Abstract
Patients with active cancer are at an increased risk of arterial and venous thromboembolism (VTE) and bleeding events. Historically, in patients with cancer, low molecular weight heparins have been preferred for treatment of VTE, whereas warfarin has been the standard anticoagulant for stroke prevention in patients with atrial fibrillation (AF). More recently, direct oral anticoagulants (DOACs) have been demonstrated to reduce the risk of venous and arterial thromboembolism in large randomized clinical trials of patients with VTE and AF, respectively, thus providing an attractive oral dosing option that does not require routine laboratory monitoring. In this review, we summarize available clinical trial data and guideline recommendations, and outline a practical approach to anticoagulation management of VTE and AF in cancer.
Keywords: anticoagulation, atrial fibrillation, bleeding, cancer, cardiooncology, venous thromboembolism
Patients with active cancer are at increased risk of arterial and venous thromboembolism (VTE) and bleeding events. As life expectancy for many cancers increases with use of more patient- and tumor-targeted therapies, safe and effective strategies to ameliorate VTE burden and in thromboprophylaxis of atrial fibrillation (AF) are much needed. Anticoagulation strategies are a cornerstone component of management in the growing subspecialty of “cardio-oncology.” The selection of patients who have an acceptable risk-benefit profile for initiation of anticoagulation is complex given individual patient goals and preferences, changing prognosis of specific cancers, common comorbidities, potential drug–drug interactions, malnourished/underweight states, and competing risks of morbidity and mortality. Historically, low molecular weight heparins (LMWH) have been preferred for cancer-associated VTE given prior studies demonstrating reduced risks of recurrent VTE compared with warfarin (1,2). Meanwhile, vitamin K antagonists (VKA) have been preferred for stroke prevention in AF and cancer (3). However, injectable anticoagulation therapies and oral therapies that require frequent blood testing are burdensome, costly, and may introduce excessive patient discomfort. Moreover, the landscape of oral anticoagulation is rapidly evolving with the introduction and widespread use of direct oral anticoagulants (DOACs) in VTE and AF. We summarize the epidemiology, mechanistic drivers, available clinical trial data, and guideline recommendations for treatment of VTE and AF in patients with cancer.
BURDEN OF VENOUS AND ARTERIAL THROMBOTIC COMPLICATIONS IN PATIENTS WITH CANCER
Patients with cancer face risks of a broad range of thromboembolic complications. Up to one-third of VTE is cancer-associated in contemporary community-based cohorts (4). In addition to the magnified risks of index VTE events (5), cancer patients are at a 2- to 9-fold increased risk of recurrence (6), and as many as one-half of VTE are incidentally detected (7). Cancer-associated VTE, whether symptomatic or incidental, is a marker of poor prognosis (8).
Patients with cancer are at increased risk of arterial thromboembolic events, especially in certain cancer types (e.g., lung cancer) at advanced stages (9), and after vasculotoxic cancer therapies (e.g., radiation). Indeed, arterial thromboses may be a heralding feature of occult cancer (10). Patients who experience arterial ischemic events face high risks of short- (11) and long-term mortality (12).
CANCER AND ATRIAL FIBRILLATION
AF occurs frequently (13) and is a common reason for cardiovascular consultation in the cancer population. Up to one-quarter of the overall AF population has comorbid cancer (14). New-onset AF is associated with higher rates of occult cancer diagnosis (15,16). However, a causal relationship is uncertain, as AF may be a risk marker for cancer and increased cancer diagnosis may be attributable to detection bias. AF in cancer identifies patients at heightened risk of adverse cardiovascular events, including heart failure (17).
MECHANISMS DRIVING THROMBOSIS AND BLEEDING IN CANCER
The etiology of dysregulated hemostasis in cancer is multifactorial and has been linked to extent of disease, tumor biology, local and systemic inflammation, cancer therapeutics, and patient-related factors (18,19) (Figure 1).
FIGURE 1. Factors Contributing to Increased Thrombotic Risks in Cancer.

Three components of Virchow’s Triad (stasis, endothelial injury, and hypercoagulability) intersect and contribute to excess cancer-associated thrombotic risks.
VENOUS AND ARTERIAL THROMBOTIC EVENT PATHOGENESIS.
Aggressive solid tumors with early metastatic potential and advanced stage are associated with higher VTE risk (19). Tumor cells elaborate procoagulants such as tissue factor–bearing circulating microparticles (20) and inflammatory cytokines (18). Tumor vascular invasion and compression contribute to endothelial damage and stasis.
Cancer type is strongly correlated with thrombotic risk (21). Specific cancer gene mutations can also predispose to VTE (e.g., JAK2 mutations via integrin activation [22]). Solid organ malignancies (Table 1) with highest thrombotic risks include pancreatic, gastric, brain, lung, ovarian, and renal cancers. All hematological cancers appear to carry a high risk of VTE. However, most cancer-associated VTE is observed with prostate, breast, and colon cancers due to higher prevalence despite lower thrombotic risks (18).
TABLE 1.
Select Cancers Associated With Thrombotic Adverse Events
| Cancers | Unique Phenotypes and Select Examples | Thrombotic Presentations | |
|---|---|---|---|
| Very high risk | Pancreas thrombosis | — | Migratory thrombophlebitis; Portal vein |
| Stomach | — | Migratory thrombophlebitis | |
| Metastatic | |||
| High risk | Gynecological | Clear cell carcinoma | Pelvic venous obstruction |
| Lung | Mucinous adenocarcinoma | ||
| Brain | High-grade gliomas | ↑ Post-operative venous thromboses | |
| Hematological | Multiple myeloma; high-grade/bulky lymphomas | ||
| Genitourinary (excluding prostate) | Renal cell carcinoma | Renal vein and caval tumor invasion and thrombosis | |
| Modest risk | Breast | Highly prevalent, with modest/low thrombotic risks | |
| Prostate | |||
| Colon | |||
Thrombotic risk categories were adapted from the validated Khorana score (21). Deep vein thrombosis and pulmonary embolism are the most frequent thrombotic events experienced by patients with cancer, but we highlight other, unique thrombotic presentations relevant to specific cancer types.
Various cancer therapies carry important treatment-related VTE risks (23) (Table 2). Surgery is associated with a 2-fold increased risk of postoperative VTE and a 4-fold increased risk of pulmonary embolism (PE)–related death in cancer. Central venous catheters (CVCs) needed during the course of therapy are associated with thrombosis. Patient-related factors, such as functional impairment, extremes of body weight, black race, advanced age, and comorbidities, are associated with elevated VTE risks (24).
TABLE 2.
Select Cancer Therapies Associated With Atrial Arrhythmias and Thrombotic Adverse Events
| Class | Anti-Cancer Mechanism | Select Drug Examples | Mechanism of Cardiovascular Toxicity |
|---|---|---|---|
| Cancer therapies associated with atrial arrhythmias | |||
| Anthracyclines | Inhibition of DNA/RNA synthesis via topoisomerase inhibition | Doxorubicin | ? Direct cardiotoxicity |
| Daunorubicin | |||
| Idarubicin | |||
| Epirubicin | |||
| Alkylating agents | Inhibition of DNA/RNA synthesis via formation of carbonium ions | Melphalan | Unknown |
| Anti-metabolites | Inhibition of DNA/RNA synthesis via acting as a pyrimidine analog | Fluorouracil | ? Ischemia |
| Interleukins | Immunotherapy | Il-2 | Inflammation |
| Bruton’s tyrosine kinase TKIs | Inhibition of Bruton’s tyrosine kinase | Ibrutinib | ? Direct kinase inhibition |
| Acalabrutinib | |||
| Immune checkpoint inhibitors | Activation of immune system | Ipilimumab | Cardiac inflammation; myocarditis, pericarditis, vasculitis |
| Nivolumab | |||
| Pembrolizumab | |||
| Cancer therapies associated with venous/arterial thrombotic risk | |||
| Platinum-based | Inhibition of DNA synthesis via formation of DNA cross-links | Cisplatin | Unknown |
| Hormonal therapy | Inhibition of estrogen signaling (activated in breast cancer) | Tamoxifen | Unknown |
| Anti-VEGF therapy | Inhibition of angiogenesis (may include either biologics or small molecule TKIs) | Bevacizumab | Endothelial damage; ? thrombotic microangiopathy |
| Sunitinib | |||
| Pazopanib | |||
| BCR-ABL TKI | Inhibition of ABL1 kinase (activated in certain leukemias) | Nilotinib | Endothelial damage |
| Ponatinib | |||
| Immunomodulators | Activation of protein degradation (specifically transcription factors activated in B-cell cancer types) | Thalidomide | Unknown |
| Lenalidomide | |||
| Pomalidomide | |||
| Proteasome inhibitors | Inhibition of protein degradation machinery | Carfilzomib | Unknown |
DNA = deoxyribonucleic acid; IL = interleukin; RNA = ribonucleic acid; TKI = tyrosine kinase inhibitor; VEGF = vascular endothelial growth factor.
