Abstract
Background
Elderly patients with gastrointestinal cancer and mental illness have significant comorbidities that can impact the quality of their care. We investigated the relationship between mental illness and frequent emergency department (ED) use in the last month of life, an indicator for poor end‐of‐life care quality, among elderly patients with gastrointestinal cancers.
Methods
We used SEER‐Medicare data to identify decedents with gastrointestinal cancers who were diagnosed between 2004 and 2013 and were at least 66 years old at time of diagnosis (median age: 80 years, range: 66–117 years). We evaluated the association between having a diagnosis of depression, bipolar disorders, psychotic disorders, anxiety, dementia, and/or substance use disorders and ED use in the last 30 days of life using logistic regression models.
Results
Of 160,367 patients included, 54,661 (34.1%) had a mental illness diagnosis between one year prior to cancer diagnosis and death. Patients with mental illness were more likely to have > 1 ED visit in the last 30 days of life (15.6% vs. 13.3%, p < 0.01). ED use was highest among patients with substance use (17.7%), bipolar (16.5%), and anxiety disorders (16.4%). Patients with mental illness who were male, younger, non‐white, residing in lower income areas, and with higher comorbidity were more likely to have multiple end‐of‐life ED visits. Patients who received outpatient treatment from a mental health professional were less likely to have multiple end‐of‐life ED visits (adjusted odds ratio 0.82, 95% confidence interval 0.78–0.87).
Conclusions
In elderly patients with gastrointestinal cancers, mental illness is associated with having multiple end‐of‐life ED visits. Increasing access to mental health services may improve quality of end‐of‐life care in this vulnerable population.
Keywords: cancer management, digestive cancer, quality of life, SEER‐Medicare
Frequent end‐of‐life emergency department (ED) use is an indicator for poor quality of care. Elderly patients with gastrointestinal malignancies and comorbid mental illness are more likely to have multiple ED visits at the end of life; however, receipt of early mental health services mitigates this risk.

1. INTRODUCTION
Patients with cancer frequently visit the emergency department (ED) at the end of life. 1 , 2 More than 10% of elderly patients use the ED multiple times in the last month of life, and this proportion has been increasing despite attempts to reduce unnecessary visits. 3 , 4 ED use and subsequent hospitalizations can be disruptive for patients and contribute significantly to the rising cost of cancer care. 5 , 6 , 7 In addition, these visits often result from a lack of sufficient symptom management and palliative care. 5 , 8 Thus, limiting the proportion of patients with frequent end‐of‐life ED visits is a well‐recognized component of delivering high‐quality and cost‐effective care. 9
While socioeconomic and demographic factors have been shown to influence end‐of‐life ED use, the impact of clinical factors such as mental illness is poorly understood. 10 Cancer patients have a high rate of mental illness, which is linked to disparities in care. 11 , 12 For instance, patients with mental illness are screened for cancer less frequently, diagnosed at a later stage, receive non‐definitive treatment more commonly, and have higher mortality rates. 13 , 14 , 15 , 16
We investigated the impact of mental illness on end‐of‐life ED use among elderly patients with gastrointestinal cancers since mental illness is prevalent in this population and it negatively influences patient care. 15 , 17 , 18 Those with gastrointestinal cancers also have a unique set of symptoms and needs, with common complications including bowel and biliary obstruction, gastrointestinal bleeding, and pain. As a result, patients with gastrointestinal cancers appear to visit the ED more frequently than those with other types of cancers. 19 Therefore, defining the relationship between mental illness and end‐of‐life ED use in this population is vital for both identifying disparities in care and establishing effective interventions for high‐risk patients.
2. MATERIALS AND METHODS
2.1. Data source
We used the Surveillance, Epidemiology and End Results (SEER)‐Medicare linked database, which provides longitudinal clinical information on Medicare beneficiaries with cancer by linking cancer incidence and survival data from population‐based SEER cancer registries with insurance claims data from Medicare. 20 Each cancer registry collects information about patient demographics, cancer diagnosis, tumor characteristics, and initial course of treatment. Medicare claims data are linked on the patient level and provide detailed information about hospital, physician, and outpatient health services for all patients included in the database. This study received institutional review board exemption from the Stanford University School of Medicine.
2.2. Patient selection
We identified patients with a first gastrointestinal malignancy diagnosed between 2004 and 2013 (Figure 1). These included individuals with primary colorectal, pancreatic, gastric, hepatic, biliary, esophageal, small bowel, anal, and other (peritoneal, retroperitoneal, or unspecified) cancers. Patients included were at least 66 years old at time of cancer diagnosis, had a recorded death date, and were continuously enrolled in Medicare Parts A and B from one year prior to cancer diagnosis until death. We excluded patients who were members of health maintenance organizations or were eligible for insurance due to disability or end stage renal disease.
