Abstract
Pain assessment that fully represents patients’ pain experiences is essential for chronic pain research and management. The traditional primary outcome measure has been a patient’s average pain intensity over a time period. In this series of three articles, we examine whether pain assessment can be enhanced by considering additional outcome measures capturing temporal aspects of pain, such as pain maxima, duration, and variability. Ecological momentary assessment (EMA) makes the assessment of such indices readily available. In this first article, we discuss the rationale for considering additional pain indices derived from EMA and examine which are most important to stakeholders. Patients (n=32), clinicians (n=20), and clinical trialists (n=20) were interviewed about their preference rankings for Average, Worst, and Least Pain, Time in High Pain, Time in No/Low Pain, Pain Variability, and Pain Unpredictability. Each stakeholder group displayed a distinct preference hierarchy for different indices, and there were few commonalities between groups. Patients favored Worst Pain and Time in High Pain, followed by Pain Variability and Unpredictability. Trialists favored Average Pain, whereas clinicians favored Worst Pain. Results suggest that multiple temporal aspects of pain are relevant for stakeholders and should be considered when evaluating the efficacy of pain management.
Keywords: Pain intensity, Ecological Momentary Assessment, Pain indices, Stakeholders
Introduction
Pain assessment that fully represents patients’ experiences with pain is essential for chronic pain research and management. Self-reports of pain intensity represent the primary outcome in most pain clinical trials and are nearly universally assessed during patient encounters [15,28,46]. However, it is widely acknowledged that current pain measurement can and should be improved [22,46]. There are various aspects of pain that can provide information of diagnostic importance and that can reflect change due to treatment. Reviews of pain assessment recommend that multiple dimensions of pain be considered as clinical endpoints, including sensory, affective, perceptual, and temporal features [16,22].
A fundamental aspect of the pain experience is that pain does not remain at the same intensity level at all times, but rather exhibits dynamic changes and temporal patterns that unfold within and across days [9,23,39]. The primary outcome variable in many clinical and research contexts has been the average of a patient’s pain intensity over a specific time period (e.g., a week). However, a focus on average pain misses dynamic patterns of pain intensity that may be important clinical targets. Prominent recommendations emphasize the need for enhancing current pain measurement by assessments of temporal dimensions of pain, such as pain maxima, frequency, duration, and variability [15,16].
To date, it is not clear which temporal aspects of pain should be assessed to achieve the greatest utility for chronic pain research and practice. The United States Food and Drug Administration (FDA) draft guidance on analgesic drug development recommends the use of worst pain levels over a time period (e.g., a day) as the primary outcome in clinical trials [57]. The use of least pain as clinical trial outcome has also been recommended [15], despite limited empirical evidence supporting these recommendations [47]. There are also other ways to characterize temporal aspects of pain intensity that have received less attention. Evidence suggests that the amount of time patients spend either in low pain or in high pain represent distinctive features of pain [27,42,48]. The amount of variability and the unpredictability of shifts in pain have been linked to psychosocial and functional outcomes [3,4,19,32,33,39], suggesting these also may be important pain indices.
This paper is the first in a series of three articles in which we aim to advance evidence-based decision-making about which aspects of pain intensity should be assessed in research and practice. We present a strategy to investigate alternative indices that characterize different temporal aspects of a patient’s pain intensity utilizing Ecological Momentary Assessments (EMA). With EMA, momentary pain intensity reports are collected multiple times daily, which allows assessment of the dynamic ebb and flow of pain in patients‟ natural environments [31,53]. We propose to use the fine-grained information provided by EMA for the construction of additional pain indices that can be used as outcome measures [43,49].
The purpose of the present study was to identify which temporal aspects of pain that can be captured with EMA are important to stakeholders (subsequent papers in this series focus on empirical examinations of EMA-derived pain indices [38,40]). Incorporating stakeholders in healthcare research is strongly promoted by regulatory agencies [13,17,25,56,59]. Previous attempts to clarify important outcomes from the patient perspective have lent support to using a variety of outcomes in addition to average pain intensity (e.g., sleep, physical functioning) [8,37,55], but temporal indices of pain have not been considered. Additionally, multiple stakeholder groups are necessary to understand commonalities and differences in perspectives about the utility of pain measures [44]. For example, knowing which aspects of pain are valued by clinicians is important for understanding how treatment decisions are made. Understanding patient and provider preferences can also facilitate patient-centered care by identifying potential mismatches in perspectives that can then be remedied. Similarly, understanding preferences of clinical trialists and how they align with patient-valued endpoints can heighten awareness of potential gaps in outcome assessments that could be improved. Accordingly, we interviewed (a) patients with chronic pain, (b) healthcare clinicians, and (c) researchers conducting pain clinical trials about their preferences for temporal indices of pain intensity.
Methods
Conceptualization of Pain Intensity Indices
Before describing the study design, we briefly introduce the conceptualization of different indices of pain intensity derived from EMA that provide the framework for the present study. EMA involves the collection of repeated momentary pain intensity ratings in patients’ everyday environments. It has long been acknowledged that EMA reduces or eliminates recall bias and enhances the ecological validity of pain data [7], and an increasing body of research has used EMA to examine determinants and within-person correlates of momentary pain experiences [31]. An additional use of EMA that has been less often considered is that repeated momentary pain intensity ratings collected over a given time period (e.g., a day, week, or month) can be summarized in different ways to create alternative outcome measures [43,49]. Here, we distinguish three basic categories of outcomes: they focus (a) on different kinds of pain level (average, worst, least), (b) on the amount of time spent in high pain and low pain, and (c) on short-term shifts and variability in pain intensity over time. We note that this list considers basic distributional characteristics of repeated pain assessments, and it is by no means exhaustive (e.g., additional temporal features such as the time to onset of pain relief [15] or the persistence of pain states [41] are not considered here; for a more detailed description of EMA-derived pain outcomes, see [49]).
Indices focusing on pain intensity levels.
The first three indices are the average amount of pain (“Average Pain”), the highest level of pain (“Worst Pain”), and the lowest level of pain (“Least Pain”) during the measurement period, parallel to those assessed using patient recall questions in the Brief Pain Inventory [11]. This does not mean, though, that EMA-derived measures of average, worst, and least pain are necessarily equivalent to those based on patients’ recall of their pain. In fact, there is considerable evidence suggesting that they measure somewhat different constructs [50,51] with it being likely that cognitive heuristics impact the recall-based measures.
Indices focusing on the amount of time spent in high or low pain.
Another method of characterizing pain intensity is shown in the next two indices: the amount of “Time in No/Low Pain” and “Time in High Pain.” These are based on the proportion of time (operationalized by EMA moments assessed) that a person’s pain intensity levels fall below a threshold of low (or “mild”) pain or exceed a threshold of high (“severe”) pain (e.g., using established cutoffs for “mild” pain and “severe” pain [6]). In contrast to the indices in the first group that do not explicitly provide any information about the duration of pain states, this second group of indices combine information about both pain intensity levels and their duration. Conceptually, these indices summarize pain experiences in ways similar to physiological (amount of time in hyperglycemia [2]) or behavioral (amount of time spent in sedentary or moderate-vigorous physical activity [30]) measures that have been developed from ambulatory assessments.
Indices focusing on pain variability.
