Abstract
Objective
The aim of the present paper was to evaluate cases of lumbar degenerative diseases treated with oblique lateral interbody fusion (OLIF) using a modified lateral approach (i.e. anteroinferior psoas exposure under direct vision) and to analyze the effect and safety of this approach.
Methods
From June 2016 to April 2019, a total of 226 patients with an average age of 65.5 ± 16.2 years (98 men and 128 women) with degenerative lumbar diseases who underwent the AIP approach of OLIF were followed up and analyzed retrospectively. Data concerning operative and clinical parameters were collected, including operative time, intraoperative estimated blood loss, duration of postoperative hospital stay, and time to ambulation after surgery. For the assessment of clinical outcomes, the visual analogue scale (VAS) score (for back pain) and the Oswestry disability index (ODI) were calculated. Complications were also recorded as surgical exposure approach‐related complications. More than 6 months after surgery, 132 patients consented to having MRI examinations to evaluate the psoas muscle atrophy when they were followed up.
Results
The mean operative time was 82.5 ± 31.6 min. The mean operative time for each segment of OLIF was 43.3 ± 15.5 min. The mean blood loss was 48.0 ± 11.6 mL. The mean blood loss for each segment of OLIF was 25.3 ± 10.1 mL. No patients needed blood transfusion intraoperatively or postoperatively. The mean hospital stay was 4.1 ± 2.1 days. All patients were followed up for 12–31 months (mean 18.2 months). Clinical assessment showed that the VAS and ODI scores at 6 months after surgery were markedly lower than the preoperative scores (P < 0.001) but did not differ from the scores at the final follow‐up (P > 0.05). There was no significant difference in percentage changes of the cross‐sectional area of the lean psoas muscle and the T2 signal intensity ratio of gross psoas to quadratus lumborum muscles between the left side (operative side) and the right side (nonoperative side) (P > 0.05). A total of 11 surgical exposure approach‐related complications were reported, with an incidence of 4.9%: transient thigh pain/numbness, psoas weakness (2.2%), sympathetic chain injury (1.3%), cage subsidence (0.9%), and segmental artery injury (0.4%). There was no permanent motor neurological deficit, and no injury of vascular, ureter or peritoneal membranes.
Conclusion
The anteroinferior psoas approach for OLIF is safe and can preserve the psoas and lumbar plexus.
Keywords: Anteroinferior, Atrophy, Lumbar, Oblique lateral interbody fusion, Psoas
We presented a modified lateral approach (i.e. anteroinferior psoas exposure under direct vision) for OLIF and found that it is safe and can preserve the psoas and the lumbar plexus.
Introduction
Lumbar interbody fusion (LIF) is a common and effective procedure for the treatment of lumbar degenerative diseases 1 , 2 . According to the direction of approach, LIF techniques include posterior LIF (PLIF), transforaminal LIF (TLIF), anterior LIF (ALIF), lateral LIF (LLIF), and oblique lateral LIF (OLIF). Each technique has unique advantages and disadvantages 3 . In 1932, Carpenter et al. 4 first reported the treatment of lumbar spondylolisthesis by ALIF approach. This technique was then applied for lumbar instability, lumbar deformity, discogenic low back pain, and posterior lumbar revision surgery. Over the past 20 years, because of the use of the retroperitoneal approach and small incision devices or endoscopic systems, surgical trauma has been significantly reduced; however, complications such as large vessel injury and retrograde ejaculation using the ALIF technique are still difficult to completely avoid 3 . In 1944, Briggs et al. 5 first reported the PLIF technique. With the development of bone grafting materials, fusion devices, and internal fixation, the technique was at one stage the most widely used LIF technique in the world. However, with people's attention on the injury of posterior paraspinal muscles, spinous processes, and the ligament complex, in 1982, Harms and Rolinger proposed the TLIF technique 6 ; decompression and fusion were performed through removal of the articular process, which not only reduced the traction of the dura mater and the nerve root but also preserved the integrity of the posterior complex. Especially in the past 20 years, with the development of tubes, endoscopes, and imaging systems, TLIF technology has increasingly involved small incisions and minimally invasive approaches, with less trauma and faster postoperative recovery. However, any posterior LIF technology cannot completely avoid damage to the lumbar posterior structures and disturbance of the spinal canal. McAfee et al. 7 (1998) and Ozgur et al. (2006) 8 reported on the minimally invasive LLIF via the psoas major muscle approach, which avoids the disadvantages of posterior fusion and reduces the risk of large blood vessel injury using ALIF; however, the risk of psoas major muscle and lumbar plexus nerve injury cannot be ignored 9 .
