Abstract
Background
Opioid use is associated with excess mortality in patients with inflammatory bowel disease (IBD). Recent data have highlighted that inpatient opioid exposure is associated with postdischarge opioid use in this population. It is unknown if preadmission use of cannabis, which is commonly used for symptom relief among patients with IBD, increases the risk for inpatient opioid exposure when patients lack access to cannabis for symptom management. We sought to determine the association between preadmission cannabis use and inpatient opioid exposure while adjusting for relevant confounders.
Methods
We performed a retrospective cohort study of adult patients hospitalized for IBD within a large academic health system from March 1, 2017, to April 10, 2018. Opioid exposure was calculated by converting the sum of administered opioid doses to intravenous morphine milligram equivalents and dividing by length of stay. We used multivariable linear regression to assess the association between cannabis use and inpatient opioid exposure while adjusting for confounders including IBD severity and preadmission opioid use.
Results
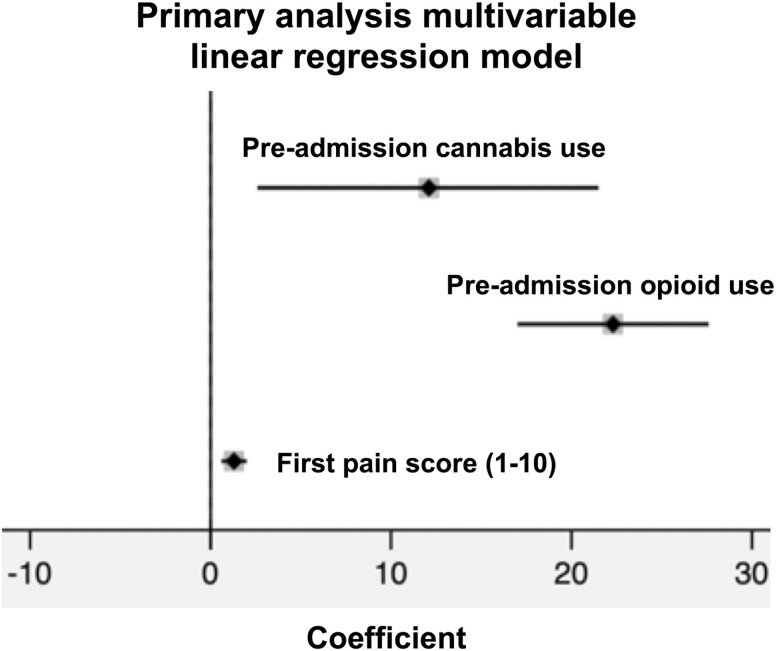
Our study included 423 IBD patients. Linear regression analysis showed a significant positive correlation between inpatient opioid exposure (intravenous morphine milligram equivalents divided by length of stay) and preadmission cannabis use (coefficient = 12.1; 95% confidence interval [CI], 2.6-21.5). Other significantly associated variables were first patient-reported pain score (coefficient = 1.3; 95% CI, 0.6-2.0) and preadmission opioid use (coefficient = 22.3; 95% CI, 17.0-27.6).
Conclusions
Cannabis use is positively correlated with inpatient opioid exposure after controlling for confounders. A personalized pain management approach should be considered to limit inpatient and possibly future opioid exposure among hospitalized patients with IBD who use cannabis.
Keywords: inflammatory bowel disease, opioids/opiates, cannabinoids, Crohn disease, ulcerative colitis
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory systemic disorder associated with a decreased quality of life and variable responses to conventional medical therapy. Opioid analgesics are commonly used to treat the pain of patients who are hospitalized for flares of IBD.1 However, opioid use is associated with excess mortality and infections in patients with IBD, and inpatient opioid exposure is associated with higher odds of postdischarge opioid use, with a dose-dependent effect.2, 3 In addition, recent data suggest that reducing inpatient opioid exposure is associated with shorter hospital length of stay (LOS) and lower readmission rates in this patient population.4 It is prudent to identify patients with IBD who may be susceptible to greater inpatient opioid use because interventions can target these high-risk groups to decrease their opioid exposure and improve outcomes.