This third category of indices summarizes the short-term shifts or fluctuations in a patient’s pain intensity over the reporting period. Indices of intraindividual variability derived from EMA and other ambulatory assessments have proven fruitful in a number of research and medical areas, including research on physiological parameters (e.g., short-term variability in ambulatory blood pressure [35], heart rate [54], blood glucose levels [34]), behaviors (e.g., reaction time variability [21]), and emotions (e.g., variability in positive and negative affect [20]). While fluctuations in experiences can be quantified numerous ways (e.g., [36]), two basic aspects were selected for this study: the magnitude (or amplitude) of intraindividual pain variations (“Pain Variability”) and the extent to which shifts in pain are expected or unexpected (“Pain Unpredictability”).
Study materials
The purpose of the stakeholder interviews was to examine which of the pain intensity indices are viewed as most important to each of the stakeholder groups and which should have the highest priority as targets for intervention. In order to convey the meaning of different pain indices that can be derived from densely repeated momentary pain reports to stakeholders who themselves may not be familiar with EMA, we created definitions and explanations of seven pain intensity indices: Average Pain, Worst Pain, Least Pain, Amount of Time in High Pain, Amount of Time in No/Low Pain, Pain Variability, and Pain Unpredictability. The descriptions were provided to all stakeholders on a sheet of paper as shown in Table 1. The definitions of all pain indices used a period of one week of momentary pain intensity ratings, since this the reporting period that is often used in retrospective recall pain assessments in clinical contexts and may also be relevant for creating variables to serve as clinical endpoints in chronic pain trials [28].
Table 1:
Descriptions of Pain Intensity Indices Provided to Stakeholders during the Interviews
| Pain Intensity Index | Definition/Explanation |
|---|---|
| Average pain intensity over a week | If we take many ratings of a patient’s pain intensity during a week, add them up and then divide by the number of ratings, this would give us an average of a patient’s pain during that week. |
| Level of pain intensity when it is at its worst during a week | If we take many ratings of a patient’s pain intensity during a week, we could see what a patient’s highest pain level was. This would indicate the level of pain intensity when it was at its worst. |
| Level of pain intensity when it is at its least during a week | If we take many ratings of a patient’s pain intensity during a week, we could see what a patient’s lowest pain level was. This would indicate the level of pain intensity when it was at its least. |
| Amount of time patient spends with no or low pain during a week | This refers to how much of the time during the week a patient didn’t feel any or felt very little pain. That is, if we were to take many ratings of a patient’s pain intensity, we could figure out the amount of time during a week that a patient had no pain or almost no pain. |
| Amount of time patient spends in high pain during a week | If we were to take many ratings of a patient’s pain intensity during the week, we could figure out the amount of time when a patient had ratings of pain intensity at very high levels. |
| How much pain intensity fluctuates or changes during a week | If we take many ratings of a patient’s pain intensity during a week, we can get a sense of how much a patient’s pain intensity varies from moment-to-moment or day-to-day over the week. That is, whether the intensity is more or less constant or how much a patient’s pain fluctuates (that is, goes up and down). |
| Amount of unpredictability of pain levels during a week | This refers to the degree to which a patient’s pain intensity changes for reasons that the patient can’t identify. If a patient doesn’t know when and why his/her pain changes, then a patient’s pain levels are unpredictable. |
Note: Shown here are the definitions and explanations provided to clinicians and clinical trialists. For patients, any reference to “a patient[‘s]” in the table was replaced with “you” or “your”.
Stakeholder recruitment
A total of 72 stakeholders (32 patients, 20 clinicians, and 20 clinical trialists) were recruited for the interviews.
Patients (n = 32) with chronic pain were recruited from a pre-existing Internet panel hosted by Survey Sampling International (SSI). Patients were required to have a self-reported condition that results in chronic pain (which was defined as moderate or greater levels of pain severity that had lasted longer than three months) and have an average pain intensity score of 4 or greater on a 10-point scale for the past week. They were also required to be age 21 or older, be able to read and speak English fluently, and be willing to provide verbal informed consent prior to participating in the study. Recruitment was stratified by gender (50% female) and age group (three groups of 21–39, 40–59, and 60+ years of age) to enhance generalizability with regards to these demographic characteristics [18]. Patients were sampled from all four geographic regions of the United States.
Clinicians (n = 20) were recruited through the American Academy of Pain Medicine (AAPM) mailing list. To be eligible to participate, clinicians were required provide medical care to patients with chronic pain for more than eight hours a week. Included were medical doctors (MDs), psychologists (PhDs), nurse practitioners (NPs), and physician assistants (PAs). Recruitment efforts covered the four geographic regions of the US (Northeast, South, Midwest, West) based on clinician zip codes. A total of 145 invitations were sent via postal mail, and follow-up phone calls were made to anyone who did not respond to the letter; the recruitment success rate was 13.8% (i.e., 20 of 145).
Clinical Trialists (n = 20) were identified through the NIH Research Portfolio Online Reporting Tools (RePORT) databases (https://report.nih.gov/searchable_public_databases/). To be eligible to participate, researchers had to have been principal investigator on at least one chronic pain clinical trial, documented on the NIH RePORTER. Recruitment was stratified by gender (50% female) and geographic location (approximately equal numbers of researchers located in Northeastern, Southern, Midwestern, and Western US regions). A total of 50 invitation emails were sent out to eligible trialists, and phone calls were made to anyone who did not respond to the email. Recruitment success rate was 40.0% (i.e., 20 of 50).
Procedures
The study was approved by the University of Southern California Institutional Review Board and informed consent was obtained from all participants. Study invitations to stakeholders consisted of a description of the study, details of participation, eligibility criteria, compensation for participation, and contact information of the research staff. Those interested in participating were screened for eligibility on the phone by members of the research team. For those who were eligible and interested in participating, a structured telephone interview (about 20–40 minutes) about the measurement of pain intensity was scheduled. Participants were sent a reminder email before their scheduled interview with an informed consent sheet and a pain measurement concepts sheet that contained the seven indices of pain intensity along with definitions of those concepts as attachments (shown in Table 1). Patient stakeholders were also mailed a set of index cards printed with the name of each pain index to visually assist with the preference ranking task. Before starting the interview, patients, clinicians, and trialists were asked if they had read the consent sheet and were verbally assessed for their comprehension of the study and their participation, and any questions they had were answered before beginning the interview. Patient stakeholders received $30 for their participation in the study. The initial level of compensation offered to clinicians and trialists was $150 and was later increased to $200 to increase participant recruitment.
Stakeholder Interviews
The interviews began with a semi-structured phase with open-ended questions to spontaneously elicit themes about pain intensity measurement that were of importance to the stakeholder and to introduce the notion of the pain intensity indices. This was followed by a structured interview phase to examine stakeholders’ subjective understanding of and experience with the seven different indices of pain intensity. Participants were asked to refer to the pain measurement concepts sheet (Table 1) during the interview. Research staff read each index out loud and then asked the participant about their perspective on how relevant the index would be as an outcome of pain treatment. Patient stakeholders were also probed for comprehension of the definitions and explanations of the pain indices to ensure that the concepts were understandable to them.
Then, stakeholders were asked to rank order the subjective importance of each pain intensity index. Patient stakeholders were asked to rank the indices in order of what they were “most hoping for as a result of treatment;” clinician and trialist stakeholders were asked to rank the indices in order of “importance for evaluating treatment outcome” (rank #1 = most important and rank #7 = least important). Participants had the option to assign the same rank to two or more indices (i.e., ties were allowed), and to set pain indices aside if they were deemed completely unimportant. They then read their list to the research staff member conducting the interview starting with the most important to the least important.
