Abstract
Aims: Although intensive statin therapy reduced cardiovascular risks, cardiovascular events have not been completely prevented. Probucol is a potent antioxidant and reduces tendon xanthomas in familial hypercholesterolemia patients despite reduction of high-density lipoprotein (HDL)-cholesterol (HDL-C). We investigated whether probucol can reduce cardiovascular events on top of conventional lipid-lowering therapy in patients with coronary heart disease (CHD).
Methods: PROSPECTIVE is a multicenter, randomized, prospective study that recruited 876 Japanese patients with CHD and dyslipidemia with a low-density lipoprotein (LDL)-cholesterol (LDL-C) level of ≥ 140 mg/dL without medication or those treated with lipid-lowering drugs. Lipid-lowering agents were administered during the study period in the control group (n = 438), and probucol 500 mg/day was added to lipid-lowering therapy in the probucol group (n = 438). Patients were randomly assigned to two treatment groups by adjusting the LDL-C level and presence of diabetes and hypertension and followed up for more than 3 years. The primary end point was a composite of cerebrovascular and cardiovascular events (cardiovascular disease death including sudden death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, hospitalization for heart failure, or coronary revascularization). The secondary end point was carotid intima-media thickness in a subset of patients.
Results: The incidence of the primary end point showed a trend to be lower in the probucol group compared with that in the control group despite reduced HDL-C without serious adverse events. Anti-atherogenic effects of probucol may be attributed to its potent antioxidative function and enhancement of reverse cholesterol transport.
Conclusion: Since there was no statistical significance between the probucol and control groups despite a marked reduction of HDL-C, further studies on the clinical outcomes of probucol on top of conventional therapy may be necessary in the future (UMIN000003307).
Keywords: Probucol, Coronary heart disease, Prevention, Reverse cholesterol transport, Antioxidants
See editorial vol. 28: 97–99
Introduction
Although intensive low-density lipoprotein (LDL)-lowering therapy using statins1), in combination with an intestinal cholesterol transporter inhibitor (ezetimibe)2) and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors such as evolocumab3) and alirocumab4), could significantly reduce the event rate of atherosclerotic cardiovascular diseases, these events have not been completely prevented. Therefore, additional effective pharmacological intervention is necessary to mitigate “residual risks” in combination with statins.
Probucol is a diphenolic compound with potent antioxidant and anti-inflammatory properties5, 6). Probucol was developed to reduce LDL-cholesterol (LDL-C) levels before the development of statins7–10) and has been used for hypercholesterolemic patients in Japan, especially those with familial hypercholesterolemia (FH) and Achilles tendon xanthomas11).
Probucol reduces LDL-C levels even in LDL receptor-deficient Watanabe heritable hyperlipidemic (WHHL) rabbits12) and in patients with homozygotes and heterozygotes of FH who are deficient in LDL receptor13–14). Thus, the LDL-C reduction by probucol was attributed to enhanced catabolism of LDL independent of an LDL receptor14) and increased excretion of cholesterol into bile15). Furthermore, probucol has a potent antioxidative effect, inhibits LDL oxidation16), and reduces skin and tendon xanthomas in patients with FH11) despite a marked reduction in HDL-C due to enhancement of cholesteryl ester transfer protein activity17) and hepatic expression of scavenger receptor class B type I (SR-BI)18). Probucol attenuated atherosclerosis in WHHL rabbits independent of its lipid-lowering effect19). Probucol, but not α-tocopherol or ubiquinone-10, protected WHHL rabbits against atherosclerosis20).
Probucol Quantitative Regression Swedish Trial (PQRST)21), a randomized controlled trial, was carried out in Sweden to elucidate whether 3 years' administration of probucol to hypercholesterolemic patients may reduce femoral atherosclerosis. Treatment of patients with probucol lowered LDL-C by 12% and HDL-C by 24%; however, lumen volume did not show a significant increase in the probucol-treated group. Thus, most of the Western countries quitted using probucol after the launch of statins. Probucol was withdrawn partly because of the reduction of serum HDL-C levels and possible QT interval prolongation, which might increase the possibility of ventricular arrhythmias.
Probucol Observational Study Illuminating Therapeutic Impact on Vascular Events (POSITIVE)22) evaluated the long-term effects of probucol treatment on the risk of cardiovascular events in 410 patients with heterozygous FH. The primary outcome was the time to the first cardiovascular event involving hospitalization, and multivariate Cox regression analysis demonstrated that the hazard ratio of probucol use was 0.13 (95% confidence interval 0.05 to 0.34; p < 0.001) in patients for secondary prevention. Although the hazard ratio was not significant in patients for primary prevention because significantly higher LDL-C levels were in the probucol group than in the non-probucol group, this retrospective study has strongly suggested that long-term treatment with probucol may prevent the secondary cardiovascular events in a very high-risk population such as FH. Thus, a current multicenter, randomized, prospective study has been designed to test the hypothesis that the addition of probucol to other lipid-lowering drugs prevents cerebrovascular and cardiovascular events in Japanese patients with coronary heart disease (CHD) and high LDL-C levels.
Patients and Protocol
Study Design and Recruiting Patients
The current PROSPECTIVE study is a randomized (1:1), prospective, open-label, multicenter clinical trial conducted in patients with hyper-LDL-cholesterolemia with a prior history of coronary events as CHD. The rationale and design of this study were already published elsewhere23). The two arms of the trial are a control group of patients on conventional lipid-lowering therapy (LLT) and a test group of patients on conventional LLT plus probucol treatment. The protocol was initially reviewed and approved by the institutional review board (IRB) of Osaka University Hospital and thereafter by the IRBs of the participating institutions. Investigators obtained IRB approval and permission from the head of each institution before conducting the study. This study is registered with UMIN (UMIN000003307, March 3, 2010). Participating facilities and study organization are described in detail in the Appendix.
The current study enrolled male and female patients older than 20 years with all of the following seven clinical statuses: 1) diagnosis of dyslipidemia with high LDL-C level (≧ 140 mg/dL) without any medication or those treated with any lipid-lowering drugs including statins before providing informed consent; 2) serum LDL-C level less than 200 mg/dL within 8 weeks before providing informed consent, as calculated by Friedewald's formula (LDL-C = total cholesterol – HDL-cholesterol – triglycerides (TG)/5); 3) history of acute myocardial infarction or angina pectoris more than 3 months before providing informed consent, old myocardial infarction, coronary artery bypass grafting (CABG) more than 3 months earlier, percutaneous coronary intervention (PCI) more than 9 months earlier, or PCI with no restenosis that was diagnosed by follow-up coronary angiography at 6–9 months after PCI; 4) normal cardiac function, mild or moderate heart failure (NYHA classification I or II); 5) older than 20 years at the time of informed consent; 6) no severe hepatic and renal dysfunction (AST < 100 IU/L, ALT < 100 IU/L, serum creatinine < 1.5 mg/dL) within 4 weeks before providing informed consent; and 7) signed written informed consent for participation in the current study. The exclusion criteria were the presence of the following clinical statuses at the time of informed consent: 1) ongoing treatment with probucol within 6 months before the time of informed consent; 2) ongoing treatment with cyclosporine; 3) history of hypersensitivity reactions to probucol; 4) diagnosis of FH based on the NICE Clinical Guideline 71 24); 5) very high TG level (> 400 mg/dL) within 8 weeks before providing informed consent; 6) markedly high HbA1c level (≧ 8%) on the most recent blood test; 7) frequent multifocal ventricular arrhythmia; 8) atrial fibrillation (Af ) including paroxysmal Af; 9) long QTc interval on a resting electrocardiogram (> 450 ms in males or > 470 ms in females); 10) congestive heart failure (NYHA III or IV) or unstable angina; 11) participation in other clinical trials; 12) women who are pregnant, are lactating, might become pregnant, or wish to become pregnant within the study period; or 13) inappropriate candidate for participation as assessed by the doctors in the current study.
Randomization and Treatment
After the inclusion and exclusion criteria were screened and patient's consent was gained, a Web-based central registration system automatically and randomly assigned patients to either the control group (conventional LLT continued) or the test group (LLT with probucol) with 1:1 allocation rate based on the registered patient's data. In randomization, LDL-C level (140 mg/dL and more vs. less than 140 mg/dL), diabetes (with vs. without), and hypertension (with vs. without) were dynamically balanced between the two groups as adjusted allocation factors. As a protocol treatment, each of the lipid-lowering agents is administered continuously during the study period in the control group, and probucol (500 mg/day, 250 mg twice daily after breakfast and dinner) was added to the LLT in the test group within 6 weeks after the registration.
End Points
The primary efficacy end point was the presence and the time from registration until the first occurrence of cerebrovascular and cardiovascular events. Cerebrovascular and cardiovascular events were recognized as follows: 1) cardiovascular death including cardiac sudden death; 2) nonfatal myocardial infarction; 3) nonfatal cerebral stroke excluding transient ischemic attack; 4) hospital admission due to unstable angina, 5) hospital admission due to heart failure; and 6) all coronary revascularizations with either PCI or CABG.
The secondary efficacy and safety end points were as follows: 1) all death; 2) all cerebrovascular and cardiovascular disease; 3) event-free survival time; 4) levels of the mean intima–media thickness (IMT) of carotid arteries and their changes; 5) levels of max IMT in common or internal carotid arteries and their changes; and 6) severe adverse events and their frequency.
Measurements and Reporting Serious Adverse Events (SAEs)
All data were input to the case report after the protocol treatment was started. During the study period, patient's background, all cerebrovascular and cardiovascular events, all adverse events, and the confirmation of being alive were determined before the protocol treatment and at 3 months; at 1, 2, and 3 years after registration; and at the time of termination and the simultaneous outcome. At the same time points, the levels of total cholesterol, TG, and HDL-C were determined by the measurement method of each facility, and the data were input to the system. High-sensitivity CRP (hsCRP) and adiponectin levels were measured at the center laboratory (SRL Inc. Tokyo, Japan) using the specimen from the facility where it was available. In maximum (max)/minimum (min) IMT of the carotid arteries, doctors and clinical technologists at each facility used the same protocol to depict the same site as much as possible using echography, submitted the images to the Imaging Evaluation Committee, which evaluated their values before the protocol treatment and at 1, 2, and 3 years after registration.