Arterial thrombosis in cancer is less well-studied compared with VTE, but both share similar risk factors. These events are likely multifactorial, with greater propensity to occur with vasculotoxic cancer therapies and greater cancer burden (25).
AF PATHOGENESIS.
Shared epidemiology and risk factors contribute to the association between cancer and AF, which both increase with age and age-related comorbidities. Pathobiologies of cancer and AF are also linked by increased sympathetic drive, anemia, pulmonary and pericardial cancer involvement, paraneoplastic processes, inflammation, and specific interventions (e.g., surgery). Certain cancer therapies may increase risk of AF (26). For instance, ibrutinib, a tyrosine kinase inhibitor, can lead to AF in 6% to 16% of treated patients (27). Broadly, proposed mechanisms driving cancer therapy–related arrhythmogenicity and AF include membrane channel–specific interactions, excess oxidative stress, and increased levels of inflammatory mediators (26).
BLEEDING PATHOGENESIS.
Increased propensity for bleeding (28) may be explained by cancer-related thrombocytopenia, disseminated intravascular coagulation, and elaboration of fibrinolytic factors by tumor cells (29). Direct invasion, especially with certain cancers (e.g., renal, gastrointestinal, melanoma), may result in increased vascular fragility. Chemotherapy-related bone marrow suppression, radiation-induced tissue damage, and post-surgical wound healing issues all increment bleeding risks.
CLINICAL DATA OF ANTICOAGULATION APPROACHES IN CANCER
LMWH have been the standard of care in treating cancer-associated VTE. Data supporting the use of LMWH over VKA are derived from 2 large randomized clinical trials (RCTs). In an initial trial of 676 cancer patients with acute VTE, 6-month treatment with dalteparin significantly reduced VTE recurrence by 52% without influencing rates of major bleeding or mortality compared with VKA (1). More recently, in 900 cancer patients with acute VTE, 6-month treatment with tinzaparin nonsignificantly reduced the risk for VTE recurrence (7.6% vs. 10.5%; p = 0.07), did not affect major bleeding or mortality, and significantly reduced nonmajor bleeding (10.9% vs. 15.3%; p = 0.004) compared with warfarin (2). Notably, the times in therapeutic range with VKA were <50% in these trials, which may have reduced VKA efficacy.
Emerging head-to-head trials of DOACs versus LMWH have recently completed or are actively underway (Table 3). Hokusai VTE Cancer was an open-label RCT that compared 6 to 12 months of the once-daily, oral factor Xa inhibitor edoxaban versus dalteparin in symptomatic or incidental VTE in 1,050 patients with cancer. Edoxaban was noninferior to dalteparin with respect to composite recurrent VTE and major bleeding (12.8% vs. 13.5%). Recurrent VTE was reduced by edoxaban compared with dalteparin (7.9% vs. 11.3%), but major bleeding was increased (6.9% vs. 4.0%), driven by higher bleeding rates in patients with gastrointestinal cancers (13.2% vs. 2.4%) (30). The Select-D Pilot trial (31) was an open-label RCT of 406 patients with cancer and VTE treated for 6 months, and showed that rivaroxaban reduced the risk of recurrent VTE (4% vs. 11%), but increased the risk of clinically-relevant nonmajor bleeding (13% vs. 2%) compared with dalteparin (31).
TABLE 3.
Available Dedicated Trials or Subsets of Landmark Phase III Trials Evaluating DOACs in Cancer
| Trials (Ref. #) | DOAC | Comparator | Duration | Active Cancer Patients | Efficacy Endpoint | Efficacy Endpoint: DOAC vs. VKA HR (95% CI) |
|
|---|---|---|---|---|---|---|---|
| Active Cancer at Enrollment | Cancer Diagnosed in Follow-Up | ||||||
| Venous thromboembolism | |||||||
| Hokusai VTE Cancer (30) | Edoxaban 60 mg once daily | Dalteparin 200 IU/kg daily for 1 month, followed by 150 IU/kg daily | 6–12 months | Edoxaban n = 522, Dalteparin n = 524 | Recurrent VTE or major bleeding | 0.97 (0.70–1.36) | N/A |
| Select-D™ Pilot (31) | Rivaroxaban 15 mg twice daily for 21 days, followed by 20 mg once daily | Dalteparin 200 IU/kg daily for 1 month, followed by 150 IU/kg daily | 6 months | Rivaroxaban n = 203, Dalteparin n = 203 | Recurrent VTE | 0.43 (0.19–0.99) | N/A |
| RE-COVER I, II (35) | Dabigatran 150 mg twice daily | Warfarin INR 2–3 | 6 months | Dabigatran n = 173, Warfarin n = 162 | Symptomatic recurrent VTE or VTE-related death | 0.74 (0.20–2.70) | 0.63 (0.20–2.0) |
| EINSTEIN PE/DVT (33) | Rivaroxaban 15 mg twice daily for 21 days, followed by 20 mg once daily | Warfarin or acenocoumarol INR 2–3 | 3, 6, or 12 months | Rivaroxaban n = 354, VKA n = 301 | Symptomatic or recurrent VTE | 0.62 (0.21–1.79) | 0.80 (0.34–1.88) |
| AMPLIFY (32) | Apixaban 10 mg twice daily for 7 days followed by 5 mg twice daily | Warfarin INR 2–3 | 6 months | Apixaban n = 88, Warfarin n = 81 | VTE/VTE-related death | 0.74 (0.20–2.70) | 0.63 (0.20–2.0) |
| Hokusai-VTE (34) | Edoxaban 60 mg once daily | Warfarin INR 2–3 | 3–12 months | Edoxaban n = 109, Warfarin n = 99 | Recurrent DVT, fatal or nonfatal PE | 0.55 (0.16–1.51) | 0.73 (0.36–1.49) |
| Atrial fibrillation | |||||||
| ENGAGE AF-TIMI 48 (39) | Edoxaban once daily | Warfarin INR 2–3 | Median follow-up 2.8 years | Edoxaban n = 758, Warfarin n = 395 | Stroke or systemic embolic event | N/A | 0.60 (0.31–1.15)* |
| ARISTOTLE (40) | Apixaban 5mg twice daily | Warfarin INR 2–3 | Median follow-up 1.8 years | Apixaban n = 76, Warfarin n = 81 | Stroke or systemic embolic event | 0 vs. 3.3 events per 100 patient-yrs | — |
Higher-dose edoxaban regimen (60/30 mg) vs. warfarin.
