Abstract
Objective
The aim of the present study was to evaluate the potential protective mechanism of icariin against oxidative damage caused by hydrogen peroxide in MC3T3‐E1 cells.
Methods
MC3T3‐E1 cells were treated with different concentrations of icariin to explore the optimal dose of icariin. MC3T3‐E1 cells were divided into groups treated with various concentrations of hydrogen peroxide (H2O2; 0, 0.1, 0.2, 0.5, 1, and 2 mM) for 24 h to induce oxidative damage and cell viability was assessed by Cell Counting Kit‐8 (CCK‐8) assay. Then, cells were divided into five groups: control, H2O2 (0.2 mM), icariin (0.1 μM) and H2O2 (0.2 mM), + icariin (0.1 μM). Cell viability was detected by CCK‐8 assay. In addition, the content of glutathione and superoxide dismutase and the activity level of malondialdehyde in these treatment groups were determined. Alkaline phosphatase (ALP) and alizarin red S (ARS) staining were also performed to measure the early and late osteogenesis, respectively. Protein expression of β‐catenin and cyclin D1 was measured by western blot assay. Then, we used an antagonist of Wnt/β‐catenin signaling pathway (DKK‐1) and western blot analysis to further explore potential mechanism.
Results
After 24 h of exposure to 0.2 mM H2O2, the viability of MC3T3‐E1 cells was significantly decreased compared to that of the control cells. We first found that icariin can promote cell proliferation of MC3T3‐E1 cells in a dose‐dependent manner, with the dosage 0.1 μM showing the best pro‐proliferative effect. Furthermore, icariin could promote the protein expression of OSX and RUNX2. The results showed that icariin can reverse the inhibitory osteogenic effects of MC3T3‐E1 caused by H2O2. In addition, icariin could increase the Wnt‐signaling related proteins. The results showed that MC3T3‐E1 cells in the H2O2 (0.2 mM) + icariin (0.1 μM) + Wnt‐signaling antagonist (DKK‐1) group had weaker ALP and ARS staining compared with that observed in the control and H2O2 (0.2 mM) + icariin (0.1 μM) groups. The ALP activity and calcium content were decreased in the 0.2 mM H2O2 + 0.1 μM icariin + DKK‐1 group compared to that observed in the 0.2 mM H2O2 + 0.1 μM icariin group.
Conclusion
The results showed that icariin can increase the viability of MC3T3‐E1 cells, reverse the oxidative stress induced by H2O2 and protect MC3T3‐E1 cells against H2O2‐induced inhibition of osteogenic differentiation, which may occur through the Wnt/β‐catenin signaling pathway.
Keywords: Icariin, H2O2, MC3T3‐E1, Osteogenesis
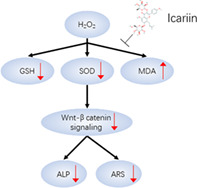
Schematic diagram illustrating the mechanism of action of icariin against oxidative damage.
Introduction
Osteoporosis is a common metabolic bone disorder that affects approximately 75 million people all over the world 1 . Osteoporosis is characterized by low bone mineral density (BMD), which predisposes individuals to an increased risk of fracture 2 . The pathogenesis of osteoporosis is yet to be fully elucidated. Previous reports have shown that estrogen deficiency, a lack of exercise, and oxidative stress play a central role in the onset and development of postmenopausal osteoporosis 3 , 4 . Oxidative stress can produce excessive reactive oxygen species (ROS) and promote osteoblast damage 5 , 6 , 7 . Furthermore, ROS accelerates the apoptosis of osteoblasts, inhibiting their differentiation and impairing bone formation 8 . Previous studies have reported that some antioxidants, such as vitamin E, vitamin C, and N‐acetylcysteine (NAC), can protect cells from damage induced by oxidative stress and are, therefore, considered to be alternative therapeutic agents for osteoporosis 9 , 10 , 11 . Therefore, increased oxidative stress may play an important role in bone loss and finally cause osteoporotic fractures. As a result, antioxidants may be used as a novel therapeutic approach in the prevention and treatment of osteoporosis.