FIGURE 1.
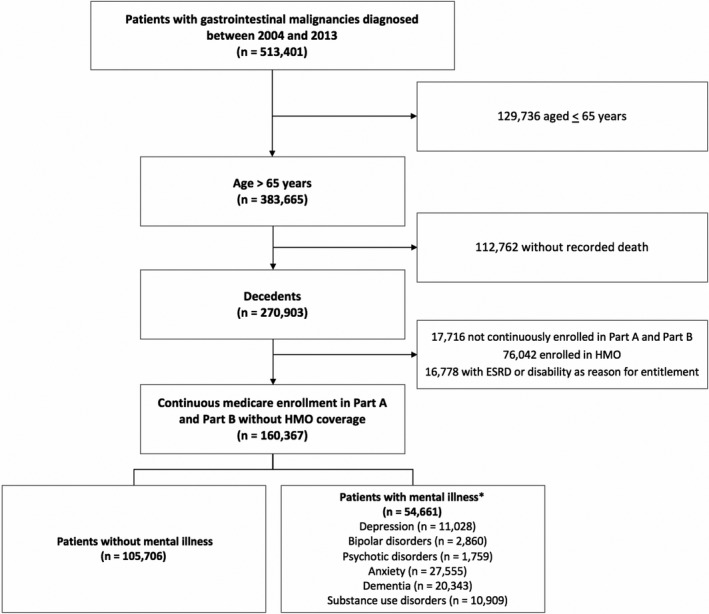
Cohort selection. *Patients with multiple mental illnesses were included in all applicable categories
2.3. Mental illness identification
Patients were classified as having a mental illness based on the presence of relevant International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes in any inpatient or outpatient claims between one year prior to cancer diagnosis and death. We included patients diagnosed with mental illness prior to cancer diagnosis since there is growing evidence that having a pre‐existing mental illness can impact the quality of cancer care patients receive, which, in turn, may impact quality of end‐of‐life care. 16 We identified patients with depression, bipolar disorders, psychotic disorders, anxiety, dementia, and substance use disorders (Table S1). We did not include patients with personality disorders in our analysis since we identified fewer than 150 patients with these disorders that met inclusion criteria. Patients with more than one mental illness were included in all applicable categories.
2.4. Patient characteristics
We extracted patient age, sex, race, Hispanic ethnicity, marital status, and census tract‐level median household income from SEER. Patient race was defined independently of Hispanic ethnicity 21 and included the following categories: White, Black, Asian or Pacific Islander, American Indian, or Alaska Native. Primary site was determined using International Classification of Diseases for Oncology Third Edition (ICD‐O‐3) topography codes. We also classified cancer stage as local, regional, or distant based on the summary stage reported in SEER. 22 Comorbidity burden was estimated from insurance claims in the year prior to cancer diagnosis using the Klabunde modification of the Charlson Comorbidity Index. 23 , 24
2.5. Identification of emergency department visits
ED visits were identified through insurance claims by using a combination of Healthcare Common Procedure Coding System (HCPCS), Berenson‐Eggers Type of Service (BETOS), and inpatient admission codes. 3 Specific codes are presented in Table S2. We identified ED visits made by all patients within the last year of life. We also specifically determined whether patients visited the ED multiple times in the last 30 days of life, an indicator for poor quality end‐of‐life cancer care. 9
To identify reasons for ED visits, we extracted the first ICD‐9‐CM diagnosis code from all relevant claims and reassigned these codes into clinically meaningful categories using the Agency for Healthcare Research and Quality (AHRQ) Clinical Classification Software.
2.6. Professional management of patients with mental illness
We classified patients as having received professional management for mental illness if they had at least one provider claim for a mental health service within a year of their first diagnosed mental illness. Table S3 includes the specific HCPCS, Center for Medicare Services (CMS) specialty, and revenue center codes that were used.
2.7. Statistical analysis
We compared baseline patient characteristics between those with and without mental illness using Pearson's chi‐squared test. We evaluated the association between various mental illnesses and multiple ED visits within the last 30 days of life using multivariable logistic regression models. We included all extracted patient characteristics as model covariates to mitigate the effect of confounding factors.
The significance level for all tests was set to 0.05. All statistical analyses were performed using R (version 3.4.3, R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
In total, 160,367 patients with gastrointestinal cancers met inclusion criteria, of whom 54,661 (34.1%) had at least one diagnosed mental illness between one year prior to cancer diagnosis and death. The cohort of patients with mental illness was more highly represented by individuals who were at least 85 years old, women, white, and unmarried, had colorectal or anal cancer, earlier stage disease, and higher comorbidity burden (Table 1).
TABLE 1.