Previous research has identified certain independent risk factors for inpatient opioid use in the IBD population, including preadmission opioid use and cigarette smoking.1 However, cannabis, with an estimated 22.2 million users in the United States, has not been investigated as a potential predictor of inpatient opioid exposure.5, 6
Individual state laws have allowed for the medical use of cannabis, which is now utilized as adjunctive therapy in several chronic illnesses, including cancer, AIDS, multiple sclerosis, and chronic pancreatitis.7-9 Although the direct therapeutic mechanisms of cannabis are unclear, the most well-studied compounds within cannabis include cannabidiol and ∆9-tetrahydrocannabinol.10, 11 These are respectively known for their psychotropic and immunomodulatory properties.10-12
With increasing public knowledge of the anti-inflammatory and analgesic effects of cannabis, several studies have shown its use for symptomatic relief of IBD.13-16 However, it is unknown whether cannabis use increases the risk for inpatient opioid exposure when patients with IBD are hospitalized and do not have access to cannabis for symptom management. We therefore sought to determine the association between preadmission cannabis use and inpatient opioid exposure while adjusting for other potential risk factors.
METHODS
Study Design and Patient Enrollment
We conducted a retrospective cohort study of patients aged ≥18 years who were hospitalized at any of 3 urban hospitals within the University of Pennsylvania Health System (UPHS) between March 1, 2017, and April 10, 2018. The health system includes 2 urban major teaching hospitals (ie, members of the Council of Teaching Hospitals) and 1 urban minor teaching hospital (ie, medical school affiliation only), as defined by the 2016 American Hospital Association Survey.17 Hospitalizations with a primary discharge diagnosis of IBD (ICD-10-CM K50x for Crohn disease [CD] and K51x for ulcerative colitis [UC]) or a secondary discharge diagnosis of IBD with an IBD-related complication (Supplementary Table 1) as the primary diagnosis were included. If a patient had multiple hospitalizations during the study period, only the first hospitalization was included to ensure that every hospitalization represented a unique patient. Inpatient demographic and clinical data, social and psychiatric history, home medications, and inpatient medication administrations were obtained from the electronic health record (EHR). This study was approved by the University of Pennsylvania Institutional Review Board.
Independent Variables
The primary independent variable assessed was preadmission cannabis use documented in the EHR at the time of hospitalization. Other categorical independent variables included CD, fistula or abscess diagnoses, pancolitis diagnosis, sex, race, cigarette smoking, psychiatric diagnoses (anxiety, depression, or bipolar disorder), opioid abuse or opioid dependence diagnosis, irritable bowel syndrome diagnosis, prior IBD-related bowel surgery as defined in earlier research,18 IBD-related bowel surgery during hospitalization, perianal or perirectal procedure during hospitalization (incision and drainage of abscess, fistulectomy, seton placement, or exam under anesthesia), and methylprednisolone administration. Any of the following medications listed on the home medication reconciliation were also assessed: opioids (codeine, hydrocodone, hydromorphone, meperidine, morphine, oxycodone, transdermal [TD] fentanyl, buprenorphine, methadone, naloxone, and naltrexone; the last 4 were included because they may identify patients with opioid use disorders), benzodiazepines (alprazolam, clonazepam, diazepam, lorazepam, and temazepam), prednisone, biologic agents (adalimumab, infliximab, certolizumab pegol, golimumab, natalizumab, vedolizumab, and ustekinumab), and immunomodulators (methotrexate, azathioprine, mercaptopurine, and tofacitinib).19 The categorization of these drugs is similar to previously published methods.3 Continuous independent variables included hospital LOS, age, Charlson comorbidity index scores without age, body mass index, serum albumin obtained during hospitalization, and first patient-reported pain scores recorded by nursing staff at the time of hospitalization (1-10 Likert scale, with 10 being the worst possible pain).
Fecal calprotectin and C-reactive protein were not assessed because of availability in <50% of the sample and inconsistency in the specific assay obtained, respectively.
Outcome
The primary outcome was inpatient opioid dose exposure. This was calculated by converting all administered opioid doses to intravenous morphine milligram equivalents (IVMMEs) and dividing the total IVMMEs by LOS in days (IVMMEs/d).20 Inpatient opioids included IV hydromorphone, IV morphine, oral (PO) hydromorphone, PO morphine, PO codeine, PO oxycodone, and TD fentanyl. We excluded IV fentanyl because it is used primarily for endoscopy or other procedural sedation at our institution. Hydrocodone and meperidine are not available at our inpatient pharmacies. Tramadol, a weak mu-opioid receptor agonist that is not associated with increased mortality in patients with IBD, was not considered an opioid in this analysis.21 We converted TD fentanyl to IVMMEs using a conversion rate of 1:100 to PO morphine.22 We excluded IV opioids administered by patient-controlled analgesia (PCA) or continuous infusions from dose calculations because the precise dose totals via these routes could not be determined. Opioids were administered at the discretion of inpatient providers based on the severity of patients’ pain. There were no established protocols for inpatient opioid therapy.