SAEs were graded according to NCI-CTCAE by each doctor. SAEs included death, events that could lead to death, events that require hospital admission or extended hospitalization, disorders, events that could lead to disorders, others that are serious as well as the former adverse events, or congenital disease or abnormality in post-generations. The efficacy and safety evaluation committee evaluated these to make recommendation of early termination or study changes to the principal investigator.
Statistical Design and Analyses
There have been no secondary prevention studies of probucol in dyslipidemic patients in Japan except for a retrospective study of patients with FH22). On sample size consideration in the study, we assumed that the morbidity of cerebrovascular and cardiovascular events in the control group would be 10.7% on the basis of the results from the statin-treated group of the Japan EPA Lipid Intervention Study (JELIS)25). In the test group, we assumed that the rate would be 5.4%, almost half of that in the statin-treated group, because the addition of probucol to conventional LLT might improve the morbidity of cardiovascular events as well as the primary prevention shown in the Fukuoka Atherosclerosis Trial (FAST)26). Sample size per group was calculated as 408 patients with a two-tailed significance level of 0.05 and a power of 0.8 in 4 years' accrual period and 3 years' follow-up. Considering that several patients will be dropped out from the analysis, we decided that the target number of patients in one group would be 430 and total target number of patients in the current study would be 860.
The patient population in statistical analysis was a full analysis set based on the intention-to-treat principle. The characteristics of patients' demographics and baseline values were compared between the two groups. For the primary efficacy end point, the presence of and time from registration until the first cerebrovascular and cardiovascular events were analyzed. By estimating event-free survival curves using the Kaplan–Meier method and the 95% confidence interval (CI) at 1, 2, and 3 years, event-free survival curves for the two groups were compared using the log-rank test stratified with random allocation factors: LDL-C level (140 mg/dL and more vs. less than 140 mg/dL), diabetes (with vs. without), and hypertension (with vs. without). The secondary end points of the period until all death and period until all cardiovascular and cerebrovascular disease and event-free survival time were analyzed in the same way as the primary end point. Levels of the mean and max IMT of carotid arteries from baseline to 1, 2, and 3 years were analyzed by a mixed-effects model with repeated measurements with baseline value (included only if the change from baseline was the response variable), allocation group, visit, and interaction between allocation group and visit as fixed effects and patient as a random effect. Comparison of groups was performed. The frequency of SAEs was summarized by preferred term and intensity, and compared using Fisher's exact test. The two-tailed significant level in statistical analysis was 0.05. All statistical analyses were done with SAS version 9.3.
Results
Patients
A total of 876 patients with CHD underwent randomization at 82 sites in Japan from June 2010 through February 2014, and after evaluations for safety and full analysis sets, 831 of 876 patients were assigned to either the control group (conventional LLT continued, 431 patients) or the test group (LLT plus probucol 500 mg/day, 400 patients) (Supplemental Fig. 1). The mean age of the patients was 70 years, and 26.6% of the patients were women; 35.9% of the patients had a history of myocardial infarction, 63.1% angina pectoris, 6.3% cerebrovascular disease, and 3.7% peripheral artery disease. Most of the patients (97.0%) qualified with an LDL-C level of less than 140 mg/dL. Of the patients, 80.3% were accompanied by hypertension and 42.0% by diabetes mellitus. The rate of use of secondary preventive therapies was high, at the trial entry, with 99.0% (unknown eight patients were treated as not used), 93.5%, 82.3%, and 34.4% of the patients taking lipid-lowering, antiplatelet, antihypertensive, and antidiabetic agents, respectively. Lipid-lowering agents included statins (92.7%) and resins (1.4%). After randomization using the LDL-C levels and the presence or absence of diabetes or hypertension, there were no significant differences in the background characteristics between the control group and the test group except for the NYHA classification (p = 0.0433) and smoking status (p = 0.0119). However, both NYHA classification and smoking status were not prognostic factors. The baseline characteristics of the patients in the two groups were well balanced except for the NYHA classification and smoking status, and these are shown in Tables 1 and 2. Lipid-lowering agents other than probucol included statins, an intestinal cholesterol transporter inhibitor, and resins. Lipid-lowering agents were continuously administrated during the study period in the control group, and probucol (500 mg/day, 250 mg twice daily) was added to LLT in the test group.
Supplemental Fig. 1.
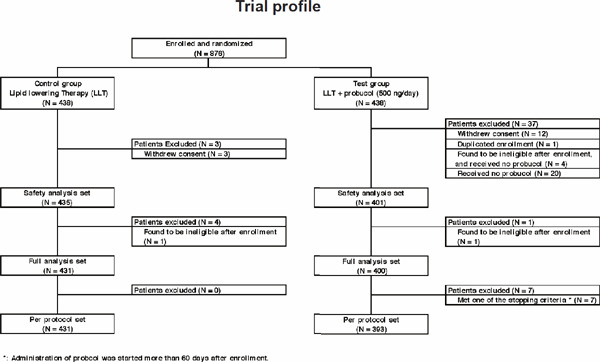
Trial profile of PROSPECTIVE
Table 1. Baseline demographics and other characteristics (qualitative variable).
| Characteristics | Control group | Test group | P value | |
|---|---|---|---|---|
| (N = 431) | (N = 400) | |||
| No. of patients (%) | ||||
| Gender | Male | 319 (74.0) | 291 (72.8) | 0.695 |
| Female | 112 (26.0) | 109 (27.3) | ||
| NYHA classification | I | 418 (97.0) | 376 (94.0) | 0.043* |
| II | 13 (3.0) | 24 (6.0) | ||
| LDL-C | < 140 mg/dL | 418 (97.0) | 388 (97.0) | 1.000 |
| ≥ 140 mg/dL | 13 (3.0) | 12 (3.0) | ||
| Diabetes mellitus | No | 250 (58.0) | 232 (58.0) | 1.000 |
| Yes | 181 (42.0) | 168 (42.0) | ||
| Hypertension | No | 89 (20.6) | 75 (18.8) | 0.542 |
| Yes | 342 (79.4) | 325 (81.3) | ||
| Cerebrovascular disease | No | 404 (93.7) | 375 (93.8) | 1.000 |
| Yes | 27 (6.3) | 25 (6.3) | ||
| Peripheral artery disease | No | 417 (96.8) | 385 (96.3) | 0.710 |
| Yes | 14 (3.2) | 15 (3.8) | ||
| Smoking status | Current smoker | 34 (7.9) | 15 (3.8) | 0.012* |
| Ex-smoker | 177 (41.1) | 193 (48.3) | ||
| Never | 219 (50.8) | 192 (48.0) | ||
| Unknown | 1 (0.2) | 0 (0.0) | ||
| Coronary heart disease | Myocardial infarction | 160 (37.1) | 138 (34.5) | 0.728 |
| Angina pectoris | 266 (61.7) | 258 (64.5) | ||
| Others | 5 (1.2) | 4 (1.0) | ||
| Unknown | 0 (0.0) | 0 (0.0) | ||
| Lipid-lowering agents | No | 2 (0.5) | 0 (0.0) | 0.500 |
| Yes | 428 (99.3) | 393 (98.3) | ||
| Statin | 404 (93.7) | 366 (91.5) | ||
| Fibrate | 18 (4.2) | 13 (3.3) | ||
| Nicotinic acid and derivatives | 2 (0.5) | 1 (0.3) | ||
| Anion exchange resin | 2 (0.5) | 4 (1.0) | ||
| Others | 56 (13.0) | 48 (12.0) | ||
| Unknown | 1 (0.2) | 7 (1.8) | ||
| Antihypertensive agents | No | 77 (17.9) | 69 (17.3) | 0.855 |
| Yes | 353 (81.9) | 331 (82.8) | ||
| Ca channel blockers | 208 (48.3) | 183 (45.8) | ||
| ARB | 186 (43.2) | 207 (51.8) | ||
| Diuretics | 47 (10.9) | 51 (12.8) | ||
| ACE inhibitors | 68 (15.8) | 58 (14.5) | ||
| β-blockers | 135 (31.3) | 141 (35.3) | ||
| Others | 28 (6.5) | 33 (8.3) | ||
| Unknown | 1 (0.2) | 0 (0.0) | ||
| Antidiabetic agents | No | 33 (7.7) | 32 (8.0) | 0.891 |
| Yes | 149 (34.6) | 137 (34.3) | ||
| Sulfonylurea | 44 (10.2) | 56 (14.0) | ||
| Insulin preparations | 16 (3.7) | 20 (5.0) | ||
| Insulin resistance improving agents | 53 (12.3) | 43 (10.8) | ||
| α-Glucosidase inhibitors | 39 (9.0) | 34 (8.5) | ||
| Others | 95 (22.0) | 77 (19.3) | ||
| Unknown | 249 (57.8) | 231 (57.8) | ||
| Antiplatelet agents | No | 30 (7.0) | 23 (5.8) | 0.482 |
| Yes | 400 (92.8) | 377 (94.3) | ||
| Cilostazol | 30 (7.0) | 21 (5.3) | ||
| Ticlopidine | 35 (8.1) | 38 (9.5) | ||
| Dipyridamole | 5 (1.2) | 2 (0.5) | ||
| Aspirin | 350 (81.2) | 334 (83.5) | ||
| Sarpogrelate | 9 (2.1) | 8 (2.0) | ||
| Clopidogrel | 124 (28.8) | 115 (28.8) | ||
| Others | 25 (5.8) | 25 (6.3) | ||
| Unknown | 1 (0.2) | 0 (0.0) | ||
In case of “yes”, multiple choices were allowed.
Fisher's exact test was used to evaluate the significance of differences between groups.
The level of significance was set at p < 0.05 (2-sided).
ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blockers; LDL-C, low-density lipoprotein cholesterol; NYHA, New York Heart Association
Table 2. Baseline demographics and other characteristics (quantitative variable).
| Characteristics | Control group |
Test group |
P value | ||
|---|---|---|---|---|---|
| N | mean ± SD or median (IQR) | N | mean ± SD or median (IQR) | ||
| Age (years) | 431 | 70.0 ± 9.4 | 400 | 70.0 ± 9.0 | 0.750 |
| Height (cm) | 431 | 160.2 ± 8.5 | 400 | 160.5 ± 8.8 | 0.561 |
| Weight (kg) | 431 | 63.2 ± 11.4 | 400 | 63.7 ± 12.0 | 0.635 |
| TC (mg/dL) | 431 | 169.5 ± 28.7 | 400 | 169.0 ± 28.6 | 0.591 |
| HDL-C (mg/dL) | 431 | 54.4 ± 14.4 | 400 | 53.6 ± 13.6 | 0.510 |
| LDL-C (mg/dL) | 431 | 90.4 ± 23.5 | 400 | 89.6 ± 22.7 | 0.511 |
| TG (mg/dL) | 431 | 107 (80–154) | 400 | 114 (83–158) | 0.302 |
| hs-CRP (ng/mL) | 69 | 570 (216–1640) | 56 | 698 (409–1585) | 0.268 |
| Adiponectin (µg/mL) | 69 | 8.60 (6.00–11.10) | 56 | 8.00 (6.05–11.55) | 0.880 |
| AST (IU/L) | 431 | 22 (18–28) | 400 | 23 (19–27) | 0.345 |
| ALT (IU/L) | 431 | 19 (15–26) | 400 | 19 (15–27) | 0.473 |
| Serum creatinine (mg/dL) | 431 | 0.87 ± 0.20 | 400 | 0.86 ± 0.21 | 0.711 |
| HbA1c (%) | 431 | 5.70 (5.30–6.20) | 400 | 5.70 (5.35–6.20) | 0.651 |
| No. of cigarettes smoked on average daily (only current smokers) | 34 | 20 (10–20) | 15 | 10 (7–20) | 0.327 |
| Time from enrollment until the first onset of CAD (months) | 429 | 48 (20–92) | 399 | 50 (20–96) | 0.461 |
Two-sample Wilcoxon test was used to evaluate the significance of differences between groups.
AST, aspartate transaminase; ALT, alanine transaminase; CAD: coronary artery disease; hs-CRP, high sensitive C-reactive protein; Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride
Withdrawal of consent occurred in 3 and 12 patients in the control and test groups, respectively. Premature permanent discontinuation of the study regimen (probucol) occurred in 21 patients in the test group. The durations of follow-up were 3.90 (3.12–4.77) (median [IQR]) years and 3.82 (3.15–4.79) years in the control group and the test group, respectively.
Lipid Data
The LDL-C levels at baseline were 90.4 ± 23.5 (mean ± standard deviation) mg/dL and 89.6 ± 22.7 mg/dL in the control group and the test group, respectively (Table 2). At 3 months, the absolute reduction of least square mean in LDL-C levels in the test group, as compared with that in the control group, was 7.3 mg/dL (95% CI, 4.7 to 9.9; p < 0.0001). The reduction in LDL-C levels was maintained over time (Fig. 1a). At 36 months, the absolute reduction of least square mean in LDL-C levels in the test group, as compared with that in the control group, was 8.5 mg/dL (95% CI, 5.4 to 11.7; p < 0.0001).
Fig. 1.
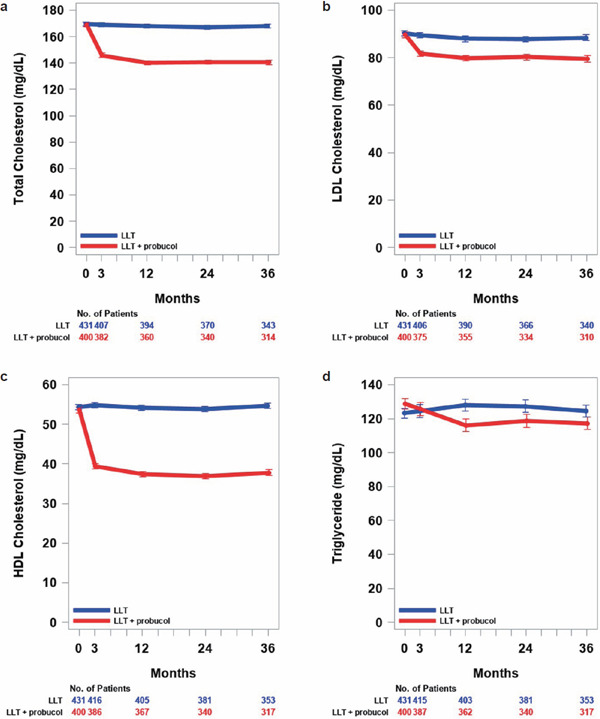
Total cholesterol (a), LDL-cholesterol (b), HDL-cholesterol (c), and triglycerides levels (d) over time by treatment group (FAS)
Measured values at each visit were shown as least square mean with bars of standard error and analyzed by mixed-effects models with repeated measures (MMRM) with allocation group, visit (at baseline and 3, 12, 24, and 36 months), and interaction between allocation group and visit as fixed effects and patient as a random effect. Comparisons between treatment groups at each visit were made using the least square mean of each group. The MMRM analyses were performed using the Kenward-Roger method for adjusting degrees of freedom in an unstructured (UN) covariance structure. Blue and red circles with bars and lines show the control group (conventional LLT continued) and the test group (LLT plus probucol), respectively.
The baseline levels in the control group and the test group were 169.5 ± 28.7 and 169.0 ± 28.6 mg/dL for total cholesterol, 54.4 ± 14.4 and 53.6 ± 13.6 mg/dL for HDL-C, and 107 (80–154) and 114 (83–158) mg/dL for TG. Total cholesterol, HDL-C, or triglycerides levels in the test group at 3 and 48 months were consistently and significantly lower than those in the control group, except for the TG levels at 3 months (no difference between the two groups) (Fig. 1b, c, and d). At 3 months, the absolute reductions of least square means in total cholesterol, HDL-C, and TG levels in the test group, as compared with those in the control group, were 22.9 mg/dL (95% CI, 19.7 to 26.2; p < 0.0001), 14.9 mg/dL (95% CI, 13.7 to 16.2; p < 0.0001), and 2.6 mg/dL (95% CI, 6.2 to 11.5; p = 0.5601), respectively. At 36 months, the absolute reductions of least square means in total cholesterol, HDL-C, and TG levels in the test group, as compared with those in the control group, were 27.5 mg/dL (95% CI, 23.8 to 31.2; p < 0.0001), 16.3 mg/dL (95% CI, 14.8 to 17.7; p < 0.0001), and 10.8 mg/dL (95% CI, 2.3 to 19.3; p = 0.0126), respectively.
Efficacy End Points
The primary end point occurred in 44 patients (10.2%) in the control group and in 31 patients (7.8%) in the test group (adjusted hazard ratio, 0.746; 95% CI, 0.471 to 1.182; stratified log-rank test, p = 0.1839) (Table 3 and Fig. 2). We observed no significant difference between the control group and the test group in the incidence of primary endpoint of cerebrovascular and cardiovascular events. Additionally, there were no significant differences in the incidence of the key composites of the primary end point of cerebrovascular and cardiovascular events; death because of cardiovascular diseases including sudden death, nonfatal myocardial infarction, nonfatal stroke excluding transient ischemic attack, hospitalization for unstable angina, and hospitalization for heart failure or coronary revascularization such as PCI or CABG after at least 3 years of follow-up. As a note, although the differences did not reach statistical significance, the numbers of patients (%) tended to be decreased in the test group, compared with those in the control group, in the incidence of the key composites of the primary end point of cerebrovascular and cardiovascular events except nonfatal stroke excluding transient ischemic attack; death because of cardiovascular diseases including sudden death (0.8 vs. 1.2), nonfatal myocardial infarction (0.3 vs. 0.9), nonfatal stroke excluding transient ischemic attack (1.3 vs. 0.9), hospitalization for unstable angina (0.8 vs. 1.4), and hospitalization for heart failure (1.3 vs. 1.6) or coronary revascularization such as PCI or CABG after at least 3 years of follow-up (4.0 vs. 5.6).
Table 3. The incidence of cerebro- and cardiovascular events.
| Control group | Test group | P value* | |
|---|---|---|---|
| (N = 431) | (N = 400) | ||
| No. of patients (%) | |||
| Cerebro- and cardiovascular events | 44 (10.2) | 31 (7.8) | 0.228 |
| Cardiovascular death including sudden cardiac death | 5 (1.2) | 3 (0.8) | 0.727 |
| Nonfatal myocardial infarction | 4 (0.9) | 1 (0.3) | 0.375 |
| Nonfatal stroke excluding TIA | 4 (0.9) | 5 (1.3) | 0.745 |
| Unstable angina requiring hospitalization | 6 (1.4) | 3 (0.8) | 0.508 |
| Heart failure requiring hospitalization | 7 (1.6) | 5 (1.3) | 0.775 |
| Coronary revascularization (PCI and CABG) | 24 (5.6) | 16 (4.0) | 0.332 |
Fisher's exact test was used to evaluate the significance of differences between groups.
The level of significance was set at p < 0.05 (2-sided).
Fig. 2.
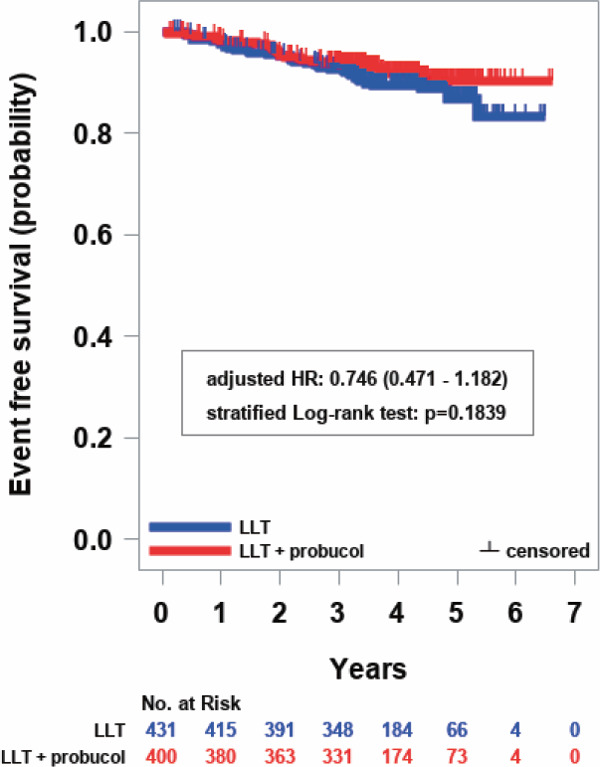
Kaplan–Meier curves for the primary efficacy end point
Survival curves were estimated using the Kaplan–Meier method, and the stratified log-rank test was performed to compare the survival rates, using levels of LDL-C, presence or absence of diabetes, and presence or absence of hypertension as the stratified factors. The level of significance was set at p < 0.05 (two-sided). The hazard ratio (HR) and 95% CI were estimated using the Cox proportional hazards model adjusted by allocation factors (levels of LDL-C, presence or absence of diabetes, and presence or absence of hypertension). Blue and red markers with bars and lines show the control group (conventional LLT continued) and the test group (LLT plus probucol), respectively.