AMPLIFY = Apixaban for the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis as First-Line Therapy; ARISTOTLE = Apixaban for Reduction in STroke and Other ThromboemboLic Events in atrial fibrillation; CI = confidence interval; DOAC = direct oral anticoagulants; DVT = deep vein thrombosis; EINSTEIN DVT = Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients with Acute Symptomatic Deep-Vein Thrombosis; EINSTEIN PE = Oral Direct Factor Xa Inhibitor Rivaroxaban in Patients with Acute Symptomatic Pulmonary Embolism; Effective Anticoagulation with factor Xa next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction study 48; Hokusai-VTE = Comparative Investigation of Low Molecular Weight (LMW) Heparin/Edoxaban Versus Heparin/Warfarin in the Treatment of Symptomatic Deep-Vein Blood Clots and/or Lung Blood Clots; HR = hazard ratio; INR = international normalized ratio; PE = pulmonary embolism; RE-COVER = Efficacy and Safety of Dabigatran Compared to Warfarin for 6 Month Treatment of Acute Symptomatic Venous Thromboembolism; VKA = vitamin K antagonist.
Data from phase III VTE trials suggest that DOACs have similar efficacy and either comparable or superior safety (with respect to clinically relevant bleeding) compared with VKA in subgroups of cancer patients (32–37) (Table 3). However, these data should be considered hypothesis-generating given that only 2% to 5% of patients had active cancers, and few patients had metastatic cancer or were on active chemotherapy (38).
Subgroup analyses of phase III trials of AF have also demonstrated consistent safety and efficacy profiles in cancer patients. An analysis of the ENGAGE AF-TIMI 48 (Effective Anticoagulation with factor Xa next Generation in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48) trial, which included 1,153 patients who developed cancer post-randomization, demonstrated preserved efficacy and safety of edoxaban compared with warfarin, regardless of cancer status (39). Apixaban had superior safety and efficacy relative to warfarin among 157 patients with active cancer and 1,079 patients with a history of cancer enrolled in the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial (40). Early observational experiences similarly have shown that rivaroxaban, when used in patients with active cancer and AF in clinical practice, was associated with low rates of ischemic stroke and clinically-relevant bleeding (41). Recent comparative effectiveness data of patients with AF and cancer consistently have shown that DOACs were associated with lower or similar risks of bleeding and stroke compared with warfarin (42).
ONGOING TRIALS TESTING NOVEL ANTICOAGULATION STRATEGIES IN CANCER
Several ongoing clinical trials (Table 4) comparing DOACs with LMWH for treatment of VTE are underway and will provide further insight into the composite efficacy and safety profile and drug-specific versus class effects of DOACs in cancer. Trials are assessing a broader range of endpoints beyond recurrent VTE and bleeding events, including patient-reported outcomes, such as treatment satisfaction and pain. CARAVAGGIO (Apixaban for the Treatment of Venous Thromboembolism in Patients With Cancer) (NCT03045406) is the largest ongoing study (planned N = 1,168) and is an open-label, multicenter, noninferiority trial designed to assess apixaban versus dalteparin in cancer-associated VTE.
TABLE 4.
Recently Completed or Ongoing DOAC Versus LMWH in VTE Treatment Studies in Patients With Cancer
| Trial | Estimated Sample Size | Planned Date of Study Completion | Phase | DOAC | Comparator | Primary Outcome |
|---|---|---|---|---|---|---|
| CONKO-11, NCT02583191 | 450 | December 2018 | Phase III | Rivaroxaban | LMWH | Patient-reported treatment satisfaction with rivaroxaban |
| CASTA-DIVA, NCT02746185 | 200 | April 2018 | Phase III | Rivaroxaban | LMWH | Symptomatic DVT, symptomatic PE, unsuspected DVT/PE, worsening pulmonary or venous obstruction within 3 months |
| PRIORITY, NCT03139487 | 176 | May 2020 | Phase II | Rivaroxaban | Dalteparin | Clinically relevant bleeding, interruption or discontinuation of anticoagulation, associated pain, or impairment of activities of daily living |
| CARAVAGGIO, NCT03045406 | 1,168 | June 2019 | Phase III | Apixaban | Dalteparin | Objectively confirmed recurrent VTE |
| ADAM VTE, NCT02585713 | 300 | November 2018 | Phase IV | Apixaban | LMWH | Major bleeding |
| COSIMO, NCT02742623 | 500 | March 2019 | Observational | Noninterventional, patients treated with standard LMWH/VKA for 4 weeks after index VTE, followed by switch to rivaroxaban | Patient-reported satisfaction with regards to use of rivaroxaban and perception of recurrent DVT/PE | |
| CANVAS, NCT02744092 | 940 | September 2019 | Interventional; phase not specified | Dabigatran, rivaroxaban, apixaban, or edoxaban | LMWH | Cumulative VTE recurrence |
ADAM VTE = Apixaban or Dalteparin in Reducing Blood Clots in Patients with Cancer Related Venous Thromboembolism; CANVAS = Direct Oral Anticoagulants versus LMWH Warfarin for VTE in Cancer; CARRAVAGIO = Apixaban for the Treatment of Venous Thromboembolism in Patients with Cancer; CASTA-DIVA = Cancer Associated Thrombosis, a Pilot Treatment Study using Rivaroxaban; CONKO-11 = Rivaroxaban in the Treatment of Venous Thromboembolism in Cancer Patients; COSIMO = Cancer-associated ThrOmboSIs - Patient-reported OutcoMes with RivarOxaban; DOAC = direct oral anticoagulants; DVT = deep vein thrombosis; LMWH = low-molecular weight heparins; PE = pulmonary embolism; VTE = venous thromboembolism.
SAFETY OF DOACs: DRUG INTERACTIONS, RENAL IMPAIRMENT, AND THROMBOCYTOPENIA DRUG INTERACTIONS.
The uptake of all DOACs is influenced by the P-glycoprotein (P-gp) system (43), while DOACs are also subject to variable metabolism via the cytochrome P450 (CYP) system (dabigatran 0%, edoxaban <4%, apixaban 15%, rivaroxaban 66%) (44,45). Cotreatment of DOACs with therapies that significantly influence these systems presents a theoretical risk for drug levels outside of the therapeutic range. Coadministration of any of the DOACs is not recommended with cancer therapies that are strong P-gp inducers or inhibitors. Several cancer and adjunctive therapies (including anti-emetics, opioids, and antibiotics) can alter CYP3A4 metabolism (46). Dabigatran or edoxaban may be preferred if a DOAC is selected in patients being treated with cancer therapies that are strong CYP3A4 inducers or inhibitors. Table 5 provides a summary of anticipated drug–drug interactions between DOACs and common cancer therapies that affect CYP3A4 metabolism and/or P-gp transport (further details in Steffel et al. [3]). Clinicians should be mindful of interactions between DOACs and investigational cancer therapies and ramifications of DOAC use on patient eligibility for clinical trials. The importance of interprofessional communication when prescribing anticoagulant therapy in cancer patients cannot be overstated.
TABLE 5.
Anticipated Drug–Drug Interactions Between Common Anticancer Drug Classes and DOACs
| P-Glycoprotein Interaction (All DOACs Affected) | CYP3A4 Interaction (Strongly Affects Rivaroxaban and Apixaban) | |
|---|---|---|
| Inhibition | • Immune-modulating agents (e.g., tacrolimus; strong to moderate to competition to none) • Tyrosine kinase inhibitors (e.g., imatinib; strong to moderate to competition to none) • Hormonal agents (e.g., abiraterone; strong to competition to none) |
• Immune modulating agents (e.g., cyclosporine; moderate to mild to competition) • Tyrosine kinase inhibitors (e.g., nilotinib; moderate to mild to competition) • Hormonal agents (e.g., bicalutamide; moderate to mild to competition to none) • Topoisomerase inhibitors (e.g., etoposide; mild to competition to none) • Anthracyclines (e.g., idarubicin; mild to competition to none) • Alkylating agents (e.g., cyclophosphamide; mild to competition to none) |
| Induction | • Anthracyclines (e.g., doxorubicin; strong to competition to none) • Antimitotic agents (e.g., vinblastine; strong to competition) • Immune-modulating agents (e.g., dexamethasone; strong to competition to none) |
• Immune-modulating agents (e.g., dexamethasone; strong to moderate to competition) • Antimitotic agents (e.g., paclitaxel; moderate to mild to competition) • Tyrosine kinase inhibitors (e.g., vemurafenib; moderate to competition) • Hormonal agents (e.g., enzalutamide; strong to competition to none) |
| Minimal to no Interaction | • Alkylating agents (e.g., bendamustine; competition to none) • Antimetabolites (e.g., methotrexate; competition to none) • Monoclonal antibodies (e.g., rituximab; none) • Platinum based agents (e.g., cisplatin; none) • Intercalating agents (e.g., bleomycin; none) |
• Monoclonal antibodies (e.g., brentuximab; competition to none) • Antimetabolites (e.g., pemetrexed; none) • Platinum based agents (e.g., oxaliplatin; none) • Intercalating agents (e.g., mitomycin C; none) |
Anticipated interactions are offered within anticancer drug classes as no interaction, competition, or potential interaction (mild, moderate, or strong). Intraclass differences exist in inhibition/induction and presence and strength of interaction. Table 4 of the 2018 European Heart Rhythm Association practical guide (3) offers more detailed drug–drug interactions and anticipated effects on DOAC drug levels.
