocitinib was permanently discontinued for both patients.25
The discontinuation rate in the 200 mg and 100 mg abrocitinib groups were respectively of 6% (n=9) and 6% (n=9), with AD, gastrointestinal disorders, inflammatory bowel disease, and vomiting as most frequent causes of treatment discontinuation.25 However, the discontinuation rate resulted higher in the placebo group (9.1%) than in the abrocitinib groups (5.8%). A dose-related reduction of median platelet count was observed in both treatment groups (100 and 200 mg), with a nadir at week 4. However, in all cases, there was a return to baseline values without requiring treatment discontinuation.25 No major cardiovascular events or venous thromboembolism or death have been reported.25
JADE MONO-2
In the JADE MONO-2 trial (NCT03575871), a multicentre, double-blind, randomised phase III trial, 391 patients suffering from moderate-to-severe AD were randomly assigned in the following treatment groups: i) monotherapy with QD abrocitinib 200 mg; ii) abrocitinib 100 mg; iii) placebo.26 The proportion of subjects at 12 weeks reaching IGA 0/1 or with an improvement of ≥2 grades from baseline and the proportion of subjects reaching EASI-75 response have been identified as primary end points. Secondary end points were the proportion of subjects with an improvement of ≥4 points from baseline in P-NRS score, and the change from baseline to week 12 in the total score of an 11-item survey created to evaluate daily symptoms of AD [Pruritus and Symptoms Assessment (PSA)].26
Three hundred and thirty patients completed the trial. After 12 weeks, abrocitinib treatment groups (both 200 mg and 100 mg groups) achieved significantly higher responses if compared to placebo group in terms of IGA 0/1 (38.1%, 28.4% vs 9.1%), EASI 75 (61.0%, 44.5%, vs 10.4%), and P-NRS ≥4-point improvement (55.3%, 23.9% vs 11.5%).26 P-NRS response was reached after a median time of 29 days in patients treated with abrocitinib 200 mg, 58 days in patients treated with 100 mg and 112 days in patients treated with placebo.26 A significant reduction in P-NRS scores in both abrocitinib groups was firstly reported at day 1.26 The reduction from baseline at week 12 in PSA total score was superior for both treatment groups compared to placebo group: −3.0, −2.4 and −0.8 for the patients treated with abrocitinib 200 mg, 100 mg group and placebo, respectively.26
In the JADE MONO-2 trial, 65.8% (n=102) of the 200 mg group, 62.7% (n=99) of the 100 mg group, and 53.8% (n=42) of the placebo group reported treatment-emergent AEs.17 The following were the most reported AEs: nausea in the 200 mg group (14.2%), nasopharyngitis in the 100mg group (12.7%), and AD in the placebo group (15.4%). In the 100 mg abrocitinib group one death was reported, which was not related to the treatment. A total of 7 serious AEs were reported in the treatment groups. Of these, 2 AEs reported in the 100mg group were considered related to treatment and were a case of herpangina and a case of pneumonia.26 Both cases required abrocitinib discontinuation. Discontinuation rates were 3.2%, 3.8%, and 12.8% of 200 mg, 100 mg, and placebo group, respectively. Headache was the most frequent reported AE in both treatment groups (100 mg, 200 mg, and placebo). A reduction in platelet counts was described in the JADE MONO-2 as well, which returned to baseline levels despite the continuation of treatment.