Through utilizing the anatomical space between the aorta and psoas muscle to access the disc space, the OLIF was the proposed solution to the approach‐related disadvantages of ALIF and LLIF 10 . First, compared with the posterior lumbar surgeries, OLIF completely preserved the posterior structures of the spine and avoided the occurrence of iatrogenic back pain 3 . Fan et al. 11 found that the minimally invasive PLIF can significantly reduce the injury and atrophy of paraspinal muscles and reduce the incidence of iatrogenic back pain compared with the traditional posterior open surgery. However, they also found that, even in the minimally invasive approach group, the paraspinal multifidus still had a little atrophy 1 year after the operation 11 . According to Danneels et al. (2000) 12 , once the paraspinal muscles degenerate, the antagonistic balance between the original trunk muscles is broken, resulting in a series of compensatory changes, which can cause secondary damage to the lumbar spine. Second, using OLIF, there is no need to open the spinal canal, so there is no interference to the nerves and postoperative neurologic complications are avoided; moreover, it can remove more intervertebral disc tissues and expand the fusion area, while the wide fusion cage can extend to the dense epiphyseal ring around the vertebral body on both sides, which increases the support strength of the fusion cage. Because of the retention of the anterior and posterior longitudinal ligaments, when the intervertebral space is distracted, it can obtain better reduction of spondylolisthesis and effective indirect decompression of the vertebral canal 3 . Compared with ALIF, OLIF not only avoids the retraction of anterior blood vessels, and reduces the risk of vascular injury and retrograde ejaculation, but also preserved the anterior longitudinal ligament and anterior annulus fibrous structure. In addition, the anterior tension band of the lumbar spine can be preserved intact. Compared with LLIF, OLIF enters through the front edge of the psoas muscle, avoiding direct damage to the psoas muscle, and can reduce the risk of lumbar plexus injury 13 .
An anatomical study measured the OLIF access corridor, which was defined at L2–5 as the left lateral border of the aorta (or iliac artery) and the anterior medial border of the psoas, with the results showing that the mean access corridor diameters in the static state were as follows: at L2–3, 18.60 mm; at L3–4, 19.25 mm; and at L4–5, 15.00 mm 14 . An MRI study also showed that the oblique corridor measurements to the L2–5 discs have the following mean distances: L2–3 = 16.04 mm, L3–4 = 14.21 mm, and L4–5 = 10.28 mm 15 . The diameter of the OLIF tube was 22 mm and the width of the PEEK cage was at least 18 mm; both of the distances were a little bigger than the anatomical access corridor. There may be vessel variation, such as segmental arteries, and ovarian/testicular veins may run close to the disc space 16 . Therefore, it is still not safe to settle the surgical tube system using just the physiological gap between major vessels and the psoas. Davis et al. 6 advised that psoas retraction can provide a generous corridor to disc space.
Therefore, in this study, we presented a modified lateral approach (i.e. anteroinferior psoas [AIP] exposure under direct vision) for OLIF and performed a retrospective chart review of patients treated with AIP OLIF at our hospital. The purpose of this study was: (i) to introduce the AIP approach; (ii) to evaluate the outcomes and complications of patients who underwent this procedure at our institute; and (iii) to discuss the advantages of this AIP approach compared to previous anterior psoas approaches.
Materials and Methods
Inclusion and Exclusion Criteria
The inclusion criteria were: (i) patients with degenerative diseases, lumbar spondylosis, spondylolisthesis, lumbar stenosis, and scoliosis confirmed by anteroposterior, lateral, oblique, and flexion–extension plain radiographs, CT scans, and MRI; (ii) patients who experienced failed conservative treatment for at least 3 months; and (iii) patients who underwent single or multilevel OLIF from L1–L5 with the AIP approach. The exclusion criteria were: (i) patients with spinal neoplasm, infection, and trauma; (ii) patients who had posterior lumbar decompression or fusion procedures; and (iii) patients with a follow‐up period of less than 1 year.
