Abstract
Objective
The purpose of this paper is to investigate the effects of senescent nucleus pulposus cell (NPC)‐derived exosomes (SNPC‐Exo) and the roles of the P53/P21 pathway on the senescence of NPC.
Methods
The senescent phenotypes of NPC were induced by interleukin‐1β treatment. SNPC‐Exo was extracted from the culture medium of senescent NPC and purified by differential centrifugation. The structure of SNPC‐Exo was identified by transmission electron microscopy and western blot analysis was used to determine the exosomal marker proteins CD63 and Tsg101. Western blot analysis was performed to determine the relative expression levels of P16, P21, and P53 in NPC. Senescence‐associated β‐galactosidase (SA‐β‐gal) staining was used to stain the senescent NPC and a phase contrast microscope was used to observe and count the SA‐β‐gal staining of NPC. The proliferation of SNPC‐Exo‐treated NPC was assessed using growth curve analysis and the colony formation assay. The cell cycle of SNPC‐Exo‐treated NPC was determined by flow cytometry. NPC were transfected with siRNA to knock down P53 and P21 expression.
Results
Interleukin‐1β‐treated NPC had a higher percentage of SA‐β‐gal positive cells (45%) than the control group (20%) and showed an increase in the relative expression of P16, P21, and P53 (P < 0.05). SNPC‐Exo were positive for exosomal marker protein CD63 and Tsg 101 and negative for calnexin, and successfully internalized as previously described. SNPC‐Exo‐treated NPC showed an increase in the relative expression of P21 and P53 (P < 0.05). Compared with the control group, the SNPC‐Exo‐treated NPC showed a lower growth rate (3 times lower on the 5th day and 2 times lower on the 7th day), fewer colony‐forming units (12.0%), and a higher percentage of SA‐β‐gal‐positive NPC (50.0%). The SNPC‐Exo‐treated NPC contained more G1 phase cells (68.0%) and fewer S phase (15.5%) cells than the control group (53.0% in G1 phase, 33.5% in S phase). The expression of P21 and P53 significantly decreased in SNPC‐exo‐treated NPC after siRNA transfection (P < 0.05), followed by a higher growth rate (2 times higher on the 5th day and 1.5 times higher on the 7th day) and lower percentage of SA‐β‐gal‐positive NPC (22.5%). Moreover, the inhibition of the P53/P21 pathway promoted the SNPC‐Exo‐treated NPC to enter the S phase (from 15.5% to 25.3%).
Conclusion
The inhibition of the P53/P21 pathway attenuated the senescence of NPC induced by SNPC‐Exo.
Keywords: Exosomes, Nucleus pulposus cells, P21, P53, Senescence
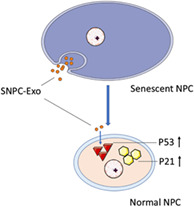
This study investigated the effects of SNPC‐Exo on the senescence of NPC. SNPC‐Exo‐treated NPC showed a senescence‐related phenotype. The inhibition of the P53/P21 pathway attenuated the senescence of NPC induced by SNPC‐Exo.
Introduction
It is reported that over 70% of adults suffer from low back pain throughout their lives, which imposes huge economic burdens and health problems on countries 1 , 2 . When intervertebral disc degeneration (IDD) occurs, NPC exhibit degeneration‐related phenotypes, like senescence and decrease of proliferative ability. The intervertebral disc is an avascular tissue, with limited capacity for self‐repair 3 . The currently available treatment options for disc degeneration can be divided into surgical therapy and physical therapy. Increasing evidence shows that traditional surgical treatment may lead to limited flexibility of the spine, changes in spinal mechanical stability, and accelerated degeneration of adjacent segments, which eventually results in spinal instability and spinal stenosis 4 , 5 . In contrast, physical therapy can only partially relieve symptoms and cannot reverse the progression of IDD. Therefore, new biological treatment modalities are receiving increased attention. Senescent NPC accelerate the process of IDD via changes in the microenvironment and promotion of intervertebral disc catabolism 3 . Inhibiting the senescence and enhancing the proliferation of NPC in the early stages of IDD is one of the effective ways of maintaining the physiological function of the intervertebral disc. However, the molecular mechanism associated with NPC senescence remains unclear.
