Abstract
To introduce a new surgery, percutaneous endoscopic unilateral laminotomy and bilateral decompression (Endo‐ULBD) using visual trepan, and investigate its efficacy and safety in elderly patients with lumbar spinal stenosis. In our retrospective study, a total of 69 patients were enrolled between March 2018 and September 2018; 31 patients were treated with Endo‐ULBD and 38 patients were treated with posterior lumbar interbody fusion surgery (PLIF). The operation time, intraoperative blood loss, and hospitalization duration were compared between the two groups. A visual analog scale (VAS) was used to evaluate the degree of pain. The Oswestry Disability Index (ODI) and European Quality of Life‐5 Dimensions (EQ‐5D) were used to evaluate lumbar function and quality of life, respectively. Lumbar X‐ray, computed tomography (CT) and magnetic resonance imaging (MRI) were performed postoperatively at different time points. MacNab's outcome assessment and perioperative complications were also documented. The surgeon completed all surgeries successfully, and all 69 patients were followed up. The operative time of the Endo‐ULBD group was 60.68 ± 0.47 min, while that of the PLIF group was 120.23 ± 10.24 min. The operative time of the Endo‐ULBD group was shorter than that of the PLIF group, and the difference was statistically significant (P < 0.001). The volume of intraoperative blood loss was 47.25 ± 0.43 mL in the Endo‐ULBD group and 256.90 ± 20.83 mL in the PILF group (P < 0.001). The length of hospital stay in the Endo‐ULBD group was 5.12 ± 1.60 days and that in the PILF group was 10.54 ± 1.82 days (P < 0.001). The VAS scores at postoperative 1 day, 3 months, 6 months, final follow‐up (Endo‐ULBD: 6.58 ± 0.65, 4.55 ± 0.54, 2.78 ± 0.24, 1.31 ± 0.78; PLIF: 7.19 ± 1.14, 4.80 ± 0.13, 2.71 ± 0.83, 1.29 ± 0.56) were significantly improved compared with those before surgery (Endo‐ULBD: 8.63 ± 0.37; PLIF: 8.31 ± 1.34). The ODI and EQ‐5D scores of lumbar function and quality of life at each time point after surgery (Endo‐ULBD ODI: 30.29% ± 0.47%, 23.35% ± 0.95%, 19.45% ± 0.81%, 10.84% ± 0.36%; EQ‐5D: 0.38 ± 0.15, 0.45 ± 0.17, 0.63 ± 0.14, 0.71 ± 0.20; PLIF ODI: 33.56% ± 1.58%, 25.69% ± 2.69%, 20.01% ± 1.49%, 10.72% ± 0.29%; EQ‐5D: 0.33 ± 0.03, 0.39 ± 0.05, 0.62 ± 0.07, 0.72 ± 0.10) were significantly improved compared with those before surgery (Endo‐ULBD: 44.56 ± 1.32, 0.33 ± 0.07; PLIF: 43.79 ± 1.91, 0.31 ± 0.09, respectively), with statistically significant differences (P < 0.05); however, there was no significant difference between the two groups at the last follow‐up (P > 0.05). At the last follow‐up, the excellent and good efficacy rate was 90.3% (28/31) in the Endo‐ULBD group and 89.4% (34/38) in the PILF group (χ2 = 0.089, P = 0.993). No mortality, irreversible nerve injury, or even paralysis occurred in either group. Endo‐ULBD for lumbar spinal stenosis has the advantages of less trauma, a shortened operation time, and rapid recovery and is an effective alternative for the treatment of lumbar spinal stenosis. Strict surgical indications, reasonable surgical plans, and experienced surgeons are important factors to ensure safety and satisfactory postoperative efficacy.
Keywords: Endoscopic, Lumbar spinal stenosis, Unilateral laminotomy and bilateral decompression (Endo‐ULBD), Visual trepan
Endo‐ULBD for LSS has the advantages of less trauma, a shortened operation time and rapid recovery and is an effective alternative for the treatment of lumbar spinal stenosis. Strict surgical indications, reasonable surgical plans and experienced surgeons are important factors to ensure safety and satisfactory efficacy.
Introduction
Lumbar spinal stenosis (LSS) is a common degenerative disease of the lumbar vertebrae in elderly patients and is characterized by neurogenic intermittent claudication, leading to continuously shortened walking distances as the disease progresses and seriously affecting patient quality of life 1 , 2 . Surgical procedures are commonly used in the treatment of lumbar spinal stenosis, including total laminectomy decompression and lumbar intervertebral fusion with pedicle screw fixation 3 . Although these approaches can effectively achieve spinal canal decompression, the operation causes great damage to the posterior column of the spine, which can lead to lumbar instability, degenerative deformity, adjacent segment disease, and other complications 4 , 5 . Therefore, it is imperative to adopt appropriate surgical methods for elderly patients with degenerative lumbar spinal stenosis.
Verbiest, a Dutch neurosurgeon, first published an original paper on spinal stenosis in 1950 6 . In 1951, O'Connell published a paper on the surgical treatment of disc herniation in which he described in detail the evolution of laminectomy, which laid a solid foundation for the future treatment of LSS 7 . In 1988, Young first reported the technique of unilateral laminectomy for bilateral decompression (ULBD) in place of expanded laminectomy, and this new approach retained the spinous process and contralateral lamina and retained most of the posterior columnar structure 8 . In 1991, McCulloch further improved the ULBD technology invented by Young, marking the maturity of ULBD technology. ULBD has achieved good clinical effect and high safety of operation. However, there is no water as the medium under the microscope. Besides,due to bleeding, the visual field of the operation is slightly poor.
