Abstract
Purpose:
Bisphosphonates reduce bone metastases in postmenopausal women with early-stage breast cancer but carry the risk of bisphosphonate-related osteonecrosis of the jaw (BRONJ). We describe risk factors for BRONJ and compare BRONJ provoked by infection or trauma with spontaneous lesions, which carry a better prognosis.
Methods:
SWOG 0307 randomized women with Stage I-III breast cancer to receive zoledronic acid (ZA), clodronate (CL), or ibandronate (IB) for three years, implemented BRONJ prevention guidelines, and collected information about dental health and development of BRONJ. All statistical tests were two-sided.
Results:
Of 6018 women, 48 developed BRONJ. Infection was present in 21 (43.8%). Median time to BRONJ was 2.1 years for ZA, 2.0 years for IB, 3.4 years for clodronate (p=0.04). BRONJ was associated with bisphosphonate type (28/2231 (1.26%) for ZA, 8/2235 (0.36%) for CL, 12/1552 (0.77%) for IB), dental calculus (OR 2.03), gingivitis (OR 2.11), moderate/severe periodontal disease (OR 2.87), and periodontitis > 4mm (OR 2.20) (p < 0.05). Of 57 lesions, BRONJ occurred spontaneously in 20 (35.1%) and was provoked by dental extraction in 20 (35.1%), periodontal disease in 14 (24.6%), denture trauma in 6 (10.5%), dental surgery in 2 (3.5%). Spontaneous BRONJ occurred more frequently at the mylohyoid ridge. There were no differences in dental disease, infection, or bisphosphonate type between spontaneous and provoked BRONJ.
Conclusion:
ZA and worse dental health were associated with increased incidence of BRONJ, with a trend toward additive risk when combined. BRONJ incidence was lower than in similar studies, with prevention strategies likely linked to this.
Clinical trial number NCT0012720.
Keywords: clinical trials, breast cancer, bisphosphonate, osteonecrosis of the jaw
INTRODUCTION
Bisphosphonates reduce the risk of bone metastases in a low-estrogen environment [1] and are recommended as adjuvant therapy in post-menopausal women with early-stage breast cancer [2–4]. Bisphosphonates inhibit the ability of osteoclasts to resorb bone, decreasing local metabolic activity and making for a less hospitable environment for metastasized cancer cells [5]. Amino-bisphosphonates, characterized by the addition of a nitrogen atom, block osteoclast activity and induce apoptosis by inhibiting farnesyl pyrophosphate synthase, an enzyme crucial to cell growth and division, while non-amino-bisphosphonates do so through formation of cytotoxic metabolites [6]. Bisphosphonates block tumor cell growth and angiogenesis, inducing apoptosis and immune system activation, and act in synergy with other anti-cancer agents [5, 7–9].
Bisphosphonate-related osteonecrosis of the jaw (BRONJ) is an uncommon but serious side effect [10]. BRONJ is defined as an area of non-healing exposed bone in the mandible or maxilla in the setting of bisphosphonate use, without exposure to radiation at the lesion site, and no other identifiable cause [11].
BRONJ can result in mouth pain, unsatisfactory diet, self-consciousness, and decreased life satisfaction [12]. Healing occurs faster in spontaneous BRONJ and BRONJ provoked by trauma and slower if provoked by dental extraction [13] and in patients with autoimmune conditions, diabetes, corticosteroid use or tobacco smokers [14–16]. Based on our clinical experience, many cases resolve in 1–3 years. Treatment can vary from basic oral hygiene to repeated antibiotics, with rare advanced cases requiring hospitalization, intravenous antibiotics, and extensive oral surgery [13].
While the pathogenesis of BRONJ is poorly elucidated, and more studies are needed in this area, it is likely multifactorial. Decreased bone turnover and ability to repair bone due to the presence of bisphosphonates results in impaired healing after injury induced by a dental procedure, trauma, or infection (as evidenced by pain, swelling, gum erythema, numbness, purulent drainage, or fistula) that necessitate bone healing to resolve [17].
BRONJ can arise spontaneously without a clear provoking factor [18,19]. Suggested causes include inflammation from microcracks [20], thin mucosal covering at the mylohyoid ridge [21], bisphosphonate toxicity alone or in combination with anti-angiogenics [22]. The oral microbiome and its interplay with the local immune response [23] and genetic differences related to immune barrier [24, 25] and osteoclast functions may play a role [26]. In animal models, intravenous bisphosphonates and tooth extraction alone [26] or in combination with inoculation by bacteria [25] or cyclophosphamide [27] resulted in BRONJ.
BRONJ is twice as likely to occur in the mandible as in the maxilla [21]. Drug-specific risk factors include bisphosphonate potency [16], intravenous administration, and cumulative dose [21, 28]. Local factors include tooth extraction [29], tooth abscess or infection [30], periodontitis [31], denture trauma, periodontal surgery, root canals, or dental implants [22]. Other risk factors include ethnicity [32] and following BRONJ prevention guidelines [33].
Conservative treatment of BRONJ with chlorhexidine rinses and antibiotics frequently resulted in resolution of symptoms, while local curettage/debridement led to recurrence or progression [31]. Thus, treatment recommendations for early-stage BRONJ emphasize conservative management. Advanced surgical techniques are effective when there is progression, extensive necrosis, osteolysis, fistula, or pathologic fracture [10]. Treatment with pentoxifylline and tocopherol has shown promise [34].
Wide resection of necrotic bone to “bleeding margins” with smoothing of sharp bony edges and primary tension-free multi-layer wound closure has shown cure rates of 80–90% for early-stage BRONJ [35]. Outcomes are similar in patients treated with conservative management and up-front surgery for early-stage BRONJ, with faster healing in the latter [36]. Based on our clinical experience, oral surgeons are more likely to utilize up-front surgery, while dentists start with more conservative treatment.