Demographic and clinical characteristics of patients with gastrointestinal cancers by mental illness status
| Characteristics | No Mental Illness (n = 105,706, 65.9%) | Any Mental Illness (n = 54,661, 34.1%) | p |
|---|---|---|---|
| disease site (%) | |||
| Colorectal | 42,702 (40.4) | 27,584 (50.5) | <0.001 |
| Pancreas | 25,912 (24.5) | 9,544 (17.5) | |
| Stomach | 10,746 (10.2) | 4,839 (8.9) | |
| Liver and intrahepatic bile ducts | 8,803 (8.3) | 4,201 (7.7) | |
| Esophagus | 6,743 (6.4) | 3,236 (5.9) | |
| Gallbladder and extrahepatic bile ducts | 5,734 (5.4) | 2,421 (4.4) | |
| Small intestine | 1,818 (1.7) | 994 (1.8) | |
| Anus | 920 (0.9) | 766 (1.4) | |
| Other | 2,328 (2.2) | 1,076 (2.0) | |
| Age (%) | |||
| 85+ | 28,214 (26.7) | 15,257 (27.9) | <0.001 |
| 75–84 | 47,301 (44.8) | 23,878 (43.7) | |
| 66–74 | 30,039 (28.5) | 15,464 (28.3) | |
| Sex (%) | |||
| Female | 51,168 (48.4) | 30,368 (55.6) | <0.001 |
| Male | 54,538 (51.6) | 24,293 (44.4) | |
| Race (%) | |||
| White | 88,800 (84.0) | 47,208 (86.4) | <0.001 |
| American Indian/Alaska Native | 485 (0.5) | 207 (0.4) | |
| Asian or Pacific Islander | 7,166 (6.8) | 2,371 (4.3) | |
| Black | 9,067 (8.6) | 4,791 (8.8) | |
| Unknown | 188 (0.2) | 84 (0.2) | |
| Ethnicity (%) | |||
| Non‐Hispanic | 99,291 (93.9) | 51,234 (93.7) | 0.115 |
| Hispanic | 6,415 (6.1) | 3,427 (6.3) | |
| Marital status (%) | |||
| Single | 7,812 (7.4) | 4,972 (9.1) | <0.001 |
| Divorced/Separated | 7,154 (6.8) | 4,553 (8.3) | |
| Married/Domestic Partner | 51,411 (48.6) | 22,767 (41.7) | |
| Widowed | 34,841 (33.0) | 19,745 (36.1) | |
| Unknown | 4,488 (4.2) | 2,624 (4.8) | |
| Household income by area (%) | |||
| Top quantile | 23,907 (22.6) | 12,518 (22.9) | <0.001* |
| 2nd quantile | 23,960 (22.7) | 12,470 (22.8) | |
| 3rd quantile | 23,903 (22.6) | 12,527 (22.9) | |
| Bottom quantile | 23,935 (22.6) | 12,491 (22.9) | |
| Unknown | 10,001 (9.5) | 4,655 (8.5) | |
| Stage (%) | |||
| Local | 22,212 (21.0) | 16,461 (30.1) | <0.001 |
| Regional | 29,343 (27.8) | 16,576 (30.3) | |
| Distant | 39,475 (37.3) | 13,883 (25.4) | |
| Unknown | 14,676 (13.9) | 7,741 (14.2) | |
| Charlson comorbidity index (%) | |||
| 0 | 48,672 (46.0) | 19,798 (36.2) | <0.001 |
| 1 | 26,793 (25.3) | 13,738 (25.1) | |
| 2 | 14,014 (13.3) | 8,511 (15.6) | |
| 3+ | 16,227 (15.4) | 12,614 (23.1) | |
p‐value was 0.97 when unknown values were excluded.
3.1. End‐of‐life ED use and associated risk factors
ED use consistently increased throughout the last year of life (Figure 2). The sharpest increase in use occurred in the last month of life, during which 51.3% of patients with and 49.1% of patients without mental illness visited the ED at least once.
FIGURE 2.