In recent years, minimally invasive endoscopic decompression technology for cervical, thoracic, and lumbar spine disorders has been widely used 9 , 10 , 11 , 12 . Some scholars reported that percutaneous endoscopic transforaminal discectomy (PETD) 13 has high safety in treatment of degenerative spinal disease and has achieved a good early clinical effect. It has also been reported in the literature that transforaminal endoscopic thoracic discectomy could be a feasible method for treating thoracic spinal stenosis 9 . Furthermore, the surgical instruments have been continuously improved and the indications of spinal endoscopic technology have been further expanded 12 , 14 , 15 , especially for the application of endoscopic visual trepan, which allows the technology to be applied in the treatment of LSS.
Endo‐ULBD surgery could achieve bilateral decompression by only decompressing the lamina on one side. The root of the spinous process and the contralateral lamina were retained in this operation, which caused little damage to the posterior column of the lumbar spine and avoided later spinal instability. Endo‐ULBD was performed under the combination of basic anesthesia and local anesthesia, which could improve the patients' comfort and relieve tension during the operation. Despite the advantages of endoscopic surgery via ULBD approach, the technique relies too much on surgical experience and intraoperative fluoroscopy. In addition, orthopaedic surgeons are more familiar with the posterior median approach, which makes this technique harder to accept and lengthens the learning curve. To solve the above problems, we introduced the procedures of Endo‐ULBD using visual trepan in elderly patients with LSS. The application of visual trepan for lamina decompression not only shortens the operative time by approximately 1 hour compared with conventional endoscopic surgery 16 , but also reduces the risk of nerve injury and improves safety by performing decompression under direct vision rather than relying on surgical experience and intraoperative fluoroscopy. Different from the previous endoscopic techniques 17 , this operation does not require multiple intraoperative fluoroscopy, but only needs to place the working channel at the root of the spinous process. The visual trepan used in this operation can not only establish the working channel, but also be used as a surgical tool to replace the Endo‐Kerrison punch. Because of its serrated front end, it can be used to remove the lamina and part of the root of the spinous process, which greatly reduces the operation time. In addition, spine surgeons are more familiar with the posterior approach, which makes this technique easier to accept, safer, and shortens the learning curve.
However, a successful percutaneous endoscopic unilateral laminotomy and bilateral decompression (Endo‐ULBD) operation for LSS requires surgeons skilled in spinal endoscopy technology, and there is little literature on this subject 12 , 15 .
The purpose of our research was to: (i) introduce a new surgical tool called visual trepan to be used in Endo‐ULBD surgery; (ii) evaluate the efficacy and feasibility of Endo‐ULBD by comparing with traditional PLIF; (iii) demonstrate the advantages of Endo‐ULBD using visual trepan.
In our study, a total of 69 patients were enrolled from March 2018 to September 2018, Endo‐ULBD using visual trepan and posterior lumbar interbody fusion operations (PLIF) were performed respectively, and the curative effects of the two operations were compared. In summary, this surgical method combined with the use of visual trepan has high safety, is short, and causes little damage, which can greatly reduce the postoperative stress response and potential complications.
The research was approved by the medical ethics committee of the Third Hospital of Henan Province. All patients and their families signed informed consent forms. The report is as follows.
Patients and Methods
Inclusion Criteria
Our inclusion criteria were as follows: (i) patients diagnosed with degenerative LSS, Schizas grade 18 B and C; bilateral lower limb symptoms with neurotic intermittent claudication <100 m with ineffective conservative treatment for more than 3 months; and central canal stenosis or lateral recess stenosis with single‐segment LSS was confirmed by CT and MRI (Fig. 1); (ii) patients who underwent Endo‐ULBD or PLIF; (iii) visual analog scale (VAS), Oswestry disability index (ODI) scale, European quality of life–5 dimensions (EQ‐5D) scores, and MacNab's criteria and complications were compared; (iv) follow‐up results of the patients were recorded; and (v) a clinical retrospective research.
Fig. 1.
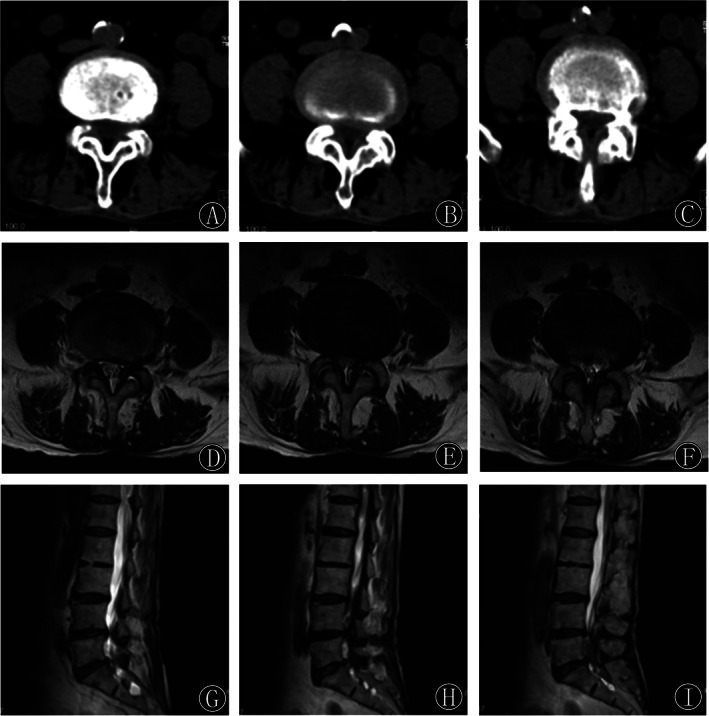
(A–C) Preoperative consecutive axial CT images showed facet joint hyperplasia and cohesion at the L4‐5 segment, resulting in spinal canal stenosis and severe compression of the dural sac and spinal roots. (D–I) Preoperative axial and sagittal T2‐weighted MRI images showed spinal stenosis at the L4‐5 segment with significant compression of the dural sac.