Research about BRONJ in post-menopausal women with early-stage breast cancer taking adjuvant bisphosphonates is limited to reports of BRONJ incidence [37–40]. In the Adjuvant Zoledronic Acid to Reduce Recurrence (AZURE) and Austrian Breast Cancer Study Group (ABCSG) trial-12 studies, both of which randomized patients with breast cancer to zoledronic acid or not, BRONJ prevention guidelines were not implemented and participants’ dental health was not explored [36–38].
The SWOG S0307 randomized controlled trial of adjuvant bisphosphonates in women with early stage breast cancer compared three bisphosphonates, zoledronic acid (ZA), ibandronate (IB), and clodronate (CL), and reported no difference in efficacy in reducing recurrence or death. S0307 reported the incidence of BRONJ in these patients after following recommended BRONJ prevention guidelines and was the first to report the incidence of BRONJ in women taking clodronate or ibandronate at doses intended to prevent breast cancer bone metastases [40]. While neither oral CL nor IB at the adjuvant dose used in S0307 are available in the United States, they are used at these doses in other countries to treat bone metastases. ZA and CL are included in guidelines as adjuvant therapy for postmenopausal women with early-stage breast cancer, and IB is supported by S0307 primary outcome data [2–4].
Given the negative influence of BRONJ on quality of life, it is especially important to elucidate time to onset and risk factors associated with BRONJ in women who are taking adjuvant bisphosphonates to prevent breast cancer recurrence. We conducted a pre-planned secondary analysis of the data collected during S0307 to determine the time to onset of and risk factors for BRONJ and compare characteristics of spontaneous and provoked BRONJ to improve BRONJ outcomes.
MATERIALS AND METHODS
In S0307, 6097 patients diagnosed with Stage I-III breast cancer who had undergone surgery and were receiving adjuvant systemic therapy were randomized to receive zoledronic acid 4mg IV monthly for 6 months, then every 3 months, clodronate 1600mg by mouth daily, or ibandronate 50mg by mouth daily for three years. Accrual started in November 15th, 2005 and completed February 1, 2010.
The diagnosis of BRONJ was made clinically when there was presence of exposed non-healing bone for at least 8 weeks without history of radiation exposure or metastasis at the lesion site without another attributable cause [10].
Informed consent was obtained from all patients and demographic and clinical information collected as described previously [40]. All patients provided written informed consent. The study was approved by the National Cancer Institute Central Institutional Review Board (IRB), as well as by IRBs of participating institutions.
A baseline Dental Examination Form was required to be completed by a dental health professional within six months of starting bisphosphonate. Instructions stated that the exam should include a visual inspection, periodontal probing, and an evaluation of BRONJ risk factors. X-rays were not required, but it was recommended that they be performed to assess the degree of periodontal involvement and endodontic (root canal) problems. The following dental health measures were collected: dental plaque levels, calculus, gingivitis, periodontitis, overall dental disease, presence of dentures, and number of teeth with deep caries, failing root canals, fractures/restorations, or endodontic treatment. Financial assistance was available for baseline dental exams for patients with severe financial need and without dental insurance. Patients were encouraged to complete planned dental procedures prior to starting the study drug, undergo regular dental exams while on study, maintain good oral hygiene, and report any dental symptoms to their oncologist and dentist. Patients who planning to undergo oral surgery were discouraged from enrollment. An off-study dental examination was required at study completion.
Participants were monitored for BRONJ with dental exams as part of routine medical care every 6–12 months. Information about BRONJ development was collected every 6 months and for up to 5 years after completing bisphosphonate treatment. For patients who developed BRONJ, completion of the Osteonecrosis Jaw (ONJ) Lesion Form was required, including the following: time since enrollment, number of lesions, lesion location, size, and evidence of infection. BRONJ was judged to be either spontaneous or provoked by one or more of: periodontal infection, dental extraction, other dental surgery, or denture trauma. Consultation with the S0307 dental health coordinator (MS) was recommended. Because it was not clear that stopping the bisphosphonate therapy would aid in ONJ healing, this was decided on a case-by-case basis.
Outcome dichotomous variable was set to development of BRONJ during follow-up. Independent dichotomous variables included: prior bisphosphonate use, chemotherapy, dental plaque, calculus, gingivitis, periodontitis, overall dental disease, creatinine. The number of teeth with deep caries, failing root canals, fractured teeth/restorations, and endodontically treated teeth was grouped into: none, 1, 2–3, > 3. All other dental health variables categorized were categorized as none/mild v. moderate/severe. Independent categorical variables included: bisphosphonate type and ethnicity.
Relative risk was calculated for all dichotomous variables, and odds ratios (OR) for categorical variables in this intent to treat analysis. Crude and adjusted ORs with 95% confidence intervals were calculated by univariate and multivariate logistic regression, respectively. Pearson’s Chi-squared/Fisher’s exact test and Student’s T-test were used to test differences in categorical and continuous variables, respectively. Logistic regression was used test independent association. Survival analysis methods include Kaplan-Meier plots, log-rank tests and Cox regression analyses. All p-values were two-sided.
All statistical calculations were made using SAS program, version v94.
