Abstract
Background
The co-occurrence of autoimmune diseases is well recognized. Though studies have suggested that eosinophilic esophagitis (EoE) is more common in patients with inflammatory bowel diseases (IBD), whether co-occurrence of EoE modifies natural history of IBD is unknown.
Methods
This was a retrospective case-control study at a referral center. Cases consisted of patients with IBD and EoE, with both diseases diagnosed using established criteria. Controls comprised patients with IBD without concomitant EoE. Two controls were selected per case and were matched for duration of IBD. Relevant covariates regarding disease presentation and natural history were extracted from the medical record and compared between the 2 groups.
Results
A total of 95 IBD-EoE cases and 190 IBD controls were included in our study. The IBD-EoE group was diagnosed with IBD at a younger age than those with IBD alone (22.3 years vs 29.0 years; P < 0.001) and were more likely to be male (80.0% vs 45.8%; P < 0.001). There were no differences in medical or surgical therapy for IBD between the 2 groups. Among those with IBD-EoE, patients for whom IBD was diagnosed first presented more commonly with dysphagia (50.8% vs 26.9%; P = 0.04) and endoscopically had evidence of esophageal rings (50.0% vs 23.1%; P = 0.02) when compared with those where EoE was diagnosed first.
Conclusion
Patients with concurrent IBD-EoE are diagnosed at a younger age and more likely to be males but have similar natural history as those without EoE. There were differences in EoE phenotype based on whether the EoE or IBD was diagnosed first.
Keywords: inflammatory bowel disease, eosinophilic esophagitis, disease co-occurrence
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are the 2 major forms of inflammatory bowel disease (IBD), affecting an estimated 2 million individuals in the United States. Though the exact mechanism of disease is unknown, there is a complex interplay of genetics and the environment that results in inappropriate immune activation and inflammation.1–5 Due to overlapping genetics and potentially common environmental exposures, it is well established that there is a clustering and co-occurrence of various immune-mediated diseases.6, 7 Inflammatory bowel disease has been associated in co-occurrence with celiac disease, psoriasis, asthma, and rheumatoid arthritis.8–13 In a large cohort, up to 21% of patients with IBD had a second immune-mediated disease.13 Concurrent immune-mediated disease may also modify the natural history of IBD. For example, the milder clinical course but increased risk of colon cancer in the setting of primary sclerosing cholangitis is well recognized.14–16 Patients with a concurrent immune-mediated disease may be more likely to need anti-TNF therapy and have worse quality of life.13
Eosinophilic esophagitis (EoE), an esophageal inflammatory disease driven by an immune response to allergens, is increasing in incidence.17 Numerous similarities exist between IBD and EoE. Both diseases have a genetic basis, are potentially influenced by similar environmental risk factors, and share similar epidemiological trends.17, 18 Additionally, those with either EoE or IBD are at higher risk of developing additional autoimmune and inflammatory diseases.19, 20 A few studies have examined the occurrence of EoE in patients with IBD. Eosinophilic esophagitis may be more common among those with IBD than in the general population.21–26 However, whether having co-occurring disease modifies the phenotype and natural history of each individual disease is unknown. We performed this study with the objectives of (1) characterizing patients with IBD with coexistent EoE and (2) comparing presentations and outcomes of this population with those with IBD alone.
METHODS
Study Population
This was a retrospective case-control study that included patients from the Partners Healthcare System, which includes 2 tertiary referral hospitals: Massachusetts General Hospital and Brigham and Women’s Hospital. Potential cases and controls were identified through a query of the Partners Healthcare Research Practice Data Registry (RPDR), which is a database of all the patients seen within the Partners Healthcare System for inpatient or outpatient care. Use of this registry has been described previously.8, 13, 27 The cases comprised patients who had been diagnosed with both IBD (UC or CD) and EoE. The controls comprised patients who had been diagnosed with IBD and had never been diagnosed with EoE. Potential cases were identified by using ICD-10 codes for CD (ICD-10: K50) or ulcerative colitis (ICD-10: K51) in combination with a diagnosis of EoE (ICD 10: K20.0). A chart review was then performed to confirm case and control status. Both IBD and EoE diagnoses were established using accepted clinical, endoscopic, and histologic criteria. For EoE, these criteria included histology demonstrating at least 15 eosinophils per high powered field (HPF) in the esophagus. Two randomly selected controls were matched to each case based on IBD duration to ensure comparability of follow-up. This study was approved by the Partners Healthcare Institutional Review Board.
