Abstract
Aims: The efficacy of antiplatelet therapy may vary among different disease subtypes. Prasugrel is generally a more potent, consistent, and fast-acting platelet inhibitor than clopidogrel. This sub-analysis of the phase III comparison of PRAsugrel and clopidogrel in Japanese patients with ischemic STROke (PRASTRO-I) trial aimed to assess the differences in efficacy of these treatments for each stroke subtype.
Methods: In the PRASTRO-I trial, a total of 3,753 patients with ischemic stroke were recruited from 224 centers throughout Japan and randomized (1:1) to prasugrel (3.75 mg/day) or clopidogrel (75 mg/day) for 96 weeks. For the sub-analysis, strokes were classified as large-artery atherosclerosis, small-artery occlusion (lacunar), stroke of other etiology, and stroke of undetermined etiology. The cumulative incidence of primary events (ischemic stroke, myocardial infarction, and death from other vascular cause) and hazard ratios (HRs) were calculated for each subgroup.
Results: For patients with large-artery atherosclerosis, the primary event incidence was 3.8% in the prasugrel group and 4.8% in the clopidogrel group (HR 0.79; 95% confidence interval [CI] 0.45–1.41). For patients with small-artery occlusion, the incidence was 3.3% in the prasugrel group and 3.9% in the clopidogrel group (HR 0.82; 95% CI 0.45–1.50). For patients with stroke of undetermined etiology, the incidence was 4.6% in the prasugrel group and 3.0% in the clopidogrel group (HR 1.56; 95% CI 0.90–2.72). The incidence of bleeding was similar across subtypes.
Conclusions: Although statistical significance was not reached, the efficacy of prasugrel was potentially different between stroke subtypes, warranting further studies.
Keywords: Stroke, Subtype, Thienopyridine, Prasugrel, Ischemic
Introduction
Antiplatelet agents are used uniformly for secondary prevention for all patients with non-cardioembolic stroke; however, their protective effects against vascular events depend on the subtype of ischemic stroke. In the SOCRATES trial, ticagrelor was more effective than aspirin in patients with > 50% stenosis of intracranial or extracranial arteries but not in patients without such stenosis1). In the CHANCE trial and sub-analysis, a combination of clopidogrel plus aspirin prevented more ischemic events than aspirin alone in patients with intracranial arterial stenosis (ICAS), although the difference was not significant2). In patients without ICAS, no difference in the incidence of ischemic events between the two treatment groups was found. These results indicate that the efficacy of antiplatelet agents in preventing ischemic events in stroke patients may differ depending on the subtype of stroke.
At present, clopidogrel is the most frequently used treatment for the secondary prevention of non-cardioembolic stroke3–5). Prasugrel is a member of the thienopyridine class of adenosine diphosphate receptor inhibitors that provides more prompt, potent, and consistent platelet inhibition than clopidogrel6). The comparison of PRAsugrel and clopidogrel in Japanese patients with ischemic STROke (PRASTRO-I) trial was one of the largest phase III trials of patients with non-cardioembolic stroke7, 8). The primary analysis of PRASTRO-I found that prasugrel (3.75 mg) was as effective as clopidogrel (75 mg) for the prevention of primary events (ischemic stroke, myocardial infarction, and death from other vascular causes) in patients with non-cardioembolic stroke. Non-inferiority was not statistically proven. However, as there are various subtypes of non-cardioembolic stroke in this population, determining the efficacy and safety of antiplatelet agents according to stroke subtype is important.
Although both the SOCRATES and CHANCE trials demonstrated that patients with atherothrombotic stroke and stenosis of intracranial or extracranial arteries benefit from drugs with a potent antiplatelet effect1, 2), these studies either compared antiplatelet agents with different mechanisms or used treatment combinations. No comparison between thienopyridine monotherapies has been reported. In addition, although the SOCRATES and CHANCE trials included patients with acute stage disease, the PRASTRO-I trial only included patients with chronic stage disease.
Aims
We conducted a sub-analysis of the PRASTRO-I trial to compare prasugrel and clopidogrel within patient subgroups defined by stroke subtype, and we also analyzed comparative safety (especially incidence of bleeding) in these subgroups.
Methods
Data Sharing
Anonymized data and materials have been made publicly available at Vivli Center for Global Clinical Research Data and can be accessed at https://vivli.org/.
Study Design and Patients
PRASTRO-I was a randomized, double-blind, active-controlled, parallel-group, multicenter (224 institutions), and phase III trial conducted in Japan from July 2011 to March 2016. The trial was registered with the Japan Pharmaceutical Information Center (JapicCTI-111582). The study design and the primary results have been published elsewhere7, 8). In brief, patients were included if they were aged ≥ 20 and < 75 years, weighed > 50kg, and experienced a non-cardioembolic ischemic stroke between 1 and 26 weeks prior to informed consent. Patients were excluded if they met any of the following criteria: presence of a cardioembolic stroke, paradoxical cerebral embolism, or asymptomatic ischemic stroke; or the presence of atrial fibrillation or other cardiovascular diseases that cause cardioembolic stroke. The full list of inclusion/exclusion criteria and prohibited drugs is provided in the Supplemental Methods.
Ethical approval was obtained from the institutional review boards, and all patients provided written informed consent. The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines.
Randomization and Masking
Eligible patients were randomized 1:1 to either prasugrel (3.75 mg/day) or clopidogrel (75 mg/day) orally once daily after breakfast using a web-based registration system, stratified by stroke subtype (using the TOAST classification)9) (Supplemental Methods). Patients, investigators, and the sponsor were blinded to treatment allocation.