RESULTS
BRONJ incidence and time to onset
Median follow-up was 7.5 years for ZA and CL and 8 years for IB (range 0–11.1 years). Of 6018 eligible women, 48 developed BRONJ (0.8%), with median duration to onset of 2.3 years (range 0.1–6.3). ONJ incidence was 1.26% (28/2231) for ZA, 0.77% (12/1552) for IB, 0.36% (8/2235) for CL (p = 0.0034), with median time to onset of 2.1 years for ZA, 2.0 years for IB and 3.4 years for CL (p = 0.0447). (Table 1, Figure 1)
Table 1:
Time to onset of BRONJ by bisphosphonate (years)
| Time to ONJ onset | CL (n = 8/2235) | IB (n = 12/1552) | ZA (n = 28/2231) | Total (n = 48) |
|---|---|---|---|---|
| Mean (95% CI) | 3.9 (3.0, 4.9) | 2.4 (1.2, 3.6) | 2.2 (1.6, 2.8) | 2.5 (2.0, 3.0) |
| Median (range) | 3.4 (2.8, 5.6) | 2.0 (0.2, 6.3) | 2.1 (0.1, 5.6) | 2.3 (0.1, 6.3) |
Figure 1:
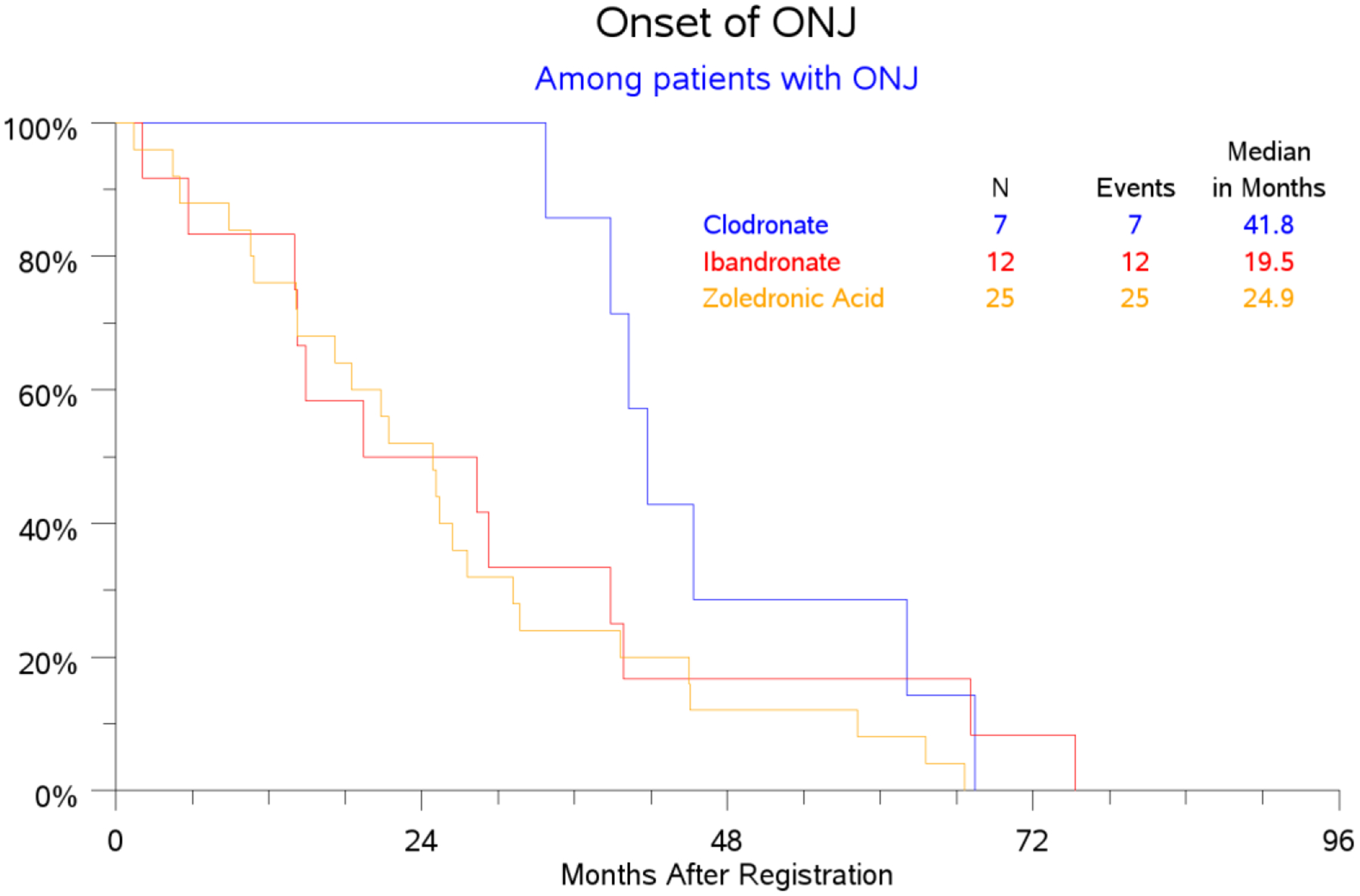
ONJ onset among patients with ONJ (months)
BRONJ characteristics and risk factors
Of the 48 patients with BRONJ, 40 (83.3%) developed one lesion, 7 (14.6%) two, and one (2.1%) three lesions. Mean size was 7.0mm (5.0–9.0). Infection was present in 21 (43.8%), absent in 22 (45.8%). Of 57 lesions, 42 (73.7%) occurred in the mandible and 14 (24.6%) in the maxilla.
BRONJ was associated with moderate/severe dental calculus, gingivitis, periodontal disease, and periodontitis > 4mm (Table 2), but not with dentures at enrollment, plaque, chemotherapy use, baseline creatinine, or ethnicity. The same variables were also associated with BRONJ at the exit dental health assessment, except for periodontitis. At the exit assessment, the BRONJ was 5/190 (2.7%) among patients with complete dentures compared to 0.8% (30/3847) among those with no or partial dentures (p = 0.002). BRONJ incidence was higher among patients who reported bisphosphonate use prior to study enrollment, presumably for osteoporosis (1.53% (5/326) compared to 0.76% (43/5692)) (p = 0.18).