Recent studies report that senescent cells secrete a pool of molecules and induce the stagnation of proliferation and senescence of surrounding cells through autocrine secretion or paracrine. The secretion of these factors is referred to as the senescence‐associated secretory phenotype (SASP) 6 . SASP has been regarded as a critical factor in the occurrence and development of age‐related diseases 7 . Some studies have indicated that senescent cells secrete not only inflammatory cytokines, chemokines, and matrix metalloproteinases but also exosomes 8 . Exosomes are double‐layered membrane vesicles secreted by cells and play a crucial role in the material exchange and information transduction between cells 9 . The content of exosomes depends on the type and state of the cells, which determines that its function varies from cell to cell. It has been widely reported that exosomes participate in tumor metastasis 10 , cell differentiation 11 , and tissue repair 12 . Exosomes also play a role in the regulation of cell senescence and proliferation. For example, exosomes secreted by ultraviolet‐induced senescent melanocytes accelerate the senescence of normal melanocytes 13 . Exosomes derived from senescent stromal cells promote the proliferation of breast cancer cells 14 . Studies have shown that NPC secrete exosomes in both humans and rats 11 , 15 . However, whether SNPC‐Exo contains senescence‐related factors and their role in the senescence of NPC remain unclear.
Several signaling pathways are involved in the process of cell senescence, and the p53/p21 pathway is one of the classical pathways 16 . P53 is a tumor suppressor. P53 is stimulated by DNA damage and telomere erosion, resulting in increased expression of cell cycle‐dependent protein kinase inhibitor P21, which causes RB dephosphorylation and pRB activation, eventually leading to cell replicative senescence 17 , 18 . It has been reported that cyclic mechanical tension induces premature senescence of NPC by activating the p53/p21 pathway 19 . In addition, inhibition of the p53/p21 pathway alleviates interleukin (IL)‐1β induced senescence of NPC 20 . Inactivation of P53 promotes the proliferation of senescent cells 17 , and P21 deficiency attenuates the process of senescence. It can be concluded that the p53/p21 pathway may be one of the main pathways regulating senescence of NPC. Therefore, regulation of the p53/p21 pathway may be a promising strategy to reverse the senescence of NPC.
There are two purposes of this study. The first is to explore the effect of SNPC‐Exo on the senescence of NPC. The second is to investigate the role of the p53/p21 pathway in the senescence of NPC. The present study provides new ideas for the treatment of IDD.
Materials and Methods
Nucleus Pulposus Cell Isolation, Culture, and Induction of Senescent Phenotypes
Nucleus pulposus cell were isolated from 12‐week‐old Sprague–Dawley rats as previously described 21 . This study was approved by the Ethics Committee of the Baoji People's Hospital and also conformed to the provision of the Declaration of Helsinki. The rats were killed under anesthesia, and the nucleus pulposus tissue was separated from the tail vertebrae under sterile conditions and cut into small pieces. Digestion with 0.1% collagenase was performed for 6 h, and the tissue suspension was centrifuged at 300 g for 5 min and resuspended in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 100 U/mL penicillin–streptomycin. The partially digested tissue was incubated at 37°C in a humidified atmosphere of 5% CO2. Passage 3 NPC were used for induction of senescent phenotypes. NPC were treated with 10 ng/mL IL‐1β (Sigma, Ohio, USA) for 4 days to establish a senescence model as previously described 22 .
Isolation of Senescent Nucleus Pulposus Cell‐Derived Exosomes
Senescent nucleus pulposus cell‐derived exosomes were isolated by differential centrifugation as previously described 23 . Fetal bovine serum was centrifuged at 120,000 g for 90 min at 4°C to remove endogenous exosomes. Senescent NPC were cultured in DMEM‐F12 containing 10% exosome‐free fetal bovine serum. After 48 h, the cell culture supernatant was collected and centrifuged at 300 g for 10 min and 2000 g for 20 min to remove the cells. The supernatant was filtered using a 0.22‐μm membrane to remove any particles larger than 0.22 μm in diameter. The exosomes were pelleted by ultracentrifugation at 120,000 g for 70 min at 4°C, washed, and resuspended in phosphate buffer solution (PBS). The exosomes were quantified by BCA protein assay and stored at −80°C for further use.
Identification of Senescent Nucleus Pulposus Cell‐Derived Exosomes using Transmission Electron Microscopy
To observe the struture of SNPC‐Exo under transmission electron microscopy, SNPC‐Exo were obtained through differential centrifugation and resuspended in PBS; 10 μg exosomes suspension was dropped onto electron microscopy copper grids and dried for 10 min. The grids were stained with 1% phosphotungstic acid for 5 min and examined by transmission electron microscope (JEM‐1400PLUS, Japan) at 100 kV.
Exosomes Labeling and Observation Under the Fluorescence Microscopy
To determine the internalization of SNPC‐Exo by NPC, SNPC‐Exo and NPC were labeled by fluorescence dyes CM‐Dil and CM‐Dio (Beyotime, Shanghai, China), respectively, as previously described 24 . Briefly, 20 μg SNPC‐Exo in 100 μL PBS were incubated with CM‐Dil in the dark for 30min, washed with PBS, ultracentrifuged at 100,000 g for 70 min to remove the non‐binding dye and resuspended in PBS. NPC were incubated with CM‐Dio in the dark for 30 min and centrifuged at 300 g for 5 min to remove any non‐binding dye. The CM‐Dil‐labeled SNPC‐Exo were incubated with CM‐Dio‐labeled NPC at 37°C for 24 h. After incubation, fluorescence images were collected using a fluorescence microscope and analyzed with the Leica Application Suite Advanced Fluorescence (LAS AF) software.
