Abstract
The response of patients with inflammatory bowel disease (IBD) to fecal microbial transplantation (FMT) has been inconsistent possibly due to variable engraftment of donor microbiota. This failure to engraft has resulted in the use of several different strategies to attempt optimization of the recipient microbiota following FMT. The purpose of our study was to evaluate the effects of two distinct microbial strategies—antibiotic pre-treatment and repeated FMT dosing—on IBD outcomes. A systematic literature review was designed and implemented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A medical librarian conducted comprehensive searches in MEDLINE, Embase, Scopus, Web of Science Core Collection, and Cochrane Library on 25 November 2019 and updated on 29 January 2021. Primary outcomes of interest included comparing relapse and remission rates in patients with IBD for a single FMT dose, repeated FMT dosages, and antibiotic pre-treatment groups. Twenty-eight articles (six randomized trials, 20 cohort trials, two case series) containing 976 patients were identified. Meta-analysis revealed that both repeated FMT and antibiotic pre-treatment strategies demonstrated improvements in pooled response and remission rates. These clinical improvements were associated with increases in fecal microbiota richness and α-diversity, as well as the enrichment of several short-chain fatty acid (SCFA)-producing anaerobes including Bifidobacterium, Roseburia, Lachnospiraceae, Prevotella, Ruminococcus, and Clostridium related species.
Keywords: inflammatory bowel disease, fecal microbial transplantation, antibiotic treatment
1. Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal tract categorized by Crohn’s disease (CD), ulcerative colitis (UC), and indeterminate colitis [1,2]. The incidence of IBD is steadily increasing worldwide [3], as are its extensive healthcare and economic burdens. While IBD is believed to involve a host’s genetic predisposition, environmental factors, and an imbalanced gut microbial community, the etiology of IBD has yet to be fully elucidated [4,5,6,7,8]. The complex pathophysiology underlying IBD has led to the current implementation of non-specific therapeutic strategies centered on systemic immunosuppression [9,10]. Despite the significant complications associated with these strategies, ongoing high rates of refractory disease remain [11,12,13] suggesting that alternative targeted approaches are needed to enhance the clinical efficacy and safety of modern IBD therapies [14].
Accumulating evidence suggests that imbalances in the gut microbiome, a highly diverse community of microorganisms that inhabits the gastrointestinal tract of humans, plays a causative role in the pathogenesis of IBD [15,16,17]. In general, gut microbial communities of patients with IBD are characterized by reduced microbial diversity, an increased abundance of aerobic pro-inflammatory bacteria, and a reduction in anaerobic bacteria that generate beneficial anti-inflammatory metabolites, such as short-chain fatty acids (SCFA). These findings have fostered growing interest in adopting microbiota-targeted strategies into the forefront of modern IBD therapeutics [18,19,20] in order to reduce the need for long-term immunosuppressants and their associated adverse complications.
Fecal microbial transplantation (FMT) is one such microbiota-targeted strategy that has shown initial promise for the management of IBD by implanting members of microbiota from healthy donors in an attempt to restore imbalances in host-microbial ecology [21]. However, clinical response of IBD to FMT has shown extensive inter-study heterogeneity [22], which might stem from the variable engraftment of donor derived microbes and the high or persistent populations of unfavorable pathobionts in the host [23,24,25,26]. In this regard, both antibiotic pre-treatments (to lessen competitive interactions) and increased frequency of FMT delivery may both enhance the engraftment of putatively beneficial microbes, correcting dysbiotic populations, and promoting clinical response and disease remission [27,28,29,30]. While several trials utilizing either antibiotic pre-treatments [31,32,33,34] or repeated FMT regimens [35,36] have been conducted in patients with IBD, no pooled analyses of these findings exist, therefore hindering the optimization of FMT-based IBD therapies.
The purpose of our study was to address this important gap in knowledge by conducting a systematic review and meta-analysis to characterize the effects of antibiotic pre-treatment and repeated FMT dosing on IBD response and remission. Our primary outcome was to compare differences in pooled relapse and remission rates between antibiotic pre-treatment and repeated FMT dosing strategies. Secondary outcomes included comparing differences in fecal microbiota composition associated with disease response and remission for these two approaches.
2. Methods
2.1. Eligibility Criteria
A systematic literature search strategy was designed using the Population, Intervention, Comparison, Outcome, and Study Design (PICOS) framework and implemented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. FMT was defined as the administration of a fecal matter solution from a healthy donor to the gastrointestinal tract of a recipient to confer a health benefit. Our inclusion criteria included studies with adults (age ≥ 18 years) that had a diagnosis for IBD and received FMT. All modalities of FMT delivery, such as colonoscopy, nasogastric tube, oral capsules, or enemas, and any regimen of antibiotic pre-treatment were included. Studies were excluded if disease was localized to the surgical pouch (i.e., pouchitis), patients had concurrent Clostridioides difficile infection, less than six patients were enrolled, or in a pediatric population. Duplicate studies, kin studies, studies using animal models, and non-English studies were also excluded.
2.2. Search Strategy
A medical librarian (JK) systematically searched the MEDLINE (via Ovid), Embase (Ovid), Scopus, Web of Science Core Collection, and Cochrane Library (via Wiley) databases on 25 November 2019 (see Supplemental Table S1 for full-text search strategy) and updated on 29 January 2021. No language or date limits were applied. To complement this approach, the research team also screened the first 200 results from Google Scholar for inclusion. Manual searches of references from included studies were further performed to identify potentially missed articles.
2.3. Study Selection
Titles and abstracts of relevant articles were first manually screened for inclusion by two independent reviewers (VM, SR). Studies meeting initial screening criteria by at least one reviewer were selected for a full text review by two independent reviewers (VM, SR) using pre-specified inclusion and exclusion criteria. Disputes were resolved by a third reviewer (JD). Data were extracted independently by two reviewers (VM, SR) into separate Excel spreadsheets and cross-examined for accuracy. Studies were then assessed for methodological quality and bias using the Newcastle-Ottawa [37] tool for cohort studies and the Cochrane Risk of Bias [38] evaluation for randomized controlled trials (RCTs).
2.4. Data Extraction
Study characteristics were evaluated for study design, year, and country of origin. Primary outcomes of interest included relapse and remission rates following FMT. Secondary outcomes included differences in fecal microbiota composition, and adverse events. Patient characteristics included age, sex, mean disease duration, type of IBD, histology disease scoring, and current medications. FMT strategy-specific variables included donor stool processing, mode of delivery, type of FMT regimen, and type and duration of antibiotic pre-treatments.
2.5. Data Synthesis
Continuous data were expressed as mean ± standard deviation (SD). For the purpose of meta-analysis, data extracted as medians and interquartile ranges were converted to mean ± SD using methods outlined by Hozo et al. [39]. Meta-analyses of pooled proportions were conducted using a random effects models by the DerSimonian-Laird method [40]. Estimates of heterogeneity were obtained from inverse-variance fixed-effect models. Pooled estimate variances were stabilized using the Freeman-Tukey Double Arcsine Transformation. Heterogeneity was assessed using the Chi-squared test with significance set at p < 0.10 and the amount of heterogeneity quantified by the I2 statistic as low <50%, moderate 50–75%, or high >75% [41]. Categorical data were assessed using either Chi-squared or Fischer’s exact tests. A two-sided α of less than 0.05 was considered statistically significant. Meta-analysis was conducted using the metaprop function in STATA (v15.1; StataCorp, College Station, TX, USA).
3. Results
3.1. Search Results and Study Characteristics
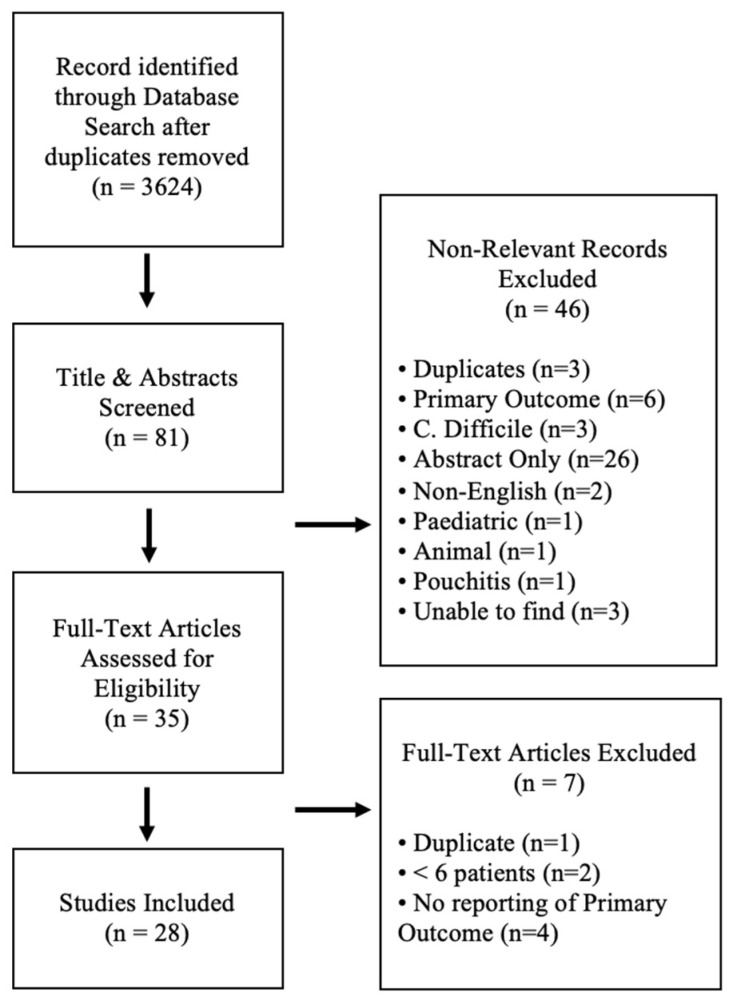
Comprehensive search of the five databases yielded a total of 4220 results, and after duplicate records were removed, 3624 articles remained (Figure 1). After initial screening of the titles and abstracts, the text of 45 articles were fully reviewed. Following full text review, 28 articles were eligible for inclusion in the final systematic review. No prior systematic reviews examining FMT outcomes with respect to antibiotic pre-treatment or repeated FMT regimens were identified. Of the included articles, six were randomized controlled trials, 20 were prospective cohort trials, and two studies were case series.
