Abstract
Objective
The aim of the present paper was to reveal the clinical differences between selective and nonselective decompression for symptomatic tandem stenosis of the cervical and thoracic spine (TSCTS).
Methods
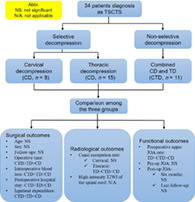
A total of 34 patients were eligible and included in the study. Among them, 8 patients underwent selective cervical decompression (CD), 15 patients underwent selective thoracic decompression (TD), and 11 patients underwent combined CD and TD (CTD) surgery. Age, sex, operative time, intraoperative blood loss, postoperative hospital stay, inpatient expenditure, preoperative upper Japanese Orthopaedic Association (JOA) rate, canal occupation rate, high‐intensity T2‐weighted image (T2WI) of the spinal cord, and preoperative and postoperative JOA scores were compared among the three groups.
Results
The CD group had shorter operative time (138.8 ± 36.1 vs 229.7 ± 95.8 vs 328.6 ± 94.8, min, P < 0.001), less intraoperative blood loss (141.3 ± 116.7 vs 496.7 ± 361.8 vs 654.6 ± 320.5, mL, P = 0.004), and shorter postoperative hospital stay (4.6 ± 1.6 vs 9.0 ± 3.5 vs 10.3 ± 6.6, days, P = 0.008), as well as lower preoperative upper JOA rate (34.1 ± 5.6 vs 53.9 ± 8.4 vs 48.2 ± 15.2, %, P = 0.001) than the TD and CTD groups. The CTD group had higher inpatient expenditure than the CD and TD groups (87,850 ± 18,379 vs 55,100 ± 12,890 vs 55,772 ± 15,715, CNY, P < 0.001). The cervical canal occupation rates were similar among different groups (P > 0.05); however, the TD group showed a higher thoracic canal occupation rate than the CD group (58.3 ± 14.7 vs 43.3 ± 12.3, %, P = 0.035). All positive levels in high‐intensity T2WI of the spinal cord were decompressed. The preoperative JOA scores as well as the postoperative JOA scores at 6 months and at last follow‐up were comparable among the three groups (P > 0.05). Similarly, the JOA recovery rate showed no significant difference among the groups (P > 0.05).
Conclusion
Selective CD or TD alone demonstrated similar clinical effectiveness to nonselective and combined CTD for TSCTS. Individualized surgical decision should be made after meticulous assessments of clinical and radiological manifestations, general patient condition, and socioeconomic factors.
Keywords: Cervical spine, Decompression, JOA score, Tandem stenosis, Thoracic spine
Symptomatic tandem stenosis of the cervical and thoracic spine (TSCTS) is rare. The clinical outcomes of selective and nonselective decompression for TSCTS have not been previously reported. Our retrospective study found that selective cervical or thoracic decompression alone demonstrated similar clinical effectiveness to nonselective and combined cervical and thoracic decompression for TSCTS.
Introduction
The term “tandem spinal stenosis” (TSS) was proposed by Dagi et al. 1 in 1987, to refer to a condition characterized by the triad of neurogenic intermittent claudication, gait disturbance, and mixed myelopathy and radiculopathy. Although the original use of the term TSS is especially directed to combined cervical and lumbar stenosis, which exhibits mixed upper and lower motor neuron deficits, TSS should be generally interpreted as any concomitant stenosis of the whole spine. Many researchers from East Asia have revealed the prevalence and characteristics of tandem ligament ossification in the cervical and thoracic spine, which represents another important form of TSS, which is referred to as “tandem stenosis of the cervical and thoracic spine” (TSCTS) 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 . Some patients with radiological stenosis are asymptomatic or have irrelevant symptoms and signs, which have little clinical significance. Therefore, the term “TSCTS” in this article indicates symptomatic TSCTS unless noted otherwise.
For symptomatic TSCTS, which causes myelopathy or myeloradiculopathy, surgical decompression is the first‐line treatment 2 , 4 , 5 , 11 . Most studies retrospectively described the clinical results of combined cervical and thoracic decompression (CTD) or compared clinical effects between one‐stage and two‐staged surgeries. Zhao et al. compared the clinical effectiveness of one‐stage (10 patients) and two‐stage (8 patients) decompression surgeries in the treatment of upper thoracic combined with multilevel cervical spinal stenosis, and found that both short‐term and long‐term improvement rates of neurological function were higher in the one‐stage group. Hu and co‐authors defined adjacent lesions treated by combined one‐stage decompression and skip lesions, which were decompressed by two‐staged procedures, and concluded that both surgical approaches were effective for the indicated populations. However, the disadvantages of combined procedures, whether one‐stage or two‐staged, are prominent. Hu and colleagues reported that combined surgery was accompanied by high rates of postoperative complications and concluded that more studies were needed before its wide application 4 . Conversely, patients with severe radiological compression may show disproportional and indistinctive clinical symptoms. Hence, the concurrent cervical and thoracic stenosis may not contribute equally to, or be simultaneously responsible for, neurological deterioration. Based on reasonable ratiocination, a selective, precise, and segmental location and decompression may exhibit acceptable, even equivalent effect compared to the traditional combined decompression surgery. Theoretically, this procedure can provide considerable benefits, such as less operative time and intraoperative blood loss, shortened hospital stay, decreased inpatient expenditure, and lower incidence of complications. Meanwhile, selective surgery complies with the surgical principles for patients who cannot tolerate extensive and time‐consuming surgeries.