JADE COMPARE
The JADE COMPARE study (NCT03720470) is a comparative study in which two dosages of abrocitinib, 100 mg and 200 mg taken orally QD were compared to placebo at 12 weeks and to dupilumab at 2 weeks in relation to decrease of itch.27 Co-primary endpoints are the proportion of patients achieved IGA 0/1 or with a reduction from baseline of ≥2 points at week 12, and the proportion of patients achieving EASI-75. The study comprised 838 patients randomized into 5 arms of treatment: 1) patients randomized to take one of the two abrocitinib dosage plus a placebo injectable every 2 weeks until week 16, and then kept on receiving only the abrocitinib for 4 weeks;27 2) patients randomized in the arm dupilumab every 2 weeks and oral placebo QD until week 16 and after only oral placebo for 4 weeks;27 3) patients randomized to the placebo arm (in both injectable and oral form) every 2 weeks until week 16, and after in the arm abrocitinib 100 mg or 200 mg QD for 4 weeks.27 At week 12 IGA 0/1 EASI75 response resulted significantly higher in abrocitinib treated groups than placebo group.27 These results were still maintained after 16 weeks. Similarly, patients treated with dupilumab reached similar outcomes when compared to placebo at both weeks 12 and 16.7 Moreover, the percentage of patients who had a clinically significant reduction in P-NRS by week 2 was higher in abrocitinib 200 mg group than dupilumab, and not statistically significantly higher, for the abrocitinib 100 mg group than dupilumab.27 From the data currently available, the JADE COMPARE study showed a safety profile of abrocitinib consistent with prior trials.27
Ongoing Clinical Trials
Phase IIA
In the JADE MOA study (NCT03915496), a multicentre, double-blind, randomised phase IIA study, the mechanism of action of abrocitinib is evaluated and correlated to efficacy outcomes, with changes from baseline in key skin and blood biomarkers in adult (≥18-year-old) participants.32 The study provides for the enrolment of 51 patients and is currently in the recruiting phase. The participants will be randomized in a 1:1:1 ratio to receive abrocitinib 200 mg once daily (QD), abrocitinib 100 mg QD, or matching placebo QD for 12 weeks. The primary endpoint is the evaluation of expression in lesional and non-lesional skin of biomarkers for general inflammation (MMP12), hyperplasia (K16), Th2 immune response (CCL17, CCL18, CCL26), and Th22 immune response (S100A8, S100A9, S100A12), from baseline to week 12.32
Phase III
In the phase III study (JADE REGIMEN; NCT03627767) the efficacy and safety of abrocitinib has been evaluated in patients suffering from moderate to severe AD, aged ≥12 years, and with a body weight ≥40 kg.28 The study included 1234 subjects. After an initial 12-week, open-label treatment with abrocitinib 200 mg QD, the patients have been randomized to receive abrocitinib 200 mg QD, 100 mg QD, or placebo QD.28 The primary endpoint is the percentage of patients that 12 weeks after the randomization showed a loss of response to abrocitinib (defined as a loss of at least 50% of the EASI) requiring rescue treatment during the blinded treatment period. The main secondary endpoints are the change of IGA, EASI, P-NRS, SCORAD, BSA, and DLQI, from baseline to all timepoints. The study enrolment has been completed, but no results are available.28
In the JADE EXTEND study (NCT03422822), multi-center, long-term extension trial, the efficacy and safety of abrocitinib has been evaluated in about 3000 atopic patients aged 12 years and older.29 Abrocitinib was administered in 2 dosages, 100 mg and 200 mg taken orally QD.29 After a 92-week initial treatment period, the patients received the same dosage treatment in open-label until drug availability in commerce. The co-primary endpoints were the evaluation of incidence of AEs during the blinded phase of the study and until availability of commercial product, the changes from baseline in clinical laboratory values, the electrocardiogram measurements, and the vital signs. The main secondary endpoints were percentage of reduction of IGA, EASI, P-NRS, and BSA from baseline to week 92. The study is currently in the enrolment phase and is expected to end in December 2023. No results are available.29
In a phase IIIb randomized clinical trial (NCT04345367), the effectiveness and safety of abrocitinib 200 mg QD is compared to dupilumab 300 mg administered by subcutaneous injection every other week.30 To date, the study is in the enrolment phase, and the randomization of about 600 subjects is planned. The primary endpoints are the change percentage in P-NRS and EASI values, from baseline to week 4. The main secondary endpoints were: 1) the percentage of patients achieving EASI90 (≥90% improvement from baseline) at week 16; 2) the percentage of participants achieving EASI75 at week 16; 3) the percentage of patients achieving IGA 0/1 or with a reduction from baseline of ≥2 points at all scheduled time points; 4) percentage of subjects with at least a 4-point improvement in the severity of P-NRS; 5) percentage reduction of BSA.30 The estimated study completion date is July 2021.