Figure 1.
PRISMA flow chart of assessed studies.
Of the 28 studies reviewed, 22 included patients with UC, four included patients with CD, and two studies assessing both UC and CD. Most studies examined disease response in patients with mild to moderate disease (n = 9 studies), with twelve studies assessing patients with severe disease (Table 1). Study duration and follow-up ranged from 4 weeks to 13 years with most studies having a follow up 12 weeks (n = 17). Five studies utilized pre-operative antibiotics prior to FMT, with only two studies utilizing the same antibiotic regiments. Nearly half of the studies included a single FMT delivery (n = 12), while the remaining trials use varied regimens.
Table 1.
Study design and FMT regimen characteristics.
| Study | Study Design |
Patients (n) | Country | Disease | Severity | FMT Delivery |
FMT Donor | FMT Dosage | FMT Frequency | Pre-Treatment Antibiotics |
Antibiotic Frequency | Total Follow-up (Weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Borody 2003 [31] | Case series | 6 | Australia | UC | Severe | Enema | Healthy donors chosen by patient | 200–300 g/200–300 mL saline | Daily for 5 days | Vancomycin (500 mg bid), metronidazole (400 mg bid), rifampicin (150 mg bid) | 7–10 days | 676 |
| Chen 2020 [42] | Prospective cohort |
9 | China | UC | Moderate-severe | Naso-jejunal | Healthy donor | 200–250 mL of fecal suspension | 3 doses at 1, 3 and 5 days | - | - | 12 |
| Chen 2020 [43] | Prospective cohort |
44 | China | UC | Mild- moderate |
Colonoscopy | Healthy donor | 150–200 g stool/1000 mL saline | ×3 in 1 week | - | - | 12 |
| Cold 2019 [44] | Prospective cohort | 7 | Denmark | UC | Active | Oral capsules |
Healthy volunteers | 12 g daily dose of 25 capsules | 25 capsules/day for 50 days | - | - | 24 |
| Costello 2019 [35] | RCT | 73 | Australia | UC | Mild- moderate |
Colonoscopy and enema | Healthy volunteers recruited by advertisement |
50 g/200 mL saline colonoscopy, 25 g/100 mL saline enema | 1× colonoscopy then 2× enemas over 1 week | - | - | 8 |
| Cui 2015 [45] | Prospective cohort |
30 | China | CD | Moderate-severe | Gastroscopy | Related or unrelated volunteer | 60 mL/100 mL saline | ×1 | - | - | 65 |
| Dang 2020 [46] | Case series | 12 | China | UC | Moderate-severe | Colonoscopy | Healthy volunteers | 15 mL bacterial pellet in 75 mL saline | multiple, exact frequency NR | - | - | 52 |
| Damman 2015 [47] | Prospective cohort |
7 | USA | UC | Mild- moderate |
Colonoscopy | Chosen by patient | Diluted with 2–3 mL saline/g of stool | ×1 | - | - | 12 |
| Ishikawa 2017 [48] | Prospective cohort |
36 | Japan | UC | Mild-severe | Colonoscopy | Spouse or relative | 150–250 g/350–500 mL saline | ×1 | Amoxicillin (1500 mg/day), Fosfomycin (3000 mg/day), metronidazole (750 mg/day) | 2 weeks until 2 days before FMT | 4 |
| Jacob 2017 [49] | Prospective cohort |
20 | USA | UC | Active | Colonoscopy | Healthy donor | 60 mL | ×1 | - | - | 4 |
| Kump 2017 [50] | Prospective cohort |
27 | Austria | UC | Mild-severe | Colonoscopy | Related or unrelated volunteer | 50 g/200–500 mL saline | 5×, 14 days apart | Vancomycin (250 mg qid), paromomycin (250 mg tid), nystatin (10 mL, 1 million IE qid) | 10 days | 13 |
| Mizuno 2017 [51] | Prospective cohort |
10 | Japan | UC | Moderate-severe | Colonoscopy | Healthy relatives | 50–300 g/50–100 mL saline | ×1 | - | - | 12 |
| Moayyedi 2015 [52] | RCT | 70 | Canada | UC | Mild-moderate | Enema | Healthy donors | 50 g/300 mL water | ×6; 0, 1, 2, 3, 4, 5, 6 weeks | - | - | 7 |
| Nishida 2017 [53] | Prospective cohort |
41 | Japan | UC | Mild-moderate | Colonoscopy | Healthy relatives | 150–200 g/500 mL saline | ×1 | - | - | 12 |
| Okahara 2020 [54] | Prospective cohort |
92 | Japan | UC | Mild-severe | Colonoscopy | Spouses and relatives | 350–500 mL filtered bacterial suspension infusion | ×1 | Amoxicillin (1500 mg/day), Fosfomycin (3000 mg/day), metronidazole (750 mg/day) | 2 weeks prior to FMT | 104 |
| Paramsothy 2017 [55] | RCT | 85 | Australia | UC | Mild-moderate | Colonoscopy and enema | Healthy volunteers recruited by advertisement |
37.5 g | ×5/week for 8 weeks | - | - | 8 |
| Rossen 2015 [56] | RCT | 50 | Finland | UC | Mild- moderate |
Nasoduodenal tube | Relatives, partner, or volunteer | 120 g | ×2; 3 weeks apart | - | - | 12 |
| Schierova 2020 [57] | Prospective cohort |
16 | Czech Republic |
UC | NR | Enema | Healthy donors | 50 g stool/150 mL saline | 5×/week for 1 week then weekly × 6 weeks | - | - | 12 |
| Sokol 2020 [58] | RCT | 17 | France | CD | NR | Colonoscopy | Healthy donors | 50–100 g/250–350 mL saline | ×1 | - | - | 24 |
| Sood 2019 [59] | Prospective cohort |
41 | India | UC | Mild- moderate |
Colonoscopy | Two healthy unrelated volunteers | NR | ×7; 0, 2, 6, 10, 14, 18, 22 weeks | - | - | 22 |
| Sood 2020 [60] | Prospective cohort |
140 | India | UC | Moderate-severe | Colonoscopy | Healthy donors | 80 g stool/ 200 mL saline | ×7; 0, 2, 6, 10, 14, 18, 22 weeks |
- | - | 30 |
| Uygun 2017 [61] | Prospective cohort |
30 | Turkey | UC | Moderate-severe | Colonoscopy | Relatives, partner, or volunteer | 120–150 g | ×1 | - | - | 12 |
| Vaughn 2016 [62] | Prospective cohort |
19 | USA | CD | Active | Colonoscopy | Healthy unrelated volunteers |
50 g/250 mL saline | ×1 | - | - | 4 |
| Vermeire 2016 [63] | Prospective cohort |
14 | Belgium | UC+ CD |
Refractory | Naso-jejunal or rectal tube | Family, friend, or partner |
200 g/400 mL saline | ×2; 2 consecutive days | - | - | 8 |
| Wang 2020 [64] | Prospective cohort |
16 | China | UC | Moderate- severe | Colonoscopy | Healthy donor | 100 g stool/ 500 mL saline |
×3; 2–3 month intervals | - | - | >24 |
| Wei 2015 [34] | Prospective cohort |
14 | China | UC+ CD |
NR | Colonoscopy or naso-jejunal tube | Healthy unrelated donor |
60 g/350 mL saline | ×1 | Vancomycin (500 mg) | Twice a day for 3 days before FMT | 4 |
| Yang 2019 [65] | RCT | 27 | China | CD | Mild- moderate |
Gastroscopy or colonoscopy | Healthy donors | 200 g in saline | ×2; 1 week apart | - | - | 2 |
| Zhang 2016 [66] | Prospective cohort |
19 | China | UC | Moderate-severe | Gastroscopy | NR | NR | ×1 | - | - | 13 |
IBD—Inflammatory Bowel Disease; FMT—Fecal Microbiota Transplantation; UC—Ulcerative Colitis; CD—Crohn’s Disease; NR—Not recorded; RCT—Double-blinded, randomized controlled trial.