Patient Information
A total of 382 consecutive patients who underwent the AIP OLIF procedure between June 2016 and April 2019 were retrospectively reviewed at our institution between June 2016 and April 2019. Based on the exclusion criteria, 56 patients were excluded from the study, bringing the final number of patients to 226 and the total number of operative levels to 431. Among these 226 patients, 61 patients received a stand‐alone procedure without any additional internal fixation and 165 patients received additional posterior pedicle screw fixation through the Wiltse approach 17 . Patient characteristics, including age, gender, diagnosis, and co‐morbidities, were collected. All patients were followed up (18.2 ± 6.1 months, range: 12–31 months). More than 6 months after surgery, 132 patients consented to having MRI examinations. The mean period of MRI follow‐up for these 132 patients was 9.1 ± 3.5 months. The patients’ demographic characteristics and procedure data are listed in Table 1.
TABLE 1.
Characteristics of the included patients
| Characteristics | |
|---|---|
| Age (y) | 65.5 ± 16.2 |
| Gender (M/F) | 98/128 |
| Surgical diagnosis (n, %) | |
| Degenerative disc disease | 28 (12.4%) |
| Spondylolisthesis | 56 (24.8%) |
| Spinal stenosis | 99 (43.8%) |
| Degenerated scoliosis | 43 (19.0%) |
| Fused segments (n, %) | |
| 1 | 95 (42.0%) |
| 2 | 77 (34.1%) |
| 3 | 34 (15.0%) |
| 4 | 20 (8.8%) |
| Fused levels (n, %) | |
| L1/L2 | 23 (5.3%) |
| L2/L3 | 72 (31.9%) |
| L3/L4 | 116 (26.9%) |
| L4/L5 | 220 (51.0%) |
| Hospital stay (d) | 4.1 ± 2.1 |
| Time of last follow up (m) | 18.2 ± 6.1 |
| Time for MRI postoperatively (m) | 9.1 ± 3.5 |
d, day; m, month; M/F, male/female; n, number; y, year.
Surgical Techniques
Anesthesia and Position
After general anesthesia, the patient was placed in the lateral decubitus position on the right side. The hip was positioned just below the table break and was gently flexed to relax the psoas muscle and the femoral nerve. A pillow was placed in between the knees and taping of the lower pelvis and uppermost hip and femur was performed to stabilize the spine and allow gentle traction of pelvis by bending the surgical bed. Anterior–posterior fluoroscopy was used to ensure that there was no rotation of the spine. The chest and hip were then taped, and lateral fluoroscopy was performed to ensure the central point of the target intervertebral disc space.
Approach and Exposure
A transverse skin incision is recommended that is approximately 4 cm in length from the midpoint of the target intervertebral disc for a one‐level procedure. For a two‐level procedure, the incision was on the middle part of the connective line between the two midpoints of adjacent disc spaces, and still approximately 4 cm in length. For a three‐level or four‐level procedure, we advise two independent 4‐cm incisions. Three muscular layers of the abdominal wall, the external oblique, the internal oblique, and the transversalis, were bluntly split along the direction of muscle fibers. Then the retroperitoneal space was bluntly dissected, and the peritoneum was mobilized anteriorly using the retractors to expose the anterior border of the psoas. The intervertebral disc was identified by retracting the anterior border of the psoas posteriorly using Cobb dissector under direct visualization and the psoas muscle was dissected from the disc surface and retracted posteriorly (Fig. 1). The guide pin, the probe, the sequential dilators, and the tube retractor were sequentially put on the surface of disc vertically, and the retractor was fixed to the upper vertebral body with a pin (Fig. 2).
Fig. 1.