Evaluation of Efficacy and Safety Endpoints
The primary efficacy endpoint was the incidence of composite events comprising ischemic stroke, myocardial infarction, and death from other vascular causes observed from the start of the study drug administration to 1 day following study drug completion or discontinuation. The primary safety event was the incidence of bleeding events, i.e., life-threatening bleeding, major bleeding, and clinically relevant bleeding.
Statistical Analysis
Sample size calculations for the main analysis have been previously described7, 8). For baseline characteristics, frequencies were calculated for categorical data, and summary statistics were used for continuous data. The cumulative incidence of primary endpoint events was calculated for the two treatment groups stratified by stroke subtype: large-artery atherosclerosis, small-artery occlusion (lacunar), other etiology, and undetermined etiology. Hazard ratios (HRs) and 95% confidence intervals (CIs) based on a Cox proportional hazard model were estimated to compare incidence rates in the two treatment groups by stroke subtype. Other efficacy events and bleeding were analyzed in a manner similar to that used for primary events. The cumulative incidence of primary events was evaluated using a Kaplan–Meier curve. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Patients
A total of 3,753 patients were randomized to treatment. Of these, 3,747 were included in the full analysis set (797 females, 21.3%). The six patients excluded were withdrawn from the trial before administration of the trial drug. The baseline characteristics for patients with different stroke subtypes are summarized in the Supplemental Table 1. Of the 3,747 patients in the full analysis set, 1,099 presented with large-artery atherosclerosis, 1,176 presented with small-artery occlusion (lacunar), 84 presented with stroke of other determined etiology, and 1,388 presented with stroke of undetermined etiology. No notable differences in the baseline characteristics between the two treatment groups were found.
Supplemental Table 1. Patient Characteristics.
| Large-artery atherosclerosis |
Small-artery occlusion (lacunar) |
Acute stroke of other determined etiology |
Stroke of undetermined etiology |
|||||
|---|---|---|---|---|---|---|---|---|
| Prasugrel | Clopidogrel | Prasugrel | Clopidogrel | Prasugrel | Clopidogrel | Prasugrel | Clopidogrel | |
| n = 553 | n = 546 | n = 583 | n = 593 | n = 35 | n = 49 | n = 714 | n = 674 | |
| Age (years), mean ± SD | 62.7 ± 8.8 | 63.1 ± 8.2 | 61.8 ± 8.0 | 62.4 ± 7.8 | 56.9 ± 13.1 | 57.5 ± 11.5 | 61.5 ± 8.8 | 62.3 ± 8.6 |
| Sex (female), n (%) | 115 (20.8) | 117 (21.4) | 105 (18.0) | 106 (17.9) | 4 (11.4) | 8 (16.3) | 162 (22.7) | 180 (26.7) |
| Body weight (kg), mean ± SD | 65.6 ± 10.4 | 65.3 ± 8.9 | 66.0 ± 10.0 | 65.6 ± 9.8 | 68.2 ± 9.4 | 67.1 ± 10.0 | 65.7 ± 11.1 | 65.1 ± 10.1 |
| Body mass index, mean ± SD | 24.5 ± 3.2 | 24.4 ± 2.8 | 24.6 ± 3.2 | 24.4 ± 3.0 | 24.2 ± 2.6 | 24.7 ± 2.9 | 24.5 ± 3.3 | 24.5 ± 3.2 |
| Blood pressure (mmHg), mean ± SD | ||||||||
| Systolic | 133.0 ± 15.6 | 133.8 ± 14.7 | 135.1 ± 13.9 | 135.3 ± 14.4 | 131.9 ± 15.0 | 131.2 ± 16.3 | 134.8 ± 14.3 | 134.7 ± 15.0 |
| Diastolic | 78.3 ± 11.0 | 78.4 ± 11.0 | 81.0 ± 10.7 | 80.4 ± 10.6 | 81.5 ± 10.2 | 77.3 ± 10.9 | 80.1 ± 10.5 | 79.8 ± 10.9 |
| Modified Rankin Scale, n (%) | ||||||||
| Grade 0 | 145 (26.2) | 124 (22.7) | 137 (23.5) | 157 (26.5) | 10 (28.6) | 16 (32.7) | 152 (21.3) | 153 (22.7) |
| Grade 1 | 267 (48.3) | 283 (51.8) | 357 (61.2) | 361 (60.9) | 20 (57.1) | 26 (53.1) | 382 (53.5) | 358 (53.1) |
| Grade 2 | 89 (16.1) | 96 (17.6) | 72 (12.3) | 64 (10.8) | 4 (11.4) | 6 (12.2) | 126 (17.6) | 107 (15.9) |
| Grade 3 | 37 (6.7) | 27 (4.9) | 11 (1.9) | 9 (1.5) | 0 (0.0) | 1 (2.0) | 40 (5.6) | 38 (5.6) |
| Grade 4 | 15 (2.7) | 16 (2.9) | 6 (1.0) | 2 (0.3) | 1 (2.9) | 0 (0.0) | 14 (2.0) | 18 (2.7) |
| History of atherosclerotic disease, n (%) | ||||||||
| Ischemic stroke | 71 (12.8) | 68 (12.5) | 84 (14.4) | 85 (14.3) | 3 (8.6) | 6 (12.2) | 60 (8.4) | 52 (7.7) |