Exclusion Criteria
Patients with lumbar instability and symptoms of lower back pain, LSS with Meyerding Grade II spondylolisthesis, multisegment stenosis with a history of lumbar surgery, infection, tumor or trauma, lower extremity neuropathy, or mental disorders were excluded.
Patient Population
According to the above criteria, a total of 69 patients were enrolled in our retrospective study between March 2018 and September 2018; 31 patients were treated with percutaneous endoscopic unilateral laminotomy and bilateral decompression (Endo‐ULBD group), and 38 patients were treated with posterior lumbar interbody fusion surgery (PLIF group). The average age of the Endo‐ULBD group was 75.52 ± 1.48 years, including 14 males and 17 females, while that of the PLIF group was 76.10 ± 0.93 years, with 16 males and 22 females. The length of disease course in the Endo‐ULBD group was 9.16 ± 0.96 months and that in the PILF group was 8.97 ±1.57 months. The follow‐up period was 20.16 ± 0.43 months in the Endo‐ULBD group and 21.22 ± 2.47 months in the PILF group. There were no significant differences in sex, age, disease course and follow‐up period, imaging classification and grade, or surgical segments between the two groups (P > 0.05).
Surgical Techniques
Endo‐ULBD Group
Anesthesia and Position
The patients were placed prone with the chest and iliac crest pads elevated, and the abdomen was suspended. Basic anesthesia combined with local anesthesia was administered to relieve the patient's nervousness.
Approach and Exposure
After positioning the patient and disinfecting the surface, an 8‐mm longitudinal incision was made 0.5–0.8 cm from the posterior midline according to the size of the patient. The scalpel incised the skin, subcutaneous tissue and deep fascia successively after the skin was anesthetized using lidocaine. X‐ray fluoroscopy was performed again to identify the lesion level by inserting the guide rod. When the working sleeve and visual trepan were inserted along the guide rod, radiofrequency electrodes and nucleus pulposus forceps were used to clean the fibrous adipose tissue on the lamina surface to expose the facet joints and superior and inferior lamina (Figs 2 and 3).
Fig. 2.
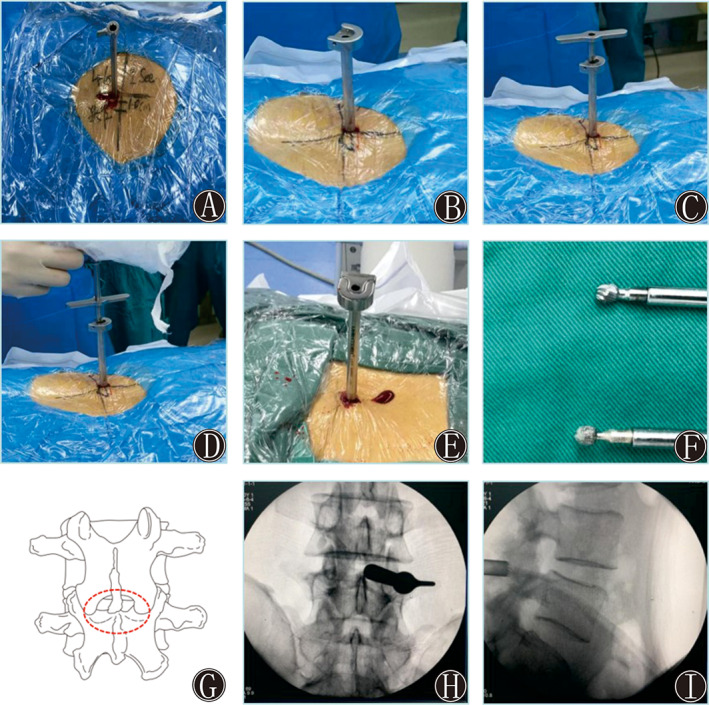
(A) The puncture and incision point were located at the intersection of the posterior median line approximately 1–1.5 cm from the lower edge of the superior lamina. (B) Insertion of the working channel. (C) Setting up the visual trepan along the working channel. (D) Placing the visual endoscopic system along the visual trepan for decompression of the lamina. (E and F) Removal of the lamina bone fragments via the visual trepan. (G) The red dotted line shows the decompression range, including the lower edge of the superior lamina, upper edge of the inferior lamina, and hypertrophy of the ligamentum flavum and articular process. (H and I) The working cannula was confirmed to be inserted into the level of L4‐5 via intraoperative anteroposterior X‐ray imaging.
Fig. 3.
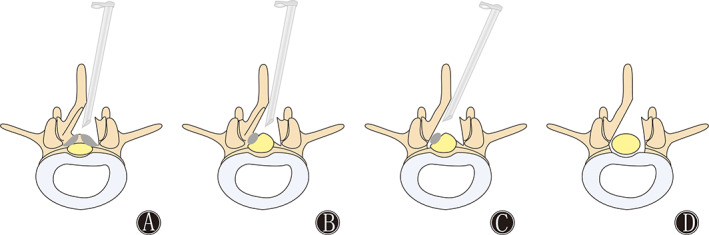
Schematic diagram of visual trepan decompression through percutaneous endoscopic unilateral laminotomy and bilateral decompression (Endo‐ULBD) for LSS. (A) The schematic diagram shows the decompression of the ipsilateral spinal canal by removing the medial edge of the lamina, hypertrophy of the ligamentum flavum, and articular process. (B) The schematic diagram shows adequate decompression of the ipsilateral spinal canal. (C) The schematic diagram shows decompression of the contralateral spinal canal by removing the medial edge of the lamina, hypertrophy of the ligamentum flavum, and articular process. (D) The schematic diagram shows enlargement of the spinal canal after decompressing the spinal cord.