Another study revealed that ROS targets the Wnt/β‐catenin signaling pathway and hinders bone formation 8 . Wnt/β‐catenin signaling plays an important role in the primary processes controlling osteogenesis, as it promotes osteoblast differentiation and mineralization 12 . Moreover, the inhibition of β‐catenin by DKK‐1 leads to elevated oxidative stress and reduced osteogenic capacity 13 . In an oxidative damage model, an aberrant WNT/β‐catenin pathway is generally observed and leads to oxidative stress 14 . Taken together, these results indicate that inhibition of the Wnt/β‐catenin signaling pathway in responding to stressors, such as oxidative stress, also suppress osteogenic differentiation.
Icariin, the major active component of Herba Epimedii (Yinyanghuo or horny goat weed), may help with postmenopausal osteoporosis (PO) treatment and prevention. Icariin has been reported to possess multiple pharmacological effects, including anti‐oxidative 15 , anti‐inflammatory 16 , and anti‐apoptotic effects 17 and anti‐osteoporosis activities 18 .
Several studies have shown that icariin has an antiosteoporosis effect in ovariectomized rats 19 , 20 , 21 . Mok et al. 22 performed an in vitro study and observed that icariin exerts anabolic effects in bone, possibly by activating estrogen receptor in a ligand‐independent manner. Another study revealed that icariin can protect cardiac cells from oxidative stress‐induced injury through stimulation of the extracellular signal‐regulated kinase (ERK) pathway 23 . Furthermore, icariin has osteogenic effects, primarily through activating Wnt signaling cascades 24 . Saud et al. 25 conducted a review to comprehensively interpret the functional role and mechanisms of icariin for cell proliferation and differentiation. They found that icariin could promote cell differentiation by targeting WNT and, finally, regulating RUNX2 expression. The protective effects and potential mechanism of icariin against oxidative damage are not yet known.
Therefore, the purpose of the present study was to examine: (i) the optimal dose of icariin for protecting against oxidative damage; (ii) whether icariin could reverse the oxidative stress induced by H2O2 and protect MC3T3‐E1 cells against the H2O2‐induced inhibition of osteogenic differentiation; and (iii) whether icariin could activate Wnt signaling cascades to protect against oxidative damage caused by H2O2.
Materials and Methods
Cell Viability Assay
Cell viability was investigated using a Cell Counting kit‐8 assay (CCK‐8, Solarbio, Beijing, China) according to the manufacturer's instructions. We seeded 1000 cells/well in 96‐well plates and then added different concentrations of H2O2 (0, 0.1, 0.5, 1, and 2 mM) and icariin (0.001, 0.01, 0.1, 1, and 10 μM). The dose of H2O2 and icariin was determined based on previous studies 26 , 27 . Then, after 24 h, 10 μL of CCK‐8 solution was added to cells in each well and incubated for 1 h. Subsequently, the absorbance was measured using an enzyme‐linked immunosorbent assay reader at 450 nm.
MC3T3‐E1 Treatment with Icariin, H2O2, or their Combination
MC3T3‐E1 cells were divided into four groups: a group not treated with icariin or H2O2 as control, a group treated with 0.2 mM H2O2 as an oxidative stress model, a group treated with 0.1 mM icariin, and a group treated with 0.2 mM H2O2 + 0.1 μM icariin. At 0, 12, 24, 36, and 48 h after treatment, 10 μL of cell counting kit‐8 (CCK‐8) solution was added to the medium, and 1 h later, the absorbance of the samples at 450 nm was measured with a microplate reader to assess the cell viability. In addition, alkaline phosphatase (ALP) and alizarin red S (ARS) staining were performed to assess the early and late stages of osteogenic differentiation of MC3T3‐E1 cells. To further elucidate the potential mechanism by which icariin regulates the osteogenesis of MC3T3‐E1 cells, the Wnt inhibitor Dickkopf‐1 (Dkk‐1) was added, at 10 μM, to the icariin and H2O2 (0.2 mM) group.
Culturing of MC3T3‐E1 Osteoblast‐Like Cells and Induction of Osteoblast Differentiation
Murine MC3T3‐E1 cells were cultured in α‐MEM (Life Technologies) containing 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin under an atmosphere with 5% CO2 at 37°C. The medium was replaced with fresh medium every 2–3 days. When the cells reached 80% confluence, the medium was replaced with osteoblast differentiation medium (α‐MEM supplemented with 10% FBS, 50 μg/mL ascorbic acid, 10 mM β‐glycerophosphate, and 10 nM dexamethasone) for 14 days, after which the cells were used for further study. This study was approved by the ethics committee of the General Hospital of Ningxia Medical University.