SA‐β‐Gal Staining Analysis
To stain and count the numbers of senescent NPC, passage 3 NPC were plated in 12‐well plates at 1.5 × 104 cells/cm2 density. Once they had reached 30%–50% confluence, the cells were washed twice with PBS and incubated with a 4% paraformaldehyde solution for 15 min. After washing with PBS 3 times, NPC were incubated overnight with SA‐β‐gal staining reagent (Beyotime, Shanghai, China) at 37°C. A phase contrast microscope was used to observe the SA‐β‐gal staining of NPC.
Colony‐Forming Units Assay of Nucleus Pulposus Cell
To count the numbers of colony‐forming units and evaluate the self‐renewal capacity of NPC, the cells were digested with trypsin, and 1 × 102 NPC were seeded per well of 6‐well plates containing DMEM‐F12 medium. After 10 days of culturing, NPC were washed twice with PBS, fixed using 4% paraformaldehyde for 15 min, and washed again with PBS 3 times. After that, crystal violet staining solution (Beyotime, Shanghai, China) was used to stain the colonies. The colonies consisting of more than 50 cells were counted under a microscope.
Cell Cycle Analysis
To determine the cell cycle of NPC, total cell‐cycle progression was assessed using EdU Flow Cytometry Assay Kits (Invitrogen, Massachusetts, USA). Single NPC suspensions were prepared in PBS, and the cells were treated with the corresponding specialized reagent. The cell pellets were incubated at 37°C for 30 min and obtained by centrifugation. Finally, the specimens were investigated by flow cytometry with FACS MoFlo (Beckman, USA).
Proliferation Assay of Nucleus Pulposus Cell
The Cell Counting Kit‐8 assay (CCK‐8, Dojindo, Tokyo, Japan) was performed to determine the proliferation rate of NPC. NPC were seeded in triplicate in 96‐well plates at a concentration of 2 × 103 cells/well in 100 μL DMEM‐F12 medium supplemented with 10% FBS. The NPC that received the corresponding treatment on days 1, 3, 5, and 7 were washed twice with PBS and incubated with 10 μL CCK‐8 reagent for 2 h at 37°C in the dark. The absorbance was measured at 450 nm, and the fold changes of absorbance relative to the control group were used to generate the cell growth curve.
Western Blot Analysis
To determine the relative protein expression of CD63, Tsg101, P16, P21 and P53, NPC and SNPC‐Exo were lysed with cell lysis buffer (Beyotime Biotechnology) containing 1% phenylmethylsulfonyl fluoride (Beyotime Biotechnology) and then centrifuged at 14,000 g at 4°C for 5 min to discard the cell debris. NPC lysates and SNPC‐Exo lysates were subjected to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, USA). The membranes were blocked using 5% non‐fat milk in TBST, incubated with specific antibodies overnight at 4°C and then washed thrice with TBST. The membranes were incubated with a horseradish peroxidase‐labeled secondary antibody (Beyotime Biotechnology dilution 1:4000) for 2 h and visualized using an Enhanced Chemiluminescence Substrate (Beyotime Biotechnology) and Image Quant Las4000. The relative expressions of the target protein to glyceraldehyde 3 phosphate dehydrogenase (GAPDH) were calculated using the Image J software. The antibodies involved are as follows: GAPDH (Beyotime 1:1000 dilution), CD63/Tsg101/Calnexin (Santa Cruz 1:200 dilution), and P16/P21/P53 (Protein TECH 1:500 dilution).
P53 and P21 siRNA Transfection
P53 and P21 siRNA were provided by Genechem (Shanghai, China). NPC were seeded in 6‐well plates and cultured until 20%–30% confluence. NPC were transfected with P53/P21siRNA or negative control following the manufacturer's instructions. The medium was changed after 12 h and upon reaching 80%–90% confluence. NPC were passaged for further use. The transfection efficiency was determined by western blot analysis.
Measurement of Relative Protein Expression
To evaluate the expression of senescence‐related protein and exosomal marker protein, the following parameters were involved for evaluation.
Expression of P16 Protein
P16 protein is an inhibitor of cyclin‐dependent kinases 4 (CDK4), one of the key enzymes in the cell division cycle. It can be the indicator of cells senescence. In this experiment, the expression of P16 could be detected by western blot analysis.
Expression of P21 Protein
P21 protein is an important member of the CDK inhibitor family. It is not only closely related to tumor suppression but can also coordinate cell senescence by inhibiting the activity of CDK complex. The expression of P21 could be detected by western blot analysis.
Expression of P53 Protein
P53 protein is a transcriptional factor that controls the start of the cell cycle. It can also regulate the senescence of cells. The expression of P53 could be detected by western blot analysis.