There were no significant differences between the control group and the test group in the secondary efficacy and safety end points either; all death, all cerebrovascular and cardiovascular disease, event-free-survival time, levels of the mean IMT of carotid arteries and their changes, or levels of max IMT in common or internal carotid arteries and their changes (Supplemental Fig. 2a–d). In the current study, the number of patients for whom ultrasonography of carotid arteries was performed was small. There may be a limitation in the accurate measurement of mean and max IMT at the same point in each patient. Therefore, the tendency of reduction of IMT in the control group and slight increase of IMT in the probucol-treated group may reflect these limitations. Furthermore, the changes of IMT may not be related to the cerebrovascular and cardiovascular event rate.
Supplemental Fig. 2.
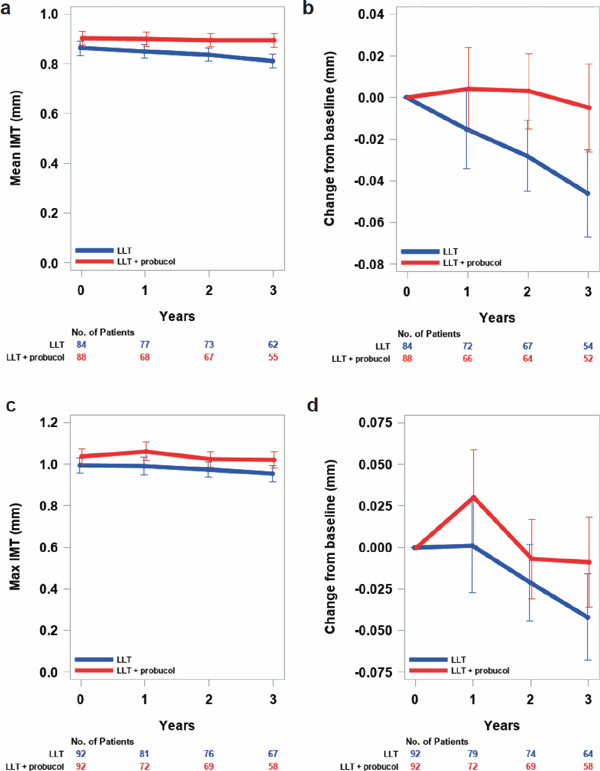
Levels in mean (a) and max (c) IMT and changes from baseline in mean (b) and max (d) IMT over time by treatment group (FAS)
Measured values at each visit were shown as least square mean with bars of standard error and analyzed using a mixed-effects models with repeated measures (MMRM) with allocation group, visit (at baseline and 12, 24, and 36 months), and interactions between allocation group and visit defined as fixed effects and patient as a random effect. Comparisons between treatment groups at each visit were made using the least square mean of each group. The MMRM analyses were performed using the Kenward–Roger method for adjusting degrees of freedom in an unstructured (UN) covariance structure.
In a limited number of patients, the serum concentrations of hsCRP and adiponectin were measured and compared between the control group and the test group. There were no significant differences in the changes of serum hsCRP and adiponectin concentrations between the control group and the test group (data not shown).
Safety
The SAEs occurred in 14 patients (3.2%; 95% CI, 1.8 to 5.3) in the control group and in 12 patients (3.0%; 95% CI, 1.6 to 5.2) in the test group (Table 4). There was no significant difference in the frequencies of SAEs between the two groups (p = 1.0000).
Table 4. Adverse events.
| Adverse events | Control group | Test group | P value | |
|---|---|---|---|---|
| (N = 435) | (N = 401) | |||
| No. of patients (%) | ||||
| Any adverse events | 203 (46.7) | 224 (55.9) | 0.009* | |
| Serious adverse events | 14 (3.2) | 12 (3.0) | 1.000 | |
| Aortic valve stenosis | 0 (0.0) | 1 (0.2) | 0.480 | |
| Haematemesis | 1 (0.2) | 0 (0.0) | 1.000 | |
| Salivary gland calculus | 0 (0.0) | 1 (0.2) | 0.480 | |
| Large intestine polyp | 1 (0.2) | 0 (0.0) | 1.000 | |
| Death | 2 (0.5) | 1 (0.2) | 1.000 | |
| Cholecystitis acute | 0 (0.0) | 1 (0.2) | 0.480 | |
| Disseminated tuberculosis | 1 (0.2) | 0 (0.0) | 1.000 | |
| Septic shock | 0 (0.0) | 1 (0.2) | 0.480 | |
| Tibia fracture | 1 (0.2) | 0 (0.0) | 1.000 | |
| Lumbar spinal stenosis | 1 (0.2) | 0 (0.0) | 1.000 | |
| Bladder cancer | 0 (0.0) | 1 (0.2) | 0.480 | |
| Breast cancer female | 0 (0.0) | 1 (0.2) | 0.480 | |
| Gastric cancer | 1 (0.2) | 2 (0.5) | 0.610 | |
| Lung cancer | 1 (0.2) | 1 (0.2) | 1.000 | |
| Malignant pleural effusion | 0 (0.0) | 1 (0.2) | 0.480 | |
| Pancreatic cancer | 1 (0.2) | 1 (0.2) | 1.000 | |
| Renal cancer | 1 (0.2) | 0 (0.0) | 1.000 | |
| Tongue neoplasm malignant stage unspecified | 1 (0.2) | 0 (0.0) | 1.000 | |
| Hepatocellular carcinoma | 1 (0.2) | 1 (0.2) | 1.000 | |
| Cerebral hemorrhage | 1 (0.2) | 0 (0.0) | 1.000 | |
| Depression | 1 (0.2) | 0 (0.0) | 1.000 | |
| Hypoxia | 1 (0.2) | 0 (0.0) | 1.000 | |
| Aspiration pneumonia | 0 (0.0) | 1 (0.2) | 0.480 | |
| Blue toe syndrome | 1 (0.2) | 0 (0.0) | 1.000 | |
Fisher's exact test was used to evaluate the significance of differences between groups.
The level of significance was set at p < 0.05 (2-sided).
The incidence of any adverse events was significantly higher in the test group than in the control group (55.9% vs 46.7%; p = 0.0085): note that the frequencies of ventricular arrhythmia and each composite of laboratory examination (other than increase in serum creatine phosphokinase) tended to be high in both groups, because all reports of no examination with the grade level of unknown were evaluated as adverse events. The incidence of ventricular arrhythmia, gastrointestinal disturbances, infectious or parasite diseases, metabolic or nutritional disturbances, benign or malignant neoplasms, mental disorders, and vascular diseases did not differ significantly between the two groups. However, the incidence of QT elongation on the electrocardiogram, a composite of laboratory examinations, was more frequent in the test group than the control group (35.9% vs. 25.3%; p = 0.0009).
Discussion
Intensive therapies with statins and PCSK9 inhibitors have been shown to significantly reduce atherosclerotic cardiovascular events; however, these events have not completely been prevented even if LDL-C levels were lowered to less than 50 mg/dL. Therefore, additional effective pharmacological therapy intervention may be necessary to mitigate “residual risks” in combination with statins. The residual risks related to dyslipidemia include increased remnant lipoproteins and reduced HDL-C levels as well as oxidative stress and inflammation associated with dyslipidemia. In the current PROSPECTIVE study, we could not demonstrate that probucol can significantly reduce cardiovascular events in CHD patients treated with statins. However, we also showed that probucol treatment in the high-risk patients treated with statins was well tolerated and safe despite reduction of serum HDL-C. Probucol reduced LDL-C by 7.3 mg/dL at 3 months compared with control therapy, which was maintained over time (Fig. 1). Therefore, there might be a possibility that the reduction of LDL-C by probucol may be one of the reasons for a tendency to lower cardiovascular events.
Although we determined the sample size of this randomized controlled trial based on our retrospective observational study, the study results may indicate that the sample size is underpowered to address the hypothesis. Other large-scale clinical studies that tried to show non-statin benefits, such as IMPROVE-IT2), FOURIER3), and ODYSSEY OUTCOMES4), have recruited more than 10,000 patients. Therefore, we might need another trial to show the benefit of probucol in high-risk patients for cardiovascular disease with a larger sample size. Indeed, Kang et al. have also tried to investigate the effect of probucol on carotid IMT and the rate of cerebrovascular and cardiovascular events in Korea and China (IMPACT Trial) with a similar regimen27). We are planning to conduct a meta-analysis by combining the data in these three countries.
Probucol was withdrawn from the market because of its HDL-C lowering effect and QT elongation. Many CETP inhibitors have been developed to increase serum HDL-C levels as well as to decrease serum LDL-C levels. However, most of CETP inhibitors such as torcetrapib28), dalcetrapib29), and evacetrapib30) that can increase serum HDL-C levels have failed to show cardiovascular risk reduction in patients treated with statins. Although anacetrapib has shown some benefits on top of statins31), the pharmaceutical company (Merck) has quitted its development possibly because of its prolonged accumulation in adipose tissues32) and relatively small event risk reduction. The failure of CETP inhibitors may be attributed partly to their strategy to increase serum HDL-C levels by inhibiting the transfer of cholesteryl ester from HDL particles, which resulted in the formation of very large cholesteryl ester-rich HDL particles with impaired antiatherogenic functions33). Thus, the strategies for increasing serum HDL-C levels have not been shown to be effective to reduce the cardiovascular risk in statin-treated patients.