Study Variables
Demographic and relevant disease covariates were obtained from the electronic medical record. Demographics included age, sex, race, and smoking status. The IBD-specific variables included disease type (UC vs CD), age of IBD diagnosis, duration of disease at the time of data collection, and need for IBD surgery. The Montreal Classification was used to characterize disease location and behavior.28 For UC, this included disease extent (proctitis, left-sided colitis, or pancolitis). For CD, this included the presence of perianal disease, disease location (ileal, colonic, ileocolonic, or upper gastrointestinal), and disease behavior (nonstricturing, nonpenetrating, stricturing, or penetrating). We also recorded information on current or previous IBD medication use including 5-aminosalicylates, immunomodulators (azathioprine and 6-mercaptopurine), methotrexate, oral corticosteroids (prednisone or budesonide), antitumor necrosis factor therapy (anti-TNF, adalimumab, infliximab, certolizumab), and anti-integrin biologics (vedolizumab). We recorded EoE presenting symptoms (dysphagia or gastroesophageal reflux disease), initial endoscopic findings (furrows, rings, exudates, and/or strictures), eosinophils per HPF at the time of diagnosis, and treatments (swallowed steroids, dietary modification, endoscopic dilation, and/or proton pump inhibitor). In cases of coexistent disease, we recorded whether EoE or IBD was diagnosed first and the interval between diagnoses. If IBD preceded EoE, we also recorded the IBD medications that were currently or previously being used at the time of EoE diagnosis.
Statistical Analysis
Continuous variables were compared using the t test, whereas categorical variables were compared using the × 2 test with the Fisher modification where appropriate. Univariate and, where indicated, multivariable regression models examined the independent effect of concomitant EoE diagnosis on IBD outcomes. In comparing cases and controls, we then performed analysis stratified by whether EoE or IBD was diagnosed first. A 2-sided P value <0.05 indicated independent statistical significance. All analysis was conducted using Stata 15.1 (StataCorp, College Station, TX).
RESULTS
Study Population
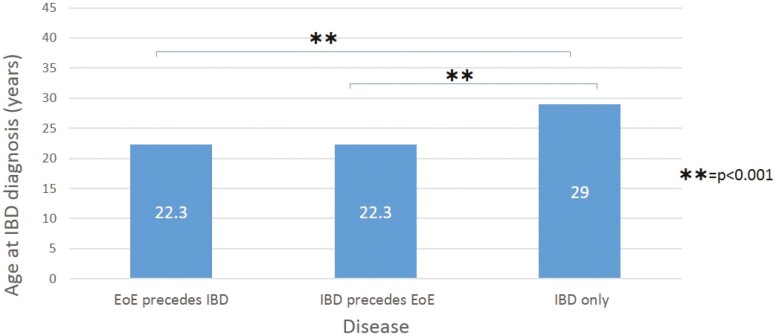
A total of 95 cases and 190 matched controls were included in the study. Table 1 compares the demographics between the IBD-EoE group and the IBD group. Confirming successful matching, IBD disease duration was similar between the 2 groups (11.1 vs 11.7 years; P = 0.60). There were significantly more males in the IBD-EoE group compared with those with IBD alone (80% vs 45.8%, P < 0.001). There were slightly fewer patients who had a history of tobacco use in the IBD-EoE group compared with the IBD group (15.8% vs 27.4%; P = 0.09). There were no differences in race between the 2 groups. Patients with coexisting IBD and EoE had an earlier age at diagnosis than those with IBD alone (22.3 years vs 29 years; P < 0.001). This was noted irrespective of whether the IBD or EoE was diagnosed first (Fig. 1).
