SUMMARY
Although wild birds are considered the main reservoir of the influenza A virus (IAV) in nature, empirical investigations exploring the interaction between the IAV prevalence in these populations and environmental drivers remain scarce. Chile has a coastline of more than 4,000 kilometers with hundreds of wetlands, which are important habitats for both resident and inter hemispheric migratory species. The aim of this study was to characterize the temporal dynamics of IAV in main wetlands in central Chile and to assess the influence of environmental variables on AIV prevalence. For that purpose, four wetlands were studied from September 2015 to June 2018. Fresh faecal samples of wild birds were collected for IAV detection by real-time RT-PCR. Furthermore, a count of wild birds present at the site was performed and environmental variables, such as temperature, rainfall, vegetation coverage (Normalized Difference Vegetation Index-NDVI) and water body size were determined. A generalized linear mixed model was built to assess the association between IAV prevalence and explanatory variables. An overall prevalence of 4.28% ± 0.28 was detected with important fluctuations among seasons, being greater during summer (OR=4.87, 95% CI 2.11 to 11.21) and fall (OR=2.59, 95% CI 1.12 to 5.97). Prevalence was positively associated with minimum temperature for the month of sampling and negatively associated with water body size measured two months before sampling, and NDVI measured three months before sampling. These results contribute to the understanding of IAV ecological drivers in Chilean wetlands providing important considerations for the global surveillance of IAV.
Keywords: Influenza virus, wild birds, NDVI, generalized linear mixed model, Chile
INTRODUCTION
Research and interest in influenza A viruses (IAV) have increased considerably in recent decades in response to highly pathogenic avian influenza outbreaks in poultry and its zoonotic potential (Hoye, Munster, Nishiura, Klaassen, & Fouchier, 2010). However, empirical investigations that include ecological and environmental drivers of IAV in wild bird habitats remain scarce, in spite of the importance of understanding the infection dynamics in wild populations to prevent the disease in humans and food-producing animals (Ferenczi et al., 2016; N. Gaidet et al., 2012).
Wild waterfowl, particularly the Anseriformes and Charadriiformes orders, have an important role in IAV ecology because they are recognized as natural reservoirs of most of the subtypes described in its low pathogenic form (LPAI) (Olsen et al., 2006; Webster, Bean, Gorman, Chambers, & Kawaoka, 1992). These birds have the potential to spread IAV when they migrate within and between continents, representing a risk for the emergence of highly pathogenic avian influenza (HPAI) outbreaks in domestic birds (Lee et al., 2015). Therefore, the epidemiology and infection dynamics of IAV are closely related to the behavior of these reservoir species, including their feeding, habitats and migratory patterns (Munster & Fouchier, 2009). In addition, the environmental conditions and landscape structure in which wild waterfowl reside may play a key role in maintaining the infection within the population (Cumming et al., 2015; Klaassen, Hoye, & Roshier, 2011).
Influenza A virus shows a marked seasonal pattern in wild birds in the northern hemisphere, with the highest virus prevalence reported in late summer and early fall prior to migration, followed by a decline during winter (Dijk et al., 2014; Munster & Fouchier, 2009; Olsen et al., 2006). However, IAV dynamics may differ in other geographical contexts, where weather conditions, landscape structure, hosts and persistence of the virus in the environment are different from the northern hemisphere. For example, research performed in Australia and Africa have shown the absence of a seasonal pattern, suggesting that the factors influencing the dynamics of infection in these areas are different from those in the northern hemisphere (Ferenczi et al., 2016; N. Gaidet et al., 2012; Mundava et al., 2016). Therefore, it is important to carry out long-term studies to determine the ecological and environmental drivers of IAV in a local context.
Studies conducted so far have consistently shown that abiotic factors such as rainfall and temperature, biotics such as wildfowl community (age, host density and species present) and amount of vegetation, and anthropogenic factors, such as land use and type of land cover, could have an important role on the prevalence of IAV in ecosystems (Cumming et al., 2015; Ferenczi et al., 2016; Gaidet, 2016; Pérez-Ramírez et al., 2012; Torrontegi et al., 2019).
Chile has a coastline of more than 4,000 kilometers, along which there are hundreds of wetlands that serve as breeding and wintering sites for both resident and migratory wild birds (García Walther, Senner, Norambuena, & Schmitt, 2017). Thousands of individuals belonging to more than 30 species travel each year from the northern hemisphere, to spend the northern hemisphere’s winter season, along the three flyways that arrive in the country (Atlantic, Central and the Pacific flyways). The families Scolopacidae (wader) and Laridae (gulls, terns and skimmers) are the ones that concentrate the largest number of species that migrate to Chile (García Walther et al., 2017; Olsen et al., 2006).
Recent studies carried out by our research group have demonstrated the presence of a wide diversity of IAV subtypes in both resident and migratory wild birds in Chile, including low pathogenic H5 and H7 strains (Jiménez-Bluhm et al., 2018). These are concerning findings given the risk of IAV transmission to poultry. In addition, circulation of IAV in backyard productive systems (BPS) and evidence of spillover from wild birds have been described (Bravo-Vasquez et al., 2016; Jimenez-Bluhm et al., 2018).
From late December 2016 to February 2017, two outbreaks of LPAI H7N6 occurred in commercial turkey farms in Central Chile, including a nearby BPS to these farms, which originated from wild birds (Jimenez-Bluhm et al., 2019). Therefore, it is necessary to improve our understanding about the epidemiology and ecological drivers that could explain the risk for transmission of influenza A viruses between wild birds and domestic poultry.
The aim of this study was to characterize the temporal dynamics of IAV in four important wetlands in central Chile between September 2015 and June 2018 and to assess the influence of environmental variables on the prevalence of the virus in these sites.
MATERIALS AND METHODS
Study area
The study was carried out in four wetlands in central Chile: Batuco, Concon, Maipo River and Cahuil (Figure 1). All sites recognized as important wild bird concentration areas in Chile were characterized (according to information obtained in previous censuses) by species diversity, number of inter hemispheric migratory species, number of resident species, and species already recognized as reservoirs of IAV. Based on these variables, a risk score was calculated for each site to focus the surveillance in high-risk areas for IAV.
Figure 1.