Measurement of Oxidative Stress
We measured the level of superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA) to assess cellular damage using three kits obtained from Nanjing Jiancheng Biochemistry (Nanjing, Jiangsu, China) following the manufacturer's instructions. Briefly, total proteins were extracted and measured at 450 nm.
Alkaline Phosphatase Staining and Activity Measurement
MC3T3‐E1 cells were induced in differentiation medium for 7 days. For ALP staining, alkaline phosphatase staining was performed with a BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime, Shanghai, China) according to the manufacturer's instructions 28 . First, we mixed 33 μL of BCIP solution, 66 μL of NBT solution and 10.1 mL of BCIP/NBT dye working solution. Then, MC3T3‐E1 cells were fixed with 4% paraformaldehyde and washed with PBS three times before being incubated with the mixed solutions for 10 minutes and washed with PBS to stop the reaction.
After incubating for 7 days, the MC3T3‐E1 cells were harvested and washed with ice‐cold PBS before being lysed by three cycles of freezing and thawing. Aliquots of supernatants from each group were used to quantitatively analyze the ALP activity using an ALP activity kit (Nanjing Jiancheng Biological Engineering Institute, China) according to the manufacturer's instructions.
Alizarin Red S Staining and Calcium Assay
MC3T3‐E1 cells were cultured in osteogenic induction medium before being rinsed with PBS, fixed with 4% paraformaldehyde, and stained with 0.2% alizarin red S solution (pH = 8.3, Solarbio, Beijing, China) for 1 h. Orange‐red staining indicated the location and intensity of calcium deposition. Calcium levels were measured with an enzyme‐linked immunosorbent assay reader at 560 nm.
Reverse Transcription‐Polymerase Chain Reaction
Total RNA was isolated using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. A PrimeScript RT Reagent Kit (TaKaRa, Japan) was used to perform reverse transcription. Reverse‐transcription polymerase chain reaction (RT‐PCR) was performed using a SYBR Premix Ex Taq II kit (TaKaRa) and detected with a Roche LightCycler 480 sequence detection system. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as a loading control for mRNA quantitation. The gene sequences of RUNX2, OSX, and β‐actin are presented in Table 1. The relative mRNA expression levels of RUNX2 and OSX were first normalized to β‐actin levels and then calculated relative to their treatment controls.
Table 1.
Gene sequences of RUNX2, OSX, and β‐actin primers
| Gene names | Forward sequence | Reverse sequence |
|---|---|---|
| RUNX2 | 5′‐AAGTGCGGTGCAAACTTTCT‐3′ | 5′‐TCTCGGTGGCTGCTAGTGA‐3′ |
| OSX | 5′‐GCCAGAAGCTGTGAAACCTC‐3′ | 5′‐GCTGCAAGCTCTCCATAACC‐3′ |
| β‐actin | 5′‐TCCTAGCACCATGAAGATC‐3′ | 5′‐AAACGCAGCTCAGTAACAG‐3′ |
Western Blot Analyses
MC3T3‐E1 cells were lysed in radioimmunoprecipitation assay buffer (Solarbio, Beijing, China) supplemented with phenylmethanesulfonyl fluoride (PMSF). The total protein content was measured using a bicinchoninic acid protein assay kit (Boster, Wuhan, China). Equivalent amounts of protein from each group of cells were separated by SDS‐PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Merck‐Millipore) for 60 min at 200 mA. After blocking for 3 h with 5% nonfat dried milk in Tris‐buffered saline supplemented with Tween (TBST), the PVDF membranes were incubated with antibodies against RUNX2 and OSX at ratios of 1:150 and 1:200, respectively, at 4°C overnight. Then, the membranes were incubated with secondary antibodies for 37°C for 1 h after washing the membrane three times with TBST. The relative expression levels of the proteins of interest were normalized to that of GAPDH.
Outcome Measures
The icariin and H2O2 dose was identified by CCK‐8 assay. Oxidative stress was measured by the level of SOD, GSH, and MDA. Early and late osteogenic capacity were assessed by ALP and ARS, respectively. Osteogenic‐related markers were measured by RT‐PCR and western blot analyses.