Table 1.
Characteristics of Patients With Inflammatory Bowel Diseases, Stratified by Presence of Coexisting Eosinophilic Esophagitis
| Characteristic | IBD-EoE (n = 95) | IBD controls (n = 190) | P |
|---|---|---|---|
| Mean age (years) (SD) | 33.4 (15.9) | 40.6 (15.1) | <0.001 |
| Age at IBD diagnosis (years) (SD) | 22.3 (13.1) | 29.0 (14.6) | <0.001 |
| Duration of IBD (years) (SD) | 11.1 (7.7) | 11.7 (9.1) | 0.6 |
| Male (%) | 76 (80) | 87 (45.8) | <0.001 |
| Caucasian (%) | 85 (89.5) | 154 (81.1) | 0.26 |
| Current or former smoking (%) | 15 (15.8) | 52 (27.4) | 0.09 |
| Crohn’s Disease (CD) | |||
| CD patients (%) | 56 (59.0) | 102 (53.7) | 0.40 |
| CD location (%) | 0.54 | ||
| Ileal | 10 (17.9) | 27 (26.5) | |
| Colonic | 16 (28.6) | 21 (20.6) | |
| Ileocolonic | 28 (50.0) | 51 (50.0) | |
| Upper | 2 (3.6) | 3 (2.9) | |
| CD behavior (%) | 0.86 | ||
| Nonstricturing, Nonpenetrating | 32 (57.1) | 62 (60.8) | |
| Stricturing | 10 (17.9) | 15 (14.7) | |
| Penetrating | 14 (25.0) | 25 (24.5) | |
| Perianal CD (%) | 13 (23.2) | 13 (12.8) | 0.09 |
| Ulcerative Colitis (UC) | |||
| UC disease extension (%) | 0.47 | ||
| Proctitis | 4 (10.3) | 10 (11.4) | |
| Left sided colitis | 11 (28.2) | 34 (38.6) | |
| Pancolitis | 24 (61.5) | 44 (50.0) | |
| IBD management | |||
| IBD related surgery (%) | 18 (19.0) | 46 (24.2) | 0.32 |
| IBD medication use (%) | |||
| 5-aminosalicylates | 77 (81.1) | 144 (75.8) | 0.10 |
| Immunomodulators | 29 (30.5) | 64 (33.7) | 0.80 |
| Methotrexate | 15 (15.8) | 20 (10.5) | 0.09 |
| Oral corticosteroids | 59 (62.0) | 99 (52.1) | 0.24 |
| Anti-tumor necrosis factor | 49 (51.6) | 81 (42.6) | 0.36 |
| Vedolizumab | 12 (12.8) | 87 (45.8) | 0.61 |
| Any biologic use | 51 (53.7) | 87 (45.8) | 0.21 |
Figure 1.
Age of IBD diagnosis of patients with concomitant inflammatory bowel disease and eosinophilic esophagitis.
IBD Disease Phenotype and Natural Behavior
Table 1 compares the IBD disease phenotype and natural behavior between the IBD-EoE and IBD groups. There was no difference in the distribution of IBD type among the 2 groups (41.0% UC vs 46.3% UC; P = 0.71). Among those diagnosed with UC, we did not find any difference in disease extent between the UC-EoE and UC groups (P = 0.47). In those diagnosed with CD, we did not find any difference in the distribution of disease location with respect to the terminal ileum, colon, or ileo-colon (P = 0.54) between the CD-EoE and CD groups. Additionally, there was no difference in disease behavior (P = 0.86). However, there was a trend toward more perianal disease in the CD-EoE group compared with the CD group (23.2% vs 12.8%; P = 0.09). There were no differences in the use of various medical therapies, including 5-aminosalicylates, immunomodulators, methotrexate, or biologics or IBD-related surgery between the 2 groups.
EoE Characteristics
On biopsy, the mean number of eosinophils per high powered field was 36.4 (range 15–130/hpf). The most common presenting EoE symptoms were gastroesophageal reflux disease (51%), dysphagia (44%), and/or chest pain (17%). Eosinophilic esophagitis was treated with swallowed steroids (38%), dietary restriction (29%), endoscopic dilation (9%), and/or proton pump inhibitors (62%).