Analysis strategy
Differences in the stakeholder preference rankings of the pain intensity indices were analyzed with rank-ordered logistic regression models (also known as the Plackett–Luce model) [29]. These models are appropriate when respondents directly compare (i.e., rank-order) several alternative test items instead of rating each of the items independently [12]. The models allow for ties (i.e., respondents giving the same rank to two or more alternatives) and unranked alternatives (where it is assumed that pain indices that were deemed irrelevant and set aside by participants are less preferred than ranked ones) [5].
A first set of models was tested separately within each stakeholder group (i.e., separate models for patients, clinicians, and clinical trialists) to examine whether the pain indices systematically differed from each other in the importance assigned to them. Within each stakeholder group, we first performed an overall (i.e., omnibus) test to test the null hypothesis that all seven pain indices were ranked equally important. Significant omnibus tests were followed up by post-hoc pairwise comparisons of importance rankings between the pain indices.
A second set of models examined whether the importance assigned to each pain index differed between the three stakeholder groups. An overall test of differences in the rankings among all three stakeholder groups was conducted first, followed by post-hoc tests comparing the importance rankings of each index between pairs of stakeholder groups. Odds ratios (OR) were computed to indicate effect sizes; in rank-ordered logistic regression models, these represent the odds that a given pain index is more preferred (i.e., receives a higher rank) by one stakeholder group compared to another group. All rank-ordered logistic regression models were conducted using the rologit command using maximum likelihood parameter estimation in STATA 15. Ties were handled using the exact marginal likelihood method. Results at p < .05 were considered statistically significant.
The quantitative analyses of preference rankings were supplemented by qualitative analyses of stakeholder interview content. Interview transcripts were entered into a qualitative data management software program (NVivo version 11) and analyzed for statements describing how relevant the indices would be as an outcome of pain treatment. Verbatim quotes from the interviews are presented to exemplify reasons for ranking a pain index as most important.
Results
Demographic and medical characteristics of patient stakeholders are shown in Table 2. The majority of patients (72%) experienced chronic back pain, arthritis, or fibromyalgia. The average years since pain diagnosis was 15.5 (range 3 – 55 years), and 59% of the patients were currently in pain treatment. Demographic and professional characteristics of clinicians and clinical trialists are shown in Table 3. Clinician stakeholders were somewhat more likely to be male (65%) and held a range of clinical professions, with a duration of clinical practice ranging from 1 to 30 years. Clinical trialists had conducted between 1 and 30 clinical trials, with a duration of clinical research ranging from 4 to 40 years.
Table 2:
Demographic and Medical Characteristics of Patient Stakeholders (n = 32)
| Patient characteristic | |
|---|---|
| Age (M, sd, range) | 50.2 ± 14.9 (23–78 years) |
| Female sex (N, %) | 16 (50%) |
| US geographic region | |
| Northeast | 6 (19%) |
| South | 14 (44%) |
| Midwest | 5 (16%) |
| West | 7 (22%) |
| Race/ethnicity | |
| White | 20 (63%) |
| Black | 6 (19%) |
| Hispanic | 6 (19%) |
| Married | 16 (50%) |
| Education | |
| Up to high school | 16 (50%) |
| Some college and more | 16 (50%) |
| Employed | 9 (28%) |
| On disability | 14 (44%) |
| Chronic pain condition | |
| Back pain | 10 (31%) |
| Arthritis/Fibromyalgia | 13 (41%) |
| Migraine | 3 (9%) |
| Neuropathic pain | 4 (13%) |
| Pain of the limbs | 2 (6%) |
| Currently in pain treatment | 19 (59%) |
| Years since pain diagnosis | 15.5 ± 15.1 (3–55 years) |
Note: Values are n (%) or mean ± standard deviation (range).
Table 3:
Demographic and Professional Characteristics of Clinician (n = 20) and Clinical Trialist (n = 20) Stakeholders
| Clinicians | Clinical Trialists | |
|---|---|---|
| Age (M, sd, range) | 43.8 ± 10.9 (31–64 years) | 52.2 ± 14.9 (30–76 years) |
| Female sex (N, %) | 7 (35%) | 10 (50%) |
| US geographic region | ||
| Northeast | 5 (25%) | 6 (30%) |
| South | 7 (35%) | 5 (25%) |
| Midwest | 2 (10%) | 5 (25%) |
| West | 6 (30%) | 4 (20%) |
| Degree/license | ||
| Medical doctor | 15 (75%) | 4 (20%) |
| Nurse practitioner | 2 (10%) | 3 (15%) |
| Physical therapist | 0 (0%) | 4 (20%) |
| Psychologist | 1 (5%) | 4 (20%) |
| Pharmacologist | 1 (5%) | 0 (0%) |
| Physician Assistant | 1 (5%) | 0 (0%) |
| Other fields (PhD) | 0 (0%) | 5 (25%) |
| Number of years in practice/research | 14.7 ± 9.1 (1–30 years) | 21.3 ± 10.0 (4–40 years) |
| Number of clinical trials conducted | 10.1 ± 8.3 (1–30 trials) | |
| Clinical trial focus Behavioral | 12 (60%) | |
| Pharmacological | 2 (10%) | |
| Combined behavioral and pharmacological | 6 (30%) |
Note: Values are n (%) or mean ± standard deviation (range).
Patient stakeholder preferences
The 32 patients assigned importance ranks to 94.0% of the indices, and 6.0% were set aside as unimportant (4 patients left 2 indices unranked and 2 patients left 3 indices unranked). Of the ranked indices, 91.9% received unique ranks (8.1% were ties).
The left panel in Figure 1 shows the indices’ mean ranks for the patient stakeholder group. Pain indices that are located within the same circle in the figure do not significantly differ from each other in their importance rankings. The omnibus test of differences in the rankings was highly significant (χ2(6) = 107.22, p < .0001). Patients ranked indices of Worst Pain and Time in High Pain as the most important (both were significantly more favored than each of the other 5 indices; all ps < .05), and they assigned indices of Least Pain and Time in No/Low Pain the lowest rankings (both were significantly less favored than each of the other 5 indices; all ps < .001). Indices of Average Pain, Pain Variability, and Pain Unpredictability were ranked between these two groups of indices. The model estimated percentages of patients giving each index the highest (#1) rank were: Worst Pain (30.93%), Time in High Pain (30.57%), Pain Variability (16.01%), Pain Unpredictability (10.72%), Average Pain (8.13%), Time in No/Low Pain (1.91%), and Least Pain (1.72%). Illustrative statements about the importance of pain indices ranked highest by at least 10% of patients are shown in Table 4.
Figure 1.