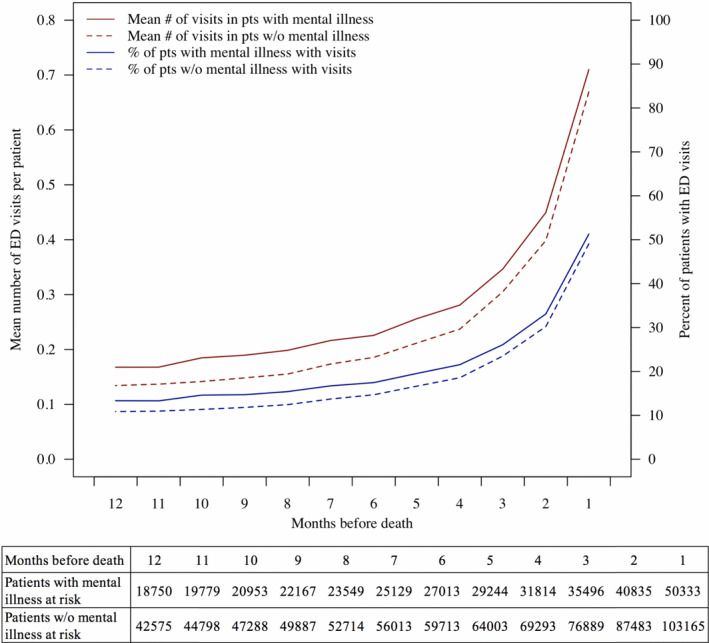
ED visits in the last year of life. Abbreviations: ED, emergency department. pts, patients. w/o, without
Patients with mental illness were more likely to visit the ED multiple times in the month before death than those without mental illness (15.6% vs. 13.3%, p < 0.01; Table 2). In particular, we observed higher odds of multiple ED visits in the last 30 days of life in patients with anxiety (adjusted odds ratio [aOR] 1.26, 95% confidence interval [CI] 1.21–1.31), substance use (aOR 1.18, 95% CI 1.12–1.24), and bipolar (aOR 1.12, 95% CI 1.01–1.24) disorders. We also examined risk factors associated with multiple end‐of‐life ED visits among patients with mental illness (Table 3). These included being male (aOR 1.17, 95% CI 1.11 – 1.23), younger (aOR 1.21, 95% CI 1.13 – 1.30), black (aOR 1.51, 95% CI 1.40 – 1.64), Asian or Pacific Islander (aOR 1.15, 95% CI 1.03 – 1.29), and Hispanic (aOR 1.20, 95% CI 1.10 – 1.32). Risk factors also included living in a lower income census tract (aOR 1.10, 95% CI 1.03 – 1.18 for third income quantile) and having a higher Charlson comorbidity index (aOR 1.26, 95% CI 1.18 – 1.34 for score ≥ 3). Furthermore, end‐of‐life ED visits were associated with pancreatic (aOR 1.10, 95% CI 1.03 – 1.18), hepatic (aOR 1.17, 95% CI 1.07 – 1.28), esophageal (aOR 1.17, 95% CI 1.06 – 1.29), biliary (aOR 1.16, 95% CI 1.03 – 1.30), small bowel cancer (aOR 1.22, 95% CI 1.03 – 1.44), and other gastrointestinal cancers (peritoneal, retroperitoneal, or unspecified; aOR 1.19, 95% CI 1.01–1.41) compared to colorectal cancer. Regional disease was associated with lower odds of having multiple end‐of‐life ED visits compared to local disease (aOR 0.91, 95% CI 0.86 – 0.97).
TABLE 2.
Impact of mental illness on having multiple ED visits in the last 30 days of life
| Risk factor | >1 ED visit in last 30 days of life | ||
|---|---|---|---|
| No. of patients (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| No mental illness (n = 105,706) | 14,100 (13.3) | Reference | Reference |
| Any mental illness (n = 54,661) | 8,531 (15.6) | 1.20 (1.17–1.24) | 1.20 (1.16–1.24) |
| Depression (n = 11,028) | 1,635 (14.8) | 1.06 (1.01–1.12) | 0.97 (0.92–1.03) |
| Bipolar disorders (n = 2,860) | 473 (16.5) | 1.21 (1.1–1.34) | 1.12 (1.01–1.24) |
| Psychotic disorders (n = 1,759) | 274 (15.6) | 1.12 (0.99–1.28) | 0.98 (0.85–1.12) |
| Anxiety (n = 27,555) | 4,522 (16.4) | 1.24 (1.2–1.29) | 1.26 (1.21–1.31) |
| Dementia (n = 20,343) | 2,903 (14.2) | 1.02 (0.97–1.06) | 1.02 (0.97–1.06) |
| Substance use disorders (n = 10,909) | 1,928 (17.7) | 1.34 (1.27–1.41) | 1.18 (1.12–1.24) |
Abbreviations: ED, emergency department; OR, odds ratio.
TABLE 3.