Comparison of Disease Characteristics Based on Initial Diagnosis in Coexisting Disease
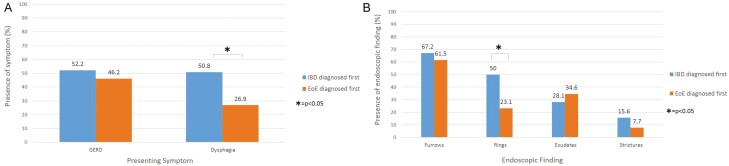
We conducted a subanalysis to compare IBD and EoE characteristics based on which disease was diagnosed first (Table 2) in those with coexisting disease. Inflammatory bowel disease was diagnosed before EoE in 69 patients (73%), with a mean duration of 7.3 years between diagnoses. In terms of IBD characteristics, there was no difference noted in IBD disease location or behavior based on whether EoE or IBD was diagnosed first. Those for whom IBD preceded EoE were more likely to have an IBD-related surgery (25% vs 0%, P = 0.004), but this was in the context of having had a longer IBD disease duration at the time of data collection. There was a trend toward more vedolizumab use in those for whom IBD was diagnosed first (17.9% vs 0%; P = 0.08). There were similar rates of other biologic use, in addition to 5-aminosalicylates, methotrexate, and immunomodulatory use between the groups. In terms of EoE characteristics, those who had IBD diagnosed before EoE tended to present more frequently with dysphagia compared with patients where EoE was diagnosed first (50.8% vs 26.9%; P = 0.04; Fig. 2A). Additionally, patients were more frequently found to have esophageal rings as an initial endoscopic finding if IBD was diagnosed before EoE (50.0% vs 23.1%; P = 0.02; Fig. 2B). There was no difference in EoE management (proton pump inhibitor, swallowed steroid, dietary restriction, and/or esophageal dilation) based on which disease was diagnosed first. There was also no difference in IBD medications used based on which disease was diagnosed first.
Table 2.
Comparison of Characteristics of Eosinophilic Esophagitis Based on Whether Diagnosis Preceded or Followed the Diagnosis of Inflammatory Bowel Diseases
| Characteristic | IBD preceded EoE (n = 67) | EoE preceded IBD (n = 26) | P |
|---|---|---|---|
| Demographics | |||
| Mean age (years) (SD) | 36.3 (16.6) | 26.2 (11.7) | 0.01 |
| Age at IBD diagnosis (years) (SD) | 22.3 (14) | 22.3 (11.1) | 0.99 |
| Duration of IBD (years) (SD) | 13.6 (7.3) | 4.2 (3.6) | <0.001 |
| Male (%) | 56 (83.6) | 19 (73.1) | 0.25 |
| Caucasian (%) | 61 (91.0) | 22 (84.6) | 0.81 |
| Current of former smoking (%) | 12 (17.9) | 3 (11.5) | 0.45 |
| Presenting Symptom (%) | |||
| Dysphagia | 34 (50.8) | 7 (26.9) | 0.04 |
| GERD | 35 (52.2) | 12 (46.2) | 0.6 |
| Initial endoscopic finding (%) | |||
| Rings | 32 (50.0) | 6 (23.1) | 0.02 |
| Furrows | 43 (67.2) | 16 (61.5) | 0.61 |
| Exudates | 18 (28.1) | 9 (34.6) | 0.54 |
| Strictures | 10 (15.6) | 2 (7.7) | 0.32 |
| Management (%) | |||
| Swallowed steroids | 27 (40.3) | 7 (26.9) | 0.36 |
| Dietary modification | 15 (22.4) | 12 (46.2) | 0.07 |
| Proton pump inhibitor | 39 (59.1) | 17 (68.0) | 0.44 |
| Endoscopic dilation | 7 (10.5) | 2 (7.7) | 0.69 |
| Past or present IBD management at the time of EoE diagnosis (%) | |||
| 5-aminosalicylates | 52 (77.6) | 24 (92.3) | 0.11 |
| Immunomodulators | 22 (32.8) | 5 (19.2) | 0.34 |
| Methotrexate | 11 (16.4) | 2 (7.7) | 0.48 |
| Oral corticosteroids | 42 (62.7) | 15 (57.7) | 0.9 |
| Anti- tumor necrosis factor | 35 (52.2) | 12 (46.2) | 0.25 |
| Vedolizumab | 12 (17.9) | 0 (0) | 0.43 |
| Any biologic | 37 (55.2) | 12 (46.2) | 0.43 |
Figure 2.