3.2. Risk of Bias Assessment
Risk of bias for cohort studies was characterized using an adjusted 7-point Newcastle-Ottawa scale of selection, comparability, and study outcome categories (Supplemental Table S2). The 19 included cohort studies demonstrated low to moderate risk of bias due to a lack of long-term follow-up greater than three months (n = 7 studies), and inadequate description or evaluation fecal microbiota changes (n = 8 studies). The six randomized trials were assessed for bias using the Cochrane Risk of Bias tool and together demonstrated low risk of bias (Supplemental Table S3).
3.3. Baseline Demographics
A total of 976 patients were identified from the 28 studies included (Table 2). Twenty-two studies included only patients with UC (n = 767), while three studies included patients with CD (n = 87) alone. The mean weighted age of all patients was 40.0 years, of which 59% were on average male with a mean weighted disease duration of 6.2 years. The proportion of patients receiving concurrent corticosteroids varied extensively from 7% to 100%. Patients with a diverse spectrum of IBD severity were included although the majority of included patients had mild-moderate disease (n = 439; 9 studies). Prior to FMT, total Mayo scores for UC activity ranged from 6.1 to 11.1 and CD activity index ranged from 275 to 345. No significant differences in clinical characteristics were observed between CD and UC patients prior to FMT.
Table 2.
Baseline characteristics of patients for included studies.
| Study | Disease | Intervention Arm | Patients (n) | Age | Sex (% male) |
Disease Duration (Years) | Ongoing Systemic Corticosteroids (%) | Total Mayo Score |
CDAI |
|---|---|---|---|---|---|---|---|---|---|
| Borody 2003 | UC | Antibiotic pre-treatment and repeated FMT | 6 | 35.8 (11.0) | 50.0 | 11.7 (5.8) | NR | NR | - |
| Chen 2020 | UC | Repeated FMT | 9 | 47.9 (10.6) | 77.8 | 5.3 (5.1) | 33.3 | 5.9 (2.0) | - |
| Chen 2020 | UC | Repeated FMT | 44 | 44.4 (15.5) | 57 | 4.6 (2.1) | 25.0 | 5.9 (2.0) | - |
| Cold 2019 | UC | Repeated FMT | 7 | 38.3 (5.8) | 71.4 | 10.8 (3.8) | NR | NR | - |
| Costello 2019 | UC | Repeated donor FMT | 38 | 38.5 (6) | 53.0 | 4.9 (4.8) | 21.0 | 7.2 (1.7) | - |
| Repeated autologous FMT | 35 | 35.0 (5.25) | 57.0 | 5.8 (2.2) | 31.0 | 7.4 (1.9) | - | ||
| Cui 2015 | CD | Single FMT | 30 | 38.0 (13.8) | 64.5 | 7.4 (5.3) | 56.7 | NR | NR |
| Damman 2015 | UC | Single FMT | 8 | 41.1 (15.5) | 25.0 | 16.6 (13.1) | NR | NR | - |
| Dang 2020 | UC | Repeated FMT | 12 | 51 (14.0) | 66.0 | NR | 41.7 | NR | - |
| Ishikawa 2017 | UC | Antibiotic pre-treatment and single FMT | 17 | 40.4 (14.2) | 76.5 | 7.8 (8.4) | 29.4 | 7.5 (1.9) | - |
| Antibiotic pre-treatment only | 19 | 44.8 (14.9) | 63.2 | 7.0 (8.0) | 47.4 | 8.2 (2.2) | - | ||
| Jacob 2017 | UC | Single FMT | 20 | 38.4 (12.6) | 60.0 | NR | 30.0 | 8.1 (2.4) | - |
| Kump 2017 | UC | Antibiotic pre-treatment and repeated FMT | 17 | 44.0 (18.0) | 82.0 | 8.0 (8.0) | 59.0 | 8.9 (1.6) | - |
| Antibiotic pre-treatment only | 10 | 36.0 (13.0) | 30.0 | 7.0 (6.0) | 30.0 | 8.1 (3.1) | - | ||
| Mizuno 2017 | UC | Single FMT | 10 | 31.8 (7.8) | 70.0 | 6.25 (3.5) | NR | 6.1 (1.0) | - |
| Moayyedi 2015 | UC | Repeated FMT | 38 | 42.2 (15.0) | 47.0 | 7.9 (5.6) | 39 | 8.2 (2.6) | - |
| Placebo | 37 | 35.8 (12.1) | 70.0 | 7.0 (6.8) | 35 | 7.9 (2.3) | - | ||
| Nishida 2017 | UC | Single FMT | 41 | 39.6 (16.9) | 68.3 | 7.6 (8.6) | 26.8 | 5.6 (2.4) | - |
| Okahara 2020 | UC | Antibiotic pre-treatment Single FMT | 55 | 40.1 (13.3) | 69.1 | 8.6 (7.4) | 43.2 | 6.3 (4.1) | - |
| Paramsothy 2017 | UC | Repeated FMT | 41 | 35.6 (5.3) | 54.0 | 5.8 (1.4) | 22.0 | 8 (0.8) | - |
| Placebo | 40 | 35.4 (4.5) | 63.0 | 5.8 (1.4) | 28.0 | 8 (0.8) | - | ||
| Rossen 2015 | UC | Single donor FMT | 23 | 42.3 (5.8) | 47.8 | 7 (NR) | 21.7 | NR | - |
| Single autologous FMT | 25 | 41 (4.5) | 44.0 | 9 (NR) | 20.0 | NR | - | ||
| Schierova 2020 | UC | Repeated FMT | 8 | 41.3 (10.1) | 50.0 | NR | 0 | 5.8 (1.7) | - |
| Medical therapy | 8 | 44.3 (10.4) | 50.0 | NR | 25.0 | 6.0 (1.5) | - | ||
| Sokol 2020 | CD | Single FMT | 8 | 31.8 (6.8) | 62.5 | 8.5 (8.1) | 100 | NR | 89 (30.5) |
| Placebo | 9 | 38.3 (6.0) | 44.4 | 11.3 (2.0) | 100 | NR | 61.5 (20.1) | ||
| Sood 2019 | UC | Repeated FMT | 41 | 36.5 (10.7) | 58.5 | 4.6 (4.2) | 100 | 8.8 (2.6) | - |
| Sood 2020 | UC | Repeated FMT | 93 | 35 (11) | 62.4 | 5.2 (4.6) | 78.5 | 8.1 (2.0) | - |
| Uygun 2017 | UC | Single FMT | 30 | 34.6 (10.3) | 46.7 | 5.3 (3.3) | NR | 11.1 (1.1) | - |
| Vaughn 2016 | CD | Single FMT | 19 | 36 (12.3) | 63.0 | 12.5 (10.6) | 42.0 | NR | NR |
| Vermeire 2016 | UC and CD | Repeated FMT | 14 | 38.6 (8.2) | 50.0 | 10.2 (7.5) | 21.4 | 8.4 (0.6) | 290 (29) |
| Wang 2020 | UC | Repeated FMT | 16 | 39.5 (4) | 62.5 | 7.5 (5.8) | NR | 9.9 (2.2) | - |
| Wei 2015 | UC and CD | Antibiotic pre-treatment and single FMT | 14 | 43.5 (16.4) | 42.9 | 4.1 (3.2) | 7.1 | 5.8 (1.9) | 345 (77.8) |
| Yang 2019 | CD | Repeated FMT | 30 | 72.2 (10.8)) | 57.9 | 1.3 (0.4) | NR | NR | 283 (131) |
| Zhang 2016 | UC | Single FMT | 19 | 39.2 (14.1) | 36.8 | 8.0 (5.8) | NR | 10.5 (1.7) | - |
Values are presented as mean +/− SD; UC—ulcerative colitis; CD—Crohn’s disease; NR—Not Recorded; CDAI—Crohn’s Disease Activity Index.
3.4. FMT Administration, Dosing, and Donor Characterization
FMT methodologies varied substantially across all studies. The most frequent mode of FMT was via colonoscopy (n = 19 studies), followed by nasoduodenal/naso-jejunal tube (n = 4 studies), enemas (n = 4 studies), gastroscopy (n = 3 studies), and oral capsules (n = 1 study). The dosage of FMT ranged from 12 g to 300 g of stool per administration with 50% (n = 10 studies) of all studies delivering multiple doses. Antibiotic pre-treatment regimens ranged from three to 14 days prior to FMT (n = 5 studies), with most studies using a combination of antibiotics (n = 4 studies) and specifically vancomycin (n = 3 studies). FMT donors of included studies were typically healthy donors unrelated to the recipients. Nine studies utilized donors that were either relatives or specifically chosen by the patients.