Statistical Analysis
The obtained data are expressed as the means ± standard deviation. Each experiment was repeated at least three times in triplicate. Statistical analyses were performed by one‐way ANOVA followed by a Bonferroni multiple comparison test for pairwise comparison. A P‐value <0.05 was considered significant.
Results
Icariin and H2O2 Dose Validation
After 24 h of exposure to 0.2 mM H2O2, the viability of MC3T3‐E1 cells (56.4% ± 5.6%) was significantly decreased compared to that of the control cells (100% ± 8%, P < 0.05, Fig. 1A). In contrast, the 0.1 μM icariin treatment significantly increased the viability of MC3T3‐E1 cells (148% ± 7%) compared to that of the control group (100% ± 8%, P < 0.05, Fig. 1B). We then treated MC3T3‐E1 cells with both H2O2 and icariin for 12, 24, 36, and 48 h to test whether icariin could alleviate H2O2‐induced cytotoxicity in MC3T3‐E1 cells. The results showed that the addition of icariin led to a significant recovery in the viability of MC3T3‐E1 cells in a time‐dependent manner (Fig. 1C).
Fig. 1.

Dose‐dependent effect of H2O2 and icariin on MC3T3‐E1 cells. (A) MC3T3‐E1 cell viability when treated with different concentrations of H2O2 (0.1 to 2 mM); (B) MC3T3‐E1 cell viability when cocultured with different doses of icariin (0.001 to 10 μM); (C) MC3T3‐E1 cell viability when cocultured with 0.2 mM H2O2 and 0.1 μM icariin from 0 to 48 h. *P < 0.05, compared with the control group.
Oxidative Stress Change
As shown in Fig. 2, the GSH (55% ± 8%) and SOD levels (59% ± 8%) in the H2O2‐treated group were significantly lower than those of the control group (GSH: 101.4% ± 11%, SOD: 105% ± 9.4%). However, the MDA level in the H2O2‐treated group (179.5% ± 9%) was significantly higher than that of control group (100% ± 9%, Fig. 2).
Fig. 2.

Icariin inhibits oxidative damage of MC3T3‐E1 cells. The glutathione (GSH) levels (A), superoxide dismutase (SOD) activity (B), and malondialdehyde (MDA) levels (C) in the control, 0.2 mM H2O2, 0.1 μM icariin, and 0.1 μM icariin combined with 0.2 mM H2O2 groups. *P < 0.05 vs control and #P < 0.05 vs 0.2 mM H2O2 treatment alone.
The groups cocultured with icariin exhibited SOD and GSH levels lower than those observed in the H2O2‐exposed group. In addition, the MDA level in the icariin and H2O2‐treated group was significantly lower than that observed in the H2O2‐treated group (P < 0.05).
Alkaline Phosphatase and Alizarin Red S Results
The H2O2‐induced inhibition of osteogenesis in MC3T3‐E1 cells in vitro was observed by microimaging of cells with ALP and ARS staining. In addition, icariin was observed to rescue the inhibition of osteogenesis mediated by H2O2. Furthermore, the quantitative analysis results of the ALP activity and calcium content in each group were in accordance with the microphotography results (Fig. 3A−C).
Fig. 3.

Icariin can reverse the inhibitory effects of H2O2 in early and late stages of MC3T3‐E1 cell osteogenic differentiation, as determined by alkaline phosphatase (ALP) staining and alizarin red S (ARS), respectively. (A) Representative ALP and ARS staining images assessed by general observation in the control, 0.2 mM H2O2, 0.1 μM icariin, and 0.1 μM icariin combined with 0.2 mM H2O2 groups and the NAC combined with 0.2 mM H2O2 group. The relative ALP activity (B) and calcium contents (C) in each group. (D) Relative mRNA expression levels of RUNX2 and OSX in the control, 0.2 mM H2O2, 0.1 μM icariin, and 0.1 μM icariin combined with 0.2 mM H2O2 groups and N‐acetylcysteine (NAC) combined with the 0.2 mM H2O2 group. *P < 0.05 vs control and #P < 0.05 vs 0.2 mM H2O2 group.
