Abstract
The beneficial effects of long-chain polyunsaturated omega-3 fatty acids (omega-3 PUFAs) in cardioprotection are widely known and generally accepted. In this literature review, we have focused on the known and postulated mechanisms of action of omega-3 PUFAs and their metabolites on various components of the haemostatic system, in particular on blood platelets and endothelium. We have also made an attempt to provide a comprehensive review of epidemiological studies with particular regard to clinical trials. Notably, the results of these studies are contradictory, and some of them failed to report the beneficial effects of taking or supplementing omega-3 PUFAs in the diet. A potential explanation, in our opinion, could be the need to use higher doses of omega-3 PUFAs and a proper ratio of omega-3 and omega-6 PUFAs. An additional problem which is difficult to solve is the use of a proper neutral placebo for interventional studies. Despite some controversies regarding the beneficial effects of supplementation of omega-3 PUFAs in cardiovascular disease, our review suggests that a promising aspect of future studies and applications is to focus on the anti-thrombotic properties of these compounds. An argument supporting this assumption is the recent use of omega-3 PUFAs as a supporting tool for the treatment of COVID-19 complications.
Keywords: omega-3, PUFA, DHA, EPA, haemostasis, platelet, endothelium, cardiovascular, COVID-19
1. Introduction
The beneficial effects of long-chain polyunsaturated omega-3 fatty acids (omega-3 PUFAs) are widely accepted based on the evidence that supplementation with omega-3 PUFAs enhances cardioprotection in patients at cardiovascular risk or with cardiovascular disease (CVD) [1,2]. It is known that CVD results, among other causes, from the hyperreactivity of blood platelets, their enhanced aggregation and dysfunction of the endothelium [3,4,5]. The mechanisms of action of omega-3 PUFAs on haemostasis are complex, and their contribution to final outcomes has not been fully elucidated [6,7,8].
Haemostasis is defined as a balanced set of defence mechanisms of an organism allowing it to maintain blood circulation and the prevention of blood loss in the case of the integrity of blood vessels being broken. Additionally, the haemostatic system plays a role in the regulation of function of the vascular bed and immune response. Major components of haemostatic system are the vessel wall (including endothelium), blood platelets, coagulation and fibrinolytic systems [9].
There is increasing scientific evidence supporting a beneficial role of polyunsaturated fatty acids (PUFAs) in the prevention and treatment of CVD. Particular cardioprotective properties are attributed to PUFAs from the omega-3 group due to their anti-aggregatory and anti-inflammatory properties [10,11,12,13]. Current studies concerning the effects of omega-3 PUFAs on haemostasis focus predominately on blood platelets and endothelial cells. This is understandable, as according to the cell theory of the regulation of haemostasis, both platelets and endothelial cells are largely responsible for the pathophysiology of cardiovascular disease. Omega-3 PUFAs are known to affect both platelets and the endothelium. Thus, the inhibition of the procoagulant activity of both cell types is believed to be one of the most important mechanisms in cardioprotection [2].
In the case of patients with CVD, it is recommended to increase the supply of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which is intended to enhance the classical pharmacological treatment. As an example, the American Heart Association (AHA) recommends a supplementation of EPA and DHA in the form of fish oil in a dose of 2–4 g/day for patients with hypertriglyceridemia [14]. For all CVD patients, it is extremely important that the number of capsules intended to be consumed daily should reflect the recommended dose of EPA and DHA. Obviously, the manufacturers are obliged to provide information on the actual content of fatty acids contained in the formulation. However, a certain discrepancy sometimes exists between the declared and actual content. There are reports suggesting that such discrepancies are greater for DHA than for EPA [15]. Additionally, do Vale et al. described methodological problems associated with investigating the cardiovascular effects of omega-3 PUFAs. These problems are mainly due to the use of inappropriate (active) placebos in studies related to omega-3 PUFAs, which highlights the importance of choosing a truly inert placebo to maintain the high reliability of randomized clinical trials [16].
2. Metabolism and Mechanism of Action of Omega-3 PUFAs
Omega-3 and omega-6 PUFAs are components of phospholipids, which are constituents of cell membranes. Due to the catalytic action of phospholipase A2 (PLA2), these fatty acids are released from the membrane and become precursors of eicosanoids, such as prostaglandins, thromboxanes, leukotrienes or lipoxins, with a plethora of biological effects [17].
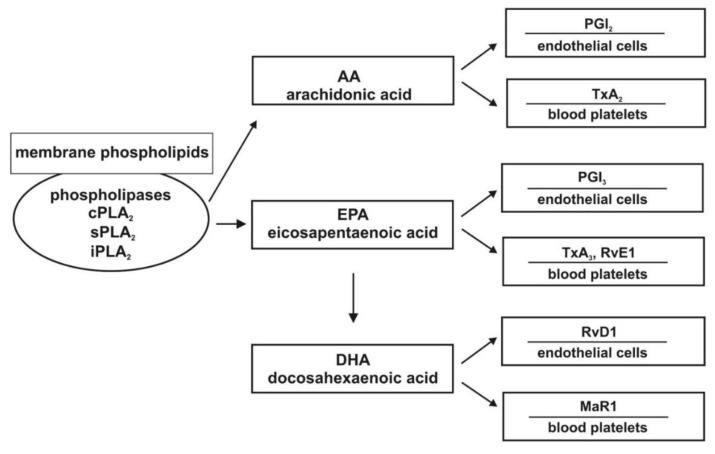
An important member of the omega-6 PUFAs is linoleic acid (LA), which is the precursor of arachidonic acid (AA) and characterized by pro-inflammatory properties. By contrast, ALA is a major fatty acid from the omega-3 group and is a precursor of EPA, which is a substrate for DHA. These fatty acids are characterized by anti-inflammatory properties [18,19,20]. The metabolic pathway of AA by cyclooxygenase (COX) results in the production of eicosanoids. The synthesis of pro-aggregatory thromboxane A2 (TXA2) takes place in blood platelets, whereas prostacyclin I2 (PGI2) is produced in the endothelium, with an antagonistic effect. Thromboxane A3 (TXA3) synthesized in platelets exhibits only slight pro-aggregatory activity. In turn, prostaglandin I3 (PGI3) produced in the endothelium possesses anti-aggregatory properties. TXA3 and PGI3 are products of the metabolism of EPA [17,18,20]. The anti-aggregatory effects of prostaglandins, especially PGI2 that has originated from AA, results from binding to the Gs protein-coupled receptor, which leads to an increase in intracellular cAMP concentration and the reduction of the synthesis of TXA2 [21,22,23,24]. Figure 1 presents the simplified metabolic pathways of AA, EPA, and DHA.
Figure 1.
Association between metabolites of unsaturated fatty acids and platelets, as well as endothelial cells. Abbreviations: PLA2: Phospholipase A2; MaR1: Maresin 1; PGI2: Prostacyclin; TXA2, TXA3: Thromboxanes; RvE1: Resolvin E1; RvD1: Resolvin D1; AA: Arachidonic acid; DHA: Docosahexaenoic acid; EPA: Eicosapentaenoic acid.
