Abstract
Nanoemulsions are gaining importance in healthcare and cosmetics sectors as a result of the unique properties of nanosized droplets, such as high surface area. Here we review nanotechnology and nanoemulsions with focus on emulsifiers and nanoemulsifiers, and applications for drugs and vaccines delivery, cancer therapy, inflammation treatment, cosmetics, perfumes, polymers, and food. We discuss nanoemulsion safety and properties, e.g., stability, emulsification, solubility, molecular number and arrangements, ionic strength, pH and temperature.
Keywords: Nanotechnology, Nanoparticles, Nanoemulsions, Emulsifiers, Applications
Introduction
Nanotechnology is developing rapidly in many sectors, especially cosmetics, pharmaceutics, agriculture and food industries (Chowdhury et al. 2017). Most of these interests are geared toward the development of lipophilic substances like fatty acids, flavors, colors, and drugs (Azmi et al. 2019). The need for using nanotechnology/nanoparticles in emulsion fabrication is crucial because emulsions have been produced from numerous ingredients and additives for so many years, creating markets and profitability. Carbohydrates, fats, proteins and other active components such as antioxidants, colorings, acidulants, flavorings, preservatives, benzoic acid, coenzyme-Q10, vitamins A and E, isoflavones, beta-carotene, citric acid, ascorbic acid, lutein, omega-3 fatty acids, minerals, among other bioactive compounds have contributed to improved taste, appearance, texture, fortification and stability, and as such innovatively inspired wide applications of nanoemulsions to foods for enhanced uptake, absorbability and bioavailability (Kumar and Sarkar 2018; Mcclements and Jafari 2018; Dasgupta et al. 2019a; Walia et al. 2019; Saini et al. 2020).
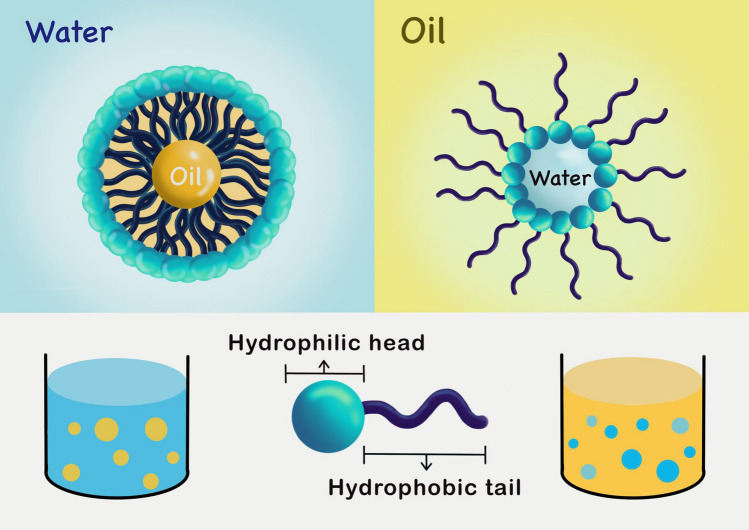
Research into nanoemulsions is extensive. These emulsions are disequilibrated systems of water-in-oil (W/O) or oil-in-water (O/W) emulsions (Fig. 1) within nanometer-range particle sizes and droplet diameters of 50–1000 nm (Yukuyama et al. 2016); otherwise, nanoemulsions are referred to as dispersed systems with ≤ 100 nm droplets (Azmi et al. 2019). Nanoemulsions are immiscible liquids consisting of oil and water forming a single phase by an emulsifier such as the surfactants and co-surfactants. The combination of these constituents confers high thermodynamics, stability and other physicochemical properties on the emulsion. Thus, the versatility of nanoemulsions becomes greater than that of conventional emulsions, including microemulsions and macroemulsions.
Fig. 1.
Oil-in-water and water-in-oil emulsions. Nanoemulsions are disequilibrated systems of water-in-oil (W/O) or oil-in-water (O/W) emulsions. They are immiscible liquids consisting of oil and water forming a single phase by an emulsifier such as the surfactants and co-surfactants, the combination of which confers high thermodynamics, stability and other physicochemical properties on the emulsion
The Federal Drug Administration (FDA) has utilized molecularity and functions of some emulsifiers as the basis of approval for their use in pharmaceutical and food industries (McClements et al. 2017). Stability, emulsification and solubility of emulsions are inspired by their surface-active nature dovetailed with molecular number and arrangements, ionic strength, pH and temperature of the mixture (McClements et al. 2017). Since the preparations of nanoemulsions and their emulsifiers are crucial and paramount to the interests of key industries, the aim of the present review is to evaluate the concept of nanotechnology, the formation and the applications of nanoemulsions in the healthcare and cosmetics industries, as well as their potentials in foods and other sectors in this present century. Further considerations were also put forward. To conduct the review, web of science (WoS), google and springer search engines were used, using the words and phrases “nano”, “nanosize”, “nanotechnology”, “emulsion”, “nanoemulsion”, “applications of nanoemulsions” and “applications of emulsions”, filtering the year range to 2000–2020.
Nanotechnology and nanoemulsions
Nanotechnology is a global concept that entails the manipulation of particles within the range of 10–1000 nm using scientific, innovative and well-characterized techniques, for improved productivity and applications in agriculture, healthcare, cosmetics, defense, energy and food sectors (Kaul et al. 2018; Feng et al. 2018; Aziz et al. 2019). The world’s nanotechnology industry could worth 80 billion US dollars and could be reaching 17% growth rate by 2024. Therefore, the potential benefits and profits are huge in this emerging market (Research and Market 2018). Application of the technology to the food industry has always been towards emulsions, encapsulation and packaging technologies, of which nanoemulsions are of utmost economic importance (Dasgupta et al. 2019b).
Nanoemulsions are formed when an emulsifier is involved in the mixture of two immiscible liquids to form stable but kinetic dispersions with droplet sizes and diameters of ≤ 100 nm and 20–200 nm, respectively. The kinetic stability is achieved when nanoparticles Brownian motion surpasses the emulsions’ gravitational forces, which then stop the particles from aggregating (Wani et al. 2018). Being very minuscule in size, nanoemulsions offer great functional potentials such as enhanced stability, surface area, optical transparency, rheology and other functions associated with innovative technologies and fortification of many aqueous-based food and beverage products (Dasgupta et al. 2019b). Regarding nanoemulsions production, common methods used include high-pressure homogenization, phase-inversion temperature, ultrasonication, high-shear mixing, solvent displacement, emulsion inversion point, bubble bursting and spontaneous emulsification—all falling under the categories of low- and high-energy techniques (Mohammadi et al. 2016; O'Sullivan et al. 2018; Azmi et al. 2019; Dasgupta et al. 2019a, b).
Increasing rate of consumption of processed foods and beverages has led to increased use of food additives and processing aids such as emulsifiers, which are meant to enhance the texture, flavor, stability and shelf-life of foods (Roca-Saavedra et al. 2018; Vo et al. 2019). The hydrophilic and hydrophobic molecular groups in emulsifiers contribute to immiscibility of the formulated liquids, which then lead to much enhanced homogeneity and stability in the final products as found in butter, mayonnaise, dressings, chocolates, ketchups and yogurts (Shah et al. 2017; Vo et al. 2019). Emulsifiers have been estimated to constitute more than 70% of the world’s approved food ingredients (Shah et al. 2017), necessitating the importance and use of nanosized emulsifiers in this century.
Emulsifiers and nanoemulsifiers for nanoemulsions
Emulsifiers are surface-active molecules widely applied in the breakage of droplets into tiny and very small droplets. Emulsifiers concentration must reach the right amounts so as to overcome the interfacial forces, and to ensure that the coating rate is rapid (Artiga-Artigas et al., 2018). This phenomenon prevents the particles from aggregating whilst enabling longer storage time and better stability. Fabricating nanoemulsions implies that the emulsifiers are well absorbed at the oil–water interface and subsequently reduce the interfacial tension in order to loosen up the particles and easily disrupt the droplets. The emulsifiers then protectively coat the oil droplets to prevent aggregation and coagulation (Dasgupta et al. 2019a).
The mixture of oil and water in the process of emulsion formulation creates two immiscible phases based on the segregation caused by coalescing globules in dispersion. The use of emulsifiers or nanoemulsifiers from the generally regarded as safe (GRAS) agents and components, such as peptides, proteins, phospholipids, micro-molecular surfactants, and polysaccharides, for the production of emulsion systems in the appropriate ratios would contribute to their efficiency and stability. Table 1 illustrates some emulsifier-stabilized nanoemulsions. This stability results from circumnavigation of processes like Oswald ripening, especially when the surfactants/emulsifiers are mixed with oil and other components in the right ratio (Gordon et al. 2003).
Table 1.