CYP3A4 = Cytochrome P450 3A4; DOAC = direct oral anticoagulants.
RENAL IMPAIRMENT.
Patients with cancer have high rates of chronic kidney disease and commonly receive nephrotoxic chemotherapeutic agents (47). Renal insufficiency also affects DOAC pharmacokinetics. Individual agents have differing renal clearance (dabigatran 80%, edoxaban 50%, rivaroxaban 33%, and apixaban 27%) (48). Appropriate renal dose adjustment is critical for patients with renal dysfunction and applies to all DOACs. In patients with progressive renal impairment, switching from a DOAC to an alternative anticoagulant with less renal clearance is generally preferred over anti-Xa level monitoring (49). It is not advisable to use DOACs in patients with creatinine clearance <15 ml/min, as drug exposure increases for all DOACs in patients with stage V chronic kidney disease.
THROMBOCYTOPENIA.
Thrombocytopenia, which commonly develops from myeloablative chemotherapy, tumor invasion of the bone marrow, and secondary immune-mediated phenomena, is another safety concern. Depending on individual thrombotic risks, anticoagulation should be avoided when platelet counts fall below 50,000 to 70,000/µl (50). Below this range, platelet transfusion or use of reduced-dose anticoagulation strategies may be considered to permit uninterrupted anticoagulation (51). Regulatory approvals of targeted reversal agents (idarucizumab, humanized antidabigatran monoclonal antibody, and andexanet alfa, recombinant modified factor Xa), mitigate some of the risk associated with DOACs. Ciraparantag, a broad nonspecific small molecule that reverses the anticoagulant effects of Xa inhibitors, direct thrombin inhibitors, unfractionated heparin, and LMWH, is currently under investigation (52).
SUMMARY OF CLINICAL PRACTICE GUIDELINES
Until recently, all guidelines recommended that cancer-associated VTE be treated with LMWH for at least 3 to 6 months (Table 6) (50,53,54). However, given encouraging interval data, the latest International Society on Thrombosis and Haemostasis guidance supports use of DOACs (with preference for edoxaban and rivaroxaban) in acute VTE and low bleeding risk if no significant drug–drug interaction is present, and LMWH in acute VTE and high bleeding risk (including intraluminal gastrointestinal and genitourinary cancers or abnormalities) (55). The National Comprehensive Cancer Network guidelines now recommend either LMWH or edoxaban (with an initial parenteral dosing with LMWH) in cancer-associated VTE (56).
TABLE 6.
Summary of Guidelines for Anticoagulation in Cancer
| EHRA 2018 | ITAC-CME 2016 | ASCO 2015 | ACCP 2016 | ISTH 2018 | NCCN 2018 | |
|---|---|---|---|---|---|---|
| VTE prophylaxis | ||||||
| Acute VTE treatment | LMWH, UFH or fondaparinux for initial treatment; LMWH for at least 3 months | LMWH preferred over VKA for at least 6 months | LMWH preferred over VKA and DOACs, treat >3 months in active cancer | If low bleeding risk and no DDIs, DOACs preferred. If high bleeding risk (especially GI/ GU), LMWH preferred. | LMWH for first 6 months in proximal DVT or PE Edoxaban with initial parenteral dosing with LMWH | |
| DOACs | Not recommended | LMWH preferred | Select DOACs (edoxaban and rivaroxaban are the only ones directly compared with LMWH thus far) preferred in low-bleeding risk, acute VTE | Edoxaban with initial parenteral dosing with LMWH or UFH Apixaban and rivaroxaban are considered other alternatives in patients who have reasons to avoid LMWH | ||
| IVC filters | Contraindication to anticoagulation or recurrence on treatment | Contraindication to anticoagulation or progression on LMWH | Contraindication to anticoagulation in proximal DVT or PE, or poor cardiopulmonary reserve | |||
| Catheter-associated thrombosis | Anticoagulation for 3 months using LMWH | Anticoagulation for >3 months or as long as catheter is in place | ||||
| Brain metastases | Not a contraindication for anticoagulation | Anticoagulation recommended for VTE in primary CNS malignancies as in other cancers | ||||
| Atrial fibrillation | No demonstrated efficacy of LMWH in thromboprophylaxis; further data required for DOACs; close examination of DDIs |
ACCP = American College of Chest Physicians; ASCO = American Society of Clinical Oncology; CNS = central nervous system; DDI = drug-drug interactions; DOACs = direct oral anticoagulants; DVT = deep vein thrombosis; EHRA = European Heart Rhythm Association; GI = gastrointestinal; GU = genitourinary; ISTH = International Society on Thrombosis and Haemostasis; ITAC-CME = International Initiative on Thrombosis and Cancer; LMWH = low-molecular weight heparins; NCCN = National Comprehensive Cancer Network; PE = pulmonary embolism; UFH = unfractionated heparin; VKA = vitamin K antagonist.
The 2018 European Heart Rhythm Association practical guide notes highlights limited high-quality data on use of LMWH in thromboprophylaxis of AF. The guide endorses shared decision-making, avoidance of strong drug–drug interactions, and further study of DOACs in comorbid cancer and AF (3). Traditional risk scores (e.g., CHA2DS2-VASc) (57) should be applied to determine individual thrombotic risks, together with cancer- and therapy-specific factors (3). Future guidelines should consider updating recommendations in AF and cancer given recent data.
UNIQUE CLINICAL DILEMMAS IN ANTICOAGULATION MANAGEMENT IN CANCER
CENTRAL VENOUS CATHETER-ASSOCIATED VTE.
Insertion of CVCs accounts for 70% of cancer-associated upper extremity deep vein thromboses (58). Whether DOACs, which inhibit only a single clotting factor, would be as effective as less-specific anticoagulants (especially agents that inhibit the contact pathway) for CVC-related VTE is unknown. Current guidelines for CVC-associated VTE in cancer patients recommend treatment with either LMWH bridged to VKA or LMWH monotherapy ≥12 weeks without requiring removal of the catheter (unless defective, nonfunctional, or infected) (50) (Table 6). Data for the use of DOACs are limited. In a small, open-label study of CVC-associated VTE, preservation of line function was 100%, but rates of bleeding were high with 12-week treatment with rivaroxaban (59). Routine prophylactic anticoagulation is not recommended for CVCs inserted in cancer patients (60).
INTERRUPTION OF ANTICOAGULATION.
Anticoagulation frequently requires interruption for surgical or interventional procedures in cancer care. Interruption of anticoagulation is associated with heightened risks of adverse cardiovascular and cerebrovascular events (61), but limited data exist regarding strategies to limit these thrombotic risks in cancer. Given the consistent increment in bleeding risk, full-dose periprocedural bridging anticoagulation with LMWH or unfractionated heparin is not routinely recommended in patients with cancer with VTE more than 3 months prior (49).
TREATMENT FAILURE AND INFERIOR VENA CAVA FILTER PLACEMENT.