Table 2:
Factors associated with BRONJ
| Variable | Ref. | OR for ONJ | 95%CI | p-value | Risk rate of ONJ in the reference group | Risk rate of ONJ in the exposure group |
|---|---|---|---|---|---|---|
| Calculus (n = 5837) | None or mild | 2.03 | (1.08, 3.81) | 0.028 | 0.67% | 1.34% |
| Gingivitis (n = 5759) | None or mild | 2.11 | (1.12, 3.98) | 0.021 | 0.65% | 1.37% |
| Periodontitis (n = 5516) | < 4 mm | 2.20 | (1.18, 4.08) | 0.013 | 0.59% | 1.30% |
| Overall periodontal disease (n = 5636) | None or mild | 2.87 | (1.49, 5.53) | 0.002 | 0.61% | 1.74% |
| Type of bisphosphonate (IB) | ZA | 0.61 | (0.31, 1.21) | 0.158 | 1.26% | 0.77% |
| Type of bisphosphonate (CL) | ZA | 0.28 | (0.13, 0.62) | 0.002 | 1.26% | 0.36% |
Eleven of the 48 patients (22.9%) developed BRONJ after finishing three years of the study drug. There was a trend toward higher risk of BRONJ in those with worsening periodontitis (though not worsening gingivitis or overall periodontal disease) compared to enrollment (p = 0.11).
BRONJ incidence ranged from 0.59–0.67% in patients with no or mild dental disease to 1.30–1.74% in patients with moderate or severe disease (Table 2). In multivariate analysis, only bisphosphonate type was associated with BRONJ (p = 0.039). The incidence of BRONJ increased in both none/mild and moderate/severe dental disease in patients who were taking ZA compared to CL and IB (p > 0.05). BRONJ incidence in patients with moderate/severe periodontitis who were taking ZA was 6/282 (2.13%) compared to 4/277 (1.44%) in patients who were taking CL and 3/188 (1.6%) in patients who were taking IB. BRONJ incidence in patients with none/mild calculus was 1.06% in patients taking ZA compared to 0.73% for IB and 0.23% for CL.
BRONJ incidence in patients with none or one deep caries was 0.61% (34/5588) compared to 3.45% (14/406) in patients with two or more. Incidence in patients with fractured teeth/restorations was 0.61% (29/4710) for those with no fractured teeth/restorations compared to 1.48% (19/1284) in one or more. Incidence was not increased in patients with failing root canals or endodontically treated teeth. (Table 3)
Table 3:
Association between dentition and BRONJ
| Baseline dental health status | ONJ (% incidence) (n = 48) | No ONJ (n = 5946) | P-value |
|---|---|---|---|
| Deep caries | <.0001 | ||
| None | 32 (0.62%) | 5144 | |
| 1 | 2 (0.49%) | 410 | |
| 2–3 | 10 (3.79%) | 254 | |
| >3 | 4 (2.82%) | 138 | |
| Fractured teeth | 0.0108 | ||
| None | 29 (0.61%) | 4681 | |
| 1 | 7 (1.35%) | 511 | |
| 2–3 | 6 (1.87%) | 314 | |
| >3 | 6 (1.34%) | 440 | |
| Endodontically treated teeth | 0.7834 | ||
| None | 27 (0.74%) | 3621 | |
| 1 | 9 (0.81%) | 1098 | |
| 2–3 | 8 (0.93%) | 854 | |
| >3 | 4 (1.1%) | 373 | |
| Failing root canals | 0.4652 | ||
| None | 46 (0.80%) | 5729 | |
| 1 | 1 (0.63%) | 159 | |
| 2–3 | 1 (2.22%) | 44 | |
| >3 | 0 (0%) | 14 | |
Characteristics of provoked and spontaneous BRONJ
BRONJ was considered provoked by dental extraction in 20 (35.1%), periodontal disease in 14 (24.6%), denture trauma in 6 (10.5%), other dental surgery in 2 (3.5%), with no cause identified in 20 lesions (35.1%). There was no difference in the amount of dental disease or bisphosphonate type between provoked and spontaneous BRONJ. Spontaneous BRONJ was significantly more likely to occur at the mylohyoid ridge (lingual and mylohyoid plate, some posterior mandible). (Tables 4 and 5) Infection was present in 13/27 (48.1%) provoked BRONJ and 7/18 (38.9%) spontaneous BRONJ (p = 0.41).