Emulsifiers in nanoemulsion systems
| Type of emulsifier | Homogenization technique | Percentage of oil used | Percentage of emulsifier used | Oil phase used | Droplet size diameter (nm) | Citation |
|---|---|---|---|---|---|---|
| a. Non-ionic | ||||||
| Polyoxyethylene sorbitan monolaurate | Microfluidization/high-pressure valve homogenizer | 0.03, 1 | 1, 10 | Sunflower oil | 117–280 | Mao et al. (2009, 2010) |
| Microfluidization | 4 | 1.5 | Corn oil, Miglyol 812 and orange oil | 140–170 | Qian et al. (2012) | |
| Microfluidization | 5 | 1–10 | Corn oil | 113–143 | Qian and McClements (2011) | |
| Microfluidization/solvent evaporation | 0.3 | 0.5 | β-Carotene in hexane | 40–260 | Tan and Nakajima (2005) | |
| Microfluidization | 10 | 1 | Thyme oil/Miglyol 812 oil | 160–176 | Chang et al. (2012) | |
| Thyme oil/corn oil | 170–196 | |||||
| Sonication | 15 | 5.6 | Flaxseed oil | 135 | Kentish et al. (2008) | |
| High-pressure valve homogenizer | 3 | 4–12 | β-Carotene in MCT oil | 160–184 | Yuan et al. (2008) | |
| Polyoxyethylene sorbitan monostearate | Catastrophic phase inversion | 20 | 10–20 | Acetem 90–50 K | 100–200 | Bilbao-Sáinz et al. (2010) |
| High-pressure valve homogenizer | 3 | 4–12 | β-Carotene in MCT oil | 161–174 | Yuan et al. (2008) | |
| Microfluidization | 5 | 0.5 | Thyme oil/corn oil | 164–196 | Ziani et al. (2011) | |
| Polyoxyethylene sorbitan monooleate | Ultrasonication | 6 | 6–24 | Basil oil | 29–41 | Ghosh et al. (2013) |
| High-pressure valve homogenizer | 20/4/1 | 1 | PCL-liquid/Lipoid S-75/α-tocopherol | 170 | Hoeller et al. (2009) | |
| High-pressure valve homogenizer | 3 | 4–12 | MCT oil | 157–178 | Yuan et al. (2008) | |
| Microfluidization | 10 | 1 | Lemon oil | 217–296 | Rao and McClements (2011) | |
| Polyoxyethylene lauryl ether | Emulsification with low energy | 40–80 | 4–10 | Isohexadecane | 26–1277 | Peng et al. (2010) |
| Decaglycerol monolaurate | Microfluidization/high-pressure valve homogenizer | 0.03, 1 | 1, 10 | β-Carotene in sunflower oil | 115–279 | Mao et al.(2009, 2010) |
| Sucrose palmitate | Ultra-high-pressure homogenization | 8/2, 10 | 1 | d-limonene, trans-cinnamaldehyde, carvacrol in sunflower oil | 130–168 | Donsì et al. (2012) |
| Sucrose laureate | High-pressure valve homogenizer | 20/4/1 | 1 | PCL-liquid/lipoid S-75 /α-tocopherol | 161 | Hoeller et al. (2009) |
| Sucrose monopalmitate | Microfluidization | 10 | 1–20 | Lemon oil | 15–120 | Rao and McClements (2011) |
| b. Ionic | ||||||
| Pluronic F-68 | Ultrasonication | 25 | 1–2.5 | Olive oil | 379 | Wulff-Pérez et al. (2009) |
| Sesame oil | 368 | |||||
| Soybean oil | 380 | |||||
| Sodium dodecyl sulfate | Microfluidization | Silicone oil | 150 | Graves et al. (2005) | ||
| Microfluidization | 5 | 1–10 | Corn oil/octadecane | 92–131 | Qian and McClements (2011) | |
| c. Polysaccharide | ||||||
| Low-methoxyl pectin, amidated low-methoxyl pectin, high-methoxyl pectin | Ultra-Turrax | 20 | 0.5–3 | Itraconazole in chloroform | 200–900 | Burapapadh et al. (2010) |
| Itraconazole in Miglyol® 812 | > 2000 | |||||
| Succinylated waxy maize starch/octenyl succinate starch | High-pressure valve homogenizer | 10 | 15 | Neobee 1053 | 140 | Donsì et al. (2011) |
| Neobee 1095 | 130 | |||||
| Microfluidization/high-pressure valve homogenizer | 1 | 10 | β-Carotene in sunflower oil | 262–674 | Mao et al. (2010) | |
| High-pressure valve homogenizer | 12 | 12 | Peppermint oil/MCT oil | 184–228 | Liang et al. (2012) | |
| Maltodextrin/H-Cap | Microfluidization/sonication | 5, 10, 15 | 30/10 (40) | Fish oil | 174–274 | Jafari et al. (2007a,b) |
| d. Protein | ||||||
| Pea protein | High-pressure valve homogenizer | 8, 10 | 3 | Sunflower oil | 184–218 | Donsì et al. (2012) |
| Whey protein isolate-maltodextrin conjugate | Emulsification with high energy/evaporation | 10, 15, 20, 30 | 1 | Thymol in hexane | 67–420 | Shah et al. (2012) |
| Soy protein | Microfluidization | 0.1 | 1 | β-Carotene in hexane | 196 | Chu et al. (2007) |
| Whey protein concentrate | Microfluidization; microfluidization/sonication | 0.1; 20, 25 | 1; 10 | β-Carotene in hexane; d-limonene | 145; 125–387 | Chu et al. (2007), Jafari et al. (2006) |
| Whey protein isolate | High-pressure valve homogenizer | 15, 30, 45 | 4.3 | Pea nut oil | 146–236 | Cortés-Muñoz et al. (2009) |
| High-energy emulsification/solvent evaporation | 10 | 1 | Corn oil | 75–121 | Lee and McClements (2010) | |
| High-pressure valve homogenizer | 0.03, 1 | 1, 10 | β-Carotene in sunflower oil | 160–373 | Mao et al. (2010) | |
| High-pressure valve homogenizer | 20 | 4.5 | α-Tocopherol in palm oil | 200–500 | Relkin et al. (2011) | |
| β-Lactoglobulin (β-lg) | Microfluidization | 5 | 1–10 | Corn oil/octadecane | 162 | Qian and McClements (2011) |
| High-pressure valve homogenizer | 20 | 1 | Soy oil | 350 | Sarkar et al. (2009) | |
| Microfluidization | 10 | 1 | Corn oil | 181 | Ahmed et al. (2012) | |
| Miglyol® 812 | 174 | |||||
| Tributyrin | 1981 | |||||
| Maize germ protein | Combined aqueous extraction–ultrafiltration method | 5 | 3 | Maize germ oil bodies | 155 | Nikiforidis et al. (2011) |
| Sodium caseinate | Microfluidization | 0.05–0.3 | 0.5–5 | β-Carotene in hexane | 17 | Chu et al. (2007) |
| Microfluidization | 5 | 1–10 | Corn oil/octadecane | 179 | Qian and McClements (2011) | |
| High-pressure valve homogenizer | 40 | 3.6 | α-Tocopherol/low melting triacylglycerols | 255–416, 293–304 | Relkin et al. (2008, 2009) | |
Over time, the formulated emulsions exhibit droplet coalescence characterized with various behavioral patterns such as heterogeneous or homogeneous growths, in which droplet sizes decrease and create early phase separation, or increase with time, respectively. Indeed, microparticulated emulsions will exhibit a higher degree of aggregation than nanoparticulated emulsions because of the irreversible droplets coalescence taking place with the former’s bigger particle size (An et al. 2014). Moreover, the properties of emulsifier used, such as functional, sensory and physicochemical characteristics, can positively or negatively affect the rheology of the nanoemulsion droplets.
The hydrophile–lipophile balance (HLB) system (hydrophilic range > 10, lipophilic range = 1–10) should be considered when determining the right nano(emulsifiers) for nanoemulsions fabrication because the HLB system has been in use for more than half of the last century to prove optimum conditions of surfactants required for emulsions fabrication with desired characteristics (Macedo et al. 2006; Azmi et al. 2019).
Several studies on colloidal and nanoemulsion systems used emulsifiers due to their better interfacial diffusive properties compared to larger biopolymers, such as proteins and polysaccharides, but their metabolism and safety are of concern in the food industry (Adjonu et al. 2014). For protein and peptide-based emulsifiers, both plant and dairy sources have been widely investigated and applied in foods because they form a protectively strong film in the formed emulsions. They later become employed in nanoemulsions (Table 1d), with the most important of them being whey proteins. These proteins comprise 20% whey and 80% caseins with very high nutritional contents based on their essential amino acids composition. The whey proteins are basically α-lactalbumin, β-lactoglobulin, bovine serum albumin, lactoferrins, and immunoglobulins, and possess amphiphilic properties that make them crucial emulsifiers in foods (Foegeding et al. 2002; Adjonu et al. 2014; Zhao and Ashaolu 2020). Under optimal control, hydrolyzation of the proteins will yield shorter peptides with fewer secondary and tertiary structures, to expose the hidden hydrophobic core (Christiansen et al. 2004; Ashaolu 2020a, b). These outcomes increase their diffusion rate and interfacial capacity when compared to their native parent proteins. Both hydrophilic and hydrophobic mixed groups generated further increase their absorption tendencies to the oil droplets surfaces, which ensure much better stability (van der Ven et al. 2001).
In brief, the advantages of nanoemulsifiers/nanoemulsions above emulsifiers include: they are small-sized droplets that have larger surface area for enhanced absorption, with much less energy requirement (Gurpreet and Singh 2018); they play active role in solubilization of lipophilic drugs and suppression of off-flavors of the drugs (Yu et al. 2012); they are considered non-toxic and non-irritant in nature (Jaiswal et al. 2015); they stabilize chemically unstable compounds by protecting them from oxidative and light degradation (Kim et al. 2001); they can substitute liposomes with vessicles (Bouchemal et al. 2004) and improve the bioavailability of a drug (Lovelyn and Attama 2011; Yu et al. 2012), while several types of nanoemulsions can be formed in the likes of creams, liquids, and sprays. On the flip side, in order to stabilize the nanodroplets, higher concentration of surfactant and cosurfactant may be required by nanoemulsifiers (Lovelyn and Attama 2011); solubilization of high-melting substances could be limited (Azmi et al. 2019); environmental parameters such as temperature and pH could affect the stability of nanoemulsifiers (Mishra et al. 2014); and the surfactants used pharmaceutical applications must be non-toxic (Mishra et al. 2014).