| TIA | 44 (8.0) | 45 (8.2) | 29 (5.0) | 25 (4.2) | 2 (5.7) | 2 (4.1) | 24 (3.4) | 21 (3.1) |
| Complication, n(%) | ||||||||
| Hypertension | 442 (79.9) | 429 (78.6) | 476 (81.6) | 509 (85.8) | 26 (74.3) | 36 (73.5) | 561 (78.6) | 536 (79.5) |
| Dyslipidemia | 405 (73.2) | 405 (74.2) | 377 (64.7) | 405 (68.3) | 22 (62.9) | 31 (63.3) | 492 (68.9) | 464 (68.8) |
| Diabetes mellitus | 195 (35.3) | 196 (35.9) | 186 (31.9) | 203 (34.2) | 7 (20.0) | 12 (24.5) | 223 (31.2) | 225 (33.4) |
| Duration from onset to the study treatment, n(%) | ||||||||
| < 4 weeks | 80 (14.5) | 81 (14.8) | 103 (17.7) | 117 (19.7) | 1 (2.9) | 9 (18.4) | 132 (18.5) | 115 (17.1) |
| ≥ 4 weeks, < 12 weeks | 274 (49.5) | 279 (51.1) | 327 (56.1) | 335 (56.5) | 24 (68.6) | 24 (49.0) | 413 (57.8) | 394 (58.5) |
| ≥ 12 weeks | 199 (36.0) | 186 (34.1) | 153 (26.2) | 141 (23.8) | 10 (28.6) | 16 (32.7) | 169 (23.7) | 165 (24.5) |
| Clopidogrel pretreatment, n (%) | ||||||||
| Yes | 185 (33.5) | 175 (32.1) | 259 (44.4) | 259 (43.7) | 13 (37.1) | 15 (30.6) | 273 (38.2) | 254 (37.7) |
| No | 368 (66.5) | 371 (67.9) | 324 (55.6) | 334 (56.3) | 22 (62.9) | 34 (69.4) | 441 (61.8) | 420 (62.3) |
| Concomitant medication | ||||||||
| PPI | 184 (33.3) | 174 (31.9) | 174 (29.8) | 179 (30.2) | 10 (28.6) | 12 (24.5) | 251 (35.2) | 209 (31.0) |
| Statin | 289 (52.3) | 290 (53.1) | 228 (39.1) | 259 (43.7) | 19 (54.3) | 23 (46.9) | 329 (46.1) | 321 (47.6) |
| ARB | 269 (48.6) | 244 (44.7) | 292 (50.1) | 312 (52.6) | 6 (17.1) | 28 (57.1) | 340 (47.6) | 320 (47.5) |
| Insulin | 20 (3.6) | 22 (4.0) | 18 (3.1) | 20 (3.4) | 0 (0.0) | 0 (0.0) | 22 (3.1) | 14 (2.1) |
ARB = angiotensin II receptor blocker, eGFR = estimated glomerular filtration rate, SD = standard deviation, PPI = proton pump inhibitor, TIA = transient ischemic attack
Incidence of Efficacy Events
The incidence of efficacy events was determined for each stroke type subgroup (Table 1). Kaplan–Meier survival analysis was used to compare the cumulative incidence of primary events for each stroke subtype in the prasugrel and clopidogrel groups (Fig. 1); this was not performed in the subgroup with acute stroke of other known etiology because no primary endpoint events in either treatment group were found. Primary events were experienced by patients with large-artery atherosclerosis (21/553 [3.8%] and 26/546 [4.8%]; HR 0.79, 95% CI 0.45–1.41), small-artery occlusion (19/583 [3.3%] and 23/593 [3.9%]; HR 0.82, 95% CI 0.45–1.50), and stroke of undetermined etiology (33/714 [4.6%] and 20/674 [3.0%]; HR 1.56, 95% CI 0.90–2.72) in the prasugrel and clopidogrel groups, respectively.
Table 1. Cumulative incidence of efficacy events in each stroke subtype group.
| Large-artery atherosclerosis, % |
Small-artery occlusion (lacunar), % |
Acute stroke of other determined etiology, % |
Stroke of undetermined etiology, % |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prasugrel | Clopidogrel | HR | Prasugrel | Clopidogrel | HR | Prasugrel | Clopidogrel | HR | Prasugrel | Clopidogrel | HR | |
| N = 553 | N = 546 | [95% CI] | N = 583 | N = 593 | [95% CI] | N = 35 | N = 49 | [95% CI] | N = 714 | N = 674 | [95% CI] | |
| Primary events | 3.8 | 4.8 | 0.79 [0.45, 1.41] |
3.3 | 3.9 | 0.82 [0.45, 1.50] |
0.0 | 0.0 | - [-, -] | 4.6 | 3.0 | 1.56 [0.90, 2.72] |
| Ischemic stroke | 3.4 | 4.6 | 0.75 [0.41, 1.36] |
3.1 | 3.7 | 0.81 [0.43, 1.51] |
0.0 | 0.0 | - [-, -] | 4.5 | 2.5 | 1.79 [0.99, 3.21] |
| Myocardial infarction | 0.4 | 0.2 | 1.92 [0.17, 21.16] |
0.2 | 0.2 | 1.02 [0.06, 16.29] |
0.0 | 0.0 | - [-, -] | 0.1 | 0.6 | 0.24 [0.03, 2.10] |
| Death from other vascular cause | 0.0 | 0.0 | - [-, -] | 0.0 | 0.0 | - [-, -] | 0.0 | 0.0 | - [-, -] | 0.0 | 0.0 | - [-, -] |
| Any stroke | 3.8 | 5.1 | 0.74 [0.42, 1.30] |
3.4 | 3.9 | 0.86 [0.47, 1.56] |
0.0 | 2.0 | - [-, -] | 4.5 | 3.1 | 1.45 [0.83, 2.51] |
| Hemorrhagic stroke | 0.4 | 0.5 | 0.65 [0.11, 3.90] |
0.3 | 0.2 | 1.92 [0.17, 21.23] |
0.0 | 2.0 | - [-, -] | 0.0 | 0.6 | - [-, -] |
CI = confidence interval, HR = hazard ratio
Fig. 1.