In contrast to CETP inhibitors, probucol has been shown to decrease serum HDL-C levels through enhancement of plasma CETP activity17, 34) and hepatic expression of SR-BI18, 35). Probucol was shown to enhance hepatic SR-BI protein expression, possibly through species-specific stabilization of the protein18). The reduction of HDL-C by probucol may be explained by both an enhanced CETP-mediated transfer of cholesteryl esters from HDL to apolipoprotein B-containing lipoproteins and an increased hepatic selective uptake of cholesteryl esters from HDL via SR-BI. Probucol also enhanced the formation of lipid-poor prebeta1-HDL involved in cellular cholesterol efflux36). The reduction of serum HDL-C may reflect the enhancement of the reverse cholesterol transport37, 38). The HDL particles from probucol-treated patients are small and poor in cholesteryl ester and were shown to possess a more potent antiatherogenic activity against foam cell formation39) and a more marked antioxidative activity partly due to an increase in antioxidative paraoxonase 1 (PON1) activity40). The LDL particles from probucol-treated patients were strongly protected from oxidation16). Probucol was also demonstrated to have anti-inflammatory effects41, 42) and enhance xanthoma regression in patients with FH despite reduction in serum HDL-C levels along with the attenuated progression of atherosclerosis in animal models43, 44).
Furthermore, clinical studies on the effects of probucol on xanthomas, restenosis after PCI, and atherosclerosis extensively reviewed in Yamashita et al.10) demonstrated antiatherogenic effects of probucol in clinical settings. As described above, a retrospective POSITIVE study22) in patients with heterozygous FH has implicated that long-term probucol treatment may reduce secondary cerebrovascular and cardiovascular events in a very high-risk population such as FH. The effect of probucol therapy on long-term survival following complete revascularization was also reported in 1,694 patients after complete revascularization with PCI and/or CABG by a propensity score analysis45), in which the use of probucol was associated with a markedly significant decrease in all-cause death in a propensity score-adjusted model (hazard ratio = 0.57; p = 0.008). In post-match patients, the risk of all-cause mortality was markedly lower in the probucol group than in the non-probucol group (hazard ratio = 0.45; p = 0.002). Although probucol use was not an independent predictor of long-term survival from cardiac death, cardiac death tended to be lower, whereas noncardiac death was significantly reduced in the probucol group than in the non-probucol group. Thus, probucol was demonstrated to significantly reduce all-cause mortality in CHD patients following complete revascularization.
A multicenter, randomized controlled trial (PICASSO)46) has recently been reported; it evaluated the efficacy and safety of cilostazol versus aspirin, with and without probucol, in patients with ischemic stroke with a high risk of cerebral hemorrhage. In this randomized, controlled, 2 × 2 factorial trial, patients with ischemic stroke with a history of or imaging findings of intracerebral hemorrhage or two or more microbleeds were enrolled in three Asian countries. Patients were randomly assigned to receive, 1) cilostazol (200 mg/day), 2) aspirin (100 mg/day), 3) cilostazol plus probucol (500 mg/day), or 4) aspirin plus probucol. The co-primary outcomes were the incidence of a composite of stroke, myocardial infarction, or vascular death and the incidence of hemorrhagic stroke. Interestingly, the incidence of vascular events was significantly lower in the probucol group than in the non-probucol group (hazard ratio 0.69; 95% CI 0.50 to 0.97; p = 0.0316). In patients with ischemic stroke at high risk of cerebral hemorrhage, cilostazol was not inferior to aspirin for the prevention of cardiovascular events, but it did not reduce the risk of hemorrhagic stroke. Thus, probucol on top of aspirin or cilostazol may be beneficial to reduce the incidence of cardiovascular events in patients with ischemic stroke.
Another important finding in the current study may be that the reduction of serum HDL-C by probucol does not increase cerebrovascular and cardiovascular events, but rather it tended to decrease them. After the report of PQRST21), the reduction of serum HDL-C by probucol was supposed to be harmful and probucol disappeared from the market in the United States and Europe. PQRST was performed in Sweden to investigate whether 3 years' treatment of hypercholesterolemic patients with probucol may affect femoral atherosclerosis. The primary end point was the change in atheroma volume estimated as a change in lumen volume of femoral artery assessed by quantitative arteriography. Probucol-treated patients showed 12% lower LDL-C and 24% lower HDL-C levels than did control subjects, but no significant change was observed in lumen volume between the probucol-treated and control groups. However, changes in atheroma volume may occur without changes in the lumen because of vessel remodeling. Therefore, we should be very careful when we evaluate the results of PQRST since probucol may retard the compensatory remodeling observed in de novo atherosclerosis in apolipoprotein E-deficient mice because of the inhibition of macrophage accumulation and decrease in MMP-2 and MMP-9 47). Furthermore, regression of xanthoma or xanthelasma may be the most well-established effect of probucol11). A close correlation was observed between the extent of regression in Achilles tendon xanthoma and the reduction of serum HDL-C levels in patients with heterozygous FH48). Probucol may also prevent lipid storage in macrophages by suppressing the uptake and stimulating the release of cholesterol and other lipids from macrophages49, 50). Thus, the reduction of serum HDL-C by probucol may not be harmful but might rather suggest the enhancement of reverse cholesterol transport via increased CETP and hepatic SR-BI. Although the current study only showed a trend of probucol to reduce cerebrovascular and cardiovascular events, other previous studies using probucol have already shown antiatherogenic effects of probucol in high-risk patients9, 10, 22, 42). Therefore, administering probucol to reduce the residual risk on top of other LLTs such as statins might be an option for such patients.
Regarding the safety issues of probucol, no significant difference was observed in the frequencies of SAEs between the probucol-treated and control groups. Additionally, our data have shown no increase in ventricular arrhythmias in probucol-treated patients in spite of the fact that probucol significantly prolonged the QT interval. Similarly, previous studies including POSITIVE22), PICASSO46), and a study by Kasai et al.45) as well as those reviewed by Yamashita and Matsuzawa9) and Yamashita et al.10) demonstrated no significant increases in severe ventricular arrhythmia. Therefore, QT prolongation by probucol may not enhance the occurrence of lethal ventricular arrhythmias.
In conclusion, in the current PROSPECTIVE study, we could not demonstrate that probucol can significantly reduce cardiovascular events in CHD patients treated with statins, but probucol showed a tendency to lower cardiovascular events. We could also demonstrate that probucol treatment in the high-risk patients treated with statins was well tolerated and safe despite a marked reduction of serum HDL-C due to enhancement of reverse cholesterol transport. The results of IMPACT Trial27) in patients with CHD performed in Korea and China with a similar protocol has been published in the same issue of J Atheroscler Thromb. However, the primary end point of IMPACT is the changes in carotid IMT, and the secondary end point is the rate of cerebrovascular and cardiovascular events. A meta-analysis of PROSPECTIVE and IMPACT in the future may elucidate whether probucol on top of conventional LLTs could prevent cerebrovascular and cardiovascular events in patients with CHD.
Acknowledgements
Shizuya Yamashita, Hidenori Arai, Hideaki Bujo, Daisaku Masuda, and Tohru Ohama were all involved in the planning and management of the study. Toshiyuki Ishibashi, Koji Yanagi, and Yasuji Doi played an important role in the recruitment of patients. Satoshi Nakagawa and Koichi Yamashiro were engaged in the management of patient recruitment and communications with participating hospitals. Kenichiro Tanabe performed statistical analyses. Masanori Fukushima was responsible for all the performances at Translational Research Center for Medical Innovation (TRI), Foundation for Biomedical Research, and Innovation at Kobe, Kobe, Hyogo, Japan. Toru Kita, Masunori Matsuzaki, Yasushi Saito, and Yuji Matsuzawa contributed as an advisory to the promotion of this study. The authors (greatly) appreciate the editorial review of the manuscript by Mikio Yoshidomi and Hideaki Kaneda at the TRI (Kobe, Japan). The TRI undertook the data management, statistical analysis, and site management. This study was funded by the Foundation for Biomedical Research and Innovation at Kobe, a third-party organization that is independent of investigators' institution and was responsible for onsite monitoring in addition to data collection, data management, and data analysis. The Foundation for Biomedical Research and Innovation at Kobe has received a research grant from several pharmaceutical companies (AstraZeneca K.K., Dai-ichi Sankyo Co, Ltd., Astellas Pharma Inc., Novartis Pharma K.K., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Sanofi K.K.), which was not specific for this study. This study was partly supported by the members of Japan Atherosclerosis Society for patient recruitment.
Conflict of Interest
Dr. Yamashita reports grants and personal fees from Kowa Company, Ltd., Bayer Yakuhin, Ltd., MSD K.K., Takeda Pharmaceutical Company, Ltd., Astellas Pharma Inc., grants from Kyowa Medex Co., Ltd., Hayashibara Co., Ltd., and personal fees from Skylight Biotec, Inc., Astellas Amgen, Sanofi, Aegerion, outside the submitted work. Dr. Arai reports personal fees from MSD, Pfizer, Kowa, Daiichi Sankyo, Astellas, during the conduct of the study. Dr. Bujo has nothing to disclose. Dr. Masuda reports grants and personal fees from Nippon Boehringer Ingelheim Co., Ltd., MSD K.K., Takeda Pharmaceutical Company, Ltd., Daiichi-Sankyo Company, Ltd., Mochida Pharmaceutical Company, Ltd., Kowa Company Ltd., Kowa Company Ltd., Kissei Pharmaceutical Co., Ltd.; grants from Otsuka Pharmaceutical Co Ltd.; personal fees from Kowa Company, Ltd., Bayer Yakuhin, Ltd, , Kyowa Medex Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Ono Pharmaceutical Company, Ltd., Astellas Pharma Inc., AstraZeneca K.K., non-financial support from Skylight Biotec, Inc., Pfizer Japan Inc., Amgen Astellas Biopharma K.K., Sanofi K.K., , grants from Shionogi & Co., Ltd., grants from Bayer Yakuhin, Ltd., grants from Sanwa Kagaku Kenkyusho Co., Ltd., grants from Astellas Pharma Inc., grants from Hayashibara Co., Ltd., Teijin Pharma Limited, Kaken Pharmaceutical Co., Ltd., outside the submitted work. Dr. Ohama reports grants from Daiichi Sankyo, MSD, outside the submitted work. Dr. Ishibashi and Dr. Yanagi have nothing to disclose. Dr. Doi is now also an employee (an occupational health physician) of Takeda Pharmaceutical Company Limited, Osaka, Japan. Mr. Nakagawa, Mr. Yamashiro, Mr. Tanabe, Dr. Kita, Dr. Matsuzaki, Dr. Saito and Dr. Fukushima have nothing to disclose. Dr. Matsuzawa reports personal fees from Teijin Pharma, Kowa, Sumitomo Dainippon Pharma, outside the submitted work.