Expression of CD63 Protein
CD63 protein is a kind of transmembrane protein. It can be a marker protein of exosomes. We determined the CD63 of exosome with western blot analysis.
Expression of Tsg101 Protein
Tsg101 is also a kind of transmembrane protein. It can also be a marker protein of exosomes. We determined the Tsg101 of exosomes with western blot analysis.
Expression of Calnexin Protein
Calnexin is an endoplasmic reticulum specific expression protein, which is not expressed in exosomes. We used western blot analysis to investigate the expression of calnexin.
Statistical Analysis
The numerical data was shown as mean ± SD. Statistical analysis was conducted using SPSS 13.0 software. Student's t‐test was used for the two group statistical analysis. Statistical analysis among multiple groups was performed by one‐way ANOVA followed by Tukey's test. P‐value <0.05 was considered to be statistically significant.
Results
The Percentage of SA‐β‐Gal Positive Cells and P16, P21, and P53 Expression in Nucleus Pulposus Cell Increased After Interleukin‐1β Treatment
Nucleus pulposus cell were successfully isolated and subcultured to passage 3 for use. SA‐β‐gal staining analysis was conducted to verify the senescent phenotypes of NPC. After treatment, the percentage of SA‐β‐gal‐positive cells was significantly increased in the IL‐1β group (45%) compared to the control group (20%) (P < 0.001) (Fig. 1A,B). Western blot analysis showed a significant increase in the expression of senescence‐associate proteins P16, P21, and P53 in IL‐1β‐treated NPC (P < 0.05) (Fig. 1C). These results confirmed the successful induction of senescent phenotypes of NPC.
Fig 1.
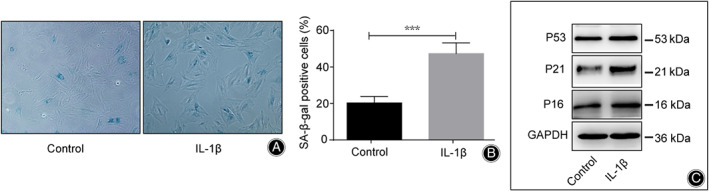
The senescence of nucleus pulposus cell (NPC) was induced with interleukin (IL)‐1β. (A) SA‐β‐gal staining of NPC with the treatment of IL‐1β. (B) Relative percentage of SA‐β‐gal‐positive cells between the IL‐1β‐treated group and the control group. All data is shown as mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. (C) Western blot analysis of P16, P21, and P53 in IL‐1Β‐treated NPC. GAPDH, glyceraldehyde 3 phosphate dehydrogenase
CD63 and Tsg 101 were Highly Expressed in Senescent Nucleus Pulposus Cell‐Derived Exosomes
Transmission electron microscopy showed that SNPC‐Exo were cup‐shaped vesicles and <100 nm in size (Fig. 2A). The SNPC‐Exo were positive for exosomal marker protein CD63 and Tsg 101 and negative for the endoplasmic reticulum‐specific expression protein calnexin (Fig. 2B). The vesicles isolated from senescent NPC culture supernatant were identified as SNPC‐Exo.
Fig 2.
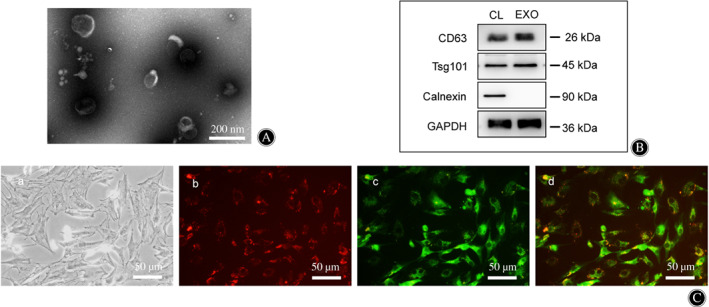
Identification of senescent nucleus pulposus cell (NPC)‐derived exosomes (SNPC‐Exo) and uptake of SNPC‐Exo by NPC. (A) Characteristics of SNPC‐Exo under transmission electron microscope. (B) Western blot analyses of exosomal protein markers CD63 and Tsg101 and negative protein calnexin. CL, NPC lysate; EXO, senescent NPC‐derived exosomes. (C) Uptake of SNPC‐Exo by NPC. SNPC‐Exo were stained with CM‐Dil (B) and NPC were stained with CM‐Dio (C). Uptake was observed by fluorescence microscope.
Senescent Nucleus Pulposus Cell‐Derived Exosomes Labeling Fluorescence was Detected in Nucleus Pulposus Cells
To detect the internalization of SNPC‐Exo by NPC, SNPC‐Exo were labeled with CM‐Dil and incubated with CM‐Dio‐labeled NPC at 37°C for 24 h. Red fluorescence spots were observed in green fluorescence‐labeled NPC under a fluorescence microscope (Fig. 2C), which confirmed that SNPC‐Exo were internalized by NPC.