Statistical Analysis
Pearson χ 2 and Kruskal-Wallis tests were used to compare characteristics between patients with IBD who used cannabis and those who did not use cannabis before hospitalization. Multivariable linear regression modeling was used to identify the relationship between preadmission cannabis use and inpatient opioid dose exposure in IVMMEs/day while adjusting for all independent variables. The final regression model was generated using stepwise backward elimination of covariates using a significance threshold of P < 0.05. A subgroup analysis was performed stratifying by IBD type. Stata/IC 15.1 (College Station, TX) was used for all analyses.
Sensitivity Analyses
Prior research has highlighted both higher rates of cannabis use among cigarette smokers and associations between cigarette smoking and inpatient opioid use in patients with IBD.1, 23, 24 We therefore included a sensitivity analysis in which cigarette smoking was maintained as an independent variable (ie, it was not eliminated based on P < 0.05) in the final linear regression model. We then removed cannabis use from this model to observe the association between cigarette smoking and inpatient opioid dose exposure.
Because of high surgical rates for our inpatients with IBD who may commonly receive postoperative PCA, we conducted a second sensitivity analysis in which we examined the correlation between preadmission cannabis use and inpatient opioid exposure among patients who did not undergo surgery during hospitalization.
RESULTS
We evaluated 423 IBD patients between March 1, 2017, and April 10, 2018. Among these patients, 34 (8.0%) had documented cannabis use before hospitalization and 325 (76.8%) received opioid medications during hospitalization. A total of 163 (38.5%) patients received opioids via PCA or continuous IV infusion, for which the associated opioid doses could not be determined or included among total opioid dose exposure. Compared with patients who did not use cannabis before hospitalization, cannabis users more commonly had CD (82.4% vs 64.8%; P = 0.04), were more commonly cigarette smokers (47.1% vs 10.1%; P < 0.01), were more commonly on biologic therapy for IBD (70.6% vs 51.7%; P = 0.03), reported higher pain scores on presentation (median 7 vs median 5; P = 0.03), and had higher total inpatient opioid dose exposure (15.7 vs. 8.0 IVMMEs/d; P = 0.04). Other patient characteristics compared by preadmission cannabis use are presented in Table 1.
Table 1.
Patient Characteristics Compared by Cannabis Use
| Characteristic | No Cannabis (n = 389) | Cannabis (n = 34) | P * |
|---|---|---|---|
| Total opioid dose exposure, IVMMEs/d, median (IQR) | 8.0 (0-24.5) | 15.7 (5.6-31.2) | 0.036 |
| Any inpatient opioid exposure, % | 75.8 | 88.2 | 0.100 |
| First pain score, 1-10, median (IQR) | 5 (0-8) | 7 (3-8) | 0.031 |
| Average pain score, 1-10, median (IQR) | 4.1 (2.2-5.8) | 5.2 (3.7-6.8) | 0.005 |
| CD (%)† | 64.8 | 82.4 | 0.038 |
| Fistula or abscess (%)† | 21.6 | 17.7 | 0.590 |
| Pancolitis (%)† | 8.0 | 5.9 | 0.663 |
| Age, y, median (IQR) | 40 (29-55) | 36.5 (29-42) | 0.128 |
| BMI, median (IQR) | 24.1 (21.1-28.5) | 22.8 (19.9-29.1) | 0.405 |
| Charlson comorbidity index, median (IQR) | 0 (0-1) | 0 (0-1) | 0.910 |
| Female (%) | 55.5 | 41.2 | 0.107 |
| Race (%) | 0.203 | ||
| White | 74.8 | 55.9 | |
| Black | 19.0 | 35.3 | |
| Asian | 2.1 | 2.9 | |
| Hispanic | 2.1 | 2.9 | |
| Other/unknown | 2.1 | 2.9 | |
| Smoking (%) | 10.1 | 47.1 | <0.001 |
| Depression (%)† | 5.9 | 8.8 | 0.498 |
| Anxiety (%)† | 4.9 | 8.8 | 0.321 |
| Bipolar (%)† | 0.3 | 0.0 | 0.767 |
| Irritable bowel syndrome (%)† | 3.1 | 0.0 | 0.299 |
| Opioid abuse or dependence (%)† | 0.8 | 2.9 | 0.210 |
| Home medication, opioid (%) | 57.3 | 61.8 | 0.615 |
| Home medication, benzodiazepine (%) | 20.6 | 20.6 | 0.997 |
| Home medication, prednisone (%) | 29.3 | 38.2 | 0.276 |
| Home medication, immunomodulator (%) | 19.0 | 20.6 | 0.824 |
| Home medication, biologic (%) | 51.7 | 70.6 | 0.034 |
| Prior IBD-related bowel surgery (%) | 46.5 | 32.4 | 0.111 |
| IBD-related bowel surgery during hospitalization (%) | 54.8 | 41.2 | 0.128 |
| Perirectal or perianal procedure during hospitalization (%) | 2.1 | 0.0 | 0.399 |
| IV methylprednisolone administered (%) | 28.8 | 20.6 | 0.308 |
| Serum albumin, g/dL, median (IQR) | 3.4 (2.8-3.9) | 3.5 (3.2-4.0) | 0.243 |
| Length of stay, d, median (IQR) | 4.2 (3.0-7.0) | 3.7 (1.6-5.9) | 0.136 |
*Determined by Kruskal-Wallis and χ 2 tests.