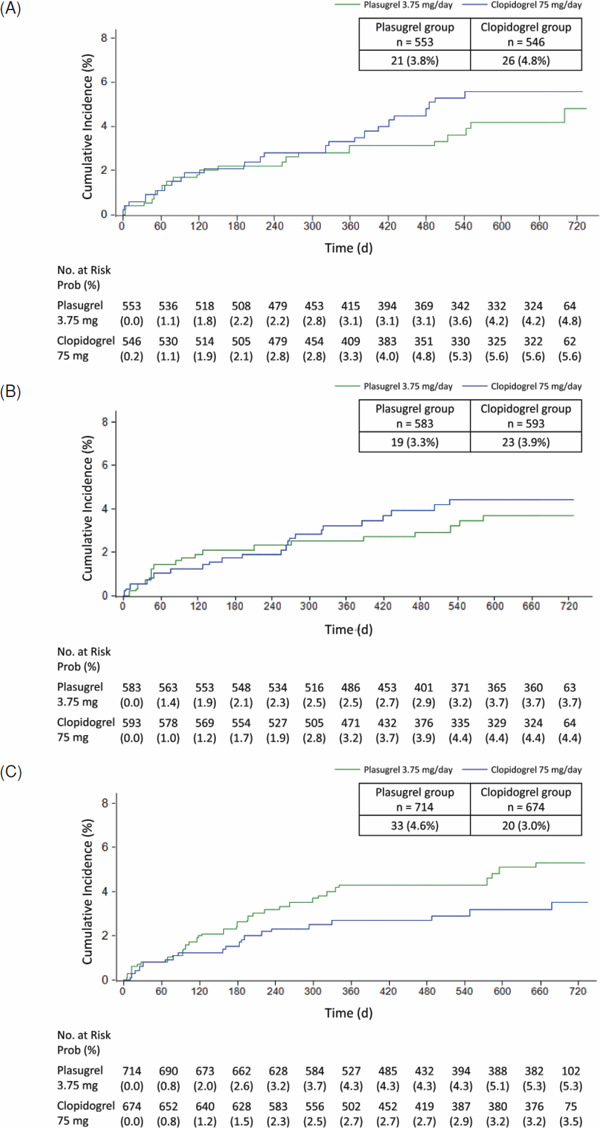
Kaplan–Meier analysis of the cumulative incidence of stroke in patients with (A) large-artery atherosclerosis, (B) small-artery occlusion, or (C) stroke of undetermined etiology
In all stroke types, the primary event was mainly ischemic stroke for patients with large-artery atherosclerosis (19/553 [3.4%] and 25/546 [4.6%]; HR 0.75, 95% CI 0.41–1.36), small-artery occlusion (18/583 [3.1%] and 22/593 [3.7%]; HR 0.81, 95% CI 0.43–1.51), and stroke of undetermined etiology (32/714 [4.5%] and 17/674 [2.5%]; HR 1.79, 95% CI 0.99–3.21) in the prasugrel and clopidogrel groups, respectively.
Bleeding Events in Each Stroke Subtype
The incidence of bleeding events (Table 2) and life-threatening bleeding was comparable in the prasugrel and clopidogrel groups for each stroke subtype. Primary events were experienced by patients with large-artery atherosclerosis (5/553 [0.9%] and 8/546 [1.5%]), small-artery occlusion (9/583 [1.5%] and 7/593 [1.2%]), and stroke of undetermined etiology (4/714 [0.6%] and 7/674 [1.0%]) in the prasugrel and clopidogrel groups, respectively. For patients with acute stroke of other determined etiology, no patients in the prasugrel group and 1/49 (2.0%) in the clopidogrel group experienced life-threatening bleeding.
Table 2. Incidence of bleeding events in each stroke subtype group.
| Large-artery atherosclerosis, % |
Small-artery occlusion (lacunar), % |
Acute stroke of other determined etiology, % |
Stroke of undetermined etiology, % |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prasugrel | Clopidogrel | HR | Prasugrel | Clopidogrel | HR | Prasugrel | Clopidogrel | HR | Prasugrel | Clopidogrel | HR | |
| N = 553 | N = 546 | Estimate | N = 583 | N = 593 | Estimate | N = 35 | N = 49 | Estimate | N = 714 | N = 674 | Estimate | |
| [95% CI] | [95% CI] | [95% CI] | [95% CI] | |||||||||
| Life-threatening bleeding | 0.9 | 1.5 | 0.61 [0.20, 1.87] |
1.5 | 1.2 | 1.25 [0.46, 3.35] |
0.0 | 2.0 | - [-, -] | 0.6 | 1.0 | 0.54 [0.16, 1.84] |
| Major bleeding | 0.0 | 0.2 | - [-, -] | 0.2 | 0.0 | - [-, -] | 2.9 | 0.0 | - [-, -] | 0.0 | 0.4 | - [-, -] |
| Clinically relevant bleeding | 4.5 | 3.3 | 1.38 [0.75, 2.52] |
6.0 | 5.4 | 1.07 [0.66, 1.73] |
8.6 | 4.1 | 2.19 [0.37, 13.12] |
4.9 | 4.6 | 1.07 [0.66, 1.74] |
| Life-threatening bleeding, major bleeding, and clinically relevant bleeding | 5.4 | 4.9 | 1.10 [0.65, 1.85] |
7.4 | 6.6 | 1.08 [0.70, 1.66] |
8.6 | 6.1 | 1.44 [0.29, 7.16] |
5.5 | 6.1 | 0.90 [0.58, 1.39] |
CI = confidence interval, HR = haard ratio
Discussion
The sub-analysis of the PRASTRO-I trial suggests that the efficacy of prasugrel, compared with that of clopidogrel, may vary according to stroke subtype. We found that prasugrel was at least as effective as clopidogrel in patients with large-artery atherosclerosis or small-artery occlusion. Since this study evaluated ischemic events in the chronic stage (from 7 days after stroke onset), the number of events was not sufficient to determine any statistical difference between prasugrel and clopidogrel.