Expression of P21 and P53 Increased and the Proliferation Rate Decreased in Senescent Nucleus Pulposus Cell‐Derived Exosome‐Treated Nucleus Pulposus Cells
To investigate the effects of SNPC‐Exo on the senescence of NPC, the expression of P16, P21, and P53 in NPC was detected by western blot analysis following SNPC‐Exo treatment for 14 days. The results showed that the expression of P21 in SNPC‐Exo‐treated NPC was 2.1 times higher than in the control group (P < 0.01) and the expression of P53 in SNPC‐Exo‐treated NPC was 2.8 times higher than in control group (P < 0.001), while there was no significant difference in P16 expression (P > 0.05) (Fig. 3A,B). Growth kinetics were measured by CCK‐8 assay. Compared with the control group, the SNPC‐Exo group showed a lower growth rate (3 times lower on the 5th day and 2 times lower on the 7th day), while there was no significant difference on the 3th day (Fig. 3C).
Fig 3.
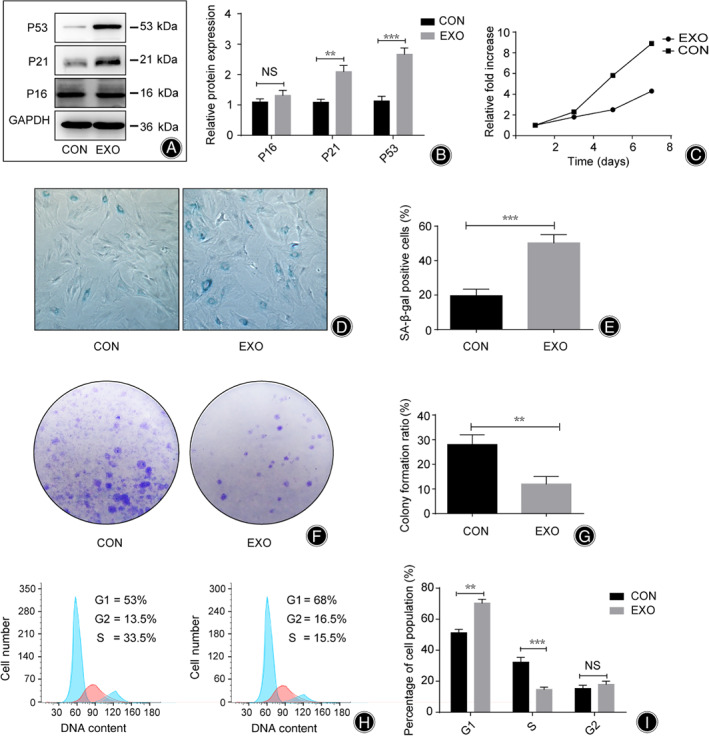
Senescent nucleus pulposus cell (NPC)‐derived exosomes (SNPC‐Exo) accelerated senescence and inhibited the proliferation of NPC. (A, B) Western blot analysis of P16, P21, and P53 in SNPC‐Exo‐treated NPC. (C) Relative fold change of absorbance between EXO group and control group at 450 nm. (D, E) Relative percentage of SA‐β‐gal positive cells between EXO group and control group. (F, G) Colony‐forming units assay of NPC. (H, I) The cell cycle phases of NPC were determined by flow cytometry analyses. CON, NPC cultured alone; EXO, NPC treated with SNPC‐Exo; NS, no significance. All data is shown as mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001.
Percentage of SA‐β‐Gal Positive Cells and G1 Phase Cells Increased and the Numbers of Colony‐Forming Units and S Phase Cells Decreased in Senescent Nucleus Pulposus Cell‐Derived Exosome‐Treated Nucleus Pulposus Cells
Senescent nucleus pulposus cell‐derived exosome‐treated NPC showed a higher percentage of SA‐β‐gal positive cells (50.0%) than the control group (20%) (P < 0.001), and smaller numbers of colony‐forming units (12.0%) than the control group (27%) (P < 0.01) (Fig. 3D–G). Flow cytometry analyses were used to determine the cell cycle phases of NPC. Approximately 53% of NPC were in the G1 phase; however, the percentage of G1 phase NPC rose to 68% after SNPC‐Exo treatment. In addition, the percentage of S phase NPC was found to have significantly dropped from 33.5% to 15.5% (P < 0.001) (Fig. 3H).