Presenting symptoms (A) and endoscopic findings (B) in patients who have been diagnosed with both inflammatory bowel diseses and eosinophilic esophagitis.
Discussion
Population-based studies have demonstrated an increasing incidence of both IBD and EoE over the past 2 decades, with changing environmental exposures on a background of genetic susceptibility playing a role. In addition to clinical co-occurrence, EoE and IBD share various similarities in disease mechanisms, genetic predisposition, and relationship to other autoimmune diseases. Both diseases may be associated with overexpression of interleukin-13 that disrupts the mucosal barrier in the gastrointestinal tract.29, 30 Like EoE, a subset of IBD patients are known to have peripheral and gastrointestinal eosinophilia.31–33 Additionally, both diseases are associated with familial clusters34 and have genetic variants that predispose an individual to developing each disease.35 Finally, EoE and IBD are similar in that that they are both associated with developing other autoimmune disorders.8, 19, 20, 36, 37 Thus, studying the interplay between these 2 diseases and the impact of each on the phenotype and clinical course of the other is important.
Our data suggest that patients who have coexisting EoE and IBD are diagnosed with IBD were predominantly male. Epidemiologic studies have shown EoE to be more prevalent in males,17, 38 which is possibly due to gender related differences in gene transcription in mast cells and eosinophils.39 However, our cases were younger and predominantly male irrespective of whether IBD or EoE was diagnosed first. This suggests that the demographics of our study population was not entirely driven by known EoE demographics and is likely reflective of true interaction between the 2 diseases.
Our patients with IBD-EoE were diagnosed with IBD approximately 7 years earlier than those without EoE. It is well established that many immune-mediated diseases can affect patients at a relatively young age. For example, type 1 diabetes mellitus is frequently diagnosed between 5 and 14 years old,40 multiple sclerosis is typically diagnosed between 20 and 40 years old,41 and systemic lupus erythematosus is typically diagnosed between 16 and 55 years old.42 Interestingly, systemic lupus erythematosus and type 1 diabetes mellitus may be more aggressive or have a more severe presentation in those diagnosed at a younger age, indicating that age may have a relation to disease behavior and natural history.43, 44 Although the typical age of diagnosis is well defined in most immune mediated diseases, our study suggests earlier diagnosis in those with coexistent disease. This is a unique finding, possibly because other studies looking at disease interaction often match controls based on age at diagnosis.20 It is unclear as to why these patients are diagnosed at a younger age. One hypothesis is that this subset of patients has a greater aggregate risk for autoimmunity, possibly by means of genetic predisposition and/or environmental exposure. Studies examining genetic burden of IBD have demonstrated a relationship between higher genetic burden of disease and early age of diagnosis. Earlier diagnosis in the EoE-IBD group could reflect the shared genetics between IBD and EoE.
To our knowledge, there are only 2 other studies that look at the interaction of IBD and EoE in terms of disease phenotype and natural history.21, 22 A recent large prospective cohort study by Limketkai et al21 demonstrated that the incidence of EoE among patients with IBD was 3- to 5-fold higher than in those without IBD. Like our study, it found that patients with coexisting IBD-EoE were predominantly male and younger. They also found that there was an increased composite risk of IBD-related complications in those who had coexisting EoE, such as a higher risk of needing systemic corticosteroids. In contrast, our study did not find significant differences in IBD phenotype and natural history. Namely, there was a similar distribution of IBD type (UC vs CD), disease extent, disease behavior, and the need for surgical management, though there was a trend toward more frequent perianal involvement in those with coexisting EoE and IBD. Fan et al22 similarly found no significant difference in IBD natural history in patients with coexisting disease.