To successfully implant the working channel into the spinal canal, we opted to decompress from the side with severe symptoms. The inner edge of the lamina was removed via the translaminar approach or through the root of the spinal process using visual trepan. The dilator was removed, and then the spinal endoscopic operating system was inserted.
Decompression
An Endo‐Kerrison punch was used to remove the hypertrophic articular osteophytes, and medulla nucleus forceps were used to remove the ligamentum flavum on the medial edge of the lower articular process. The ligamentum flavum was explored and separated to expose the compressed nerve root and narrow spinal canal (Figs 2 and 3A,B). A visual trepan was used to grind the base of the spinous process to the contralateral recess, and the same method was used for contralateral decompression. Contralateral spinal canal decompression was performed according to the above method (Fig. 3C). To completely decompress the spinal canal, all sections with stenosis needed to be fully decompressed. Careful examination of the bilateral dural sac edges and bilateral exploration of the nerve root were required under endoscopy (Fig. 3D).
Close
Finally, radiofrequency electrodes were used to stop the bleeding. Bilateral spinal canal enlargement was considered satisfactory, and the nerve root and dural sac were fully decompressed with good pulsation. After the removal of the working channel, the surgical incision was sutured without drainage.
PLIF Group
Anesthesia and Position
Tracheal intubation was performed under general anesthesia, and the patient was positioned prone on the operating table.
Approach and Exposure
C‐arm fluoroscopy was applied to locate the responsible segments, and a posterior median incision was used to separate and expose the upper and lower lamina, articular process, and transverse process of the target segment.
Decompression
The pedicle screw was accurately placed, and the lamina, hyperplastic articular process, and ligamentum flavum were removed according to the surgical plan. Bone fragments were removed and made into bone particles for preservation. After releasing the nerve, the dural sac and nerve root were retracted to the normal side, the fibrous annulus was cut, the nucleus pulposus and endplate cartilage of the intervertebral disc were removed, and then the bone particles were implanted.
Fusion and Fixation
The cage was filled with fragmented bone and placed into the intervertebral space. Fluoroscopy confirmed that the pedicle screws and cage were positioned well, the connecting rods were placed, and the pedicle screws and rods were fixed with vertebral body reduction, compression, and tightening screws. A drainage tube was placed at the surgical incision; depending on the drainage condition, the tube was usually removed after 3 days and when the drainage volume was less than 50 mL per day.
Postoperative Management
After the operation, antiphlogistic, analgesic, and neurologic drugs and nutritional support were provided. The patient was asked for a waist circumference measurement and to start getting out of bed postoperatively.
Outcome Measures
The operation time, intraoperative blood loss, hospitalization days, and complications were documented and compared between the two groups. VAS, ODI, and EQ‐5D scores were recorded at 1 week, 3 months, and 2 years postoperatively. Anteroposterior and lateral lumbar X‐ray, CT, three dimensional (3D) reconstructions, and lumbar magnetic resonance imaging (MRI) scans were also examined postoperatively.
Visual Analogue Scale
VAS is used to assess the degree of pain. The specific method is: draw a 10‐cm horizontal line on the paper, one end of the horizontal line is 0, indicating no pain; the other end is 10, indicating severe pain; the middle part indicates varying degrees of pain. A score of 0 means no pain. A score below 3 indicates mild pain and can be tolerated. A score of 4–6 indicates that the patient has pain that affects sleep and is bearable. A score of 7–10 indicates that the patient has increasingly intense pain, which is unbearable, affecting appetite and sleep. And the lower the score is, the lighter the pain.
Oswestry Disability Index
ODI is a scale commonly used to evaluate low back pain and dysfunction in the world. The ODI score questionnaire contains 10 items, including lumbar and leg pain, self‐care ability of daily living, lifting, walking, standing, sitting, sexual behavior, sleeping, social life, and traveling. Each observation item is divided into six levels according to its degree, with 0 indicating normal and 5 indicating limited function. The calculation formula = (total score / (questions×5)) × 100%. Mild dysfunction (0%–20%); moderate dysfunction (21%–40%); severe dysfunction (41%–60%); disability (61%–80%), and either long‐term bedridden, or exaggerating the impact of pain on his or her life (81%–100%).
EQ‐5D Score
EQ‐5D 19 , 20 is used to evaluate quality of life, its health description system consists of five sections: mobility, self‐care ability of daily living, daily activities, pain or discomfort, and anxiety or depression. Each section contains three ranks: no difficulties, some or moderate difficulties, and extreme difficulties. EQ‐5D is a vertical visual scale that is 20 cm long. A score of 100 at the top represents the best health in mind and a score of 0 at the bottom represents the worst health in mind. The summary scores derived from EQ‐5D to the time trade‐off (TTO) represent a person's health utility. The highest EQ‐5D score was 1, and the higher the score was, the better quality of life.
MacNab's Criteria
MacNab's criteria is used to assess the clinical efficacy at the final follow‐up after the operation 21 : poor: intermittent claudication, pain is not reduced or even worsened, and the need to take opioid analgesics seriously affects the daily life and work of the patient; fair: the patient's clinical symptoms and pain significantly improved, but the patient still needed to take nonsteroidal drugs, which affected the patient's daily life and work; good: the clinical symptoms and pain disappeared, the patient could resume daily work and they did not need to take analgesic drugs; and excellent: the clinical symptoms and pain completely disappeared, the patient could resume daily work and activities and recovered neurological function. Excellent and good efficacy rate = (excellent cases + good cases)/all cases × 100%.