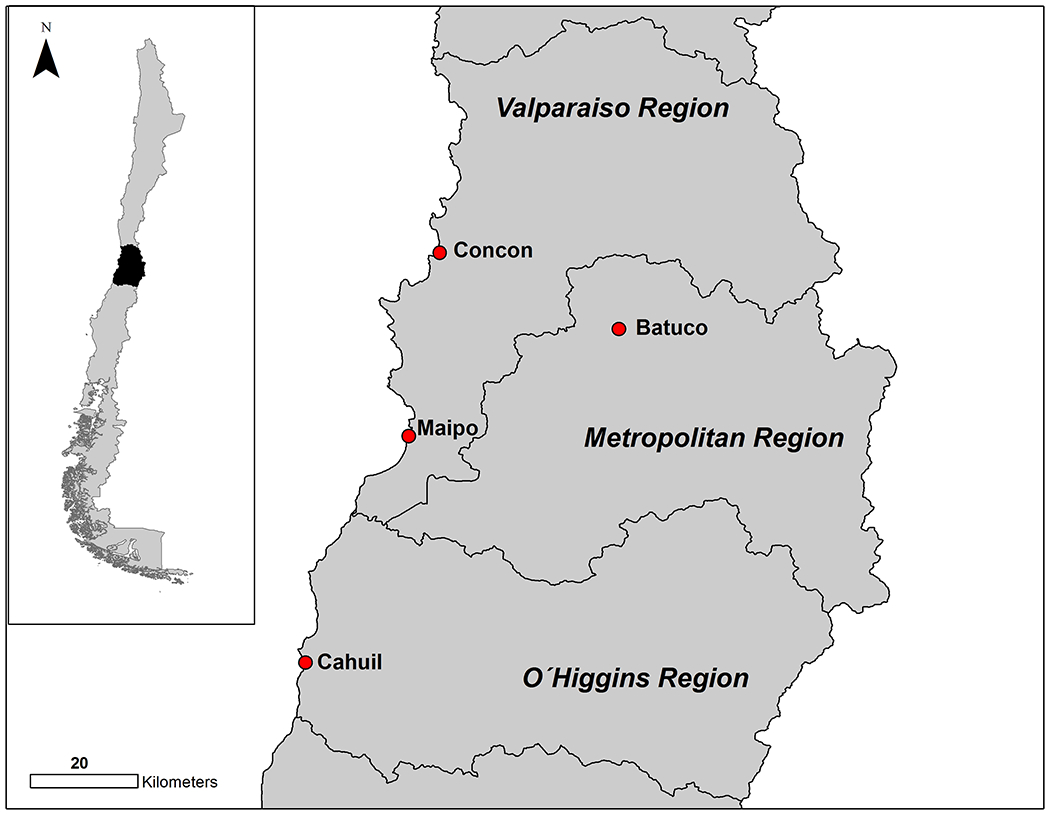
Geographical location of the study sites
The four sites selected corresponded to those with the highest risk score within the wetlands of the central zone. Concon, Maipo and Cahuil are estuarine wetlands, with hydrophilic and shrubby vegetation. Batuco, on the other hand, corresponds to a lagoon located in the intermediate depression of the Santiago basin, formed by sclerophyllous scrub-type vegetation.
All these wetlands are considered important concentration sites of resident waterfowl species, especially ducks such as Yellow-billed teal (Anas flavirostris), Yellow-billed pintail (Anas georgica), Cinnamon teal (Anas cyanoptera cyanoptera) and Red shoveler (Anas platalea). In addition, from October to March, a large number of interhemispheric migratory birds belonging to the Pacific flyway use these wetlands as wintering or stopover sites, being the families Scolopacidae and Laridae the ones that concentrate a greater number of species, principally in coastal wetlands (Concón, Maipo and Cahuil) (Table S1).
Sample collection
Faecal sampling and collection of ecological and environmental variables were carried out from September 2015 to June 2018 at previously mentioned study sites.
Sampling was performed at regular intervals, approximately once a month in Batuco, Concón and Cahuil, and every two months in Maipo. In each visit, only fresh fecal samples of wild birds were collected for influenza A virus detection.
The number of fresh feces present at a site was unknown. For sample size calculation, we assumed that at least 1,000 fresh feces per site were present. In order to detect a prevalence of 1.5% (Jiménez-Bluhm et al., 2018) with 95% confidence, 178 samples were needed in each visit (Dohoo, Martin, & Stryhn, 2009).
| Equation 1: |
where:
n = required sampling size
N = population size
∝ = 1-confidence level
D = estimated minimum number of positive samples
Prior to fecal sample collection, suitable sampling sites (wild bird roosting locations) were identified through observation or with the help of trained local staff. In order to minimize the probability of sampling the same individual’s feces twice, samples were collected uniformly by line transects throughout the area where a flock was observed. Approximately three to four parallel transects were performed at each sampling point through which the samples were collected. Each transect was run only once per sampling occasion.
Limited samples were taken from each flock, with several sampling points at each study site. The number of places sampled each month were determined by the flood level of the wetlands and by the number of birds present at the roosting areas. Approximately three to five different points were sampled at each visit.
Finally, samples were placed in tubes with universal transport medium (Copan® Universal Transport Medium, UTM™). Samples were kept at 4°C from the time of their collection to their arrival to the Laboratory of Veterinary Epidemiology (Faculty of Veterinary Science, University of Chile). Then, samples were stored at −80 ° C until analysis.
Influenza A virus detection
Influenza A virus RNA was detected using an influenza A PCR targeting the highly conserved matrix gene. The collected samples were processed individually, and molecular analyses were performed at the Laboratory of Veterinary Epidemiology (Biological Safety Level II), belonging to the Faculty of Veterinary Science of the University of Chile.
Viral RNA was extracted in a biological safety cabinet class II, from 50 μl of the swab sample with a commercial kit (ThermoFisher kit MagMAX™-96) following the manufacturer’s instructions. RNA was amplified using real time reverse transcriptase PCR (rRT-PCR) in a Mx3000P™ Stratagene Thermocycler (Agilent Technologies), with TaqMan® Fast Virus 1-Step Master Mix (ThermoFisher) and primers/probe specific for the influenza M gene (WHO, 2009). The reaction mixture consisted of 3 μL of RNA, 0.6 μL of each InfA forward and reverse primers, 0.4 μL Inf A probe, 5 μL of TaqMan® and 10.4 μL of water. Samples with a fluorescence cycle threshold (Ct) value ≤38 were considered positive (Shu et al., 2011).
Ecological and environmental variables
The ecological and environmental variables evaluated in this study were grouped into three categories (Table 1):
Table 1.
Definition of ecological and environmental variables
| Variables | Definition | |
|---|---|---|
| Wild bird community | Total abundance | Total number of birds present at the time of sampling |
| Species richness | Number of species present at the time of sampling | |
| Abundance of migrants | Number of migratory birds present at the time of sampling | |
|
Landscape |
Vegetation coverage |
Mean NDVI for the month of sampling, one month before, two months before and three months before sampling. |
| Water body size (km2) | Water body size for the month of sampling, one month before, two months before and three months before sampling. | |
| Meteorological data | Maximum monthly temperature (°C) | Monthly mean of maximum daily temperature for the month of sampling, one month before, two months before and three months before sampling. |
| Minimum monthly temperature (°C) | Monthly mean of minimum daily temperature for the month of sampling, one month before, two months before and three months before sampling. | |
| Total monthly rainfall (mm) | Total rainfall at the month of sampling, one month before, two months before and three months before sampling. | |
Wild bird community:
At each visit and prior to sampling, a count of wild birds present at the site was performed during morning hours. A point counting approach with several experienced observers was used (Bibby, Burgess, Hill, & Mustoe, 2000). The sampling site was georeferenced using a global positioning system (Garmin GPS Map 62s) and then a count and identification of birds within approximately 150 m radius of each sampling site was performed for a period of approximately 30-40 minutes (Bibby et al., 2000). These data were used to estimate total abundance, species richness and abundance of migratory birds present at the time of sampling. Total abundance of individuals was defined as the total number of individuals counted at the time of sampling and richness as the number of species identified in each sampling.
Landscape variables:
The vegetative cover of the wetland and the size of the water body of each site was measured monthly, matching the measurement with the sampling month at each site. In addition, to assess a cumulative effect between landscape changes and prevalence, these variables were also collected one month, two months and three months before the sampling month. Images of the LANDSAT 7-ETM and 8-OLI satellites were used, according to the years and sites of study. These images were downloaded from the website of the United States Geological Survey (USGS EarthExplorer) and processed using ENVI® software.
Normalized Difference Vegetation Index (NDVI) was used as an indicator of the vegetation cover. The NDVI is closely correlated with photosynthetic mass calculated from the red/near-infrared reflectance ratio. NDVI values range from −1 to 1, where negative values correspond to an absence of vegetation (Rouse, Haas, Schell, & Deering, 1974).