Our study demonstrated that in those with coexistent disease, EoE may present differently based on whether IBD or EoE was diagnosed first. In most of our cases, IBD was diagnosed before EoE, which is consistent with the Fan et al study, which found IBD to be diagnosed before EoE 92% of the time.22 Fan et al further demonstrated that patients with coexistent disease did not have significant eosinophils present on esophageal biopsy taken at the time of IBD diagnosis. This confirmed that EoE was truly absent at the time of initial IBD diagnosis, which we suspect is the case in our study.22
In our patients with coexistent disease, those who had IBD that preceded EoE were more likely to present with dysphagia and esophageal rings than those who had EoE before IBD. Although we did not have a control group of patients who were diagnosed with EoE without IBD, we can compare our patient’s disease characteristics to known EoE cohorts. In our study, patients with EoE diagnosed before IBD presented with dysphagia about 27% of the time. Dysphagia is much more common in other EoE cohorts,38, 45 with some studies showing dysphagia in up to 90% of adults with EoE. Lower rates of dysphagia are typically seen in children with EoE.46 Endoscopically, we had relatively fewer patients with esophageal strictures compared with known EoE cohorts.40, 47 Additionally, our patients for whom EoE was diagnosed before IBD were less likely to have esophageal rings. Though some differences may be due to a different threshold for evaluation in patients under the care of a gastroenterologist for their IBD, this would not be applicable to the cohort in which EoE was diagnosed first, which is where these differences were more striking. Our findings suggest that perhaps activation of different immunologic pathways in those with concomitant IBD and EoE when compared with those without IBD may modify phenotype and natural history of EoE in this population, but further prospective and mechanistic studies are needed to confirm this.
We readily acknowledge several limitations to our study. Though this is the largest study examining this question, the sample size remains limited, and larger cohorts may more robustly define differences between the 2 groups. Further, to fully understand the complex relationship between IBD and EoE, larger studies are needed that incorporate genetic and environmental data. Additionally, the study was retrospective, and even though all outcomes were confirmed by chart review, the follow-up may have been insufficiently complete for all patients. However, one would expect this not to be systematically different between cases and controls. Further studies are also needed to examine the impact of IBD on EoE presentation and course by including EoE control populations. It is also important in such studies to describe if concomitant IBD modifies the response to EoE to different therapeutic mechanisms including proton-pump inhibitor therapy and corticosteroids.
In summary, we demonstrate that patients with coexisting IBD and EoE tend to be predominantly male and diagnosed with IBD at a young age compared with non-EoE IBD controls. However, there is no significant impact of EoE on disease phenotype or long-term disease complications. Further study of the interplay between these 2 diseases are important to shed light on common mechanisms and optimal treatment strategies in the setting of co-occurrence.
Author Contribution: MM and AA contributed to the study design, statistical analysis, drafting of the manuscript, critical revision, and approval of the final manuscript. MM conducted data extraction, and AA supervised the study. All authors had full access to all the data in the study and approved the final manuscript.
Supported by: This work was supported by the National Institutes of Health (R03 DK112909), the Crohn’s and Colitis Foundation, and the Chleck Family Foundation.
Conflicts of Interest: AA has served on scientific advisory boards for Sun Pharma and Kyn Therapeutics and has received research support from Pfizer. MM has no conflicts to declare.