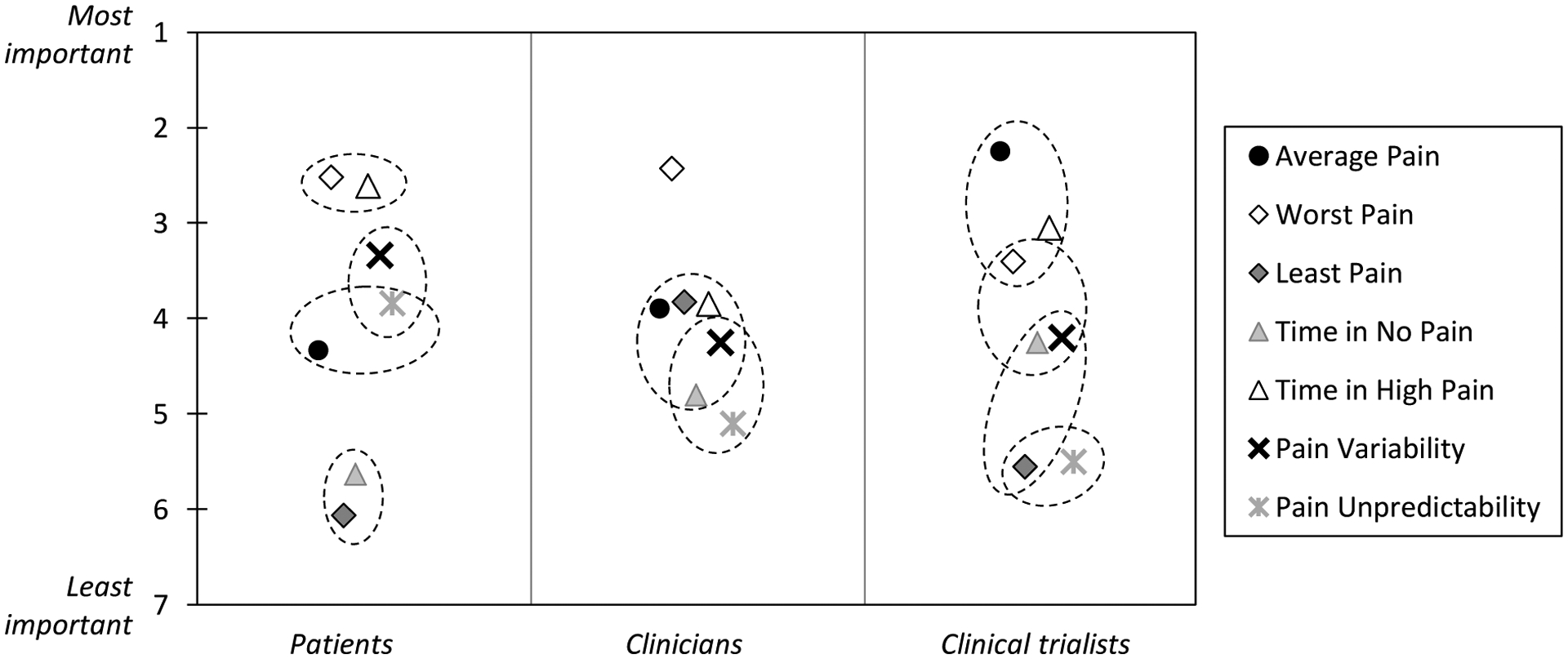
Mean importance rankings of pain indices in each stakeholder group. Rankings for indices that are located within the same circle do not significantly differ from each other within a stakeholder group (p > .05).
Table 4:
Patient Stakeholder Quotes Illustrating Reasons for Ranking a Pain Index as Most Important
| Pain index % patients ranking it most important | Patient Quotes |
|---|---|
| Worst Pain 30.9% | “Once you’ve reached that plateau, it’s very hard to come off it. Once your pain reaches that level, it is hard to get it back down to where it is manageable.” “Nobody really wants to be in pain. Your goal is not to have any pain at all but of course you’re not gonna achieve that anyway. But you really want to get rid of the intense pain.” |
| Time in High Pain 30.6% | “I can live with the little or moderate pain….I have a high pain threshold anyway….if I can deal with the amount of time in high pain better, then I can spend more time walking around, going out, being more sociable, rather than having to sit down a lot.” |
| Pain Variability 16.0% | “The reason it’s more important is because I can wake up with a 4 and by the end of the day it’s an 8 … whatever the reason the pain fluctuates….there’s no consistency in my pain….how it fluctuates during the day….there is so much change in the fluctuation in my day-to-day pain….it’s more stress than just the pain.” |
| Pain Unpredictability 10.7% | “Cause you never know how you’re gonna feel from day to day, every day of the week, when you get up in the morning,….if the day is gonna go bad or if you will be depressed, you can’t do anything.” |
Clinician preferences
Of the 20 clinicians, 19 ranked all pain indices without ties. One clinician left 3 indices (Average Pain, Time in High Pain, and Time in No/Low Pain) unranked as unimportant, and ranked 2 indices (Worst Pain and Least Pain) as tied.
The omnibus test for differences between the rankings in the clinician stakeholder group was highly significant (χ2(6) = 24.80, p < .001). As shown in the middle panel of Figure 1, the single most favorable pain index for clinicians was Worst Pain, which received significantly higher rankings than all of the other indices (all ps < .05). The remaining 6 indices were assigned similar ranks, even though Pain Unpredictability was ranked significantly less important than Average Pain, Least Pain, and Time in High Pain (ps < .05). The percentages of clinicians giving each index the highest (#1) rank were: Worst Pain (34.47%), Least Pain (15.08%), Average Pain (14.01%), Time in High Pain (13.79%), Pain Variability (9.97%), Time in No/Low Pain (7.76%), Pain Unpredictability (4.92%). Clinicians‟ reasons for assigning pain indices the highest rank are shown in Table 5 (limited to indices ranked most important by at least 10% of clinicians).
Table 5:
Clinician Stakeholder Quotes Illustrating Reasons for Ranking a Pain Index as Most Important
| Pain index % clinicians ranking it most important | Clinician Quotes |
|---|---|
| Worst Pain 34.5% | “I guess maybe that’s … really when I need to make a treatment change. You know, if we have whatever treatment we’re testing and they still have fairly high worst pain, our treatment’s not very good or we need to sequence treatments differently or we need to add on, you know, combine treatments. So, it tells me as a clinician, … we need to do something different.” “I can tell from those answers if there might be anything that … provokes it and makes it worse so I can use it as a jumping-off point so I can ask them, ‘At that time?’, ‘What were you doing?’ or ‘What position were you in when it was at its worst?’” |
| Least Pain 15.1% | “Least pain can open up questions as to what gets them there. It is useful for that. It can tell us how effective whatever medications we are using, when they’re most effective, if it gets them down to that or if an intervention that we’ve performed gets them down to the least amount of pain. Especially if we’re doing some sort of diagnostic process, if they get down to 0 out of 10 or 1 out of 10 and they are usually at a 9 out of 10, you know, that does tell us that whatever we’re doing has at least some effectiveness for whatever period of time they’re in that least amount of pain. I think the least amount of pain we need that for medical necessity purposes and things like that, so it is a useful measure.” |
| Average Pain 14.0% | “It’s like if you can only ask one thing, how can that not be what you ask. If you literally only had one question, which you don’t. I guess I was kind of thinking about it in terms of importance if I can ask one question. That’s what I’d want to know…. I mean I think it reflects that’s what their own subjective report is of their experience. Again, if you only have one data point, it’s not a rich data point, but it tells you something for that individual.” |
| Time in High Pain 13.8% | “So in terms of judging or evaluating whether treatments are effective, I definitely wanna know when would be their worst and when they’re more severe or unable to function or do anything. And so those questions are probably more important to me, and so the amount of time they were in high pain.” |
Clinical trialist preferences
The 20 trialists assigned ranks to 95.0% of the indices, and 5.0% were set aside as unimportant (2 trialists did not rank 1 index; 2 trialists did not rank 2 and 3 indices, respectively). There were no ties in trialists’ preference rankings.