To delineate water bodies, Modified Normalized Difference Water Index (MNDWI) was used. The MNDWI is derived from the Normalized Difference Water Index (NDWI) defined by McFeeters (McFeeters, 1996) which uses middle infrared instead of near infrared. In this way, MNDWI can enhance water features removing built-up land noise as well as vegetation and soil noise (Xu, 2006). MNDWI values range from −1 to 1, where zero represents the discrimination threshold. Values greater than zero correspond to water (Xu, 2006).
Meteorological data:
Each study site was characterized in terms of average monthly temperatures and accumulated monthly rainfall for each month of sampling. To investigate the cumulative effect of rainfall and temperature on AIV prevalence, these variables were also collected one month, two months and three months before the sampling month. The information was obtained from the nearest weather station to each study site, belonging to Agrometeorological Network (Agromet) of the Agricultural Research Institute (INIA) (http://agromet.inia.cl/index.php), complemented with information available from the Chilean Meteorological Office (http://www.meteochile.cl/PortalDMC-web/index.xhtml).
Statistical Analysis
A generalized linear mixed model (GLMM) was built to assess the association between IAV prevalence and the explanatory variables recorded (Table 3). We used the number of IAV positive samples at each sampling in relation to sample size (prevalence) as the response variable. IAV prevalence and 95% CIs were estimated for each month of sampling at each study site, resulting in 118 prevalence estimates over almost three years.
Table 3:
Bivariable associations between IAV prevalence and explanatory variables
| Variables | Categories | Estimate | p-value | OR | 95% CI |
|---|---|---|---|---|---|
| Season | Winter | Reference | <0.001 | 0.007 | (0.003-0.002) |
| Autumn | 0.9546 | 0.025 | 2.59 | (1.12-5.97) | |
| Spring | 0.6264 | 0.152 | 1.87 | (0.79-4.41) | |
| Summer | 1.5842 | <0.001 | 4.87 | (2.11-11.21) | |
| Abundance of wild birds | Low (< 525) | Reference | |||
| High (≥ 525) | 0.3714 | 0.234 | 1.45 | (0.79- 2.67) | |
| Species richness | Low (< 22 sp) | Reference | |||
| High (≥ 22 sp) | 0.11 | 0.726 | 1.12 | (0.60-2.06) | |
| Abundance of migrants | Low (< 233) | Reference | |||
| High (≥ 233) | 0.8496 | 0.00474 | 2.34 | (1.30 −4.22) | |
| NDVI | Low (< 0.19) | Reference | |||
| High (≥ 0.19) | −0.6672 | 0.0402 | 1.95 | (1.03- 3.69) | |
| NDVI 1 month bs | Low (< 0.17) | Reference | |||
| High (≥ 0.17) | −0.5936 | 0.043 | 0.55 | (0.31-0.98) | |
| NDVI 2 month bs | Low (< 0.16) | Reference | |||
| High (≥ 0.16) | −0.7743 | 0.016 | 0.46 | (0.25- 0.87) | |
| NDVI 3 month bs | - | −3.364 | <0.001 | 0.03 | (0.006- 0.21) |
| Water body size (km2) | Low (< 0.67) | Reference | |||
| High (≥ 0.67) | −0.7489 | 0.021 | 0.47 | (0.25 −0.89) | |
| Water body size 1 month bs | Low (< 0.39) | Reference | |||
| High (≥ 0.39) | −0.7261 | 0.013 | 0.48 | (0.27 −0.86) | |
| Water body size 2 month bs | Low (< 0.79) | Reference | |||
| High (≥ 0.79) | −1.0491 | <0.001 | 0.35 | (0.19- 0.65) | |
| Water body size 3 month bs | Low (< 0.56) | Reference | |||
| High (≥ 0.56) | −0.9203 | 0.003 | 0.39 | (0.22- 0.73) | |
| Maximum temperature (°C) | Low (< 19.34) | Reference | |||
| High (≥ 19.34) | −0.2814 | 0.429 | 0.75 | (0.38- 1.52) | |
| Maximum temperature 1 month bs | Low (< 19.20) | Reference | |||
| High (≥ 19.20) | 0.07918 | 0.823 | 1.08 | (0.54- 2.16) | |
| Maximum temperature 2 month bs | Low (< 19.34) | Reference | |||
| High (≥ 19.34) | −0.27 | 0.446 | 0.76 | (0.38- 1.53) | |
| Maximum temperature 3 month bs | Low (< 19) | Reference | |||
| High (≥ 19) | −0.01998 | 0.949 | 0.98 | (0.53- 1.81) | |
| Minimum temperature (°C) | Low (< 8.7) | Reference | |||
| High (≥ 8.7) | 1.2655 | <0.001 | 3.55 | (2.03 −6.19) | |
| Minimum temperature 1 month bs | Low (< 8.7) | Reference | |||
| High (≥ 8.7) | 1.2254 | <0.001 | 3.41 | (1.90 −6.07) | |
| Minimum temperature 2 month bs | Low (< 8.5) | Reference | |||
| High (≥ 8.5) | 0.8214 | 0.007 | 3.4 | (1.87- 6.19) | |
| Minimum temperature 3 month bs | Low (< 8.4) | Reference | |||
| High (≥ 8.4) | 0.02618 | 0.933 | 1.03 | (0.55 −1.89) | |
| Rainfall (mm) | Low (< 6.1) | Reference | |||
| High (≥ 6.1) | −0.528 | 0.087 | 0.6 | (0.32- 1.07) | |
| Rainfall 1 month bs | Low (< 6.6) | Reference | |||
| High (≥ 6.6) | −0.9064 | 0.003 | 0.4 | (0.22 −0.73) | |
| Rainfall 2 month bs | Low (< 5.5) | Reference | |||
| High (≥ 5.5) | −0.4087 | 0.192 | 0.66 | (0.35- 1.23) | |
| Rainfall 3 month bs | Low (< 6.6) | Reference | |||
| High (≥ 6.6) | −0.6086 | 0.048 | 0.54 | (0.29- 0.99) | |
bs: before sampling
Total abundance of wild birds, species richness, abundance of migrants, NDVI, water body size, maximum monthly temperature, minimum monthly temperature, total monthly rainfall, and season were used as explanatory variables. The season variable was categorized according to the southern hemisphere season when samples were collected: March–May (fall); June–August (winter); September–November (spring); December–February (summer). Since the season when the samples were taken was correlated with the environmental variables collected, it was decided to exclude season from the final multivariable model.
Overall, 118 sampling events were done in several areas within four sites (Batuco, Concón and Cahuil, and Maipo). To account for the potential lack of independence between samplings within a sampling event, we included the sampling event as random effects in the multivariable model. Site was not included as a random effect in the final multivariable mixed model since the intraclass correlation was close to zero, which resulted in convergence problems. The exclusion of the site reduced the Akaike Information Criteria (AIC) of the final model (Zuur Alain F., Ieno Elena N., Walker Neil, Saveliev Anatoly A., 2009).
The unconditional association between IAV prevalence and each of the recorded explanatory variables was assessed in a first screening (Table 2). Variables associated with the outcome at a liberal p-value of <0.15 were selected for inclusion in the multivariable model.
Table 2.