Appendix
Inclusion Criteria
This study included male and female patients older than 20 years with all of the following eight clinical statuses:
diagnosis of dyslipidemia with high LDL-C level (≧ 140 mg/dL) without any medication;
treatment using any lipid-lowering drugs including statins for more than 8 weeks before providing informed consent;
serum LDL-C level less than 200 mg/dL within 8 weeks before providing informed consent, as calculated by Friedewald's formula (LDL-C = total cholesterol – HDL-cholesterol – triglycerides (TG)/5);
history of acute myocardial infarction or angina pectoris more than 3 months before providing informed consent, old myocardial infarction, CABG more than 3 months earlier, PCI more than 9 months earlier, or PCI with no restenosis that was diagnosed by follow-up coronary angiography at 6–9 months after PCI;
normal cardiac function and mild or moderate heart failure (NYHA classification I or II);
older than 20 years at the time of informed consent;
no severe hepatic and renal dysfunction (AST < 100 IU/L, ALT < 100 IU/L, and serum creatinine < 1.5 mg/dL) within 4 weeks before providing informed consent; and
signed written informed consent for participation in this study.
Exclusion Criteria
The exclusion criteria were the presence of the following clinical statuses at the time of informed consent:
ongoing treatment with probucol within 6 months before the time of informed consent;
ongoing treatment with cyclosporine;
history of hypersensitivity reactions to probucol
diagnosis of FH based on the NICE Clinical Guideline1);
very high TG level (> 400 mg/dL) within 8 weeks before providing informed consent;
markedly high HbA1c level (≧ 8%) on the most recent blood test;
frequent multifocal ventricular arrhythmia;
Af including paroxysmal Af;
long QTc interval on a resting electrocardiogram (> 450 ms in males or > 470 ms in females);
congestive heart failure (NYHA III or IV) or unstable angina;
participation in other clinical trials;
women who are pregnant, are lactating, might become pregnant, or wish to become pregnant within the study period; and
inappropriate candidate for participation as assessed by the doctors in the current study.
Serious Adverse Events (SAEs)
SAEs were designated as death, events that could lead to death, events that require hospital admission or extended hospitalization, disorders, events that could lead to disorders, others that are serious as well as the former adverse events, or congenital disease or abnormality in post-generations.
End Points
The primary efficacy end point was the presence and the time from registration until the first occurrence of cerebrovascular and cardiovascular events, as follows:
cardiovascular death including cardiac sudden death;
nonfatal myocardial infarction;
nonfatal cerebral stroke excluding transient ischemic attack;
hospital admission due to unstable angina;
hospital admission due to heart failure; and
all coronary revascularizations with either PCI or CABG.
The secondary efficacy and safety end points are as follows:
all death;
all cerebrovascular and cardiovascular disease;
event-free survival time;
levels of the mean IMT of carotid arteries and their changes;
levels of max IMT in common or internal carotid arteries and their changes; and
severe adverse events and their frequency.
Statistical Design and Analysis
As shown in the randomized prospective study of primary prevention for common carotid atherosclerosis in Japan using probucol, the Fukuoka Atherosclerosis Trial (FAST)2) (a randomized prospective study), the morbidity of cerebrovascular and cardiovascular events within 2 years of the observation period was 2.4% in the probucol-treated group, which was half that in the lipid-lowering agent-treated group (4.8%). At the same time, there are no secondary prevention studies in dyslipidemic patients who had a prior history of cardiovascular events in Japan, except for the report evaluating the effect of probucol on secondary prevention in patients with FH3). Therefore, when we referred to the result of the Japan EPA Lipid Intervention Study (JELIS)4) that compared the effect of eicosapentaenoic acid (EPA) in addition to statins on the secondary prevention of cardiovascular events, the morbidity of cardiovascular events in patients treated with statins (pravastatin or simvastatin) for the secondary prevention of cardiovascular diseases was 10.7% for 5 years.
On sample size consideration in the study, we assumed that the morbidity of cerebrovascular and cardiovascular events in the control group would be 10.7% on the basis of the results from the statin-treated group of the JELIS study. In the test group, we assume that the rate would be 5.4%, almost half of that in the statin-treated group because the addition of probucol to conventional LLT might improve the morbidity of cardiovascular events as well as the primary prevention shown in the FAST study2). On the condition, sample size per group was calculated as 408 patients with a two-tailed significance level of 0.05 and a power of 0.8 in 4 years' accrual period and 3 years' follow-up. Considering that several patients will be dropped out from the analysis, we decided that the target number of patients in one group would be 430 and total target number of patients in this study would be 860.
The patient population in statistical analysis was a full analysis set based on the intention-to-treat principle. The characteristics of patients' demographics and baseline values were compared between the two groups. For the primary efficacy end point, the presence of and the time from registration until the first cerebrovascular and cardiovascular events were analyzed. By estimating event-free survival curves using the Kaplan–Meier method and the 95% confidence interval at 1, 2, and 3 years, event-free survival curves for the two groups were compared using the log-rank test stratified with random allocation factors: LDL-C level (140 mg/dL and more vs. less than 140 mg/dL), diabetes (with vs. without), and hypertension (with vs. without). The secondary end points of the period until all death and period until all cardiovascular and cerebrovascular disease and event-free survival time were analyzed in the same way as the primary end point. Levels of the mean- and max-IMT of carotid arteries from baseline to 1, 2, and 3 years were analyzed by a mixed-effects model with repeated measurements with baseline value (included only if the change from baseline was the response variable), allocation group, visit, and interaction between allocation group and visit as fixed effects and patient as a random effect. Comparison of groups was performed. Frequency of SAEs was summarized by preferred term and intensity, and compared using Fisher's exact test. The two-tailed significant level in statistical analysis was 0.05. All statistical analyses were be done with SAS version 9.3.
Study Organization and Their Works
Executive Committee:
Principal Investigator: Shizuya Yamashita. Professor, Department of Cardiovascular Medicine, Department of Community Medicine, Osaka University Graduate School of Medicine, Osaka, Japan (Present address: Department of Cardiology, Rinku General Medical Center, Izumisano, Osaka, Japan)
Vice Principal Investigator:
Hidenori Arai. President, The National Center for Geriatrics and Gerontology, Obu, Japan.
Hideaki Bujo. Professor, Department of Clinical-Laboratory and Experimental-Research Medicine, Toho University, Sakura Medical Center, Sakura, Japan
The executive committee is working on the study design, execution, protocol amendments, and the supervision of the study. It must adjust the various problems that might occur during the execution of the study and review the process of the study at appropriate time points to maintain the safety of patients and the integrity of the study.
Project Director: Daisaku Masuda. Assistant Professor, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, Osaka, Japan (Present address: Department of Cardiology, Rinku General Medical Center, Izumisano, Osaka, Japan)
Project Manager: Tohru Ohama. Assistant Professor, Department of Dental Anesthesiology, Osaka University Graduate School of Dentistry, Suita, Japan
The project director manages the review of the protocol in the IRB of Osaka University Hospital, communicates the approved protocol to participating institutions, and addresses every unexpected complication that might occur during the execution of the study by making various adjustments for more appropriate progression.
Advisory Board:
Yuji Matsuzawa. President, Sumitomo Hospital, Osaka, Japan
Toru Kita. President, Kobe City Medical Center General Hospital, Kobe, Japan (Present address: President, Kobe City College of Nursing, Kobe, Hyogo, Japan)
Yasushi Saito. Professor Emeritus, Graduate School of Medicine, Chiba University, Chiba, Japan
Masunori Matsuzaki. Professor Emeritus, Yamaguchi University Graduate School of Medicine, Ube, Japan
The advisory board provides advice regarding the appropriate management and the scientific significance of the study.
Protocol Committee:
Yoji Nagai. President, Translational Research Center for Medical Innovation, Foundation for Biomedical Research and Innovation at Kobe, Japan
Mariko Harada-Shiba. Director, Department of Molecular Innovation in Lipidology, National Cerebral and Cardiovascular Center Research Institute, Suita, Japan
Tohru Ohama, Assistant Professor, Department of Dental Anesthesiology, Osaka University Graduate School of Dentistry, Suita, Japan
The protocol committee creates the protocol, examines the need for the protocol revision if the probability for protocol revision appears during the execution of the study, and reports it to the principal investigator.
Imaging Evaluation Committee:
Hiroyuki Daida. Professor, Department of Cardiology, Juntendo University School of Medicine, Tokyo, Japan
Masao Moroi. Associate Professor, Department of Cardiology, Toho University Ohashi Medical Center, Tokyo, Japan
Hiroshi Matsuo. President, Matsuo Clinic, Yao, Japan
Toshimasa Fujiwara. Medical Director, Chibaken Saiseikai Narashino Hospital, Narashino, Japan
Trial Statistician: Kenichiro Tanabe. Translational Research Center for Medical Innovation, Foundation for Biomedical Research and Innovation at Kobe, Kobe, Japan
Data Center: Translational Research Informatics Center, Foundation for Biomedical Research and Innovation at Kobe, Kobe, Japan
The study data center is independent of all committees and is planning to maintain and review all study data.
Efficacy and Safety Evaluation Committee:
Chairperson: Jun Sasaki*. Professor, Graduate School of Health and Welfare Sciences, International University of Health and Welfare Graduate School, Ohtawara, Japan
*Deceased
Seiji Umemoto. Deputy Director, Center for Clinical Research, Yamaguchi University Hospital, Ube, Japan
Shigeyuki Matsui. Professor, Department of Data Sciences, The Institute of Statistical Mathematics, Tachikawa, Japan
Hidenao Fukuyama. Professor, Human Brain Research Center, Graduate School of Medicine Kyoto University, Kyoto, Japan
The efficacy and safety evaluation committee evaluates the following reports to recommend early termination or study changes to the principal investigator: 1) the report of severe adverse events that is sent from the principal investigator at appropriate time points; 2) related reports from other studies such as papers and conference presentations at an appropriate time point; 3) the report of study progress from the data center every 3 months during the registration period or every 6 months after registration; and 4) total results of the safety information.