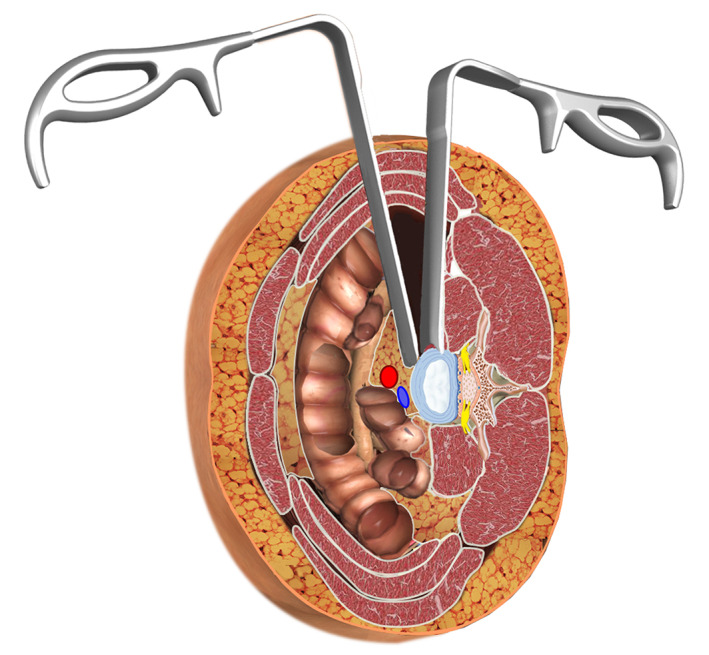
Illustration of anteroinferior psoas exposure using special retractors. The left retractor blocks the abdominal structures without retraction; the right retractor rides across the surface of the bulge disc. The psoas muscle was dissected from the disc surface and retracted posteriorly.
Fig. 2.
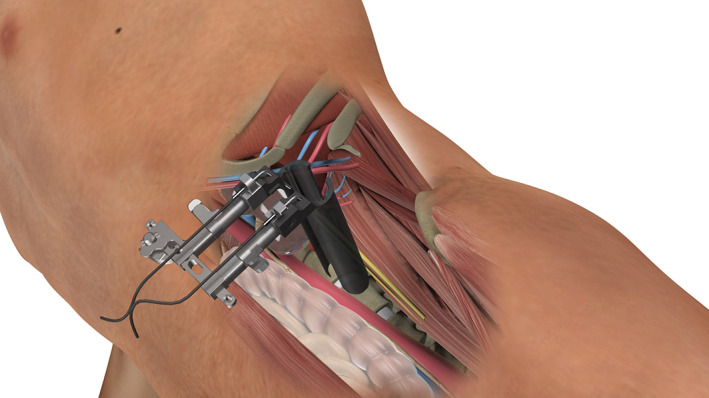
Illustration of the tube retractor placement. The tube was fixed at the left side of the target disc and made the psoas muscle retract posteriorly.
Discectomy and Interbody Fusion
Discectomy was performed and a peek cage (Clydesdale Spinal System; Medtronic Sofamor Danek, Minneapolis, MN, USA) filled with artificial bone (Wright, Tennessee, USA) was inserted vertically into the intervertebral space. After anterior fusion, some patients were turned to the prone position to undergo posterior pedicle screw instrumentation if necessary.
Clinical Assessment
Data for operative and clinical parameters were collected: operative time, intraoperative estimated blood loss, duration of postoperative hospital stay, and time to ambulation after surgery. Complications were also recorded as surgical exposure approach‐related complications.
Low Back Pain Evaluation
The degree of low back pain was evaluated using a visual analogue scale (VAS) score. The degree of low back pain was evaluated in all patients using a visual analogue graduated scale. The score criteria were as follows: no pain: 0; mild pain, tolerable, not affecting sleep: 1 to 3; moderate pain, mildly affecting sleep, still tolerable: 4 to 6; severe pain, unbearable pain, pain resulting in inability to sleep or waking up from sleep: 7 to 10.
Assessment of Disability
The Oswestry disability index (ODI) is a principal outcome measure designed to evaluate patient progress in routine clinical practice. It is a self‐administered questionnaire divided into 10 sections: pain intensity, personal care, lifting, walking, sitting, standing, sleeping, sex life, social life, and traveling. Each section is scored on a scale from 0 to 5, with 5 representing the greatest disability. The index is calculated by dividing the total score by the total possible score and then multiplying the results by 100. The intervals of 0%–20%, 21%–40%, 41%–60%, 61%–80%, and 81%–100% were considered mild dysfunction, moderate dysfunction, severe dysfunction, disability, and long‐term bedridden, respectively.
MRI Evaluation of Psoas Muscle
MRI was performed on a 1.5 Tesla System (GE Signa Excite; GE Healthcare, Milwaukee, Wisconsin) preoperatively and at the follow‐up more than 6 months postoperatively. All images were obtained using a T2‐weighted fast spin echo pulse sequence, with matrix size 255 × 512, field of view 240 × 240 mm, band width 120 Hz/Px, and echo factor 15. Slice thickness was 4 mm and the interslice gap was 1 mm. Patients were placed supine with a pillow positioned underneath the knees, ensuring that they were lying symmetrically with weight evenly distributed across both sides. The experienced musculoskeletal radiologists used anatomic markers and locating lines on sagittal plane scans to select the most similar preoperative and follow‐up axial images, at the same spinal level, for comparison.