3. Omega-3 PUFAs and Blood Platelets
EPA and DHA play an important role in maintaining physiological haemostasis and, due to their antiplatelet and anticoagulant properties, reduce the risk of occurrence of cardiovascular events. EPA and DHA are incorporated into the cell membrane, altering its fluidity and thus regulating haemostasis, including thrombin generation [25,26,27]. EPA and DHA exert inhibitory effects on platelet aggregation. LA and ALA, in turn, compete with each other for enzymes (desaturases and elongases) essential for further metabolic processes. A higher dietary intake of ALA in comparison with LA leads to an enhanced synthesis of EPA and DHA metabolites, which results in the higher production of TXA3 than the pro-aggregatory TXA2. The possible effect is also an inhibition of cyclooxygenases as well as a direct antagonistic influence on the receptor for prostaglandin H2-TXA2. Prostaglandin H2 is a precursor of other prostaglandins and thromboxanes [9].
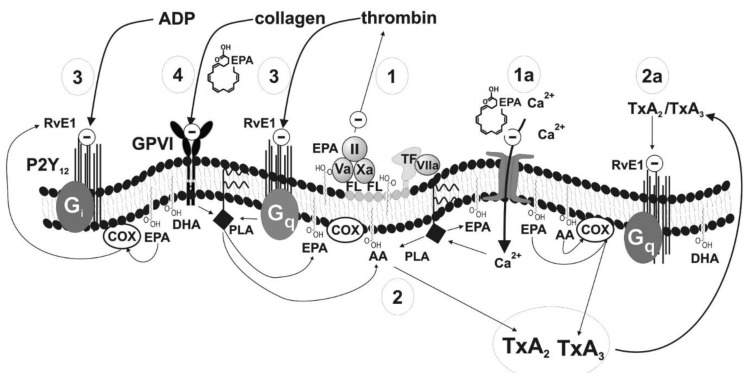
The presented mechanisms of the effects of omega-3 PUFAs (Figure 2) are described briefly below on the basis of published data (Table 1). Summarizing the information given in the table, it is widely accepted that blood platelets play an important role in haemostasis not only due to activation and aggregation but also as they are involved in thrombin generation. Thrombin is a strong platelet agonist but is also involved directly in thrombus formation. The excessive activation and aggregation of platelets can result in adverse cardiovascular events such as myocardial infarction or ischaemic stroke. Omega-3 PUFAs could enhance conventional therapy via the regulation of blood platelet function [28].
Figure 2.
Mechanism of antiplatelet effects of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and their metabolites. Abbreviations: II-Va-Xa: Factors of prothrombin complex; AA: Arachidonic acid; COX: Cyclooxygenase; FL: Membrane phospholipids; GPVI: Collagen receptor; Giq: G proteins; PLA: Phospholipase; P2Y12: ADP receptor; TF: Tissue factor; TXA2, TXA3: Thromboxanes; RvE1: Resolvin E1.
Table 1.
Potential mechanisms of antiplatelet effects of EPA and DHA.
| Number in Figure 2 | Haemostasis Component | Potential Mechanism | Effect | Strength of Evidence | Reference |
|---|---|---|---|---|---|
| 1 | Prothrombinase complex (factors Va, Xa, II) on the surface of blood platelets |
Decrease in cell membrane fluidity resulting in poor availability of procoagulant phospholipids (phosphatidylserine) | Slower conversion of prothrombin into thrombin; inhibition of coagulation cascade and platelet aggregation | Low | Larson [26] |
| 1a | Ionotropic calcium channels in plasma membrane | Decrease in cell membrane fluidity resulting in reduced influx of Ca2+ | Inhibition of blood platelets activation | Low | Kacik [31] |
| 2 | Synthesis of TxA2 | Competition between AA and EPA for enzymes essential for further metabolic steps | Inhibition of TxA2 synthesis leading to a production of TxA3 having weak pro-aggregatory properties; inhibition of TXA2-dependent platelet activation | High | DeFilippis Adili, Bäck [28,32,33] |
| 2a | Synthesis of TxA2 | Metabolite of EPA–resolvin binding to TxA2 receptor | Inhibition of TXA2-dependent platelet activation | Moderate | Sheikh, Bäck [33,34] |
| 3 | Receptor for ADP and receptor for thrombin | Metabolite of EPA–resolvin binding to receptors for ADP and thrombin | Inhibition of TXA2-and thrombin-dependent platelet activation | Moderate | Fredman, Dona [30,35] |
| 4 | Receptor GPVI (collagen receptor) | Blocking of the receptor (detailed mechanism unknown) or inhibition of platelet reactivity in a glycoprotein VI-dependent manner via activation of protein kinase A | Inhibition of collagen-dependent platelet activation | Low | Larson [36] Yamaguchi [8] |
Special attention should be paid to resolvins, which are so-called pro-resolving mediators (also termed specialized pro-resolving mediators (SPMs)) with anti-inflammatory properties. The substrates for their production are both EPA (resolvins E) and DHA (resolvins D). They contribute to the reduction of acute inflammatory response as well as inhibit thromboxane-induced platelet aggregation [29,30]. Additionally, DHA is a substrate for the generation of anti-inflammatory agents such as marisins. These substances, together with resolvins D, are classified as docasonoids [18,20]. In a recent publication, Yamaguchi et al. demonstrated that DHA 12-LOX oxylipins inhibited platelet reactivity in a glycoprotein VI-dependent manner due to the activation of protein kinase A. DHA and its 12-LOX-derived oxylipins also affected collagen-induced platelet aggregation. DHA and its oxylipins, 11-HDHA and 14-HDHA, were shown to regulate agonist-induced platelet aggregation [8].
4. Omega-3 PUFAs and Endothelium
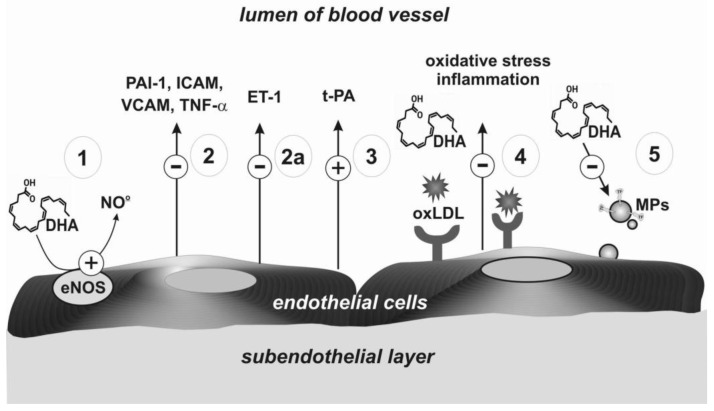
The beneficial effects of AA, EPA, and DHA metabolites have also been observed on endothelial cells. This is mainly due to the stimulating nitric oxide (NO) production [37,38]. The mechanisms are presented in Figure 3 and described in Table 2. The data summarised in Table 2 suggest that omega-3 PUFAs demonstrate a protective effect on the endothelium via both the NO synthesis and antioxidant, as well as anti-inflammatory properties. Altogether, this is important for the prevention and limiting of the development of atherosclerosis, and in consequence in the prevention of myocardial infarction and ischaemic stroke [38].
Figure 3.