Prevalence of influenza A virus by site and season
| SITE | WINTER | SPRING | SUMMER | AUTUMN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence % | Prevalence % | Prevalence % | Prevalence % | |||||||||
| Pos | N | (95% CI) | Pos | N | (95% CI) | Pos | N | (95% CI) | Pos | N | (95% CI) | |
| CONCON | 8 | 1146 | 0.70 (0.32-1.42) | 66 | 1195 | 5.66 (4.43- 7.19) | 349 | 1477 | 23.63 (21.5-25.89) | 88 | 1710 | 5.15 (4.17- 6.33) |
| BATUCO | 21 | 1338 | 1.57 (0.99- 2.43) | 42 | 1597 | 2.63 (1.92-3.57) | 46 | 1676 | 2.74 (2.03-3.67) | 21 | 1714 | 1.23 (0.78- 1.90) |
| MAIPO | 6 | 570 | 1.05 (0.43- 2.39) | 9 | 758 | 1.19 (0.58-2.32) | 47 | 942 | 4.99 (3.73- 6.63) | 93 | 1522 | 6.11 (4.98-7.46) |
| CAHUIL | 15 | 1332 | 1.13 (0.65-1.89) | 33 | 1537 | 2.15 (1.51-3.04) | 58 | 1669 | 3.48 (2.67-4.49) | 27 | 1518 | 1.78 (1.19- 2.71) |
| TOTAL | 50 | 4386 | 1.14 (0.85- 1.51) | 150 | 5087 | 2.95 (2.51-3.46) | 500 | 5764 | 8.67 (7.97-9.44) | 229 | 6464 | 3.54 (3.11-4.03) |
Pos: number of AIV positive samples, N: number of analyzed samples, CI:95% confidence interval.
The linearity of continuous explanatory variables against the log odds of IAV positivity was assessed visually. Non-linear variables were categorized using the median.
A forward stepwise inclusion of variables guided by AIC was performed to build the final multivariable mixed model.
Multicollinearity was assessed using the variance inflation factor (VIF). Variables with VIF > 3 were candidates for exclusion from the model (Zuur Alain F., Ieno Elena N., Walker Neil, Saveliev Anatoly A., 2009). Among correlated variables, we selected the variable that was most strongly associated with the response variable for inclusion in the multivariable model.
All analyses were conducted using R statistical software (R Core Team, 2018). Models were run with the ‘glmer’ function in the ‘lme4’ package in R, and ‘emmeans’ package to perform a Tukey adjusted pairwise comparison of marginal means.
To test model fit, we used the ‘DHARMa’ package in R. This package uses a simulation-based approach to create readily interpretable scaled (quantile) residuals for fitted (generalized) linear mixed models (Hartig, 2019). We made residual plots and to support the visual inspection of the residuals a test for over/underdispersion was performed (Figure S1 and Figure S2).
RESULTS
IAV prevalence and seasonality
A total of 21,701 fecal samples were collected, of which 929 (4.28%) were positive for influenza virus M gene by rRT-PCR.
Over the three years of sampling, the prevalence of IAV varied widely, showed an apparent seasonal pattern, with a peak in the summer months and with a low but constant prevalence during the other months of the year (Figure 2). Percentage of positivity and sampling effort by site and season are presented in Table 2.
Figure 2.
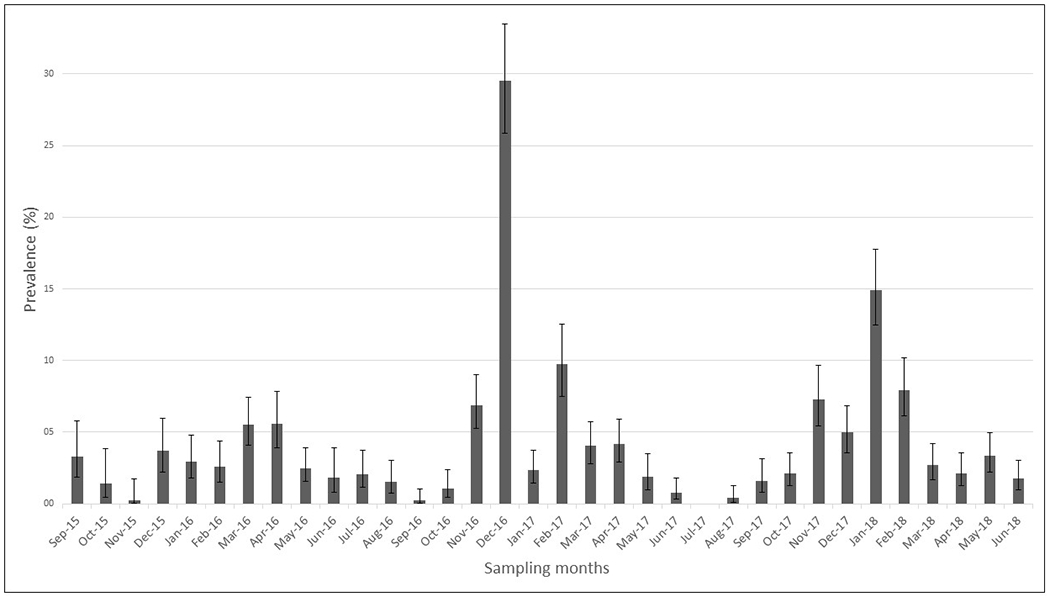
Monthly prevalence (95% CI) of influenza A virus between September 2015 and June 2018
The proportion of positive samples was greater during summer (OR=4.87, 95% CI 2.11 to 11.21) and autumn (OR=2.59, 95% CI 1.12 to 5.97) compared to winter (Table 3). No significant difference in the proportion of positive samples was observed between spring and winter (OR=1.87, 95% CI 0.79 to 4.41).
Ecological and environmental factors related to influenza A virus prevalence
Bird numbers fluctuated widely among sampling occasions at the sites studied, especially in Concón and Maipo wetlands, where a greater number of individuals was observed during the summer and early autumn months mainly due to the arrival of migratory birds at these sites (Figure S3).
The number of species observed during the study period was similar among the four wetlands. However, during the summer and autumn months, coastal sites presented a significant percentage of migratory species (Table S1), highlighting species belonging to the families Scolopacidae and Laridae (Table S2). On the other hand, in Batuco (continental wetland), the highest proportion of species corresponded to resident birds (Table S1) belonging to the family Anatidae and various species of passerines (Table S2).
Landscape and meteorological variables also varied widely among sampling occasions at the sites studied. A summary of these variables by site and season are presented in Table S2. However, only NDVI, water body size and minimum temperature were associated with IAV positivity in the wetlands (Table 4). Rainfall and maximum temperatures were not significantly associated with IAV status.
Table 4.
Multivariable model results of the association between IAV prevalence and environmental variables
| Variables | Categories | Estimate | p-value | OR | 95% CI |
|---|---|---|---|---|---|
| (Intercept) | −3.4013 | <0.001 | 0.03 | (0.02- 0.05) | |
| NDVI 3 months bs | - | −2.7074 | 0.0043 | 0.06 | (0.01- 0.43) |
| Minimum temperature (°C) | Low (< 8.7) | Reference | |||
| High (≥ 8.7) | 0.7828 | 0.0051 | 2.19 | (1.26- 3.78) | |
| Water body size 2 months bs (km2) | Low (< 0.79) | Reference | |||
| High (≥ 0.79) | −0.5904 | 0.05 | 0.55 | (0.31- 0.99) | |
bs: before sampling
NDVI in the wetlands was generally higher during spring-summer months than autumn-winter months. Batuco and Maipo wetlands showed the most extreme variation in NDVI during the study period (Figure 3) with the larger values of vegetation coverage representing greener surfaces. On the other hand, the amount of water in the wetlands increased during the winter and spring months and was lower during the summer and fall. Batuco was the wetland that experienced the greatest variations in the water mirror area. Figure 4 shows the months in which the greatest variations in water body size occurred (lowest and highest) during the study period in Batuco and Maipo wetlands.