Statistical Analysis
Summary statistics for normally distributed quantitative variables are expressed as the means and standard deviations; categorical data are summarized by ratios and percentages. The differences in the mean values of continuous variables between time points were compared using one‐way analysis of variance and LSD tests. P < 0.05 is considered statistically significant. All statistical analysis were performed using SPSS (Chicago, IL) 22.0.
Results
Clinical Characteristics
The surgeon completed all surgeries successfully, and all 69 cases were followed up. The average operative time of the Endo‐ULBD group was 60.68 ± 0.47 min, and that of the PLIF group was 120.23 ± 10.24 min. The operative time of the Endo‐ULBD group was shorter than that of PLIF group, and the difference was statistically significant (P < 0.001). The volume of intraoperative blood loss was 47.25 ± 0.43 mL in the Endo‐ULBD group and 256.90 ± 20.83 mL in the PILF group. The difference was statistically significant (P < 0.001). The length of hospital stay in the Endo‐ULBD group was 5.12 ± 1.60 days and that in the PILF group was 10.54 ± 1.82 days. The difference was statistically significant (P < 0.001) (Table 1).
TABLE 1.
Patient demographics and clinical baseline characteristics
| Items | Endo‐ULBD (n = 31) | PLIF group (n = 38) | P value |
|---|---|---|---|
| Sex (Male/Female) | 14/17 | 16/22 | 0.065 |
| Age (mean ± SD, years) | 75.52 ± 1.48 | 76.10 ± 0.93 | 0.051 |
| Period (mean ± SD, months) | 9.16 ± 0.96 | 8.97 ± 1.57 | 0.558 |
| Classification (cases) | 0.282 | ||
| CCS | 9 | 16 | |
| LRS | 6 | 3 | |
| Mixed type | 16 | 19 | |
| Schizas classification (cases) | 0.670 | ||
| Grade B | 18 | 19 | |
| Grade C | 13 | 19 | |
| Segment (cases) | 0.251 | ||
| L2‐3 | 2 | 4 | |
| L3‐4 | 4 | 5 | |
| L4‐5 | 22 | 19 | |
| L5S1 | 3 | 10 | |
| Operation time (mean ± SD, min) | 60.68 ± 0.47 | 120.23 ± 10.24 | 0.000 |
| EBL (mean ± SD, mL) | 47.25 ± 0.43 | 256.90 ± 20.83 | 0.000 |
| Hospitalization stay (mean ± SD, days) | 5.12 ± 1.60 | 10.54 ± 1.82 | 0.000 |
| Follow‐up period (mean ± SD, months) | 20.16 ± 0.43 | 21.22 ± 2.47 | 0.827 |
CCS, central canal stenosis; EBL, estimated blood loss; Endo‐ULBD, percutaneous endoscopic unilateral laminectomy for bilateral decompression; LRS, lateral recess stenosis.
VAS and ODI
After surgery, all indicators improved significantly over time, and the pair wise comparison showed statistically significant differences between time points (P < 0.05). The VAS scores at postoperative 1 day, 3 months, 6 months, final follow‐up (Endo‐ULBD: 6.58 ± 0.65, 4.55 ± 0.54, 2.78 ± 0.24, 1.31 ± 0.78; PLIF: 7.19 ± 1.14, 4.80 ± 0.13, 2.71 ± 0.83, 1.29 ± 0.56) were significantly improved compared with those before surgery (Endo‐ULBD: 8.63 ± 0.37; PLIF: 8.31 ± 1.34). The ODI of lumbar function and quality of life at postoperative 1 day, 3 months, 6 months, final follow‐up (Endo‐ULBD: 30.29% ± 0.47%, 23.35% ± 0.95%, 19.45% ± 0.81%, 10.84% ± 0.36%; PLIF: 33.56% ± 1.58%, 25.69% ± 2.69%, 20.01% ± 1.49%, 10.72% ± 0.29%) were significantly improved compared with those before surgery (Endo‐ULBD: 44.56% ± 1.32%; PLIF: 43.79% ± 1.91%)(P<0.05). The VAS and ODI scores at 1 day and 3 months after surgery in the Endo‐ULBD group were lower than those in the PILF group, and the differences were statistically significant (P < 0.05). However, there was no significant difference at the final follow‐up (P > 0.05) (Table 2) .
TABLE 2.
Comparison of the VAS and ODI score at different time points between the two groups
| Time | VAS | ODI | ||
|---|---|---|---|---|
| Endo‐ULBD | PLIF | Endo‐ULBD | PLIF | |
| Preoperation | 8.63 ± 0.37 b , c , d | 8.31 ± 1.34 b , c , d | 44.56 ± 1.32 b , c , d | 43.79 ± 1.91 b , c , d |
| Postoperation 1 day | 6.58 ± 0.65 a , c , d | 7.19 ± 1.14 a , c , d , e | 30.29 ± 0.47 a , c , d | 33.56 ± 1.58 a , c , d , e |
| Postoperation 3 months | 4.55 ± 0.54 a , b , d | 4.80 ± 0.13 a , d , e | 23.35 ± 0.95 a , b , d | 25.69 ± 2.69 a , d , e |
| Postoperation 6 months | 2.78 ± 0.24 a , b , c | 2.71 ± 0.83 a , b , c | 19.45 ± 0.81 a , b , c | 20.01 ± 1.49 a , b , c |
| Final follow‐up | 1.31 ± 0.78 a , b , c , d | 1.29 ± 0.56 a , b , c , d | 10.84 ± 0.36 a , b , c , d | 10.72 ± 0.29 a , b , c , d |
EQ‐5D, European Quality of Life‐5 Dimensions; ODI, Oswestry Disability Index; VAS, visual analog scale.
