Abstract
3D printing is an evolving technology which has a potential application in the treatment pediatric forearm fractures. Very little has been published with regard to 3D casting in children. We present two cases in which upper extremity fractures in pediatric patients were treated by wearing a custom made 3D printed cast. At latest follow-up at least one year post-injury, the clinical outcomes were excellent.
Orthopaedic surgeons may benefit from familiarizing themselves with the potential of 3D printing technology and utilizing its current applications, as well as devising future applications, in clinical practice.
Key Words: Immobilization, Innovation, 3D printing, Pediatric, Trauma
Introduction
Additive manufacturing or three-dimensional (3D) printing represents a process by which objects are created in a layer by layer fashion. While 3D printing was developed in the 1980’s, the medical application of this technology has started to blossom in recent years. The field of musculoskeletal medicine is particularly suited for this technology, and current applications include the generation of orthotics, prosthetics, and custom total joint arthroplasty implants, among others (1). While the technology is presently available, there is little data related to the use of 3D printed orthotics in the clinical setting for upper extremity pediatric fractures. We present two cases of pediatric patients treated with custom made 3D printed casts.
Case presentation
Statement of Consent
A parent for each patient was informed that data concerning the case would be submitted for publication and each parent agreed to such.
Case 1
A 3 year old right hand dominant boy fell from a height of four feet onto his dominant arm. Immediate swelling and pain were noted around the elbow. On physical examination, he had tenderness and significant swelling at the elbow joint with guarding on examination. No neurovascular compromise was noted. Elbow x-rays revealed anterior and posterior fat pad signs, but no discrete fracture noted. The patient was placed in a long arm splint. Repeat x-ray evaluation one week later demonstrated a non-displaced (type I) supracondylar fracture. At that time, the arm was scanned and a stereolithography (STL) file was generated and modeled. [Figure 1]. The 3D cast was printed and applied once the swelling decreased ten days post injury, 3 days after scanning occurred [Figure 2]. On evaluation four weeks later, the 3D cast was removed. The skin was intact, and the patient was found to be non-tender at the fracture site. X-rays revealed satisfactory healing of the fracture. On follow-up evaluation fourteen months later, the patient is asymptomatic with full range of motion and normal function.
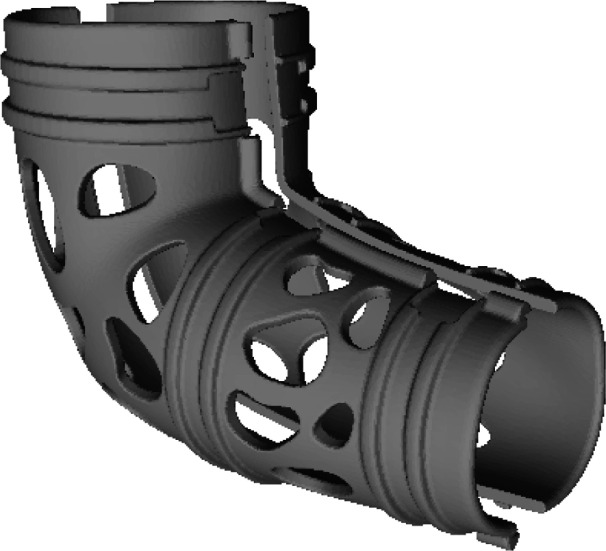
Figure 1.
STL file of cast
Figure 2.