†Identified using appropriate ICD-10 codes on active medical problem list.
BMI indicates body mass index; IQR, interquartile range.
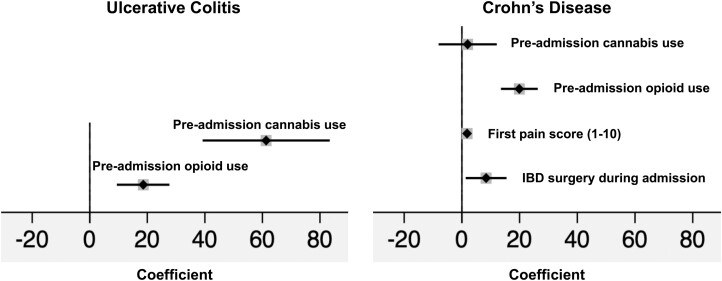
Linear regression analysis (Fig. 1) showed a significant positive correlation between inpatient opioid dose exposure (IVMMEs/d) and preadmission cannabis use (coefficient = 12.1; 95% confidence interval [CI], 2.6-21.5). Other variables significantly correlated with inpatient opioid dose exposure were first patient-reported pain score (coefficient = 1.3; 95% CI, 0.6-2.0) and preadmission opioid use (coefficient = 22.3; 95% CI, 17.0-27.6). We also performed a subgroup analysis by IBD type (Fig. 2). Among patients with UC, preadmission cannabis use (coefficient = 61.3; 95% CI, 39.2-83.4) and preadmission opioid use (coefficient = 18.6; 95% CI, 9.4-27.7) were significantly correlated with inpatient opioid dose exposure. Among patients with CD, preadmission cannabis use was not significantly correlated with inpatient opioid dose exposure (coefficient = 2.0; 95% CI, –8.1 to 12.1). Variables that were significantly correlated with inpatient opioid dose exposure were first patient-reported pain score (coefficient = 1.8; 95% CI, 0.9-2.7), preadmission opioid use (coefficient = 19.9; 95% CI, 13.5-26.3), and IBD-related surgery during hospitalization (coefficient = 8.4; 95% CI, 1.3-15.5).
Figure 1.
Primary analysis forest plot of risk factors for inpatient opioid dose exposure in IVMMEs/day after multivariable linear regression. Preadmission cannabis use coefficient = 12.1; 95% CI, 2.6-21.5; preadmission opioid use coefficient = 22.3; 95% CI, 17.0-27.6; first pain score coefficient = 1.3; 95% CI, 0.6-2.0. Nonsignificant covariates removed via stepwise backward elimination using P < 0.05.
Figure 2.
Subgroup analysis forest plots of risk factors for inpatient opioid dose exposure in IVMMEs/day after multivariable linear regression and stratification by IBD type. UC model: preadmission cannabis use coefficient = 61.3; 95% CI, 39.2-83.4; preadmission opioid use coefficient = 18.6; 95% CI, 9.4-27.7. CD model: preadmission cannabis use coefficient = 2.0; 95% CI, –8.1 to 12.1; preadmission opioid use coefficient = 19.9; 95% CI, 13.5-26.3; first pain score coefficient = 1.8; 95% CI, 0.9-2.7; IBD surgery during admission coefficient = 8.4; 95% CI, 1.3-15.5. Nonsignificant covariates removed via stepwise backward elimination using P < 0.05.