Measurements were taken with Image J 1.46 (National Institutes of Health, http://rsbweb.nih.gov/ij/download.html) with software for embedded region of interest and grayscale histogram evaluation. To determine the lean psoas muscle cross‐sectional area (CSA), the region of interest (ROI) was drawn around the psoas muscles bilaterally, taking care to avoid nearby fat, bony structures, and other soft tissues. The sum of CSA of bilateral lean psoas was calculated. To determine the mean signal intensity of psoas, the ROI was drawn around the outer perimeter of the muscle unilaterally, to include any areas of intramuscular fat. The mean signal intensity of one piece of gross psoas muscle was determined bilaterally on T2‐weighted axial images. The intensity of this signal was evaluated quantitatively using the grayscale histogram software included in the Image J package, in which a higher score means greater intensity. The mean signal intensity of the quadratus lumborum muscle was also evaluated in the same axial images from an approximate 30 mm2 circular region of interest at the center of the muscle. The signal intensity ratio of gross psoas to quadratus lumborum was calculated.
Three experienced spine surgeons, blinded to the operative side, analyzed the selected axial scans on two occasions, 2 weeks apart, using the same protocol. Interobserver and intraobserver repeatability were calculated using the intraclass correlation coefficient (ICC) (3, 1) formula 18 . The ICC for interobserver and intraobserver repeatability ranged from 0.86 to 0.95. Because these ICC indicated good intraobserver and interobserver reliability, the mean of the readings was used.
Statistical Analysis
The measurement data were expressed as mean ± standard deviation. Student's t‐test was used to evaluate the differences for VAS and ODI scores preoperatively and postoperatively, and to compare postoperative PCSA and T2 ratio changes compared to preoperative measurements between the operative side and nonoperative sides. All statistical analyses were performed using SPSS 20.0 software. P < 0.05 was considered significant.
Results
General Results
The mean operative time was 82.5 ± 31.6 min (range, 30–390 min). The mean operative time for each segment of OLIF was 43.3 ± 15.5 min (range, 30–97.5 min). The mean blood loss was 48.0 ± 11.6 mL (range, 15–110 mL). The mean blood loss for each segment of OLIF was 25.3 ± 10.1 mL (range, 10–50 mL). No patients needed blood transfusion intraoperatively or postoperatively. The mean hospital stay was 4.1 ± 2.1 days (range, 2–10 days).
Low Back Pain Evaluation
All patients were available for the 6‐month postoperative and final follow‐up low back pain assessments using VAS (Table 2). The VAS score was 6.5 ± 1.6 preoperatively, 1.6 ± 0.8 at 6 months postoperatively, and 1.3 ± 0.6 at final follow‐up postoperatively. The VAS score at 6 months after surgery was markedly lower than the preoperative score, with a mean improvement of 75.4% (P < 0.001), but did not differ from the score at the final follow‐up (P > 0.05).
TABLE 2.
Patient clinical outcome data
| Preoperative | Postoperative 6 months | Final follow‐up | |
|---|---|---|---|
| VAS | 6.5 ± 1.6 | 1.6 ± 0.8* | 1.3 ± 0.6** |
| ODI | 62.6 ± 22.1 | 13.9 ± 5.3* | 12.1 ± 4.1** |
ODI, Oswestry disability index; VAS, visual analogue scale.
*Indicates the comparison between preoperative and postoperative 6 months. **Indicates the comparison between postoperative 6 months and final follow up. * P < 0.001 and ** P > 0.05.
Disability Evaluation
The ODI score was 62.6 ± 22.1 preoperatively, 13.9 ± 5.3 at 6 months postoperatively, and 12.1 ± 4.1 at final follow‐up postoperatively (Table 2). The ODI scores at 6 months after surgery were markedly lower than the preoperative scores, with a mean improvement of 77.8% (P < 0.001), but did not differ from the score at the final follow‐up (P > 0.05).