The omnibus test for differences among the indices was highly significant also for trialists (χ2(6) = 39.86, p < .0001). The Average Pain index was most favored by this group of stakeholders, and received significantly higher rankings than most other indices (all ps < .05, except for Worst Pain, and Time in High Pain, see right panel in Figure 1). Additionally, indices of Least Pain and Pain Variability were ranked significantly less important than Average Pain, Worst Pain, and Time in High Pain by trialists (ps < .001). The percentages of trialists giving each index the highest (#1) rank were: Average Pain (31.45%), Time in High Pain (22.25%), Worst Pain (17.91%), Time in No/Low Pain (9.88%), Pain Variability (9.65%), Least Pain (4.99%), Pain Unpredictability (3.86%). Trialists‟ perspectives of indices they ranked highest are shown in Table 6.
Table 6:
Clinical trialist stakeholder quotes illustrating reasons for ranking a pain index as most important
| Pain index % trialists ranking it most important | Trialist Quotes |
|---|---|
| Average Pain 31.5% | “I’m most favorable about the average, because again you’re aggregating and trying to assess a chronic pain condition. That seems to better capture the patient’s pain experience. Yeah, I guess there’s some familiarity with that concept, so you like what you know…. I think it better captures sort of the overall pain experience for someone in chronic pain. So, I think that’s why I like it the most.” “Average pain, despite the highs and lows, worst and least pain, to me, it’s a better snapshot if you want to get sort of a summary of the pain experience of a person. That’s why I rate average pain as number one.” “It’s really a very common measure that’s used in clinical trials. I would say the majority of trials that are evaluating any kind of analgesic typically uses this. It’s validated and so, you know, it’s very common.” |
| Time in High Pain 22.3% | “I think that it’s helpful to know when people’s peak pain was, if we’re taking a day as a time frame. It’s important [to] know when the peak pain was, but it’s more important to know how long they spent in peak pain because if they have spasms they’re going to have. they’re just going to keep reporting 10 out of 10 for your whole study, and you’re not going to see any treatment related reductions. If they continue to experience even one spasm a day, you’re not going to see change. But let’s say that you give them a therapeutic where it really reduced the number of spasms a day, the experience, the amount of time they spent in 10 out of 10, it increased their functioning, they’re able to get back to work, they’re able to do some yard work and those kinds of things. This is clearly a patient who got better. ” |
| Worst Pain 14.0% | “Just to see how bad it gets for them and just kinda get an understanding of how severe it can be for them.” |
Comparison between stakeholder groups
We now consider the same data from another perspective by comparing the importance rankings among stakeholder groups. Figure 2 shows the comparison of mean importance rankings of the indices between the three stakeholder groups (stakeholder groups that are located within the same circle do not significantly differ in their importance rankings of a given pain index). The overall omnibus test of group differences in the rankings was highly significant (χ2(18) = 172,38, p < .0001). Comparing patient and clinician rankings, patients ranked indices of Least Pain (OR = 0.11, p < .001) and Time in No/Low Pain (OR = 0.28, p < .01) as significantly less important, whereas they ranked Time in High Pain (OR = 3.63, p < .001), Pain Variability (OR = 2.50, p < .05), and Pain Unpredictability (OR = 3.56, p < .01) as more important than clinicians. Comparing patients to clinical trialists, patients ranked indices of Average Pain (OR = 0.27, p < .01), Least Pain (OR = 0.38, p < .05), and Time in No/Low Pain (OR = 0.19, p < .001) as significantly less important, whereas they ranked Worst Pain (OR = 2.50, p < .05), Pain Variability (OR = 2.38, p < .05), and Pain Unpredictability (OR = 4.34, p < .001) as more important than trialists. Comparing clinicians with trialists, clinicians ranked the Average Pain index as significantly less important (OR = 0.36, p < .05), and Least Pain as significantly more important (OR = 3.34, p < .01) than trialists.
Figure 2.
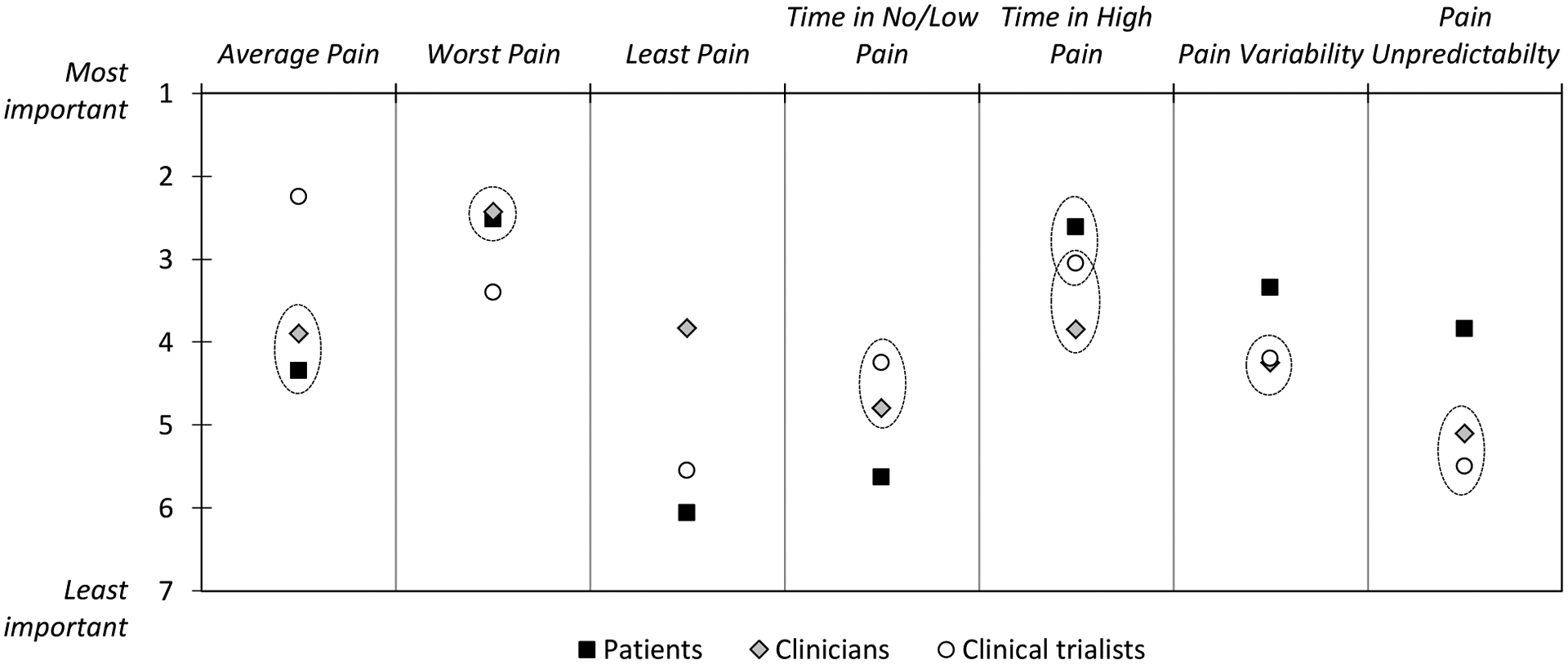
Comparison of importance rankings between stakeholder groups. Stakeholder groups that are located within the same circle do not significantly differ from each other in their importance rankings of a given pain index (p > .05).