REFERENCES
- 1. Lamb CA, Kennedy NA, Raine T, et al. ; IBD guidelines eDelphi consensus group . British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. [DOI] [PubMed] [Google Scholar]
- 3. Beaugerie L, Sokol H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J Gastroenterol. 2012;18:3806–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blumberg R, Cho J, Lewis J, et al. Inflammatory bowel disease: an update on the fundamental biology and clinical management. Gastroenterology. 2011;140:1701–1703. [DOI] [PubMed] [Google Scholar]
- 5. Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1–20. [DOI] [PubMed] [Google Scholar]
- 6. Wu X, Chen H, Xu H. The genomic landscape of human immune-mediated diseases. J Hum Genet. 2015;60:675–681. [DOI] [PubMed] [Google Scholar]
- 7. Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. [DOI] [PubMed] [Google Scholar]
- 8. Oxford EC, Nguyen DD, Sauk J, et al. Impact of coexistent celiac disease on phenotype and natural history of inflammatory bowel diseases. Am J Gastroenterol. 2013;108:1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Festen EA, Goyette P, Green T, et al. A meta-analysis of genome-wide association scans identifies IL18RAP, PTPN2, TAGAP, and PUS10 as shared risk loci for Crohn’s disease and celiac disease. PLoS Genet. 2011;7:e1001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu JZ, van Sommeren S, Huang H, et al. ; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium . Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet. 2009;10:43–55. [DOI] [PubMed] [Google Scholar]
- 12. Li YR, Li J, Zhao SD, et al. Meta-analysis of shared genetic architecture across ten pediatric autoimmune diseases. Nat Med. 2015;21:1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conway G, Velonias G, Andrews E, et al. The impact of co-existing immune-mediated diseases on phenotype and outcomes in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ananthakrishnan AN, Beaulieu DB, Ulitsky A, et al. Does primary sclerosing cholangitis impact quality of life in patients with inflammatory bowel disease? Inflamm Bowel Dis. 2010;16:494–500. [DOI] [PubMed] [Google Scholar]
- 15. Loftus EV Jr, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Navaneethan U, Venkatesh PG, Jegadeesan R, et al. Comparison of outcomes for patients with primary sclerosing cholangitis associated with ulcerative colitis and Crohn’s disease. Gastroenterol Rep (Oxf). 2016;4:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. 2018;154:319–332.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molina-Infante J, Schoepfer AM, Lucendo AJ, et al. Eosinophilic esophagitis: what can we learn from Crohn’s disease? United European Gastroenterol J. 2017;5:762–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterson K, Firszt R, Fang J, et al. Risk of autoimmunity in EoE and families: a population-based cohort study. Am J Gastroenterol. 2016;111:926–932. [DOI] [PubMed] [Google Scholar]
- 20. Halling ML, Kjeldsen J, Knudsen T, et al. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017;23:6137–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Limketkai BN, Shah SC, Hirano I, et al. Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective population-based analysis. Gut. 2019;68:2152–2160. [DOI] [PubMed] [Google Scholar]
- 22. Fan YC, Steele D, Kochar B, et al. Increased prevalence of esophageal eosinophilia in patients with inflammatory bowel disease. Inflamm Intest Dis. 2019;3:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhatia EG, Cass AL, Markowitz JE. Mo1871 markedly increased prevalence of inflammatory bowel disease among pediatric and adolescent patients with eosinophilic esophagitis. Gastroenterology. 2014;146. doi: 10.1016/s0016-5085(14)62462-8. [DOI] [Google Scholar]
- 24. Mcintire M, Saboorian H, Genta R. Eosinophilic esophagitis is associated with an increased prevalence of inflammatory bowel disease. Am J Gastroenterol. 2013;108. doi: 10.14309/00000434-201310001-00024. [DOI] [Google Scholar]
- 25. Capucilli P, Cianferoni A, Spergel J. OR071 Prevalence of coexisting diagnoses in patients with eosinophilic esophagitis in a large pediatric population. Ann Allergy Asthma Immunol. 2017;119. doi: 10.1016/j.anai.2017.08.053. [DOI] [PubMed] [Google Scholar]