Mechanisms of endothelium regulation mediated by EPA, DHA, and their metabolites. Abbreviations: ET-1: Endothelin 1; eNOS: Endothelial synthase of nitric oxide; ICAM: Intercellular adhesion molecule; MPs: Microparticles; oxLDL: Oxidized low-density lipoproteins; NO: Nitric oxide; PAI-1: Plasminogen activator inhibitor 1; t-PA: Tissue plasminogen activator; TNF-α: Tumour necrosis factor-α; VCAM: Vascular cell adhesion molecule.
Table 2.
Potential mechanisms of effects of EPA and DHA on endothelium.
| Number in Figure 3 | Haemostasis Component | Potential Mechanism | Effect | Strength of Evidence | Reference |
|---|---|---|---|---|---|
| 1 | Endothelial synthase of nitric oxide (eNOS) | Higher production of NO | Vasodilatation effect | High | Łacheta, Yamagata [39,40] |
| 2 | Adhesive receptors | Decrease of expression of adhesive molecules (VCAM, ICAM); reduced expression of tumor necrosis factor alpha (TNF-α) and PAI-1 | High | Wang, Liu [41,42] |
|
| 2a | Protein synthesis | Decreased synthesis of endothelin-1 | Anticoagulation effects | Moderate | Yamagata [40] |
| 3 | Receptor for t-PA | Higher level of t-PA | Fibrinolytic effects | Low | Din [43] |
| 4 | Receptor for oxLDL | Regulation of expression of the receptor for oxLDL | Inhibition of formation of oxidized LDL | High | Chen [44] |
| 5 | Inhibition of microparticles formation | Lower expression of tissue factor | Reduction of oxidative stress, inhibition of thrombin generation | Low | Qureshi [45] |
5. Epidemiological Studies and Clinical Trials
In the literature, there are many publications reporting the effects of omega-3 PUFAs supplementation in cardiovascular disease. The use of omega-3 PUFAs in the prevention of cardiac incidents (both primary and secondary) was addressed by relatively novel randomized clinical trials such as: JELIS [46], VITAL [47], STRENGTH [48], and ASCEND [49]. Other studies investigated not only the benefits of supplementation but also the potential risks [16,50,51]. However, since in this review we focus mainly on the effects of omega-3 PUFAs on haemostasis, below we will describe epidemiological studies concerning this particular issue.
The hypothesis that omega-3 PUFAs can affect haemostasis originated on the basis of the observations of a population of Inuits—native inhabitants of Greenland [52]—in whom a reduced reactivity of blood platelets, longer bleeding time, and a lowered ratio of thromboxanes to anti-aggregatory prostacyclins was found. The typical diet of Inuits is known for its high consumption of long-chain omega-3 PUFAs, especially DHA and EPA derived from fatty marine fish. The above-described hypothesis was confirmed in clinical studies [53].
In the work of McEwen et al., CVD patients and healthy controls were administered a 4-week supplementation of omega-3 PUFAs (640 mg/day). Interestingly, the reactivity and activation of blood platelets were lowered in healthy donors but not in CVD patients. The conclusion, and the suggestion of the authors, is the need to use higher doses of omega-3 PUFAs in these patients [23]. The concept of the application of higher doses of omega-3 PUFAs has been confirmed in a paper by Li et al. They reported that a supplementation of EPA as high as 6 g/day is required to reduce platelet adhesion [54].
Several studies carried out in humans have demonstrated that omega-3 PUFAs have antiplatelet properties. A meta-analysis of 15 randomised controlled trials provided evidence that omega-3 PUFAs inhibited blood platelet aggregation [22]. Marine omega-3 PUFAs were also observed to overcome aspirin resistance (acetylsalicylic acid (ASA)) [28,55]. In studies on laboratory animals, it was demonstrated that ASA enhanced the antiplatelet effect of fish oil [56]. In healthy but overweight men, 3 g of omega-3 PUFAs administered for 4 weeks lowered the concentrations of fibrinogen, thrombin, and factor V. Interestingly, these effects occurred mainly in subjects who were carriers of alpha-chain fibrinogen polymorphism [57]. It was found that, in a double-blind placebo-controlled study in 30 healthy subjects taking 2.52 g/day of omega-3 PUFAs as compared with 1.26 g/day for 5 weeks, the group with a higher dose of omega-3 PUFAs displayed decreased plasma viscosity, red blood cell rigidity, and systolic blood pressure [58]. In the study performed on healthy adults, it was reported that fish oil (equivalent to 6 g of EPA/day), but not vegetable oil, significantly reduced platelet adhesion [54]. In another work, it was found that supplementation with 3.6 g of omega-3 PUFAs from fish oil lowered platelet aggregation, whereas 25 g of soy lecithin (providing 1.5 g omega-6 and 0.5 g omega-3 PUFAs) enhanced platelet reactivity [59]. The omega-6/omega-3 ratio in platelets is also positively correlated with platelet adhesion both in resting platelets, as well as after the activation of platelets by agonists such as ADP and thrombin. Therefore, it seems that higher doses of marine omega-3 PUFAs result in more effective antithrombotic benefits.
In the trial REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial), 8179 patients with hypertriglyceridemia and co-existing diabetes mellitus or additional cardiovascular disorders were studied. The patients were treated with statins and randomized in a double-blind manner to either icosapent ethyl at 4 g/day (2 g twice daily with meals) or placebo groups [60]. Despite the fact that this trial did not analyse the effect of icosapentethyl on haemostasis, the results of this study could be treated as a suggestion of the need to use high doses of EPA derivatives. In other studies, a reduction of the incidence of adverse cardio-vascular events of 25% was observed [6,60,61]. On the other hand, in another double-blind trial on patients with hypertension and diabetes, it was found that only DHA significantly reduced platelet aggregation induced with pro-coagulatory factors [62].
It is noteworthy that the proper ratio of omega-6 and omega-3 PUFAs in a diet is also very important [63]. It was demonstrated that a high omega-6/omega-3 ratio enhanced platelet aggregation. It seems that omega-6 PUFAs have a pro-coagulatory and pro-inflammatory activity. LA, belonging to the omega-6 group, lowers low-density lipoprotein (LDL) levels. On the other hand, it increases the predisposition of LDL to oxidation. This is a dangerous process, leading to a higher risk of coronary artery disease. It is believed that omega-3 and omega-6 PUFAs should be balanced when they are taken in a diet in a ratio of about 1 to 1 [64].
Supplementation with omega-3 and omega-6 PUFAs is used to limit the progression of cardiovascular disease and is applied both in primary as well as secondary prevention [28]. Experimental studies also suggest that EPA and DHA inhibit platelet aggregation in vitro. However, DHA exerts a stronger effect on the platelet function [28]. Despite the fact that epidemiological data suggest a diet rich in omega-3 PUFAs is effective in CVD prevention, due to the anti-inflammatory and anti-platelet activity [28], some authors question the effectiveness of such a supplementation [50,65,66]. The lack of unambiguous recommendations regarding the supplementation results also from the controversial results of studies investigating the effect of omega-3 PUFAs on haemostasis. The review of the literature reveals that not all the publications confirm the beneficial effects of omega-3 PUFAs on haemostasis. Poreba et al. investigated the impact of the daily supplementation of 2 g omega-3 PUFAs in patients with advanced atherosclerosis and diabetes on platelet function and fibrinolysis, as well as thrombus formation. The study did not yield any positive results [67]. Similarly, this effect was not demonstrated in healthy volunteers [68].