Cancer is a major risk factor for anticoagulation failure. Switching to LMWH or dose escalation (in LMWH-treated patients) are guideline-supported strategies, but have not been examined in RCTs among cancer patients. Insertion of inferior vena cava (IVC) filters may be considered in cancer patients with contraindications to anticoagulation or evidence of progression or recurrence with LMWH (Table 6). However, RCTs examining IVC filter placement in cancer are lacking. Recurrent VTE while on therapeutic LMWH identifies patients at very high short-term mortality risk (1,62). Therefore, quality of life and patient preference are important when determining treatment approach in this high-risk subset.
VENA CAVA THROMBOSIS.
Extensive vena cava thrombosis is a relatively uncommon, but morbid complication, occurring in the setting of certain cancers (e.g., renal cell carcinoma, gastrointestinal), abdominal surgery, and/or unretrieved IVC filters (63). Anticoagulation is the mainstay of therapy with limited data on use of DOACs; adjunctive interventions including surgical thrombectomy may be required in select cases (64).
DOSING IN UNDERWEIGHT CANCER PATIENTS.
Weight loss is a common feature of cancer. Dose reduction by 50% is recommended in body weight ≤60 kg for apixaban in treatment of AF in patients age ≥80 years and/or serum creatinine ≥1.5 mg/dl. Similarly, a 50% dose reduction for body weight ≤60 kg is recommended for edoxaban in patients with AF (except in the United States) and VTE (globally). Due to lack of validated dosing guidelines, monitoring anti-Xa levels in an attempt to prevent overdosing in underweight patients is not routinely recommended. However, measurement of drug concentrations and/or anti-Xa levels may be considered in emergencies (e.g., when timing of last dose is unknown or if overdosing is suspected) or in specific circumstances (e.g., coadministration with cancer therapies with uncertain pharmacokinetic interactions or in underweight patients <50 kg) under the guidance of a coagulation expert (3).
GASTROINTESTINAL MALIGNANCIES.
High-risk bleeding is a major concern when initiating anticoagulation in cancer patients. DOACs are incompletely absorbed upon ingestion (65) and may have direct topical anticoagulant effects during gastrointestinal transit (66). Indeed, early gastrointestinal or genitourinary bleeding after DOAC initiation should prompt clinicians to pursue routine interrogation of these bleeding sites for occult cancer (66). Edoxaban (60/ 30 mg) had a higher rate of gastrointestinal bleeding than warfarin in Hokusai VTE Cancer (30), a signal that was also seen with rivaroxaban, dabigatran 150 mg, and edoxaban 60/30 mg in their respective large phase III trials in patients with AF (67). However, the rates of gastrointestinal bleeding with dabigatran 110 mg and apixaban were similar to that of warfarin in RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) and ARISTOTLE (67), respectively, whereas gastrointestinal bleeding was significantly reduced with the 30/15 mg regimen of edoxaban compared with warfarin in ENGAGE AF-TIMI 48 (67). Regimens that do not increase gastrointestinal bleeding risk may be preferred in high-risk cancer patients, although evidence is lacking in advanced gastrointestinal malignancies, and neither the lower-dose edoxaban regimen of 30/15 mg once daily (globally) nor dabigatran 110 mg twice daily (in the United States) are approved for stroke prevention in AF. PRIORITY (A Randomized Phase II Study to Compare the Safety and Efficacy of Dalteparin vs. Rivaroxaban for Cancer-associated Venous Thromboembolism) (NCT03139487) is an open-label, multicenter, phase II study comparing rivaroxaban versus dalteparin in treatment of acute VTE in patients with advanced upper gastrointestinal, hepatobiliary, and pancreatic cancers with a primary outcome of clinically-relevant bleeding. In addition, in cancer patients with gastrectomy or small bowel resections, absorption of DOACs may be affected.
INTRACRANIAL METASTASES.
Intracranial hemorrhage (ICH) is the most feared complication of anticoagulation. A retrospective study of 293 patients with brain metastases showed no increased risk of ICH in patients receiving therapeutic doses of LWMH versus no anticoagulation at 1 year (68); however, generalizability of this experience to all cancer types is uncertain. Therapeutic anticoagulation in patients with known brain metastases is not contraindicated according to several professional societies (53,54); however, it is generally avoided if attendant hemorrhage is present. Although no definitive guidance is provided for DOACs, a ~50% reduction in ICH compared with VKAs in broader populations (67) suggests that they may be a reasonable option in the setting of brain metastases. Now that reversal agents are available for DOACs, and since these drugs have rapid onset/offset, trials of DOACs in patients with brain metastases (or primary brain cancer) would be desirable.
THROMBOPROPHYLAXIS OF VTE IN CANCER PATIENTS
Because of the increased risk of VTE in cancer patients, especially during hospitalization (69) and in those undergoing oncologic surgery or receiving ambulatory chemotherapy, thromboprophylaxis for primary prevention of VTE has been an important clinical and research focus. In the ENOXACAN (Enoxaparin and Cancer) II study, extended-duration (1-month) thromboprophylaxis with enoxaparin significantly reduced VTE risk in patients undergoing laparotomy for abdominal or pelvic malignancy (70). Extended post-discharge thromboprophylaxis with LMWH or DOACs for acutely ill medical patients (including cancer) decreases recurrent VTE, but at the expense of potentially increased bleeding risks (71). Betrixaban, a once daily oral factor Xa inhibitor, was recently approved for this indication (72).
Dedicated risk scores have been developed to identify patients who are at heightened risk for cancer-related VTE (73). Two recent trials evaluated the use of DOACs as thromboprophylaxis in at-risk ambulatory patients (defined as Khorana scores ≥2) initiating systemic cancer treatment. In the CASSINI (A Study to Evaluate the Efficacy and Safety of Rivaroxaban Venous Thromboembolism [VTE] Prophylaxis in Ambulatory Cancer Participants) trial (74), 841 patients were randomized to lower-dose rivaroxaban 10 mg once daily versus placebo for 180 days. Rates of the composite VTE or VTE-related death (6.0% vs. 8.8%; p = 0.10) and of major bleeding (2.0% vs. 1.0%; p = 0.27) were similar between rivaroxaban and placebo, respectively. In AVERT (Apixaban to Prevent Venous Thromboembolism in Patients with Cancer) (75), 574 patients randomized to lower-dose apixaban 2.5 mg twice daily or placebo were followed for 6 months. Apixaban significantly reduced composite VTE events (4.2% vs. 10.2%; p < 0.001), but significantly increased rates of major bleeding (3.5% vs. 1.8%; p = 0.046) compared with placebo. Taken together, these trials validate the use of the Khorana score, a 6-point score incorporating cancer type, blood counts, and body mass index, in identifying patients at risk for venous and arterial thrombotic events. Ambulatory patients undergoing chemotherapy with intermediate-to-high VTE risk (Khorana scores ≥2) who are at low bleeding risk should be considered for thromboprophylaxis with DOACs (Central Illustration).
CENTRAL ILLUSTRATION. Approach to Anticoagulation in Patients With Active Cancer.