Moreover, the use of nanoemulsifiers is currently limited. Adjonu et al. (2014) quite well noted certain potentials of whey protein hydrolysates as emulsifying agents in nanoemulsions. Other than protein- or peptide-based nanoemulsifiers, other groups of molecules are described in Table 1. The continuous studies on nanoemulsions and its use in food applications require a whole lot of further in-depth analyses and critical evaluations, especially for their safety validation.
Nanoemulsion applications
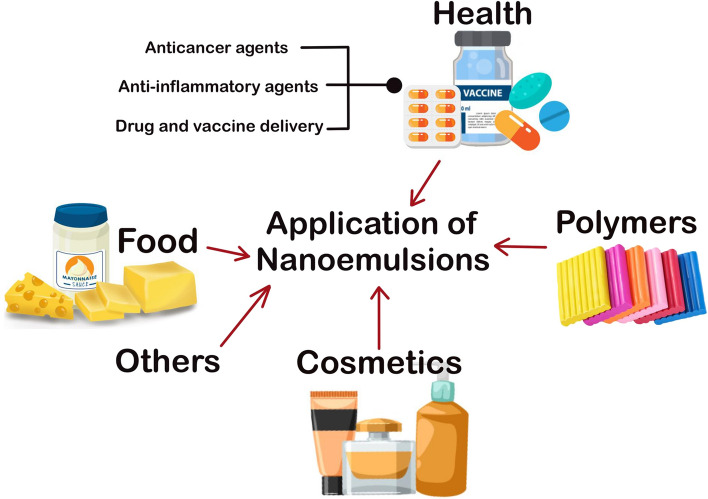
Recent studies on the applications of nanoemulsions are described below, schematically presented in Fig. 2 and captured in Tables 2 and 3. The present coronavirus disease (COVID-19) pandemic has called for a more robust and holistic approach to solving health-related issues, the highest priority of the modern human civilization.
Fig. 2.
An overview of nanoemulsions applications. Nanoemulsions are not only used in foods, they are now potent targets in healthcare delivery, cosmetics, polymers, among others. The physicochemical properties of nanoemulsions like stability, emulsification, solubility, ionic strength, pH and temperature are indicative of their functional purposes and utilization in several sectors other than the food industry
Table 2 .
Composition, fabrication methods and applications of nanoemulsions
| Citation | Composition | Fabrication method | Application/activity |
|---|---|---|---|
| Arredondo-Ochoa et al. (2017) | Beeswax–starch nanoemulsions; oil-in-water | Microfluidization with Tween-80 | Antimicrobial (against R. stolonifer, C. gloeosporioides, B. cinerea, and S. saintpaul); applied as edible coatings for food preservation |
| Bakshi et al. (2018) | Non-irritant topical formulation for topical delivery of heparinoid | Homogenization with high pressure | Therapeutic agent for superficial thrombophlebitis |
| Gharibzahedi and Mohammadnabi (2017) | Jujube gum with nettle essential oil; oil-in-water | Homogenization with Tween-40 | Antimicrobial; fabrication of jujube gum edible coating for Beluga sturgeon fillets |
| Salim et al. (2018) | Ibuprofen nanoemulsions | Phase inversion composition | Good stability; topical uses |
| Nirmal et al. (2018) | Lemon myrtle and anise myrtle essential oil in water | Ultrasonication | Antibacterial; good stability |
| Noori et al. (2018) | Sodium caseinate and ginger essential oil; oil-in-water | Ultrasonication with Tween-80 | Antimicrobial (L. monocytogenes and S. typhimurium); chicken breast fillet coating |
| Prakash et al. (2019) | Linalool-based nanoemulsions; oil-in-water | Ultrasonication with Tween-80 | Antibacterial (against S. typhimurium); antibacterial agent |
| Zhang et al. (2019) | Docosahexaenoic acid and eicosapentaenoic acid; oil-in-water | Emulsion phase inversion with Tween-80 and 85 | Antioxidant effect from tea polyphenols and good stability; applications in food fortification meant for commercial purpose |
| Lu et al. (2018) | Citral essential oil | Ultrasonication | Antimicrobial; utilizable in cosmetics, pharmaceutics and agrochemicals |
| Park et al. (2019) | Nanoemulsion powder consisting turmeric extract; oil-in-water | High-speed homogenization, ultrasonication and spray drying with Tween-80 | Antioxidant effect and good stability in gastric model; enhanced shelf-life of fortified milk for 3 weeks |
| Teo et al. (2017) | Phenytoin-loaded alkyd | Phase inversion method | Applied to topical wound healing |
| Farshi et al. (2019) | Cumin seed oil, corn oil, whey protein isolate-guar gum; oil-in-water | Ultrasonication and homogenization with Tween-80 | Antifungal (against A. flavus); food preservative |
| Kaci et al. (2018) | Coenzyme Q10 | Sonication | Topical uses |
| Pongsumpun et al. (2020) | Cinnamon essential oil; oil-in-water | Ultrasonication with Tween-80 | Antifungal (against A. niger, R. arrhizus, C. gloeosporioides and Penicillium sp.); food and agricultural uses |
| Sari et al. (2015) | Curcumin and medium chain triglyceride oil | Ultrasonication | Improved bioaccessibility and stability; applicable in functional foods |
| Majeed et al. (2016) | Purity gum ultra, canola and clove oil; water-in-oil | High speed homogenization | Antibacterial against Gram-positive strains; antimicrobial agent |
| Rebolleda et al. (2015) | Wheat bran oil-based nanoemulsions | Ultrasonication with Span-80 and Tween-80 | Good stability, antioxidant and tyrosinase inhibitory activities; Usable in functional foods |
| Gundewadi et al. (2018) | Sapindus extract and basil oil; oil-in-water | Ultrasonication with saponin | Antimicrobial (against P. chrysogenum and A. flavus); applied in food safety against food spoilage pathogens |
| Anjali et al. (2012) | Neem oil from A. indica and non-ionic surfactant (Tween 20) | Ultrasonication with Tween 20 | Active against Culex quinquefasciatus; usable as a larvicidal agent |
Table 3 .
Composition, fabrication methods and applications of nanoemulsions in foods
| Citation | Composition | Fabrication method | Application/activity |
|---|---|---|---|
| Arredondo-Ochoa et al. (2017) | Beeswax–starch nanoemulsions; oil-in-water | Microfluidization with Tween-80 | Antimicrobial (against R. stolonifer, C. gloeosporioides, B. cinerea, and S. saintpaul); applied as edible coatings for food preservation |
| Gharibzahedi and Mohammadnabi (2017) | Jujube gum with nettle essential oil; oil-in-water | Homogenization with Tween-40 | Antimicrobial; fabrication of jujube gum edible coating for Beluga sturgeon fillets |
| Nirmal et al. (2018) | Lemon myrtle and anise myrtle essential oil in water | Ultrasonication | Antibacterial; good stability |
| Noori et al. (2018) | Sodium caseinate and ginger essential oil; oil-in-water | Ultrasonication with Tween-80 | Antimicrobial (L. monocytogenes and S. typhimurium); chicken breast fillet coating |
| Prakash et al. (2019) | Linalool-based nanoemulsions; oil-in-water | Ultrasonication with Tween-80 | Antibacterial (against S. typhimurium); antibacterial agent |
| Zhang et al. (2019) | Docosahexaenoic acid and eicosapentaenoic acid; oil-in-water | Emulsion phase inversion with Tween-80 and 85 | Antioxidant effect from tea polyphenols and good stability; applications in food fortification meant for commercial purpose |
| Park et al. (2019) | Nanoemulsion powder consisting turmeric extract; oil-in-water | High-speed homogenization, ultrasonication and spray drying with Tween-80 | Antioxidant effect and good stability in gastric model; enhanced shelf-life of fortified milk for 3 weeks |
| Farshi et al. (2019) | Cumin seed oil, corn oil, whey protein isolate-guar gum; oil-in-water | Ultrasonication and homogenization with Tween-80 | Antifungal (against A. flavus); food preservative |
| Pongsumpun et al. (2020) | Cinnamon essential oil; oil-in-water | Ultrasonication with Tween-80 | Antifungal (against A. niger, R. arrhizus, C. gloeosporioides and Penicillium sp.); food and agricultural uses |
| Sari et al. (2015) | Curcumin and medium chain triglyceride oil | Ultrasonication | Improved bioaccessibility and stability; applicable in functional foods |
| Majeed et al. (2016) | Purity gum ultra, canola and clove oil; water-in-oil | High speed homogenization | Antibacterial against Gram-positive strains; antimicrobial agent |
| Rebolleda et al. (2015) | Wheat bran oil-based nanoemulsions | Ultrasonication with Span-80 and Tween-80 | Good stability, antioxidant and tyrosinase inhibitory activities; Usable in functional foods |
| Gundewadi et al. (2018) | Sapindus extract and basil oil; oil-in-water | Ultrasonication with saponin | Antimicrobial (against P. chrysogenum and A. flavus); applied in food safety against food spoilage pathogens |
Health
Drugs
Nanoemulsions have the ability to dissolve nonpolar active compounds, a characteristic paramount to being choice of use as drug and bioactive compounds delivery systems. Both oral and parenteral delivery routes are employable, as the latter has been utilized in supplying required nutrients, controlled drug release, and vaccine delivery. Systemic antibacterial, antifungal, antiparasitic and antimicrobial activities of nanoemulsions have been reported on E. coli, S. aureus, Candida spp., Dermatophytes spp., Plasmodium bergheii, among others (Singh and Vingkar 2008; Mansour et al. 2009).