In general, patients with ischemic stroke due to large-artery atherosclerosis often experience the same stroke etiology at recurrence. The numerically lower rates of ischemic stroke events caused by large-artery atherosclerosis observed in PRASTRO-I with prasugrel suggest that inhibition of the purinergic receptor P2Y12 also exhibits the potential to reduce atherosclerotic vascular events in large-artery atherosclerosis. Further study is required to examine the efficacy of prasugrel in the acute stage and in patients at a high risk of a transient ischemic attack.
Large-Artery Atherosclerosis
Although antiplatelet therapy is effective in the prevention of non-cardioembolic ischemic stroke, most previous studies have not included an analysis based on ischemic stroke subtype. In the ProFESS trial, clopidogrel monotherapy appeared to show a nonsignificant trend toward stroke prevention compared with the combination of aspirin and dipyridamole in patients with large-artery atherosclerosis (9.4% vs 10.6%)10). In a subgroup analysis of the SOCRATES trial in patients with ipsilateral atherosclerotic stenosis, ticagrelor was superior to aspirin for the prevention of ischemic events in patients with acute ischemic stroke or transient ischemic attack1). These studies suggest the importance of inhibition of the P2Y12 receptor for the prevention of ischemic stroke in patients with large-artery atherosclerosis. In the TRITON-TIMI 38 trial, prasugrel significantly reduced thrombotic events, such as cardiovascular death, myocardial infarction, and stroke, compared with clopidogrel in patients with acute coronary syndrome (ACS) scheduled to undergo percutaneous coronary intervention11). Although the mechanisms of vascular occlusion in ischemic stroke are more heterogeneous than that in ACS, large-artery ischemic stroke shares a common feature with ACS. The incidence of ischemic stroke due to large-artery atherosclerosis in the large-artery atherosclerosis subgroup was 1.8% (10/553) in the prasugrel group and 2.9% (16/546) in the clopidogrel group (data not shown in Results). However, since the PRASTRO-I study evaluated ischemic events in the chronic stage (from 7 days after stroke onset), the number of events was not sufficient to determine any statistical difference in a subgroup of patients with large-artery atherosclerosis. The SOCRATES and CHANCE trials demonstrated that patients with atherothrombotic stroke and stenosis of intra- or extra-cranial arteries benefit from potent antiplatelet therapy in the acute stage1, 2). Thus, further study is required to determine the efficacy of prasugrel in the acute stage.
The J-STARS trial investigated the effects of statin treatment in patients with non-cardioembolic ischemic stroke and showed that statins prevent stroke recurrence significantly more than control treatment in patients with ischemic stroke due to large-artery atherosclerosis12). Therefore, the expectation exists that concomitant therapy with statins and potent antiplatelet agents may be more useful in preventing atherosclerosis-related ischemic events; however, a further large-scale study including acute stage patients is necessary to determine any statistical difference.
Small-Artery Occlusion (Lacunar)
The findings of the present study suggest that prasugrel was at least as effective as clopidogrel in patients with small-artery occlusion. There are three main etiologies: atheroma of parent arteries (usually middle cerebral artery) or perforating arterioles, embolism from the heart or carotid arteries, and intrinsic small vessel disease (lipohyalinosis or fibrinoid necrosis) 13). These etiologies suggest that there would be no benefit from potent antiplatelet regimens as was shown in the SOCRATES trial1). The only randomized clinical trial testing the efficacy of antiplatelet therapies for lacunar infarction was SPS3, which showed that long-term aspirin and clopidogrel treatment doubled the risk of bleeding versus aspirin monotherapy without reducing the risk of stroke recurrence in patients with recent lacunar stroke14). Other studies showed that lacunar strokes carry a lower risk of recurrence but a higher risk of hemorrhagic stroke than other stroke subtypes14–16). In this study, the incidence of hemorrhagic stroke was comparable in the prasugrel (0.3%) and clopidogrel (0.2%) groups, although the lack of increase in hemorrhagic stroke in any subtype of stroke in this study may have been a result of reducing the dose of prasugrel to 3.75 mg for Japanese patients.