Table 4:
Location of provoked and spontaneous BRONJ
| Site (one patient could have > 1) | Provoked (n = 27) | Spontaneous (n = 18) |
|---|---|---|
| Maxilla: Anterior | 0 | 1 |
| Maxilla: Posterior | 2 | 1 |
| Maxilla: Buccal | 2 | 0 |
| Maxilla: Lingual | 0 | 1 |
| Maxilla: Alveolar ridge | 4 | 0 |
| Maxilla: Palate | 2 | 0 |
| Mandible: Anterior | 6 | 0 |
| Mandible: Posterior | 12 | 8 |
| Mandible: Buccal | 1 | 1 |
| Mandible: Lingual, mylohyoid plate | 7 | 12 |
| Mandible: Alveolar ridge | 6 | 2 |
Table 5:
Bisphosphonate type and periodontal disease association with provoked and spontaneous BRONJ
| Variable | Provoked ONJ (n = 27) | Spontaneous ONJ (n = 18) | P-value |
|---|---|---|---|
| Bisphosphonate type | |||
| Clodronate | 4 (15%) | 3 (17%) | 0.27 |
| Ibandronate | 9 (33%) | 2 (11%) | |
| Zoledronic acid | 14 (52%) | 13 (72%) | |
| Calculus (missing = 2) | 0.46 | ||
| None | 2 (8%) | 4 (22%) | |
| Mild | 15 (60%) | 8 (44%) | |
| Moderate | 5 (20%) | 5 (28%) | |
| Severe | 3 (12%) | 1 (6%) | |
| Gingivitis (missing = 3) | 0.53 | ||
| None | 6 (24%) | 8 (47%) | |
| Mild | 9 (36%) | 6 (35%) | |
| Moderate | 5 (20%) | 1 (6%) | |
| Severe | 3 (12%) | 1 (6%) | |
| Generalized | 2 (8%) | 1 (6%) | |
| Periodontitis (missing = 6) | 0.25 | ||
| < 4mm | 11 (52%) | 13 (72%) | |
| 4–6mm | 7 (33%) | 2 (11%) | |
| >6mm | 3 (14%) | 3 (17%) | |
| Periodontal disease (missing = 5) | 0.035 | ||
| None | 8 (36%) | 12 (67%) | |
| Mild | 7 (32%) | 0 | |
| Moderate | 5 (23%) | 4 (22%) | |
| Severe | 2 (9%) | 2 (11%) | |
DISCUSSION
BRONJ incidence and time to onset
While we did not evaluate adherence to oral bisphosphonates, the percentage of patients completing three years of therapy was similar, with 63% for ZA, 61% for IB, and 57% for CL [40].
The incidence of BRONJ was 1.26% (28/2231) in women taking ZA 4mg IV monthly for 6 months, then every 3 months for three years after a median follow-up of 7.5–8 years. This dose is higher than the current recommended dosing of adjuvant ZA for post-menopausal women with early-stage breast cancer, 4mg IV every 6 months for 2–5 years [2–4]. In the AZURE trial, where ZA 4mg IV was given monthly for 6 months, then every 3 months for 8 doses, followed by every 6 months for five doses, BRONJ incidence was 1.4% (26/1681) after 5 years [36] and 1.8% after 10 years [38]. There were no reports of BRONJ in the ABCSG trial-12, where half of the 1,803 premenopausal women with early stage breast cancer received ZA 4mg IV every 6 months for three years and were followed for a median of 5.2 years [38]. In a meta-analysis of nine prospective clinical trials where ZA was given as adjuvant therapy in women with early-stage breast cancer, 0.33% (13/3987) developed BRONJ [41]. In all but two trials, ZA was administered at 4mg IV every 6 months. Three trials had a follow-up period of one year, whereas BRONJ can occur three years after bisphosphonate discontinuation [42].
The incidence of BRONJ with oral clodronate 1600mg daily was 0.36% (8/2235). In the National Surgical Adjuvant Breast and Bowel Project protocol B-34 study, one possible case of BRONJ (0.06%) was observed among 1662 women randomized to receive clodronate 1600mg daily for three years with median follow-up of 7.6 years. Adherence in the clodronate arm was 56% [43]. In another study of adjuvant CL 1600mg daily for two years with a 5.5-year follow-up, no cases of BRONJ were reported, likely due to shorter exposure and smaller sample size of 530 [44]. Examining patients for BRONJ in a prospective manner could have resulted in more accurate recognition of lesions in S0307.
The incidence of BRONJ with ibandronate 50mg daily was 0.78% (12/1552). Prior studies have examined BRONJ prevalence in women taking ibandronate 150mg by mouth monthly for osteoporosis. Time to BRONJ onset was similar between ZA and IB and significantly shorter than for CL, likely because both are more potent amino-bisphosphonates [6]. Median time to onset of two years for ZA was similar to that of studies with similar or more frequent dosing done in the metastatic setting [45].
S0307 is the first to report median time to BRONJ onset with oral ibandronate and clodronate at the adjuvant dosing schedule, at 2.0 and 3.4 years, respectively. In a study of patients taking ibandronate 150mg monthly for osteoporosis, median time to BRONJ onset was 2.1 years [45].
Since time to BRONJ onset for ZA or IB did not depend on frequency of administration or dose, respectively, compared to other studies, time to BRONJ onset is likely initially related to the potency of the amino-bisphosphonate, though accumulation of bisphosphonate in bone is likely important.
Risk factors for BRONJ
Our study is the first to describe risk factors associated with BRONJ in women with early-stage breast cancer who were taking adjuvant bisphosphonates to prevent bone metastases while following BRONJ prevention guidelines. While all subjects were required to have dental exams at the start of the study and encouraged to complete planned dental procedures prior to study entry, we did not require that all “necessary” dental treatments be carried out and did not track their outcome. However, it was likely that ongoing BRONJ surveillance and support from dental providers resulted in improved dental health and decreased risk of BRONJ.
Similar to other studies, we found that ZA carries a higher risk of BRONJ compared to CL and IB [16, 21, 28], that BRONJ was more likely to occur in the mandible [21], and that tooth extraction, dental trauma, dentures, and periodontitis were risk factors for BRONJ [22, 29–31]. We found that gingivitis and dental calculus were associated with increased BRONJ incidence, with increasing severity associated with higher incidence. Similarly, deep caries and fractured teeth/restorations increased the risk on BRONJ. If not treated, progression could lead to infection, necessitating dental interventions that could result in BRONJ. Consistent with this, we found a trend toward a higher risk of BRONJ among patients with progressive periodontitis on the exit dental exam. We found a trend toward additive risk for BRONJ when more potent bisphosphonates were combined with more severe dental disease. Thus, drug accumulation in alveolar bone, when combined with relative potencies of each bisphosphonate and a subsequent insult to bone or mucosa helps explain BRONJ incidence and risk.