Discussion
Methodological developments in the measurement of pain intensity using EMA offer a fine-grained understanding of patients’ pain experience in daily life. Technological advances using smart-phones and web-based assessments have paved the way to integrate EMA into routine care and into clinical trials [26], making it increasingly feasible to obtain EMA-derived pain outcomes in many applied settings. With the vast opportunities for creating new outcome measures assessing temporal features of pain, it is important to consider the needs and perspectives of stakeholders and to ascertain which aspects of pain are most relevant to them. The rationale for stakeholder input is abundantly evident in the shift to include the patient perspective in pain clinical trial development, which argues that changes in symptoms that the patient views as important to their health and well-being should not be overlooked [15]. Similarly, understanding clinician and trialist preferences is important given their significant role in clinical decision making and the selection of primary endpoints in clinical trials.
Our results showed that stakeholders had differing opinions about the presented alternative pain intensity indices and their relevance for measuring successful treatment. Only a few stakeholders thought that some of the indices should be set aside as completely irrelevant. We observed a number of significant differences in the importance rankings within each stakeholder group. However, there were few commonalities in the hierarchy of preferences for certain pain indices between the groups. Worst Pain was deemed most important by both patients and clinicians, whereas clinical trialists favored the Average Pain index as the most important outcome. Patients also attributed relatively high importance to the Variability and Unpredictability of their pain, as well as the amount of Time in High Pain, which was not mirrored in clinicians’ and trialists’ views.
Both patients and clinicians ranked Worst Pain as most important. Whereas patients told us that Worst Pain levels indicate exacerbations that are especially difficult to manage, clinicians found the Worst Pain index particularly informative for medical decision-making in that it helped them to determine whether treatment modifications are necessary. Although trialists ranked the importance of Worst Pain significantly lower than patients and clinicians, the index was still among the top three pain intensity outcomes among trialists. The high ranking of Worst Pain is consistent with shifts by regulatory agencies such as the FDA to endorse worst pain as a clinical endpoint in clinical trials that are submitted to support indications for analgesic medications [57]. Solicitation of patient input is strongly encouraged by regulatory agencies, and our finding supports the importance of Worst Pain as an important outcome from the stakeholder perspective.
The preference for Average Pain among pain clinical trialists is in line with the historical focus on average pain as a primary outcome in research. In fact, several of the interviewed trialists expressed that familiarity with Average Pain was a primary reason for their preference for the measure. Empirically, patient retrospective self-reports of Average Pain have amply demonstrated responsiveness to detecting improvements associated with pain treatment [15,22]. Whereas trialists also acknowledged the importance of the other pain indices, patients and clinicians ranked Average Pain only as moderately important relative to the other indices. This supports the view that a broader range of pain intensity outcome measures beyond Average Pain should be considered.
Indices of Pain Variability and Pain Unpredictability were viewed as having only low importance among clinicians and trialists. In contrast, patients attributed significantly more importance to these indices. A growing body of empirical research suggests that having more pain variability is associated with greater emotional distress and functioning limitations [1,39,60]. Moreover, several studies have found that pain variability plays an important role in patients‟ retrospective summary impressions of their pain, in that patients evaluate pain overall as more severe if it exhibited pronounced fluctuations over time [24,41,52]. Unpredictability of shifts in pain (e.g., whether pain predictably occurs after a specific trigger versus without warning) has been associated with central nervous system performance and functional outcomes [3,32,33]. These empirical findings are echoed by patients‟ perspectives in the present study in that they noted that having variable and unpredictable pain is especially distressing and disruptive to daily activities.
Finally, an interesting finding was that the Time in High Pain index was significantly more favored by patients than by clinicians, whereas indices of Least Pain and Time in No/Low Pain were significantly more favored by clinicians than by patients. Given that spending as much time in no or low pain as possible should be highly desirable, it may be surprising that this index was not viewed more favorably by patients. In our interviews, clinicians stated that indices of Least Pain and Time in No/Low Pain are informative to guide treatment strategies because they indicate when treatment is moving in the right direction. By contrast, it might be speculated that patients are resistant to report assessments of Time in No/Low Pain because they are afraid that their pain and suffering will not be taken seriously enough if they acknowledge times without pain [58]. Such discrepancies in patient and clinician views of treatment outcomes may go undetected in clinical care. These data suggest assessment of different pain intensity indices in clinical settings may enhance the therapeutic process by identifying relevant treatment goals for patients and clinicians and by providing an opportunity for developing a shared understanding of priority targets in pain management.
This study has several limitations that need to be considered. First, while recruitment for the interviews stratified by demographic criteria ensured sampling from diverse geographic regions within the U.S., the sample sizes were modest in each stakeholder group, and recruitment response rates were modest for clinicians and trialists, threatening the representativeness of the samples. Larger sample sizes would have been required to conduct subgroup analyses within the stakeholder groups, for example, to examine differences in preference rankings across patients’ medical conditions or functioning levels, across clinicians with different professional backgrounds, and across researchers with different clinical trial foci. In prior research, preferences for relevant domains of patient-reported outcomes from larger sample sizes were obtained using web-surveys [55]. However, we believe that the interview methodology used in this study had advantages given that the different pain indices may have been unfamiliar to respondents. Through the interview process, it was ensured that respondents understood what they were being asked and had the opportunity to thoroughly compare the different alternatives.
Second, by asking respondents to rank the indices in order of importance rather than using traditional rating scales, our results can only speak to the relative importance of the indices and we cannot gauge the absolute level of the indices‟ perceived value. We selected this strategy because it is cognitively less demanding and greatly reduces uniform biases such as acquiescence responding [10]; in fact, prior research has found that patient preference ratings for outcome measures can lack discrimination with most areas rated as very important [55].
Third, our patient sample consisted of respondents recruited from an Internet panel who self-reported their medical conditions, and we did not obtain doctor confirmation of their diagnosis. As is typical for recruitment from Internet panels, we were unable to determine the response rate for recruited patients. The results obtained in the present study may not generalize to other samples, such as patients recruited from clinical settings. Some previous interview studies examining preferred outcome domains of pain treatment have focused on patients treated at tertiary-care facilities [8,37]. Even though we did not verify diagnoses, we believe that the detailed interviews where respondents discussed their pain made it unlikely that respondents were not experiencing chronic pain, and our selection may have been more representative of the broader population of individuals experiencing chronic pain.
Fourth, we included the index of Pain Unpredictability in the qualitative interviews because of its potential relevance for understanding patients’ pain experience. However, this index cannot be easily captured from real-time pain intensity ratings alone. In order to construct such an index, momentary information about the participant’s expectations or uncertainty about future pain events would be required. This index was not further examined in the remaining articles of this series, because those studies were secondary data analyses of existing EMA studies on pain intensity that did not include expectations or uncertainty ratings. Similarly, the list of pain indices considered in this study is not exhaustive. For example, Pain After Waking up represents a potentially relevant construct that can be readily discerned from typical EMA protocols; in lieu of unpredictability, this concept will be examined in the remaining studies of this series.
Finally, the recruited trialists were researchers conducting NIH-funded clinical trials, most of which were behavioral in nature (e.g., cognitive behavioral therapy). Given the FDA’s guidance recommending the use of Worst Pain ratings in clinical trials for analgesic medications, preference rankings of the pain indices might have been different among researchers conducting pharmacological trials.
