Highlights
-
•
Companion diagnostics and precision medicine.
-
•
FDA approved companion diagnostics.
-
•
Drug-diagnostic codevelopment.
-
•
Biomarkers, drugs, clinical indications, and analytical platforms.
-
•
Regulatory paths and status.
Keywords: Companion diagnostics, Biomarkers, Premarket application, Laboratory developed test, Precision medicine, Personalized medicine
Abstract
Predictive biomarker is an important element in the realization of precision medicine and with the introduction of the drug-diagnostic codevelopment model, the number of regulatory approved companion diagnostics (CDx) have steadily increased. This short perspective is based on an analysis of the FDA List of Cleared or Approved Companion Diagnostic Devices and focus on the biomarkers, drugs, clinical indications, analytical platforms, regulatory paths and status related to the different assays. By the end of 2020, the total number of CDx assays approved by the FDA had reached 44. These assays are almost exclusively linked to different hematological and oncological drugs. Without an accurate and reliable CDx assay these drugs will lose their value. The analytical platforms are diverse and cover technologies like immunohistochemistry, in situ hybridization, polymerase chain reaction, next generation sequencing, and imaging. CDx assays are high risk devices and the regulatory path almost exclusively requires submission of a Premarket Application (PMA); however, a relatively large group of the CDx assays is PMA approved Laboratory Developed Test.
Introduction
With the development of trastuzumab (Herceptin), a new era in drug development began. Not only was it a scientific and medical achievement but it also paved the way for the drug-diagnostic codevelopment model, where a predictive biomarker assay is developed in parallel to the drug [1, 2]. In September 1998, the Food and Drug Administration (FDA) simultaneously granted approval to trastuzumab and the HER2 immunohistochemical (IHC) assay, HercepTest (Dako), through a new coordinated procedure [3]. Without an accurate and reliable companion diagnostics (CDx) assay, most targeted anti-cancer drugs lose their value. Over the past more than 20 years, an increasing number of drugs have been developed and launched with a predictive biomarker assay, and the number of FDA approved CDx assays have steadily increased [3]. In this short perspective, an analysis based on the FDA List of Cleared or Approved Companion Diagnostic Devices is presented [4]. This analysis focus on the biomarkers, drugs, clinical indications, analytical platforms/technologies, regulatory paths and status related to the different CDx assays.
FDA approved companion diagnostics
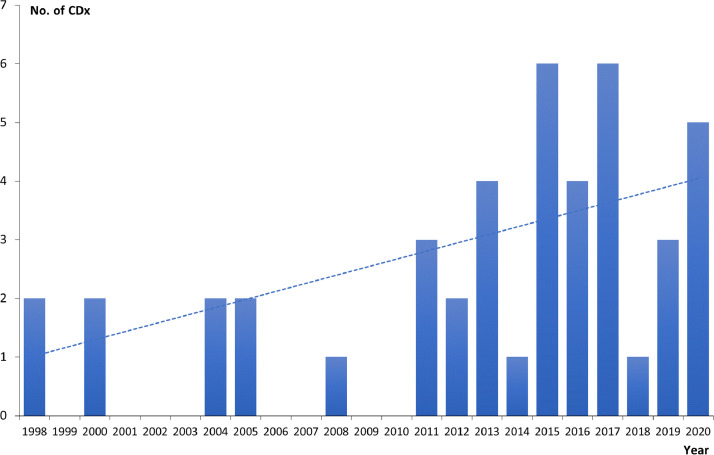
In 2014, the FDA issued a regulatory guidance document on CDx, which defines this type of assay as an in vitro diagnostic device (IVD) that provides information that is essential for the safe and effective use of a corresponding therapeutic product. The use of a CDx is stipulated in both the assay instructions for use and in the labeling of the corresponding therapeutic product, including the labeling of any generic equivalents of the therapeutic product [5]. This means that testing with a CDx assay is mandatory and must be performed before the specific therapeutic product can be prescribed to the patient. At the end of 2020, the total number of CDx assays approved by the FDA was 44 [4]. Fig. 1 shows the CDx approvals by year since the first approval in 1998. Up to and including 2010, the FDA had only approved 9 CDx assays but since then, the development has taken off with 35 approvals for the past 10 years. Nearly all these assays are linked to hematological and oncological drugs and reflects the increased number of targeted anti-cancer therapies approved in the same period of time. Fig. 1 also emphasizes the trend observed over the past more than 20 years with respect to a more frequent use of the drug-diagnostic codevelopment model in anti-cancer drug development [6].