Completed 3D printed cast
Case 2
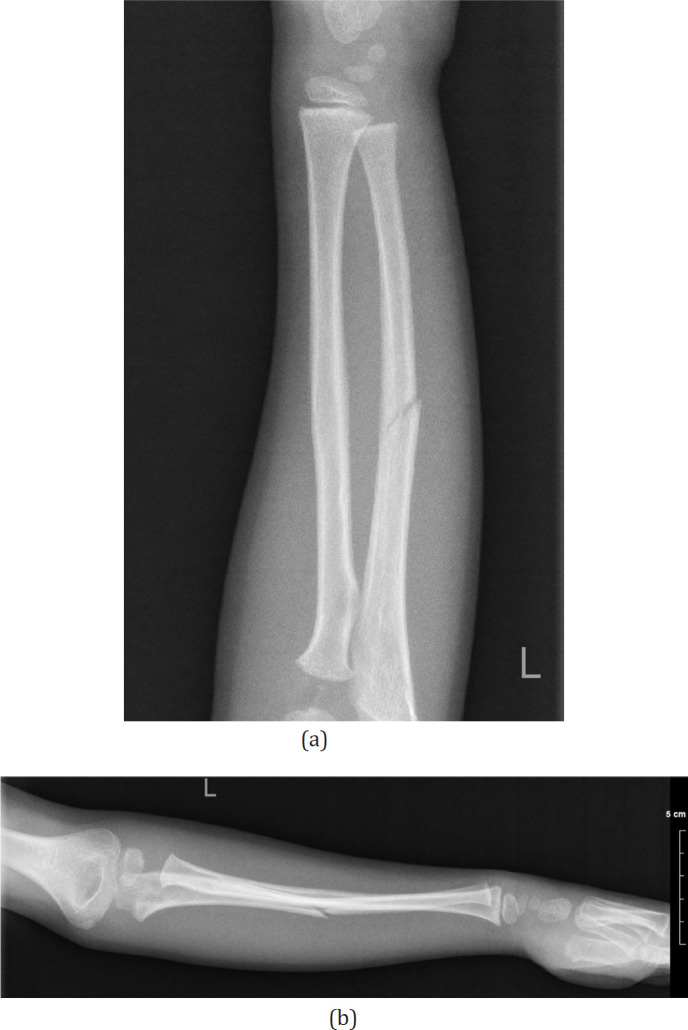
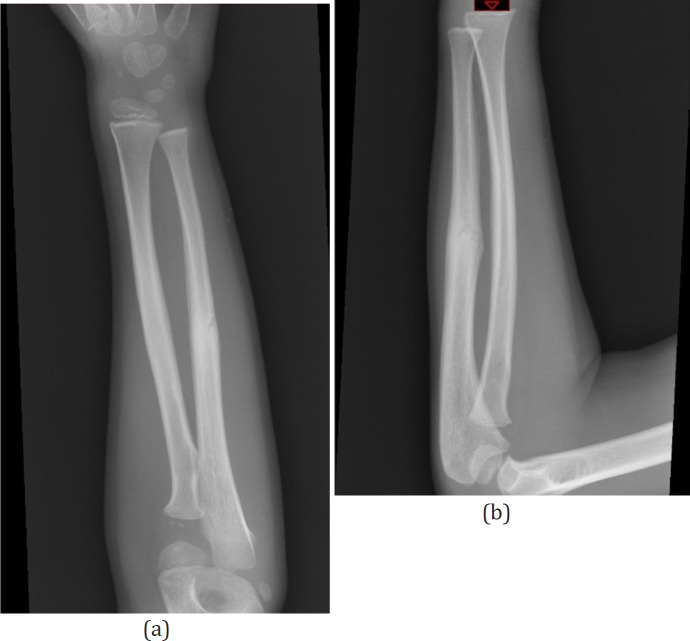
A 6 year old right hand dominant boy fell from monkey bars onto his left arm, subsequently developing forearm swelling and pain. On evaluation, he had marked discomfort over the ulna, but no wrist or shoulder pain. On physical exam, there was ecchymosis and tenderness to palpation at the mid forearm, with guarding to pronation and supination. Forearm x-rays revealed a minimally displaced ulnar shaft fracture [Figures 3a; b]. The patient was placed in a splint for one week to allow for swelling to subside. When he returned one week later, his forearm was scanned, shortly thereafter a 3D cast was manufactured and applied [Figure 4]. He remained in the cast for four additional weeks. Complete union of the fracture was confirmed radiographically at five weeks [Figures 5a; b]. Upon removal of the printed cast, the skin was intact and the patient was non-tender in the forearm. At follow-up evaluation 12 months after the injury, the patient is asymptomatic with full range of motion and normal function.