Cerebro- and Cardiovascular Events Evaluation Committee:
Takeshi Kimura. Professor, Department of Cardiovascular Medicine, Graduate School of Medicine, Kyoto University, Kyoto, Japan
Akira Sumitsuji. Associate Professor, Department of Advanced Cardiovascular Therapeutics, Osaka University Graduate School of Medicine, Suita, Japan
Kazuo Kitagawa. Professor, Department of Neurology, Graduate School of Medicine, Tokyo Women's Medical University, Tokyo, Japan
The cerebro- and cardiovascular events evaluation committee determines whether the reported problem corresponds to the cerebro- and cardiovascular event recognized as a primary end point in this study unless it appears to be clearly relevant.
Participating Faculties:
Tsutomu Saito (Saito Naika clinic), Daisuke Hotta (Hokkaido Cardiovascular Hospital), Takashi Saito (Akita Kousei Medical Center), Toshiyuki Ishibashi (Ohara Hospital), Megumu Kanno (Southern TOHOKU research Institute for Neuroscience), Eisuke Miura (The Hoshi General Hospital), Takayuki Owada (Japanese Red Cross Society Fukushima Hospital), Shunichi Murano (Tochigi Medical Center), Tadashi Suzuki (Fujioka General Hospital), Fumio Naganuma (Tsurugaya Hospital), Hiroshi Oshima (Hikari Hospital), Hiroshi Kamiyama (Kamiyama Clinic), Eiichi Okamoto (Okamoto Clinic), Yuichi Sato (Health Park Clinic), Takafumi Koga (Hidaka Hospital), Koichi Tomaru (Tomaru Naika Clinic), Tsuyoshi Uchiyama (Tsunoda Hospital), Etsuo Yamaguchi (Yamaguchi Naika Iin), Shinichi Momomura (Jichi Medical University Saitama Medical Center), Yoshio Kobayashi (Chiba University Hospital), Ichiro Tatsuno (Toho University Sakura Medical Center), Koichi Taira (Seirei Sakura Citizen Hospital), Kenya Yamazaki (Matsudo City General Hospital), Hirotoshi Ohmura (Juntendo University Hospital), Kaoru Sugi (Toho University Ohashi Medical Center), Seijiro Mori (Tokyo Metropolitan Geriatric Hospital), Akira Tanaka (Kobayashi Hospital), Hisao Hara (National Center for Global Health and Medicine), Atsushi Hirayama (Nihon University Itabashi Hospital), Masahiro Ishikawa (Nippon Medical School Musashi Kosugi Hospital), Tohru Minamino (Niigata University Medical & Dental Hospital), Masaharu Urakaze (Kamiichi General Hospital), Shinya Minatoguchi (Gifu University), Tomoya Onodera (Shizuoka City Shimizu Hospital), Harumitsu Yamamoto (National Hospital Organization Higashinagoya National Hospital), Toyoaki Murohara (Nagoya University Hospital), Satoru Usami (Taigenkai Hospital), Takashi Konishi (Japanese Red Cross Society Otsu Hospital), Takafumi Tsuji (Cardiovascular Medicine Kusatsu Heart Center), Shigeru Ikeguchi (Shiga General Hospital), Yutaka Tadano (Koto Memorial Hospital), Tsuneaki Kawashima (Tango Central Hospital), Masaharu Akao (National Hospital Organization Kyoto Medical Center), Koh Ono (Kyoto University Hospital), Yutaka Nagano (The Japan Baptist Hospital), Koji Yanagi (Kenporen Osaka Central Hospital), Yasuji Doi (Saiseikai Senri Hospital), Shizuya Yamashita (Osaka University Hospital), Daisaku Masuda (Sousei Hospital), Masaru Tanaka (Japanese Red Cross Society Osaka Hospital), Kunihiko Nagai (Ikeda City Hospital), Tsutomu Nakagawa (Toyonaka Municipal Hospital), Hisatoyo Hiraoka (Sumitomo Hospital), Yoshiyuki Nagai (Rinku General Medical Center), Mitsuo Matsuda (Kishiwada City Hospital), Yuzuru Takano (Japan Community Health care Organization Hoshigaoka Medical Center), Yoshihiro Yamamoto (Kansai Medical University Medecal Center), Shunichi Miyazaki (Kindai University Hospital), Ichiro Shiojima (Kansai Medical University Hospital), Masaharu Takeuchi (Medical Corporation Kyoujinkai Komatsu Hospital), Kenshi Fujii (Sakurabashi Watanabe Hospital), Mariko Harada-Shiba (National Cerebral and Cardiovascular Center), Takashi Yoshino (Suita Municipal Hospital), Ryuji Nohara (Kitano Hospital Tazuke Kofukai Medical Research Institute), Yukihiko Ueda (Hirakata Kohsai Hospital), Yasunori Ueda (Osaka Police Hospital), Kenichi Hirata (Kobe University Hospital), Takao Maruyama (Kawasaki Hospital), Yasunaka Makino (Hyogo Prefectural Nishinomiya Hospital), Shuichi Nozaki (Kawanishi City Hospital), Yutaka Furukawa (Kobe City Medical Center General Hospital), Yoshiki Takatsu (Hyogo Prefectural Amagasaki Hospital), Tohru Masuyama (Hyogo College of Medicine), Masaaki Uematsu (Kansai Rosai Hospital), Hideharu Akagi (Kinan Hospital), Setsu Ohara (Wakayama National Hospital), Toshiharu Kawamoto (National Hospital Organization Kure Medical Center), Genshi Egusa (Egusa Genshi Clinic), Yasuki Kihara (Hiroshima University Hospital), Takayuki Okamura (Yamaguchi University Hospital), Junichi Funada (National Hospital Organization Ehime Medical Center), Yasunori Sawayama (Japanese Red Cross Society Fukuoka Hospital)
References
- 1). Cholesterol Treatment Trialists' (CTT) Collaboration, Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A: Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet, 2015; 385: 1397-1405 [DOI] [PubMed] [Google Scholar]
- 2). Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE-IT Investigators: Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med, 2015; 372: 2387-2397 [DOI] [PubMed] [Google Scholar]
- 3). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators: Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med, 2017; 4; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 4). Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators: Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med, 2018; 379: 2097-2107 [Google Scholar]
- 5). Parthasarathy S, Young SG, Witztum JL, Pittman RC, Steinberg D: Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest, 1986; 77: 641-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Fruebis J, Gonzalez V, Silvestre M, Palinski W: Effect of probucol treatment on gene expression of VCAM-1, MCP-1, and M-CSF in the aortic wall of LDL receptor-deficient rabbits during early atherogenesis. Arterioscler Thromb Vasc Biol, 1997; 17: 1289-1302 [DOI] [PubMed] [Google Scholar]
- 7). Barnhart JW, Sefranka JA, McIntosh DD: Hypocholesterolemic effect of 4,4′-(isopropylidenedithio)-bis(2,6-di-t-butylphenol) (probucol). Am J Clin Nutr, 1970; 23: 1229-1233 [DOI] [PubMed] [Google Scholar]
- 8). Heel RC, Brogden RN, Speight TM, Avery GS: Probucol: a review of its pharmacological properties and therapeutic use in patients with hypercholesterolaemia. Drugs, 1978; 15: 409-428 [DOI] [PubMed] [Google Scholar]
- 9). Yamashita S, Matsuzawa Y: Where are we with probucol: a new life for an old drug? Atherosclerosis, 2009; 207: 16-23 [DOI] [PubMed] [Google Scholar]
- 10). Yamashita S, Masuda D, Matsuzawa Y: Did we abandon probucol too soon? Curr Opin Lipidol, 2015; 26: 304-316 [DOI] [PubMed] [Google Scholar]
- 11). Yamamoto A, Matsuzawa Y, Yokoyama S, Funahashi T, Yamamura T, Kishino B: Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol, 1986; 57: 29H-35H [DOI] [PubMed] [Google Scholar]
- 12). Naruszewicz M, Carew TE, Pittman RC, Witztum JL, Steinberg D: A novel mechanism by which probucol lowers low density lipoprotein levels demonstrated in the LDL receptor-deficient rabbit. J Lipid Res, 1984; 25: 1206-1213 [PubMed] [Google Scholar]
- 13). Durrington PN, Miller JP: Double-blind, placebo-controlled, cross-over trial of probucol in heterozygous familial hypercholesterolaemia. Atherosclerosis, 1985; 55: 187-194 [DOI] [PubMed] [Google Scholar]
- 14). Baker SG, Joffe BI, Mendelsohn D, Seftel HC: Treatment of homozygous familial hypercholesterolaemia with probucol. S Afr Med J, 1982; 62: 7-11 [PubMed] [Google Scholar]
- 15). Tawara K, Tomikawa M, Abiko Y: Mode of action of probucol in reducing serum cholesterol in mice. Jpn J Pharmacol, 1986; 40: 123-133 [DOI] [PubMed] [Google Scholar]
- 16). Cristol LS, Jialal I, Grundy SM: Effect of low-dose probucol therapy on LDL oxidation and the plasma lipoprotein profile in male volunteers. Atherosclerosis, 1992; 97: 11-20 [DOI] [PubMed] [Google Scholar]
- 17). Franceschini G, Sirtori M, Vaccarino V, Gianfranceschi G, Rezzonico L, Chiesa G, Sirtori CR: Mechanisms of HDL reduction after probucol. Changes in HDL subfractions and increased reverse cholesteryl ester transfer. Arteriosclerosis, 1989; 9: 462-469 [DOI] [PubMed] [Google Scholar]
- 18). Hirano K, Ikegami C, Tsujii K, Zhang Z, Matsuura F, Nakagawa-Toyama Y, Koseki M, Masuda D, Maruyama T, Shimomura I, Ueda Y, Yamashita S: Probucol enhances the expression of human hepatic scavenger receptor class B type I, possibly through a species-specific mechanism. Arterioscler Thromb Vasc Biol, 2005; 25: 2422-2427 [DOI] [PubMed] [Google Scholar]
- 19). Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C: Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A, 1987; 84: 5928-5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Bräsen JH, Koenig K, Bach H, Kontush A, Heinle H, Witting PK, Ylä-Herttuala S, Stocker R, Beisiegel U: Comparison of the effects of alpha-tocopherol, ubiquinone-10 and probucol at therapeutic doses on atherosclerosis in WHHL rabbits. Atherosclerosis, 2002; 163: 249-259 [DOI] [PubMed] [Google Scholar]