The studies performed by our group demonstrated that a higher consumption of omega-3 PUFAs had a significant inhibitory effect on inflammatory markers, whereas blood platelet reactivity was not affected [69,70]. However, the lack of a significant effect of omega-3 PUFAs on the platelet function in these studies can be explained by the insufficient consumption of omega-3 PUFAs (EPA + DHA below 1 g/day). This hypothesis is in accordance with the results of Sing et al., who reported that supplementation of EPA and DHA in a dose of 1.24 g/day inhibited the inflammatory response, which manifested as a reduced level of C-reactive protein in serum [71]. Assuming the hypothesis that an anti-aggregatory effect of omega-3 PUFAs is mediated also via a mechanism mediated by resolvins, it seems that a diet or a supplementation should contain higher doses than those described above for the substrates of resolvins, i.e., EPA and DHA. With regards to the anti-aggregatory potential, the most interesting resolvin appears to be resolvin E1 (RvE1) [29,72]. RvE1 is a metabolite of EPA and was found to inhibit ADP-induced platelet aggregation [35].
The diet supplementation of patients suffering from coronary artery disease with omega-3 PUFAs in a dose of 2 g/day was found to have significant effects on the endothelium function or the secretion of tissue plasminogen activator (t-PA), which plays a role in a plasmin production, limiting thrombus formation [43]. In another randomized study, patients with peripheral artery disease were supplemented with either fish oil for 6 weeks (with a daily dose of EPA and DPA of 850–882 mg) or a placebo. The result was that no significant effect of this supplementation was observed with respect to the blood platelet activity, endothelium function, as well as inflammatory markers [73]. He et al. evaluated the influence of the consumption of fried and unfried fish as well as the consumption of omega-3 PUFAs on the endothelium function and inflammatory marker levels. It was found that the intake of omega-3 PUFAs was associated with a lower level of pro-inflammatory interleukin-6 (IL-6), irrespective of the remaining parameters evaluated in the patients. On the other hand, lower levels of C-reactive protein (CRP) and IL-6 were associated with the consumption of unfried fish, whereas the consumption of the fried fish resulted in a decrease of intercellular adhesion molecule (ICAM) expression. Therefore, it seems that the consumption of fish and omega-3 PUFAs leads to a cardioprotective effect resulting from the influence on the endothelium, as well as to a lowering of the level of inflammatory markers and expression of adhesive proteins [37].
The normal function of the vascular endothelium plays an important role in the prevention of arterial disorders such as atherosclerosis. A dysfunction of the endothelium is associated with an impaired generation of NO, which normally exerts a vasodilatory effect. The protective effects of statins—drugs applied to treat dyslipidaemia as well as EPA—were demonstrated in [74]. In this study, the dual effect of statins (a metabolite of atorvastatin (ATM)) and EPA was assessed on the condition of an endothelium subjected to oxidative stress. It was found that the combined therapy yielded more beneficial results in comparison to ATM or EPA alone. Moreover, the same effect was not observed when EPA was replaced by DHA or when using other drugs which lower triglycerides levels, e.g., fenofibrate or gemfibrozil. This finding seems to be of special importance for disorders in which endothelium dysfunction leads to the development of cardiovascular disease [74].
The list of epidemiological studies and clinical trials, including author, year, type of study, source and dose of omega-3 PUFAs, duration of supplementation or observation, outcome, and commentary is presented in Supplementary Table S1.
Adverse Effects
Due to the effects of omega-3 PUFAs on haemostasis, there is a potential risk of bleeding.
With regards to a risk of possible bleeding as an adverse effect of omega-3 PUFAs supplementation (individual or used as an enhancement of standard therapy), currently no clinically relevant indications have been suggested [75]. It has been shown that there is no higher risk of bleeding as a post-surgical complication in patients taking only fish oil and no additional anticoagulants [76]. Interestingly, there is a study on the possible occurrence of arrhythmias as an effect of supplementation. The authors suggest a need to precisely document the occurrence of arrhythmias in both present and future trials in order to assess the possible adverse effects of supplementation on nonfatal arrhythmias [77].
6. Novel Perspectives of Omega-3 PUFAs
Despite some controversies regarding the cardiovascular effects of omega-3 PUFAs, mainly regarding their role in dyslipidaemias and ventricular arrhythmias [16,50,67], there is increasing evidence of their anti-thrombotic properties. The work of Bonutti et al. described the synergistic anti-thrombotic effect of a combined therapy using aspirin and fish oil in the prevention of venous thromboembolism (VTE) after surgery in patients suffering from primary total knee arthroplasty. The results of this study suggest that the simultaneous application of aspirin and fish oil can be a good approach for the prevention of thrombosis in patients after surgery [78]. Recently, the anti-thrombotic and anti-inflammatory activity of omega-3 PUFAs has been observed in patients at risk of VTE [79,80]. According to the study of Isaksen et al., a high intake of marine omega-3 PUFAs in the diet was associated with a reduced risk of VTE after unprovoked index events [81]. In another study, it was demonstrated that omega-3 PUFAs consumption leads to a lower risk of both VTE and recurrent VTE [82]. There is also a discussion of the ability of omega-3 PUFAs to decrease the risk of deep-vein thrombosis and pulmonary embolism after surgical procedures [80,83].
Currently, there are a few publications analysing the antithrombotic properties of omega-3 PUFAs in patients hospitalized due to COVID-19 [84,85]. It is known that coagulopathy is frequently observed in many—especially severe—cases of COVID-19 and manifests as disseminated intravascular coagulation (DIC). The International Society of Thrombosis and Haemostasis (ISTH) has suggested the introduction of a new category for the early phase of sepsis-associated disseminated intravascular coagulation, called “sepsis-induced coagulopathy” (SIC) [85]. Currently, there are no results clearly suggesting the beneficial effects of omega-3 PUFAs in COVID-19 patients. However, it seems likely that the dietary intake of omega-3 PUFAs and/or their metabolites can exert positive effects contributing to the prevention and management of cardiovascular and thrombotic complications in patients suffering from COVID-19. Omega-3 PUFAs can affect and modulate some of the adverse effects of the immune overresponse, limit coagulopathy, and affect cell signalling and gene expression [86]. Omega-3 PUFAs are well known to have antithrombotic, anti-inflammatory, and pro-resolving properties that can yield positive effects in COVID-19 patients [87]. Since beneficial effects of fish oil/omega-3 PUFAs in limiting thrombosis have been confirmed in many disorders and clinical settings, it now appears logical to investigate the potential role of fish oil/omega-3 PUFAs supplementation as an adjunct to pharmacotherapy in COVID-19 patients at risk of vascular thrombotic complications [84].
7. Concluding Remarks
In the presence of some contradictory results, we suggest a novel potential use of fish oil. One of the aims of this paper was to indicate a new possibility—the support of a traditional anticoagulant treatment with the supplementation of EPA and DHA contained in fish oil. We believe it is a promising but still insufficiently explored research field. It seems likely that EPA and DHA being the sources of immunomodulatory metabolites (suppressing the inflammatory response) [88] could increase the effectiveness of antiplatelet drugs [89].