Figure 3 a,b.
Forearm injury films
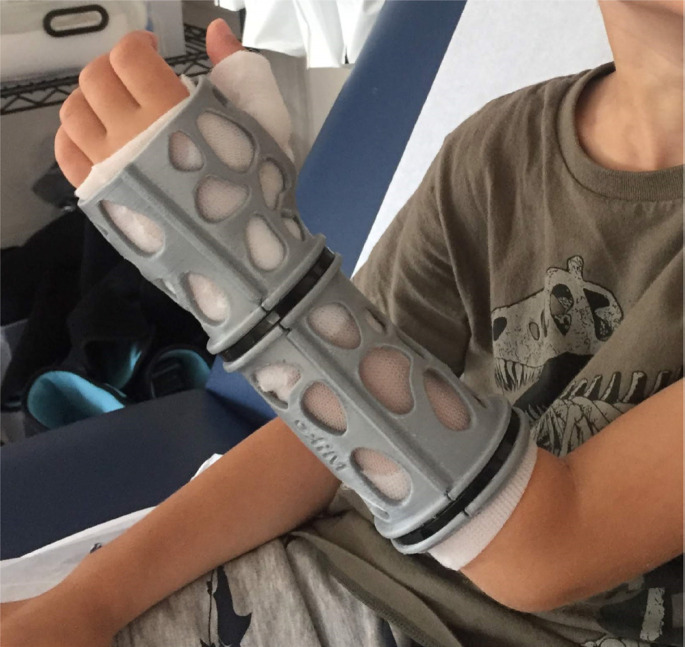
Figure 4.
Completed 3D printed cast
Figure 5a, b.
X-rays demonstrating healed fracture
Discussion
Various methods of immobilization for pediatric upper extremity fractures are currently in use (2, 3). Fiberglass has largely replaced plaster due to its material strength, yet difficulties with comfort, hygiene, and the need for cast changes remain a frequent problem in this patient population. DiPaolo et al. reviewed a study group of 1135 upper and lower extremity casts in a pediatric population (4). It was found that 60 casts (5.3%) required an unplanned change. Twenty-eight were changed for wetness, 20 for wear/breakage, 2 for skin irritation, and 10 for other reasons including objects in the cast and patient self-removal. The complications associated with traditional casts in the pediatric population, in addition to the aforementioned causes, can include cast-saw injuries, pressure sores, ill-fitting casts, and rarely, compartment syndrome (5, 6). It is possible that the implementation of custom made 3D casts can minimize the risk of many of these complications.
Splinting can be appropriate even in the case of more significant orthopaedic injuries. Boutis, et al. found that a molded orthoplast splint was as effective as a cast in the treatment of mildly displaced fractures of the forearm in children (7).The group consisted of patients with greenstick fractures angulated less than 15 degrees, or transverse fractures of the radius. The difference in fracture angulation was not significant, nor was grip strength, range of motion or complications between the cast and the splint group. Similarly, a prospective randomized trial by Grafstein et al. evaluated splinting versus casting in an adult population with displaced Colles fractures requiring closed reduction. It was found that functional outcomes were similar in those patients treated to conclusion with a splint or a circumferential cast. However, it was noted that the sugar-tong splint had a slightly higher risk of displacement than the volar-dorsal splint or the cast (8). The implementation of 3D printing is being explored in the manufacturing of both casts and splints alike, with a great deal of promise (9).
3D printed orthoses offer an opportunity to use digital technology to improve the patient’s experience while providing appropriate immobilization. By scanning the limb, the fit of the cast is made to match the exact anatomy of the patient [Figure 6]. This avoids pressure points and allows for open areas over wounds or incisions. Interestingly, a 3D cast model has been developed which incorporates a bone stimulator directly into its design (10). There may be reasonable evidence to support the role of bone stimulators in fracture care, however it can be cumbersome to incorporate these devices into a traditional plaster cast (11, 12). Manufacturing a 3D printed cast which can seamlessly accommodate a bone stimulator is merely one example of the unique ways in which this technology can be implemented and optimized in the field of orthopaedics. The casts in this report were made of poly-lactic acid (PLA), which is one of the most commonly used materials in 3D printing. Of note, it has also been documented that materials such as high density polyethylene and polypropylene have been implemented in the manufacturing of 3D casts (10).