Stroke of Undetermined Etiology
In the stroke of undetermined etiology subgroup, the incidence of thrombotic events was numerically higher in the prasugrel group than that in the clopidogrel group. From these results, patients with stroke of undetermined etiology did not seem to be good candidates for more potent antiplatelet therapy. Cerebral infarction with an unknown cause may include patients with embolic factors, such as latent paroxysmal atrial fibrillation, patent foramen ovale, and latent malignant tumor, which are difficult to detect in screening. Given that these conditions would be refractory to antiplatelet therapy, their presence could mask differences in efficacy between antiplatelet therapies. Of the subgroups within stroke of undetermined etiology, two patients in the prasugrel group (versus none in the clopidogrel group) for whom the cerebral infarction event was a cardiogenic cerebral embolism were found. We excluded cardioembolic stroke, paradoxical cerebral embolism, asymptomatic ischemic stroke by magnetic resonance imaging, and atrial fibrillation. However, for paroxysmal atrial fibrillation, which can be detected by long-term repeated Holter electrocardiogram in up to 22% of patients17), and for patent foramen ovale, which accounts for up to 40% of all ischemic strokes18), anticoagulant therapy is indicated, and antiplatelet agents would be ineffective. Thus, the incidence of ischemic events will vary according to the source of thrombus/embolus in this category, and the increased ischemic event rate with prasugrel may be explained by the complicated etiology of thrombus/embolus formation.
Bleeding Events
The incidence of bleeding events in the prasugrel and clopidogrel groups was comparable in all stroke subtypes. In the TRITON-TIMI 38 trial, significant increases in bleeding events were observed with prasugrel 10 mg versus clopidogrel with concomitant use of aspirin from the third day of treatment19). However, in the PRASTRO-I trial, no such trend was observed for any subtype. Monotherapy with prasugrel 3.75 mg, which is approximately one-third of the dose used outside of Japan, is considered to demonstrate a safety profile similar to that of clopidogrel in all patients regardless of stroke subtype.
Limitations
First, the PRASTRO-I trial was not designed to be powered for this prespecified subgroup analysis, and therefore, the sample size for each subtype was small. As there was an inadequate follow-up period in the second year, and statistical significance was not achieved, our observations should be confirmed in a larger study. Second, the results of this study cannot be generalized to women or older patients because the study enrolled relatively few women (21.3%) and only patients aged < 75 years.
Conclusion
Our findings suggest that prasugrel was at least as effective as clopidogrel in patients with large-artery atherosclerosis or small-artery occlusion in the chronic stage. Patients with stroke of undetermined etiology did not seem to be good candidates for more potent antiplatelet therapy. These results may contribute to how antiplatelet drugs are appropriately considered for stroke patients. Further study is therefore needed to determine the efficacy of prasugrel in the acute stage and in patients at a high risk of a transient ischemic attack.
Acknowledgements
We deeply appreciate the contributions of all the investigators and clinical/research staff involved in the present study. We thank Susan E Cottrell, PhD, and Michelle Belanger, MD, of Edanz Medical Writing for providing medical writing support, which was funded by Daiichi Sankyo Co., Ltd through EMC K.K. according to Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
Abbreviations
- PRASTRO-I
PRAsugrel and clopidogrel in Japanese patients with ischemic STROke trial
- HR
hazard ratio
- CI
confidence interval
- iCAS
intracranial arterial stenosis
- SOCRATES
Acute Stroke Or Transient IsChemic Attack TReated With Aspirin or Ticagrelor and Patient OutcomES trial
- CHANCE
Clopidogrel in High-risk Patients With Acute Non-disabling Cerebrovascular Events trial
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
- ProFESS
Prevention Regimen for Effectively Avoiding Second Strokes trial
- TRITON-TIMI 38
TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction
- ACS
acute coronary syndrome
- J-STARS
The Japan Statin Treatment Against Recurrent Stroke trial
- SPS3
Secondary Prevention of Small Subcortical Strokes trial
Funding Source
The present study was sponsored by Daiichi Sankyo.
Conflicts of Interest Statement
AO reports personal fees from Daiichi Sankyo. TK reports grants and personal fees from Daiichi Sankyo; personal fees from Bayer Yakuhin; and grants from Sanofi, Takeda Pharmaceutical, Chugai Pharmaceutical, Astellas Pharma, Nippon Boehringer Ingelheim, MSD, Bristol-Myers Squibb, EA Pharma, Shionogi, Mitsubishi Tanabe Pharma, Eisai, Pfizer, Torii Pharmaceutical, Otsuka Pharmaceutical, and Asahi Kasei Medical. KT reports personal fees from Daiichi Sankyo, Bayer Yakuhin, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim. KK reports grants and personal fees from Takeda Pharmaceutical, Nippon Boehringer Ingelheim, Sumitomo Dainippon Pharma, Astellas Pharma, Kyowa Kirin, Otsuka Pharmaceutical, Bayer Yakuhin, Sanofi, and Daiichi Sankyo; personal fees from Mitsubishi Tanabe Pharma, Shionogi, Pfizer, and Bristol-Myers Squibb; and grants from AstraZeneca and Eisai. TN reports personal fees from Daiichi Sankyo. HY reports personal fees from Bayer Yakuhin, Daiichi Sankyo, Nippon Boehringer Ingelheim, and Stryker Japan; grants from Bristol-Myers Squibb; and funding not related to the present work from the Japan Cardiovascular Research Foundation. SU reports personal fees from Bayer Yakuhin, Nippon Boehringer Ingelheim, Daiichi Sankyo, and Bristol-Myers Squibb. MM reports grants and personal fees from Daiichi Sankyo, Sanofi, Bayer Yakuhin, Takeda Pharmaceutical, Otsuka Pharmaceutical, Nippon Boehringer Ingelheim, Sumitomo Dainippon Pharma, and Bristol-Myers Squibb; and personal fees from Novartis Pharma, Mochida Pharmaceutical, Kyowa Kirin, Nihon Medi-Physics, MSD, Pfizer, Shionogi, Mitsubishi Tanabe Pharma, and Eisai. KM reports personal fees from Bayer Yakuhin and Bristol-Myers Squibb. MN reports personal fees from Daiichi Sankyo and Sanofi. YI reports grants from Daiichi Sankyo. TS and KA are employees of Daiichi Sankyo. SU, NT, and IN have nothing to disclose.