Compared with preoperative value, P < 0.05;
Compared with the 1 day postoperative value, P < 0.05;
Compared with the 3 month postoperative value, P < 0.05;
Compared with the 6 months postoperative value, P < 0.05;
Compared with the Endo‐ULBD group, P < 0.05.
EQ‐5D Score
The EQ‐5D scores of lumbar function and quality of life at postoperative 1 day, 3 months, 6 months, final follow‐up (Endo‐ULBD: 0.38 ± 0.15, 0.45 ± 0.17, 0.63 ± 0.14, 0.71 ± 0.20; PLIF: 0.33 ± 0.03, 0.39 ± 0.05, 0.62 ± 0.07, 0.72 ± 0.10) were significantly improved compared with those before surgery (Endo‐ULBD: 0.33 ± 0.07; PLIF: 0.31 ± 0.09; P<0.05). EQ‐5D scores at 1 day and 3 months after surgery were higher than those in the PILF group, and the differences were statistically significant (P < 0.05). However, there was no significant difference at the final follow‐up (P > 0.05) (Table 3).
TABLE 3.
Changes in the EQ‐5D score at baseline and at each postoperative time point in the two groups (mean ± SD)
| Groups | Preoperation | Postoperation 1 d | Postoperatio 3 months | Postoperation 6 months | Final follow‐up | Statistics value |
|---|---|---|---|---|---|---|
| Endo‐ULBD | 0.33 ± 0.07 | 0.38 ± 0.15 | 0.45 ± 0.17 | 0.63 ± 0.14 | 0.71 ± 0.20 |
F = 8.120 P = 0.000 |
| PLIF | 0.31 ± 0.09 | 0.33 ± 0.03 | 0.39 ± 0.05 | 0.62 ± 0.07 | 0.72 ± 0.10 |
F = 8.990 P = 0.000 |
| Statistics value |
t = 1.01 P = 0.315 |
t = 2.01 P < 0.05 |
t = 2.07 P < 0.05 |
t = 0.38 P = 0.701 |
t = 0.27 P = 0.789 |
– |
MacNab's Criteria
At the final follow‐up, according to MacNab's criteria, 16 outcomes were considered excellent (51.6%), 12 were good (38.7.0%), two were fair (6.5%), and one was poor (3.2%). The excellent and good efficacy rate was 90.3% in the Endo‐ULBD group. The patient with poor efficacy underwent posterior lumbar decompression, fusion, and internal fixation. In the PILF group, 20 outcomes (52.6%) were considered excellent, 14 cases (36.8%) good, and three (7.9%) were fair. There were no significant differences between the two groups (χ 2 = 0.089, P = 0.993).
Radiographical Findings
In these two groups, postoperative CT and MRI showed that the stenosis of the spinal canal was obviously enlarged and the compressed nerve root was fully decompressed compared with the findings on preoperative images, as shown in Fig. 4.
Fig. 4.
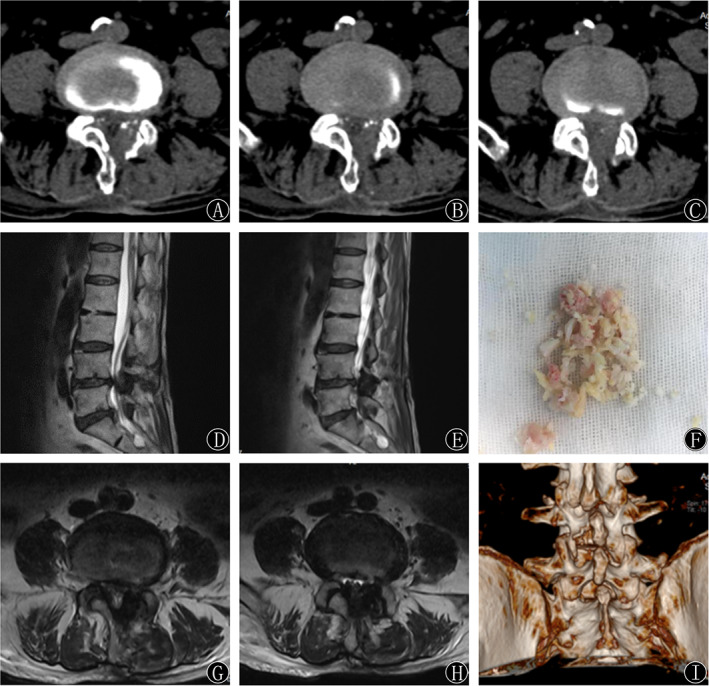
(A‐H) Sagittal MRI, axial MRI, and CT showed satisfactory decompression effects 1 day postoperatively. The medial edge of the lamina, hypertrophy of the ligamentum flavum, and articular process were removed, and the spinal cord was decompressed. (I) A round‐shaped laminotomy at the L4‐5 segment was shown on the 3D reconstruction of the CT scan.
Complications
In the Endo‐ULBD group that used visual trepan, cerebrospinal fluid leakage following a dural tear during the operation occurred in one case. Pain in the back and extremities was exacerbated in one case, which was significantly relieved after the administration of anti‐inflammatory and analgesic agents for 1 week. Two patients had transient sensory disorders and decreased muscle strength in the lower extremities after surgery, and the symptoms were relieved with nerve pain medications, nutritional support, and rehabilitation physiotherapy for 2–3 weeks. In the PILF group, there was one case of nerve root injury (the patient recovered within 1 week after adjusting the poor nail position), three cases of poor incision healing, and two cases of cerebrospinal fluid leakage. During the follow‐up period, no serious complications, such as death, lumbar surgical site infection, or nerve injury, occurred in either group.