Risk factors associated with having multiple ED visits in the last 30 days of life among patients with mental illness
| Risk factor | >1 ED visit in last 30 days of life OR (95% CI) | |
|---|---|---|
| Disease site | Colorectal | 1.0 (Ref) |
| Pancreas | 1.1 (1.03–1.18) | |
| Stomach | 1.06 (0.97–1.16) | |
| Liver and intrahepatic bile ducts | 1.17 (1.07–1.28) | |
| Esophagus | 1.17 (1.06–1.29) | |
| Gallbladder and extrahepatic bile ducts | 1.16 (1.03–1.3) | |
| Small intestine | 1.22 (1.03–1.44) | |
| Anus | 1.08 (0.87–1.31) | |
| Other | 1.19 (1.01–1.41) | |
| Age | 85+ | 1.0 (Ref) |
| 75–84 | 1.14 (1.07–1.21) | |
| 66–74 | 1.21 (1.13–1.3) | |
| Sex | Female | 1.0 (Ref) |
| Male | 1.17 (1.11–1.23) | |
| Race | White | 1.0 (Ref) |
| American Indian/Alaska Native | 0.99 (0.6–1.42) | |
| Asian or Pacific Islander | 1.15 (1.03–1.29) | |
| Black | 1.51 (1.4–1.64) | |
| Unknown | 0.81 (0.4–1.47) | |
| Ethnicity | Non‐Hispanic | 1.0 (Ref) |
| Hispanic | 1.2 (1.1–1.32) | |
| Marital status | Single | 1.0 (Ref) |
| Divorced/Separated | 0.98 (0.88–1.09) | |
| Married/Domestic Partner | 1.06 (0.98–1.16) | |
| Widowed | 1.04 (0.96–1.14) | |
| Unknown | 1.12 (0.99–1.28) | |
| Household income by area | Top quantile | 1.0 (Ref) |
| 2nd quantile | 1.04 (0.97–1.12) | |
| 3rd quantile | 1.1 (1.03–1.18) | |
| Bottom quantile | 1.07 (1–1.15) | |
| Unknown | 0.96 (0.87–1.06) | |
| Stage | Local | 1.0 (Ref) |
| Regional | 0.91 (0.86–0.97) | |
| Distant | 0.98 (0.92–1.05) | |
| Unknown | 0.71 (0.66–0.78) | |
| Charlson comorbidity index | 0 | 1.0 (Ref) |
| 1 | 1.1 (1.04–1.17) | |
| 2 | 1.1 (1.02–1.18) | |
| 3+ | 1.26 (1.18–1.34) | |
| Mental illness | Depression | 0.99 (0.91–1.08) |
| Bipolar disorders | 1.14 (1.02–1.28) | |
| Psychotic disorders | 1.01 (0.88–1.16) | |
| Anxiety | 1.23 (1.13–1.34) | |
| Dementia | 1 (0.92–1.1) | |
| Substance use disorders | 1.15 (1.05–1.26) | |
| Multiple (vs. single) | 0.94 (0.85–1.05) | |
Abbreviations: ED, emergency department; OR, odds ratio.
3.2. Common reasons for end‐of‐life ED visits
Abdominal pain was the most common reason for ED visits in the last month of life, comprising 7.5% of all visits for patients with mental illness and 9.6% for those without (Table 4). Lower respiratory disease, malaise and fatigue, hypovolemia, septicemia, pneumonia, gastrointestinal hemorrhage, and liver disease were other common diagnoses shared between both groups.
TABLE 4.
Most common reasons for ED visits in the last 30 days of life
| Primary Diagnosis for ED Visit | Percent of all ED Visits in Cohort |
|---|---|
| Any Mental Illness | |
| Abdominal pain | 7.5% |
| Lower respiratory disease a | 5.5% |
| Pneumonia (except that caused by TB or STD) | 4.6% |
| Septicemia (except in labor) | 4.3% |
| Hypovolemia | 4.2% |
| Malaise and fatigue | 3.9% |
| Hemorrhage of gastrointestinal tract | 2.7% |
| Liver diseases a | 2.5% |
| Urinary tract infections | 2.1% |
| Congestive heart failure (non‐hypertensive) | 2.1% |
| No Mental Illness | |
| Abdominal pain | 9.6% |
| Lower respiratory disease a | 5.3% |
| Malaise and fatigue | 4.6% |
| Hypovolemia | 4.5% |
| Septicemia (except in labor) | 3.3% |
| Pneumonia (except that caused by TB or STD) | 3.3% |
| Hemorrhage of gastrointestinal tract | 3.0% |
| Liver diseases a | 2.8% |
| Nausea and vomiting | 2.5% |
| Intestinal obstruction without hernia | 2.4% |
Abbreviations: ED, emergency department.
other and unspecified type.
3.3. Impact of professional management of mental illness on end‐of‐life ED use
Patients who received professional management for mental illness were less likely than those who did not to have visited the ED multiple times in the last 30 days of life (13.7% vs 16.3%, p < 0.01; Table 5). Receiving mental health services was significantly associated with reduced odds of multiple end‐of‐life ED visits (aOR 0.82, 95% CI 0.78–0.87).
TABLE 5.