In our opinion, the recommended duration of the supplementation highly depends on the type and objective of the study and obviously on the group of the patients/subjects. In this paper, we have discussed both studies investigating the mechanisms of action as well studies focused on determining beneficial effects on health. For EPA and DHA, it is necessary to obtain maximum metabolite concentrations (2–4 h) [88], as well as to determine the duration of exposure for specific research subjects. The supplementation time among healthy adults should be in the range of 15 days [59] to 6 weeks [90].
While assessing the effect of fish oil supplementation on hemostatic parameters, platelet viability (10 days) and short half-lives of clotting factors (up to 5 days) should be taken into consideration. Supplementation in this case (minimum 4 g per day) should last no less than 2 weeks. On the other hand, assessing the effect of EPA/DHA metabolites on vascular endothelium, we recommend supplementation to last 6 weeks.
For the analysis of omega-3 PUFAs effects on health, the time of the supplementation should be significantly longer. Clinical trials ranged on average from 4 weeks to 6 years of observation (with a 90-day intervention), and in some cases the observation lasted even 10 years [81]. An analysis of the available literature suggests that the minimum duration of supplementation should be no less than 6 weeks.
With regards to a dose of omega-3 PUFAs required for the supplementation, it is assumed that fish oil effectively inhibits platelet adhesion, obtaining a maximum beneficial effect, with a daily amount of EPA of about 6 g [28,54,59]. Based on the results of the REDUCE-IT study (including icosapent ethyl at 4 g/day) [60] and the efficacy of a single dose of 4.5 g/day [88], we assume that supplementation should be no lower than 4 g/day. In the study by Souza et al., plasma specialized pro-resolving mediator (SPM) levels were analyzed in healthy volunteers. An increase in SPM concentration was observed as a function of dose and duration of supplementation, obtaining a peak within 2–4 h after supplementation. A significant supplementation effect on the organism’s immune response was noted at a dose level of 4.5 g [88]. The importance of the proper dose of omega-3 PUFAs is also evident in the light of the recent meta-analysis by Bernasconi et al., who demonstrated that supplementation with omega-3 PUFAs resulted in a decreased risk of myocardial infarction. Such a trend was not observed in the case of cardiovascular events. In both cases, these observations were dependent on the supplemented doses of EPA and DHA that ranged from 400 to 5500 mg/day (data from 40 studies). The risk of myocardial infarction was significantly reduced by 9% for each additional dose of 1 g per day. Omega-3 PUFAs seem to be much more effective in a population with higher cardiac risk [51].
In general, in our opinion, patients being on a Western diet characterized by generally lower omega-3 PUFAs intake may require a higher dose of PUFAs (>4 g/day EPHA) and 6-week supplementation in order to obtain a significant outcome of omega-3 PUFAs effects on haemostasis.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/5/2394/s1.
Author Contributions
Concept of the paper, literature search, preparing figures and tables, J.G.; writing a first draft of the manuscript, P.S.; editing and revision of the manuscript, writing the final version of the manuscript, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Lodz, Poland (503/6-020-01/503-61-001-19-00).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siscovick D.S., Barringer T.A., Fretts A.M., Wu J.H.Y., Lichtenstein A.H., Costello R.B., Kris-Etherton P.M., Jacobson T.A., Engler M.B., Alger H.M., et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease. Circulation. 2017;135:e867–e884. doi: 10.1161/CIR.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Innes J.K., Calder P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020;21:1362. doi: 10.3390/ijms21041362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Incalza M.A., D’Oria R., Natalicchio A., Perrini S., Laviola L., Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Tsoupras A., Lordan R., Zabetakis I. The Impact of Nutrition and Statins on Cardiovascular Diseases. Academic Press; Cambridge, MA, USA: 2019. Inflammation and Cardiovascular Diseases; pp. 53–117. [Google Scholar]
- 5.Khodadi E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2019;20:1–10. doi: 10.1007/s12012-019-09555-4. [DOI] [PubMed] [Google Scholar]
- 6.Mason R.P., Libby P., Bhatt D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arter. Thromb. Vasc. Biol. 2020;40:1135–1147. doi: 10.1161/ATVBAHA.119.313286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adili R., Voigt E.M., Bormann J.L., Foss K.N., Hurley L.J., Meyer E.S., Veldman A.J., Mast K.A., West J.L., Whiteheart S.W., et al. In vivo modeling of docosahexaenoic acid and eicosapentaenoic acid-mediated inhibition of both platelet function and accumulation in arterial thrombi. Platelets. 2019;30:271–279. doi: 10.1080/09537104.2017.1420154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi A., Stanger L., Freedman C.J., Standley M., Hoang T., Adili R., Tsai W., Van Hoorebeke C., Holman T.R., Holinstat M. DHA 12-LOX-derived oxylipins regulate platelet activation and thrombus formation through a PKA-dependent signaling pathway. J. Thromb. Haemost. 2020 doi: 10.1111/jth.15184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capurso A., Capurso C. Principles of Nutrigenetics and Nutrigenomics. Elsevier; Amsterdam, The Netherlands: 2020. Hemostasis and Thrombosis; pp. 361–369. [Google Scholar]
- 10.Bowen K.J., Harris W.S., Kris-Etherton P.M. Omega-3 Fatty Acids and Cardiovascular Disease: Are There Benefits? Curr. Treat. Options Cardiovasc. Med. 2016;18:1–16. doi: 10.1007/s11936-016-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe Y., Tatsuno I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020;27:183–198. doi: 10.5551/jat.50658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thota R.N., Ferguson J.J.A., Abbott K.A., Dias C.B., Garg M.L. Science behind the cardio-metabolic benefits of omega-3 polyunsaturated fatty acids: Biochemical effectsvs. clinical outcomes. Food Funct. 2018;9:3576–3596. doi: 10.1039/C8FO00348C. [DOI] [PubMed] [Google Scholar]
- 13.Manson J.E., Bassuk S.S., Cook N.R., Lee I.-M., Mora S., Albert C.M., Buring J.E., VITAL Research Group Vitamin D, Marine n-3 Fatty Acids, and Primary Prevention of Cardiovascular Disease Current Evidence. Circ. Res. 2020;126:112–128. doi: 10.1161/CIRCRESAHA.119.314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skulas-Ray A.C., Wilson P.W., Harris W.S., Brinton E.A., Kris-Etherton P.M., Richter C.K., Jacobson T.A., Engler M.B., Miller M., Robinson J.G., et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation. 2019;140:e673–e691. doi: 10.1161/CIR.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 15.Osadnik K., Jaworska J. Analysis of ω-3 fatty acid content of polish fish oil drug and dietary supplements. Acta Pol. Pharm. Drug Res. 2016;73:875–883. [PubMed] [Google Scholar]