Similar to one study [28] and contrary to another [16], we did not find any association between BRONJ and chemotherapy. Animal models where BRONJ was induced by cyclophosphamide and ZA[27] suggest that the specific chemotherapy, dose, and timing would be especially critical for BRONJ development. Unlike a study that found increased BRONJ risk in Caucasian patients, we did not find an association between BRONJ and ethnicity [32]. We did not find a link between renal adverse events and BRONJ, likely due to stopping bisphosphonates in Grade 3 or 4 renal toxicity.
Characteristics of provoked and spontaneous BRONJ
Twenty (30.7%) BRONJ lesions were spontaneous, compared to 0–50% in other studies [18]. As we did not train community dentists to evaluate whether BRONJ was spontaneous or provoked, a higher rate of spontaneous BRONJ could be due to incomplete data collection [18]. Our participants may have better dental health than the general population, resulting in a higher rate of spontaneous BRONJ.
Spontaneous BRONJ was more likely to occur at the mylohyoid ridge, consistent with our clinical experience. We expected provoked and spontaneous BRONJ to differ with respect to risk factors given faster healing of spontaneous BRONJ in one study [19] and in our clinical experience. However, provoked and spontaneous BRONJ did not differ in terms of dental health or bisphosphonate type. This could be due to BRONJ prevention measures that resulted in a more even distribution of risk factors or failure to capture differences between these as a result of BRONJ characterization done without specific training.
Limitations
Our results are limited by the small number of patients with BRONJ (likely in part due to following BRONJ prevention guidelines) and lack of specific training about BRONJ for community dentists who treated our patients. Results were limited by an older definition of BRONJ that required bone to be exposed (11). In our view, the data still provide useful insights into this significant complication of adjuvant bisphosphonate use.
CONCLUSION
S0307 is the first study to report BRONJ incidence and time to onset in a prospective cohort of women with early-stage breast cancer randomized to adjuvant ZA, IB, or CL to prevent bone metastases. While dental disease has long been implicated as risk factor for BRONJ, our study is the first to involve a comprehensive dental health assessment, implement BRONJ prevention measures, and measure the association between dental disease severity and BRONJ incidence. More advanced dental disease led to increased BRONJ risk; there was a trend toward additive risk when it was combined with more potent bisphosphonates. We are not aware of other prospective multi-center studies of bisphosphonates that have incorporated a similar degree of dental assessment and have found similar associations.
Given the variable progression and time to resolution of BRONJ, randomized controlled studies comparing outcomes in patients with early-stage BRONJ receiving conservative vs early surgical treatment are urgently needed. Future studies that rely on community dentists for early identification and treatment of provoked and spontaneous BRONJ should provide uniform training to community dentists to ensure internal validity of the collected data. It would be important to design protocols that followed standards of care for preventing BRONJ, trained and supported medical and dental providers in preventing BRONJ, and measured dental health, including type and site of disease, treatment received and its outcome, and adherence to regular dental check-ups throughout the length of the study.
Spontaneous and provoked BRONJ represent a spectrum of disease severity, and it would be important to determine whether patients with spontaneous BRONJ would be good candidates for conservative treatment given their tendency for faster healing. Given their proven efficacy, current recommendations for BRONJ prevention should be implemented widely and refined further to reflect recent research related to BRONJ prevention in patients who are taking bisphosphonates and require tooth extractions [33]. Given our finding of a higher risk of BRONJ in patients with moderate/severe periodontitis, gingivitis, calculus, deep caries, fractured teeth/restorations, and a trend toward higher risk of BRONJ with worsening periodontitis, treatment and follow-up of these conditions should be encouraged.
Acknowledgements
We would like to thank the patients who participated in SWOG 0307 and their families. We would also like to thank the Southwest Oncology Group (SWOG) for statistical support and the National Cancer Institute, the National Institutes of Health, the Breast Cancer Research Foundation, The Susan G. Komen Foundation, Berlex, Roche/Genentech, Novartis for funding this trial.
Funding
This project has been funded with federal funds from NIH/NCI CA180888, CA180819, CA180820, CA180821, CA180868, CA180863, as well as with funds from the Breast Cancer Research Foundation, Komen, Berlex, Roche/Genentech, Novartis.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: Dr. Ingle reports grants from U.S. National Cancer Institute, during the conduct of the study. Dr. Falkson reports grants from Seattle Genetics, grants from Pfizer, other from Biotheranostics, other from Agendia, grants from Genentech/Roche, other from ExactSciences/OncotypeDx, outside the submitted work. Dr. Barlow reports grants from the National Cancer Institute, during the conduct of the study. Dr. Hortobagyi reports grants and personal fees from Novartis, outside the submitted work. Dr. Gralow reports other from Roche/Genentech, other from Novartis, other from Radius, other from Astra Zeneca, other from Pfizer, other from Puma, other from Immunomedics, other from Seattle Genetics, other from Sandoz/Hexal AG, outside the submitted work. All other authors declare no conflicts of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. All patients provided written informed consent. The study was approved by the National Cancer Institute Central Institutional Review Board (IRB), as well as by IRBs of participating institutions and monitored by an independent data safety monitoring committee.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication Does not apply
Availability of data and material Does not apply
Code availability Does not apply
Contributor Information
Darya Kizub, The Everett Clinic, Everett, Washington, United States..
Jieling Miao, SWOG Statistical Center, Seattle, Washington, United States..
Mark M. Schubert, Fred Hutchinson Cancer Research Center, Seattle, WA; University of Washington School of Dentistry, Seattle, Washington, United States..
Alexander H.G. Paterson, Tom Baker Cancer Center, Calgary, Alberta, Canada..