Expression of P53 and P21 Decreased Significantly After siRNA Transfection
Based on the previous results showing higher expression levels of P21 and P53 in SNPC‐Exo‐treated NPC, and no significant difference in P16 expression, in this study, we focused on the function of P21 and P53. siRNA transfection was used to inhibit the expression of P21 and P53 in NPC, to explore the roles of P21 and P53 in NPC proliferation and senescence. After P21 siRNA transfection, the expression of P21 in the siRNA group was 0.5 times that of the negative group (P < 0.01) and 0.4 times that of the control group (P < 0.001), while there was no significant change between the negative group and the control group (P > 0.05) (Fig. 4A,B). After P53 siRNA transfection, the expression of P53 in the siRNA group was 0.25 times that of the negative group (P < 0.001) and the control group (P < 0.001), while there was no significant change between the negative group and the control group (P > 0.05) (Fig.4A,C).
Nucleus Pulposus Cells Showed a Higher Growth Rate, Higher S Phase Percentage, and Lower Percentage of SA‐β‐Gal‐Positive Cells after siRNA Transfection
After siRNA transfection, the percentage of SA‐β‐gal positive cells was lower in the EXO+siP53 group (22.5%) and the EXO+siP21 group (19.8%) than in the EXO group (46.7%) and the EXO+NC group (51.8%) (P < 0.001), while there was no significant change between the EXO+siP53 group and the EXO+siP21 group or the EXO group and the EXO+NC group (P > 0.05) (Fig. 4C,D). Compared with the EXO group and the EXO+NC group, the EXO+siP53 group and the EXO+siP21 group showed a higher growth rate (approximately 2 times higher on the 5th day and 1.5 times higher on the 7th day) (P < 0.001), while there was no significant difference on the 3th day (Fig. 4E). In addition, there was no significant change between the EXO+siP53 group and the EXO+siP21 group or among the control group, the EXO group, and the EXO+NC group (P > 0.05) (Fig. 4E). After siRNA transfection, the percentage of G1 phase NPC was lower in the EXO+siP53 group (60.5%) and the EXO+siP21 group (62.8%) than in the EXO group (70.7%) and the EXO+NC group (72.8%) (P < 0.05), while there was no significant change between the EXO+siP53 group and the EXO+siP21 group or the EXO group and the EXO+NC group (P > 0.05) (Fig. 4F). However, the percentage of S phase NPC was higher in the EXO+siP53 group (25.3%) and the EXO+siP21 group (24.2%) than in the EXO group (16.3%) and the EXO+NC group (15.5%) (P < 0.05), while there was no significant change between the EXO+siP53 group and the EXO+siP21 group or the EXO group and the EXO+NC group (P > 0.05) (Fig. 4F).
Fig 4.
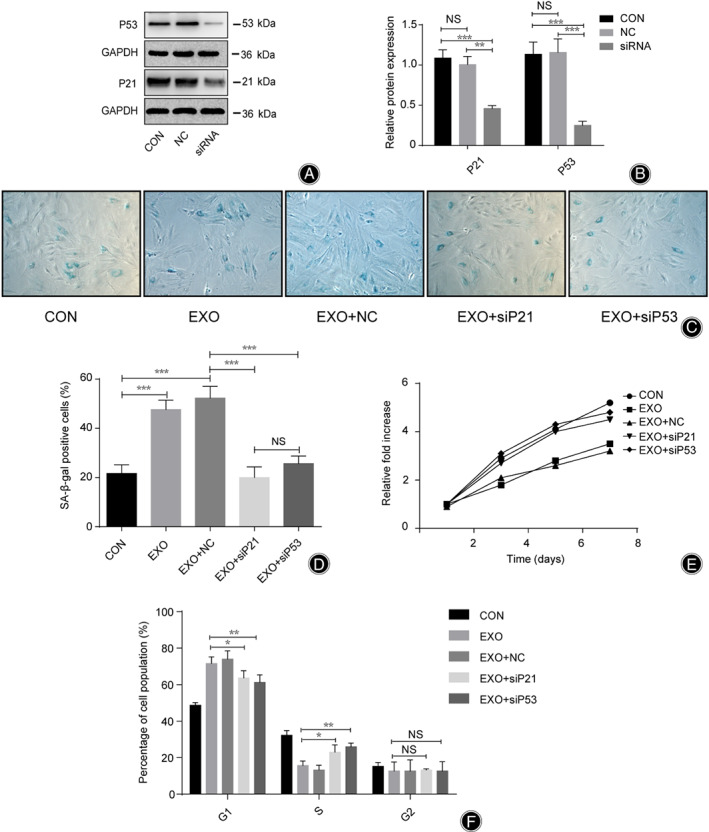
P21 and P53 regulated the proliferation and senescence of nucleus pulposus cell (NPC) senescent NPC‐derived exosomes (SNPC‐Exo) treatment. (A, B) The expression of P21 and P53 in NPC was inhibited by siRNA transfection. (C, D) Relative percentage of SA‐β‐gal positive cells among groups after corresponding treatments. (E) Relative fold change of absorbance among groups after Corresponding treatments at 450 nm. (F) The cell cycle phases of NPC were determined by flow cytometry analyses. CON, NPC cultured alone; EXO, NPC treated with SNPC‐Exo; EXO+siP21, NPC treated with SNPC‐Exo and P21 siRNA; EXO+siP53, NPC treated with SNPC‐Exo and P53 siRNA; NC, NPC treated with siRNA negative control; NS, no significance; siRNA, NPC treated with P21 or P53; siRNAEXO+NC, NPC treated with SNPC‐Exo and siRNA negative control. All data are shown as mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001.