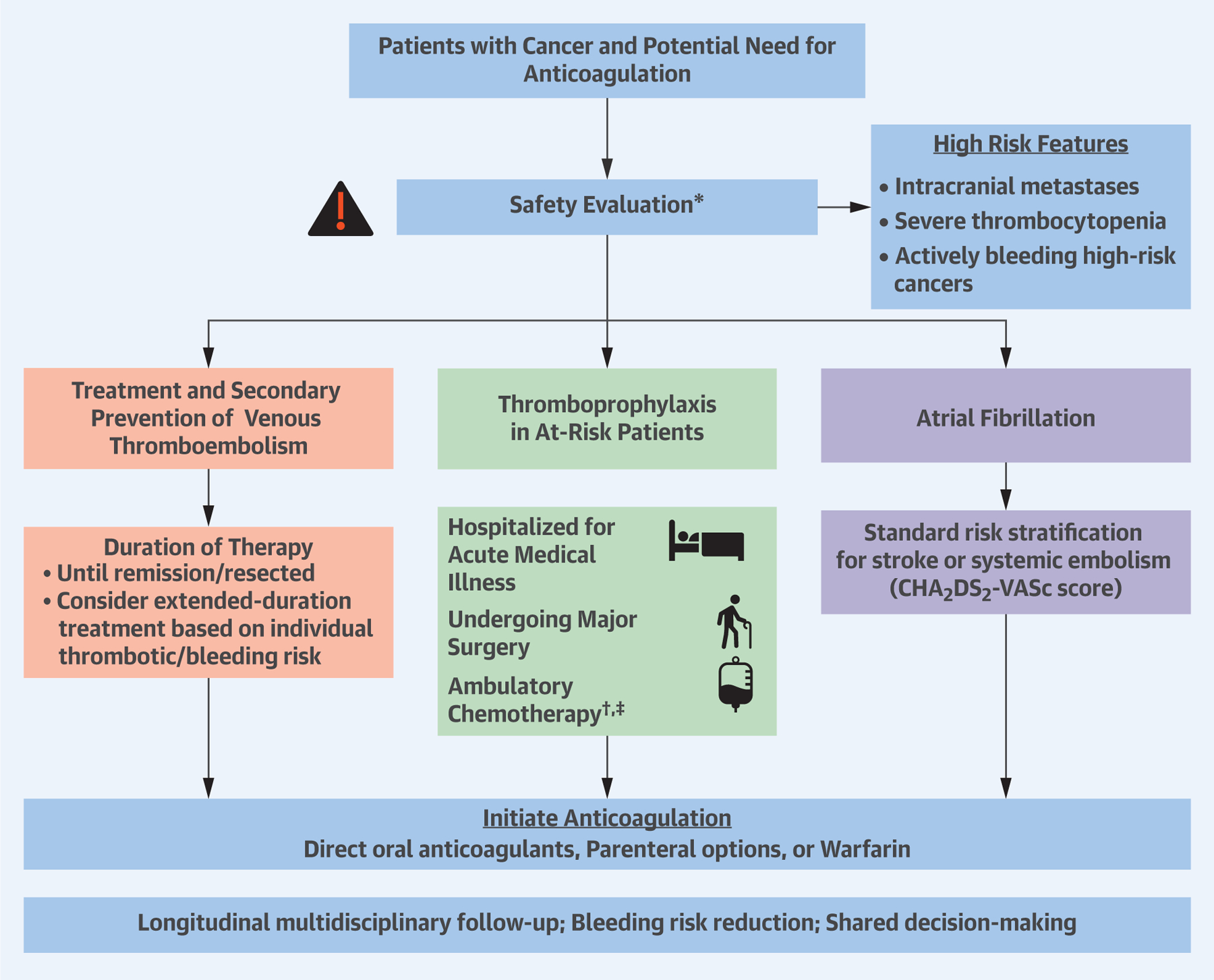
*For all patients, measure safety laboratory studies, assess potential drug–drug interactions, determine individual patient bleeding risks and preferences, and use bleeding reduction strategies. †Patients with multiple myeloma receiving thalidomide or lenalidomide-based regimens with chemotherapy and/or dexamethasone should receive thromboprophylaxis with aspirin or low molecular weight heparins (LMWH) for low-risk patients and LMWH for high-risk patients. ‡The Khorana score (21) is a validated risk score to assess thrombotic risk among ambulatory patients with cancer. The score ranges from 0 to 6 and is based on site of cancer (2 points for stomach, pancreas; 1 point for lung, lymphoma, gynecological, genitourinary excluding prostate), 1 point for platelet counts ≥350,000 per mm3, 1 point for leukocyte count ≥11,000 per mm3, 1 point for hemoglobin <10 g/dl or use of erythropoiesis-stimulating agents, and 1 point for body mass index ≥35 kg/m2. Khorana scores ≥2 identify patients at intermediate-to-high risk for venous thromboembolism who may benefit from thromboprophylaxis with direct oral anticoagulants.
HOW LONG SHOULD CANCER-ASSOCIATED VTE BE TREATED?
Data for therapeutic anticoagulation in the treatment of cancer-associated VTE beyond 6 months are limited (76,77), but suggest that LMWH therapy up to 12 months is generally safe. However, in practice, therapeutic anticoagulation is often continued in high-risk patients (e.g., widely metastatic disease and ongoing chemotherapy). In broader cohorts, “step-down” extended-duration treatment with lower-intensity DOACs after initial therapy for acute VTE has been shown to significantly decrease risk of recurrent VTE without associated increased risk of bleeding (78); this approach may be appropriate in select cancer patients.
PERSONALIZED ASSESSMENT OF THROMBOSIS RISK IN THE ERA OF PRECISION ONCOLOGY
The introduction of novel targeted cancer therapies has had diverse and unique adverse cardiovascular effects (23). In the future, a personalized approach is needed for preventative and therapeutic anticoagulation approaches in cancer patients. Recent trials have supported the use of personalized VTE risk score scores (e.g., Khorana score) and tailored anticoagulation management by cancer type (e.g., cautious DOAC use in intraluminal gastrointestinal cancers).
PATIENT AND PROVIDER PREFERENCES RELATED TO ANTICOAGULANT CHOICE
Ultimately, multidisciplinary care that accounts for individualized risk factors, patient preferences, and periodic clinical reassessment is warranted to identify the optimal anticoagulation regimen (Central Illustration). The acute phase of VTE (79) and major complications of AF (80) negatively affect quality of life. LMWH (in VTE) (81) and long-term oral anticoagulation (in AF) (82) are viewed as a necessary and acceptable trade-offs to prevent thrombotic complications; minimal interference with cancer treatment is a major priority among cancer patients (83). In current practice, approximately one-half of patients with cancer-associated VTE receive warfarin, 40% receive LMWH, and a minority receive DOACs (84). Similarly, 15% of patients with AF and cancer are prescribed DOACs (vs. VKA) in current practice (85); early cardiology involvement has been associated with higher prescription fill rates (86). Given the high financial burden of global cancer care, cost-effectiveness analyses to compare anticoagulation strategies would be desirable.
SUMMARY AND FUTURE DIRECTIONS
Anticoagulation management of cancer patients should be determined with longitudinal multidisciplinary follow-up with frequent clinical reassessment. Ongoing RCTs will further our understanding of the optimal antithrombotic approach to management of VTE and AF in patients with cancer. Future studies are needed to address challenges unique to cancer patients, including use in CVC-associated thrombosis, safety and efficacy in underweight patients, protocols for thrombocytopenia, and use in malignancies with potential for high-risk bleeding (e.g., brain metastases, luminal gastrointestinal cancers). It is likely that anticoagulation decisions in cancer patients will remain highly individualized even as high-quality clinical data amount, given variation in patient preferences, thrombotic and bleeding risk factors, and disease activity. Nonetheless, DOACs represent a convenient and patient-centric anticoagulation strategy with emerging data supporting their safety and efficacy in the care of cancer patients.
HIGHLIGHTS.
Patients with active cancer face higher risks of arterial and venous thromboembolism (VTE), atrial arrhythmias, and bleeding events.
Historically, in patients with cancer, low-molecular weight heparins have been preferred for treatment of VTE, while warfarin has been the standard anticoagulant for stroke prevention in atrial fibrillation.
Select direct oral anticoagulants have now been shown to safely prevent thrombotic events in recent clinical trials, and present an attractive oral dosing option for patients with cancer.
Multidisciplinary care that accounts for individualized bleeding and thrombotic risks, drug-drug interactions, patient preferences, and periodic clinical reassessment is warranted to identify the optimal anticoagulation strategy for patients with cancer.