Discussion
Introduction of Endo‐ULBD using Visual Trepan
With the rapid development of endoscopic decompression technology, there are increasingly more indications for endoscopic decompression surgery 11 , 12 . Furthermore, the improvements to surgical instruments have made endoscopic surgery more accurate and safer, as well as increasingly in line with the concept of surgical decompression 14 , 15 . Therefore, we investigated the safety and efficacy of Endo‐ULBD using visual trepan in elderly patients with LSS. This procedure could fully obtain a clear visual field of the dorsal structure of the nerve tissue and decompress the dorsal structure of the nerve tissue, including the hypertrophic ligament tissue and the cohesive proliferative articular process, under visual monitoring. The ventral compressors such as intervertebral disc tissues or posterior longitudinal ligaments could even be treated by pushing the nerve tissues inwards 17 . In addition, the contralateral compressors could also be removed by adjusting the angle of the working channel, as shown in Fig. 3.
Clinical Effectiveness and Safety of Endo‐ULBD using Visual Trepan
Our study showed that in terms of early postoperative lumbar function, Endo‐ULBD is superior to traditional PLIF surgery, which may be due to the small incision and limited access to the injury site with endoscopy. On the other hand, the PLIF surgical incision is large, and the local tissue is separated, pulled and stretched by instruments, resulting in damage to the paraspinal muscles and surrounding soft tissue; thus, in terms of the early postoperative lumbar function evaluation and the VAS, ODI, and EQ‐5D scores 2 days and 3 months after the operation, Endo‐ULBD is superior to the traditional PLIF surgery (P < 0.05), showing the advantages of less trauma and a quick recovery after the operation. From 3 months after the operation to the final follow‐up, with postoperative rehabilitation exercise and the recovery of lumbar function, the ligaments and muscles at the operation sites were reconstructed and recovered, and the lumbar strength and stability gradually recovered in the PLIF group 3 months after the operation. The VAS, ODI, and EQ‐5D scores tended to be consistent between the two groups (P > 0.05).
Comparison with Traditional PLIF Procedure
Moreover, compared with traditional PLIF and MIS‐TLIF reported in the literature 22 , Endo‐ULBD for LSS also required a smaller incision and led to a faster recovery. In addition, this approach reduced the operation cost while retaining the motion segment without internal fixation.
Indications of Endo‐ULBD using Visual Trepan
Regarding surgical indications, since Endo‐ULBD is performed through a posterior median approach, it is particularly suitable for cases of LSS in which the main pathological features are hypertrophy of the ligamentum flavum and proliferation of the articular process. Therefore, for lateral recess decompression in LSS patients with intervertebral foramen stenosis, we recommend the lateral endoscopic technique 23 , 24 .
Advantages and Methods of Local Anesthesia in Endo‐ULBD Surgery
Endo‐ULBD was performed under the combination of basic anesthesia and local anesthesia, which could improve the patients' comfort and relieve tension during the operation. The intravenous medications were as follows: dexmedetomidine: 200 μg diluted to 50 mL, load dose: 0.5 μg/kg (patients with weight of 50 kg, 25 mL/h, infusion for 15 min); maintenance dose: 0.4–0.6 μg·kg−1·h−1 (5–7.5 mL/h, patients with weight of 50 kg); and sufentanil: 5–10 μg intravenous injection. Local anesthesia was performed with normal saline (NS) 20 mL + a lidocaine hydrochloride injection 15 mL. This strategy of anesthesia provided a safe and effective method for elderly patients with basic diseases who could not tolerate general anesthesia.
Complications
Although this method had many advantages in the treatment of LSS, it also had surgical risks and complications. Dural tears, cerebrospinal fluid leakage, intraoperative hemorrhage, and nerve injury were common complications. After the operation, a drainage tube was preserved, which was not removed until the volume of drainage was <20 mL/d, and intravenous fluids were also administered. However, no symptoms of low cranial pressure were found. For patients with deteriorating back and extremity pain, we administered anti‐inflammatory and analgesic drugs, which significantly relieved the symptoms after 1 week. Two patients had transient sensory disorders and decreased muscle strength in the lower extremity after surgery, and the symptoms were relieved with nerve pain drugs, nutritional support, and rehabilitation physiotherapy for 2–3 weeks. Therefore, an excellent endoscopic decompression is the basis for a successful operation.
Limitations
In addition, this study was a retrospective study, some biases could not be excluded, and the sample size was relatively small; a multicenter prospective study with a large sample size is needed for further clarification.
Conclusions
Endo‐ULBD has the advantages of less trauma, a shortened operation time, and rapid recovery and is an effective alternative for lumbar spinal stenosis. Strict surgical indications, reasonable surgical plans, and experienced surgeons are important factors to ensure safety and satisfactory postoperative efficacy.
ACKNOWLEDGMENTS
The authors declare that there is no conflict of interests. This study was supported by the National Natural Science Foundation of China (Grant No.81874017 and 81960403); the Fundamental Research Funds for the Central Universities (lzujbky‐2019‐kb16); Lanzhou Science and Technology Plan Program (2018‐3‐52, 2017‐4‐88); Doctoral Research Fund of Lanzhou University Second Hospital (ynbskyjj2015‐2‐9); Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2017‐QN12,CY2017‐ZD02); Natural Science Foundation of Gansu Province of China (17JR5RA188); Joint Project of Medical Science and Technology of Henan Province (LHGJ20190859); Overseas Research and Training Project of Health Science and Technology Talents in Henan Province (HWYX 2019159).