- 16.Vale F.M.D., Diógenes M.J., Barbacena H.A. Controversies about the cardiovascular effects of OM3FA. Did inappropriate placebos skew clinical trial results? Pharmacol. Res. 2021;164:105368. doi: 10.1016/j.phrs.2020.105368. [DOI] [PubMed] [Google Scholar]
- 17.Piper K., Garelnabi M. Eicosanoids: Atherosclerosis and cardiometabolic health. J. Clin. Transl. Endocrinol. 2020;19:100216. doi: 10.1016/j.jcte.2020.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saini R.K., Keum Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 19.Shahidi F., Ambigaipalan P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018;9:345–381. doi: 10.1146/annurev-food-111317-095850. [DOI] [PubMed] [Google Scholar]
- 20.Endo J., Arita M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016;67:22–27. doi: 10.1016/j.jjcc.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Miller G.J. Dietary fatty acids and the haemostatic system. Atherosclerosis. 2005;179:213–227. doi: 10.1016/j.atherosclerosis.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Gao L.-G., Cao J., Mao Q.-X., Lu X.-C., Zhou X.-L., Fan L. Influence of omega-3 polyunsaturated fatty acid-supplementation on platelet aggregation in humans: A meta-analysis of randomized controlled trials. Atherosclerosis. 2013;226:328–334. doi: 10.1016/j.atherosclerosis.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 23.McEwen B., Morel-Kopp M.-C., Chen W., Tofler G., Ward C. Effects of Omega-3 Polyunsaturated Fatty Acids on Platelet Function in Healthy Subjects and Subjects with Cardiovascular Disease. Semin. Thromb. Hemost. 2013;39:25–32. doi: 10.1055/s-0032-1333309. [DOI] [PubMed] [Google Scholar]
- 24.Paes A.M.D.A., Gaspar R.S., Fuentes E., Wehinger S., Palomo I., Trostchansky A. Lipid Metabolism and Signaling in Platelet Function. Single Mol. Single Cell Seq. 2019;1127:97–115. doi: 10.1007/978-3-030-11488-6_7. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto M., Hossain S., Shido O. Docosahexaenoic acid but not eicosapentaenoic acid withstands dietary cholesterol-induced decreases in platelet membrane fluidity. Mol. Cell. Biochem. 2006;293:1–8. doi: 10.1007/s11010-006-0164-x. [DOI] [PubMed] [Google Scholar]
- 26.Larson M.K., Tormoen G.W., Weaver L.J., Luepke K.J., Patel I.A., Hjelmen C.E., Ensz N.M., McComas L.S., Mccarty O.J.T. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am. J. Physiol. Physiol. 2013;304:C273–C279. doi: 10.1152/ajpcell.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwen B., Morel-Kopp M.-C., Tofler G., Ward C. The Effect of Omega-3 Polyunsaturated Fatty Acids on Fibrin and Thrombin Generation in Healthy Subjects and Subjects with Cardiovascular Disease. Semin. Thromb. Hemost. 2015;41:315–322. doi: 10.1055/s-0034-1395352. [DOI] [PubMed] [Google Scholar]
- 28.Adili R., Hawley M., Holinstat M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018;139:10–18. doi: 10.1016/j.prostaglandins.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowak J.Z. Przeciwzapalne „prowygaszeniowe” pochodne wielonienasyconych kwasów tłuszczowych omega 3 i omega 6* Anti-inflammatory pro-resolving derivatives of omega-3 and omega-6 polyunsaturated fatty acids. Postepy Hig. Med. Dosw. 2010;64:115–132. [PubMed] [Google Scholar]
- 30.Dona M., Fredman G., Schwab J.M., Chiang N., Arita M., Goodarzi A., Cheng G., Von Andrian U.H., Serhan C.N. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kacik M., Olivan-Viguera A., Köhler R. Modulation of KCa3.1 Channels by Eicosanoids, Omega-3 Fatty Acids, and Molecular Determinants. PLoS ONE. 2014;9:e112081. doi: 10.1371/journal.pone.0112081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeFilippis A., Rai S.N., Cambon A., Miles R., Jaffe A.S., Moser A.B., O Jones R., Bolli R., Schulman S.P. Fatty acids and TxA2 generation, in the absence of platelet-COX-1 activity. Nutr. Metab. Cardiovasc. Dis. 2014;24:428–433. doi: 10.1016/j.numecd.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bäck M. Omega-3 fatty acids in atherosclerosis and coronary artery disease. Futur. Sci. OA. 2017;3:FSO236. doi: 10.4155/fsoa-2017-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheikh O., Hei A.G.V., Battisha A., Hammad T., Pham S., Chilton R. Cardiovascular, electrophysiologic, and hematologic effects of omega-3 fatty acids beyond reducing hypertriglyceridemia: As it pertains to the recently published REDUCE-IT trial. Cardiovasc. Diabetol. 2019;18:1–12. doi: 10.1186/s12933-019-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fredman G., Van Dyke T.E., Serhan C.N. Resolvin E1 Regulates Adenosine Diphosphate Activation of Human Platelets. Arter. Thromb. Vasc. Biol. 2010;30:2005–2013. doi: 10.1161/ATVBAHA.110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larson M.K., Shearer G.C., Ashmore J.H., Anderson-Daniels J.M., Graslie E.L., Tholen J.T., Vogelaar J.L., Korth A.J., Nareddy V., Sprehe M., et al. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot. Essent. Fat. Acids. 2011;84:93–98. doi: 10.1016/j.plefa.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He K., Liu K., Daviglus M.L., Jenny N.S., Mayer-Davis E., Jiang R., Steffen L., Siscovick D., Tsai M., Herrington D. Associations of Dietary Long-Chain n-3 Polyunsaturated Fatty Acids and Fish With Biomarkers of Inflammation and Endothelial Activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am. J. Cardiol. 2009;103:1238–1243. doi: 10.1016/j.amjcard.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamagata K. Prevention of Endothelial Dysfunction and Cardiovascular Disease by n-3 Fatty Acids-Inhibiting Action on Oxidative Stress and Inflammation. Curr. Pharm. Des. 2020;26:3652–3666. doi: 10.2174/1381612826666200403121952. [DOI] [PubMed] [Google Scholar]
- 39.Łacheta D., Olejarz W., Włodarczyk M., Nowicka G. Effect of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) on the regulation of vascular endothelial cell function. Postępy Higieny i Medycyny Doświadczalnej. 2019;73:467–475. doi: 10.5604/01.3001.0013.5064. [DOI] [Google Scholar]
- 40.Yamagata K. Docosahexaenoic acid regulates vascular endothelial cell function and prevents cardiovascular disease. Lipids Health Dis. 2017;16:1–13. doi: 10.1186/s12944-017-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T.-M., Chen C.-J., Lee T.-S., Chao H.-Y., Wu W.-H., Hsieh S.-C., Sheu H.-H., Chiang A.-N. Docosahexaenoic acid attenuates VCAM-1 expression and NF-κB activation in TNF-α-treated human aortic endothelial cells. J. Nutr. Biochem. 2011;22:187–194. doi: 10.1016/j.jnutbio.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Liu K.-L., Yang Y.-C., Yao H.-T., Chia T.-W., Lu C.-Y., Li C.-C., Tsai H.J., Lii C.-K., Chen H.-W. Docosahexaenoic acid inhibits inflammation via free fatty acid receptor FFA4, disruption of TAB2 interaction with TAK1/TAB1 and downregulation of ERK-dependent Egr-1 expression in EA.hy926 cells. Mol. Nutr. Food Res. 2016;60:430–443. doi: 10.1002/mnfr.201500178. [DOI] [PubMed] [Google Scholar]