The RT‐PCR results are presented in Fig. 3D and showed that compared to those in the control group, RUNX2 and OSX mRNA levels in the H2O2‐treated group were significantly decreased. Furthermore, compared with the control group, the icariin group exhibited increased RUNX2 and OSX mRNA levels.
Wnt‐Signaling Pathway Change
Compared with the control group, the protein levels of β‐catenin and cyclin D1 in the H2O2‐treated MC3T3‐E1 group were significantly decreased (P< 0.05). Furthermore, compared with the control group, cells simultaneously treated with icariin and H2O2 had a similar β‐catenin and cyclin D1, with significance (P < 0.05, Fig. 4A and B).
Fig. 4.

Icariin activates the WNT/β‐catenin signaling pathway. (A). Quantitative analysis β‐catenin and cyclin D1 protein expression levels in each group (B). **P < 0.01 vs control, ##P < 0.01 vs 0.2 mM H2O2 treatment alone.
Our results showed that the calcium content was similar in the control and the 0.2 mM H2O2 + 0.1 μM icariin groups. However, the calcium content was decreased in the 0.2 mM H2O2 + 0.1 μM icariin + DKK‐1 group compared to that observed in the 0.2 mM H2O2 + 0.1 μM icariin group (Fig. 5A and B).
Fig. 5.

Inhibiting Wnt/β‐catenin signaling pathway with DKK1‐inhibited osteogenic differentiation of MC3T3‐E1. (A). Quantitative analysis of the calcium contents (B) and alkaline phosphatase (ALP) activity (C) of each group. *P < 0.05 vs control and #P < 0.05 vs 0.2 mM H2O2 and DKK‐1 treatment alone.
Osteogenic‐Related Protein Expression
As shown in Fig. 6, the relative expression of OSX and RUNX2 was significantly decreased, by 47.1% and 59.1%, respectively, in the 0.2 mM H2O2 + 0.1 μM icariin + DKK‐1 group than that observed in the 0.2 mM H2O2 + 0.1 μM icariin group (P < 0.05). In addition, the relative expression of OSX and RUNX2 was increased by 61.7% and 68.2%, respectively, in the 0.2 mM H2O2 + 0.1 μM icariin group than in the control group (P < 0.05).
Fig. 6.

DKK‐1 decreased RUNX2 and OSX protein level. A, Western blot analysis of RUNX2 and OSX expression in the control, 0.2 mM H2O2, and 0.1 μM icariin treatment groups and the group treated with of 0.2 mM H2O2, 0.1 μM icariin, and DKK‐1. B, Relative protein expression of RUNX2 and OSX normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH). *P < 0.05 vs control and #P < 0.05 vs 0.2 mM H2O2 and DKK‐1 treatment alone.
Discussion
Our results showed that icariin has protective effects against the H2O2‐induced inhibition of osteogenic differentiation in MC3T3‐E1 cells, primarily through the Wnt‐signaling pathway. First, we identified the optimal concentrations of H2O2 and icariin using the CCK‐8 assay. Then, we measured GSH, SOD, and MDA levels in these groups. We observed that icariin attenuated H2O2‐induced oxidative stress in MC3T3‐E1 cells. Furthermore, we revealed that icariin could activate Wnt signaling‐relevant proteins and promote osteogenesis. In the present study, we identified a mechanism for the icariin‐mediated regulation of the H2O2‐induced inhibition of osteogenic differentiation of osteoblasts. This is the first study to show the involvement of Wnt‐related genes in the oxidative stress response to icariin.
Oxidative stress greatly influences the viability, proliferation, and survival of osteocytes 29 . In this study, we used 0.2 mM H2O2 to generate a model of oxidative damage according to a previous study 30 . We observed that after treatment with 0.2 mM H2O2, the viability and osteogenic differentiation capacity of cells was decreased. Furthermore, we assessed GSH, SOD, and MDA levels to confirm the oxidative stress model 31 . The results showed that the GSH and SOD levels in the H2O2‐exposed group were significantly lower than those observed in the control group. In addition, the MDA levels in the icariin and H2O2‐treated group were significantly lower than those observed in the H2O2‐exposed group. MDA is closely associated with cell damage, and we observed that the MDA levels were significantly increased in the 0.2 mM H2O2 treatment group compared to those observed in the control group.