- 26. Talathi S, Knight T, Dimmitt R, et al. Concurrent eosinophilic esophagitis in pediatric patients with inflammatory bowel disease. Ann Allergy Asthma Immunol. 2019;123:313–316. [DOI] [PubMed] [Google Scholar]
- 27. Ananthakrishnan AN, Huang H, Nguyen DD, et al. Differential effect of genetic burden on disease phenotypes in Crohn’s disease and ulcerative colitis: analysis of a North American cohort. Am J Gastroenterol. 2014;109:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dellon ES, Kim EM, Randall C, et al. Sa1833 – mucosal eosinophilia is an independent predictor of vedolizumab efficacy in inflammatory bowel disesase (IBD). Gastroenterology. 2019;156. doi: 10.1016/s0016-5085(19)37900-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suttor VP, Chow C, Turner I. Eosinophilic esophagitis with Crohnʼs disease. Am J Gastroenterol. 2009;104:794–795. [DOI] [PubMed] [Google Scholar]
- 31. Davis BP, Stucke EM, Khorki ME, et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1:e86355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Click B, Anderson AM, Koutroubakis IE, et al. Peripheral eosinophilia in patients with inflammatory bowel disease defines an aggressive disease phenotype. Am J Gastroenterol. 2017;112:1849–1858. [DOI] [PubMed] [Google Scholar]
- 33. Beauvais JC, Aroniadis OC, Lukin DJ. Peripheral eosinophilia as a predictor of outcomes in inflammatory bowel disease: an 11-year retrospective study. Gastroenterology. 2017;152. doi: 10.1016/s0016-5085(17)31466-x. [DOI] [Google Scholar]
- 34. Collins MH, Blanchard C, Abonia JP, et al. Clinical, pathologic, and molecular characterization of familial eosinophilic esophagitis compared with sporadic cases. Clin Gastroenterol Hepatol. 2008;6:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology. 2015;148:1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jensen ET, Martin CF, Shaheen NJ, et al. Su1852 high prevalence of co-existing autoimmune conditions among patients with eosinophilic esophagitis. Gastroenterology. 2013;144. doi: 10.1016/s0016-5085(13)61817-x. [DOI] [Google Scholar]
- 37. Capucilli PS, Cianferoni A, Grundmeier RW, et al. A comparison of comorbid diagnoses in children with and without eosinophilic esophagitis in a large primary care population. J Allergy Clin Immunol. 2018;141. doi: 10.1016/j.jaci.2017.12.440. [DOI] [PubMed] [Google Scholar]
- 38. Kapel RC, Miller JK, Torres C, et al. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316–1321. [DOI] [PubMed] [Google Scholar]
- 39. Gonsalves N, Berdnikovs S, Schroeder H, et al. Gender-specific differences in the molecular signatures of adult Eosinophilic Oesophagitis. Clin Exp Allergy. 2017;47:969–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patterson CC, Dahlquist G, Soltesz G, et al. Variation and trends in incidence of childhood diabetes in Europe. Lancet. 2000;355:873–876. [PubMed] [Google Scholar]
- 41. Ghezzi A. Clinical characteristics of multiple sclerosis with early onset. Neurol Sci. 2004;25(Suppl 4):S336–S339. [DOI] [PubMed] [Google Scholar]
- 42. Ballou SP, Khan MA, Kushner I. Clinical features of systemic lupus erythematosus: differences related to race and age of onset. Arthritis Rheum. 1982;25:55–60. [DOI] [PubMed] [Google Scholar]
- 43. Tucker LB, Uribe AG, Fernández M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus. 2008;17:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sabbah E, Savola K, Ebeling T, et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care. 2000;23:1326–1332. [DOI] [PubMed] [Google Scholar]
- 45. Sgouros SN, Bergele C, Mantides A. Eosinophilic esophagitis in adults: a systematic review. Eur J Gastroenterol Hepatol. 2006;18:211–217. [DOI] [PubMed] [Google Scholar]
- 46. Shaheen NJ, Mukkada V, Eichinger CS, et al. Natural history of eosinophilic esophagitis: a systematic review of epidemiology and disease course. Dis Esophagus. 2018;31. doi: 10.1093/dote/doy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pasha SF, DiBaise JK, Kim HJ, et al. Patient characteristics, clinical, endoscopic, and histologic findings in adult eosinophilic esophagitis: a case series and systematic review of the medical literature. Dis Esophagus. 2007;20:311–319. [DOI] [PubMed] [Google Scholar]