3.5. Response and Remission Rates for Repeated FMT Regimens
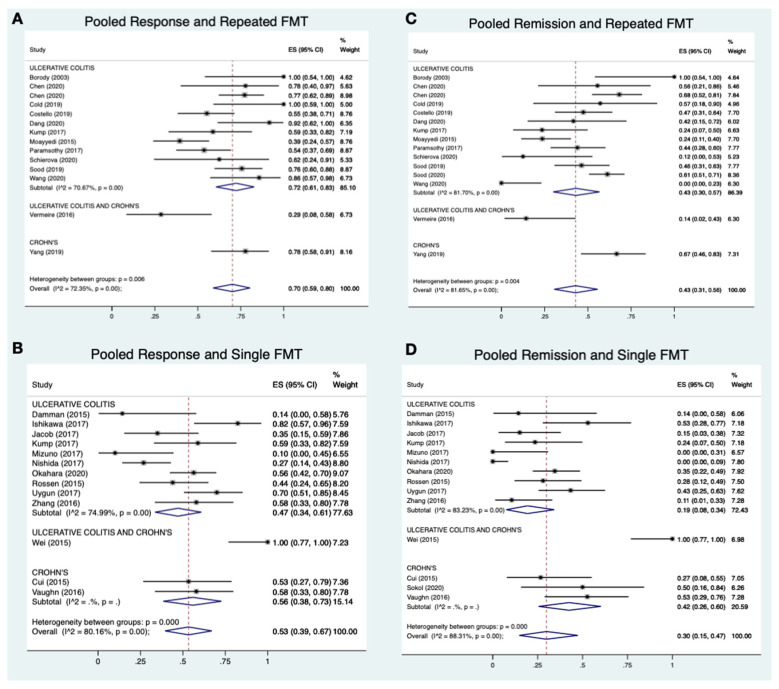
Of the 976 patients included, 41.9% (n = 409) were treated with a single FMT and 30.0% (n = 229) with repeated FMT (Table 1 and Table 3). Meta-analysis of all included studies revealed that repeated FMT studies had higher pooled response rates (15 studies; 70%; 95% CI 59–80%; I2 = 72%; Figure 2A) than those with single FMT (13 studies; 53%; 95% CI 39–67%; I2 = 80%; Figure 2B). Pooled remission rates for studies with multiple FMTs (15 studies; 43%; 95% CI 31–56%; I2 = 82%; Figure 2C) were also higher than for studies with a single FMT (13 studies; 30%; 95% CI 15–47%; I2 = 88%; Figure 2D).
Table 3.
Response and remission rates for included studies.
| Study | Intervention Arm | Follow-Up at Response/Remission (Weeks) |
Patients (n) | Response (%) | Remission (%) |
|---|---|---|---|---|---|
| Borody 2003 | Antibiotic pre-treatment and repeated FMT | 676 | 6 | 6 (100%) | 6 (100%) |
| Chen 2020 | Repeated FMT | 2 weeks for response 12 weeks for remission |
9 | 7 (77.8%) | 5 (55.6%) |
| Chen 2020 | Repeated FMT | 12 | 44 | 34 (77.3%) | 30 (68.2%) |
| Cold 2019 | Repeated FMT | 24 | 7 | 7 (100%) | 4 (57.1%) |
| Costello 2019 | Repeated donor FMT | 8 | 38 | 21 (55%) | 18 (47%) |
| Repeated autologous FMT | 8 | 35 | 8 (23%) | 6 (17%) | |
| Cui 2015 | Single FMT | 12–72 | 15 | 8 (53.3%) | 4 (26.7%) |
| Dang 2020 | Repeated FMT | 52 | 12 | 11 (91.7%) | 5 (41.7%) |
| Damman 2015 | Single FMT | 4 | 7 | 1 (14.3%) | 1 (14.3%) |
| Ishikawa 2017 | Antibiotic pre-treatment and single FMT | 4 | 17 | 14 (82.3%) | 9 (52.9%) |
| Antibiotic pre-treatment only | 4 | 19 | 13 (68.4%) | 3 (15.8%) | |
| Jacob 2017 | Single FMT | 4 | 20 | 7 (35%) | 3 (15%) |
| Kump 2017 | Antibiotic pre-treatment and repeated FMT | 13 | 17 | 10 (59%) | 4 (24%) |
| Antibiotic pre-treatment only | 13 | 10 | 1 (10%) | 0 (0%) | |
| Mizuno 2017 | Single FMT | 12 | 10 | 1 (10%) | 0 (0%) |
| Moayyedi 2015 | Repeated FMT | 7 | 38 | 15 (39%) | 9 (24%) |
| Placebo | 7 | 37 | 9 (24%) | 2 (5%) | |
| Nishida 2017 | Single FMT | 8 | 41 | 11 (26.8%) | 0 (0%) |
| Okahara 2020 | Single FMT | 4 | 55 | 31 (56.3%) | 19 (34.5%) |
| Paramsothy 2017 | Repeated FMT | 8 | 41 | 22 (54%) | 18 (44%) |
| Placebo | 8 | 40 | 9 (23%) | 8 (20%) | |
| Rossen 2015 | Repeated donor FMT | 12 | 23 | 11 (47.8%) | 7 (30.4%) |
| Repeated autologous FMT | 12 | 25 | 13 (52.0%) | 8 (32.0%) | |
| Schierova 2020 | Repeated FMT | 12 | 8 | 5 (62.5%) | 1 (12.5%) |
| Sokol 2020 | Single FMT | 24 | 8 | NR | 4 (50%) |
| Sood 2019 | Repeated FMT | 22 | 41 | 31 (75.6%) | 19 (46.3%) |
| Sood 2020 | Repeated FMT | 30 | 93 | NR | 57 (61.3%) |
| Uygun 2017 | Single FMT | 12 | 30 | 21 (70%) | 13 (43.3%) |
| Vaughn 2016 | Single FMT | 4 | 19 | 11 (58%) | 10 (53%) |
| Vermeire 2016 | Repeated FMT | 6 weeks for response 8 weeks for remission |
14 | 4 (50%) | 2 (14.3%) |
| Wang 2020 | Repeated FMT | >6 mo | 16 | 14 (87.5%) | 0 (0%) |
| Wei 2015 | Antibiotic pre-treatment and single FMT | 4 | 14 | 14 (100%) | 14 (100%) |
| Yang 2019 | Repeated FMT | 2 | 27 | 21 (77.8%) | 18 (66.7%) |
| Zhang 2016 | Single FMT | 13 | 19 | 11 (57.9%) | 2 (10.5%) |
Figure 2.
A-2D: Meta-analysis of pooled response and remission rate for repeated vs. single FMT.
Subgroup analysis of UC studies revealed more pronounced differences in pooled response (12 studies; 72%; 95% CI 61–83%; I2 = 71% vs. 10 studies; 47%; 95% CI 34–61%; I2 = 75%) and remission rates (12 studies; 43%; 95%CI 30–57%; I2 = 82% vs. 10 studies; 19%; 95% CI 8–34%; I2 = 83%) when comparing repeated and single FMT regimens, respectively.
Taken together, pooled response and remission rates were more favorable for patients receiving repeated FMT regimens than single FMT alone. Heterogeneity for all pooled analyses was high with all I2 values greater than 70%.
3.6. Response and Remission Rates for Antibiotic Pre-Treatments
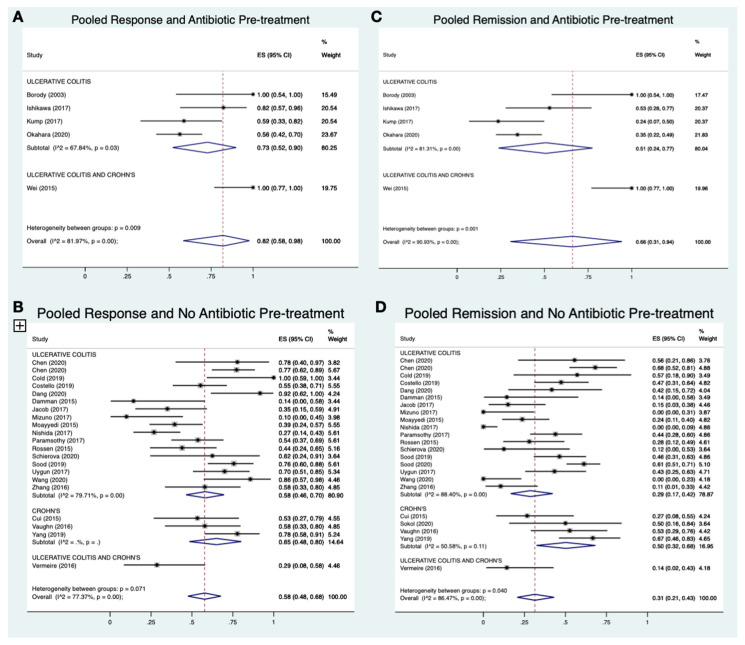
Antibiotics were not frequently administered as pre-treatments, with only 11.2% (n = 109) of patients receiving an antibiotic regimen prior to FMT. Meta-analysis of included studies revealed that pooled response rates for antibiotic pre-treatment (five studies; 82%; 95% CI 58–98%; I2 = 82%; Figure 3A) were higher than for no pre-treatment (23 studies; 58%; 95% CI 48–68%; I2 = 77%; Figure 3B). Likewise, antibiotic pre-treatment was also associated with improved remission rates (five studies; 66%; 95%CI 31–94%; I2 = 91%; Figure 3C) when compared to no pre-treatment (23 studies; 31%; 95%CI 21–43%; I2 = 86%; Figure 3D).