To date, a multicentre clinical trial (NCT04564755) provides access to abrocitinib treatment, to adolescent (from 12 years of age) and adult patients with moderate to severe AD who have inadequate treatment options with available and approved medicated topical and systemic therapies.31 The participants will receive abrocitinib 100 mg or 200 mg orally QD.31 No further information is currently available.31
Conclusion
Selective JAK1 inhibitors seem to be effective and safe for AD treatment, with good oral bioavailability as well as a lack of immunogenicity, addressing some of the limitations of biologic drugs. Abrocitinib, among others, could change how to treat our moderate to severe AD patients. Indeed, the efficacy is significantly higher than placebo and seems to be comparable or higher than that of dupilumab, a monoclonal antibody largely used in patients with moderate-to-severe AD. Furthermore, abrocitinib improved pruritus firstly starting after 2 days of treatment. This rapid effect may increase adherence to treatment in patients before gaining clinical control of the disease. The safety and tolerability profile was acceptable; in fact, only few patients dropped-out during clinical trials. However, to evaluate its most appropriate role in AD treatment, it will be essential to have long-term real-life data and head-to-head studies for different JAK inhibitors and biologic drugs.
Funding Statement
There is no funding to report.
Disclosure
MN has acted as speaker, consultant and advisory board member for Sanofi, Abbvie, Leo Pharma and Novartis.
GF has been principal investigator in clinical trials sponsored by and/or and has received personal fees from AbbVie, Abiogen, Almirall, Celgene, Eli-Lilly, Leo Pharma, Novartis, Sanofi, and UCB.
CP has acted as investigator, speaker, consultant, and advisory board member for AbbVie, Eli Lilly, Novartis, Pfizer and Sanofi.
The authors report no other potential conflicts of interest for this work.
References
- 1.Sidbury R, Kodama S. Atopic dermatitis guidelines: diagnosis, systemic therapy, and adjunctive care. Clin Dermatol. 2018;36:648–652. doi: 10.1016/j.clindermatol.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 2.Torres T, Ferreira EO, Gonçalo M, Mendes-Bastos P, Selores M, Filipe P. Update on atopic dermatitis. Acta Med Port. 2019;32:606–613. doi: 10.20344/amp.11963 [DOI] [PubMed] [Google Scholar]
- 3.Napolitano M, Ruggiero A, Fontanella G, Fabbrocini G, Patruno C. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2020;e14475. [DOI] [PubMed] [Google Scholar]
- 4.Szalus K, Trzeciak M, Nowicki RJ. JAK-STAT inhibitors in atopic dermatitis from pathogenesis to clinical trials results. Microorganisms. 2020;8:E1743. doi: 10.3390/microorganisms8111743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He H, Guttman-Yassky E. JAK inhibitors for atopic dermatitis: an update. Am J Clin Dermatol. 2019;20:181–192. doi: 10.1007/s40257-018-0413-2 [DOI] [PubMed] [Google Scholar]
- 6.Czarnowicki T, Krueger JG, Guttman-Yassky E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol. 2017;139:1723–1734. doi: 10.1016/j.jaci.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Czarnowicki T, Gonzalez J, Shemer A, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136:104–115. doi: 10.1016/j.jaci.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 8.Werfel T, Allam J-P, Biedermann T, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:336–349. doi: 10.1016/j.jaci.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 9.Guttman-Yassky E, Waldman A, Ahluwalia J, Ong PY, Eichenfield LF. Atopic dermatitis: pathogenesis. Semin Cutan Med Surg. 2017;36:100–103. doi: 10.12788/j.sder.2017.036 [DOI] [PubMed] [Google Scholar]
- 10.Chu CY. Treatments for childhood atopic dermatitis: an update on emerging therapies. Clin Rev Allergy Immunol. 2020. doi: 10.1007/s12016-020-08799-1 [DOI] [PubMed] [Google Scholar]
- 11.Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139:S65–S76. doi: 10.1016/j.jaci.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutowska-Owsiak D, Schaupp AL, Salimi M, et al. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp Dermatol. 2012;21:104–110. doi: 10.1111/j.1600-0625.2011.01412.x [DOI] [PubMed] [Google Scholar]