- 21). Walldius G, Erikson U, Olsson AG, Bergstrand L, Hådell K, Johansson J, Kaijser L, Lassvik C, Mölgaard J, Nilsson S, Schäfer-Elinder L, Stenport G, Holme I: The effect of probucol on femoral atherosclerosis: the Probucol Quantitative Regression Swedish Trial (PQRST). Am J Cardiol, 1994; 74: 875-883 [DOI] [PubMed] [Google Scholar]
- 22). Yamashita S, Bujo H, Arai H, Harada-Shiba M, Matsui S, Fukushima M, Saito Y, Kita T, Matsuzawa Y: Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb, 2008; 15: 292-303 [DOI] [PubMed] [Google Scholar]
- 23). Yamashita S, Masuda D, Ohama T, Arai H, Bujo H, Kagimura T, Kita T, Matsuzaki M, Saito Y, Fukushima M, Matsuzawa Y; PROSPECTIVE Study Group: Rationale and design of the PROSPECTIVE trial: Probucol Trial for Secondary Prevention of Atherosclerotic Events in Patients with Prior Coronary Heart Disease. J Atheroscler Thromb, 2016; 23: 746-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Marks D, Thorogood M, Neil HA, Humphries SE: A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis, 2003; 168: 1-14 [DOI] [PubMed] [Google Scholar]
- 25). Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K; Japan EPA lipid intervention study (JELIS) Investigators: Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]
- 26). Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, Kashiwagi S, Hayashi J: Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol, 2002; 39: 610-616 [DOI] [PubMed] [Google Scholar]
- 27). Kang HJ, Kim MH, Sung J, Kim SH, Kim CH, Park JE, Ge J and Oh BH: On behalf of IMPACT on IMT investigators: Effect of probucol and/or cilostazol on carotid intima media thickness in patients with coronary heart disease: a randomized, multicenter, multinational study. J Atheroscler Thromb, 2021; 28: 124-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Sear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B; ILLUMINATE Investigators: Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med, 2007; 357: 2109-2122 [DOI] [PubMed] [Google Scholar]
- 29). Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS; dal-OUTCOMES Investigators: Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med, 2012; 367: 2089-2099 [DOI] [PubMed] [Google Scholar]
- 30). Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE; ACCELERATE Investigators: Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med, 2017; 376: 1933-1942 [DOI] [PubMed] [Google Scholar]
- 31). HPS3/TIMI55-REVEAL Collaborative Group, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ: Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med, 2017; 377: 1217-1227 [DOI] [PubMed] [Google Scholar]
- 32). Krishna R, Gheyas F, Liu Y, Hagen DR, Walker B, Chawla A, Cote J, Blaustein RO, Gutstein DE: Chronic administration of anacetrapib is associated with accumulation in adipose and slow elimination. Clin Pharmacol Ther, 2017; 102: 832-840 [DOI] [PubMed] [Google Scholar]
- 33). Ishigami M, Yamashita S, Sakai N, Arai T, Hirano K, Hiraoka H, Kameda-Takemura K, Matsuzawa Y: Large and cholesteryl ester-rich high-density lipoproteins in cholesteryl ester transfer protein (CETP) deficiency can not protect macrophages from cholesterol accumulation induced by acetylated low-density lipoproteins. J Biochem, 1994; 116: 257-262 [DOI] [PubMed] [Google Scholar]
- 34). Franceschini G, Chiesa G, Sirtori CR: Probucol increases cholesteryl ester transfer protein activity in hypercholesterolaemic patients. Eur J Clin Invest, 1991; 21: 384-388 [DOI] [PubMed] [Google Scholar]
- 35). Hong SC, Zhao SP, Wu ZH: Effect of probucol on HDL metabolism and class B type I scavenger receptor (SR-BI) expression in the liver of hypercholesterolemic rabbits. Int J Cardiol, 2007; 115: 29-35 [DOI] [PubMed] [Google Scholar]
- 36). Miida T, Seino U, Miyazaki O, Hanyu O, Hirayama S, Saito T, Ishikawa Y, Akamatsu S, Nakano T, Nakajima K, Okazaki M, Okada M: Probucol markedly reduces HDL phospholipids and elevated prebeta1-HDL without delayed conversion into alpha-migrating HDL: putative role of angiopoietin-like protein 3 in probucol-induced HDL remodeling. Atherosclerosis, 2008; 200: 329-335 [DOI] [PubMed] [Google Scholar]
- 37). Yamashita S, Matsuzawa Y: Re-evaluation of cholesteryl ester transfer protein function in atherosclerosis based upon genetics and pharmacological manipulation. Curr Opin Lipidol, 2016; 27: 459-472 [DOI] [PubMed] [Google Scholar]
- 38). Yamashita S, Ruscica M, Macchi C, Corsini A, Matsuzawa Y, Sirtori CR: Cholesteryl ester transfer protein: An enigmatic pharmacology - Antagonists and agonists. Atherosclerosis, 2018; 278: 286-298 [DOI] [PubMed] [Google Scholar]
- 39). Ishigami M, Yamashita S, Sakai N, Hirano K, Arai T, Maruyama T, Takami S, Koyama M, Kameda-Takemura K, Matsuzawa Y: High-density lipoproteins from probucol-treated patients have increased capacity to promote cholesterol efflux from mouse peritoneal macrophages loaded with acetylated low-density lipoproteins. Eur J Clin Invest, 1997; 27: 285-292 [DOI] [PubMed] [Google Scholar]
- 40). Inagaki M, Nakagawa-Toyama Y, Nishida M, Nakatani K, Nakaoka H, Kawase M, Kawase R, Tsubakio-Yamamoto K, Masuda D, Ohama T, Matsuyama A, Ishigami M, Komuro I, Yamashita S: Effect of probucol on antioxidant properties of HDL in patients with heterozygous familial hypercholesterolemia. J Atheroscler Thromb, 2012; 19: 643-656 [DOI] [PubMed] [Google Scholar]
- 41). Zucoloto AZ, Manchope MF, Staurengo-Ferrari L, Pinho-Ribeiro FA, Zarpelon AC, Saraiva ALL, Cecílio NT, Alves-Filho JC, Cunha TM, Menezes GB, Cunha FQ, Casagrande R, Verri WA Jr: Probucol attenuates lipopolysaccharide-induced leukocyte recruitment and inflammatory hyperalgesia: effect on NF-κB activation and cytokine production. Eur J Pharmacol, 2017; 809: 52-63 [DOI] [PubMed] [Google Scholar]
- 42). Li T, Chen W, An F, Tian H, Zhang J, Peng J, Zhang Y, Guo Y: Probucol attenuates inflammation and increases stability of vulnerable atherosclerotic plaques in rabbits. Tohoku J Exp Med, 2011; 225: 23-34 [DOI] [PubMed] [Google Scholar]
- 43). Yoshikawa T, Mitani K, Kotosai K, Nozako M, Miyakoda G, Yabuuchi Y: Antiatherogenic effects of cilostazol and probucol alone, and in combination in low density lipoprotein receptor-deficient mice fed with a high fat diet. Horm Metab Res, 2008; 40: 473-478 [DOI] [PubMed] [Google Scholar]
- 44). Li S, Liang J, Niimi M, Bilal Waqar A, Kang D, Koike T, Wang Y, Shiomi M, Fan J: Probucol suppresses macrophage infiltration and MMP expression in atherosclerotic plaques of WHHL rabbits. J Atheroscler Thromb, 2014; 21: 648-658 [DOI] [PubMed] [Google Scholar]
- 45). Kasai T, Miyauchi K, Kubota N, Kajimoto K, Amano A, Daida H: Probucol therapy improves long-term (> 10-year) survival after complete revascularization: a propensity analysis. Atherosclerosis, 2012; 220: 463-469 [DOI] [PubMed] [Google Scholar]
- 46). Kim BJ, Lee EJ, Kwon SU, Park JH, Kim YJ, Hong KS, Wong LKS, Yu S, Hwang YH, Lee JS, Lee J, Rha JH, Heo SH, Ahn SH, Seo WK, Park JM, Lee JH, Kwon JH, Sohn SI, Jung JM, Navarro JC, Kang DW; PICASSO investigators: Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): a multicentre, randomised controlled trial. Lancet Neurol, 2018; 17: 509-518 [DOI] [PubMed] [Google Scholar]
- 47). Wu BJ, Di Girolamo N, Beck K, Hanratty CG, Choy K, Hou JY, Ward MR, Stocker R: Probucol [4,4′-[(1-methylethylidene) bis(thio)]bis-[2,6-bis(1,1-dimethylethyl)phenol]] inhibits compensatory remodeling and promotes lumen loss associated with atherosclerosis in apolipoprotein E-deficient mice. J Pharmacol Exp Ther, 2007; 321: 477-484 [DOI] [PubMed] [Google Scholar]
- 48). Matsuzawa Y, Yamashita S, Funahashi T, Yamamoto A, Tarui S: Selective reduction of cholesterol in HDL2 fraction by probucol in familial hypercholesterolemia and hyperHDL2 cholesterolemia with abnormal cholesteryl ester transfer. Am J Cardiol, 1988; 62: 66B-72B [DOI] [PubMed] [Google Scholar]
- 49). Yamamoto A, Takaichi S, Hara H, Nishikawa O, Yokoyama S, Yamamura T, Yamaguchi T: Probucol prevents lipid storage in macrophages. Atherosclerosis, 1986; 62: 209-217 [DOI] [PubMed] [Google Scholar]
- 50). Yamamoto A, Matsuzawa Y, Yokoyama S, Funahashi T, Yamamura T, Kishino B: Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol, 1986; 57: 29H-35H [DOI] [PubMed] [Google Scholar]
References of Appendix
- 1). Marks D, Thorogood M, Neil HA, Humphries SE: A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis, 2003; 168: 1-14 [DOI] [PubMed] [Google Scholar]
- 2). Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, Kashiwagi S, Hayashi J: Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST). J Am Coll Cardiol, 2002; 39: 610-616 [DOI] [PubMed] [Google Scholar]
- 3). Yamashita S, Bujo H, Arai H, Harada-Shiba M, Matsui S, Fukushima M, Saito Y, Kita T, Matsuzawa Y: Long-term probucol treatment prevents secondary cardiovascular events: a cohort study of patients with heterozygous familial hypercholesterolemia in Japan. J Atheroscler Thromb, 2008; 15: 292-303 [DOI] [PubMed] [Google Scholar]
- 4). Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K; Japan EPA lipid intervention study (JELIS) Investigators: Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet, 2007; 369: 1090-1098 [DOI] [PubMed] [Google Scholar]