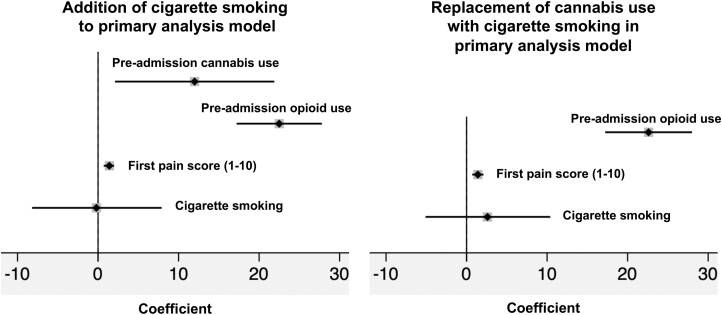
We performed a sensitivity analysis to further explore the interaction between cigarette smoking and cannabis use and their effects on inpatient opioid exposure. We observed that a higher proportion of cigarette smokers used cannabis than did noncigarette smokers (29.1% vs 5.0%; P < 0.01). A linear regression analysis was performed in which cigarette smoking was maintained as an independent variable in the final linear regression model regardless of P value. Cigarette smoking was not significantly associated with inpatient opioid dose exposure (coefficient = –0.2; 95% CI, –8.2 to 7.9), and its presence in the model did not affect the association between preadmission cannabis use and inpatient opioid dose exposure (coefficient = 12; 95% CI, 2.1-21.9) (Fig. 3). When cannabis use was removed from this model, inpatient opioid dose exposure was positively but not significantly associated with cigarette smoking (coefficient = 2.6; 95% CI, –5.1 to 10.4) (Fig. 3).
Figure 3.
Sensitivity analysis forest plots assessing interaction between cigarette smoking and cannabis use and their effects on inpatient opioid dose exposure in IVMMEs/day. Addition of cigarette smoking to primary analysis model: preadmission cannabis use coefficient = 12.0; 95% CI, 2.1-21.9; preadmission opioid use coefficient = 22.5; 95% CI, 17.2-27.8; first pain score coefficient = 1.4; 95% CI, 0.7-2.0; cigarette smoking coefficient = –0.2; 95% CI, –8.2 to 7.9. Replacement of cannabis use with cigarette smoking in primary analysis model: preadmission opioid use coefficient = 22.6; 95% CI, 17.2-28.0; first pain score coefficient = 1.4; 95% CI, 0.7-2.1; cigarette smoking coefficient = 2.6; 95% CI, –5.1 to 10.4. Nonsignificant covariates removed via stepwise backward elimination using P < 0.05.
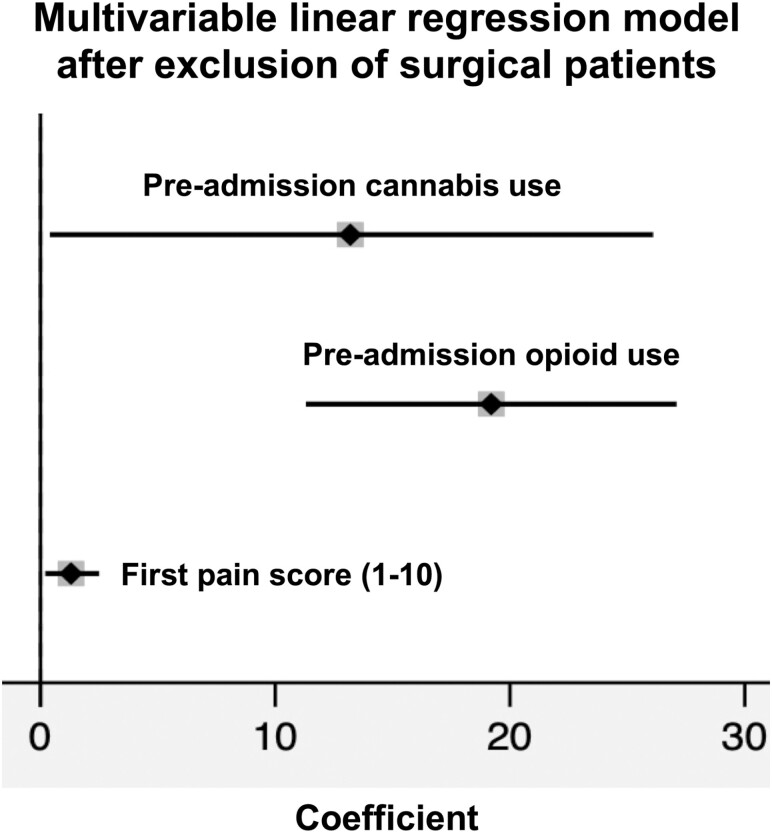
In a second sensitivity analysis, we examined the impact of preadmission cannabis use on inpatient opioid exposure among patients with IBD who did not require surgery during hospitalization. For this population (n = 196), 20 (10.2%) patients used cannabis, 137 (69.6%) patients received inpatient opioids, and 18 (9.2%) patients received opioids via PCA or continuous IV infusion. A greater proportion of patients who used cannabis received inpatient opioids compared with those who did not use cannabis (95.0% vs 67.1%; P = 0.01). Multivariable linear regression showed results similar to the primary analysis (Fig. 4).