Fig. 1.
FDA approval of companion diagnostic (CDx) assays by year. The total number of approvals by the end of 2020 are 44.
An overview of biomarkers, drugs, and indications linked to the FDA approved CDx assays is found in Table 1. So far, the drug-diagnostic codevelopment model has largely been based on a ‘one drug one biomarker’ scenario, which is also the situation for most of the drugs listed in the Table 1. Deferasirox (Exjade) is the only non-anti-cancer drug listed, which is an iron chelator indicated for treatment of patients with non-transfusion-dependent thalassemia. The assay linked to this drug is FerriScan, which the FDA describe as a liver iron concentration imaging CDx [4]. The efficacy of the different drug-diagnostic combinations with respect to clinical outcome can be found in the Prescribing Information for the individual drugs, under the Clinical Studies section [4,7].
Table 1.
List of biomarkers, drugs, and indications for the FDA approved companion diagnostics [4].
| Biomarker | Drugs* | Indications |
|---|---|---|
| ALK/ALK | Alectinib Brigatinib Ceritinib Crizotinib |
Non-small cell lung cancer |
| BCR-ABL1 | Nilotinib | Chronic myeloid leukemia |
| BRAF | Cobimetinib Dabrafenib Encorafenib Trametinib Vemurafenib |
Colorectal cancer Melanoma Non-small cell lung cancer |
| BRCA1/BRCA2 | Niraparib Olaparib Rucaparib Talazoparib |
Breast cancer Ovarian cancer Pancreatic cancer Prostate cancer |
| EGFR/EGFR | Afatinib Cetuximab Dacomitinib Erlotinib Gefitinib Osimertinib Panitumumab |
Colorectal cancer Non-small cell lung cancer |
| EZH2 | Tazemetostat | Follicular lymphoma tumor |
| FGFR2 | Pemigatinib | Cholangiocarcinoma |
| FGFR3 | Erdafitinib | Urothelial cancer |
| FLT3 | Midostaurin Gilteritinib |
Acute myelogenous leukemia |
| HER2/HER2 | Trastuzumab Pertuzumab Trastuzumab emtansine |
Breast cancer Gastric and gastroesophageal cancer |
| HRR | Olaparib | Prostate cancer |
| IDH1 | Ivosidenib | Acute myeloid leukemia |
| IDH2 | Enasidenib | Acute myeloid leukemia |
| c-KIT | Imatinib | Gastrointestinal stromal tumors |
| KIT | Imatinib | Aggressive systemic mastocytosis |
| MET | Capmatinib | Non-small cell lung cancer |
| NTRK1/2/3 | Larotrectinib | Solid tumors with NTRK gene fusion |
| PDGFRB | Imatinib | Myelodysplastic syndrome/Myeloproliferative Disease |
| PIK3CA | Alpelisib | Breast cancer |
| PD-L1 | Atezolizumab Nivolumab Nivolumab + Ipilimumab Pembrolizumab |
Breast cancer Cervical cancer Esophageal squamous cell carcinoma Gastric or gastroesophageal junction adenocarcinoma Head and neck squamous cell carcinoma Non-small cell lung cancer Triple-Negative Breast Carcinoma Urothelial carcinoma |
| RAS (KRAS/NRAS) | Cetuximab Panitumumab |
Colorectal cancer |
| RET | Pralsetinib | Non-small cell lung cancer |
| ROS1 | Crizotinib | Non-small cell lung cancer |
| Software for MRI | Deferasirox | Nontransfusion-dependent thalassemia |
| TMB-H | Pembrolizumab | Solid tumors with mutational burden-high (TMB-H) |
| TP53 | Venetoclax | B-cell chronic lymphocytic leukemia |
Not all drugs are regulatory approved for the listed indications. Further information on the indications for the individual drug is found at: https://www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools.