Figure 3:
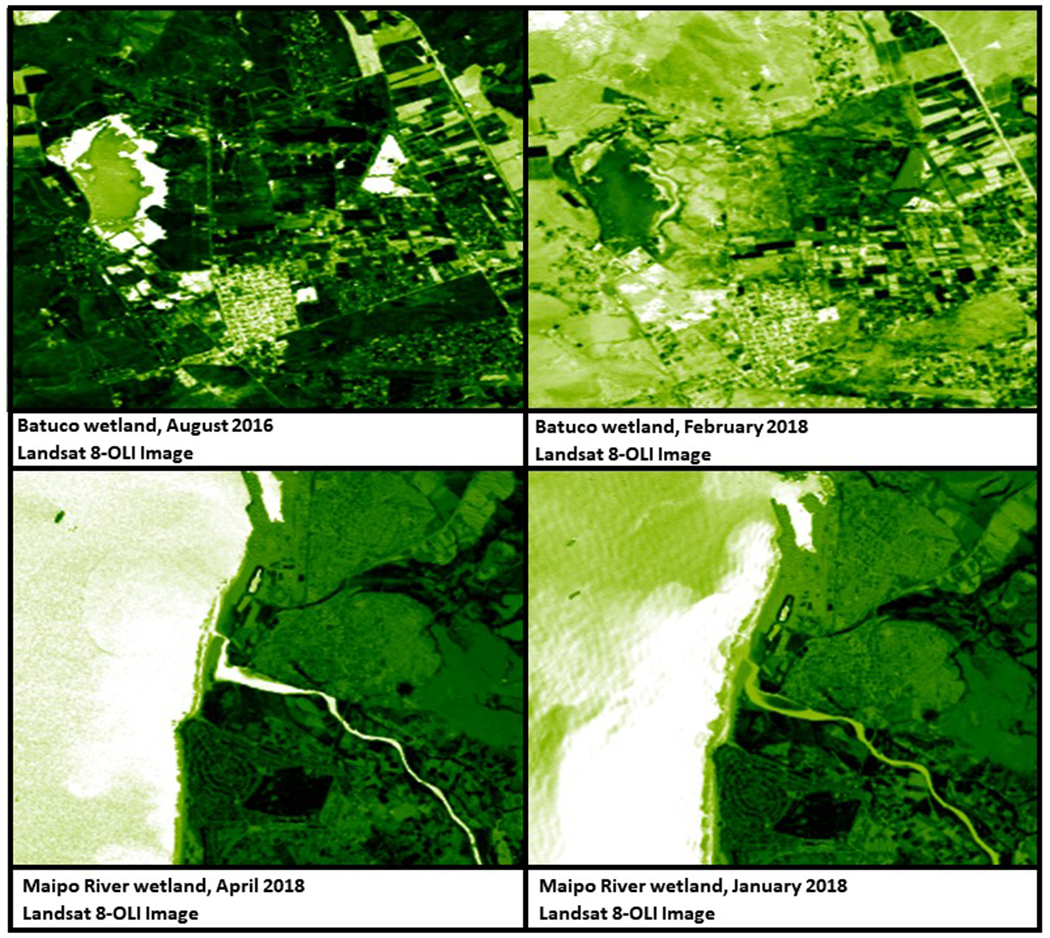
NDVI images in Batuco and Maipo River wetlands in the most extreme months of sampling.
Figure 4:

Water body size in Batuco and Maipo River wetlands in the most extreme variation months of sampling.
The final multivariable model showed that IAV prevalence was positively associated with minimum temperature for the month of sampling and negatively associated with water body size measured two months before sampling and NDVI measured three months before sampling (Table 4). None of the variables included in “wild bird community” category was retained in the final multivariable model.
DISCUSSION
This is the first study in South America to explore ecological and environmental factors related to the prevalence of IAVs in wetlands and adds to the still scarce information about environmental variables influencing IAVs epidemiology in Chilean ecosystems. Our results indicate that environmental variables such as water body size, NDVI and minimum temperature were related to virus positivity in the studied wetlands.
The overall prevalence during the study period 4.28% [95% CI:4.01% - 4.56%] was similar to data reported in a previous surveillance study conducted in wetlands in central and northern Chile (Jiménez-Bluhm et al., 2018) and other Mediterranean climate countries, where faecal samples were also analyzed. In these studies, the overall prevalence fluctuated between 1.7% and 5.43% (Ferenczi et al., 2016; Pérez-Ramírez et al., 2012; Torrontegi et al., 2019).
Because continuous sampling was conducted at regular intervals at all sites over three years of the study, a seasonal pattern in prevalence was identified, with a markedly higher prevalence in the summer and fall months compared to winter. No significant differences in the proportion of positive samples was observed between spring and winter, with a low but constant prevalence during these seasons. These results are similar to those found in a previous study of prevalence conducted in Chile, where summer/fall season had a higher positivity than winter/spring season (Jiménez-Bluhm et al., 2018). However, it is important to emphasize that in that study sampling was not done at regular intervals in all sites.
In South America, most of the studies in wild birds have focused on the overall prevalence and characterization of IAV isolates (Afanador-Villamizar, Gomez-Romero, Diaz, & Ruiz-Saenz, 2017; Hurtado & Vanstreels, 2016; Jiménez-Bluhm et al., 2018; Nelson et al., 2016). Only a few studies have documented IAV positivity over the seasons of the year in wild birds (de Araujo et al., 2014; Ghersi et al., 2009; Jiménez-Bluhm et al., 2018). A study conducted in Perú identified positive samples at the beginning and end of the migratory season but was unable to detect viruses throughout the year (Ghersi et al., 2009). Also in Brazil, in a four-year surveillance study, IAV was only detected in ruddy turnstones in November, at the beginning of the wintering period (de Araujo et al., 2014). However, more long-term studies are needed to evaluate infection patterns.
The increased prevalence in the summer months found in our study may be explained by a higher concentration of immunologically naïve young individuals post-breeding. The breeding season for most resident species at the sites begins in the spring. Therefore, during the summer months there is a large concentration of young individuals in the wetlands who are susceptible to infection. This situation has already been well documented in other studies conducted mainly in the northern hemisphere (Dijk et al., 2014; Munster & Fouchier, 2009; Olsen et al., 2006). In addition, the peak of IAV infection in summer coincides with the time of greatest congregation of northern hemisphere migrants at the sites, who may introduce new strains of IAV into the local community, encouraging viral transmission and amplifying local IAV circulation. It has already been demonstrated that a wide variety of IAV strains are circulating in Chile in wild birds, many of which belong to the North American lineage, suggesting that Chile may be a possible point of confluence where North and South American IAVs intermix (Jiménez-Bluhm et al., 2018).
Similar prevalence between winter and spring indicates the annual persistence of IAV at these study sites and allow us to speculate that at these sites there is an endemic cycle of avian influenza virus that circulates within the resident bird community maintaining infection and may peak when the density of juveniles increases in conjunction with migratory birds from the northern hemisphere, creating the conditions for virus reassortment and emergence of new strains, that could represent a risk for both, animal and human health. Resident species such as Yellow-billed teals (Anas flavirostris) and Yellow-billed pintails (Anas georgica) have already been identified as primary hosts of viruses circulating in Chilean wetlands (Jiménez-Bluhm et al., 2018) and may have an important role in the perpetuation of the virus in the study sites.
This situation has already been described by other authors, who noted the importance of non-migratory species in the avian influenza virus maintenance cycle in wetlands (Stallknecht, Brown, & Swayne, 2008). In tropical Africa, for example, the prevalence of IAV infection in wild birds is low but constant throughout the year, even in months when migrants are absent. However, with the arrival of migrants, the local circulation of the virus is amplified (Caron et al., 2011; Gaidet, 2016; Mundava et al., 2016).