Supplemental Methods
1. Full list of inclusion/exclusion criteria
Inclusion Criteria:
Eligible patients had to satisfy criteria (1) through (5) to be enrolled in this study:
(1) Age of 20 years to less than 75 years at the time of informed consent
(2) Body weight exceeding 50 kg
(3) At the time of informed consent, 1 to 26 weeks had to have passed since the last onset of cerebral infarction (irrespective of whether the onset was the first or a recurrent event, or whether neurologic signs and symptoms lasted for 24 hours or more)
(4) Head diagnostic imaging (magnetic resonance imaging [MRI] taken between the last stroke and the end of the pretreatment period) identified the infarct lesion that probably caused the last stroke episode
(5) Type of treatment: in- and out-patient care (subjects were allowed to change the type of care during the study)
Exclusion Criteria:
Patients were excluded from participating in the study when one or more of the following conditions were applicable by the time study treatment started:
(1) Modified Rankin Scale score of 5 and greater
(2) Contraindication for head MRI scans
(3) Cardiogenic cerebral embolism, paradoxical cerebral embolism, or asymptomatic cerebral infarction
(4) Cardiovascular disease that may cause atrial fibrillation or cardiogenic cerebral embolism (prosthetic valve, left ventricular thrombus, dilated cardiomyopathy, or another high-risk source of embolism as defined by the TOAST classification)
(5) Dual therapy with aspirin and an adenosine diphosphate receptor antagonist because of a history of coronary stent placement for the treatment of acute coronary syndrome (unstable angina, myocardial infarction) or for another reason
(6) Patients who underwent cerebral revascularization (e.g., carotid endarterectomy, carotid artery stenting, bypass grafting) for treatment of cerebral infarction or transient ischemic attack, and required management with multiple antiplatelet drugs
(7) Intracerebral hemorrhage or a history thereof. Patients with asymptomatic microhemorrhages that were only detectable by MRI were not included in this category.
(8) Subarachnoid hemorrhage or a high risk thereof (e.g., patients with an untreated, unruptured cerebral aneurysm not smaller than 5 mm in diameter)
(9) Bleeding or a high risk of bleeding (e.g., hemorrhagic infarction, vitreous hemorrhage, retinal hemorrhage, hemoptysis, hematemesis, hematuria, hematochezia, melena, hemorrhoidal hemorrhage, congenital and acquired hemorrhagic disease, coagulation disorder, platelet disorder). The term “hemorrhagic infarction” as used herein relates to the presence of a non-regressing massive hemorrhagic infarction.
-
(10) Hypertensive patients with poorly controlled blood pressure who met either of the following conditions:
- For patients within 4 weeks after the last episode of ischemic attack: resting seated systolic or diastolic blood pressure of at least 180 mmHg or 110 mmHg, respectively
- For patients over 4 weeks after the last episode of ischemic attack: resting seated systolic or diastolic blood pressure of at least 160 mmHg or 100 mmHg, respectively
- For inpatients, resting recumbent blood pressure values were also taken into consideration.
Antihypertensive therapies were performed according to the criteria for the prevention of recurrent cerebral infarction in chronic patients recommended in the Japanese Guidelines for the Management of Stroke 2009. The blood pressure treatment target was < 140/90 mmHg.
- (11) Patients with a severe blood disorder that fell under one or more of the following criteria:
- Red blood cell count < 3,000,000/µL
- Hemoglobin < 9.5 g/dL
- White blood cell count < 3,000/µL
- Neutrophil count < 1,500/µL
- Platelet count < 75,000/µL
- (12) Severe hepatic disorder, i.e., patients with fulminant hepatitis, hepatic cirrhosis, hepatic tumor, or one corresponding to one or more of the following criteria:
- Total bilirubin ≥ 3.0 mg/dL
- Aspartate aminotransferase ≥ 2.5 times the institutional upper limit of normal range or ≥ 100 U/L
- Alanine aminotransferase ≥ 2.5 times the institutional upper limit of normal range or ≥ 100 U/L
- Alkaline phosphatase ≥ 2.5 times the institutional upper limit of normal range
(13) Severe renal disorder (i.e., serum creatinine value not below 2 mg/dL, or qualitative proteinuria grade of 2+ or higher)
(14) Severe heart failure (New York Heart Association functional classes III and IV)
(15) Severe arrhythmia (e.g., atrioventricular conduction disturbance grades II and III)
(16) Patients with malignant tumors
(17) Patients with a history of thrombotic thrombocytopenic purpura or agranulocytosis
(18) Patients scheduled to receive one or more of the prohibited concomitant drugs during the period in which they were not allowed
(19) Patients with a history of severe adverse drug reactions to ticlopidine hydrochloride or clopidogrel sulphate
(20) Patients who participated in a clinical study of CS-747 or CS-747S and were assigned to take the study drug in the past
(21) Patients who received any study drug within 12 weeks before providing informed consent to participate in this clinical study
(22) Patients planning to undergo surgery during the study period
(23) Patients who were pregnant, lactating, possibly pregnant, and wishing to become pregnant during the study period
(24) Other patients judged unsuitable for enrolment in this clinical study by the investigators
2. Prohibited and Permitted Concomitant drugs
Patients did not use other antiplatelet agents, anticoagulants, thrombolytic agents, or acidic nonsteroidal anti-inflammatory drugs during the study. Concomitant use of other drugs, including proton pump inhibitors, was allowed.