Nanoemulsions are more advantageous than the conventional emulsions and other systems since their droplet sizes are below the micrometers range, and thus they easily pass the stringency of intravenous administration of drugs. The parenteral administration of nanoemulsions employed in nutrition of vitamins and other bioactive substances, attest to the merits they possess over other systems as their transit time, absorption, and efficacy are highly improved, while drug toxicity is reduced. Therefore, they are perfect drug delivery systems for antimicrobials, diuretics, steroids, and hormones (Sonneville-Aubrun et al. 2004; Singh and Vingkar 2008; Quintão et al. 2013).
Vaccines
Of equal importance is the delivery of vaccines by nanoemulsions because it is gaining much wider attention from researchers. An attenuated organism is delivered onto the surface of a mucosal in order to elicit an immune response. Nanoemulsions are used in this case as adjuvants to deliver proteins onto the mucosal surface in order to instigate rapid absorption of antigen presenting cells. Physical adsorption, encapsulation (with or without coating and targeting), and conjugation (with chemical or targeting) mechanisms are often used to load antigens into nanocarriers. In any of the mechanisms, antigens are encapsulated in nanocarriers, while the nanoparticles degrade in vivo (Zhao et al. 2014). The vaccines can be very effective and spontaneous, irrespective of the site they are introduced to in the body. For instance, the immunity of genital mucosa might be guaranteed by administering the vaccines to the nasal mucosal (Berkowitz and Goddard 2009).
Vaccine adjuvants of oil/water emulsions have notable prospects, as found in ASO3 pandemic flu and recombinant HIV gp 120 nanoemulsion-mixed vaccines (Akhter et al. 2008; Reed et al. 2009). The validation of these studies are still warranted. Moreover, the composition of emulsion, antigenicity and adjuvant specificity are paramount factors to consider when designing nanoemulsion-based vaccines. The essence of this is to ensure safety and effective immunological benefits.
Inflammation
Nanoemulsions could also be employed in anti-inflammatory functions. Free radicals are released by enzymes, toxic metabolites of pathogens, and inflammation mediators such as polymorphonuclear lymphocytes, leading to chronic inflammation. These same enzymes and metabolites deprive host cells of their required nutrients for growth. However, the mix of emulsifiers and oils was reportedly significant in phytochemicals absorption and treatment of inflammatory bowel disease (IBD), i.e., Crohn’s disease and ulcerative colitis (Yen et al. 2018) and periodontitis, a chronic inflammatory disease that erodes teeth’s supporting structures (Aithal et al. 2018).
Inflammatory bowel disease (IBD) is often characterized with inflamed intestinal wall where the colonic and rectal mucosa are impacted. This is continuous for ulcerative colitis while it may be transmural and discontinuous in Crohn’s disease. Host’s lifestyle, genetic make-up, oxidative stress, pathogenic attack, immune responses, and drastic changes of inflammatory mediator levels are factors associated with IBD (Head and Jurenka 2003). Meanwhile, some studies have shown the usefulness of phytochemicals in treating periodontitis and IBD, including diterpenoid and quercetin, respectively (Geoghegan et al. 2010). Due to their low water solubility, they are less bio-available and are poorly absorbed orally. To increase their absorbability, both phytochemicals were improved with emulsions (Azuma et al. 2002).
Cancer
Abnormal cell proliferation due to genetic coding errors generate cancer cells. Active angiogenesis and vascular density occur in order to utilize the blood supply for tumor tissues growth, and is supported by a microenvironment of the extracellular matrix, adipocytes, pericytes, immune cells, and others (Ganta et al. 2014). The use of anticancer drugs may result in inadequate solubility, toxicity to non-cancer cells, and poor selectivity of target cancer cells, while chemotherapy drugs may not be ideal in the long run due to their action on every form of proliferative cells, including hair follicles, bone marrow, red blood cells, gut epithelial cells, and lymphatic cells (Qiao et al. 2010; Mahato 2017).
Poor solubility and hydrophobicity of most anticancer drugs connote their inability to reach or effect their action on cancer cells (Sareen et al. 2012). This is where nanoemulsions are essential, as they proffer solubility to hydrophobic drugs and their stability. The resultant effect is that cancerous cells are selectively targeted earlier, leading to a high rate of successful cancer treatment. For instance, nanoemulsions could be engineered using specific ligands to target cells, tissues, or organs, all to improve the status quo in cancer therapy (Mahato 2017). Nanoparticles easily conjugate with multifunctional moieties, as found in nanoemulsions, aimed at drug delivery for cancer therapy via diagnostic means and imaging. Indeed, Tiwari et al. (2006) studied and used lipid-rich nanoemulsions containing fatty acids (omega-3 and omega-6 fatty acids, linoleic acid), non-glucose-based calories, and vitamins E and K as colloidal carriers for chemotherapeutic drugs, whilst using diagnostic and imaging techniques. Although the in vitro study was promising, the need for a validation and safety studies cannot be undermined. Table 2 offers more examples of nanoemulsion applications in healthcare delivery.
Cosmetics and others
Nanoemulsions could be traced to other applications such as complex matter developments and cosmetics, based on their liquid–liquid affinity to macromolecular moieties, minute size and large surface area. As building blocks of polymers, nanoemulsions could use their hydrophobic monomeric units in the droplets to generate polymers, with vast examples demonstrated in the studies of Asua (2002), Landfester (2006) and Gupta et al. (2016). Interestingly, from the development of varying protein shell structures with silicone oil-based nanoemulsions (Chang et al. 2008), amphiphilic photoreactive surfactants (de Oliveira et al. 2011), silica nanospheres (Wu et al. 2013), photoinduced and thermoreactive polymers or organogels (Helgeson et al. 2012), essential oils-based products (Barradas and e Silva 2020), suspended magnetic nanoparticles (Primo et al. 2007), to erythrocyte-like composite hydrogels (An et al. 2013), nanoemulsions have been studied or applied.
Moreover, nanoemulsions could be used in composite and crystal formulations on the premise of size specificity and accuracy of active pharmaceutical ingredients and other products, involving a low energy-requiring process (Eral et al. 2014). Indeed, nanosized oil-loaded droplets ensure penetration of the stratum corneum, making them highly useful in alcohol-free perfume formulations (Rai et al. 2018; Barradas and e Silva 2020). Noteworthy to mention is that some oils used in nanoemulsions proffer some benefits that should not be undermined, and could be useful in the encapsulation of volatile components like aromatic compounds and essential oils.
On another note, the surface area and small size of nanoemulsion droplets increase their propensity to lesser viscousness, making them utilizable in the cosmetics sector as they could more efficiently deliver active substances to the skin. They are no flocculants, coalescing or creaming agents, and demonstrate little or no sediments, making their applications far more important than conventional emulsions in cosmetology. In this regard, Vellozia squamata and Opuntia ficusindica (L.) Mill hydroglycolic extracts were studied and applied to fabricate nanoemulsion-based moisturizers, creams, anti-ageing agents (Quintão et al. 2013; Ribeiro et al. 2015). Similarly, oils like coconut, polyethylene glycol octyl phenyl ether and polyethylene glycol hydrogenated castor oil were included in nanoemulsions for cosmeceutical applications (Pengon et al. 2018).
Furthermore, the use of nano-gel technique for trans-epidermal water loss minimization, dermal protection, and efficacious active ingredients penetration has been suggested for inclusion in moisteners, anti-ageing creams and various sun care formulations (Mansour et al. 2009). For instance, Kemira nano-gel is a nanoemulsion-based patented cosmetics system meant to attain skin smoothness by enhancing high penetrative capacity of active ingredients and dermal cells production (Guglielmini 2008). Another patented example is that of L’Oreal (Paris, France), using nanoemulsion-based phosphoric acid fatty acid esters in cosmetics and pharmaceutical products, among others (Shah et al. 2010). Table 2 presents more on the overview of use of nanoemulsions in cosmetics and other non-food applications.
Food
Nanoemulsions have proven quite useful in bioavailability, bioactivity, digestibility, stability, safety, quality, and sensory enhancements of food components and natural extracts, such as lycopene-solubilized and β-carotene-based nanoemulsions (Nedovic et al. 2011; Bakshi et al. 2018), based on their wide surface area and small droplets. To remain stable, formulation of nanoemulsions requires the presence of an emulsifier, often acting as not only an additive but a preservative of the food products and/or the active components. As an example, surface-active molecules were introduced during the fabrication of basil oil nanoemulsions, and were found to exert antimicrobial activity against certain food spoiling fungi, including Aspergillus flavus and Penicillium chrysogenum (Gundewadi et al. 2018). A similar study was carried out on avocado oil-based nanoemulsions stabilized with Quillaja saponins (QS) where thermal stability was reportedly increased as QS was incorporated in the emulsions, a merit in the cause of safe and sterile emulsion-based food products development (Teo et al. 2017; Riquelme et al. 2019).