Figure 6.
Scanning a child’s forearm
The scanning process takes about 5 minutes, the computer modeling about 15 minutes, and the printing about 6-7 hours. The casts are radiolucent. Upfront costs are $3,000-4,000 for the printer and $500-5,000 for the scanner. The software used for computer modeling is usually free and the modeling can be performed in most personal computers. The scanner used is commercially available and uses light and multiple cameras to scan object surfaces. The scanning process does not involve ionizing radiation, but rather visible light. The scanner acquires the data while connected to a personal computer and generates a Standard Tessellation Language (STL) file, which defines the surface geometry of the scanned object. The STL file is then imported into the modeling software for editing, which is typically a computer aided design (CAD) software. The design model is then output to the 3D printer for manufacturing. There have been programable modeling tools developed which reduce the amount of required manual operations in CAD, simplifying the modeling process for clinicians and alleviating the need for complex operations or extensive training. These tools also significantly reduce the time required to model the orthoses (9).
The 3D orthosis can be fixed or removable based on the patient. Cast padding can be placed on the skin prior to administration. This allows more breathability, decreased perspiration, and potentially less irritation from the orthosis itself. The time for application should be after maximal swelling which typically occurs three to five days post-injury. In our practice, pediatric patients that present with acute wrist fractures and substantial swelling are splinted initially for several days to allow for swelling to subside. In the subsequent visit, the cast (conventional or 3D printed) is applied, which allows for enough time to generate the 3D cast. In patients who present subacutely or in those with minimal swelling, the patient is temporarily splinted, given the 3D casts cannot be applied during the first visit but the scanning process would occur. In this setting, the fitting for the 3D cast would occur 1-2 days after the initial visit.
If a 3D orthosis is selected for treatment, it can be used for the entirety of the fracture healing episode. This could decrease the need for multiple cast changes. The orthosis can be removed for hygiene and physical therapy visits if the physician deems appropriate. A fixed 3D orthosis can even be easily converted to a removable one at the end of treatment.
There is a paucity of data regarding treatment of pediatric upper extremity fractures with 3D printed casts. Guida et al. developed a protocol to manufacture customized, 3D printed orthoses in a hospital setting (13). Eighteen children were successfully treated with the 3D printed cast with high patient satisfaction. The device was significantly lighter than a plaster cast and allowed for ventilation. The fabrication cost was comparable to a conventional plaster cast. Graham et al. evaluated the functionality of 3D printed orthoses compared to conventional immobilization in adults (14). While immobilized in either a short arm fiberglass cast or a 3D printed orthosis, volunteers were assessed with both the Jebson Hand Function Test and Patient-Rated Wrist Evaluation (PRWE). Those in the 3D orthosis were faster at completing tasks in the JHFT, and the scores were much lower in the PRWE. After wearing the cast or 3D orthosis, outcomes of patient satisfaction, comfort and perceived function were superior in the 3D orthosis group. The mean wear burden was “moderate” in the fiberglass cast group, compared with “no hassle” in the 3D cast group. Chen et al. published a report of ten patients, consisting of adults with distal radius fractures, treated with a 3D printed cast (15). No loss of reduction occurred, nor did any orthosis break. After two weeks of application of the 3D printed device, 100% of patients opted to use the 3D printed cast instead of a plaster cast.
3D printing is an evolving technology which has a potential application in the treatment of pediatric forearm fractures. Both of the patients in this report healed successfully without displacement, skin problems, or need for cast changes. At latest follow-up at least one year post-injury, the clinical outcomes were excellent. 3D printed casts may allow for greater patient satisfaction and reduced complications in both the adult and pediatric patient populations, and as this technology becomes further integrated into medicine, and within orthopaedics specifically, there will likely be more data published to support its use. Orthopaedic surgeons may benefit from familiarizing themselves with the potential of 3D printing technology and utilizing its current applications, as well as devising future applications for clinical practice.