Impact of professional management of mental illness on having multiple ED visits in the last 30 days of life
| Professional management for mental illness | >1 ED visit in last 30 days of life (n = 8,531) | ||
|---|---|---|---|
| No. of patients (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Received treatment (n = 14,885) |
2,044 (13.7) |
0.82 (0.77–0.86) | 0.82 (0.78–0.87) |
| Did not receive treatment (n = 39,776) |
6,487 (16.3) |
Reference | Reference |
Abbreviations: ED, emergency department; OR, odds ratio.
4. DISCUSSION
Elderly patients with gastrointestinal cancer and mental illness have a high degree of comorbidity that can impact the quality of their cancer care and compromise outcomes. In our population‐based analysis, we found that having a mental illness was associated with receipt of poorer quality end‐of‐life care among these patients. Specifically, patients with mental illness were more likely to use the ED multiple times in their last month of life. Further, we found that receipt of early mental health services mitigates this risk, highlighting the importance of effective mental health support and interventions in this population.
End‐of‐life ED visits can be disruptive for patients during a time when most patients prefer home‐based care and less aggressive interventions. 25 , 26 There is increasing recognition that these visits signal poor quality cancer care. For instance, the American Society of Clinical Oncology supports a quality measure aimed at reducing the proportion of patients with multiple ED visits in the last 30 days of life. 9 From a health care system perspective, these visits and subsequent hospitalizations significantly contribute to cancer costs, as the majority of health care expenditures in the last month of life stem from acute inpatient care. 7 Therefore, addressing disparities in end‐of‐life care, such as those that exist for patients with mental illness, may improve care quality and reduce costs.
We suspect that mental illness impacts end‐of‐life ED use through several different biological, social, and behavioral pathways. For instance, having a mental illness influences which treatments patients receive, whether through patient or physician choice, and this has downstream consequences on health. 13 , 15 Patients with mental illness also frequently have poor social support, which makes it difficult to navigate the healthcare system and adhere to treatments and medical appointments. 27 , 28 Furthermore, psychological distress can complicate medical decision‐making and lead to receiving high intensity end‐of‐life interventions. 29 Finally, patients with mental illness are less likely to participate in advance care planning, which is important for clarifying preferences about end‐of‐life care. 30
Specifically, we found that anxiety, bipolar, and substance use disorders were associated with multiple ED visits in the last month of life. In contrast, depression, psychotic disorders, and dementia were not associated with frequent ED use at this stage. This variability in impact is likely related to multiple factors. Patients with particular mental illnesses experience unique barriers to accessing care and often respond to symptoms such as pain differently, which can influence subsequent use of emergency services. 31 For instance, increased anxiety appears to be closely linked with decreased pain tolerance, 32 and abdominal pain was the most common reason for ED visits in our study. Further studies are needed to better understand differences between these disorders with respect to end‐of‐life care; however, our study specifies a subset of cancer patients with mental illness who are at highest risk for ED use at the end of life and thus require greater attention from health care providers.
Additional factors associated with ED use are race, gender, socioeconomic status and comorbidity burden. Black patients were more likely to have multiple ED visits prior to death. Asian, Pacific Islander, and Hispanic patients also had a higher risk of frequent visits. This may be partly driven by patient preference; however, it may also reflect structural disparities, including access to palliative services. 33 , 34 Given the strong evidence for racial and ethnic disparities in end‐of‐life cancer care, 35 , 36 minority patients with mental illness represent a particularly high‐risk group. Younger age, male sex, living in a lower income area, and higher comorbidity were also associated with having multiple end‐of‐life ED visits among those with mental illness, which in consistent with previous studies. 10
Furthermore, those with pancreatic, hepatic, esophageal, biliary, and small bowel cancer were more likely to visit the ED compared to patients with colorectal cancer. This may be related to the aggressiveness of various cancer subtypes, as shorter post‐diagnosis survival time is associated with more intense end‐of‐life care 37 ; however, we also found that patients with regional disease are less likely to use the ED frequently at the end of life than those with local disease. Thus, it appears that the relationship between aggressiveness of disease and end‐of‐life care is complex and likely involves various factors such as post‐diagnosis survival time and symptom burden. These results stratify risk among cancer patients with mental illness and should inform interventions in this population.
Most encouragingly, we found that patients who receive mental health services soon after being diagnosed with a mental illness are less likely to require ED use prior to death. This is a promising avenue for intervention since cancer patients with mental illness have a low rate of accessing mental health services. 12 Thus, it is important for oncologists to partner with mental health providers to better screen for and address mental illness among patients with cancer.