MRI Evaluation of Psoas Muscle (n = 132)
The CSA of lean psoas muscle at the operative level of the left side was 637.8 ± 331.6 mm2 preoperatively and 651.2 ± 373.6 mm2 postoperatively; the percentage change was 2.10% ± 3.12%. The CSA of lean psoas muscle at the operative level of the right side was 654.3 ± 367.2 mm2 preoperatively and 670.1 ± 352.9 mm2 postoperatively; the percentage change was 2.41% ± 3.18%. Based on MRI, at the follow‐up, the CSA of lean psoas muscle at the operative level had increased a little on both sides (Table 3, Fig. 3). There was no significant difference in percentage changes between the left side (operative side) and the right side (nonoperative side) (P = 0.531). The T2 signal intensity ratio of gross psoas to quadratus lumborum at the operative level of the left side was 1.21 ± 0.15 preoperatively and 1.23 ± 0.11 postoperatively; the percentage change was 1.65% ± 3.01%. The T2 signal intensity ratio of gross psoas to quadratus lumborum at the operative level of the right side was 1.20 ± 0.25 preoperatively and 1.22 ± 0.13 postoperatively; the percentage change was 1.68% ± 5.26%. The T2 signal intensity ratio of gross psoas to quadratus lumborum had increased a little on both sides at the follow‐up with MRI (Table 3, Fig. 3). There was also no significant difference in percentage changes between the left side and right side (P = 0.682).
TABLE 3.
MRI evaluation of psoas muscle
| Left side | Right side | P | |||||
|---|---|---|---|---|---|---|---|
| Preoperative | Follow‐up | Change (%) | Preoperative | Follow‐up | Change (%) | ||
| PCSA | 637.8 ± 331.6 | 651.2 ± 373.6 | 2.10 ± 3.12 | 654.3 ± 367.2 | 670.1 ± 352.9 | 2.41 ± 3.18 | 0.531 |
| T2 ratio | 1.21 ± 0.15 | 1.23 ± 0.11 | 1.65 ± 3.01 | 1.20 ± 0.25 | 1.22 ± 0.13 | 1.68 ± 5.26 | 0.682 |
PCSA, cross‐sectional area of lean psoas muscle; T2 ratio, T2‐weighted signal intensity ratio of gross psoas to quadratus lumborum muscle.
Fig. 3.
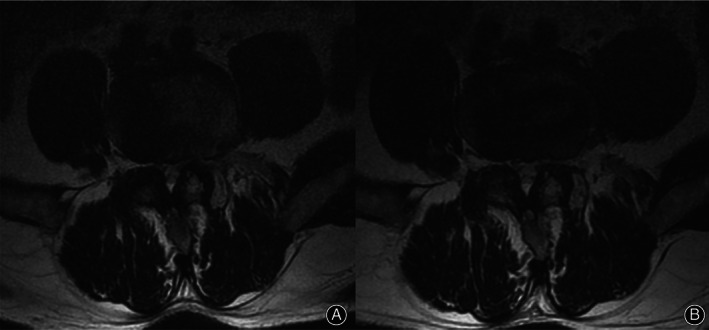
Representative T2‐axial MRI showing the preservation of the left (surgical side) psoas using the anteroinferior psoas technique. (A) Preoperative. (B) Postoperatively, there was no edema or fatty infiltration changes at the surgical side (left side) in the psoas muscle.
Complications
Table 4 shows the reported surgical exposure approach‐related complications. A total of 11 complications were reported, with an incidence of 4.9%: transient thigh pain/numbness, psoas weakness (2.2%), sympathetic chain injury (1.3%), cage subsidence (0.9%), and segmental artery injury (0.4%). There was no permanent motor neurological deficit and no injury of vascular, ureter or peritoneal membranes.
TABLE 4.
Surgical exposure approach‐related complications (4 cases, 5.6%)
| Complications | N (%) |
|---|---|
| Transient thigh pain/numbness, psoas weakness (neurological injury) | 5 (2.2%) |
| Sympathetic chain injury (neurological injury) | 3 (1.3%) |
| Cage subsidence | 2 (0.9%) |
| Segmental artery injury | 1 (0.4%) |
Discussion
Surgical Data and Clinical Outcome
In this study, we proposed a modified lateral approach: anteroinferior psoas (AIP) exposure under direct vision for OLIF. The perioperative data, such as operative time, blood loss, blood transfusion, and hospital stay, were similar to those of previous studies 10 , 19 . The clinical outcome, evaluated by VAS and ODI score, showed good clinical effects of this AIP OLIF procedure.