Mark Clemons, Ottawa Hospital Research Institute, Ottawa, Ontario, Canada.
Elizabeth C. Dees, University of North Carolina, Chapel Hill, North Carolina, United States..
James N. Ingle, Mayo Clinic, Rochester, Minnesota, United States..
Carla I. Falkson, Wilmot Cancer center at the University of Rochester, Rochester, New York, United States.
William E. Barlow, SWOG Statistical Center, Seattle, Washington, United States..
Gabriel N. Hortobagyi, University of Texas MD Anderson Cancer Center, Houston, Texas, United States..
Julie R. Gralow, University of Washington School of Medicine, Seattle, Washington, United States..
REFERENCES
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386(10001):1353–1361. [DOI] [PubMed] [Google Scholar]
- 2.Dhesy-Thind S, Fletcher GG, Blanchette PS et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. Clin Oncol. 2017;35(18):2062–2081. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019. August 1;30(8):1194–1220. [DOI] [PubMed] [Google Scholar]
- 4.Hadji P, Coleman RE, Wilson C, Powles TJ, Clézardin P, Aapro M, Costa L, Body JJ, Markopoulos C, Santini D, Diel I, Di Leo A, Cameron D, Dodwell D, Smith I, Gnant M, Gray R, Harbeck N, Thurlimann B, Untch M, Cortes J, Martin M, Albert US, Conte PF, Ejlertsen B, Bergh J, Kaufmann M, Holen I. Adjuvant bisphosphonates in early breast cancer: consensus guidance for clinical practice from a European Panel. Ann Oncol. 2016. March;27(3):379–90. [DOI] [PubMed] [Google Scholar]
- 5.Guise T. Examining the metastatic niche: targeting the microenvironment. Semin Oncol. 2010;37 Suppl 2:S2–14. [DOI] [PubMed] [Google Scholar]
- 6.Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006;12(20 Pt 2):6222s–6230s. [DOI] [PubMed] [Google Scholar]
- 7.Guo RT, Cao R, Liang PH, et al. Bisphosphonates target multiple sites in both cis- and transprenyltransferases. Proc Natl Acad Sci U S A. 2007;104(24)10022–10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res. 2000;15(11):2211–2221. [DOI] [PubMed] [Google Scholar]
- 9.Clezardin P Bisphosphonates antitumor activity: an unrevealed side of a multifaceted drug class. Bone. 2011;48(1):71–79. [DOI] [PubMed] [Google Scholar]
- 10.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–7. [DOI] [PubMed] [Google Scholar]
- 11.Ruggiero SL, Dodson TB, Fantasia J et al. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–56. [DOI] [PubMed] [Google Scholar]
- 12.Miksad RA, Lai KC, Dodson TB et al. Quality of life implications of bisphosphonate-associated osteonecrosis of the jaw. Oncologist. 2011;16(1):121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melea PI, Melakopoulos I, Kastritis E et al. Conservative treatment of bisphosphonate-related osteonecrosis of the jaw in multiple myeloma patients. Int J Dent. 2014;2014:427273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Ryan FS, Lo JC. Bisphosphonate-related osteonecrosis of the jaw in patients with oral bisphosphonate exposure: clinical course and outcomes. J Oral Maxillofac Surg. 2012;70(8):1844–53. [DOI] [PubMed] [Google Scholar]
- 15.Lee LW, Hsiao SH, Chen LK. J Formos Med Assoc. Clinical treatment outcomes for 40 patients with bisphosphonates-related osteonecrosis of the jaws. 2014;113(3):166–72. [DOI] [PubMed] [Google Scholar]
- 16.Assaf AT, Smeets R, Riecke B et al. Incidence of bisphosphonate-related osteonecrosis of the jaw in consideration of primary diseases and concomitant therapies. Anticancer Res. 2013;33(9):3917–24. [PubMed] [Google Scholar]
- 17.Aghaloo T, Hazboun R, Tetradis S. Pathophysiology of Osteonecrosis of the Jaws. Oral Maxillofac Surg Clin North Am. 2015;27(4):489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichardo SE, van Merkesteyn JP. Bisphosphonate related osteonecrosis of the jaws: spontaneous or dental origin? Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(3):287–92. [DOI] [PubMed] [Google Scholar]
- 19.O’Ryan FS, Lo JC. Bisphosphonate-related osteonecrosis of the jaw in patients with oral bisphosphonate exposure: clinical course and outcomes. J Oral Maxillofac Surg. 2012;70(8):1844–53. [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Landayan ME, Lee JY et al. Role of microcracks in the pathogenesis of bisphosphonate-related osteonecrosis of the jaw. Clin Oral Investig. 2016;20(8):2251–2258. [DOI] [PubMed] [Google Scholar]
- 21.Estilo CL, Van Poznak CH, Wiliams T et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13(8):911–20. [DOI] [PubMed] [Google Scholar]
- 22.Ngamphaiboon N, Frustino JL, Kossoff EB, Sullivan MA, O’Connor TL. 1. Osteonecrosis of the jaw: dental outcomes in metastatic breast cancer patients treated with bisphosphonates with/without bevacizumab. Clin Breast Cancer. 2011;11(4):252–7. [DOI] [PubMed] [Google Scholar]