- 14.Cotter DG, Schairer D, Eichenfield L. Emerging therapies for atopic dermatitis: JAK inhibitors. J Am Acad Dermatol. 2018;78:S53–S62. doi: 10.1016/j.jaad.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 15.Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat Immunol. 2017;18:374–384. doi: 10.1038/ni.3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira S, Guttman-Yassky E, Torres T. Selective JAK1 inhibitors for the treatment of atopic dermatitis: focus on upadacitinib and abrocitinib. Am J Clin Dermatol. 2020;21:783–798. doi: 10.1007/s40257-020-00548-6 [DOI] [PubMed] [Google Scholar]
- 17.Crowley EL, Nezamololama N, Papp K, Gooderham MJ. Abrocitinib for the treatment of atopic dermatitis. Expert Rev Clin Immunol. 2020;16:955–962. doi: 10.1080/1744666X.2021.1828068 [DOI] [PubMed] [Google Scholar]
- 18.Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2:e24137. doi: 10.4161/jkst.24137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napolitano M, Marasca C, Fabbrocini G, Patruno C. Adult atopic dermatitis: new and emerging therapies. Expert Rev Clin Pharmacol. 2018;11:867–878. doi: 10.1080/17512433.2018.1507734 [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues MA, Torres T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J Dermatolog Treat. 2020;31:33–40. doi: 10.1080/09546634.2019.1577549 [DOI] [PubMed] [Google Scholar]
- 21.Napolitano M, Fabbrocini G, Cinelli E, Stingeni L, Patruno C. Profile of baricitinib and its potential in the treatment of moderate to severe atopic dermatitis: a short review on the emerging clinical evidence. J Asthma Allergy. 2020;13:89–94. doi: 10.2147/JAA.S206387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.New oral treatment for moderate to severe atopic dermatitis. Available from: https://www.ema.europa.eu/en/news/new-oral-treatment-moderate-severe-atopic-dermatitis. Accessed January18, 2021.
- 23.Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371–1379. doi: 10.1001/jamadermatol.2019.2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peeva E, Hodge MR, Kieras E, et al. Evaluation of a Janus kinase 1 inhibitor, PF-04965842, in healthy subjects: a Phase 1, randomized, placebo-controlled, dose-escalation study. Br J Clin Pharmacol. 2018;84:1776–1788. doi: 10.1111/bcp.13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, Phase 3 trial. Lancet. 2020;396:255–266. doi: 10.1016/S0140-6736(20)30732-7 [DOI] [PubMed] [Google Scholar]
- 26.Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863–873. doi: 10.1001/jamadermatol.2020.1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Study evaluating efficacy and safety of PF-04965842 and dupilumab in adult subjects with moderate to severe atopic dermatitis on background topical therapy (JADE compare). Available from: https://www.clinicaltrials.gov/ct2/show/NCT03720470?term=NCT03720470&draw=2&rank=1. Accessed January18, 2021.
- 28.Study to investigate efficacy and safety of PF-04965842 in subjects aged 12 years and over with moderate to severe atopic dermatitis with the option of rescue treatment in flaring subjects. Available from: https://www.clinicaltrials.gov/ct2/show/NCT03627767?term=abrocitinib&cond=Atopic+Dermatitis&draw=2&rank=5. Accessed January18, 2021.
- 29.Study to evaluate efficacy and safety of PF-04965842 with or without topical medications in subjects aged 12 years and older with moderate to severe atopic dermatitis (JADE EXTEND). Available from: https://www.clinicaltrials.gov/ct2/show/NCT03422822?term=abrocitinib&cond=Atopic+Dermatitis&draw=2&rank=3. Accessed January18, 2021.
- 30.Study of abrocitinib compared with dupilumab in adults with moderate to severe atopic dermatitis on background topical therapy. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04345367?term=abrocitinib&cond=Atopic+Dermatitis&draw=2&rank=2. Accessed January18, 2021.
- 31.Abrocitinib expanded access protocol in adolescents and adults with moderate to severe atopic dermatitis. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04564755?term=abrocitinib&cond=Atopic+Dermatitis&draw=2&rank=1. Accessed January18, 2021.
- 32.Study evaluating the mechanism of action of PF-04965842 monotherapy for moderate-to-severe atopic dermatitis (JADE MOA). Available from: https://www.clinicaltrials.gov/ct2/show/NCT03915496?term=abrocitinib&cond=Atopic+Dermatitis&draw=2&rank=4. Accessed January18, 2021.