Figure 4.
Sensitivity analysis forest plot of risk factors for inpatient opioid dose exposure in IVMMEs/day after multivariable linear regression and exclusion of patients who underwent inpatient surgery. Preadmission cannabis use coefficient = 13.2; 95% CI, 0.4-26.1; preadmission opioid use coefficient = 19.2; 95% CI, 11.3-27.1; first pain score coefficient = 1.3; 95% CI, 0.2-2.5. Nonsignificant covariates removed via stepwise backward elimination using P < 0.05.
Discussion
In this study, we showed that more than 75% of patients hospitalized for flares of IBD received opioids. Preadmission cannabis use was positively correlated with total inpatient opioid dose exposure after controlling for IBD history and severity, patient demographics, psychiatric comorbidities, preadmission opioid use, patient-reported pain scores, and other confounders. This association seems to be driven by patients with UC. We observed a similar correlation between cannabis use and inpatient opioid exposure in our nonsurgical population. Other independent predictors of inpatient opioid dose exposure included first patient-reported pain scores and preadmission opioid use, which are consistent with previous research investigating risk factors of opioid use in IBD.1, 3
Although prior work by Long et al1 showed a positive association between cigarette smoking and inpatient opioid exposure in the IBD population, we found a similar but nonsignificant association only when excluding cannabis use from our regression analysis. When both cannabis use and cigarette smoking were maintained in the same regression model, only cannabis use was significantly correlated with inpatient opioid dose exposure. Given the significantly higher rates of cannabis use among cigarette smokers noted in this study (29.1% in smokers vs 5.0% in nonsmokers; P < 0.01) and others, it is possible that prior associations between cigarette smoking and inpatient opioid requirements are driven by the concomitant use of cannabis, which is believed to provide symptomatic relief in many patients with IBD who may lack access to this therapeutic option in the inpatient setting.23 Cannabis use should therefore be considered as a relevant confounder in any analysis of IBD hospitalization outcomes where cigarette smoking is evaluated as a potential risk factor.
Our study has several strengths. Unlike prior research assessing risk factors for any degree of inpatient opioid use among patients with IBD, our study is the first to focus on the risk factors correlated with higher quantified inpatient opioid dose exposure, which has been associated with postdischarge opioid use in this population.3 We also chose a patient selection strategy that identified individuals hospitalized specifically for flares of IBD; this reduced the likelihood that patients received opioids for management of non-IBD-related pain.18, 25 Because of growing concerns of pain in the absence of objective IBD activity, our study also adjusted for important psychosocial confounders of inpatient pain requirements, including patient-reported pain scores, preadmission opioid and benzodiazepine use, and comorbid psychiatric illness, irritable bowel syndrome, or opioid abuse or dependence diagnoses.26-28
This study has several limitations. The 3-hospital system in which this study took place includes 2 urban major teaching hospitals and 1 urban minor teaching hospital. Although the range in urban hospital teaching status may provide greater variability in inpatient IBD acuity than other single-center studies, it is unknown if our results are generalizable to rural settings.
This retrospective study utilized data obtained from the EHR, which relies on correct ICD-10 coding and is subject to documentation omissions and errors. The EHR precluded precise dose calculations from opioid infusions or PCA, underestimating total opioid dose exposures in our primary analysis. However, our sensitivity analysis excluding surgical patients minimized this underestimation because less than 10% of this population received opioids via infusion or PCA. Because our health system provides tertiary IBD care, we could not quantify the extent or duration of patients’ prehospitalization opioid use. Whereas the use of cannabis is included as part of the history and physical template for all admissions to our hospitals, accurate completion of this documentation was dependent on the admitting providers. To the extent that this misclassification of cannabis use was nondifferential, we have underestimated the magnitude of association. If the documentation of cannabis use were more likely to occur in patients with more pain (ie, those more likely to receive opioids), then we may have overestimated the association. However, the large magnitude of effect observed in this study suggests that such bias would be unlikely to completely eliminate the association. Prospective studies with standardization of cannabis use documentation, including documentation of frequency and usual route of consumption, are thus needed to confirm our results.
Our study is also limited because of potential confounding by indication. Cannabis users in this study had higher pain scores on presentation (median = 7 for cannabis users vs median = 5 for nonusers; 1-10 Likert scale), possibly because of altered perceptions of pain in this population or greater IBD severity that was refractory to other medical therapies. These patients may experience chronic pain, which promotes opioid use.29 However, we controlled for pain scores and attempted to control for disease severity by assessing outpatient IBD therapy, prednisone use, prior IBD surgeries, abscess, fistula or pancolitis diagnoses, LOS, nutritional status (albumin and body mass index), IBD-related surgery, perirectal procedures, or IV methylprednisolone use during the hospitalization. With the exception of higher rates of biologic therapy, patients who used cannabis did not have increased markers of IBD severity compared with patients who did not use cannabis. However, the relatively small sample of cannabis users (n = 34) may be insufficient to detect differences in these factors.