In our model, none of the variables related to the wild bird community (total abundance, species richness and abundance of migratory species) were significantly associated with positivity to the virus in the study sites. However, these variables were only based on the count of birds at the sampling points. For future studies it would be important to consider variables related to the composition of the bird assemblages at each site, determining the dominant species at the different sampling occasions that could influence the prevalence. In addition, it would be important to consider the number of juvenile individuals and the density of birds on each sampling occasion, variables that have been significant in other studies (Dijk et al., 2014; Gaidet, 2016).
A study in Australia also showed that IAV dynamics are not simply a function of bird numbers. The age structure of the population is a key element to maintain infection (Ferenczi et al., 2016). In addition, the environmental conditions in which birds reside may also play an important role in their exposure to infection and in the persistence of the virus in the wetlands (Klaassen et al., 2011). In our study, vegetation, water body size and minimum temperature were the main environmental factors related to the positivity of the IAV on the studied wetlands.
Vegetative coverage three months before sampling (measured by NDVI) decreased the odds of IAV positivity. These results demonstrate that environmental effects are not always immediately expressed in biological processes, thus there may be a cumulative effect (Ferenczi et al., 2016). Low NDVI values three months before sampling indicate lighter vegetation at the sites studied, favouring a greater congregation of individuals in areas where the vegetation provides food and shelter and therefore a greater risk of IAV transmission (Si et al., 2010). In studies conducted in Europe, the vegetation surrounding wetlands has been recognized as an important driver in the presentation dynamics of the IAV (Pérez-Ramírez et al., 2012; Si et al., 2010).The same was observed in Africa and the Middle East, where the occurrence of H5N1 HPAI has been associated with areas with large seasonal variation in NDVI values, indicating that the spatial distribution of H5N1 HPAI cases in these areas would be related to specific environmental characteristics, generating an "environmental fingerprint" for the presentation of the virus (Williams & Peterson, 2009).
During the study period, all wetlands showed significant variations in their surface area flooded by water, being more extreme in Batuco wetland. In our model, water body size measured two months before sampling was negatively associated to IAV prevalence, probably because host aggregation in small flooded areas favors infection. These results are similar to those found in Afro-tropical regions, where the extreme variations experienced by the wetlands during the dry season, directly affect the congregation of birds and therefore the dynamics of infection of the IAV (Nicolas Gaidet, 2016). While in Africa these extreme variations are mainly due to rainfall, it is not clear what produces such variations in the wetlands studied. In South America, El Niño-Southern Oscillation (ENSO) is the main driver of interannual climate extremes, which has been associated in recent years with unprecedented warming and a larger extent of extreme drought in areas such as Amazonia (Jiménez-Muñoz et al., 2016). Central Chile’s climate is also marked by a significant ENSO influence, which has been linked to the current drought indices in the country (Oertel, Meza, & Gironás, 2020). This ENSO effect, combined with human extraction activities, could be affecting the flooded area of the wetlands.
In Australia, ENSO also has a significant influence on climatic conditions. In this country, a study showed a positive long term effect of ENSO related rainfall on IAV prevalence in waterfowl (Ferenczi et al., 2016). Therefore, it would be interesting to evaluate the effect of this variable on IAV prevalence in Chilean wetlands through longer-term studies.
The water body size effect measured two months before sampling, could also be explained by an environmental persistence of the virus at the sites. Several studies have suggested that environmental reservoirs play an important role in maintenance of IAV in wild bird population (Breban, Drake, Stallknecht, & Rohani, 2009; Roche et al., 2009; Rohani, Breban, Stallknecht, & Drake, 2009). The persistence of the virus in water can vary widely depending on the viral strain and the physicochemical characteristics of the water, such as temperature, pH and salinity (Brown, Goekjian, Poulson, Valeika, & Stallknecht, 2009; D E Stallknecht & Brown, 2009). Although in this study an effort was made to take data corresponding to the physicochemical characteristics of the water, it was not possible to obtain this information from the beginning of the sampling period. Therefore, these variables were not included in the model. However, it is necessary to explore these environmental variables in future IAV surveillance studies, because it has been determined that environmental persistence would also play an important role in the evolutionary biology and genetic diversity of avian influenza viruses (Roche et al., 2014).
We included temperature in our analysis, because it has been indicated as an important factor influencing LPAI virus prevalence in wild birds. In the northern hemisphere, a higher probability of infection was associated with lower air temperatures (Farnsworth et al., 2012; Fuller et al., 2010; Papp et al., 2017). In our study prevalence was positively related with the minimum temperature for the month of sampling. The air minimum temperature in the wetlands fluctuated between 2°C and 12° during the sampling period. Prevalence was higher in the months that had a temperature higher than 8°C as a monthly average. The effect of temperature on persistence of IAV in water has been well studied in laboratory conditions (Brown et al., 2009; Lebarbenchon et al., 2011; David E. Stallknecht, Goekjian, Wilcox, Poulson, & Brown, 2010). Those studies showed that virus durability (quantified by the time required to reduce infectivity by 90%) at temperatures of 10°C fluctuated between 15 and 90 days. Therefore, the relationship between site positivity and temperature may be associated with the survival of the virus in the environment. In addition, a higher minimum temperature can encourage the congregation of birds at the sites. As described in wetlands in Europe where high minimum temperatures have been associated with HPAI outbreaks in wild birds (Si et al., 2010).
In this study, we used environmental samples to determine the IAV positivity at the sites. The use of fecal sampling for IAV surveillance in wild birds has proven to be a rapid, cost-effective an non-invasive sampling technique for determining virus presence in wetlands (Pannwitz, Wolf, & Harder, 2009; Tracey, 2010). Furthermore, this technique allows to not discriminate arbitrarily between the species of birds that are sampled. As an example, capturing and hunting birds for sampling may narrow the sample down to a specific species while the role of other bird species that are involved in the epidemiology of the virus in a given location may be missed (Pannwitz et al., 2009; Torrontegi et al., 2019). Consequently, while most of the research related with IAV has focused on Anseriformes and Charadriiformes, , other bird species may play a role in maintaining IAV in Chilean wetlands. Although one of the main disadvantages of this sampling strategy is an inevitable loss of species-specific information on individual samples, this loss can be significantly reduced if ornithologic observation and a systematic bird count at the samplings site is performed 15–30 min prior to sampling (Pannwitz et al., 2009). In addition, the droppings from several wild bird species can be recognized from size, shape, and color. However, it is necessary to consider support of molecular techniques to assess species identification (Pannwitz et al., 2009; Torrontegi et al., 2019). Although this study did not include the identification of the sampled species, it is important to note that this data is available for future studies, in which a barcoding analysis will be used to identify the species that were positive for the virus.
CONCLUSION
Although the study of the ecological and environmental drivers of IAV infection in wild birds are very complex, long-term research that includes these aspects are necessary for the understanding of the epidemiology of the virus in these populations.
The prevalence of IAV in central Chilean wetlands is not constant over the year, but changes according to the season, being higher in the summer and fall months. Environmental factors driving this prevalence include minimum temperature, NDVI and size of the wetland water body. Our results constitute a unique contribution to the understanding of the prevalence variations of IAV in Chilean wetlands and its associations with environmental drivers, providing important considerations for the global surveillance of IAV in wild birds.