3. Classification of Stroke Subtype
Patients were stratified by stroke subtype based on the TOAST classification.1 Strokes were classified as large-artery atherosclerosis, small-artery occlusion (lacunar), acute stroke of other determined etiology, or stroke of undetermined etiology.
References
- 1). Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, Hill MD, Jonasson J, Kasner SE, Ladenvall P, Minematsu K, Molina CA, Wang Y, Wong KSL, Johnston SC; SOCRATES Steering Committee and Investigators: Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol, 2017; 16: 301-310 [DOI] [PubMed] [Google Scholar]
- 2). Liu L, Wong KS, Leng X, Pu Y, Wang Y, Jing J, Zou X, Pan Y, Wang A, Meng X, Wang C, Zhao X, Soo Y, Johnston SC, Wang Y; CHANCE Investigators: Dual antiplatelet therapy in stroke and ICAS: Subgroup analysis of CHANCE. Neurology, 2015; 85: 1154-1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Ogawa A: The Japan Stroke Society: Committee on guidelines for the management of stroke. Japanese guidelines for the management of stroke 2015. Kyowa Kikaku, Tokyo, 2015 [Google Scholar]
- 4). Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease: Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2014; 45: 2160-2236 [DOI] [PubMed] [Google Scholar]
- 5). Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group: 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis, 2016; 252: 207-274 [DOI] [PubMed] [Google Scholar]
- 6). Wiviott SD, Trenk D, Frelinger AL, O'Donoghue M, Neumann FJ, Michelson AD, Angiolillo DJ, Hod H, Montalescot G, Miller DL, Jakubowski JA, Cairns R, Murphy SA, McCabe CH, Antman EM, Braunwald E; PRINCIPLE-TIMI 44 Investigators: Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation, 2007; 116: 2923-2932 [DOI] [PubMed] [Google Scholar]
- 7). Nagao T, Toyoda K, Kitagawa K, Kitazono T, Yamagami H, Uchiyama S, Tanahashi N, Matsumoto M, Minematsu K, Nagata I, Nishikawa M, Nanto S, Abe K, Ikeda Y, Ogawa A: A noninferiority confirmatory study of prasugrel versus clopidogrel in Japanese patients with ischemic cerebrovascular disease: rationale and study design for a randomized controlled trial. Expert Opin Pharmacother, 2018; 19: 529-535 [DOI] [PubMed] [Google Scholar]
- 8). Ogawa A, Toyoda K, Kitagawa K, Kitazono T, Nagao T, Yamagami H, Uchiyama S, Tanahashi N, Matsumoto M, Minematsu K, Nagata I, Nishikawa M, Nanto S, Abe K, Ikeda Y; PRASTRO-I Study Group: Comparison of prasugrel and clopidogrel in patients with non-cardioembolic ischaemic stroke: a phase 3, randomised, non-inferiority trial (PRASTRO-I). Lancet Neurol, 2019; 18: 238-247 [DOI] [PubMed] [Google Scholar]
- 9). Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd: Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 1993; 24: 35-41 [DOI] [PubMed] [Google Scholar]
- 10). Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vandermaelen C, Voigt T, Weber M, Yoon BW; PRoFESS Study Group: Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med, 2008; 359: 1238-1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM; TRITON-TIMI 38 Investigators: Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med, 2007; 357: 2001-2015 [DOI] [PubMed] [Google Scholar]
- 12). Hosomi N, Nagai Y, Kohriyama T, Ohtsuki T, Aoki S, Nezu T, Maruyama H, Sunami N, Yokota C, Kitagawa K, Terayama Y, Takagi M, Ibayashi S, Nakamura M, Origasa H, Fukushima M, Mori E, Minematsu K, Uchiyama S, Shinohara Y, Yamaguchi T, Matsumoto M; J-STARS Collaborators: The Japan Statin Treatment Against Recurrent Stroke (J-STARS): A Multicenter, Randomized, Openlabel, Parallel-group Study. EBioMedicine, 2015; 2: 1071-1078 [Google Scholar]
- 13). Shi Y, Wardlaw JM: Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol, 2016; 1: 83-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Benavente OR, Hart RG, McClure LA, McClure LA, Szychowski JM, Coffey CS, Pearce LA: Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med, 2012; 367: 817-825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Amarenco P, Lavallee PC, Labreuche J, Albers GW, Bornstein NM, Canhão P, Caplan LR, Donnan GA, Ferro JM, Hennerici MG, Molina C, Rothwell PM, Sissani L, Školoudík D, Steg PG, Touboul PJ, Uchiyama S, Vicaut É, Wong LK; TIAregistry.org Investigators: One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med, 2016; 374: 1533-1542 [DOI] [PubMed] [Google Scholar]
- 16). Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ; MATCH investigators: Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet, 2004; 364: 331-337 [DOI] [PubMed] [Google Scholar]
- 17). Higgins P, MacFarlane PW, Dawson J, McInnes GT, Langhorne P, Lees KR: Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial. Stroke, 2013; 44: 2525-2531 [DOI] [PubMed] [Google Scholar]
- 18). Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR, Wolf PA: Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol, 1989; 25: 382-390 [DOI] [PubMed] [Google Scholar]
- 19). Antman EM, Wiviott SD, Murphy SA, Voitk J, Hasin Y, Widimsky P, Chandna H, Macias W, McCabe CH, Braunwald E: Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol, 2008; 51: 2028-2033 [DOI] [PubMed] [Google Scholar]
Supplemental Reference
- 1). Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd: Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke, 1993; 24: 35-41 [DOI] [PubMed] [Google Scholar]