As far as we know, no author has proposed the concept of selective decompression for TSCTS and further compared the clinical outcomes between selective and non‐selective decompressions. One of the explanations is the preferential distribution in the East Asian population of the ligamental ossification diseases, especially ossification of the posterior longitudinal ligament (OPLL) and ossification of the ligamentum flavum (OLF), which were predominant pathological types of TSCTS. In contrast, the low occurrence of concurrent symptomatic stenosis of both the cervical and the thoracic spine restricts the necessary amount of patients in the cohort study.
The purposes of this retrospective cohort study were: (i) to evaluate the effect of nonselective combined CTD surgery for TSCTS; (ii) to compare the clinical effectiveness of selective decompression (cervical decompression alone [CD] or thoracic decompression alone [TD]) with nonselective decompression (two‐staged or one‐stage CTD); and (iii) to evaluate the feasibility of and indication for selective surgery for the challenging situation when dealing with TSCTS patients.
Methods
Patient Selection
We screened patients who were diagnosed with symptomatic TSCTS and underwent selective or nonselective decompression surgeries at our hospital between July 2010 and May 2018. The inclusion criteria were: (i) symptoms or signs of cervical and thoracic myelopathy; (ii) concurrent cervical and thoracic canal stenosis and spinal cord compression confirmed with CT and MRI; (iii) intact medical documents, radiological images, and follow‐up records; and (iv) intensive follow‐up for at least 1 year. The exclusion criteria included: (i) contraindications which hindered surgical interventions under general anesthesia; (ii) severe comorbidities which should be addressed prior to the surgical decompression; and (iii) the patient subjectively refused surgical decompression. This protocol was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University and informed consent was obtained from all participants.
A total of 34 patients, comprising 18 men and 16 women, were included in the final cohort. Demographic data from medical records, such as sex, age, duration of symptoms, surgical procedure, intraoperative blood loss, operative time, duration of hospital stay, and inpatient expenditure, were routinely collected. The patients were subsequently divided into three groups according to the surgical procedure (Fig. 1). Specifically, 8 patients underwent selective cervical decompression only (CD group), 15 patients underwent selective thoracic decompression only (TD group), and 11 patients underwent combined one‐stage or two‐staged cervical and thoracic decompression (CTD group).
Fig. 1.
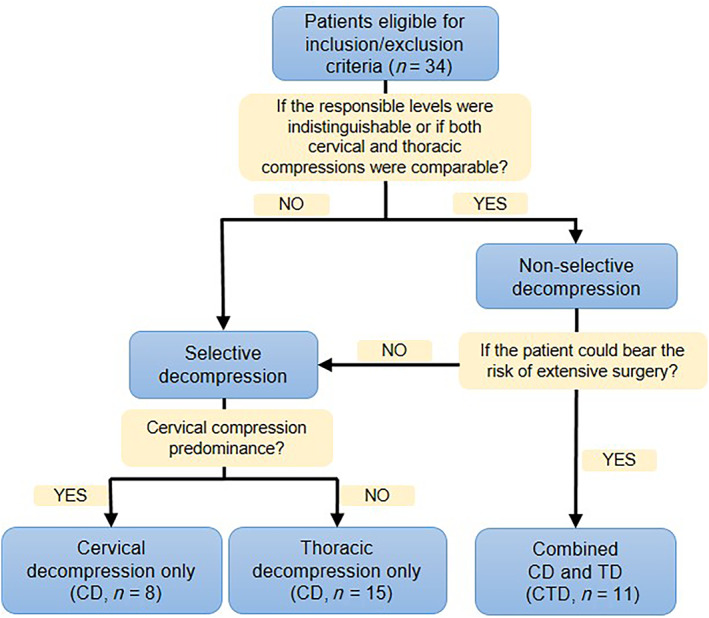
Flow diagram of patient grouping and principles for grouping.
Surgical Procedures
The surgical strategy for TSCTS varies from one surgeon to another. Nevertheless, some common surgical principles or guidelines were followed (Fig. 1). Nonselective CTD was indicated for patients in whom the putative symptom‐associated level was indistinguishable or who had comparable cervical compression and thoracic compression. Patients who were deemed able to tolerate the procedure and were well informed of the risk of the combined surgery were considered eligible. For multi‐level cervical and concomitant upper thoracic stenosis, one‐stage posterior decompression was recommended. For two‐staged surgeries, the interval between two surgeries was <3 months. Selective CD or TD was considered if the patient could not bear the risk of extensive surgery and if the predominant cervical or thoracic compression could be determined on the basis of clinical and radiological evidence. For example, for patients who manifested severe symptoms of the lower extremities but slight symptoms of the upper extremities, TD was recommended. Otherwise, CD was the first‐choice surgery.
For cervical surgery, the standard procedure, including anterior cervical discectomy and fusion, anterior cervical corpectomy and fusion, or posterior expansive open‐door laminoplasty, was individually implemented according to the common principles 12 . For thoracic surgery, laminectomy was regularly performed to achieve posterior decompression. If laminectomy alone was deemed insufficient, an additional anterior decompression procedure was meticulously performed with neurophysiological monitoring.