Supplementary Material
Supplementary Figure 1. Scaled residual plot and dispersion test for seasonality GLMM using DHARMAa package
Supplementary Figure 2. Scaled residual plot and dispersion test for GLMM final model using DHARMAa packag
Supplementary Figure 3. Total number of migratory and resident birds by site between September 2015 and June 2018.
ACKNOWLEDGEMENTS
We would like to acknowledge Pam Freiden, Bridgett Sharp, Brandi Livingston and Nicolas Bravo for technical assistance. This research was supported by Fondecyt grant 1191747 to CHW and NIAID contract HHSN272201400006C to SSC and CHW.
Footnotes
ETHICAL STATEMENT
Ethical Statement is not applicable. Sample collection or questionnaires from animals/human has not been gathered to perform this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Afanador-Villamizar A, Gomez-Romero C, Diaz A, & Ruiz-Saenz J (2017). Avian influenza in Latin America: A systematic review of serological and molecular studies from 2000-2015. PLoS ONE, 12(6), 1–21. 10.1371/journal.pone.0179573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby C, Burgess N, Hill D, & Mustoe S (2000). Bird Census Techniques (2nd edn). London UK: Academic Press. [Google Scholar]
- Bravo-Vasquez N, Di Pillo F, Lazo A, Jiménez-Bluhm P, Schultz-Cherry S, & Hamilton-West C (2016). Presence of influenza viruses in backyard poultry and swine in El Yali wetland, Chile. Preventive Veterinary Medicine, 134, 211–215. 10.1016/j.prevetmed.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Breban R, Drake JM, Stallknecht DE, & Rohani P (2009). The Role of Environmental Transmission in Recurrent Avian Influenza Epidemics. PLoS Computational Biology, 5(4). 10.1371/journal.pcbi.1000346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Goekjian G, Poulson R, Valeika S, & Stallknecht DE (2009). Avian influenza virus in water: Infectivity is dependent on pH, salinity and temperature. Veterinary Microbiology, 136(1–2), 20–26. 10.1016/j.vetmic.2008.10.027 [DOI] [PubMed] [Google Scholar]
- Caron A, Abolnik C, Mundava J, Gaidet N, Burger CE, Mochotlhoane B, … Cumming GS (2011). Persistence of low pathogenic avian influenza virus in waterfowl in a southern African ecosystem. EcoHealth, 8(1), 109–115. 10.1007/s10393-010-0356-4 [DOI] [PubMed] [Google Scholar]
- Cumming GS, Abolnik C, Caron A, Gaidet N, Grewar J, Hellard E, … Reynolds C (2015). A social–ecological approach to landscape epidemiology: geographic variation and avian influenza. Landscape Ecology, 30(6), 963–985. 10.1007/s10980-015-0182-8 [DOI] [Google Scholar]
- de Araujo J, de Azevedo Júnior SM, Gaidet N, Hurtado RF, Walker D, Thomazelli LM, … Durigon EL (2014). Avian Influenza Virus (H11N9) in Migratory Shorebirds Wintering in the Amazon Region, Brazil. PLoS ONE, 9(10), e110141. 10.1371/journal.pone.0110141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk JGB van, Hoye BJ, Verhagen JH, Nolet BA, Fouchier RAM, & Klaassen M (2014). Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. Journal of Animal Ecology, 83(1), 266–275. 10.1111/1365-2656.12131@10.1111/(ISSN)1365-2656.MOLECOL_JANE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohoo I, Martin W, & Stryhn H (2009). Veterinary Epidemiologic Research (2nd ed.; V. Inc., Ed.). Prince Edward Island, Canada. [Google Scholar]
- Farnsworth ML, Miller RS, Pedersen K, Lutman MW, Swafford SR, Riggs PD, & Webb CT (2012). Environmental and demographic determinants of avian influenza viruses in waterfowl across the contiguous United States. PLoS ONE, 7(3). 10.1371/journal.pone.0032729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M, Beckmann C, Warner S, Loyn R, O’Riley K, Wang X, & Klaassen M (2016). Avian influenza infection dynamics under variable climatic conditions, viral prevalence is rainfall driven in waterfowl from temperate, south-east Australia. Veterinary Research, 47(1), 23. 10.1186/s13567-016-0308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller TL, Saatchi SS, Curd EE, Toffelmier E, Thomassen HA, Buermann W, … Smith TB (2010). Mapping the risk of avian influenza in wild birds in the US. BMC Infectious Diseases, 10(1), 187. 10.1186/1471-2334-10-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidet N, Caron A, Cappelle J, Cumming GS, Balança G, Hammoumi S, … Dodman T (2012). Understanding the ecological drivers of avian influenza virus infection in wildfowl: A continental-scale study across Africa. Proceedings of the Royal Society B: Biological Sciences, 279(1731), 1131–1141. 10.1098/rspb.2011.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidet Nicolas. (2016). Ecology of Avian Influenza Virus in Wild Birds in Tropical Africa. Avian Diseases, 60(1s), 296–301. 10.1637/11149-051115-review [DOI] [PubMed] [Google Scholar]
- García Walther J, Senner N, Norambuena H, & Schmitt F (2017). Atlas de las aves playeras de Chile: Sitios importantes para su conservación. Santiago: Universidad Santo Tomás. [Google Scholar]
- Ghersi BM, Blazes DL, Icochea E, Gonzalez RI, Kochel T, Tinoco Y, … Montgomery JM (2009). Avian influenza in wild birds, central coast of Peru. Emerging Infectious Diseases, 15(6), 935–938. 10.3201/eid1506.080981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig F (2019). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. R package version 0.2.6. Retrieved from http://florianhartig.github.io/DHARMa/
- Hoye BJ, Munster VJ, Nishiura H, Klaassen M, & Fouchier RAM (2010). Surveillance of wild birds for avian influenza virus. Emerging Infectious Diseases, 16(12), 1827–1834. 10.3201/eid1612.100589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado R, & Vanstreels RET (2016). Avian Influenza in Wild Birds from South America: Review, Implications and Perspectives. Exploratory Research and Hypothesis in Medicine, 1(4), 62–74. 10.14218/erhm.2016.00014 [DOI] [Google Scholar]
- Jimenez-Bluhm P, Bravo-Vasquez N, Torchetti MK, Killian ML, Livingston B, Herrera J, … Hamilton-West C (2019). Low pathogenic avian influenza (H7N6) virus causing an outbreak in commercial Turkey farms in Chile. Emerging Microbes and Infections, 8(1), 479–485. 10.1080/22221751.2019.1595162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Bluhm P, Di Pillo F, Bahl J, Osorio J, Schultz-Cherry S, & Hamilton-West C (2018). Circulation of influenza in backyard productive systems in central Chile and evidence of spillover from wild birds. Preventive Veterinary Medicine, 153, 1–6. 10.1016/j.prevetmed.2018.02.018 [DOI] [PubMed] [Google Scholar]
- Jiménez-Bluhm P, Karlsson EA, Freiden P, Sharp B, Di Pillo F, Osorio JE, … Schultz-Cherry S (2018). Wild birds in Chile Harbor diverse avian influenza A viruses. Emerging Microbes & Infections, 7(1), 1–4. 10.1038/s41426-018-0046-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Muñoz JC, Mattar C, Barichivich J, Santamaría-Artigas A, Takahashi K, Malhi Y, … Schrier G Van Der. (2016, September 8). Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015-2016. Scientific Reports, Vol. 6, pp. 1–7. 10.1038/srep33130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen M, Hoye BJ, & Roshier DA (2011). Identifying crucial gaps in our knowledge of the life-history of avian influenza viruses—an Australian perspective. Emu - Austral Ornithology, 111(2), 103–112. 10.1071/MU10042 [DOI] [Google Scholar]