Acknowledgments
Dr. Vaduganathan has received support from the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (National Institutes of Health/National Center for Advancing Translational Sciences Award UL 1TR002541); and has served on the advisory boards for Amgen, AstraZeneca, Bayer AG, and Baxter Healthcare. Dr. Qamar has received support from a National Heart, Lung, and Blood Institute T32 postdoctoral training grant (T32HL007604) and the American Heart Association Strategically Focused Research Network in Vascular Disease grants (18SFRN3390085 and 18SFRN33960262). Dr. Moslehi serves as a consultant or in an advisory role for Bristol-Myers Squibb, Daiichi-Sankyo, Novartis, Pfizer, Regeneron, Takeda, Myokardia, and Ipsen; and has received research funding from the NIH (R56 HL141466), Bristol-Myers Squibb, and Pfizer. Dr. Piazza has received research grant support from EKOS, a BTG International group company, Bristol-Myers Squibb, Daiichi-Sankyo, Bayer, Portola, and Janssen. Dr. Giugliano has received research grants and honoraria for CME programs from Daiichi-Sankyo and the American College of Cardiology; and has served as a compensated consultant for Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, Merck, Portola, and Pfizer. Dr. Mosarla has reported that she has no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- CVC
central venous catheter
- DOAC
direct oral anticoagulant agent
- LMWH
low-molecular weight heparins
- RCT
randomized clinical trial
- VTE
venous thromboembolism
Footnotes
Listen to this manuscript’s audio summary by Editor-in-Chief Dr. Valentin Fuster on JACC.org.
REFERENCES
- 1.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349: 146–53. [DOI] [PubMed] [Google Scholar]
- 2.Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA 2015;314:677–86. [DOI] [PubMed] [Google Scholar]
- 3.Steffel J, Verhamme P, Potpara TS, et al. The 2018 EHRA practical guide on the use of NOACs in patients with atrial fibrillation. Eur Heart J 2018; 39:1330–93. [DOI] [PubMed] [Google Scholar]
- 4.Puurunen MK, Gona PN, Larson MG, Murabito JM, Magnani JW, O’Donnell CJ. Epidemiology of venous thromboembolism in the Framingham Heart Study. Thromb Res 2016;145: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen AT, Katholing A, Rietbrock S, Bamber L, Martinez C. Epidemiology of first and recurrent venous thromboembolism in patients with active cancer. A population-based cohort study. Thromb Haemost 2017;117:57–65. [DOI] [PubMed] [Google Scholar]
- 6.Chee CE, Ashrani AA, Marks RS, et al. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: a population-based cohort study. Blood 2014;123: 3972–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Es N, Bleker SM, Di Nisio M. Cancer-associated unsuspected pulmonary embolism. Thromb Res 2014;133 Suppl 2:S172–8. [DOI] [PubMed] [Google Scholar]
- 8.Faller N, Limacher A, Mean M, et al. Predictors and causes of long-term mortality in elderly patients with acute venous thromboembolism: a prospective cohort study. Am J Med 2017;130: 198–206. [DOI] [PubMed] [Google Scholar]
- 9.Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol 2017;70:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundboll J, Veres K, Horvath-Puho E, Adelborg K, Sorensen HT. Risk and prognosis of cancer after lower limb arterial thrombosis. Circulation 2018;138:669–77. [DOI] [PubMed] [Google Scholar]
- 11.Brenner B, Bikdeli B, Tzoran I, et al. Arterial ischemic events are a major complication in cancer patients with venous thromboembolism. Am J Med 2018;131:1095–103. [DOI] [PubMed] [Google Scholar]
- 12.Grilz E, Konigsbrugge O, Posch F, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica 2018;103:1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol 2014;63:945–53. [DOI] [PubMed] [Google Scholar]
- 14.Melloni C, Shrader P, Carver J, et al. Management and outcomes of patients with atrial fibrillation and a history of cancer: the ORBIT-AF registry. Eur Heart J Qual Care Clin Outcomes 2017;3:192–7. [DOI] [PubMed] [Google Scholar]
- 15.Conen D, Wong JA, Sandhu RK, et al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol 2016;1:389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sorensen HT. Atrial fibrillation as a marker of occult cancer. PLoS One 2014; 9:e102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu YF, Liu CJ, Chang PM, et al. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol 2013;165:355–7. [DOI] [PubMed] [Google Scholar]
- 18.Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost 2017;117:219–30. [DOI] [PubMed] [Google Scholar]
- 19.Piazza G Venous thromboembolism and cancer. Circulation 2013;128:2614–8. [DOI] [PubMed] [Google Scholar]
- 20.Thaler J, Ay C, Pabinger I. Clinical significance of circulating microparticles for venous thromboembolism in cancer patients. Hamostaseologie 2012;32:127–31. [DOI] [PubMed] [Google Scholar]
- 21.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edelmann B, Gupta N, Schnoder TM, et al. JAK2-V617F promotes venous thrombosis through beta1/beta2 integrin activation. J Clin Invest 2018; 128:4359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 2016;375: 1457–67. [DOI] [PubMed] [Google Scholar]
- 24.Ashrani AA, Gullerud RE, Petterson TM, Marks RS, Bailey KR, Heit JA. Risk factors for incident venous thromboembolism in active cancer patients: a population based case-control study. Thromb Res 2016;139:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronson D, Brenner B. Arterial thrombosis and cancer. Thromb Res 2018;164 Suppl 1:S23–8. [DOI] [PubMed] [Google Scholar]
- 26.Buza V, Rajagopalan B, Curtis AB. Cancer treatment-induced arrhythmias: focus on chemotherapy and targeted therapies. Circ Arrhythm Electrophysiol 2017;10. [DOI] [PubMed] [Google Scholar]
- 27.Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood 2016;128: 138–40. [DOI] [PubMed] [Google Scholar]
- 28.Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res 2014;133 Suppl 2:S49–55. [DOI] [PubMed] [Google Scholar]
- 29.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia 2002;4: 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018; 378:615–24. [DOI] [PubMed] [Google Scholar]
- 31.Young A, Marshall A, Thirlwall J, et al. Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism: results of the Select-D™ Pilot Trial. Blood 2017; 130:625.28546143 [Google Scholar]
- 32.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost 2015;13: 2187–91. [DOI] [PubMed] [Google Scholar]
- 33.Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol 2014;1:e37–46. [DOI] [PubMed] [Google Scholar]
- 34.Raskob GE, van Es N, Segers A, et al. Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematol 2016;3:e379–87. [DOI] [PubMed] [Google Scholar]
- 35.Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost 2015;114:150–7. [DOI] [PubMed] [Google Scholar]
- 36.van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014;124:1968–75. [DOI] [PubMed] [Google Scholar]
- 37.Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest 2015;147:475–83. [DOI] [PubMed] [Google Scholar]
- 38.Ay C, Kamphuisen PW, Agnelli G. Antithrombotic therapy for prophylaxis and treatment of venous thromboembolism in patients with cancer: review of the literature on current practice and emerging options. ESMO Open 2017;2:e000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fanola CL, Ruff CT, Murphy SA, et al. Efficacy and safety of edoxaban in patients with active malignancy and atrial fibrillation: analysis of the ENGAGE AF-TIMI 48 Trial. J Am Heart Assoc 2018; 7:e008987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melloni C, Dunning A, Granger CB, et al. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and a history of cancer: insights from the ARISTOTLE Trial. Am J Med 2017;130:1440–8.e1. [DOI] [PubMed] [Google Scholar]
- 41.Laube ES, Yu A, Gupta D, et al. Rivaroxaban for stroke prevention in patients with nonvalvular atrial fibrillation and active cancer. Am J Cardiol 2017;120:213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah S, Norby FL, Datta YH, et al. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv 2018;2:200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wessler JD, Grip LT, Mendell J, Giugliano RP. The P-glycoprotein transport system and cardiovascular drugs. J Am Coll Cardiol 2013;61: 2495–502. [DOI] [PubMed] [Google Scholar]
- 44.Qamar A, Vaduganathan M, Greenberger NJ, Giugliano RP. Oral anticoagulation in patients with liver disease. J Am Coll Cardiol 2018;71:2162–75. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Quesada CJ, Giugliano RP. Comparison of the phase III clinical trial designs of novel oral anticoagulants versus warfarin for the treatment of nonvalvular atrial fibrillation: implications for clinical practice. Am J Cardiovasc Drugs 2014;14:111–27. [DOI] [PubMed] [Google Scholar]
- 46.Short NJ, Connors JM. New oral anticoagulants and the cancer patient. Oncologist 2014;19: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Launay-Vacher V, Oudard S, Janus N, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer 2007;110:1376–84. [DOI] [PubMed] [Google Scholar]
- 48.Levy JH, Spyropoulos AC, Samama CM, Douketis J. Direct oral anticoagulants: new drugs and new concepts. J Am Coll Cardiol Intv 2014;7: 1333–51. [DOI] [PubMed] [Google Scholar]
- 49.Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv 2018;2:3257–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE Disease: CHEST guideline and expert panel report. Chest 2016;149: 315–52. [DOI] [PubMed] [Google Scholar]
- 51.Samuelson Bannow BT, Lee A, Khorana AA, et al. Management of cancer-associated thrombosis in patients with thrombocytopenia: guidance from the SSC of the ISTH. J Thromb Haemost 2018;16:1246–9. [DOI] [PubMed] [Google Scholar]
- 52.Levy JH, Douketis J, Weitz JI. Reversal agents for non-vitamin K antagonist oral anticoagulants. Nat Rev Cardiol 2018;15:273–81. [DOI] [PubMed] [Google Scholar]
- 53.Farge D, Bounameaux H, Brenner B, et al. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2016;17:e452–66. [DOI] [PubMed] [Google Scholar]
- 54.Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update 2014. J Clin Oncol 2015;33: 654–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khorana AA, Noble S, Lee AYY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost 2018;16:1891–4. [DOI] [PubMed] [Google Scholar]
- 56.National Comprehensive Cancer Network. NCCN Guidelines for Cancer-Associated Venous Thromboembolic Disease. Available at: https://www.nccn.org/Common/FileManager.ashx?fileManagerId=b0461271-ae6f-455a-bbb9-a637326abe49. Accessed January 2, 2019.