Radiological Assessment
The preoperative cervical and thoracic radiographs, CT scans, and MRI scans were reviewed. The radiological patterns of canal stenosis were identified and classified as OPLL, OLF, spinal spondylosis, and disc herniation. OPLL was further divided into four subgroups (continuous, segmental, localized, and mixed) according to a well‐established classification 13 . The affected segments in each patient were recorded to determine the distribution of stenosis. Because the incidence of diffuse idiopathic skeletal hyperostosis (DISH) was elevated in the population with ligament ossification, the concomitant DISH was also counted 3 , 14 , 15 .
For cervical and thoracic stenosis, the canal occupation rate was calculated to represent the degree of stenosis, as previously reported by other authors 16 , 17 . In brief, the maximum stenosis in the axial CT plane was selected, and ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to measure the canal occupation rate (defined as the relative occupied area divided by the canal area). Each measurement was repeated three times. The average was adopted as the final value. Furthermore, high‐intensity T2‐weighted images (T2WI) were obtained and analyzed.
Clinical Effectiveness
The Japanese Orthopaedic Association (JOA) score 18 and JOA recovery rate 19 , which are widely used to evaluate functional status in patients with cervical spondylotic myelopathy, were recorded at different time points (preoperatively, 6 months postoperatively, and at the last follow‐up). According to Hirabayashi's definition 19 , the JOA recovery rate (%) was calculated as (postoperative JOA score – preoperative JOA score)/(17 – preoperative JOA score) × 100. On the basis of the JOA recovery rate, the final JOA recovery was graded as follows: excellent (75%–100%), good (50%–74%), fair (25%–49%), and unchanged or deteriorated (<25%). In addition, we defined the preoperative upper JOA rate (%), which indicated the relative severity of cervical versus thoracic compression, as preoperative upper‐limb JOA score (maximum six points)/total JOA score (maximum 17 points) × 100.
Surgery‐related complications, including but not limited to neurological deterioration, dura tear and cerebrospinal fluid leakage, infection, instrument failure, and reoperation, were documented at follow‐up in all groups.
Statistical Analysis
IBM SPSS version 19 software (Armonk, NY, USA) was used for statistical analysis. Numerical variables are presented as mean ± standard deviation. For the comparison of preoperative and postoperative JOA scores, a paired Student's t‐test was used. Comparison among the three groups was conducted using the χ2‐test for dichotomous variables and analysis of variance for numerical variables if the results of the homogeneity of variance test were not different among groups. If equal variance was not assumed, the rank‐sum test was thereafter performed with the Kruskal–Wallis test for comparing all three independent groups and the Mann–Whitney test for comparing two groups. The significance level was set as 0.05.
Results
General Outcomes
The demographic and clinical outcomes of the included patients are listed in Table 1. Compared with the preoperative JOA score (10.5 ± 2.4), the postoperative JOA scores at 6 months (14.3 ± 2.0) and at the last follow‐up (14.8 ± 1.8) showed significant improvements (both P < 0.001).
TABLE 1.
Demographic data of included patients
| Variables | Mean | SD | 95% CI | Minimum, Maximum |
|---|---|---|---|---|
| Age, years | 61.4 | 8.8 | [58.4, 64.5] | [44,81] |
| Duration of symptoms, months | 20.7 | 38.4 | [7.3, 34.1] | [0.5,180] |
| Number of decompressed segments | 2.7 | 1.6 | [2.1, 3.2] | [1,6] |
| Intraop blood loss, mL | 531.8 | 555.5 | [337.9, 725.6] | [20,3000] |
| Operative time, minutes | 240.3 | 109.8 | [202.0, 278.6] | [90,570] |
| Duration in hospital, days | 14.3 | 6.9 | [11.9, 16.7] | [8,27] |
| Follow‐up, months | 49.6 | 24.7 | [41.0, 58.2] | [17,111] |
| Preop JOA | 10.5 | 2.4 | [9.7, 11.3] | [6,14] |
| JOA at 6 months postop | 14.3 | 2 | [13.6, 15.0] | [9,17] |
| JOA at follow‐up | 14.8 | 1.8 | [14.2, 15.4] | [9,17] |
| JOA recovery rate at 6 months postop, % | 57.9 | 27.1 | [48.5, 67.4] | [0,100] |
| JOA recovery rate at last follow‐up, % | 64.5 | 27.3 | [55.0, 74.1] | [0,100] |
CI, confidence interval; JOA, Japanese Orthopaedic Association; SD, standard deviation.
JOA recovery rate = (postop JOA – preop JOA)/(17 – preop JOA) × 100%.
After grouping the patients according to surgical procedures, the demographic data (sex and age) and follow‐up time were not significantly different (P > 0.05, Table 2). However, operation‐related variables showed plausible differences. Generally, the CD group showed shorter operative time, less intraoperative blood loss, and shorter postoperative hospital stay, as well as a lower preoperative upper JOA rate than the TD and CTD groups (P < 0.05). Furthermore, the CTD group showed an even longer operative time than the TD group (P < 0.05). Accordingly, the CTD group had higher inpatient expenditure than the CD and TD groups (P < 0.05), whereas the CD and TD groups had comparable inpatient expenditure (P > 0.05).