In summary, we have presented a number of alternative pain intensity indices from momentary data that can be useful in research and clinical practice. The first step in exploring the qualities of these indices was an examination of stakeholders’ views of the indices. Our stakeholder findings indicate that assessment of treatment outcomes may be augmented by considering alternative indices in addition to Average Pain. They also show significant differences in the hierarchy of preferences between the stakeholder groups. Understanding and addressing these differences may improve patient-centered care and shared decision-making in the clinical setting and foster a better alignment of clinical endpoints with patient-valued outcomes in chronic pain treatment studies.
More broadly, our elicitation of stakeholder preferences is supported by an increasing number of healthcare researchers, policy makers, and funding agencies that consider preferences as an important component in the process of identifying relevant patient-oriented outcomes [14,44,45]. Of course, the present findings must be combined with and substantiated by empirical evidence of the validity and utility of different EMA-derived pain indices. This may facilitate understanding of the phenomenology of chronic pain and the mechanisms linking pain with everyday emotional, physical, and social functioning. Additionally, if indices of pain intensity are differentially impacted by treatment, this may augment detection of treatment effects relative to what is afforded by examining pain intensity as a single undifferentiated construct. These issues are addressed in subsequent articles in this series. Ultimately, these data will provide more informed decisions about the selection of most appropriate pain outcome measures.
HIGHLIGHTS.
We introduce several indices of pain intensity that can be derived from EMA
Patients, providers, and clinical trialists were interviewed about the EMA indices
Each stakeholder group had a distinct preference hierarchy for different indices
Multiple temporal characteristics of pain intensity are relevant for stakeholders
Perspective.
Examining which aspects of pain are most important to measure from the perspective of different stakeholders can facilitate efforts to include all relevant treatment outcomes. Our study suggests that multiple temporal aspects of pain intensity are important to stakeholders. This should be considered when evaluating the efficacy of pain management.
Acknowledgments
We would like to thank the patients, clinicians, and clinical trialists for their participation in this study and for sharing their perspectives with us.
This work was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR066200; A.A.S. and S.S., principal investigators).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
A.A.S. is a Senior Scientist with the Gallup Organization and a consultant with IQVIA and Adelphi Values, Inc. The remaining authors have no conflict of interest to declare.
References
- [1].Affleck G, Tennen H, Urrows S, Higgins P: Individual differences in the day-to-day experience of chronic pain: a prospective daily study of rheumatoid arthritis patients. Health Psychol 10:419–426, 1991 [DOI] [PubMed] [Google Scholar]
- [2].Agiostratidou G, Anhalt H, Ball D, Blonde L, Gourgari E, Harriman KN, Kowalski AJ, Madden P, McAuliffe-Fogarty AH, McElwee-Malloy M: Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 40:1622–1630, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Allen K, Bosworth H, Coffman C, Jeffreys A, Oddone E, Yancy W: Associations of frequent predictable and unpredictable pain with functional and psychological outcomes. Osteoarthritis and Cartilage 21:S263–S264, 2013 [Google Scholar]
- [4].Allen KD: The value of measuring variability in osteoarthritis pain. J Rheumatol 34:2132–2133, 2007 [PubMed] [Google Scholar]
- [5].Allison PD, Christakis N: Logit models for sets of ranked items, in Marsden PV (ed): Sociological Methodology. Vol. 24. Oxford: Blackwell, 1994, pp 123–126 [Google Scholar]
- [6].Boonstra AM, Preuper HRS, Balk GA, Stewart RE: Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. PAIN® 155:2545–2550, 2014 [DOI] [PubMed] [Google Scholar]
- [7].Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA: The accuracy of pain and fatigue items across different reporting periods. Pain 139:146–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Casarett D, Karlawish J, Sankar P, Hirschman K, Asch DA: Designing pain research from the patient’s perspective: What trial end points are important to patients with chronic pain? Pain medicine 2:309–316, 2001 [DOI] [PubMed] [Google Scholar]
- [9].Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R: The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of clinical epidemiology 63:1179–1194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cheung MW-L, Chan W: Reducing uniform response bias with ipsative measurement in multiple-group confirmatory factor analysis. Structural Equation Modeling 9:55–77, 2002 [Google Scholar]
- [11].Cleeland CS, Ryan KM: Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 23:129–138, 1994 [PubMed] [Google Scholar]
- [12].Craig BM, Busschbach JJ, Salomon JA: Modeling ranking, time trade-off and visual analogue scale values for EQ-5D health states: A review and comparison of methods. Med Care 47:634–641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Wit M, Abma T, Koelewijn-van Loon M, Collins S, Kirwan J: Involving patient research partners has a significant impact on outcomes research: a responsive evaluation of the international OMERACT conferences. BMJ open 3:e002241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dirksen CD: The use of research evidence on patient preferences in health care decision-making: issues, controversies and moving forward. Expert review of pharmacoeconomics & outcomes research 14:785–794, 2014 [DOI] [PubMed] [Google Scholar]
- [15].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J: Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113:9–19, 2005 [DOI] [PubMed] [Google Scholar]
- [16].Fillingim RB, Loeser JD, Baron R, Edwards RR: Assessment of chronic pain: Domains, methods, and mechanisms. The Journal of Pain 17:T10–T20, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Frank L, Basch E, Selby JV: The PCORI perspective on patient-centered outcomes research. Jama 312:1513–1514, 2014 [DOI] [PubMed] [Google Scholar]
- [18].Gobo G; 2004. Sampling, representativeness and generalizability. In: Seale C, Gobo G, Gubrium JF, Silverman Deditors. Qualitative Research Practice. London: SAGE Publications Ltd, pp. 405–426. [Google Scholar]
- [19].Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ: Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis and Rheumatism 52:3670–3674, 2005 [DOI] [PubMed] [Google Scholar]
- [20].Houben M, Van Den Noortgate W, Kuppens P: The relation between short-term emotion dynamics and psychological well-being: A meta-analysis. Psychological bulletin 141:901–930, 2015 [DOI] [PubMed] [Google Scholar]
- [21].Hultsch DF, MacDonald SW, Dixon RA: Variability in reaction time performance of younger and older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 57:P101–P115, 2002 [DOI] [PubMed] [Google Scholar]
- [22].Jensen MP, Karoly P: Self-report scales and procedures for assessing pain in adults, in Turk DC, Melzack R (eds): Handbook of pain assessment. New York: Guilford Press, 2001, pp 15–34 [Google Scholar]
- [23].Jensen MP, McFarland CA: Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 55:195–203, 1993 [DOI] [PubMed] [Google Scholar]
- [24].Kikuchi H, Yoshiuchi K, Miyasaka N, Ohashi K, Yamamoto Y, Kumano H, Kuboki T, Akabayashi A: Reliability of recalled self-report on headache intensity: investigation using ecological momentary assessment technique. Cephalalgia 26:1335–1343, 2006 [DOI] [PubMed] [Google Scholar]
- [25].Kirwan JR, de Wit M: Patients as partners: Building on the experience of Outcome Measures in Rheumatology (OMERACT). Arthritis & Rheumatology 68:1334–1336, 2016 [DOI] [PubMed] [Google Scholar]