References
- 1.Mulford JS, Babazadeh S, Mackay N. Three-dimensional printing in orthopaedic surgery: review of current and future applications. ANZ journal of surgery. 2016;86(9):648–53. doi: 10.1111/ans.13533. [DOI] [PubMed] [Google Scholar]
- 2.Williams KG, Smith G, Luhmann SJ, Mao J, Gunn JD, Luhmann JD. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatric emergency care. 2013;29(5):555–9. doi: 10.1097/PEC.0b013e31828e56fb. [DOI] [PubMed] [Google Scholar]
- 3.Witney-Lagen C, Smith C, Walsh G. Soft cast versus rigid cast for treatment of distal radius buckle fractures in children. Injury. 2013;44(4):508–13. doi: 10.1016/j.injury.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 4.DiPaola MJ, Abzug JM, Pizzutillo PD, Herman MJ. Incidence and etiology of unplanned cast changes for fractures in the pediatric population. Journal of Pediatric Orthopaedics. 2014;34(6):643–6. doi: 10.1097/BPO.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen S, McDowell M, Schlechter J. Casting: Pearls and pitfalls learned while caring for children’s fractures. World journal of orthopedics. 2016;7(9):539. doi: 10.5312/wjo.v7.i9.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samora JB, Samora WP, Dolan K, Klingele KE. A quality improvement initiative reduces cast complications in a pediatric hospital. Journal of pediatric orthopedics. 2018;38(2):e43–9. doi: 10.1097/BPO.0000000000001117. [DOI] [PubMed] [Google Scholar]
- 7.Boutis K, Willan A, Babyn P, Goeree R, Howard A. Cast versus splint in children with minimally angulated fractures of the distal radius: a randomized controlled trial. Cmaj. 2010;182(14):1507–12. doi: 10.1503/cmaj.100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grafstein E, Stenstrom R, Christenson J, Innes G, MacCormack R, Jackson C, et al. A prospective randomized controlled trial comparing circumferential casting and splinting in displaced Colles fractures. Canadian Journal of Emergency Medicine. 2010;12(3):192–200. doi: 10.1017/s1481803500012239. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Tanaka H. Rapid customization system for 3D-printed splint using programmable modeling technique–a practical approach. 3D printing in medicine. 2018;4(1):1–21. doi: 10.1186/s41205-018-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin H, Shi L, Wang D. A rapid and intelligent designing technique for patient-specific and 3D-printed orthopedic cast. 3D printing in medicine. 2016;2(1):1–0. doi: 10.1186/s41205-016-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riboh JC, Leversedge FJ. The use of low-intensity pulsed ultrasound bone stimulators for fractures of the hand and upper extremity. Journal of Hand Surgery. 2012;37(7):1456–61. doi: 10.1016/j.jhsa.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Lou S, Lv H, Li Z, Zhang L, Tang P. The effects of low-intensity pulsed ultrasound on fresh fracture: A meta-analysis. Medicine (Baltimore) 2017;96(39):e8181. doi: 10.1097/MD.0000000000008181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guida P, Casaburi A, Busiello T, Lamberti D, Sorrentino A, Iuppariello L, et al. An alternative to plaster cast treatment in a pediatric trauma center using the CAD/CAM technology to manufacture customized three-dimensional-printed orthoses in a totally hospital context: a feasibility study. Journal of Pediatric Orthopaedics B. 2019;28(3):248–55. doi: 10.1097/BPB.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 14.Graham J, Wang M, Frizzell K, Watkins C, Beredjiklian P, Rivlin M. Conventional vs 3-dimensional printed cast wear comfort. HAND. 2020;15(3):388–92. doi: 10.1177/1558944718795291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YJ, Lin H, Zhang X, Huang W, Shi L, Wang D. Application of 3D–printed and patient-specific cast for the treatment of distal radius fractures: initial experience. 3D Printing in Medicine. 2017;3(1):1–9. doi: 10.1186/s41205-017-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]