Figure 3.
A-3D: Meta-analysis of pooled response and remission rate for antibiotic pre-treatment vs. no pre-treatment.
The favorable effect of antibiotic pre-treatment on pooled response (four studies; 73%; 95% CI 52–90%; I2 = 68% vs. 17 studies; 58%; 95% CI 48–70%; I2 = 80%) and remission rates (four studies; 51%; 95% CI 24–77%; I2 = 81% vs. 18 studies; 29%; 95% CI 17–42%; I2 = 88%) was also observed on subgroup analysis of UC studies.
Similar to the repeated FMT analysis, heterogeneity for the pooled proportion analyses of antibiotic pre-treatment was high.
3.7. Fecal Microbiota Compositional Changes Following FMT
3.7.1. Overview of Microbiota Reporting of Included Studies
Although FMT aims to shift the gut microbial communities of patients with IBD, only 64% of studies (n = 18 studies) characterized the recipient’s fecal microbiota following FMT and only two studies directly assessed associations between IBD remission and fecal microbiota compositional changes (Table 4). Further, no study directly compared microbial changes of antibiotic pre-treatment vs. no pre-treatment or repeated FMT vs. single-dose FMT. Only five studies provided donor microbial characterization. The majority of studies (n = 14 studies) used 16 s rRNA gene amplicon sequencing methods, with three studies using whole-genome sequencing and one using Bacteroides HSP60 sequencing.
Table 4.
Effect of fecal microbial transplant therapy on microbiota composition.
| Study | Methods | Donor Microbiota Differences vs. Recipient |
Recipient Microbiota Changes Following FMT |
Recipient Microbiota Changes Associated with Response/Remission |
|---|---|---|---|---|
| Borody 2003 | NR | NR | NR | NR |
| Chen 2020 | NR | NR | NR | NR |
| Chen 2020 | 16 s rRNA | ↑ α—diversity (Shannon, Chao1) |
↑ α—diversity (Shannon, Chao1) ↑ F. Prausnitzii |
NR |
| Cold 2019 | 16 s rRNA | NR | No change in α—diversity (Shannon, Simpson) |
NR |
| Costello 2019 | 16 s rRNA | NR | ↑ α—diversity (operational taxonomic units—OTUs) ↑ Peptococcus niger, ↑ Faecalicoccus pleomorphus, ↑ Olsenella sp., ↑ Acidaminococcus intestini, ↑ Prevotella copri, ↑ Clostridium methylpentosum, ↑ Allistipes indistinctus, ↑ Odoribacter splanchnicus ↓ Anaerostipescaccae, ↓ Clostridium aldenense |
NR |
| Cui 2015 | NR | NR | NR | NR |
| Damman 2015 | Metagenomic Shotgun Sequencing |
NR | No significant difference in α diversity (Shannon) ↑ Actinobacteria, ↑ Bacteroidetes (Prevotella copri) |
NR |
| Dang 2020 | NR | NR | NR | NR |
| Ishikawa 2017 | 16 s rDNA | NR | ↑ Bacteroidetes | NR |
| Jacob 2017 | 16 s rRNA | NR | ↑ α—diversity (OTUs, Shannon) Change in β—diversity (Bray-Curtis) towards donor |
NR |
| Kump 2017 | 16 s rRNA | ↑ unclassified Ruminococcus sp., ↑ Akkermansia muciniphila |
No change in α—diversity (Chao1) Change in β—diversity (Bray-Curtis) towards donor |
↑ Akkermansia, ↓ Dialister sp. Change in β—diversity (Bray-Curtis) towards donor in responders |
| Mizuno 2017 | 16 s rRNA | NR | No significant difference in diversity or composition | NR |
| Moayyedi 2015 | 16 s rRNA | ↑ Lachnospiraceae, ↑ Ruminococcus |
Change in β—diversity (Bray-Curtis) towards donor | Change in β—diversity (Bray-Curtis) towards donor |
| Nishida 2017 | 16 s rRNA | ↑ Bifidobacterium | No significant difference in α—diversity (Shannon) or β—diversity (Bray-Curtis) between responders and non-responders | NR |
| Okahara 2020 | HSP60 Bacteroidetes Sequencing |
NR | Increase in similarity of Bacteroidetes species to donor |
↑ Bacteroides uniformis, ↑ Parabacteroides distasonis, ↑ Bacteroides dorei |
| Paramsothy 2017 | 16 s rRNA shotgun sequencing | NR | ↑ α—diversity (OTUs, Shannon) Shift towards donor at OTU level ↑ Prevotella spp., ↓ Bacteroides spp. |
↑Barnesiella spp., ↑ Parabacteroides spp., ↑ Clostridium cluster IV, ↑ Ruminococcus spp. |
| Rossen 2015 | 16 s rRNA | NR | ↑ α—diversity (OTUs, Shannon) ↑ Clostridium clusters IV, XIVa, XVIII ↓ Bacteroidetes |
NR |
| Schierova 2020 | 16 sRNA | NR | No difference in α—diversity (Shannon, Chao1, Faith’s phylogenetic diversity) or β—diversity |
↑ Lachnospiraceae, ↑ Ruminococcaeae, ↑ Clostridaceae, ↑ Bifidobacteriaceae, ↑ Coriobacteriaceace ↑Faecalibacterium ↑ Blautia, ↑ Coriobacteria, ↑ Collinsella, ↑ Slackia, ↑ Bifidobacterium |
| Sokol 2020 | 16 s rRNA | NR | Transient ↑ α—diversity (Shannon, Chao1) Trend towards change in β—diversity (Bray-Curtis, Sorensen similarity index) between donor/recipient correlated |
Sorensen index similarity showing improved engraftment; ↑ Ruminococcaecea, ↑ Coprococcus, ↑ Desulfovibrio |
| Sood 2019 | NR | NR | NR | NR |
| Sood 2020 | NR | NR | NR | NR |
| Uygun 2017 | NR | NR | NR | NR |
| Vaughn 2016 | Whole-genome shotgun sequencing | NR | ↑ α—diversity (Shannon) ↑ Bacteroides cellulosilyticus, ↑ Bilophila unclassified, ↑ Desulfovibrio piger, ↑ Bilophila wadsorthia, ↑ Clostridium leptum, ↑ Odoribacter splanchnicus, ↑ Bacteroides dorei, ↑ Parasutterella excrementihominis, ↑ Lachnospiraceae bacterium 7 1 58FAA, ↑ Eubacterium ventriosum, ↑ Burkholderiales bacterium 1 1 47, ↑ Dorea longicatena, ↑ Alistipes finegoldii ↓ Coprobacillus unclassified, ↓ Bacteroides massiliensis, ↓ Ruminococcus lactaris, ↓ Veillonella dispar, ↓ Lachnospiraceae bacterium 5 1 57FAA, ↓ Bifidobacterium adolescentis, ↓ Bacteroides vulgatus, ↓ Bacteroides ovatus, ↓ Streptococcus parasanguinis, ↓ Streptococcus salivarius, ↓ Clostridium scindens |
Change in β—diversity (Bray-Curtis) towards donor in responders |
| Vermeire 2016 | 16 s DNA | ↑ α—diversity (OTUs) | ↑ α—diversity (OTUs), ↑ Roseburia, Oscillibacter, ↑ unclassified Lachnospiraceae, ↑ unclassified Ruminococcaceae |
NR |
| Wang 2020 | NR | NR | NR | NR |
| Wei 2015 | NR | NR | NR | NR |
| Yang 2019 | 16 s RNA | NR | ↑ α—diversity (OTUs, Shannon) | NR |
| Zhang 2016 | NR | NR | NR | NR |
NR—Not recorded.
3.7.2. Changes in Alpha and Beta Diversity Following FMT
Of these 18 studies, nine (50%) reported an increase in microbial richness and α-diversity following FMT, as estimated by the abundance of operational taxonomic units (OTUs), Chao1, Simpson and Shannon indices. Six studies reported no change in α-diversity after FMT. Changes in β-diversity evaluated using Bray-Curtis dissimilarity were reported in five studies, with the majority (n = 4 studies) showing that the microbial ecology of FMT recipients underwent shifts towards those of their respective donors. Within these four studies, increased engraftment was associated with improved clinical outcomes.
In terms of specific bacterial shifts, the effects of FMT were shown to be highly variable (Table 4). Nonetheless, 15 of the 18 studies (83%) that evaluated for shifts in specific gut microbial taxa reported increases in the abundance of anaerobes purported to produce health promoting anti-inflammatory SCFAs, such as Bifidobacterium, Roseburia, Lachnospiraceae, Prevotella, Ruminococcus, and Clostridium related species.