In summary, we identified a positive correlation between preadmission cannabis use and inpatient opioid dose exposure. Although cannabis users account for a small fraction of hospitalized patients with IBD who receive opioids, they likely represent a higher-risk group that is susceptible to greater opioid exposure and possibly subsequent complications. Therefore, more aggressive pain management early in the hospital course using nonopioid alternatives such as acetaminophen, celecoxib, dicyclomine, and hyoscyamine could be emphasized for users of cannabis. Many of these analgesics have been shown to be relatively safe and effective in IBD with opioid-sparing effects.4, 30, 31 In addition, because impulsivity and depressive symptoms have been shown to be more common among patients with IBD who self-medicate their symptoms with cannabis, inpatient psychiatric and/or psychological interventions and assistance with coping mechanisms may be beneficial.23 Moreover, it should be emphasized to patients that although cannabis has been shown to improve some symptoms of IBD, it has not been shown to induce clinical remission or mucosal healing.32 As such, continued use may mask ongoing inflammation and bowel damage. With increasing concerns regarding complications of opioid therapy in the IBD population, a personalized and holistic symptom management approach should be implemented to limit inpatient and possibly future opioid exposure among patients with IBD who use cannabis.
Supplementary Material
Author contributions: RSD: study concept and design, acquisition of data, statistical analysis, analysis and interpretation of data, drafting of manuscript. SP: acquisition of data, critical revision of manuscript for important intellectual content. CKS: acquisition of data, analysis and interpretation of data. JDL: analysis and interpretation of data, critical revision of manuscript for important intellectual content. SM: analysis and interpretation of data, critical revision of manuscript for important intellectual content. GRL: analysis and interpretation of data, critical revision of manuscript for important intellectual content.
Supported by: Grant T32-DK007740 from National Institute of Diabetes and Digestive and Kidney Diseases (training grant for Sonali Palchaudhuri).
Conflicts of interest: Gary Lichtenstein has received compensation for research/grant support, support for lectures, and for scientific advisory committee participation from the following companies: Abbott, Actavis, Alaven, CellCeutrix, Celgene, Clinical Advances in Gastroenterology, Ferring, Gastro-Hep Communications, Gilead, Hospira, Ironwood, Janssen Orthobiotech, Luitpold/American Regent, Merck, McMahon Publishing, Pfizer Pharmaceuticals, Prometheus Laboratories, Romark, Salix Pharmaceuticals, Santarus, Shire Pharmaceuticals, Slack, Springer Science and Business Media, Takeda, UCB, and Up-To-Date. James Lewis has received consulting honoraria from Pfizer, Gilead, UCB, Janssen Orthobiotech, Celgene, AbbVie, Samsung Bioepis, Bridge Biotherapeutics, Merck, Eli Lilly, Bristol-Myers Squibb, Arena Pharmaceuticals, and Takeda. He has received research funding from Takeda, Janssen, and Nestle Health Sciences.
REFERENCES
- 1. Long MD, Barnes EL, Herfarth HH, et al. Narcotic use for inflammatory bowel disease and risk factors during hospitalization. Inflamm Bowel Dis. 2012;18:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107:1409–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dalal RS, Palchaudhuri S, Snider CK, et al. Exposure to intravenous opioids is associated with future exposure to opioids in hospitalized patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2019. pii: S1542-3565(19)31504-6. doi: 10.1016/j.cgh.2019.12.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4. Dalal RS, Palchaudhuri S, Snider C, et al. A multimodal intervention reduces intravenous opioid exposure among hospitalized patients with inflammatory bowel disease. Paper presented at: American College of Gastroenterology Annual Scientific Meeting; October 29, 2019;San Antonio, TX. [Google Scholar]
- 5.Center for Behavioral Health Statistics and Quality. 2015 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration. 2016. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed January 15, 2020. [Google Scholar]
- 6. UCLA Medical Marijuana Research. Distribution of Medical Marijuana Dispensaries from 2007 to 2014, Los Angeles, CA 2015. http://www.uclamedicalmarijuanaresearch.com/sites/default/files/MMD_ResearchBrief1v5.pdf. Accessed January 15, 2020. [Google Scholar]
- 7. Gerich ME, Isfort RW, Brimhall B, et al. Medical marijuana for digestive disorders: high time to prescribe? Am J Gastroenterol. 2015;110:208–214. [DOI] [PubMed] [Google Scholar]
- 8. Belendiuk KA, Baldini LL, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barlowe TS, Koliani-Pace JL, Smith KD, et al. Effects of medical cannabis on use of opioids and hospital visits by patients with painful chronic pancreatitis. Clin Gastroenterol Hepatol. 2019;17:2608–2609.e1. [DOI] [PubMed] [Google Scholar]
- 10. Kaplan BL, Springs AE, Kaminski NE. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem Pharmacol. 2008;76:726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Sickle MD, Duncan M, Kingsley PJ, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. [DOI] [PubMed] [Google Scholar]
- 13. Storr M, Devlin S, Kaplan GG, et al. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014;20:472–480. [DOI] [PubMed] [Google Scholar]
- 14. Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:891–896. [DOI] [PubMed] [Google Scholar]
- 15. Ravikoff Allegretti J, Courtwright A, Lucci M, et al. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2809–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weiss A, Friedenberg F. Patterns of cannabis use in patients with inflammatory bowel disease: a population based analysis. Drug Alcohol Depend. 2015;156:84–89. [DOI] [PubMed] [Google Scholar]
- 17.American Hospital Association. Data collection methods: the gold standard for hospital trend analysis. Accessed April 24, 2020.http://www.ahadata.com/data-collection-methods/.