TABLE 2.
Detailed data and statistical results of patients in different groups
| CD group (n = 8) | TD group (n = 15) | CTD group (n = 11) | P‐value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 5 | 8 | 5 | 0.763 |
| Female | 3 | 7 | 6 | |
| Age, years | 55.9 ± 7.1 | 63.1 ± 9.1 | 63.2 ± 8.4 | 0.122 |
| Follow‐up, months | 56.0 ± 26.7 | 54.0 ± 26.1 | 38.9 ± 19.3 | 0.22 |
| Operative time, minutes | 138.8 ± 36.1 | 229.7 ± 95.8* | 328.6 ± 94.8***## | 0.000 |
| Intraop blood loss, mL | 141.3 ± 116.7 | 496.7 ± 361.8* | 654.6 ± 320.5** | 0.004 |
| Postop hospital stay, days | 4.6 ± 1.6 | 9.0 ± 3.5** | 10.3 ± 6.6* | 0.008 † |
| Inpatient expenditure, CNY | 55100 ± 12890 | 55772 ± 15715 | 87850 ± 18379***### | 0.000 |
| Preop upper JOA rate, % | 34.1 ± 5.6 | 53.9 ± 8.4*** | 48.2 ± 15.2** | 0.001 |
| Cervical canal occupation rate, % | 37.7 ± 12.3 | 38.9 ± 15.2 | 41.1 ± 9.7 | 0.850 |
| Thoracic canal occupation rate, % | 43.3 ± 12.3 | 58.3 ± 14.7* | 54.5 ± 9.1 | 0.035 |
| Increased T2 cervical spinal cord | ||||
| Negative cases | 5 | 15 | 5 | N/A |
| Positive cases | 3 | 0 | 6 | |
| Increased T2 thoracic spinal cord | ||||
| Negative cases | 8 | 10 | 8 | N/A |
| Positive cases | 0 | 5 | 3 | |
| Preop JOA | 11.0 ± 2.5 | 10.3 ± 1.9 | 10.3 ± 2.9 | 0.776 |
| JOA at 6 m postop | 14.4 ± 0.9 | 14.1 ± 2.6 | 14.5 ± 1.8 | 0.964 † |
| JOA at last follow‐up | 15.1 ± 1.2 | 14.7 ± 2.2 | 14.6 ± 1.5 | 0.785 |
| JOA recovery rate at 6 m postop, % | 48.6 ± 23.9 | 60.0 ± 31.2 | 61.8 ± 23.7 | 0.543 |
| JOA recovery rate at last follow‐up, % | 65.1 ± 29.2 | 67.1 ± 27.0 | 60.6 ± 28.6 | 0.843 |
| Recovery grade at the last follow‐up | ||||
| Excellent | 4 | 6 | 4 | N/A |
| Good | 2 | 7 | 5 | |
| Fair | 1 | 1 | 1 | |
| Unchanged/deteriorated | 1 | 1 | 1 | |
| Complications | ||||
| Dural tears | 0 | 4 | 2 | N/A |
| Hypoalbuminemia | 0 | 1 | 0 | |
| Neurological deficit deterioration | 0 | 1 | 0 | |
| Reoperation | 1 | 0 | 0 |
CD, cervical decompression; CTD, cervical and thoracic decompression; JOA, Japanese Orthopaedic Association; N/A, not applicable; TD, thoracic decompression.
Preop upper JOA rate = preop upper limb JOA scores/total JOA scores×100%.
Canal occupation rate = intra‐canal occupation area/canal area×100%.
JOA recovery rate = (postop JOA − preop JOA)/(17 − preop JOA) × 100%.
χ2‐test for dichotomous variables and ANOVA for numerical variables unless indicated.
*P < 0.05, **P < 0.01, ***P < 0.001 vs C group; ##P < 0.01, ###P < 0.001 vs T group.
Indicates Kruskal–Wallis test for comparing three groups and further Mann–Whitney test for comparing each two groups if P < 0.05.
Radiological Outcomes
The patterns of both cervical and thoracic stenosis are shown in Table 3. The predominant pattern of cervical stenosis was OPLL (29 of 34 cases, 85.3%) followed by cervical spondylosis (8 cases, 23.5%). For thoracic stenosis, OLF was the most common etiology of compression (33 cases, 97.1%), followed by OPLL (10 cases, 29.4%) and disc herniation (4 cases, 11.8%). Nine patients (26.5%) had combined OLF and OPLL in the thoracic spine. Among all patients, 7 (20.6%) presented typical characteristics of DISH.
TABLE 3.