The use of nanoemulsions to stabilize essential oils in foods also assist with overcoming their high volatility, hydrophobicity, and reactivity with other food components, which could reduce their applications in the food sector. The stabilized oils or nanoemulsions thus improve the antioxidant and antimicrobial activities of the processed essential oils, enhancing their compatibility, solubility, stability, physicochemical equilibrium, and behaviors (Lawrence and Rees 2000; Bai et al. 2016; Ghasemi et al. 2018; Prakash et al. 2019). Other studies that have applied nanoemulsions in foods include corn oil-based β-carotene nanoemulsions at 300 nm (Borba et al. 2019), sensory efficient nanoemulsions constituted with Brazilian propolis extracts (Seibert et al. 2019), improved digestible curcumin nanoemulsions, and nanoemulsion-based fortified beverages with vitamin D3 (Golfomitsou et al. 2018; Maurya and Aggarwal 2019). The possibilities of nanoemulsions in foods and food products seem limitless. More examples are presented in Table 3.
Conclusion
Based on their physicochemical and functional properties, nanoemulsions have very promising multisectorial uses in healthcare, food, polymer manufacturing and cosmetics industries. Therefore, they have gained prominent attention in the scientific community. Recent uses of beeswax-starch, jujube gum, sodium caseinate, turmeric extract, linalool, docosahexaenoic and eicosapentaenoic acid, cumin seed oil, whey protein isolate, and oils like cinnamon, lemon and anise myrtle essential oils in nanoemulsion formulations (Arredondo-Ochoa et al. 2017; Gharibzahedi and Mohammadnabi 2017; Nirmal et al. 2018; Noori et al. 2018; Prakash et al. 2019; Zhang et al. 2019; Park et al. 2019; Farshi et al. 2019; Pongsumpun et al. 2020) have shown high potentials in the food industry and for the general well being. They can also deliver phytochemicals and other bioactive components in the food industry (Mahmood et al. 2017). In addition, nanoemulsions also have multifarious prospects in non-food applications shown recently in inflammatory and periodontitis treatment, agrochemical, cosmetics and pharmaceutics, texturizing agents and creams, non-steroidal anti-inflammatory drug, drug and vaccine delivery, cosmeceutical applications and alcohol-free perfume formulations (Aithal et al. 2018; Teo et al. 2017; Bakshi et al. 2018; Lu et al. 2018; Kaci et al. 2018; Salim et al. 2018; Pengon et al. 2018; Rai et al. 2018; Shaker et al. 2019; Kumar et al. 2019; Barradas and e Silva 2020).
Formulating nanoemulsions often employ emulsifiers/surfactants and nanoparticles, which have raised eyebrows regarding their safety because they accumulate both in the environment and in the human body (Bajpai et al. 2018). Any engineered nanoparticulates or materials attract some degree of attention due to limited comprehension of their mechanisms and health consequences, a major reason to delay further implementation of nanotechnology in the food industry (Loira et al. 2020). For instance, long-term exposure to silver nanoparticles could lead to cell damage and inflammation via oxidative stress reactions (Gaillet and Rouanet 2015; Smolkova et al. 2015). Thus, it is required that the safety of nanoparticles-based systems such as nanoemulsions must be ascertained, along with their physicochemical characteristics in order to help with tactile conclusions and policies of regulatory bodies.
In addition, the methods and conditions associated with nanoemulsions production will affect their stability and other physicochemical properties, with corresponding impacts on their applications. Hence, specificity is key. Solubility, availability, activity, acceptability and polarity would be noteworthy concerns to look at, when developing nanoemulsions. Considering all these factors would inadvertently affect the cost of production for much larger commercial applications. Conclusively, nanoemulsions indeed could be utilized in several sectors other than the food industry.
Acknowledgements
The author expresses his profound gratitude to the Institute of Research and Development, Duy Tan University, Da Nang, Vietnam.
Authors' contributions
TJA conceptualized, researched, compiled and analyzed data, as well as drafted, edited and revised the manuscript.
Funding
No external funding was received for this work.
Availability of data and material
No dataset were generated in this study.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The author has no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adjonu R, Doran G, Torley P, et al. Whey protein peptides as components of nanoemulsions: A review of emulsifying and biological functionalities. J Food Eng. 2014;122:15–27. doi: 10.1016/j.jfoodeng.2013.08.034. [DOI] [Google Scholar]
- Ahmed K, Li Y, McClements DJ, et al. Nanoemulsion-and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012;132(2):799–807. doi: 10.1016/j.foodchem.2011.11.039. [DOI] [Google Scholar]
- Aithal GC, Nayak UY, Mehta C, et al. Localized in situ nanoemulgel drug delivery system of quercetin for periodontitis: development and computational simulations. Molecules. 2018;23(6):1363. doi: 10.3390/molecules23061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter S, Jain GK, Ahmad FJ, et al. Investigation of nanoemulsion system for transdermal delivery of domperidone: ex-vivo and in vivo studies. Curr Nanosci. 2008;4(4):381–390. doi: 10.2174/157341308786306071. [DOI] [Google Scholar]
- An HZ, Safai ER, Eral HB, et al. Synthesis of biomimetic oxygen-carrying compartmentalized microparticles using flow lithography. Lab Chip. 2013;13(24):4765–4774. doi: 10.1039/C3LC50610J. [DOI] [PubMed] [Google Scholar]
- An Y, Yan X, Li B, et al. Microencapsulation of capsanthin by self-emulsifying nanoemulsions and stability evaluation. Eur Food Res Technol. 2014;239(6):1077–1085. doi: 10.1007/s00217-014-2328-3. [DOI] [Google Scholar]
- Anjali CH, Sharma Y, Mukherjee A, et al. Neem oil (Azadirachta indica) nanoemulsion—a potent larvicidal agent against Culex quinquefasciatus. Pest Manag Sci. 2012;68(2):158–163. doi: 10.1002/ps.2233. [DOI] [PubMed] [Google Scholar]
- Arredondo-Ochoa T, García-Almendárez BE, Escamilla-García M, et al. Physicochemical and antimicrobial characterization of beeswax–starch food-grade nanoemulsions incorporating natural antimicrobials. Int J Mol Sci. 2017;18(12):2712. doi: 10.3390/ijms18122712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiga-Artigas M, Guerra-Rosas MI, Morales-Castro J, et al. Influence of essential oils and pectin on nanoemulsion formulation: a ternary phase experimental approach. Food hydrocoll. 2018;81:209–219. doi: 10.1016/j.foodhyd.2018.03.001. [DOI] [Google Scholar]
- Ashaolu TJ. Applications of soy protein hydrolysates in the emerging functional foods: a review. Int J Food Sci Technol. 2020;55(2):421–428. doi: 10.1111/ijfs.14380. [DOI] [Google Scholar]
- Ashaolu TJ. Soy bioactive peptides and the gut microbiota modulation. Appl Microbiol Biotechnol. 2020;104:9009–9017. doi: 10.1007/s00253-020-10799-2. [DOI] [PubMed] [Google Scholar]
- Asua JM. Miniemulsion polymerization. Prog Polym Sci. 2002;27(7):1283–1346. doi: 10.1016/S0079-6700(02)00010-2. [DOI] [Google Scholar]
- Aziz ZAA, Mohd-Nasir H, Setapar M, et al. Role of nanotechnology for design and development of cosmeceutical: application in makeup and skin care. Front Chem. 2019;7:739. doi: 10.3389/fchem.2019.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi NAN, Elgharbawy AA, Motlagh SR, et al. Nanoemulsions: factory for food, pharmaceutical and cosmetics. Processes. 2019;7(9):617. doi: 10.3390/pr7090617. [DOI] [Google Scholar]
- Azuma K, Ippoushi K, Ito H, et al. Combination of lipids and emulsifiers enhances the absorption of orally administered quercetin in rats. J Agric Food Chem. 2002;50(6):1706–1712. doi: 10.1021/jf0112421. [DOI] [PubMed] [Google Scholar]
- Bai L, Huan S, Gu J, et al. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016;61:703–711. doi: 10.1016/j.foodhyd.2016.06.035. [DOI] [Google Scholar]
- Bajpai VK, Kamle M, Shukla S, et al. Prospects of using nanotechnology for food preservation, safety, and security. J Food Drug Anal. 2018;26(4):1201–1214. doi: 10.1016/j.jfda.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi P, Jiang Y, Nakata T, et al. Formulation development and characterization of nanoemulsion-based formulation for topical delivery of heparinoid. J Pharm Sci. 2018;107(11):2883–2890. doi: 10.1016/j.xphs.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Barradas TN, e Silva KGDH. Nanoemulsions of essential oils to improve solubility, stability and permeability: a review. Environ Chem Lett. 2020 doi: 10.1007/s10311-020-01142-2. [DOI] [Google Scholar]
- Berkowitz AC, Goddard DM. Novel drug delivery systems: future directions. J Neurosci Nurs. 2009;41(2):115–120. doi: 10.1097/jnn.0b013e318193458b. [DOI] [PubMed] [Google Scholar]
- Bilbao-Sáinz C, Avena-Bustillos RJ, Wood DF, et al. Nanoemulsions prepared by a low-energy emulsification method applied to edible films. J Agric Food Chem. 2010;58(22):11932–11938. doi: 10.1021/jf102341r. [DOI] [PubMed] [Google Scholar]