The most common reasons that patients with mental illness visited the ED in our analysis were abdominal pain, respiratory distress, infection, hypovolemia, and malaise and fatigue. These results signal additional opportunities to intervene, as visits made for poorly controlled symptoms are likely preventable with effective outpatient strategies. 2 , 8 In particular, expanding access to palliative and supportive services may help reduce unnecessary end‐of‐life ED visits. 10 Furthermore, early palliative care has also been shown to improve quality of life, reduce caregiver burden and distress, decrease aggressive end‐of‐life interventions, and even increase survival. 38 , 39 , 40
This study has several important limitations. First, we used administrative data to conduct our analysis. In particular, we used diagnosis codes in insurance claims to detect mental illness, which can lead to patient misclassification. 41 Second, this is a retrospective analysis and is therefore susceptible to selection bias and confounding; however, this study design is well‐suited for assessing patterns of end‐of‐life care across populations as it allows for efficient identification of terminally ill patients. 42 Third, we did not have access to important factors that influence end‐of‐life care, such as patient preferences, advance care planning, and social support in our data. Finally, our cohort consisted of Medicare beneficiaries with gastrointestinal cancers, which limits the generalizability of our study.
5. CONCLUSIONS
Elderly patients with gastrointestinal cancers and comorbid mental illness have an increased risk of visiting the ED multiple times at the end of life. Improving access to mental health services reduces this risk and is therefore an important component of delivering high‐quality care. Future studies should investigate barriers to accessing mental health services among this population.
ETHICAL APPROVAL STATEMENT
This study received institutional review board exemption from the Stanford University School of Medicine.
CONFLICT OF INTEREST
Erqi Pollom has received honorarium from Accuray.
AUTHOR CONTRIBUTIONS
Mehr Kashyap: Conceptualization, data curation, methodology, investigation, writing‐original draft, and writing‐reviewing and editing. Jeremy P. Harris: Data curation, and writing‐reviewing and editing. Daniel T. Chang: Conceptualization, methodology, investigation, and writing‐reviewing and editing. Erqi L. Pollom: Conceptualization, methodology, investigation, writing‐original draft, and writing‐reviewing and editing.
Supporting information
Table S1‐S3
Funding Information
None.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available through the Surveillance, Epidemiology and End Results (SEER)‐Medicare linked database. Restrictions apply to the availability of these data, which were used under license for this study. Data are available at https://healthcaredelivery.cancer.gov/seermedicare/.
REFERENCES
- 1. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. JCO. 2004;22(2):315‐321. [DOI] [PubMed] [Google Scholar]
- 2. Barbera L, Taylor C, Dudgeon D. Why do patients with cancer visit the emergency department near the end of life? CMAJ. 2010;182(6):563‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: is it a quality‐of‐care Issue? J Clin Oncol. 2008;26(23):3860‐3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Handley NR, Schuchter LM, Bekelman JE. Best practices for reducing unplanned acute care for patients with cancer. JOP. 2018;14(5):306‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? results of a 2008 population study in north carolina. JCO. 2011;29(19):2683‐2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the united states: 2010–2020. JNCI Journal of the National Cancer Institute. 2011;103(2):117‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chastek B, Harley C, Kallich J, Newcomer L, Paoli CJ, Teitelbaum AH. Health care costs for patients with cancer at the end of life. JOP. 2012;8(6S):75s‐80s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Earle CC, Park ER, Lai B, Weeks JC, Ayanian JZ, Block S. Identifying potential indicators of the quality of end‐of‐life cancer care from administrative data. JCO. 2003;21(6):1133‐1138. [DOI] [PubMed] [Google Scholar]
- 9. QOPI‐Related Measures | ASCO Practice Central . Accessed January 19, 2020. https://practice.asco.org/quality‐improvement/quality‐programs/quality‐oncology‐practice‐initiative/qopi‐related‐measures
- 10. Henson LA, Gao W, Higginson IJ, et al. Emergency department attendance by patients with cancer in their last month of life: a systematic review and meta‐analysis. JCO. 2014;33(4):370‐376. [DOI] [PubMed] [Google Scholar]
- 11. Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751‐757. [DOI] [PubMed] [Google Scholar]
- 12. Kadan‐Lottick NS, Vanderwerker LC, Block SD, Zhang B, Prigerson HG. Psychiatric disorders and mental health service use in patients with advanced cancer. Cancer. 2005;104(12):2872‐2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52(1):106‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haskins CB, McDowell BD, Carnahan RM, et al. Impact of preexisting mental illness on breast cancer endocrine therapy adherence. Breast Cancer Res Treat. 2019;174(1):197‐208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baillargeon J, Kuo Y‐F, Lin Y‐L, Raji MA, Singh A, Goodwin JS. Effect of mental disorders on diagnosis, treatment, and survival of older adults with colon cancer. J Am Geriatr Soc. 2011;59(7):1268‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prasad SM, Eggener SE, Lipsitz SR, Irwin MR, Ganz PA, Hu JC. Effect of depression on diagnosis, treatment, and mortality of men with clinically localized prostate cancer. J Clin Oncol. 2014;32(23):2471‐2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;2004(32):57‐71. [DOI] [PubMed] [Google Scholar]