- Lebarbenchon C, Yang M, Keeler SP, Ramakrishnan MA, Brown JD, Stallknecht DE, & Sreevatsan S (2011). Viral Replication, Persistence in Water and Genetic Characterization of Two Influenza A Viruses Isolated from Surface Lake Water. PLoS ONE, 6(10), e26566. 10.1371/journal.pone.0026566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-H, Torchetti MK, Winker K, Ip HS, Song C-S, & Swayne DE (2015). Intercontinental Spread of Asian-Origin H5N8 to North America through Beringia by Migratory Birds. Journal of Virology, 89(12), 6521–6524. 10.1128/jvi.00728-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeeters SK (1996). The use of the Normalized Difference Water Index (NDWI) in the delineation of open water features. International Journal of Remote Sensing, 17(7), 1425–1432. 10.1080/01431169608948714 [DOI] [Google Scholar]
- Mundava J, Caron A, Garine-Wichatitsky M, Abolnik C, Mundy P, & Gaidet N (2016). The role of breeding phenology and aggregation of waterfowl on avian influenza dynamics in southern Africa. Ibis, 158(4), 762–775. 10.1111/ibi.12404 [DOI] [Google Scholar]
- Munster VJ, & Fouchier RAM (2009). Avian influenza virus: Of virus and bird ecology. Vaccine, 27(45), 6340–6344. 10.1016/j.vaccine.2009.02.082 [DOI] [PubMed] [Google Scholar]
- Nelson MI, Pollett S, Ghersi B, Silva M, Simons MP, Icochea E, … Bausch DG (2016). The genetic diversity of influenza A viruses in wild birds in Peru. PLoS ONE, 11(1), 1–10. 10.1371/journal.pone.0146059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel M, Meza FJ, & Gironás J (2020). Observed trends and relationships between ENSO and standardized hydrometeorological drought indices in central Chile. Hydrological Processes, 34(2), 159–174. 10.1002/hyp.13596 [DOI] [Google Scholar]
- Olsen B, Munster VJ, Wallensten A, Waldenström J, Osterhaus ADME, & Fouchier RAM (2006, April 21). Global patterns of influenza A virus in wild birds. Science, Vol. 312, pp. 384–388. 10.1126/science.1122438 [DOI] [PubMed] [Google Scholar]
- Pannwitz G, Wolf C, & Harder T (2009). Active Surveillance for Avian Influenza Virus Infection in Wild Birds by Analysis of Avian Fecal Samples from the Environment. In Journal of Wildlife Diseases (Vol. 45). [DOI] [PubMed] [Google Scholar]
- Papp Z, Clark RG, Parmley EJ, Leighton FA, Waldner C, & Soos C (2017). The ecology of avian influenza viruses in wild dabbling ducks (Anas spp.) in Canada. PLOS ONE, 12(5), e0176297. 10.1371/journal.pone.0176297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Ramírez E, Acevedo P, Allepuz A, Gerrikagoitia X, Alba A, Busquets N, … Höfle U (2012). Ecological Factors Driving Avian Influenza Virus Dynamics in Spanish Wetland Ecosystems. PLoS ONE, 7(11), e46418. 10.1371/journal.pone.0046418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Retrieved from https://www.r-project.org/.
- Roche B, Drake JM, Brown J, Stallknecht DE, Bedford T, & Rohani P (2014). Adaptive Evolution and Environmental Durability Jointly Structure Phylodynamic Patterns in Avian Influenza Viruses. PLoS Biology, 12(8), e1001931. 10.1371/journal.pbio.1001931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche B, Lebarbenchon C, Gauthier-Clerc M, Chang CM, Thomas F, Renaud F, … Guégan JF (2009). Water-borne transmission drives avian influenza dynamics in wild birds: The case of the 2005–2006 epidemics in the Camargue area. Infection, Genetics and Evolution, 9(5), 800–805. 10.1016/j.meegid.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Rohani P, Breban R, Stallknecht DE, & Drake JM (2009). Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proceedings of the National Academy of Sciences of the United States of America, 106(25), 10365–10369. 10.1073/pnas.0809026106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse JW, Haas R ., Schell J ., & Deering DW (1974). Monitoring vegetation systems in the Great Plains with ERTS. NASA special publication. [Google Scholar]
- Shu B, Wu KH, Emery S, Villanueva J, Johnson R, Guthrie E, … Lindstrom S (2011). Design and performance of the CDC real-time reverse transcriptase PCR Swine Flu Panel for detection of 2009 A (H1N1) pandemic influenza virus. Journal of Clinical Microbiology, 49(7), 2614–2619. 10.1128/JCM.02636-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Wang T, Skidmore AK, de Boer WF, Li L, & Prins HHT (2010). Environmental factors influencing the spread of the highly pathogenic avian influenza H5N1 virus in wild birds in Europe. Ecology and Society, 15(3), 26. 10.5751/ES-03622-150326 [DOI] [Google Scholar]
- Stallknecht DE, & Brown JD (2009). Tenacity of avian influenza viruses. Rev. Sci. Tech. Off. Int. Epiz, 28(1), 59–67. Retrieved from https://pdfs.semanticscholar.org/4556/0521cf6246e4852e1ccffd0936e9fadc80bc.pdf [DOI] [PubMed] [Google Scholar]
- Stallknecht, David E, Brown JD, & Swayne DE (2008). Ecology of Avian Influenza in Wild Birds. In Avian Influenza (pp. 43–58). [Google Scholar]
- Stallknecht, David E, Goekjian VH, Wilcox BR, Poulson RL, & Brown JD (2010). Avian Influenza Virus in Aquatic Habitats: What Do We Need to Learn? Avian Diseases Digest, 5(s1), e99–e100. 10.1637/9200-876009-digest.1 [DOI] [PubMed] [Google Scholar]
- Torrontegi O, Alvarez V, Acevedo P, Gerrikagoitia X, Höfle U, & Barral M (2019). Long-term avian influenza virus epidemiology in a small Spanish wetland ecosystem is driven by the breeding Anseriformes community. Veterinary Research, 50(1), 4. 10.1186/s13567-019-0623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey JP (2010). Risk-based surveillance of avian influenza in Australia’s wild birds. Wildlife Research, 37(2), 134. 10.1071/WR09152 [DOI] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, & Kawaoka Y (1992, March 1). Evolution and ecology of influenza A viruses. Microbiological Reviews, Vol. 56, pp. 152–179. 10.1128/mmbr.56.1.152-179.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2009). CDC protocol of realtime RTPCR for influenza A(H1N1). Geneva, Switzerland. [Google Scholar]
- Williams RA, & Peterson AT (2009). Ecology and geography of avian influenza (HPAI H5N1) transmission in the Middle East and northeastern Africa. International Journal of Health Geographics, 8(1), 47. 10.1186/1476-072X-8-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H (2006). Modification of normalised difference water index (NDWI) to enhance open water features in remotely sensed imagery. International Journal of Remote Sensing, 27(14), 3025–3033. 10.1080/01431160600589179 [DOI] [Google Scholar]
- Zuur Alain F., Ieno Elena N., Walker Neil, Saveliev Anatoly A., S. GM (2009). Mixed Effects Models and Extensions in Ecology with R (Springer, Ed.). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Scaled residual plot and dispersion test for seasonality GLMM using DHARMAa package
Supplementary Figure 2. Scaled residual plot and dispersion test for GLMM final model using DHARMAa packag
Supplementary Figure 3. Total number of migratory and resident birds by site between September 2015 and June 2018.