- Borba CM, Tavares MN, Macedo LP, et al. Physical and chemical stability of β-carotene nanoemulsions during storage and thermal process. Food Res Int. 2019;121:229–237. doi: 10.1016/j.foodres.2019.03.045. [DOI] [PubMed] [Google Scholar]
- Bouchemal K, Briançon S, Perrier E, et al. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J pharm. 2004;280(1–2):241–251. doi: 10.1016/j.ijpharm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Burapapadh K, Kumpugdee-Vollrath M, Chantasart D, et al. Fabrication of pectin-based nanoemulsions loaded with itraconazole for pharmaceutical application. Carb Polym. 2010;82(2):384–393. doi: 10.1016/j.carbpol.2010.04.071. [DOI] [Google Scholar]
- Chang CB, Knobler CM, Gelbart WM, et al. Curvature dependence of viral protein structures on encapsidated nanoemulsion droplets. ACS Nano. 2008;2(2):281–286. doi: 10.1021/nn700385z. [DOI] [PubMed] [Google Scholar]
- Chang Y, McLandsborough L, McClements DJ. Physical properties and antimicrobial efficacy of thyme oil nanoemulsions: influence of ripening inhibitors. J Agric Food Chem. 2012;60(48):12056–12063. doi: 10.1021/jf304045a. [DOI] [PubMed] [Google Scholar]
- Chowdhury P, Gogoi M, Borchetia S, et al. Nanotechnology applications and intellectual property rights in agriculture. Environ Chem Lett. 2017;15(3):413–419. doi: 10.1007/s10311-017-0632-4. [DOI] [Google Scholar]
- Christiansen KF, Vegarud G, Langsrud T, et al. Hydrolyzed whey proteins as emulsifiers and stabilizers in high-pressure processed dressings. Food Hydrocoll. 2004;18(5):757–767. doi: 10.1016/j.foodhyd.2003.12.002. [DOI] [Google Scholar]
- Cortés-Muñoz M, Chevalier-Lucia D, Dumay E. Characteristics of submicron emulsions prepared by ultra-high pressure homogenisation: effect of chilled or frozen storage. Food hydrocoll. 2009;23(3):640–654. doi: 10.1016/j.foodhyd.2008.07.023. [DOI] [Google Scholar]
- Dasgupta N, Ranjan S, Gandhi M. Nanoemulsion ingredients and components. Environ Chem Lett. 2019;17(2):917–928. doi: 10.1007/s10311-018-00849-7. [DOI] [Google Scholar]
- Dasgupta N, Ranjan S, Gandhi M. Nanoemulsions in food: market demand. Environ Chem Lett. 2019;17(2):1003–1009. doi: 10.1007/s10311-019-00856-2. [DOI] [Google Scholar]
- de Oliveira RJ, Brown P, Correia GB, et al. Photoreactive surfactants: a facile and clean route to oxide and metal nanoparticles in reverse micelles. Langmuir. 2011;27(15):9277–9284. doi: 10.1021/la202147h. [DOI] [PubMed] [Google Scholar]
- Donsì F, Annunziata M, Vincensi M, et al. Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J Biotechnol. 2012;159(4):342–350. doi: 10.1016/j.jbiotec.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Donsì F, Wang Y, Huang Q. Freeze–thaw stability of lecithin and modified starch-based nanoemulsions. Food Hydrocoll. 2011;25(5):1327–1336. doi: 10.1016/j.foodhyd.2010.12.008. [DOI] [Google Scholar]
- Eral HB, López-Mejías V, O’Mahony M, et al. Biocompatible alginate microgel particles as heteronucleants and encapsulating vehicles for hydrophilic and hydrophobic drugs. Crystal Growth Des. 2014;14(4):2073–2082. doi: 10.1021/cg500250e. [DOI] [Google Scholar]
- Farshi P, Tabibiazar M, Ghorbani M, et al. Whey protein isolate-guar gum stabilized cumin seed oil nanoemulsion. Food Biosci. 2019;28:49–56. doi: 10.1016/j.fbio.2019.01.011. [DOI] [Google Scholar]
- Feng J, Zhang Q, Liu Q et al (2018) Application of nanoemulsions in formulation of pesticides. In: Nanoemulsions. Academic Press, pp 379–413. 10.1016/B978-0-12-811838-2.00012-6
- Foegeding EA, Davis JP, Doucet D, et al. Advances in modifying and understanding whey protein functionality. Trends Food Sci Technol. 2002;13(5):151–159. doi: 10.1016/s0924-2244(02)00111-5. [DOI] [Google Scholar]
- Gaillet S, Rouanet JM. Silver nanoparticles: their potential toxic effects after oral exposure and underlying mechanisms—a review. Food Chem Toxicol. 2015;77:58–63. doi: 10.1016/j.fct.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Ganta S, Talekar M, Singh A, et al. Nanoemulsions in translational research—opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech. 2014;15(3):694–708. doi: 10.1208/s12249-014-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan F, Wong RWK, Rabie ABM. Inhibitory effect of quercetin on periodontal pathogens in vitro. Phytother Res. 2010;24(6):817–820. doi: 10.1002/ptr.3014. [DOI] [PubMed] [Google Scholar]
- Gharibzahedi SMT, Mohammadnabi S. Effect of novel bioactive edible coatings based on jujube gum and nettle oil-loaded nanoemulsions on the shelf-life of Beluga sturgeon fillets. Int J Biol Macromol. 2017;95:769–777. doi: 10.1016/j.ijbiomac.2016.11.119. [DOI] [PubMed] [Google Scholar]
- Ghasemi S, Jafari SM, Assadpour E, et al. Nanoencapsulation of d-limonene within nanocarriers produced by pectin-whey protein complexes. Food Hydrocoll. 2018;77:152–162. doi: 10.1016/j.foodhyd.2017.09.030. [DOI] [Google Scholar]
- Ghosh V, Mukherjee A, Chandrasekaran N. Ultrasonic emulsification of food-grade nanoemulsion formulation and evaluation of its bactericidal activity. Ultrason Sonochem. 2013;20(1):338–344. doi: 10.1016/j.ultsonch.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Golfomitsou I, Mitsou E, Xenakis A, et al. Development of food grade O/W nanoemulsions as carriers of vitamin D for the fortification of emulsion based food matrices: a structural and activity study. J Mol Liq. 2018;268:734–742. doi: 10.1016/j.molliq.2018.07.109. [DOI] [Google Scholar]
- Gordon EM, Cornelio GH, Lorenzo CC, et al. First clinical experience using a ‘pathotropic’injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int J Oncol. 2003;24(1):177–185. doi: 10.3892/ijo.24.1.177. [DOI] [PubMed] [Google Scholar]
- Graves S, Meleson K, Wilking J, et al. Structure of concentrated nanoemulsions. J Chem Phys. 2005;122(13):134703. doi: 10.1063/1.1874952. [DOI] [PubMed] [Google Scholar]
- Guglielmini G. Nanostructured novel carrier for topical application. Clin Dermatol. 2008;26(4):341–346. doi: 10.1016/j.clindermatol.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Gundewadi G, Sarkar DJ, Rudra SG, et al. Preparation of basil oil nanoemulsion using Sapindus mukorossi pericarp extract: physico-chemical properties and antifungal activity against food spoilage pathogens. Indus Crops Prod. 2018;125:95–104. doi: 10.1016/j.indcrop.2018.08.076. [DOI] [Google Scholar]
- Gupta A, Eral HB, Hatton TA, et al. Nanoemulsions: formation, properties and applications. Soft Mat. 2016;12(11):2826–2841. doi: 10.1039/C5SM02958A. [DOI] [PubMed] [Google Scholar]
- Gurpreet K, Singh SK. Review of nanoemulsion formulation and characterization techniques. Indian J Pharm Sci. 2018;80(5):781–789. doi: 10.4172/pharmaceutical-sciences.1000422. [DOI] [Google Scholar]
- Head KA, Jurenka JS. Inflammatory bowel disease part 1: ulcerative colitis–pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2003;8(3):247–283. [PubMed] [Google Scholar]
- Helgeson ME, Moran SE, An HZ, et al. Mesoporous organohydrogels from thermogelling photocrosslinkable nanoemulsions. Nat Mater. 2012;11(4):344–352. doi: 10.1038/nmat3248. [DOI] [PubMed] [Google Scholar]
- Hoeller S, Sperger A, Valenta C. Lecithin based nanoemulsions: a comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int J Pharm. 2009;370(1–2):181–186. doi: 10.1016/j.ijpharm.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Optimization of nano-emulsions production by microfluidization. Eur Food Res Technol. 2007;225(5–6):733–741. doi: 10.1007/s00217-006-0476-9. [DOI] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J Food Eng. 2007;82(4):478–488. doi: 10.1016/j.foodeng.2007.03.007. [DOI] [Google Scholar]
- Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–127. doi: 10.1007/s13205-014-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaci M, Belhaffef A, Meziane S, et al. Nanoemulsions and topical creams for the safe and effective delivery of lipophilic antioxidant coenzyme Q10. Colloids Surf B Biointerf. 2018;167:165–175. doi: 10.1016/j.colsurfb.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Kaul S, Gulati N, Verma D, et al. Role of nanotechnology in cosmeceuticals: a review of recent advances. J Pharm. 2018 doi: 10.1155/2018/3420204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish S, Wooster TJ, Ashokkumar M, et al. The use of ultrasonics for nanoemulsion preparation. Innov Food Sci Emerg Technol. 2008;9(2):170–175. doi: 10.1016/j.ifset.2007.07.005. [DOI] [Google Scholar]
- Kim CK, Cho YJ, Gao ZG. Preparation and evaluation of biphenyl dimethyl dicarboxylate microemulsions for oral delivery. J Control Rel. 2001;70(1–2):149–155. doi: 10.1016/s0168-3659(00)00343-6. [DOI] [PubMed] [Google Scholar]
- Kumar DL, Sarkar P. Encapsulation of bioactive compounds using nanoemulsions. Environ Chem Lett. 2018;16(1):59–70. doi: 10.1007/s10311-017-0663-x. [DOI] [Google Scholar]