We used ALP and ARS staining to assess the early and late osteogenesis capacity of cells, respectively. When cocultured with H2O2, ALP and ARS staining were significantly decreased. We observed that, compared with the control group, icariin could enhance ALP and ARS staining. These results indicated that icariin could rescue osteogenesis caused by oxidative damage. Wu et al. 32 controlled the release of icariin for the treatment of bone defects and observed that icariin has an antiosteoporotic effect that promotes bone defect repair. Wang et al. 33 conducted a review on the clinical practice of icariin for PO and revealed that it was effective in preventing osteoporosis with relatively few side effects. Previous studies have shown that icariin has a regulatory effect on oxidative stress in vascular endothelial cells by inhibiting endoplasmic reticulum stress 34 . Ding et al. 29 showed that mangiferin can inhibit oxidative damage via the BMP‐2/Smad‐1 signaling pathway. Similarly, a study conducted by Xia et al. 35 revealed that mangiferin can protect osteoblasts against oxidative damage by targeting ERK5 and Nrf2. Taken together, these data suggest that antioxidant components protect bone loss by reducing oxidative stress.
Wnt‐β‐catenin signaling plays an important role in protection against oxidative damage induced apoptosis in MC3T3‐E1 cells 36 . DKK‐1 is an upstream antagonist of Wnt components 37 . DKK‐1 was used to inactivate the Wnt/β‐catenin pathway 38 . We observed that when the H2O2 and icariin‐treated cells were treated with DKK‐1, ARS staining and ALP staining decreased. In addition, we observed that in the presence of DKK‐1, Runx‐2, and OSX expression were decreased. Icariin can protect osteoblasts against oxidative damage by modulating Wnt signaling. Wang et al. 24 revealed that icariin increased the mRNA and protein expression levels of β‐catenin and cyclin D1, and these effects were inhibited by the Wnt/β‐catenin pathway inhibitor Dickkopf‐1. Liu et al. 39 showed that icariin could stimulate osteoblastic differentiation via the Wnt/β‐catenin pathway. In addition, Zofkova et al. 40 conducted a review and suggested that icariin could stimulate Wnt/β‐catenin signaling and regulate the osteoblast activity.
Conclusion
In summary, in this study, we demonstrated that icariin increases the viability of MC3T3‐E1 cells, reverses the oxidative stress induced by H2O2, and protects MC3T3‐E1 cells against the H2O2‐induced inhibition of osteogenic differentiation, which may occur through the Wnt signaling pathway. Future studies should focus on the specific mechanism of icariin in promoting osteogenesis in MC3T3‐E1 cells.
Disclosure: The authors declare that they have no competing interests.
References
- 1. Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C. The epidemiology of osteoporosis. Br Med Bull, 2020, 133: 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol, 2014, 142: 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou Q, Zhu L, Zhang D, et al. Oxidative stress‐related biomarkers in postmenopausal osteoporosis: a systematic review and meta‐analyses. Dis Markers, 2016, 2016: 7067984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao Z, Ma X, Ma J, Sun X, Li F, Lv J. Naringin enhances endothelial progenitor cell (EPC) proliferation and tube formation capacity through the CXCL12/CXCR4/PI3K/Akt signaling pathway. Chem Biol Interact, 2018, 286: 45–51. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Q, Zhao L, Shen Y, He Y, Cheng G, Yin M. Curculigoside protects against excess‐iron‐induced bone loss by attenuating Akt‐FoxO1‐dependent oxidative damage to mice and osteoblastic MC3T3‐E1 cells. Oxid Med Cell Longev, 2019, 2019: 9281481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Y, Bai B, Zhang Y. Bone abnormalities in young male rats with iron intervention and possible mechanisms. Chem Biol Interact, 2018, 279: 21–26. [DOI] [PubMed] [Google Scholar]