- 57.Hu WS, Lin CL. Impact of atrial fibrillation on the development of ischemic stroke among cancer patients classified by CHA2DS2-VASc score-a nationwide cohort study. Oncotarget 2018;9: 7623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernardi E, Pesavento R, Prandoni P. Upper extremity deep venous thrombosis. Semin Thromb Hemost 2006;32:729–36. [DOI] [PubMed] [Google Scholar]
- 59.Davies GA, Lazo-Langner A, Gandara E, et al. A prospective study of rivaroxaban for central venous catheter associated upper extremity deep vein thrombosis in cancer patients (Catheter 2). Thromb Res 2018;162:88–92. [DOI] [PubMed] [Google Scholar]
- 60.Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev 2011: CD006649. [DOI] [PubMed]
- 61.Cavallari I, Ruff CT, Nordio F, et al. Clinical events after interruption of anticoagulation in patients with atrial fibrillation: an analysis from the ENGAGE AF-TIMI 48 trial. Int J Cardiol 2018; 257:102–7. [DOI] [PubMed] [Google Scholar]
- 62.Carrier M, Le Gal G, Cho R, Tierney S, Rodger M, Lee AY. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost 2009;7:760–5. [DOI] [PubMed] [Google Scholar]
- 63.Kraft C, Schuettfort G, Weil Y, et al. Thrombosis of the inferior vena cava and malignant disease. Thromb Res 2014;134:668–73. [DOI] [PubMed] [Google Scholar]
- 64.Alkhouli M, Morad M, Narins CR, Raza F, Bashir R. Inferior vena cava thrombosis. J Am Coll Cardiol Intv 2016;9:629–43. [DOI] [PubMed] [Google Scholar]
- 65.Aisenberg J, Chatterjee-Murphy P, Friedman Flack K, et al. Gastrointestinal bleeding with edoxaban versus warfarin: results from the ENGAGE AF-TIMI 48 Trial. Circ Cardiovasc Qual Outcomes 2018;11:e003998. [DOI] [PubMed] [Google Scholar]
- 66.Flack KF, Desai J, Kolb JM, et al. Major gastrointestinal bleeding often is caused by occult malignancy in patients receiving warfarin or dabigatran to prevent stroke and systemic embolism from atrial fibrillation. Clin Gastroenterol Hepatol 2017;15:682–90. [DOI] [PubMed] [Google Scholar]
- 67.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- 68.Donato J, Campigotto F, Uhlmann EJ, et al. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: a matched cohort study. Blood 2015; 126:494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piazza G, Rao AF, Nguyen TN, et al. Venous thromboembolism in hospitalized patients with active cancer. Clin Appl Thromb Hemost 2013;19: 469–75. [DOI] [PubMed] [Google Scholar]
- 70.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975–80. [DOI] [PubMed] [Google Scholar]
- 71.Tao DL, Bien JY, DeLoughery TG, Shatzel JJ. Extended thromboprophylaxis with direct oral anticoagulants for medical patients: a systematic review and meta-analysis. Blood 2017;129: 653–5. [DOI] [PubMed] [Google Scholar]
- 72.Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med 2016; 375:534–44. [DOI] [PubMed] [Google Scholar]
- 73.Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol 2018;5:e289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med 2019;21;380:720–8. [DOI] [PubMed] [Google Scholar]
- 75.Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2019;380: 711–9. [DOI] [PubMed] [Google Scholar]
- 76.Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost 2015;13: 1028–35. [DOI] [PubMed] [Google Scholar]
- 77.Jara-Palomares L, Solier-Lopez A, Elias-Hernandez T, et al. Tinzaparin in cancer associated thrombosis beyond 6 months: TiCAT study. Thromb Res 2017;157:90–6. [DOI] [PubMed] [Google Scholar]
- 78.Vasanthamohan L, Boonyawat K, Chai-Adisaksopha C, Crowther M. Reduced-dose direct oral anticoagulants in the extended treatment of venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost 2018;16: 1288–95. [DOI] [PubMed] [Google Scholar]
- 79.Dewilde S, Lloyd AJ, Holm MV, Lee AY. Quality of life of patients experiencing cancer-associated thrombosis. Value Health 2015;18:A397–8. [Google Scholar]
- 80.Dudink E, Erkuner O, Berg J, et al. The influence of progression of atrial fibrillation on quality of life: a report from the Euro Heart Survey. Europace 2018;20:929–34. [DOI] [PubMed] [Google Scholar]
- 81.Seaman S, Nelson A, Noble S. Cancer-associated thrombosis, low-molecular-weight heparin, and the patient experience: a qualitative study. Patient Prefer Adherence 2014;8:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilke T, Bauer S, Mueller S, Kohlmann T, Bauersachs R. Patient preferences for oral anticoagulation therapy in atrial fibrillation: a systematic literature review. Patient 2017;10: 17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noble S, Matzdorff A, Maraveyas A, Holm MV, Pisa G. Assessing patients’ anticoagulation preferences for the treatment of cancer-associated thrombosis using conjoint methodology. Haematologica 2015;100:1486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khorana AA, Yannicelli D, McCrae KR, et al. Evaluation of US prescription patterns: Are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res 2016;145:51–3. [DOI] [PubMed] [Google Scholar]
- 85.Ording AG, Horvath-Puho E, Adelborg K, Pedersen L, Prandoni P, Sorensen HT. Thromboembolic and bleeding complications during oral anticoagulation therapy in cancer patients with atrial fibrillation: a Danish nationwide population-based cohort study. Cancer Med 2017;6:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Neal WT, Claxton JS, Sandesara PB, et al. Provider specialty, anticoagulation, and stroke risk in patients with atrial fibrillation and cancer. J Am Coll Cardiol 2018;72:1913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