- 23.Sedghizadeh PP, Yooseph S, Fadrosh DW et al. Metagenomic investigation of microbes and viruses in patients with jaw osteonecrosis associated with bisphosphonate therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(6):764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalyan S, Wang J, Quabius ES et al. Systemic immunity shapes the oral microbiome and susceptibility to bisphosphonate-associated osteonecrosis of the jaw. J Transl Med. 2015;13:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mawardi H, Giro G, Kajiya M et al. A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J Dent Res. 2011;90(11):1339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Ko YJ, Kim JY et al. Genetic investigation of bisphosphonate-related osteonecrosis of jaw (BRONJ) via whole exome sequencing and bioinformatics. PLoS One. 2015;10(2):e0118084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroshima S, Sasaki M, Nakajima K, Tamaki S, Hayano H, Sawase T. Prevalence of bisphosphonate-related osteonecrosis of the jaw-like lesions is increased in a chemotherapeutic dose-dependent manner in mice. Bone. 2018;112:177–186 [DOI] [PubMed] [Google Scholar]
- 28.Boonyapakorn T, Schirmer I, Reichart PA, Sturm I, Massenkeil G. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol. 2008;44(9):857–69. [DOI] [PubMed] [Google Scholar]
- 29.Lungu AE, Lazar MA, Tonea A, Rotaru H, Roman RC, Badea ME. Observational study of the bisphosphonate-related osteonecrosis of jaws. Clujul Med. 2018;91(2):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelaz A, Junquera L, Gallego L et al. Epidemiology, pharmacology and clinical characterization of bisphosphonate-related osteonecrosis of the jaw. A retrospective study of 70 cases. Acta Otorrinolaringol Esp 2015;66(3):139–47. [DOI] [PubMed] [Google Scholar]
- 31.Saussez S, Javadian R, Hupin C et al. Bisphosphonate-related osteonecrosis of the jaw and its associated risk factors: a Belgian case series. Laryngoscope. 2009;119(2):323–9. [DOI] [PubMed] [Google Scholar]
- 32.Quispe D, Shi R, Burton G. Osteonecrosis of the jaw in patients with metastatic breast cancer: ethnic and socio-economic aspects. Breast J. 2011;17(5):510–3. [DOI] [PubMed] [Google Scholar]
- 33.Beth-Tasdogan NH, Mayer B, Hussein H, Zolk O. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst Rev. 2017;10:CD012432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heifetz-Li JJ, Abdelsamie S, Campbell CB, Roth S, Fielding AF, Mulligan JP. Systematic review of the use of pentoxifylline and tocopherol for the treatment of medication-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019. November;128(5):491–497.e2. [DOI] [PubMed] [Google Scholar]
- 35.Bodem JP, Schaal C, Kargus S et al. Surgical management of bisphosphonate-related osteonecrosis of the jaw stages II and III. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(4):367–72. [DOI] [PubMed] [Google Scholar]
- 36.Lee LW, Hsiao SH, Chen LK. J Formos Med Assoc. Clinical treatment outcomes for 40 patients with bisphosphonates-related osteonecrosis of the jaws. 2014;113(3):166–72. [DOI] [PubMed] [Google Scholar]
- 37.Rathbone EJ, Brown JE, Marshall HC et al. Osteonecrosis of the jaw and oral health-related quality of life after adjuvant zoledronic acid: an adjuvant zoledronic acid to reduce recurrence trial subprotocol (BIG01/04). J Clin Oncol. 2013;31(21):2685–91. [DOI] [PubMed] [Google Scholar]
- 38.Coleman RE, Collinson M, Gregory W, Marshall H, Bell R, Dodwell D, Keane M, Gil M, Barrett-Lee P, Ritchie D, Bowman A, Liversedge V, De Boer RH, Passos-Coelho JL, O’Reilly S, Bertelli G, Joffe J, Brown JE, Wilson C, Tercero JC, Jean-Mairet J, Gomis R, Cameron D. Benefits and risks of adjuvant treatment with zoledronic acid in stage II/III breast cancer. 10 years follow-up of the AZURE randomized clinical trial (BIG 01/04). J Bone Oncol. 2018. September 27;13:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gnant M, Mlineritsch B, Stoeger H et al. ; Austrian Breast and Colorectal Cancer Study Group, Vienna, Austria. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011. July;12(7):631–41. [DOI] [PubMed] [Google Scholar]
- 40.Gralow JR, Barlow WE, Paterson AHG, Miao JL, Lew DL, Stopeck AT, Hayes DF, Hershman DL, Schubert MM, Clemons M, Van Poznak CH, Dees EC, Ingle JN, Falkson CI, Elias AD, Messino MJ, Margolis JH, Dakhil SR, Chew HK, Dammann KZ, Abrams JS, Livingston RB, Hortobagyi GN. Phase III randomized trial of bisphosphonates as adjuvant therapy in breast cancer: S0307. J Natl Cancer Inst. 2019. October 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mauri D, Valachis A, Polyzos IP, Polyzos NP, Kamposioras K, Pesce LL. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: a meta-analysis. Breast Cancer Res Treat. 2009;116(3):433–9. [DOI] [PubMed] [Google Scholar]
- 42.Jung SY, Suh HS, Park JW, Kwon JW. Drug holiday patterns and bisphosphonate-related osteonecrosis of the jaw. Oral Dis. 2019;25(2):471–480 [DOI] [PubMed] [Google Scholar]
- 43.Paterson AH, Anderson SJ, Lembersky BC, et al. Oral clodronate for adjuvant treatment of operable breast cancer (National Surgical Adjuvant Breast and Bowel Project protocol B-34): a multicentre, placebo-controlled, randomised trial. Lancet Oncol. 2012;13(7):734–742. doi: 10.1016/S1470-2045(12)70226-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powles T, Paterson A, McCloskey E et al. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026]. Breast Cancer Res. 2006;8(2):R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fung P, Bedogni G, Bedogni A et al. Time to onset of bisphosphonate-related osteonecrosis of the jaws: a multicentre retrospective cohort study. Oral Dis. 2017;23(4):477–483. [DOI] [PubMed] [Google Scholar]