Patterns of compression in different groups
| CD group (n = 8) | TD group (n = 15) | CTD group (n = 11) | Total (n = 34) | ||
|---|---|---|---|---|---|
| Cervical | OPLL | 5 | 15 | 9 | 29 |
| Localized | 2 | 4 | 4 | 10 | |
| Segmental | 3 | 3 | 2 | 8 | |
| Continuous | 0 | 1 | 3 | 4 | |
| Mixed | 0 | 7 | 0 | 7 | |
| Spondylosis | 3 | 2 | 3 | 8 | |
| Thoracic | OPLL | 2 | 5 | 3 | 10 |
| Localized | 2 | 3 | 0 | 5 | |
| Segmental | 0 | 0 | 1 | 1 | |
| Continuous | 0 | 0 | 1 | 1 | |
| Mixed | 0 | 2 | 1 | 3 | |
| OLF | 8 | 15 | 10 | 33 | |
| OPLL+OLF | 2 | 5 | 2 | 9 | |
| Disc herniation | 0 | 3 | 1 | 4 | |
| DISH | 1 | 5 | 1 | 7 |
CD, cervical decompression; CTD, cervical and thoracic decompression; DISH, diffuse idiopathic skeletal hyperostosis; OLF, ossification of ligamentum flavum; OPLL, ossification of posterior longitudinal ligament; TD, thoracic decompression.
The segmental distribution of stenosis (Fig. 2) showed that the most affected cervical spine was C5 (30 cases, 88.2%), followed by the adjacent C6 (28 cases, 82.4%) and C4 (24 cases, 70.6%). For the thoracic spine, the upper (T2–4) and lower (T10–12) thoracic spine were the main involved levels. These trends were similar among the different groups.
Fig. 2.
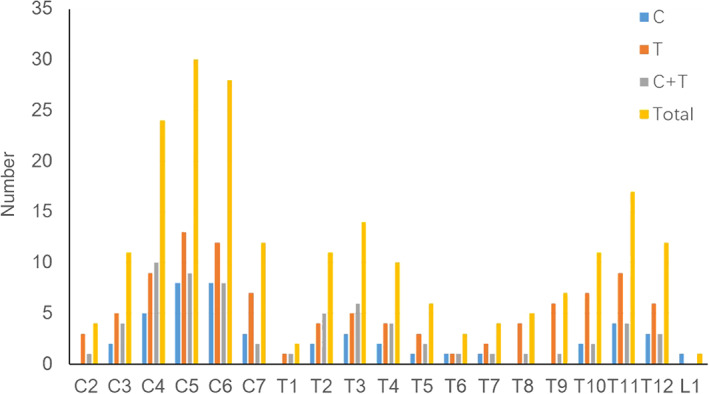
Segmental distribution of tandem stenosis of the cervical and thoracic spine in different groups.
The cervical canal occupation rates were similar among all groups (Table 2). However, the TD group showed a higher thoracic canal occupation rate than the CD group (P < 0.05). Although the number of cases was too small to perform a statistical analysis, the high‐intensity T2WI of the spinal cord revealed that all cervical‐positive cases were in the CD or CTD group and all thoracic‐positive cases were in the TD or CTD group. In other words, all positive levels in high‐intensity T2WI of the spinal cord were decompressed (Fig. 3).
Fig. 3.
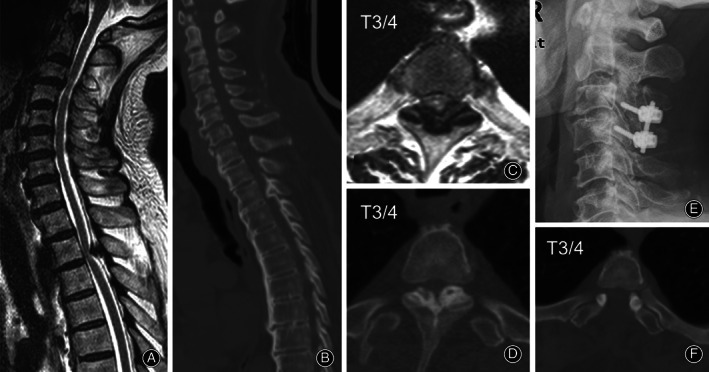
A 76‐year‐old man (Case 24) complained of numbness and weakness of the right extremities for 20 years. Brain CT and MRI scans had excluded a diagnosis of intracranial vascular disease. Positive signs included the sensory level at the sternoxiphoid plane and a positive Hoffmann's sign for both sides. MRI (A and C) and CT scan (B and D) revealed a dynamic compression at the C3/4 level and a static compression from ossification of the ligamentum flavum at the T3/4 level. High T2 signals of the spinal cord were observed at both levels (A and C). Combined C3/4 open‐door laminoplasty with additional lateral mass screw fixation and fusion and one‐staged T3/4 laminectomy were conducted. Postoperative cervical plain film (E) and thoracic CT scan (F) are presented. The Japanese Orthopaedic Association score was improved from 14 preoperatively to 17 at follow‐up.
Recovery Outcomes
The preoperative JOA scores were comparable among the three groups (P > 0.05, Table 2). Interestingly, however, neither the postoperative JOA score at 6 months nor that at the last follow‐up showed a significant difference among the groups (P > 0.05). The JOA recovery rate at 6 months or at the last follow‐up similarly showed no significant difference among the groups (P > 0.05). The number of cases with “excellent” plus “good” JOA recovery grade were 6 (75%) in the CD group, 13 (86.7%) in the TD group, and 9 (81.8%) in the CTD group. The most common complication was dura tear (6 of 34, 17.6%), all cases of which occurred in the TD group. Only 1 patient in the CD group required reoperation because of gradually deteriorating compression in the thoracic spine. Hypoalbuminemia and immediate neurological deterioration were found in 1 patient.