Discussion
Brief Mechanism of Nucleus Pulposus Cell Senescence
Nucleus pulposus cell senescence is one of the key factors of disc degeneration 25 . Currently, the underlying mechanism of NPC senescence is not fully understood. Telomere erosion, oxidative stress, cytokines, and DNA damage are closely related to senescence of intervertebral disc cells 26 , 27 , 28 . Disc degeneration is characterized by decreased proliferating ability; hence, it is difficult to replenish the active NPC by cell proliferation. In addition, senescent NPC secrete pro‐inflammatory cytokines, extracellular matrix proteases, and chemokines that promote the transition from anabolism to catabolism in the disc, which is known as SASP 3 . Therefore, it is important to explore the molecular mechanism of NPC senescence in the prevention and treatment of IDD.
Function of Exosomes on Cell Senescence
Exosomes contain various cellular components, such as proteins, lipids, and nucleic acids, which are key factors in intercellular communication. Interferon‐induced transmembrane protein 3 in exosomes derived from senescent human fetal foreskin can contribute to the induction of cellular senescence in surrounding cells 29 . It has been suggested that exosomes secreted from senescent cells might contribute to senescence‐associated pathologies. In this study, a model of senescent NPC was established in vitro, and SNPC‐Exo was successfully extracted and identified. The internalization of SNPC‐Exo by NPC was observed under a fluorescence microscope, which suggested that senescent NPC in the degenerative disc may affect the surrounding cells by releasing exosomes. With the treatments of SNPC‐Exo, normal NPC showed a degeneration‐related phenotype, specifically in the reduced capacity of proliferation and colony formation and increased expression of P53 and P21. In addition, more NPC stayed in the G1 phase in the SNPC‐Exo group than in the control group. These results suggested that SNPC‐Exo may possess bioactive substances derived from senescent NPC and played a role in promoting senescence of surrounding normal NPC. SNPC‐Exo can be regarded as a new SASP factor. Interestingly, there was no significant difference between the P16 SNPC‐Exo group and the control group, which indicated that the p16–pRB pathway was not involved in this process.
Pathways Involved in the Senescence of Nucleus Pulposus Cells
Cellular senescence is mainly controlled by the p53/p21 pathway and the p16–pRB tumor suppression pathway. The p53/p21 pathway is activated by telomere erosion, decreased telomerase activity, and DNA damage, which induces replicative senescence in disc cells. The p16–pRb pathway is activated by various stimuli, including oxidative stress, pro‐inflammatory cytokines, and high glucose levels, to mediate the stress‐induced premature senescence of disc cells 3 , 30 . However, the molecular signaling pathways involved in cellular senescence depend on induction factors, cell types, and species. The results of this experiment indicated that under the treatment of SNPC‐Exo, the expression of P53 and P21 in NPC increased, and P16 did not show any significant change. Previous studies have reported that the expression of the p53/p21 pathway increases during senescence of NPC, which is consistent with the results of this study. To further explore the role of the p53/p21 pathway in the senescence of NPC following SNPC‐Exo treatment, siRNA transfection was performed to knock down the expression of the p53/p21 pathway in NPC. p53/p21 knockdown significantly decreased the percentage of SA‐β‐gal positive NPC and enhanced NPC proliferation. In addition, the p53/p21 pathway knockdown regulated the cell cycle and prompted NPC to enter the S phase. These results demonstrate that inhibition of the p53/p21 pathway can reverse the senescence and promote the proliferation of NPC following SNPC‐Exo treatment.
Summary of Results
Although this study explains the mechanism of NPC senescence to some extent, there are some limitations in this study. First, the model of cell senescence in vitro is different from that in vivo, resulting in different types and quantities of exosome contents. Second, the functional substances carried by SNPC‐Exo were not detected. Therefore, further experiments are needed to investigate the components of SNPC‐Exo. In conclusion, this study demonstrated that SNPC‐Exo played an important role in IDD development by promoting senescence and inhibiting the proliferation of NPC through the regulation of the p53/p21 pathway. SNPC‐Exo can be considered a supplement of the SASP factor in NPC, providing new ideas for the prevention and treatment of IDD.
Disclosure: All of the authors have no conflict of interest in this research. Each author has made an important scientific contribution to the study and has assisted with the drafting or revising of the manuscript.