- [26].Krishna S, Boren SA, Balas EA: Healthcare via cell phones: a systematic review. Telemedicine and e-Health 15:231–240, 2009 [DOI] [PubMed] [Google Scholar]
- [27].Krueger AB, Stone AA: Assessment of pain: a community-based diary survey in the USA. Lancet 371:1519–1525, 2008 [DOI] [PubMed] [Google Scholar]
- [28].Litcher-Kelly L, Martino SA, Broderick JE, Stone AA: A Systematic Review of Measures Used to Assess Chronic Musculoskeletal Pain in Clinical and Randomized Controlled Clinical Trials. The Journal of Pain 8:906–913, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Marden JI: Analyzing and Modeling Rank Data. London: Chapman & Hall, 1995. [Google Scholar]
- [30].Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP: Amount of time spent in sedentary behaviors in the United States, 2003–2004. American journal of epidemiology 167:875–881, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].May M, Junghaenel DU, Ono M, Stone AA, Schneider S: Ecological Momentary Assessment Methodology in Chronic Pain Research: A Systematic Review. The Journal of Pain 19:699–716, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meulders A, Vansteenwegen D, Vlaeyen JW: Women, but not men, report increasingly more pain during repeated (un) predictable painful electrocutaneous stimulation: Evidence for mediation by fear of pain. PAIN® 153:1030–1041, 2012 [DOI] [PubMed] [Google Scholar]
- [33].Moseley GL, Brhyn L, Ilowiecki M, Solstad K, Hodges PW: The threat of predictable and unpredictable pain: differential effects on central nervous system processing? Australian Journal of Physiotherapy 49:263–267, 2003 [DOI] [PubMed] [Google Scholar]
- [34].Pai Y-W, Lin C-H, Lee I-T, Chang M-H: Variability of fasting plasma glucose and the risk of painful diabetic peripheral neuropathy in patients with type 2 diabetes. Diabetes & metabolism 44:129–134, 2018 [DOI] [PubMed] [Google Scholar]
- [35].Parati G, Ochoa JE, Lombardi C, Bilo G: Assessment and management of blood-pressure variability. Nature Reviews Cardiology 10:143–155, 2013 [DOI] [PubMed] [Google Scholar]
- [36].Ram N, Gerstorf D: Time-structured and net intraindividual variability: tools for examining the development of dynamic characteristics and processes. Psychology and aging 24:778–791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Robinson ME, Brown JL, George SZ, Edwards PS, Atchison JW, Hirsh AT, Waxenberg LB, Wittmer V, Fillingim RB: Multidimensional success criteria and expectations for treatment of chronic pain: the patient perspective. Pain medicine 6:336–345, 2005 [DOI] [PubMed] [Google Scholar]
- [38].Schneider S, Junghaenel DU, Broderick JE, Ono M, May M, Stone AA: Indices of pain intensity derived from ecological momentary assessments and their relationships with patient functioning: an indivdiual patient data meta-analysis. Journal of Pain, (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schneider S, Junghaenel DU, Keefe FJ, Schwartz JE, Stone AA, Broderick JE: Individual differences in the day-to-day variability of pain, fatigue, and well-being in patients with rheumatic disease: Associations with psychological variables. Pain 153:813–822, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schneider S, Junghaenel DU, Ono M, Broderick JE, Stone AA: Detecting treatment effects in clinical trials with different indices of pain intensity derived from ecological momentary assessment. Journal of Pain, (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schneider S, Junghaenel DU, Ono M, Stone AA: Temporal dynamics of pain: an application of regime-switching models to ecological momentary assessments in patients with rheumatic diseases. Pain 159:1346–1358, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schneider S, Stone AA: Distinguishing between frequency and intensity of health-related symptoms from diary assessments. J Psychosom Res 77:205–212, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schneider S, Stone AA: Ambulatory and diary methods can facilitate the measurement of patient-reported outcomes. Quality of life research 25:497–506, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Selby JV, Beal AC, Frank L: The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA 307:1583–1584, 2012 [DOI] [PubMed] [Google Scholar]
- [45].Selby JV, Forsythe L, Sox HC: Stakeholder-driven comparative effectiveness research: an update from PCORI. Jama 314:2235–2236, 2015 [DOI] [PubMed] [Google Scholar]
- [46].Smith SM, Hunsinger M, McKeown A, Parkhurst M, Allen R, Kopko S, Lu Y, Wilson HD, Burke LB, Desjardins P: Quality of pain intensity assessment reporting: ACTTION systematic review and recommendations. The Journal of Pain 16:299–305, 2015 [DOI] [PubMed] [Google Scholar]
- [47].Smith SM, Jensen MP, He H, Kitt R, Koch J, Pan A, Burke LB, Farrar JT, McDermott MP, Turk DC: A Comparison of the Assay Sensitivity of Average and Worst Pain Intensity in Pharmacologic Trials: An ACTTION Systematic Review and Meta-Analysis. The Journal of Pain 19:953–960, 2018 [DOI] [PubMed] [Google Scholar]
- [48].Smith WR, Bauserman RL, Ballas SK, McCarthy WF, Steinberg MH, Swerdlow PS, Waclawiw MA, Barton BA, Hydro IMS: Climatic and geographic temporal patterns of pain in the Multicenter Study of Hydroxyurea. Pain 146:91–98, 2009 [DOI] [PubMed] [Google Scholar]
- [49].Stone AA, Broderick JE, Schneider S, Schwartz JE: Expanding options for developing outcome measures from momentary assessment data. Psychosomatic medicine 74:387–397, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stone AA, Broderick JE, Schwartz JE: Validity of average, minimum, and maximum end-of-day recall assessments of pain and fatigue. Contemporary clinical trials 31:483–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stone AA, Broderick JE, Shiffman SS, Schwartz JE: Understanding recall of weekly pain from a momentary assessment perspective: absolute agreement, between- and within-person consistency, and judged change in weekly pain. Pain 107:61–69, 2004 [DOI] [PubMed] [Google Scholar]
- [52].Stone AA, Schwartz JE, Broderick JE, Shiffman SS: Variability of momentary pain predicts recall of weekly pain: a consequence of the peak (or salience) memory heuristic. Personality and Social Psychology Bulletin 31:1340–1346, 2005 [DOI] [PubMed] [Google Scholar]
- [53].Stone AA, Shiffman S: Ecological momentary assessment (EMA) in behavorial medicine. Annals of Behavioral Medicine 16:199–202, 1994 [Google Scholar]
- [54].Tracy LM, Ioannou L, Baker KS, Gibson SJ, Georgiou-Karistianis N, Giummarra MJ: Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 157:7–29, 2016 [DOI] [PubMed] [Google Scholar]
- [55].Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S: Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. PAIN® 137:276–285, 2008 [DOI] [PubMed] [Google Scholar]
- [56].US Department of Health and Human Services Food and Drug Administration: Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. Available at: http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. Accessed September 3, 2018, 2009.
- [57].US Department of Health and Human Services Food and Drug Administration: Guidance for Industry: Analgesic Indications: Developing Drug and Biological Products. Available at https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm384691.pdf. Accessed April 26, 2019, 2014.
- [58].Werner A, Malterud K: It is hard work behaving as a credible patient: encounters between women with chronic pain and their doctors. Social science & medicine 57:1409–1419, 2003 [DOI] [PubMed] [Google Scholar]
- [59].Williamson P, Altman D, Blazeby J, Clarke MJ, Gargon E: The COMET (Core Outcome Measures in Effectiveness Trials) initiative. Trials 12 (Suppl 1):A70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zakoscielna KM, Parmelee PA: Pain variability and its predictors in older adults: depression, cognition, functional status, health, and pain. Journal of Aging and Health 25:1329–1339, 2013 [DOI] [PubMed] [Google Scholar]