- 7. Wang T, Han C, Tian P, Li PF, Ma XL. Role of teriparatide in glucocorticoid‐induced osteoporosis through regulating cellular reactive oxygen species. Orthop Surg, 2018, 10: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li YP, Wu B, Liang J, Li F. Isopsoralen ameliorates H(2)O(2)‐induced damage in osteoblasts via activating the Wnt/β‐catenin pathway. Exp Ther Med, 2019, 18: 1899–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou X, Wang Z, Ni Y, Yu Y, Wang G, Chen L. Suppression effect of N‐acetylcysteine on bone loss in ovariectomized mice. Am J Transl Res, 2020, 12: 731–742. [PMC free article] [PubMed] [Google Scholar]
- 10. Li W, Zhang S, Liu J, Liu Y, Liang Q. Vitamin K2 stimulates MC3T3‐E1 osteoblast differentiation and mineralization through autophagy induction. Mol Med Rep, 2019, 19: 3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akçay H, Kuru K, Tatar B, Şimşek F. Vitamin E promotes bone formation in a distraction osteogenesis model. J Craniofac Surg, 2019, 30: 2315–2318. [DOI] [PubMed] [Google Scholar]
- 12. Nardone V, D'asta F, Brandi ML. Pharmacological management of osteogenesis. Clinics (Sao Paulo), 2014, 69: 438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lou J, Han D, Yu H, Yu G, Jin M, Kim SJ. Cytoprotective effect of taurine against hydrogen peroxide‐induced oxidative stress in UMR‐106 cells through the Wnt/β‐catenin signaling pathway. Biomol Ther (Seoul), 2018, 26: 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang W, Tang L, Wang G, et al. Molecular hydrogen protects human melanocytes from oxidative stress by activating Nrf2 signaling. J Invest Dermatol, 2020, 140: 2230–2241.e9. [DOI] [PubMed] [Google Scholar]
- 15. Park BK, Lee JH, Seo HW, et al. Icariin protects against radiation‐induced mortality and damage in vitro and in vivo. Int J Radiat Biol, 2019, 95: 1094–1102. [DOI] [PubMed] [Google Scholar]
- 16. El‐Shitany NA, Eid BG. Icariin modulates carrageenan‐induced acute inflammation through HO‐1/Nrf2 and NF‐kB signaling pathways. Biomed Pharmacother, 2019, 120: 109567. [DOI] [PubMed] [Google Scholar]
- 17. Ho MX, Poon CC, Wong KC, Qiu ZC, Wong MS. Icariin, but not Genistein, exerts osteogenic and anti‐apoptotic effects in osteoblastic cells by selective activation of non‐genomic ERα signaling. Front Pharmacol, 2018, 9: 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu T, Xiong Y, Luu S, et al. The shared KEGG pathways between icariin‐targeted genes and osteoporosis. Aging (Albany NY), 2020, 12: 8191–8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Zuo H, Liu X, Xiong J, Pei X. The antiosteoporosis effect of icariin in ovariectomized rats: a systematic review and meta‐analysis. Cell Mol Biol (Noisy‐le‐Grand), 2017, 63: 124–131. [DOI] [PubMed] [Google Scholar]
- 20. Hu J, Mao Z, He S, et al. Icariin protects against glucocorticoid induced osteoporosis, increases the expression of the bone enhancer DEC1 and modulates the PI3K/Akt/GSK3β/β‐catenin integrated signaling pathway. Biochem Pharmacol, 2017, 136: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang D, Ju C, Liu Y, Xu F, Wang Z, Wang D. Therapeutic effect of icariin combined with stem cells on postmenopausal osteoporosis in rats. J Bone Miner Metab, 2018, 36: 180–188. [DOI] [PubMed] [Google Scholar]
- 22. Mok SK, Chen WF, Lai WP, et al. Icariin protects against bone loss induced by oestrogen deficiency and activates oestrogen receptor‐dependent osteoblastic functions in UMR 106 cells. Br J Pharmacol, 2010, 159: 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song YH, Cai H, Zhao ZM, et al. Icariin attenuated oxidative stress induced‐cardiac apoptosis by mitochondria protection and ERK activation. Biomed Pharmacother, 2016, 83: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Wang R, Zhang F. Icariin promotes the proliferation and differentiation of osteoblasts from the rat mandible by the Wnt/betacatenin signalling pathway. Mol Med Rep, 2018, 18: 3445–3450. [DOI] [PubMed] [Google Scholar]