- 43.Din J.N., Sarma J., Harding S.A., Lyall K., Newby D.E., Flapan A.D. Effect of ω-3 fatty acid supplementation on endothelial function, endogenous fibrinolysis and platelet activation in patients with a previous myocardial infarction: A randomised controlled trial. BMJ Open. 2013;3:e003054. doi: 10.1136/bmjopen-2013-003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H. EPA and DHA attenuate ox-LDL-induced expression of adhesion molecules in human coronary artery endothelial cells via protein kinase B pathway. J. Mol. Cell. Cardiol. 2003;35:769–775. doi: 10.1016/S0022-2828(03)00120-2. [DOI] [PubMed] [Google Scholar]
- 45.Qureshi A., AlTamimy R., El Habhab A., El Itawi H., Farooq M., Zobairi F., Hasan H., Amoura L., Kassem M., Auger C., et al. Ageing enhances the shedding of splenocyte microvesicles with endothelial pro-senescent effect that is prevented by a short-term intake of omega-3 PUFA EPA:DHA 6:1. Biochem. Pharmacol. 2020;173:113734. doi: 10.1016/j.bcp.2019.113734. [DOI] [PubMed] [Google Scholar]
- 46.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 47.Manson J.E., Cook N.R., Lee I.-M., Christen W., Bassuk S.S., Mora S., Gibson H., Albert C.M., Gordon D., Copeland T., et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholls S.J., Lincoff A.M., Bash D., Ballantyne C.M., Barter P.J., Davidson M.H., Kastelein J.J.P., Koenig W., McGuire D.K., Mozaffarian D., et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: Rationale and design of the STRENGTH trial. Clin. Cardiol. 2018;41:1281–1288. doi: 10.1002/clc.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ascend Study Collaborative Group. Bowman L., Mafham M., Wallendszus K., Stevens W., Buck G., Barton J., Murphy K., Aung T., Haynes R., et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018;379:1540–1550. doi: 10.1056/NEJMoa1804989. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y., Bo Y., Liu Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019;18:1–14. doi: 10.1186/s12944-019-1035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernasconi A.A., Wiest M.M., Lavie C.J., Milani R.V., Laukkanen J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes. Mayo Clin. Proc. 2021;96:304–313. doi: 10.1016/j.mayocp.2020.08.034. [DOI] [PubMed] [Google Scholar]
- 52.Dyerberg J., Bang H. Hæmostatic function and platelet polyunsaturated fatty acids in eskimos. Lancet. 1979;314:433–435. doi: 10.1016/S0140-6736(79)91490-9. [DOI] [PubMed] [Google Scholar]
- 53.Holub B.J. Dietary fish oils containing eicosapentaenoic acid and the prevention of atherosclerosis and thrombosis. Can. Med Assoc. J. 1988;139:377–381. [PMC free article] [PubMed] [Google Scholar]
- 54.Li X.L., Steiner M. Dose response of dietary fish oil supplementations on platelet adhesion. Arter. Thromb. A J. Vasc. Biol. 1991;11:39–46. doi: 10.1161/01.ATV.11.1.39. [DOI] [PubMed] [Google Scholar]
- 55.Mărginean A., Bănescu C., Scridon A., Dobreanu M. Anti-platelet Therapy Resistance—Concept, Mechanisms and Platelet Function Tests in Intensive Care Facilities. J. Crit. Care Med. 2016;2:6–15. doi: 10.1515/jccm-2015-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gong Y., Lin M., Piao L., Li X., Yang F., Zhang J., Xiao B., Zhang Q., Song W.-L., Yin H., et al. Aspirin enhances protective effect of fish oil against thrombosis and injury-induced vascular remodelling. Br. J. Pharmacol. 2014;172:5647–5660. doi: 10.1111/bph.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanschoonbeek K., Feijge M.A., Paquay M., Rosing J., Saris W., Kluft C., Giesen P.L., De Maat M.P., Heemskerk J.W. Variable Hypocoagulant Effect of Fish Oil Intake in Humans. Arter. Thromb. Vasc. Biol. 2004;24:1734–1740. doi: 10.1161/01.ATV.0000137119.28893.0b. [DOI] [PubMed] [Google Scholar]
- 58.Bach R., Schmidt U., Jung F., Kiesewetter H., Hennen B., Wenzel E., Schieffer H., Bette L., Heyden S. Effects of Fish Oil Capsules in Two Dosages on Blood Pressure, Platelet Functions, Haemorheological and Clinical Chemistry Parameters in Apparently Healthy Subjects. Ann. Nutr. Metab. 1989;33:359–367. doi: 10.1159/000177559. [DOI] [PubMed] [Google Scholar]
- 59.Carletto A., Guarini P., Galvani S., Biasi D., Bellavite P., Corrocher R., Andrioli G. Differential Effects of Dietary Supplementation with Fish Oil or Soy Lecithin on Human Platelet Adhesion. Thromb. Haemost. 1999;82:1522–1527. doi: 10.1055/s-0037-1614865. [DOI] [PubMed] [Google Scholar]
- 60.Bhatt D.L., Miller M., Brinton E.A., Jacobson T.A., Steg P.G., Ketchum S.B., Doyle R.T., Juliano R.A., Jiao L., Granowitz C., et al. REDUCE-IT USA: Results from the 3146 Patients Randomized in the United States. Circulation. 2020;141:367–375. doi: 10.1161/CIRCULATIONAHA.119.044440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baum S.J., Scholz K.P. Rounding the corner on residual risk: Implications of REDUCE-IT for omega-3 polyunsaturated fatty acids treatment in secondary prevention of atherosclerotic cardiovascular disease. Clin. Cardiol. 2019;42:829–838. doi: 10.1002/clc.23220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodman R.J., A Mori T., Burke V., Puddey I.B., Barden A., Watts G.F., Beilin L.J. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis. 2003;166:85–93. doi: 10.1016/S0021-9150(02)00307-6. [DOI] [PubMed] [Google Scholar]
- 63.Candela C.G., López L.M.B., Kohen V.L. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: Nutritional recommendations. Nutrición Hospitalaria. 2011;26:323–326. doi: 10.1590/S0212-16112011000200013. [DOI] [PubMed] [Google Scholar]
- 64.DiNicolantonio J.J., Okeefe J. Importance of maintaining a low omega-6/omega-3 ratio for reducing platelet aggregation, coagulation and thrombosis. Open Hear. 2019;6:e001011. doi: 10.1136/openhrt-2019-001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miśkowiec D., Kasprzak J.D. Postępy farmakoterapii w prewencji chorób serca–skuteczne leki, nieskuteczne suplementy. Dane z ostatnich kongresów AHA i ACC. Folia Cardiol. 2019;14:648–654. [Google Scholar]