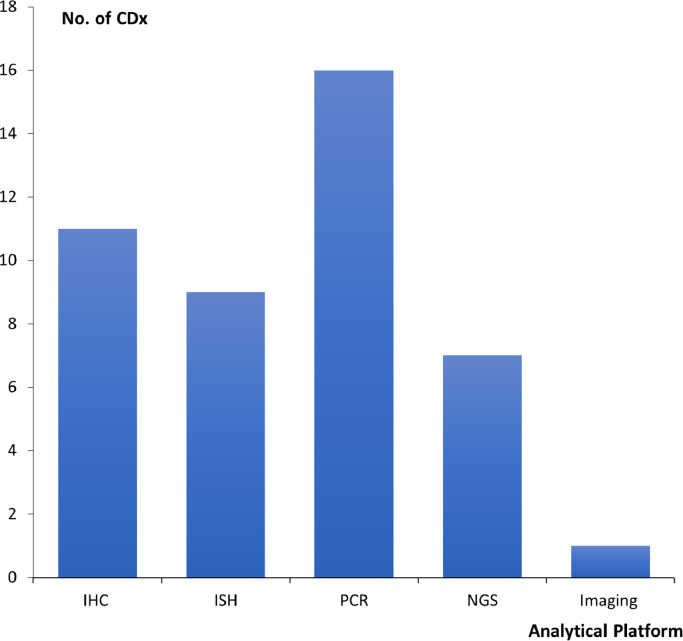
Looking at the analytical platforms for the different CDx assays, IHC and in situ hybridization (ISH) were the dominating technologies until 2011, when the first CDx based on the polymerase chain reaction (PCR) method was approved, namely the cobas 4800 BRAF V600 Mutation Test (Roche Molecular Systems). This is an assay used for detection of the BRAF V600E mutation in patients with melanoma, who might be candidates for treatment with vemurafenib (Zelboraf) [4]. So far, the PCR technology makes up the largest group of CDx assays. As it appears from Fig. 2, a total of 16 PCR based assays have obtained approval, which correspond to 36% of all the FDA approved CDx assays.
Fig. 2.
FDA approved companion diagnostic (CDx) assays by analytical platform/technology (N = 44). IHC, Immunohistochemistry; ISH, in situ hybridization; PCR, polymerase chain reaction; NGS, next generation sequencing.
Within the last five years, next generation sequencing (NGS) has also made its way as a CDx platform. The first assay based on this technology to be approved by the FDA was the FoundationFocus CDxBRCA Assay (Foundation Medicine), which is an assay for detection of BRCA1 and BRCA2 alterations in tumor tissue from patients with ovarian cancer, who might be candidates for treatment with rucaparib (Rubraca). Currently, seven different NGS based assays have been approved by the FDA as CDx, as shown in Fig. 2.
Within the last couple of year, we have seen tumor agnostic drugs been approved by the FDA, such as larotrectinib (Vitrakvi, Bayer/ Loxo Oncology) and recently, the FoundationOne CDx assay (Foundation Medicine) obtained approval for testing of NTRK gene fusion in patients with solid tumors [8,4]. Similarly, for pembrolizumab (Keytruda) in relation to the indication of high tumor mutational burden and here, the FoundationOne CDx assay has likewise obtained FDA approval for testing of patients with solid tumors [9,4].
Regulatory paths and status
Most CDx assays are high-risk devices and classified as Class III, which requires submission of a Premarket Application (PMA). Compared to other types of IVD submissions such as a 510(k), the PMA requires the most comprehensive documentation level, which further underlines the critical role of CDx assays as treatment decision tools. Submission of a PMA has also been the situation for nearly all the FDA approved CDx assays, except for a few, which include the MRDx BCR-ABL Test and FerriScan, both of which are Class II devices. The MRDx BCR-ABL Test (MolecularMD Corporation) is a PCR based assay for detection of BCR-ABL1 transcripts in chronic myeloid leukemia patients, who might be candidates for treatment with nilotinib (Tasigna). The approval of the MRDx BCR-ABL Test and FerriScan was based on a 510(k) Application [4]. Furthermore, the two CDx assays, KIT D816V Mutation Detection by PCR for Gleevec Eligibility (ARUP Laboratories) and PDGFRB FISH for Gleevec Eligibility (ARUP Laboratories) have both been approved through a humanitarian device exemption (HDE) application. An approval according to the HDE program follows a similar thinking as known from the Orphan Drug Act and is reserved for rare diseases or conditions with a low patient prevalence [10].