- 25. Saud B, Malla R, Shrestha K. A review on the effect of plant extract on mesenchymal stem cell proliferation and differentiation. Stem Cells Int, 2019, 2019: 7513404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun LJ, Li C, Wen XH, et al. Icariin stimulates hFOB 1.19 osteoblast proliferation and differentiation via OPG/RANKL mediated by the estrogen receptor. Curr Pharm Biotechnol, 2020, 21. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27. Fu JY, Jing Y, Xiao YP, Wang XH, Guo YW, Zhu YJ. Astaxanthin inhibiting oxidative stress damage of placental trophoblast cells in vitro. Syst Biol Reprod Med, 2020: 1–10. [DOI] [PubMed] [Google Scholar]
- 28. Chao C, Li F, Tan Z, Zhang W, Yang Y, Luo C. miR‐195 inhibited abnormal activation of osteoblast differentiation in MC3T3‐E1 cells via targeting RAF‐1. Exp Cell Res, 2018, 362: 293–301. [DOI] [PubMed] [Google Scholar]
- 29. Ding LZ, Teng X, Zhang ZB, Zheng CJ, Chen SH. Mangiferin inhibits apoptosis and oxidative stress via BMP2/Smad‐1 signaling in dexamethasone‐induced MC3T3‐E1 cells. Int J Mol Med, 2018, 41: 2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi BD, Lim DS, Lee SY, et al. Thymosin beta4 reduces H(2)O(2) induced oxidative stress in MC3T3‐E1 cells on titanium surface. J Nanosci Nanotechnol, 2018, 18: 893–897. [DOI] [PubMed] [Google Scholar]
- 31. Samarghandian S, Azimi‐Nezhad M, Farkhondeh T, Samini F. Anti‐oxidative effects of curcumin on immobilization‐induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother, 2017, 87: 223–229. [DOI] [PubMed] [Google Scholar]
- 32. Wu Y, Cao L, Xia L, et al. Evaluation of osteogenesis and angiogenesis of icariin in local controlled release and systemic delivery for calvarial defect in ovariectomized rats. Sci Rep, 2017, 7: 5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Z, Wang D, Yang D, Zhen W, Zhang J, Peng S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos Int, 2018, 29: 535–544. [DOI] [PubMed] [Google Scholar]
- 34. Wang FY, Jia J, Song HH, Jia CM, Chen CB, Ma J. Icariin protects vascular endothelial cells from oxidative stress through inhibiting endoplasmic reticulum stress. J Integr Med, 2019, 17: 205–212. [DOI] [PubMed] [Google Scholar]
- 35. Xia G, Li X, Zhu X, Yin X, Ding H, Qiao Y. Mangiferin protects osteoblast against oxidative damage by modulation of ERK5/Nrf2 signaling. Biochem Biophys Res Commun, 2017, 491: 807–813. [DOI] [PubMed] [Google Scholar]
- 36. Qi HH, Bao J, Zhang Q, et al. Wnt/β‐catenin signaling plays an important role in the protective effects of FDP‐Sr against oxidative stress induced apoptosis in MC3T3‐E1 cell. Bioorg Med Chem Lett, 2016, 26: 4720–4723. [DOI] [PubMed] [Google Scholar]
- 37. Ma S, Wang DD, Ma CY, Zhang YD. microRNA‐96 promotes osteoblast differentiation and bone formation in ankylosing spondylitis mice through activating the Wnt signaling pathway by binding to SOST. J Cell Biochem, 2019, 120: 15429–15442. [DOI] [PubMed] [Google Scholar]
- 38. Tang Y, Shen J, Zhang F, Yang FY, Liu M. Human serum albumin attenuates global cerebral ischemia/reperfusion‐induced brain injury in a Wnt/beta‐catenin/ROS signaling‐dependent manner in rats. Biomed Pharmacother, 2019, 115: 108871. [DOI] [PubMed] [Google Scholar]
- 39. Liu Y, Huang L, Hao B, Li R, et al. Use of an osteoblast overload damage model to probe the effect of icariin on the proliferation, differentiation and mineralization of MC3T3‐E1 cells through the Wnt/beta‐catenin signalling pathway. Cell Physiol Biochem, 2017, 41: 1605–1615. [DOI] [PubMed] [Google Scholar]
- 40. Zofkova I, Blahos J. New molecules modulating bone metabolism – new perspectives in the treatment of osteoporosis. Physiol Res, 2017, 66: S341–s347. [DOI] [PubMed] [Google Scholar]