- Kumar M, Bishnoi RS, Shukla AK, et al. Techniques for formulation of nanoemulsion drug delivery system: a review. Prev Nutr Food Sci. 2019;24(3):225. doi: 10.3746/pnf.2019.24.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfester K. Synthesis of colloidal particles in miniemulsions. Annu Rev Mater Res. 2006;36:231–279. doi: 10.1146/annurev.matsci.36.032905.091025. [DOI] [Google Scholar]
- Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Del Rev. 2000;45(1):89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McClements DJ. Fabrication of protein-stabilized nanoemulsions using a combined homogenization and amphiphilic solvent dissolution/evaporation approach. Food Hydrocoll. 2010;24(6–7):560–569. doi: 10.1016/j.foodhyd.2010.02.002. [DOI] [Google Scholar]
- Liang R, Xu S, Shoemaker CF, et al. Physical and antimicrobial properties of peppermint oil nanoemulsions. J Agric Food Chem. 2012;60(30):7548–7555. doi: 10.1021/jf301129k. [DOI] [PubMed] [Google Scholar]
- Loira I, Morata A, Escott C, et al. Applications of nanotechnology in the winemaking process. Eur Food Res Technol. 2020 doi: 10.1007/s00217-020-03519-7. [DOI] [Google Scholar]
- Lovelyn C, Attama AA. Current state of nanoemulsions in drug delivery. J Biomater Nanobiotechnol. 2011;2(05):626. doi: 10.4236/jbnb.2011.225075. [DOI] [Google Scholar]
- Lu WC, Huang DW, Wang CC, et al. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J Food Drug Anal. 2018;26(1):82–89. doi: 10.1016/j.jfda.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo JP, Fernandes LL, Formiga FR, et al. Micro-emultocrit technique: a valuable tool for determination of critical HLB value of emulsions. AAPS PharmSciTech. 2006;7(1):146–152. doi: 10.1208/pt070121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahato R. Nanoemulsion as targeted drug delivery system for cancer therapeutics. J Pharm Sci pharmacol. 2017;3(2):83–97. doi: 10.1166/jpsp.2017.1082. [DOI] [Google Scholar]
- Mahmood Z, Jahangir M, Liaquat M, et al. Potential of nano-emulsions as phytochemical delivery system for food preservation. Pak J Pharm Sci. 2017;30(6):2259–2263. [PubMed] [Google Scholar]
- Majeed H, Liu F, Hategekimana J, et al. Bactericidal action mechanism of negatively charged food grade clove oil nanoemulsions. Food Chem. 2016;197:75–83. doi: 10.1016/j.foodchem.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Mansour HM, Rhee YS, Wu X. Nanomedicine in pulmonary delivery. Int J Nanomed. 2009;4:299. doi: 10.2147/IJN.S4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Xu D, Yang J, et al. Effects of small and large molecule emulsifiers on the characteristics of β-carotene nanoemulsions prepared by high pressure homogenization. Food Technol Biotechnol. 2009;47(3):336–342. [Google Scholar]
- Mao L, Yang J, Xu D, et al. Effects of homogenization models and emulsifiers on the physicochemical properties of β-carotene nanoemulsions. J Dispers Sci Technol. 2010;31(7):986–993. doi: 10.1080/01932690903224482. [DOI] [Google Scholar]
- Maurya VK, Aggarwal M. A phase inversion based nanoemulsion fabrication process to encapsulate vitamin D3 for food applications. J Steroid Biochem Mol Biol. 2019;190:88–98. doi: 10.1016/j.jsbmb.2019.03.021. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Jafari SM (2018) General aspects of nanoemulsions and their formulation. In: Nanoemulsions. Academic Press, pp 3–20. 10.1016/B978-0-12-811838-2.00001-1
- McClements DJ, Bai L, Chung C. Recent advances in the utilization of natural emulsifiers to form and stabilize emulsions. Ann Rev Food Sci Technol. 2017;8:205–236. doi: 10.1146/annurev-food-030216-030154. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Soni GC, Mishra RP. A review article on nanoemulsion. World J Pharm Pharm Sci. 2014;3(9):258–274. doi: 10.7897/2230-8407.0913. [DOI] [Google Scholar]
- Mohammadi A, Jafari SM, Esfanjani AF, et al. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016;190:513–519. doi: 10.1016/j.foodchem.2015.05.115. [DOI] [PubMed] [Google Scholar]
- Nedovic V, Kalusevic A, Manojlovic V, et al. An overview of encapsulation technologies for food applications. Proc Food Sci. 2011;1:1806–1815. doi: 10.1016/j.profoo.2011.09.265. [DOI] [Google Scholar]
- Nikiforidis CV, Karkani OA, Kiosseoglou V. Exploitation of maize germ for the preparation of a stable oil-body nanoemulsion using a combined aqueous extraction–ultrafiltration method. Food Hydrocoll. 2011;25(5):1122–1127. doi: 10.1016/j.foodhyd.2010.10.009. [DOI] [Google Scholar]
- Nirmal NP, Mereddy R, Li L, et al. Formulation, characterisation and antibacterial activity of lemon myrtle and anise myrtle essential oil in water nanoemulsion. Food Chem. 2018;254:1–7. doi: 10.1016/j.foodchem.2018.01.173. [DOI] [PubMed] [Google Scholar]
- Noori S, Zeynali F, Almasi H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control. 2018;84:312–320. doi: 10.1016/j.foodcont.2017.08.015. [DOI] [Google Scholar]
- O'Sullivan JJ, Drapala KP, Kelly AL, et al. The use of inline high-shear rotor-stator mixing for preparation of high-solids milk protein-stabilised oil-in-water emulsions with different protein: fat ratios. J Food Eng. 2018;222:218–225. doi: 10.1016/j.jfoodeng.2017.10.015. [DOI] [Google Scholar]
- Park SJ, Hong SJ, Garcia CV, et al. Stability evaluation of turmeric extract nanoemulsion powder after application in milk as a food model. J Food Eng. 2019;259:12–20. doi: 10.1016/j.jfoodeng.2019.04.011. [DOI] [Google Scholar]
- Peng LC, Liu CH, Kwan CC, et al. Optimization of water-in-oil nanoemulsions by mixed surfactants. Colloids Surf A: Physicochem Eng Asp. 2010;370(1–3):136–142. doi: 10.1016/j.colsurfa.2010.08.060. [DOI] [Google Scholar]
- Pengon S, Chinatangkul N, Limmatvapirat C, et al. The effect of surfactant on the physical properties of coconut oil nanoemulsions. Asian J Pharm Sci. 2018;13(5):409–414. doi: 10.1016/j.ajps.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongsumpun P, Iwamoto S, Siripatrawan U. Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrasonics Sonochem. 2020;60:104604. doi: 10.1016/j.ultsonch.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Prakash A, Vadivel V, Rubini D, et al. Antibacterial and antibiofilm activities of linalool nanoemulsions against Salmonella typhimurium. Food Biosci. 2019;28:57–65. doi: 10.1016/j.fbio.2019.01.018. [DOI] [Google Scholar]
- Primo FL, Michieleto L, Rodrigues MA, et al. Magnetic nanoemulsions as drug delivery system for Foscan®: skin permeation and retention in vitro assays for topical application in photodynamic therapy (PDT) of skin cancer. J Mag Mag Mat. 2007;311(1):354–357. doi: 10.1016/j.jmmm.2006.10.1183. [DOI] [Google Scholar]
- Qian C, McClements DJ. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocoll. 2011;25(5):1000–1008. doi: 10.1016/j.foodhyd.2010.09.017. [DOI] [Google Scholar]
- Qian C, Decker EA, Xiao H, et al. Nanoemulsion delivery systems: influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012;135(3):1440–1447. doi: 10.1016/j.foodchem.2012.06.047. [DOI] [PubMed] [Google Scholar]
- Qiao W, Wang B, Wang Y, et al. Cancer therapy based on nanomaterials and nanocarrier systems. J Nanomaterials. 2010 doi: 10.1155/2010/796303. [DOI] [Google Scholar]
- Quintão FJ, Tavares RS, Vieira-Filho SA, et al. Hydroalcoholic extracts of Vellozia squamata: study of its nanoemulsions for pharmaceutical or cosmetic applications. Rev Bras Farmacogn. 2013;23(1):101–107. doi: 10.1590/S0102-695X2013005000001. [DOI] [Google Scholar]
- Rai VK, Mishra N, Yadav KS, et al. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Control Rel. 2018;270:203–225. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- Rao J, McClements DJ. Food-grade microemulsions, nanoemulsions and emulsions: fabrication from sucrose monopalmitate & lemon oil. Food hydrocoll. 2011;25(6):1413–1423. doi: 10.1016/j.foodhyd.2011.02.004. [DOI] [Google Scholar]
- Rebolleda S, Sanz MT, Benito JM, et al. Formulation and characterisation of wheat bran oil-in-water nanoemulsions. Food Chem. 2015;167:16–23. doi: 10.1016/j.foodchem.2014.06.097. [DOI] [PubMed] [Google Scholar]
- Reed SG, Bertholet S, Coler RN, et al. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30(1):23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Relkin P, Jung JM, Ollivon M. Factors affecting vitamin degradation in oil-in-water nano-emulsions. J Therm Anal Cal. 2009;98(1):13. doi: 10.1007/s10973-009-0340-9. [DOI] [Google Scholar]
- Relkin P, Shukat R, Bourgaux C, et al. Nanostructures and polymorphisms in protein stabilised lipid nanoparticles, as food bioactive carriers: contribution of particle size and adsorbed materials. Proc Food Sci. 2011;1:246–250. doi: 10.1016/j.profoo.2011.09.039. [DOI] [Google Scholar]
- Relkin P, Yung JM, Kalnin D, et al. Structural behaviour of lipid droplets in protein-stabilized nano-emulsions and stability of α-tocopherol. Food Biophys. 2008;3(2):163–168. doi: 10.1007/s11483-008-9064-9. [DOI] [Google Scholar]
- Research and Market (2018) Global nanotechnology market outlook 2024. Report ID: 4991720. https://www.researchandmarkets.com/reports/4536705/global-nanotechnology-market-outlook-2018-2024. Web assessed on 22nd December 2020.