- 66.Abdelhamid A.S., Brown T.J., Brainard J.S., Biswas P., Thorpe G.C., Moore H.J., Deane K.H., Summerbell C.D., Worthington H.V., Song F., et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020;3:CD003177. doi: 10.1002/14651858.CD003177.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poreba M., Mostowik M., Siniarski A., Golebiowska-Wiatrak R., Malinowski K.P., Haberka M., Konduracka E., Nessler J., Undas A., Gajos G. Treatment with high-dose n-3 PUFAs has no effect on platelet function, coagulation, metabolic status or inflammation in patients with atherosclerosis and type 2 diabetes. Cardiovasc. Diabetol. 2017;16:1–11. doi: 10.1186/s12933-017-0523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagge A., Schött U., Kander T. High-dose omega-3 fatty acids have no effect on platelet aggregation or coagulation measured with static and flow-based aggregation instruments and Sonoclot; an observational study in healthy volunteers. Scand. J. Clin. Lab. Investig. 2018;78:539–545. doi: 10.1080/00365513.2018.1516477. [DOI] [PubMed] [Google Scholar]
- 69.Sut A., Pytel M., Zadrożny M., Golański J., Rozalski M. Polyphenol-rich diet is associated with decreased level of inflammatory biomarkers in breast cancer patients. Roczniki Państwowego Zakładu Higieny. 2019;70:177–184. doi: 10.32394/rpzh.2019.0068. [DOI] [PubMed] [Google Scholar]
- 70.Sut A., Chizynski K., Rozalski M., Golanski J. Dietary intake of omega fatty acids and polyphenols and its relationship with the levels of inflammatory markers in men with chronic coronary syndrome after percutaneous coronary intervention. Kardiol. Pol. 2020;78:117–123. doi: 10.33963/kp.15078. [DOI] [PubMed] [Google Scholar]
- 71.Song J., Hu M., Li C., Yang B., Ding Q., Wang C., Mao L. Dose-dependent effects of fish oil on cardio-metabolic biomarkers in healthy middle-aged and elderly Chinese people: A double-blind randomized controlled trial. Food Funct. 2018;9:3235–3243. doi: 10.1039/C7FO01566F. [DOI] [PubMed] [Google Scholar]
- 72.Hasturk H., Abdallah R., Kantarci A., Nguyen D., Giordano N., Hamilton J., Van Dyke T.E. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arter. Thromb. Vasc. Biol. 2015;35:1123–1133. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackay I., Ford I., Thies F., Fielding S., Bachoo P., Brittenden J. Effect of Omega-3 fatty acid supplementation on markers of platelet and endothelial function in patients with peripheral arterial disease. Atherosclerosis. 2012;221:514–520. doi: 10.1016/j.atherosclerosis.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 74.Mason R.P., Dawoud H., Jacob R.F., Sherratt S.C., Malinski T. Eicosapentaenoic acid improves endothelial function and nitric oxide bioavailability in a manner that is enhanced in combination with a statin. Biomed. Pharmacother. 2018;103:1231–1237. doi: 10.1016/j.biopha.2018.04.118. [DOI] [PubMed] [Google Scholar]
- 75.Wachira J.K., Larson M.K., Harris W.S. n-3 Fatty acids affect haemostasis but do not increase the risk of bleeding: Clinical observations and mechanistic insights. Br. J. Nutr. 2014;111:1652–1662. doi: 10.1017/S000711451300425X. [DOI] [PubMed] [Google Scholar]
- 76.Carr J.A. Role of Fish Oil in Post-Cardiotomy Bleeding: A Summary of the Basic Science and Clinical Trials. Ann. Thorac. Surg. 2018;105:1563–1567. doi: 10.1016/j.athoracsur.2018.01.041. [DOI] [PubMed] [Google Scholar]
- 77.Parish S., Mafham M., Offer A., Barton J., Wallendszus K., Stevens W., Buck G., Haynes R., Collins R., Bowman L., et al. Effects of Omega-3 Fatty Acid Supplements on Arrhythmias. Circulation. 2020;141:331–333. doi: 10.1161/CIRCULATIONAHA.119.044165. [DOI] [PubMed] [Google Scholar]
- 78.Bonutti P.M., Sodhi N., Patel Y.H., Sultan A.A., Khlopas A., Chughtai M., Kolisek F.R., Williams N., Mont M.A. Novel venous thromboembolic disease (VTED) prophylaxis for total knee arthroplasty—Aspirin and fish oil. Ann. Transl. Med. 2017;5:S30. doi: 10.21037/atm.2017.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Isaksen T., Evensen L.H., Johnsen S.H., Jacobsen B.K., Hindberg K., Braekkan S.K., Hansen J.-B. Dietary intake of marine n-3 polyunsaturated fatty acids and future risk of venous thromboembolism. Res. Pract. Thromb. Haemost. 2019;3:59–69. doi: 10.1002/rth2.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng X., Jia R., Li Y., Liu T., Wang Z. Omega-3 fatty acids reduce post-operative risk of deep vein thrombosis and pulmonary embolism after surgery for elderly patients with proximal femoral fractures: A randomized placebo-controlled, double-blind clinical trial. Int. Orthop. 2020;44:2089–2093. doi: 10.1007/s00264-020-04610-0. [DOI] [PubMed] [Google Scholar]
- 81.Isaksen T., Evensen L.H., Brækkan S.K., Hansen J.-B. Dietary Intake of Marine Polyunsaturated n-3 Fatty Acids and Risk of Recurrent Venous Thromboembolism. Thromb. Haemost. 2019;119:2053–2063. doi: 10.1055/s-0039-1697663. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y., Ding J., Guo H., Liang J., Li Y. Associations of Fish and Omega-3 Fatty Acids Consumption With the Risk of Venous Thromboembolism. A Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2020;7:614784. doi: 10.3389/fnut.2020.614784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shigemura T. Letter to the editor regarding “Omega-3 fatty acids reduce post-operative risk of deep vein thrombosis and pulmonary embolism after surgery for elderly patients with proximal femoral fractures: A randomized placebo-controlled, double-blind clinical trial” by Zheng et al. Int. Orthop. 2020;44:2809. doi: 10.1007/s00264-020-04776-7. [DOI] [PubMed] [Google Scholar]
- 84.Merritt R.J., Bhardwaj V., Jami M.M. Fish oil and COVID-19 thromboses. J. Vasc. Surgery Venous Lymphat. Disord. 2020;8:1120. doi: 10.1016/j.jvsv.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rogero M.M., Leão M.D.C., Santana T.M., Pimentel M.V.D.M., Carlini G.C., da Silveira T.F., Gonçalves R.C., Castro I.A. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic. Biol. Med. 2020;156:190–199. doi: 10.1016/j.freeradbiomed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Darwesh A.M., Bassiouni W., Sosnowski D.K., Seubert J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharmacol. Ther. 2021;219:107703. doi: 10.1016/j.pharmthera.2020.107703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsoupras A., Lordan R., Zabetakis I. Thrombosis and COVID-19: The Potential Role of Nutrition. Front. Nutr. 2020;7:583080. doi: 10.3389/fnut.2020.583080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Souza P.R., Marques R.M., Gomez E.A., Colas R.A., De Matteis R., Zak A., Patel M., Collier D.J., Dalli J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses. Circ. Res. 2020;126:75–90. doi: 10.1161/CIRCRESAHA.119.315506. [DOI] [PubMed] [Google Scholar]
- 89.Hosogoe N., Ishikawa S., Yokoyama N., Kozuma K., Isshiki T. Add-on Antiplatelet Effects of Eicosapentaenoic Acid with Tailored Dose Setting in Patients on Dual Antiplatelet Therapy. Int. Hear. J. 2017;58:481–485. doi: 10.1536/ihj.16-430. [DOI] [PubMed] [Google Scholar]
- 90.Skeaff C., Holub B. The effect of fish oil consumption on platelet aggregation responses in washed human platelet suspensions. Thromb. Res. 1988;51:105–115. doi: 10.1016/0049-3848(88)90054-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.