References
- 1. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet, 2012, 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician, 2012, 85: 343–350. [PubMed] [Google Scholar]
- 3. Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle, 2016, 15: 1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saragiotto BT, Machado GC, Ferreira ML, et al. Paracetamol for low back pain. Cochrane Database Syst Rev, 2016, 6: D12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindback Y, Tropp H, Enthoven P, et al. PREPARE: pre‐surgery physiotherapy for patients with degenerative lumbar spine disorder: a randomized controlled trial protocol. BMC Musculoskelet Disord, 2016, 17: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coppe JP, Patil CK, Rodier F, et al. Senescence‐associated secretory phenotypes reveal cell‐nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol, 2008, 6: 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Deursen JM. The role of senescent cells in ageing. Nature, 2014, 509: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Misawa T, Tanaka Y, Okada R, et al. Biology of extracellular vesicles secreted from senescent cells as senescence‐associated secretory phenotype factors. Geriatr Gerontol Int, 2020, 20: 539–546. [DOI] [PubMed] [Google Scholar]
- 9. Toh WS, Lai RC, Hui J, et al. MSC exosome as a cell‐free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol, 2017, 67: 56–64. [DOI] [PubMed] [Google Scholar]
- 10. Fu Q, Zhang Q, Lou Y, et al. Correction: primary tumor‐derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via SMAD3 in liver cancer. Oncogene, 2019, 38: 5740–5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lan WR, Pan S, Li HY, et al. Inhibition of the Notch1 pathway promotes the effects of nucleus pulposus cell‐derived exosomes on the differentiation of mesenchymal stem cells into nucleus pulposus‐like cells in rats. Stem Cells Int 2019: 8404168, 2019, 1, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoon YM, Lee JH, Song KH, et al. Melatonin‐stimulated exosomes enhance the regenerative potential of chronic kidney disease‐derived mesenchymal stem/stromal cells via cellular prion proteins. J Pineal Res, 2020, 68: e12632. [DOI] [PubMed] [Google Scholar]
- 13. Sha J, Arbesman J, Harter ML. Premature senescence in human melanocytes after exposure to solar UVR: an exosome and UV‐miRNA connection. Pigment Cell Melanoma Res, 2020, 33: 671–684. [DOI] [PubMed] [Google Scholar]
- 14. Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun, 2017, 8: 15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu K, Li HY, Yang K, et al. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in‐vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res Ther, 2017, 8: 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campisi J. Cellular senescence as a tumor‐suppressor mechanism. Trends Cell Biol, 2001, 11: 27–31. [DOI] [PubMed] [Google Scholar]
- 17. Beausejour CM, Krtolica A, Galimi F, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J, 2003, 22: 4212–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1‐phase progression. Genes Dev, 1999, 13: 1501–1512. [DOI] [PubMed] [Google Scholar]
- 19. Feng C, Yang M, Zhang Y, et al. Cyclic mechanical tension reinforces DNA damage and activates the p53‐p21‐Rb pathway to induce premature senescence of nucleus pulposus cells. Int J Mol Med, 2018, 41: 3316–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang M, Peng Y, Liu W, Zhou M, Meng Q, Yuan C. Sirtuin 2 expression suppresses oxidative stress and senescence of nucleus pulposus cells through inhibition of the p53/p21 pathway. Biochem Biophys Res Commun, 2019, 513: 616–622. [DOI] [PubMed] [Google Scholar]
- 21. Risbud MV, Guttapalli A, Stokes DG, et al. Nucleus pulposus cells express HIF‐1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem, 2006, 98: 152–159. [DOI] [PubMed] [Google Scholar]
- 22. Che H, Li J, Li Y, et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. elife, 2020, 9: e52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun W, Zhao C, Li Y, et al. Osteoclast‐derived microRNA‐containing exosomes selectively inhibit osteoblast activity. Cell Discovery, 2016, 2: 16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan Y, Jiang W, Tan Y, et al. hucMSC exosome‐derived GPX1 is required for the recovery of hepatic oxidant injury. Mol Ther, 2017, 25: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther, 2007, 9: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dimozi A, Mavrogonatou E, Sklirou A, et al. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater, 2015, 30: 102–103. [DOI] [PubMed] [Google Scholar]
- 27. Jeong SW, Lee JS, Kim KW. In vitro lifespan and senescence mechanisms of human nucleus pulposus chondrocytes. Spine J, 2014, 14: 499–504. [DOI] [PubMed] [Google Scholar]
- 28. Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. A role for TNFalpha in intervertebral disc degeneration: a non‐recoverable catabolic shift. Biochem Biophys Res Commun, 2013, 433: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borghesan M, Fafian‐Labora J, Eleftheriadou O, et al. Small extracellular vesicles are key regulators of non‐cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep, 2019, 27: 3956–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthr Cartil, 2016, 24: 398–408. [DOI] [PubMed] [Google Scholar]