Surgical Technique
The small skin incision, deep surgical site, and adjacent vessels, nerves, and ureter make it difficult to establish the work channel, so the surgical exposure approach is the core procedure for anterior or lateral lumbar spinal surgery. According to a previous technical report 10 and the surgical technique handbook from Medtronic, the work channel was built by using the finger or handheld retractors to protect the peritoneal membrane and retract retroperitoneal fat, and the probe, sequential dilators, and tube retractor were inserted onto the surface of the target disc space. However, it is limited to retracting the anterior structures, such as the peritoneal membrane and vessels, and anterior structure retraction is not useful for exposing the target disc because the lateral annulus fibrosus is usually totally covered by the psoas muscle when the patient is in lateral decubitus position during the operation. An anatomical study 14 showed that mild psoas retraction without significant tendon disruption can make the corridor to the disc space obviously enlarged. In this study, we applied the anteroinferior psoas approach under direct vision to expose the surgical disc; we found that this approach makes it easy to build the work channel.
Complications
A systematic review and meta‐analysis 20 including 20 studies and 1874 patients for analysis of complications showed that the incidence of surgical exposure‐related complications was 27.1%, including transient thigh or groin numbness/pain (8.7%), transient hip flexor weakness (5.7%), permanent motor neurological deficit (1.0%), sympathetic plexus injury (5.4%), major vascular injury (1.8%), peritoneal (bowel) injury (1.9%), urological injury (1.1%), and hematoma (1.5%). Our results showed that the incidence of exposure approach‐related complications was 4.9%, and there was no injury of the ureter and peritoneal membranes. The direct visual exposure and the psoas muscle retraction helped to avoid injury to anterior structures.
Neurological injury to the lumbar plexus and psoas muscle injury are the two greatest risks of LLIF; they have the potential to result in sensory and motor deficits and hip flexion weakness. OLIF is the proposed solution to the approach‐related disadvantages of LLIF. In this approach, the anatomic space between the aorta and psoas muscle is used to access the disc space. However, according to previous studies, the OLIF procedure is still associated with a mild rate of neurological injury and psoas muscle injury. In a 28‐patient cohort, Fujibayashi et al. 21 encountered transient leg weakness in 7.1% and transient numbness in 21.4% of patients. DiGiorgio et al. 22 reported a 6.1% incidence of transient thigh numbness in their 49‐patient cohort. In this study, the incidence of transient thigh pain/numbness and psoas weakness was 2.2%. The psoas muscle splitting and retraction (i.e. the AIP exposure approach) can alleviate muscle and nerve squeezing when the trials and implant are placed orthogonally across the disc space. The MRI results in this study showed that the psoas muscle has no injury or atrophy change after surgery, which proved that the AIP approach is helpful for muscle and nerve preservation.
Limitations
The current study has some limitations. First, it is a retrospective study from a single center, in which the results were reviewed by the authors. Further multicenter and prospective studies should be carried out. Second, it is a technical report and case series; the advantages of the AIP approach really need to be demonstrated in a comparison study. Finally, the follow‐up period was relatively short, and the sample size was small. A longer follow‐up period with a larger number of cases is still necessary to evaluate the definitive effect of the AIP approach for OLIF.
Conclusion
We presented a modified lateral approach (i.e. AIP exposure under direct vision for OLIF), and the results showed that it has low surgical exposure approach‐related complications and can preserve the psoas and the lumbar plexus. However, further randomized controlled trial studies with large sample sizes should be conducted.
Acknowledgments
This work was sponsored by the National Natural Science Fund of China (81472064 and 81672150), the Zhejiang Medical and Health Science and Technology Project (2018KY117 and 2019ZD041), New Talent in Medical field of Zhejiang Province, and the Fundamental Research Funds for the Central Universities.