Discussion
Since the first description of combined cervical and lumbar spondylosis by Teng and Papatheodorou 20 , as well as the subsequent TSS nomenclature by Dagi et al. 1 , the clinical manifestation and treatment of this entity have been well discussed 21 . However, TSCTS, as another special type of extended TSS, has been less addressed in the literature from disease epidemiology to treatment experience. This may be partially due to the low morbidity and particular etiology of this condition 10 . In their morphological study of 1072 cadaveric specimens, Bajwa et al. 22 demonstrated that the prevalence of concomitant congenital cervical and thoracic stenosis was 1%. Nevertheless, as our results showed, tandem ligament ossification is the main etiological origin of TSCTS. This was in accordance with the results of previous radiological studies 3 , 7 , 8 , 9 . Fujimori et al. 3 obtained whole‐spine CT scans in 1500 Japanese patients and found that of cervical OPLL cases, 13% had thoracic OPLL, 34% had thoracic OLF, and 36% had DISH. Liang et al. 8 observed that approximately 50% of asymptomatic OPLL cases coexisted with thoracic OLF on CT scans from 2000 Chinese patients. Kawaguchi et al. 6 found that 53.4% of patients with cervical OPLL had coexisting OPLL in the thoracic and/or lumbar spine. In another report 7 , the same team confirmed that 64.6% of the patients with cervical OPLL had OLF, with C5 being the most frequently affected segment of OPLL and OLF being predominant in the upper and lower thoracic spine. These findings were verified by our results. Considering the slow progression of ligament ossification, and it is understandable that patients with symptomatic TSCTS are much fewer in number than those with radiological TSCTS 9 . As coexisting thoracic stenosis deceptively involves upper neuron deficit, routine CT or MRI evaluation of the thoracic spine in patients with cervical OPLL is strongly recommended to screen for this overlooked but vital comorbidity.
Although the imaging characteristics of TSCTS have been elucidated in detail, the surgical treatment and prognosis of symptomatic TSCTS, which are more crucial for spine surgeons, are rarely reported in the literature, possibly owing to race/region specificity and low morbidity. To our knowledge, almost all reports on surgical treatments have focused on combined CTD, or the comparison between one‐stage and two‐staged decompression surgery 2 , 4 , 5 , 11 . Theoretically, combined decompression surgery is the ideal strategy for symptomatic TSCTS (Fig. 3). However, taking into account the increased complications of this extensive and nonselective procedure, the balance of benefit and risk needs to be carefully evaluated. Hu et al .4 reviewed 16 patients with TSCTS who underwent one‐stage combined decompression. Up to 62.5% of the patients had cerebrospinal fluid leakage, whereas immediate neurological deterioration occurred in 37.5%, with a reoperation rate of 25%. In addition, concurrent radiological cervical spine compression and thoracic spine compression do not equally contribute to clinical deterioration, which means that selective CD or TD may be viable, and even sufficient, to achieve satisfactory symptomatic relief (Figs 4 and 5). Our results correspondingly showed that all three groups obtained similar clinical outcomes (postoperative JOA score, JOA recovery rate, and JOA recovery grade). Only one patient in the CD group needed an additional operation for the remaining compression. Notably, the CTD group had significantly prolonged operative time and higher inpatient expenditure than both the CD and TD groups. This finding should not be neglected when making the surgical plan.
Fig. 4.
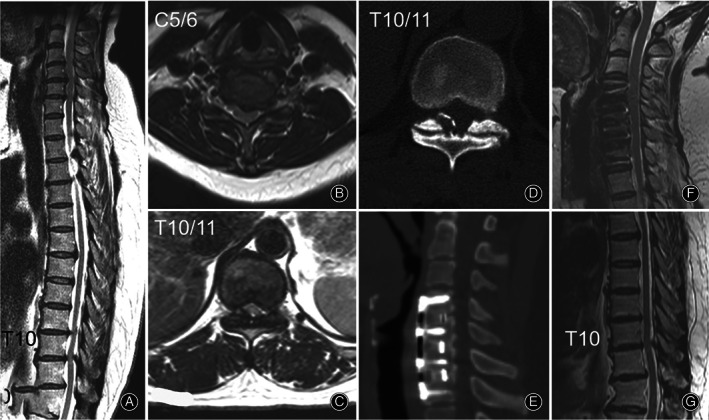
A 49‐year‐old woman (Case 20) felt moderate numbness and mild weakness of both legs for 2 years. Hypoesthesia of both legs was found on physical examination. The bilateral biceps, patellar, and Achilles tendon reflex were all hyperactive. Hoffmann's sign and Babinski's sign were positive for both sides. Preoperative MRI (A, B, and C) and CT scan (D) confirmed tandem spinal cord compression from both the cervical (C3–6) and the thoracic spine (T10–11). A selective three‐level anterior cervical discectomy and fusion procedure was chosen for this patient. At 2‐year follow‐up, the Japanese Orthopaedic Association score was 15 compared to 13 preoperatively. The surgical segments showed solid fusion on CT scan (E) and full decompression on MRI (F), although the image at T10/11 remained almost unchanged (G).
Fig. 5.