3.7.3. Recipient and Donor Microbial Ecology Associated with IBD Outcomes
Findings from the two studies that assessed associations between IBD remission and fecal microbiota compositional and functional changes were also variable. Parmsothy et al. provided the best assessment of bacterial taxa and corresponding metabolic pathways related to specific IBD outcomes. Following intensive multi-donor FMT, patients with sustained remission had increased relative abundance of Eubacterium halii, Roseburia inulivorans, and Ruminococcus while those who relapsed had higher proportions of Fusobacterium, Escherichia, and Prevotella. Metabolomics of remission patients further revealed increased activation of metabolic pathways associated with the biosynthesis of SCFAs and secondary bile acids. In addition, only one study by Kump and colleagues explored the role of donor microbiota with respect to IBD outcomes following FMT. Patients that received donor fecal microbiota of greater bacterial richness and α-diversity (assessed by OTU abundance and Shannon diversity) and with increased Ruminococcus and Akkermansia abundances were shown to have higher rates of IBD remission.
3.8. Reported Adverse Events
Overall, FMT in patients with IBD was shown to be safe and well tolerated. Frequently reported symptoms related to FMT included a transient self-limiting fever alleviated with paracetamol, and non-specific transient gastrointestinal symptoms such as abdominal discomfort, bloating, nausea, vomiting, and diarrhea (Table 5). Of 26 studies that reported serious adverse events, 13 patients with UC required colectomies and one required hospitalization due to disease progression. One patient also contracted Clostridioides difficile requiring a colectomy and one patient contracted cytomegalovirus infection seven weeks after FMT. Overall, the reported serious adverse events were suggested by the authors to be unrelated to the FMT therapy. No patient receiving FMT intervention in the included studies suffered mortality.
Table 5.
Adverse events and interventions reported for included studies.
| Study | FMT or Antibiotic Treatment Delivery and Frequency |
Patients (n) | Adverse Events Per Patient | Action |
|---|---|---|---|---|
| Borody 2003 | Daily enema for 5 days | 6 | NR | NR |
| Chen 2020 | Naso-jejunal 3 doses at 1, 3 and 5 days | 9 | Mild bloating (n = 3) Treatment failure (n = 1) |
Colectomy (n = 1) |
| Chen 2020 | Colonoscopy ×3 in 1 week | 44 | NR | NR |
| Cold 2019 | 25 oral capsules per day for 50 days | 7 | No adverse events | No adverse events |
| Costello 2019 | Single donor FMT (colonoscopy and 2 enemas over a week) | 38 | After 8 weeks: Worsening colitis (n = 1) C. difficile infection (n = 1) Pneumonia (n = 1) New anemia (n = 1) Mild elevation of alkaline phosphatase (n = 2) and alanine aminotransferase (n = 1) |
Colectomy (n = 1) |
| Single autologous FMT (colonoscopy and 2 enemas over a week) | 35 | After 8 weeks: Worsening colitis (n = 2) New anemia (n = 2) Mild elevation of alanine aminotransferase (n = 3) |
NR | |
| 61 | After 12 months: Worsening colitis (n = 13) Infections (n = 8) New psoriatic arthritis (n = 2) Entero-pathic arthritis (n = 1) Crohn’s disease (n = 1) Allergy to infliximab (n = 1) Weight gain (n = 13) Weight loss (n = 8) |
Colectomy (n = 9) | ||
| Cui 2015 | Single gastroscopy | 30 | Fever (n = 2)—1–6 h after FMT Increased diarrhea (n = 7)—1–6 h after FMT |
NR |
| Damman 2015 | Single colonoscopy | 7 | Abdominal cramping, increase in stool output (NR)—immediately after FMT Abdominal pain (n = 1)—after 5 days |
None |
| Ishikawa 2017 | Single colonoscopy | 21 | Transient borborygmus (n = 10)—during or soon after FMT | Resolved after end of treatment (n = 10) |
| Antibiotic pre-treatment only | 20 | Nausea and watery diarrhea—after antibiotic treatment (n = 8) | Discontinued antibiotic treatment (n = 3) | |
| Jacob 2017 | Single colonoscopy | 20 | Fever (n = 1) Chills (n = 1) Fatigue/malaise (n = 4) Abdominal pain (n = 3) Anorexia (n = 1) Diarrhea (n = 2) Constipation (n = 1) Transient febrile response (n = 1) Increase in Mayo score (n = 2)—at week 4 |
Conservative care Anti-TNF alpha blockade therapy or colectomy |
| Kump 2017 | Colonoscopy (5 times, 14 days apart) | 17 | Worsening colitis (n = 1)—after day 3 | Required additional therapy (n = 1) |
| Antibiotic pre-treatment only | 10 |
C. difficile infection (n = 3)—after 14 days Antibiotic-associated diarrhea (n = 1) Worsening colitis (n = 1) |
Required additional therapy (n = 5) | |
| Mizuno 2017 | Single colonoscopy | 10 | Worsening colitis (n = 6) | |
| Moayyedi 2015 | Enema (once per week for 6 weeks) | 38 | Patchy inflammation and rectal abscess (n = 2) Abdominal discomfort (n = 1) C. difficile infection (n = 1)—after end of study |
Antibiotic therapy (n = 2) |
| Placebo | 37 | Worsening colitis (n = 1) Patchy inflammation and rectal abscess (n = 1) |
Colectomy (n = 1) Antibiotic therapy (n = 1) |
|
| Nishida 2017 | Single colonoscopy | 41 | No adverse events | |
| Okahara 2020 | Single colonoscopy | 55 | Nausea (n = 20) | None |
| Paramsothy 2017 | Colonoscopy and enema (×5 per week for 8 weeks) | 41 | Infection-related adverse event (n = 10) Serious adverse event (n = 2) Abdominal pain (n = 12) Colitis (n = 10) Flatulence (n = 10) Bloating (n = 8) Upper respiratory tract infection (n = 7) Headache (n = 4) Dizziness (n = 3) Fever (n = 3) Rash (n = 3) |
Colectomy (n = 1), intravenous corticosteroid therapy (n = 1) |
| Placebo | 40 | Infection-related adverse event (n = 14) Serious adverse event (n = 1) Abdominal pain (n = 11) Colitis (n = 9) Flatulence (n = 8) Bloating (n = 11) Upper respiratory tract infection (n = 6) Headache (n = 2) Dizziness (n = 3) Fever (n = 2) |
Hospitalization (n = 1) | |
| Rossen 2015 | Donor FMT by nasoduodenal tube (twice, 3 weeks apart) | 23 | Discomfort with tube placement (n = 1) Fever (n = 2) Nausea (n = 2) Diarhea (n = 5) Headache (n = 1) Vomited fecal infusion (n = 2) Vomiting (n = 1) Abdominal pain (n = 1) Transient borborygmus (n = 4) Mild constipation (n = 1) |
|
| Autologous FMT by nasoduodenal tube (twice, 3 weeks apart) | 25 | Discomfort with tube placement (n = 1) Nausea (n = 1) Malaise (n = 1) Diarrhea (n = 1) Headache (n = 1) Abdominal cramps (n = 6) Abdominal pain (n = 4) Transient borborygmus (n = 8) Dizziness (n = 1) Cytomegalovirus infection (n = 1)—7 weeks after the first FMT; unrelated to treatment |
Ganciclovir (n = 1) | |
| 50 | Severe small bowel Crohn’s disease (n = 1) Abdominal pain (n = 1)—after 11 weeks Cervix carcinoma (n = 1)—after 6 weeks; unrelated to treatment |
Antibiotics (n = 1) | ||
| Schierova 2020 | Enema 5× for first week then weekly × 6 weeks | 8 | No adverse events | None |
| Sokol 2020 | Single colonoscopy | 8 | Gastroenteritis (n = 2) Transient asthenia (n = 1) Cutaneous abscess (n = 1) |
Self-limiting |
| Sood 2019 | Colonoscopy at 0, 2, 6, 10, 14, 18, 22 weeks | 41 | After FMT, at 0 weeks: Abdominal discomfort (n = 26) Abdominal distension (n = 14) Fever (n = 4) Worsening diarrhea (n = 4) Flatulence (n = 2) Fatigue (n = 2) |
Symptoms were self-limiting Oral rehydration solution (n = 4) |
| Sood 2020 | Colonoscopy at 0, 2, 6, 10, 14, 18, 22 weeks | 93 | Abdominal discomfort (n = 28) Flatulence (n = 12) Borborygmi (n = 10) Low grade fever (n = 8) Diarrhea (n = 7) |
Self-limiting |
| Uygun 2017 | Single colonoscopy | 30 | Nausea, vomiting, abdominal pain, diarrhea (n = 7) | NR |
| Vaughn 2016 | Single colonoscopy | 19 | Hives (n = 1) | Oral steroids (n = 1) |
| Vermeire 2016 | Naso-jejunal or rectal tube (twice one day, then the following day) | 14 | High fever (n = 4)—few hours after FMT Vomited and pneumonia (n = 1)—after FMT |
Paracetamol (n = 4) Broad-spectrum antibiotics (n = 1) |
| Wang 2020 | Colonoscopy ×3; 2–3 month intervals |
16 | None | None |
| Wei 2015 | Single colonoscopy or naso-jejunal tube | 14 | Intolerance with FMT (n = 1) Moderate fever (n = 2)—after FMT |
Self-limiting |
| Yang 2019 | Gastroscopy or colonoscopy (twice, one week apart) | 31 | Nausea (n = 1) Reflux (n = 4) Belching (n = 2) Diarrhea (n = 10) Constipation (n = 1) Fever (n = 2) Aggravation of abdominal pain (n = 5) Abdominal distension (n = 3) |
NR |
| Zhang 2016 | Single endoscopy | 19 | Transient increased diarrhea (n = 7) Mild skin pruritus (n = 1) Borborygmus (n = 2) |
- |
NR—Not recorded.