- Ribeiro RCDA, Barreto SMAG, Ostrosky EA, et al. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) Mill extract as moisturizing agent. Molecules. 2015;20(2):2492–2509. doi: 10.3390/molecules20022492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme N, Zúñiga RN, Arancibia C. Physical stability of nanoemulsions with emulsifier mixtures: replacement of tween 80 with quillaja saponin. LWT. 2019;111:760–766. doi: 10.1016/j.lwt.2019.05.067. [DOI] [Google Scholar]
- Roca-Saavedra P, Mendez-Vilabrille V, Miranda JM, et al. Food additives, contaminants and other minor components: effects on human gut microbiota—a review. J Physiol Biochem. 2018;74(1):69–83. doi: 10.1007/s13105-017-0564-2. [DOI] [PubMed] [Google Scholar]
- Saini A, Panwar D, Panesar PS, et al. Encapsulation of unctional ingredients in lipidic nanocarriers and antimicrobial applications: a review. Environ Chem Lett. 2020 doi: 10.1007/s10311-020-01109-3. [DOI] [Google Scholar]
- Salim N, García-Celma MJ, Escribano E, et al. Formation of nanoemulsion containing ibuprofen by PIC method for topical delivery. Mat Today: Proc. 2018;5:172–179. doi: 10.1016/j.matpr.2018.08.062. [DOI] [Google Scholar]
- Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. Int J Pharm Invest. 2012;2(1):12. doi: 10.4103/2230-973X.96921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari TP, Mann B, Kumar R, et al. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015;43:540–546. doi: 10.1016/j.foodhyd.2014.07.011. [DOI] [Google Scholar]
- Sarkar A, Goh KK, Singh RP, et al. Behaviour of an oil-in-water emulsion stabilized by β-lactoglobulin in an in vitro gastric model. Food Hydrocoll. 2009;23(6):1563–1569. doi: 10.1016/j.foodhyd.2008.10.014. [DOI] [Google Scholar]
- Seibert JB, Bautista-Silva JP, Amparo TR, et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019;287:61–67. doi: 10.1016/j.foodchem.2019.02.078. [DOI] [PubMed] [Google Scholar]
- Shah B, Ikeda S, Davidson PM, et al. Nanodispersing thymol in whey protein isolate-maltodextrin conjugate capsules produced using the emulsion—evaporation technique. J Food Eng. 2012;113(1):79–86. doi: 10.1016/j.jfoodeng.2012.05.019. [DOI] [Google Scholar]
- Shah P, Bhalodia D, Shelat P. Nanoemulsion: a pharmaceutical review. Syst Rev Pharm. 2010 doi: 10.4103/0975-8453.59509. [DOI] [Google Scholar]
- Shah R, Kolanos R, DiNovi MJ, et al. Dietary exposures for the safety assessment of seven emulsifiers commonly added to foods in the United States and implications for safety. Food Add Cont Part A. 2017;34(6):905–917. doi: 10.1080/19440049.2017.1311420. [DOI] [PubMed] [Google Scholar]
- Shaker DS, Ishak RA, Ghoneim A, et al. Nanoemulsion: a review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci Pharm. 2019;87(3):17. doi: 10.3390/scipharm87030017. [DOI] [Google Scholar]
- Singh KK, Vingkar SK. Formulation, antimalarial activity and biodistribution of oral lipid nanoemulsion of primaquine. Int J Pharm. 2008;347(1–2):136–143. doi: 10.1016/j.ijpharm.2007.06.035. [DOI] [PubMed] [Google Scholar]
- Smolkova B, El Yamani N, Collins AR, et al. Nanoparticles in food. Epigenetic changes induced by nanomaterials and possible impact on health. Food Chem Toxicol. 2015;77:64–73. doi: 10.1016/j.fct.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Sonneville-Aubrun O, Simonnet JT, L’alloret F. Nanoemulsions: a new vehicle for skincare products. Adv Colloid Interface Sci. 2004;108:145–149. doi: 10.1016/j.cis.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Tan CP, Nakajima M. β-Carotene nanodispersions: preparation, characterization and stability evaluation. Food Chem. 2005;92(4):661–671. doi: 10.1016/j.foodchem.2004.08.044. [DOI] [Google Scholar]
- Teo SY, Yew MY, Lee SY, et al. In vitro evaluation of novel phenytoin-loaded alkyd nanoemulsions designed for application in topical wound healing. J Pharm Sci. 2017;106(1):377–384. doi: 10.1016/j.xphs.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Tan YM, Amiji M. Preparation and in vitro characterization of multifunctional nanoemulsions for simultaneous MR imaging and targeted drug delivery. J Biomed Nanotechnol. 2006;2(3–4):217–224. doi: 10.1166/jbn.2006.038. [DOI] [Google Scholar]
- van der Ven C, Gruppen H, de Bont DB, et al. Emulsion properties of casein and whey protein hydrolysates and the relation with other hydrolysate characteristics. J Agric Food Chem. 2001;49(10):5005–5012. doi: 10.1021/jf010144c. [DOI] [PubMed] [Google Scholar]
- Vo TD, Lynch BS, Roberts A. Dietary exposures to common emulsifiers and their impact on the gut microbiota: is there a cause for concern? Compr Rev Food Sci Food Saf. 2019;18(1):31–47. doi: 10.1111/1541-4337.12410. [DOI] [PubMed] [Google Scholar]
- Walia N, Dasgupta N, Ranjan S, et al. Methods for nanoemulsion and nanoencapsulation of food bioactives. Environ Chem Lett. 2019;17(4):1471–1483. doi: 10.1007/s10311-019-00886-w. [DOI] [Google Scholar]
- Wani TA, Masoodi FA, Jafari SM et al (2018) Safety of nanoemulsions and their regulatory status. In: Nanoemulsions. Academic Press, pp 613–628. https://www.sciencedirect.com/science/book/9780128118382. Accessed 2 Dec 2020
- Wu SH, Hung Y, Mou CY. Compartmentalized hollow silica nanospheres templated from nanoemulsions. Chem Mater. 2013;25(3):352–364. doi: 10.1016/B978-0-12-811838-2.00019-9. [DOI] [Google Scholar]
- Wulff-Pérez M, Torcello-Gómez A, Gálvez-Ruíz MJ, et al. Stability of emulsions for parenteral feeding: Preparation and characterization of o/w nanoemulsions with natural oils and Pluronic f68 as surfactant. Food Hydrocoll. 2009;23(4):1096–1102. doi: 10.1016/j.foodhyd.2008.09.017. [DOI] [Google Scholar]
- Yen CC, Chen YC, Wu MT, et al. Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. Int J Nanomed. 2018;13:669. doi: 10.2147/IJN.S154824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Li C, Xu J, et al. Highly stable concentrated nanoemulsions by the phase inversion composition method at elevated temperature. Langmuir. 2012;28(41):14547–14552. doi: 10.1021/la302995a. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Gao Y, Zhao J, et al. Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res Int. 2008;41(1):61–68. doi: 10.1016/j.foodres.2007.09.006. [DOI] [Google Scholar]
- Yukuyama MN, Ghisleni DDM, Pinto TDJA, et al. Nanoemulsion: process selection and application in cosmetics—a review. Int J Cosmet Sci. 2016;38(1):13–24. doi: 10.1111/ics.12260. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang F, Fan Z, et al. DHA and EPA nanoemulsions prepared by the low-energy emulsification method: process factors influencing droplet size and physicochemical stability. Food Res Int. 2019;121:359–366. doi: 10.1016/j.foodres.2019.03.059. [DOI] [PubMed] [Google Scholar]
- Zhao C, Ashaolu TJ. Bioactivity and safety of whey peptides. LWT. 2020 doi: 10.1016/j.lwt.2020.109935. [DOI] [Google Scholar]
- Zhao L, Seth A, Wibowo N, et al. Nanoparticle vaccines. Vaccine. 2014;32(3):327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- Ziani K, Chang Y, McLandsborough L, et al. Influence of surfactant charge on antimicrobial efficacy of surfactant-stabilized thyme oil nanoemulsions. J Agric Food Chem. 2011;59(11):6247–6255. doi: 10.1021/jf200450m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No dataset were generated in this study.
Not applicable.