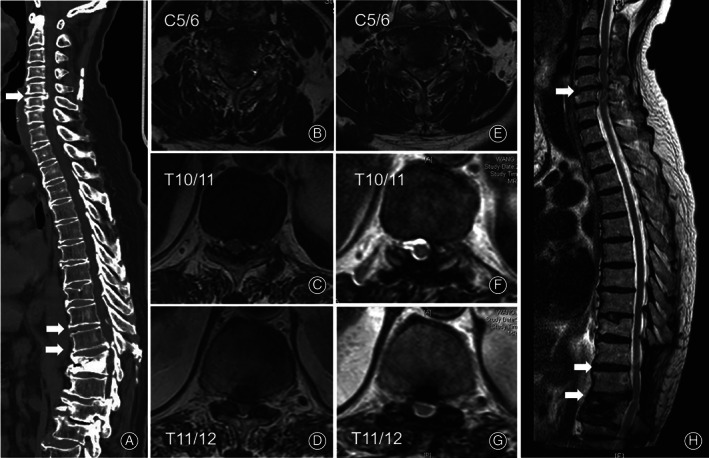
A 74‐year‐old man (Case 10) complained of weakness and numbness of the lower extremities for half a month. The patient suffered from untreated pulmonary fibrosis and rheumatoid arthritis for more than 10 years. Physical examination revealed hypoesthesia and weakened muscle strength of both legs. The patellar tendon reflexes were hyperactive for both sides. Hoffmann's sign and Babinski's sign were bilaterally positive. Preoperative CT (A) and MRI (B‐D) showed tandem spinal cord compression from ligament ossification of both the cervical (C5–6) and the thoracic spine (T10–12). A selective thoracic laminectomy was performed considering his comorbidities. Postoperative MRI images (E–H) confirmed fully decompressed thoracic spine but unchanged cervical spine. At 45‐month follow‐up, the Japanese Orthopaedic Association score was elevated to 14 from 9 points and the patient felt satisfied with his condition.
As mentioned above, preoperative evaluation of feasibility from both the clinical and radiological viewpoints is important when choosing selective surgery. The preoperative upper JOA rate, which is a simple clinical indicator, indicated relative residual function of the upper limbs and reflected the severity of cervical versus thoracic compression in the context of TSCTS in this study. The higher the preoperative upper JOA rate, the greater the upper‐limb functional reserve, and the greater the loss of lower‐limb, bowel, and bladder function. The canal occupation rate, which is an indicator of the degree of radiological compression, was proved to be positively related to myelopathy 17 . Another radiological sign of myelopathy is high T2WI signal intensity in the spinal cord 23 , 24 , 25 , 26 . In this study, all positive levels in high‐intensity T2WI were surgically decompressed. If the putative symptom‐associated level was still difficult to determine, or if the cervical and thoracic spine showed comparable compression, nonselective surgery was considered.
This study had inevitable limitations. First, the number of patients was small. Therefore, some variables could not be statistically analyzed. Second, the surgical procedures did not further distinguish anterior and posterior decompression in the CD or TD group, or one‐stage and two‐staged decompression in the CTD group, because of the small sample size. Finally, more assessment tools, such as Short Form‐36 and Nurick's classification, should be adopted to obtain more valid evidence.
In conclusion, selective CD or TD alone demonstrated similar clinical effectiveness to nonselective and combined CTD for TSCTS. Individualized surgical decisions should be made after meticulous assessments of clinical and radiological manifestations, general patient condition, and socioeconomic factors. The risks and disadvantages of both selective and nonselective decompression procedures should be discussed in detail with the patient, and a close follow‐up is warranted regardless of the selected surgical procedure.
Grant Sources: This study was financially supported by the National Natural Science Foundation of China (No. 81902209).
Disclosure: All authors declare no conflicts of interest.
References
- 1. Dagi TF, Tarkington MA, Leech JJ. Tandem lumbar and cervical spinal stenosis. Natural history, prognostic indices, and results after surgical decompression. J Neurosurg, 1987, 66: 842–849. [DOI] [PubMed] [Google Scholar]
- 2. Chen Y, Chen DY, Wang XW, Lu XH, Yang HS, Miao JH. Single‐stage combined decompression for patients with tandem ossification in the cervical and thoracic spine. Arch Orthop Trauma Surg, 2012, 132: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 3. Fujimori T, Watabe T, Iwamoto Y, Hamada S, Iwasaki M, Oda T. Prevalence, concomitance, and distribution of ossification of the spinal ligaments: results of whole spine CT scans in 1500 Japanese patients. Spine (Phila Pa 1976), 2016, 41: 1668–1676. [DOI] [PubMed] [Google Scholar]
- 4. Hu P, Yu M, Liu X, Liu Z, Jiang L, Chen Z. One‐staged combined decompression for the patients with cervico‐thoracic tandem spinal stenosis. Eur Spine J, 2017, 26: 374–381. [DOI] [PubMed] [Google Scholar]
- 5. Hu PP, Yu M, Liu XG, Liu ZJ, Jiang L. Surgeries for patients with tandem spinal stenosis in cervical and thoracic spine: combined or staged surgeries? World Neurosurg, 2017, 107: 115–123. [DOI] [PubMed] [Google Scholar]
- 6. Kawaguchi Y, Nakano M, Yasuda T, Seki S, Hori T, Kimura T. Ossification of the posterior longitudinal ligament in not only the cervical spine, but also other spinal regions: analysis using multidetector computed tomography of the whole spine. Spine (Phila Pa 1976), 2013, 38: E1477–E1482. [DOI] [PubMed] [Google Scholar]