4. Discussion
To our knowledge, we present the first systematic review and meta-analysis evaluating the effects of antibiotic pre-treatment and repeated FMT approaches on improving response in patients with IBD response. Notably, our meta-analysis revealed that repeated FMT and antibiotic pre-treatment were associated with improvements in both pooled IBD response and pooled remission rates. These improvements were associated with key changes in fecal microbial composition such as increased bacterial richness, α-diversity and relative abundance of anaerobes purported to produce SCFAs. Taken together, our findings are novel in that they highlight the potential of these microbiota-targeted strategies to optimize the efficacy of FMT for the management of IBD.
Our findings are in agreement with previous systematic reviews and meta-analyses examining the impact of FMT as a therapy for IBD. In 2014, Colman et al. first identified a lack of literature characterizing FMT treatment efficacy despite publications investigating FMT therapy for IBD more than doubling since 2012 [64]. The systematic review and meta-analysis of 18 studies consisting of 122 IBD patients by Colman and colleagues further revealed that the pooled proportion of patients achieving clinical remission was 36.2% (95% CI 17.4–60.4%). The authors concluded that, while FMT demonstrated variable efficacy, further rigorously designed RCTs were needed to determine efficacy, with a particular need for studies that investigate the effects of FMT frequency and route of administration. More recently, Imdad et al. conducted a 2018 Cochrane review examining FMT therapy on IBD response and remission [65]. Four studies with a total of 277 UC patients were identified and revealed an improved clinical response (RR 1.70; 95% CI 0.98–2.95) and endoscopic remission (RR 2.96; 95% CI 1.60–5.48) for patients receiving FMT vs. placebo. These systematic reviews were, however, limited by a lack of high-quality RCTs and standardized fecal microbiota analysis. Our study addresses a number of these gaps by evaluating both high-quality RCTs and cohort studies, which allowed us to specifically characterize the impact of FMT frequency and antibiotic pre-treatment on IBD outcomes.
Repeated FMT strategies have been employed with variable success in a number of different clinical entities thought to be associated with imbalances in host-microbial ecology [67,68,69]. Perhaps the most compelling evidence for repeated FMT is observed in the Clostridiodes difficile infection (CDI) literature. In a recent systematic review and meta-analysis by Baunwall et al., repeated FMT was found to be superior to single-dose FMT in management of recurrent CDI (91% vs. 84%) [69]. Similarly, El-Salhy et al. demonstrated an increased clinical efficacy for repeated FMT dosing in patients with irritable bowel syndrome, albeit in a small case series of 10 patients [68]. Lastly, in a double-blinded placebo-controlled pilot trial, repeated FMT in patients with obesity and metabolic syndrome demonstrated successful engraftment of donor derived microbes, but without any clinical improvements in host metabolic parameters [67]. These inconsistencies are in large part due to the dramatic study heterogeneity with respect to donor selection, FMT preparation and route of delivery, as well as underlying differences in host-gut microbiome interactions implicated in disease pathophysiology [70]. Notwithstanding, our study findings indeed suggest that repeated FMT dosing provides a promising approach to improve IBD outcomes by facilitating donor microbe engraftment, increase α-diversity, and promote SCFA producing taxa.
Ongoing debate exists regarding the pre-treatment of recipients with antibiotics prior to FMT to increase efficacy [71,72]. Conceptually, antibiotic pre-treatment helps provides a proverbial ecological clean slate for the engraftment of donor microbes by freeing up otherwise occupied niches. Elegant work by Ji et al. compared antibiotic pre-treatment versus bowel cleansing or no pre-treatment in mice prior to FMT. The authors demonstrated that FMT efficacy was dependent on the number of niches available for donor microbe engraftment [73]. Further, they found that antibiotic pre-treatment proved to be the most effective strategy for enhancing host gut microbiota reprogramming by increasing donor microbe colonization. Work by Freitag et al., on the other hand, demonstrated that antibiotic pretreatment prior to FMT in mice had only minor effects on overall donor microbial engraftment [71]. Antibiotics disrupted pre-FMT host microbial communities, yet only select donor-derived bacterial taxa such as Bifidobacterium were increased and no improvements in overall similarity to the donor microbiota were noted. Indeed, questions remain regarding the optimal antibiotic regimens required to make niches accessible, which niches should be targeted for FMT re-colonization, and whether the potential benefit surpasses the potential harm associated with antibiotic resistance and CDI. While our findings are promising as they show improvements in IBD remission and relapse for groups receiving antibiotic pre-treatment prior to FMT, further studies are needed that evaluate the mechanisms and implications of similar approach on IBD.
We acknowledge that our systematic review and meta-analysis has a number of important limitations. Pooled analysis of our primary outcomes demonstrated a high degree of heterogeneity and does not allow for direct comparison of effect size associated with either repeated FMT or antibiotic pre-treatment regiments. The heterogeneity of our results was extensive and, in a large part, due to differences in study design, FMT regimens and individualized responses to FMT. In general, the administration and preparation of FMT is not standardized with practice patterns varying dramatically. Major differences in route of delivery, donor selection, dosing rationale, and antibiotic pre-treatment regimen are all likely to promote inter-study heterogeneity in our review. Follow-up timeframes also ranged from two weeks to 13 years, with nearly half of the studies having a follow up <3 months. This may have introduced a bias towards more favorable clinical response and remission rates following FMT therapy. Therefore, arguments can be made that, given the immense variability of such disparate study interventions, more focused inclusion criteria are warranted in future studies. As this is the first IBD review to evaluate repeated FMT and antibiotic pretreatment concepts, we elected a priori to broadly include all potentially relevant literature in order to highlight current limitations and to allow for explorative hypothesis generation.
Correlations regarding outcomes and antibiotic pre-treatment should also be interpreted with caution given the small proportion of patients within included studies and the lack of direct comparison with patients receiving FMT alone. Histologic assessments pre- and post- FMT were also not consistently reported across studies hindering our ability to evaluate the histologic effects of FMT on disease activity, or the effects of FMT on mucosal adherent bacterial communities. The findings of our review also heavily favored patients with UC and are therefore less generalizable to CD. Additionally, consistent reporting and analysis of fecal microbiota compositional data for both donors and patients were not reported across all studies, which limits the ability to elucidate potential underlying features of the gut microbiome important for optimizing clinical efficacy. Finally, our literature search revealed a number of abstracts and protocols not ultimately published as final manuscripts, which is indicative of publication bias in the FMT literature.
Despite these limitations, our study provides the first systematic review and meta-analysis that evaluates the impact of two key microbial-based strategies which optimize the efficacy of FMT on IBD outcomes. Results of this study may have a number of important implications. Firstly, we demonstrate that repeated FMT dosing and antibiotic pre-treatment approaches have a promising role in optimizing IBD remission and response rates following FMT. Second, results of this study also highlight a need for standardization of FMT therapy protocols (donor, dose, delivery, and pre-treatment) and reporting of microbial data as the lack of this data seen in current practices preclude meaningful meta-analysis of microbial ecology. Lastly, additional high quality randomized trials are needed which directly compare these two strategies in order to help overcome the high degree of heterogeneity in present studies and to elucidate the mechanisms through which these improved outcomes occur. Only through such standardization practices can we eventually bring tailored microbial transplant therapies from the forefront of current IBD research to standard clinical practice.
5. Conclusions
Repeated fecal microbial transplantation and antibiotic pre-treatment engraftment strategies in patients with IBD were associated with improvements in pooled response and remission rates following FMT. These improvements were associated with an increase in fecal microbiota richness, α-diversity, and several SCFA-producing anaerobic taxa. Further standardization of FMT therapies is required to bring microbial-targeted therapies based on FMT from the forefront of current IBD research to modern clinical practice.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/5/959/s1, Table S1: Full-text search strategy of included databases; Table S2: Newcastle-Ottawa scale for assessing risk of bias for included cohort studies; Table S3: Cochrane risk of bias assessment for included randomized trials.
Author Contributions
Conceptualization, V.M., J.D., S.R.; methodology, V.M., J.D., S.R., J.Y.K., E.C.D., K.L.M.; software, V.M., S.R., J.D.; validation, V.M., S.R., E.C.D.; formal analysis, V.M., S.R., J.D.; data curation, V.M., S.R.; writing—original draft preparation, V.M., J.Y.K., S.R., K.L.M.; writing—review and editing, V.M., J.D., J.Y.K., E.C.D., K.L.M.; supervision, K.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
Mocanu is funded by the Canadian Institute of Health Research (CIHR) through a Vanier doctoral scholarship.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the systematic review study design.
Informed Consent Statement
Informed consent was waived for this study as this was a systematic review.