- 18. Dalal RS, Vajravelu RK, Lewis JD, et al. Hospitalization outcomes for inflammatory bowel disease in teaching vs nonteaching hospitals. Inflamm Bowel Dis. 2019;25:1974–1982. [DOI] [PubMed] [Google Scholar]
- 19. Marren AS. Tofacitinib is not a biologic. Ann Gastroenterol. 2017;30:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MedCalc . Narcotic Equivalence Converter. 2019. http://www.medcalc.com/narcotics.html. Accessed December 15, 2019. [Google Scholar]
- 21. Burr NE, Smith C, West R, et al. Increasing prescription of opiates and mortality in patients with inflammatory bowel diseases in England. Clin Gastroenterol Hepatol. 2018;16:534–541.e6. [DOI] [PubMed] [Google Scholar]
- 22. Donner B, Zenz M, Tryba M, et al. Direct conversion from oral morphine to transdermal fentanyl: a multicenter study in patients with cancer pain. Pain. 1996;64:527–534. [DOI] [PubMed] [Google Scholar]
- 23. Hansen TM, Sabourin BC, Oketola B, et al. Cannabis use in persons with inflammatory bowel disease and vulnerability to substance misuse. Inflamm Bowel Dis. 2020;26:1401–1406. [DOI] [PubMed] [Google Scholar]
- 24. Hindocha C, Shaban ND, Freeman TP, et al. Associations between cigarette smoking and cannabis dependence: a longitudinal study of young cannabis users in the United Kingdom. Drug Alcohol Depend. 2015;148:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ananthakrishnan AN, McGinley EL, Binion DG. Does it matter where you are hospitalized for inflammatory bowel disease? A nationwide analysis of hospital volume. Am J Gastroenterol. 2008;103:2789–2798. [DOI] [PubMed] [Google Scholar]
- 26. Edwards JT, Radford-Smith GL, Florin TH. Chronic narcotic use in inflammatory bowel disease patients: prevalence and clinical characteristics. J Gastroenterol Hepatol. 2001;16:1235–1238. [DOI] [PubMed] [Google Scholar]
- 27. Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100:2225–2229. [DOI] [PubMed] [Google Scholar]
- 28. Hanson KA, Loftus EV Jr, Harmsen WS, et al. Clinical features and outcome of patients with inflammatory bowel disease who use narcotics: a case-control study. Inflamm Bowel Dis. 2009;15:772–777. [DOI] [PubMed] [Google Scholar]
- 29. Shah A, Hayes CJ, Martin BC. Factors influencing long-term opioid use among opioid naive patients: an examination of initial prescription characteristics and pain etiologies. J Pain. 2017;18:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makharia GK. Understanding and treating abdominal pain and spasms in organic gastrointestinal diseases: inflammatory bowel disease and biliary diseases. J Clin Gastroenterol. 2011;45(Suppl):S89–S93. [DOI] [PubMed] [Google Scholar]
- 31. Sandborn WJ, Stenson WF, Brynskov J, et al. Safety of celecoxib in patients with ulcerative colitis in remission: a randomized, placebo-controlled, pilot study. Clin Gastroenterol Hepatol. 2006;4:203–211. [DOI] [PubMed] [Google Scholar]
- 32. Naftali T, Bar-Lev Schleider L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11:1276–1280.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.