Several of the CDx assays on the FDA List of Cleared or Approved Companion Diagnostic Devices are laboratory developed test (LDT). In spite of this, they have all been cleared through submission of a PMA. A total of nine CDx assays, mainly PCR or NGS based, are LDT and listed in Table 2. For all these assays, it is clearly stated in the intended use that the assay can only be used at a single specified laboratory site [4].
Table 2.
Laboratory Developed Test (LDT) assays approved by the FDA as companion diagnostic (CDx).
| LDT CDx Assay | Platform/Technology |
|---|---|
| BRACAnalysis CDx (Myriad Genetic Laboratories) | PCR |
| KIT D816V Mutation Detection by PCR for Gleevec Eligibility (ARUP Laboratories)* | PCR |
| PDGFRB FISH for Gleevec Eligibility (ARUP Laboratories)* | FISH |
| FoundationFocus CDxBRCA Assay (Foundation Medicine) | NGS |
| FoundationOne CDx (Foundation Medicine) | NGS |
| LeukoStrat CDx FLT3 Mutation Assay (Invivoscribe Technologies) | PCR |
| Myriad myChoice CDx (Myriad Genetic Laboratories) | NGS |
| Guardant360 CDx (Guardant Health) | NGS |
| FoundationOne Liquid CDx (Foundation Medicine) | NGS |
Abbreviations: FISH, fluorescence in situ hybridization; PCR, polymerase chain reaction; NGS, next generation sequencing.
Approved through a humanitarian device exemption.
Conclusion
Predictive biomarker is an important element in the realization of precision medicine but looking at the number of CDx assays approved by the FDA over the last more than 20 years, it is relatively modest. However, within the past 8–10 years, the number of CDx assays has increased, which is a trend expected to continue in the years to come. CDx assays are classified as high-risk devices, which requires substantial documentations for its clinical predictive properties and assay quality, which might be one of the reasons for the relatively low number of FDA approvals.
CRediT authorship contribution statement
Jan Trøst Jørgensen: Conceptualization, Methodology, Software, Data curtion, Writing – original draft, Visualization, Writing – review & editing.
Declaration of Competing Interest
Jan Trøst Jørgensen has worked as a consultant for Agilent Technologies, Euro Diagnostica, Oncology Venture, Azanta, Alligator Biosciences, and Leo Pharma and has given lectures at meetings sponsored by AstraZeneca, Merck Sharp & Dohme, and Roche.
References
- 1.Hayes D.F. HER2 and Breast Cancer - A Phenomenal Success Story. N. Engl. J. Med. 2019;381:1284–1286. doi: 10.1056/NEJMcibr1909386. [DOI] [PubMed] [Google Scholar]
- 2.Sawyers C.L. Herceptin: a first assault on oncogenes that launched a revolution. Cell. 2019;179:8–12. doi: 10.1016/j.cell.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Jørgensen J.T. Companion and complementary diagnostics: clinical and regulatory perspectives. Trends Cancer. 2016;2:706–712. doi: 10.1016/j.trecan.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 4.FDA. List of cleared or approved companion diagnostic devices (In Vitro and Imaging Tools). Update: 11/16/2020. ( https://www.fda.gov/medical-devices/vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-vitro-and-imaging-tools ). Accessed January 12, 2021
- 5.FDA. Guidance for industry and food and drug administration staff. In vitro companion diagnostic devices. 2014 ( https://www.fda.gov/media/81309/download ). Accessed January 14, 2021
- 6.Jørgensen J.T., Hersom M. Clinical and regulatory aspects of companion diagnostic development in oncology. Clin. Pharmacol. Ther. 2018;103:999–1008. doi: 10.1002/cpt.955. [DOI] [PubMed] [Google Scholar]
- 7.FDA. Drugs@FDA: FDA-Approved Drugs. (https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=206995). Accessed February 17, 2021.
- 8.Drilon A., Laetsch T.W., Kummar S. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marabelle A., Fakih M., Lopez J. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 10.FDA. Humanitarian Device Exemption. Update: 09/05/2019 (https://www.fda.gov/medicaldevices/deviceregulationandguidance/howtomarketyourdevice/premarketsubmissions/humanitariandeviceexemption/default.htm). Accessed January 15, 2021.