- 7. Kawaguchi Y, Nakano M, Yasuda T e a. Characteristics of ossification of the spinal ligament; incidence of ossification of the ligamentum flavum in patients with cervical ossification of the posterior longitudinal ligament – analysis of the whole spine using multidetector CT. J Orthop Sci, 2016, 21: 439–445. [DOI] [PubMed] [Google Scholar]
- 8. Liang H, Liu G, Lu S, et al. Epidemiology of ossification of the spinal ligaments and associated factors in the Chinese population: a cross‐sectional study of 2000 consecutive individuals. BMC Musculoskelet Disord, 2019, 20: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park JY, Chin DK, Kim KS, Cho YE. Thoracic ligament ossification in patients with cervical ossification of the posterior longitudinal ligaments: tandem ossification in the cervical and thoracic spine. Spine (Phila Pa 1976), 2008, 33: E407–E410. [DOI] [PubMed] [Google Scholar]
- 10. Park MS, Moon SH, Kim TH, et al. Asymptomatic stenosis in the cervical and thoracic spines of patients with symptomatic lumbar stenosis. Global Spine J, 2015, 5: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao BL, Ji C, Jiang JJ, Yin RF. Clinical effectiveness of treatment of combined upper thoracic spinal stenosis and multilevel cervical spinal stenosis with different posterior decompression surgeries. Int J Surg, 2018, 55: 220–223. [DOI] [PubMed] [Google Scholar]
- 12. Boody BS, Lendner M, Vaccaro AR. Ossification of the posterior longitudinal ligament in the cervical spine: a review. Int Orthop, 2019, 43: 797–805. [DOI] [PubMed] [Google Scholar]
- 13. Tetreault L, Nakashima H, Kato S, et al. A systematic review of classification systems for cervical ossification of the posterior longitudinal ligament. Global Spine J, 2019, 9: 85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mader R, Verlaan JJ, Buskila D. Diffuse idiopathic skeletal hyperostosis: clinical features and pathogenic mechanisms. Nat Rev Rheumatol, 2013, 9: 741–750. [DOI] [PubMed] [Google Scholar]
- 15. Nishimura S, Nagoshi N, Iwanami A, et al. Prevalence and distribution of diffuse idiopathic skeletal hyperostosis on whole‐spine computed tomography in patients with cervical ossification of the posterior longitudinal ligament: a multicenter study. Clin Spine Surg, 2018, 31: E460–E465. [DOI] [PubMed] [Google Scholar]
- 16. Feng FB, Sun CG, Chen ZQ. Progress on clinical characteristics and identification of location of thoracic ossification of the ligamentum flavum. Orthop Surg, 2015, 7: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsunaga S, Nakamura K, Seichi A, et al. Radiographic predictors for the development of myelopathy in patients with ossification of the posterior longitudinal ligament: a multicenter cohort study. Spine (Phila Pa 1976), 2008;33: 2648–2650. [DOI] [PubMed] [Google Scholar]
- 18. Lebl DR, Hughes A, Cammisa FP Jr, O'Leary PF. Cervical spondylotic myelopathy: pathophysiology, clinical presentation, and treatment. HSS J, 2011, 7: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976), 1981, 6: 354–364. [DOI] [PubMed] [Google Scholar]
- 20. Teng P, Papatheodorou C. Combined cervical and lumbar spondylosis. Arch Neurol, 1964, 10: 298–307. [DOI] [PubMed] [Google Scholar]
- 21. Overley SC, Kim JS, Gogel BA, Merrill RK, Hecht AC. Tandem spinal stenosis: a systematic review. JBJS Rev, 2017, 5: e2. [DOI] [PubMed] [Google Scholar]
- 22. Bajwa NS, Toy JO, Ahn NU. Is congenital bony stenosis of the cervical spine associated with congenital bony stenosis of the thoracic spine? An anatomic study of 1072 human cadaveric specimens. J Spinal Disord Tech, 2013, 26: E1–E5. [DOI] [PubMed] [Google Scholar]
- 23. Ando K, Imagama S, Kobayashi K, et al. Comparative study of surgical treatment and nonsurgical follow up for thoracic ossification of the posterior longitudinal ligament: radiological and clinical evaluation. Spine (Phila Pa 1976), 2017, 42: 407–410. [DOI] [PubMed] [Google Scholar]
- 24. Chang H, Song KJ, Kim HY, Choi BW. Factors related to the development of myelopathy in patients with cervical ossification of the posterior longitudinal ligament. J Bone Joint Surg Br, 2012, 94: 946–949. [DOI] [PubMed] [Google Scholar]
- 25. Matsunaga S, Sakou T. Ossification of the posterior longitudinal ligament of the cervical spine: etiology and natural history. Spine (Phila Pa 1976), 2012, 37: E309–E314. [DOI] [PubMed] [Google Scholar]
- 26. Nouri A, Martin AR, Mikulis D, Fehlings MG. Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus, 2016, 40: E5. [DOI] [PubMed] [Google Scholar]