Disclosure: The authors declare no conflict of interest. No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1. de Kunder SL, Rijkers K, Caelers IJMH, de Bie RA, Koehler PJ, van Santbrink H. Lumbar interbody fusion: a historical overview and a future perspective. Spine (Phila Pa 1976), 2018, 43: 1161–1168. [DOI] [PubMed] [Google Scholar]
- 2. Mummaneni PV, Dhall SS, Eck JC, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 11: interbody techniques for lumbar fusion. J Neurosurg Spine, 2014, 21: 67–74. [DOI] [PubMed] [Google Scholar]
- 3. Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI‐TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg, 2015, 1: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carpener N. Spondylolisthesis. Br J Surg, 1932, 19: 374–386. [Google Scholar]
- 5. Briggs H, Milligan PR. Chip fusion of the low back following exploration of the spinal canal. J Bone Joint Surg Am, 1944, 26: 125–130. [Google Scholar]
- 6. Harms J, Rolinger H. A one‐stager procedure in operative treatment of spondylolistheses: dorsal traction‐reposition and anterior fusion (author's transl). Z Orthop Ihre Grenzgeb, 1982, 120: 343–347. [DOI] [PubMed] [Google Scholar]
- 7. McAfee PC, Regan JJ, Geis WP, et al. Minimally invasive anterior retroperitoneal approach to the lumbar spine: emphasis on the lateral BAK. Spine (Phila Pa 1976), 1998, 23: 1476–1484. [DOI] [PubMed] [Google Scholar]
- 8. Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar inter‐body fusion. Spine J, 2006, 6: 435–443. [DOI] [PubMed] [Google Scholar]
- 9. Joseph JR, Smith BW, La Marca F, Park P. Comparison of complication rates of minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion: a systematic review of the literature. Neurosurg Focus, 2015, 39: E4. [DOI] [PubMed] [Google Scholar]
- 10. Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1‐L5 (OLIF25) and at L5‐S1 (OLIF51) and evaluation of complication and fusion rates. Spine J, 2017, 17: 545–553. [DOI] [PubMed] [Google Scholar]
- 11. Fan S, Hu Z, Zhao F, et al. Multifidus muscle changes and clinical effects of one‐level posterior lumbar interbody fusion: minimally invasive procedure versus conventional open approach. Eur Spine J, 2010, 19: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, de Cuyper HJ, Danneels L. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J, 2000, 9: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu DS, Walker CT, Godzik J, Turner JD, Smith W, Uribe JS. Minimally invasive anterior, lateral, and oblique lumbar interbody fusion: a literature review. Ann Transl Med, 2018, 6: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davis TT, Hynes RA, Fung DA, et al. Retroperitoneal oblique corridor to the L2‐S1 intervertebral discs in the lateral position: an anatomic study. J Neurosurg Spine, 2014, 21: 785–793. [DOI] [PubMed] [Google Scholar]
- 15. Molinares DM, Davis TT, Fung DA. Retroperitoneal oblique corridor to the L2‐S1 intervertebral discs: an MRI study. J Neurosurg Spine, 2016, 24: 248–255. [DOI] [PubMed] [Google Scholar]
- 16. Orita S, Inage K, Sainoh T, et al. Lower lumbar segmental arteries can intersect over the intervertebral disc in the oblique lateral interbody fusion approach with a risk for arterial injury: radiological analysis of lumbar segmental arteries by using magnetic resonance imaging. Spine (Phila Pa 1976), 2017, 42: 135–142. [DOI] [PubMed] [Google Scholar]
- 17. Wiltse LL. The paraspinal sacrospinalis‐splitting approach to the lumbar spine. Clin Orthop Relat Res, 1973, 91: 48–57. [DOI] [PubMed] [Google Scholar]
- 18. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull, 1979, 86: 420–428. [DOI] [PubMed] [Google Scholar]
- 19. Tannoury T, Kempegowda H, Haddadi K, Tannoury C. Complications associated with minimally invasive anterior to the psoas (ATP) fusion of the lumbosacral spine. Spine (Phila Pa 1976), 2019, 44: E1122–E1129. [DOI] [PubMed] [Google Scholar]
- 20. Walker CT, Farber SH, Cole TS, et al. Complications for minimally invasive lateral interbody arthrodesis: a systematic review and meta‐analysis comparing prepsoas and transpsoas approaches. J Neurosurg Spine, 2019, 30: 446–460. [DOI] [PubMed] [Google Scholar]
- 21. Fujibayashi S, Hynes RA, Otsuki B, Kimura H, Takemoto M, Matsuda S. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine (Phila Pa 1976), 2015, 40: E175–E182. [DOI] [PubMed] [Google Scholar]
- 22. DiGiorgio AM, Edwards CS, Virk MS, Mummaneni PV, Chou D. Stereotactic navigation for the prepsoas oblique lateral lumbar interbody fusion: technical note and case series. Neurosurg Focus, 2017, 43: E14. [DOI] [PubMed] [Google Scholar]