Disclosure: The authors declare that there is no conflict of competing financial or non‐financial interests.
References
- 1. Otani K, Kikuchi S, Yabuki S, et al. Lumbar spinal stenosis has a negative impact on quality of life compared with other comorbidities: an epidemiological cross‐sectional study of 1862 community‐dwelling individuals. ScientificWorldJournal, 2013, 590652: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schroeder GD, Kurd MF, Vaccaro AR. Lumbar spinal stenosis: how is it classified?. J Am Acad Orthop Surg, 2016, 24: 843–852. [DOI] [PubMed] [Google Scholar]
- 3. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine, 2014, 21: 179–186. [DOI] [PubMed] [Google Scholar]
- 4. Radcliff K, Curry P, Hilibrand A, et al. Risk for adjacent segment and same segment reoperation after surgery for lumbar stenosis: a subgroup analysis of the spine patient outcomes research trial (SPORT). Spine (Phila Pa 1976), 2013, 38: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan I, Bydon M, Archer KR, et al. Impact of occupational characteristics on return to work for employed patients after elective lumbar spine surgery. Spine J, 2019, 19: 1969–1976. [DOI] [PubMed] [Google Scholar]
- 6. Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br, 1954, 36: 230–237. [DOI] [PubMed] [Google Scholar]
- 7. O'connell JE. Protrusions of the lumbar intervertebral discs, a clinical review based on five hundred cases treated by excision of the protrusion. J Bone Joint Surg Br, 1951, 33: 8–30. [DOI] [PubMed] [Google Scholar]
- 8. Young S, Veerapen R, O'Laoire SA. Relief of lumbar canal stenosis using multilevel subarticular fenestrations as an alternative to wide laminectomy: preliminary report. Neurosurgery, 1988, 23: 628–633. [DOI] [PubMed] [Google Scholar]
- 9. Xiaobing Z, Xingchen L, Honggang Z, et al. "U" route transforaminal percutaneous endoscopic thoracic discectomy as a new treatment for thoracic spinal stenosis. Int Orthop, 2019, 43: 825–932. [DOI] [PubMed] [Google Scholar]
- 10. An B, Li XC, Zhou CP, et al. Percutaneous full endoscopic posterior decompression of thoracic myelopathy caused by ossification of the ligamentum flavum. Eur Spine J, 2019, 28: 492–501. [DOI] [PubMed] [Google Scholar]
- 11. Palmer S, Turner R, Palmer R. Bilateral decompression of lumbar spinal stenosis involving a unilateral approach with microscope and tubular retractor system. J Neurosurg, 2002, 97: 213–217. [DOI] [PubMed] [Google Scholar]
- 12. Park SM, Kim GU, Kim HJ, et al. Is the use of a unilateral Biportal endoscopic approach associated with rapid recovery after lumbar Decompressive laminectomy? A preliminary analysis of a prospective randomized controlled trial. World Neurosurg, 2019, 128: e709–e718. [DOI] [PubMed] [Google Scholar]
- 13. Liang JQ, Chen C, Zhao H. Revision surgery after percutaneous endoscopic Transforaminal discectomy compared with primary open surgery for symptomatic lumbar degenerative disease. Orthop Surg, 2019, 11: 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hwa Eum J, Hwa Heo D, Son SK, Park CK. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine, 2016, 24: 602–607. [DOI] [PubMed] [Google Scholar]
- 15. Zhao W, Yang S, Diao WB, Yan M, Wu WJ, Luo F. Using visual trepan to treat single segment ossification of the Ligamentum Flavum under endoscopy. Orthop Surg, 2019, 11: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGrath LB, White‐Dzuro GA, Hofstetter CP. Comparison of clinical outcomes following minimally invasive or lumbar endoscopic unilateral laminotomy for bilateral decompression. J Neurosurg Spine, 2019, 11: 1–9. [DOI] [PubMed] [Google Scholar]
- 17. Ruetten S, Komp M, Merk H, Godolias G. A new full‐endoscopic technique for cervical posterior foraminotomy in the treatment of lateral disc herniations using 6.9‐mm endoscopes: prospective 2‐year results of 87 patients. Minim Invasive Neurosurg, 2007, 50: 219–226. [DOI] [PubMed] [Google Scholar]
- 18. Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine (Phila Pa 1976), 2010, 35: 1919–1924. [DOI] [PubMed] [Google Scholar]
- 19. Brooks R. Euro Qol: the current state of play. Health Policy, 1996, 37: 53–72. [DOI] [PubMed] [Google Scholar]
- 20. Liu GG, Wu H, Li M, Gao C, Luo N. Chinese time trade‐off values for EQ‐5D health states. Value Health, 2014, 17: 597–604. [DOI] [PubMed] [Google Scholar]
- 21. Macnab I. Negative disc exploration. An analysis of the causes of nerve‐root involvement in sixty‐eight patients. J Bone Joint Surg Am, 1971, 53: 891–903. [PubMed] [Google Scholar]
- 22. Choi DJ, Kim JE. Efficacy of Biportal endoscopic spine surgery for lumbar spinal stenosis. Clin Orthop Surg, 2019, 11: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shin SH, Bae JS, Lee SH, Keum HJ, Kim HJ, Jang WS. Transforaminal endoscopic decompression for lumbar spinal stenosis: a novel surgical technique and clinical outcomes. World Neurosurg, 2018, 114: e873–e882. [DOI] [PubMed] [Google Scholar]
- 24. Chen X, Qin R, Hao J, et al. Percutaneous endoscopic decompression via transforaminal approach for lumbar lateral recess stenosis in geriatric patients. Int Orthop, 2019, 43: 1263–1269. [DOI] [PubMed] [Google Scholar]