- 18. Song H, Zhu J, Lu D, et al. Psychiatric morbidity and its impact on surgical outcomes for esophageal and gastric cancer patients: a nationwide cohort study. Oncotarget. 2017;8(46):81305‐81314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caterino JM, Adler D, Durham DD, et al. Analysis of diagnoses, symptoms, medications, and admissions among patients with cancer presenting to emergency departments. JAMA Netw Open. 2019;2(3):e190979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER‐medicare data: content, research applications, and generalizability to the united states elderly population. Med Care. 2002;40(8):IV3‐IV18. [DOI] [PubMed] [Google Scholar]
- 21. About Race. The United States Census Bureau; 2020. Accessed November 26, 2020. https://www.census.gov/topics/population/race/about.html
- 22. Young J, Roffers S, Ries L, Fritz A, Hurlbut A, eds. SEER Summary Staging Manual – 2000: Codes and Coding Instructions. Washington, DC: National Cancer Institute; 2001. [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 24. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258‐1267. [DOI] [PubMed] [Google Scholar]
- 25. Higginson IJ, Sen‐Gupta GJA. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med. 2000;3(3):287‐300. [DOI] [PubMed] [Google Scholar]
- 26. Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end‐of‐life care intensity explained by patient preferences?: a study of the US medicare population. Med Care. 2007;45(5):386‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anuk D, Özkan M, Kizir A, Özkan S. The characteristics and risk factors for common psychiatric disorders in patients with cancer seeking help for mental health. BMC Psychiatry. 2019;19(1):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Given BA, Given CW, Kozachik S. Family support in advanced cancer. CA Cancer J Clin. 2001;51(4):213‐231. [DOI] [PubMed] [Google Scholar]
- 29. Fujisawa D, Temel JS, Traeger L, et al. Psychological factors at early stage of treatment as predictors of receiving chemotherapy at the end of life. Psycho‐Oncology. 2015;24(12):1731‐1737. [DOI] [PubMed] [Google Scholar]
- 30. Cai X, Cram P, Li Y. Origination of medical advance directives among nursing home residents with and without serious mental illness. Psychiatr Serv. 2011;62(1):61‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woods A, Willison K, Kington C, Gavin A. Palliative care for people with severe persistent mental illness: a review of the literature. The Canadian Journal of Psychiatry. 2008;53(11):725‐736. [DOI] [PubMed] [Google Scholar]
- 32. Michaelides A, Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med. 2019;131(7):438‐444. [DOI] [PubMed] [Google Scholar]
- 33. Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end‐of‐life treatment. J GEN INTERN MED. 2009;24(6):695‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cole AP, Nguyen D‐D, Meirkhanov A, et al. Association of care at minority‐serving vs non–minority‐serving hospitals with use of palliative care among racial/ethnic minorities with metastatic cancer in the united states. JAMA Netw Open. 2019;2(2):e187633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor JS, Rajan SS, Zhang N, et al. End‐of‐life racial and ethnic disparities among patients with ovarian cancer. JCO. 2017;35(16):1829‐1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karanth S, Rajan SS, Sharma G, Yamal J‐M, Morgan RO. Racial‐ethnic disparities in end‐of‐life care quality among lung cancer patients: a SEER‐medicare–based study. J Thorac Oncol. 2018;13(8):1083‐1093. [DOI] [PubMed] [Google Scholar]
- 37. Tang ST, Wu S‐C, Hung Y‐N, Chen J‐S, Huang E‐W, Liu T‐W. Determinants of aggressive end‐of‐life care for taiwanese cancer decedents, 2001 to 2006. JCO. 2009;27(27):4613‐4618. [DOI] [PubMed] [Google Scholar]
- 38. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small‐cell lung cancer. N Engl J Med. 2010;363(8):733‐742. [DOI] [PubMed] [Google Scholar]
- 39. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun V, Grant M, Koczywas M, et al. Effectiveness of an interdisciplinary palliative care intervention for family caregivers in lung cancer. Cancer. 2015;121(20):3737‐3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Davis KAS, Sudlow CLM, Hotopf M. Can mental health diagnoses in administrative data be used for research? a systematic review of the accuracy of routinely collected diagnoses. BMC Psychiatry. 2016;16:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Earle CC, Ayanian JZ. Looking back from death: the value of retrospective studies of end‐of‐life care. JCO. 2006;24(6):838‐840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
The data that support the findings of this study are available through the Surveillance, Epidemiology and End Results (SEER)‐Medicare linked database. Restrictions apply to the availability of these data, which were used under license for this study. Data are available at https://healthcaredelivery.cancer.gov/seermedicare/.