Conflicts of Interest
The authors have no conflict to disclose.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Loftus E.V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Nikolaus S., Schreiber S. Diagnostics of Inflammatory Bowel Disease. Gastroenterology. 2007;133:1670–1689. doi: 10.1053/j.gastro.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology. 2012;142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Palmela C., Chevarin C., Xu Z., Torres J., Sevrin G., Hirten R., Barnich N., Ng S.C., Colombel J.F. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. 2018;67:574–587. doi: 10.1136/gutjnl-2017-314903. [DOI] [PubMed] [Google Scholar]
- 5.Kim D.H., Cheon J.H. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017;17:25–40. doi: 10.4110/in.2017.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H., Tremaroli V., Backhed F. Linking Microbiota to Human Diseases: A Systems Biology Perspective. Trends Endocrinol. Metab. 2015;26:758–770. doi: 10.1016/j.tem.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Wu G.D., Lewis J.D. Analysis of the human gut microbiome and association with disease. Clin. Gastroenterol. Hepatol. 2013;11:774–777. doi: 10.1016/j.cgh.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIlroy J., Ianiro G., Mukhopadhya I., Hansen R., Hold G.L. The gut microbiome in inflammatory bowel disease-avenues for microbial management. Aliment. Pharmacol. Ther. 2018;47:26–42. doi: 10.1111/apt.14384. [DOI] [PubMed] [Google Scholar]
- 9.Philpott J., Ashburn J., Shen B. Efficacy of vedolizumab in patients with antibiotic and anti-tumor necrosis alpha refractory pouchitis. Inflamm. Bowel Dis. 2017;23:E5–E6. doi: 10.1097/MIB.0000000000000992. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm S.M., Love B.L. Management of patients with inflammatory bowel disease: Current and future treatments. Clin. Pharm. 2017;3:83–92. doi: 10.1211/CP.2017.20202316. [DOI] [Google Scholar]
- 11.Furfaro F., Fiorino G., Allocca M., Gilardi D., Danese S. Emerging therapeutic targets and strategies in Crohn’s disease. Expert Rev. Gastroenterol. Hepatol. 2016;10:735–744. doi: 10.1586/17474124.2016.1142372. [DOI] [PubMed] [Google Scholar]
- 12.Abu-Sbeih H., Wang Y. Management Considerations for Immune Checkpoint Inhibitor-Induced Enterocolitis Based on Management of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019 doi: 10.1093/ibd/izz212. [DOI] [PubMed] [Google Scholar]
- 13.Torres J., Danese S., Colombel J. New therapeutic avenues in ulcerative colitis: Thinking out of the box. Gut. 2013;62:1642–1652. doi: 10.1136/gutjnl-2012-303959. [DOI] [PubMed] [Google Scholar]
- 14.Chande N., Costello S.P., Limketkai B.N., Parker C.E., Nguyen T.M., Macdonald J.K., Feagan B.G. Alternative and Complementary Approaches for the Treatment of Inflammatory Bowel Disease: Evidence From Cochrane Reviews. Inflamm. Bowel Dis. 2019;26:843–851. doi: 10.1093/ibd/izz223. [DOI] [PubMed] [Google Scholar]
- 15.Maharshak N., Cohen N.A., Reshef L., Tulchinsky H., Gophna U., Dotan I. Low enteric microbial diversity in patients with ulcerative colitis after pouch surgery having a mature normal ileal pouch may be predictive of pouchitis. J. Crohn’s Colitis. 2016;10:S490. doi: 10.1093/ecco-jcc/jjw019. [DOI] [Google Scholar]
- 16.Ishikawa D., Osada T., Sasaki T., Kuwahara-Arai K., Haga K., Shibuya T., Kodani T., Hiramatsu K., Watanabe S. Alterations of intestinal microbiota in ulcerative colitis patients treated with sequential antibiotic combination and faecal microbiota transplantation. J. Crohn’s Colitis. 2015;9:S364–S365. doi: 10.1093/ecco-jcc/jju027. [DOI] [Google Scholar]
- 17.Zuo T., Ng S.C. The Gut Microbiota in the Pathogenesis and Therapeutics of Inflammatory Bowel Disease. Front. Microbiol. 2018;9:2247. doi: 10.3389/fmicb.2018.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pigneur B., Sokol H. Fecal microbiota transplantation in inflammatory bowel disease: The quest for the holy grail. Mucosal Immunol. 2016;9:1360–1365. doi: 10.1038/mi.2016.67. [DOI] [PubMed] [Google Scholar]
- 19.Gallo A., Passaro G., Gasbarrini A., Landolfi R., Montalto M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J. Gastroenterol. 2016;22:7186–7202. doi: 10.3748/wjg.v22.i32.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng W., Shen J., Bo T., Peng L., Xu H., Nasser M.I., Zhuang Q., Zhao M. Cutting Edge: Probiotics and Fecal Microbiota Transplantation in Immunomodulation. J. Immunol. Res. 2019;2019:1603758. doi: 10.1155/2019/1603758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weingarden A.R., Vaughn B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8:238–252. doi: 10.1080/19490976.2017.1290757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paramsothy S., Paramsothy R., Kamm M.A., Kaakoush N.O., Mitchell H.M., Rubin D.T., Castano-Rodriguez N. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. Gastroenterology. 2017;152:S1007. doi: 10.1016/S0016-5085(17)33417-0. [DOI] [PubMed] [Google Scholar]
- 23.Khoruts A., Rank K.M., Newman K.M., Viskocil K., Vaughn B.P., Hamilton M.J., Sadowsky M.J. Inflammatory Bowel Disease Affects the Outcome of Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection. Clin. Gastroenterol. Hepatol. 2016;14:1433–1438. doi: 10.1016/j.cgh.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna S., Vazquez-Baeza Y., Gonazlez A., Weiss S., Schmidt B., Muniz-Pedrogo D.A., Rainey J.F., Kammer P., Nelson H., Sadowsky M., et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C-difficile infection affected by underlying inflammatory bowel disease. Microbiome. 2017;5:1–8. doi: 10.1186/s40168-017-0269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoruts A., Sadowsky M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016;13:508–516. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa D., Takahashi M., Ito S., Okahara K., Haga K., Kamei M., Nomura O., Shibuya T., Osada T., Nagahara A. Eradication of dysbiotic indigenous species by multiple antibiotic pre-treatment contribute to effective faecal microbiota transplantation. J. Crohn’s Colitis. 2018;12:S553. doi: 10.1093/ecco-jcc/jjx180.990. [DOI] [Google Scholar]
- 28.Ishikawa D., Sasaki T., Takahashi M., Okahara K., Ito S., Haga K., Shibuya T., Osada T., Nagahara A. Combination therapy of fresh fecal microbial transplantation and triple-antibiotic therapy for ulcerative colitis. Am. J. Gastroenterol. 2019;114:S14. doi: 10.14309/01.ajg.0000578280.72223.d3. [DOI] [Google Scholar]
- 29.Shimizu H., Ohnishi E., Arai K., Takeuchi I., Kamura H., Hata K. Outcome of the repetitive fecal microbiota transplantation using fecal solution prepared under the anaerobic condition following the antibiotic pretreatment in eight children with ulcerative colitis. Inflamm. Bowel Dis. 2019;25:S21–S22. doi: 10.1093/ibd/izy393.048. [DOI] [Google Scholar]
- 30.Blesl A., Rainer F., Wurm P., Durdevic M., Petritsch W., Wenzl H., Baumann-Durchschein F., Posch A., Streit A., Gorkiewicz G., et al. Predictors of non-response to repeated faecal microbiota transplantation in patients with therapy refractory ulcerative colitis. J. Crohn’s Colitis. 2019;13:S412. doi: 10.1093/ecco-jcc/jjy222.718. [DOI] [Google Scholar]
- 31.Borody T.J., Warren E.F., Leis S., Surace R., Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J. Clin. Gastroenterol. 2003;37:42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa D., Okahara K., Ito S., Takahashi M., Haga K., Nomura K., Shibuya T., Nagahara A. Efficacy of combination of fresh fecal microbiota transplantation and triple-antibiotic therapy for ulcerative colitis. Inflamm. Bowel Dis. 2019;25:S73. doi: 10.1093/ibd/izy393.183. [DOI] [Google Scholar]
- 33.Ishikawa D., Takahashi M., Okahara K., Ito S., Haga K., Shibuya T., Osada T., Nagahara A. Efficacy of combination therapy of fresh faecal microbiota transplantation and triple-antibiotic therapy for ulcerative colitis. J. Crohn’s Colitis. 2019;13:S301. doi: 10.1093/ecco-jcc/jjy222.511. [DOI] [Google Scholar]
- 34.Wei Y., Zhu W., Gong J., Guo D., Gu L., Li N., Li J. Fecal Microbiota Transplantation Improves the Quality of Life in Patients with Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2015;2015:517597. doi: 10.1155/2015/517597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costello S.P., Hughes P.A., Waters O., Bryant R.V., Vincent A.D., Blatchford P., Katsikeros R., Makanyanga J., Campaniello M.A., Mavrangelos C., et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA. 2019;321:156–164. doi: 10.1001/jama.2018.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sood A., Mahajan R., Singh A., Midha V., Mehta V., Narang V., Singh T., Singh Pannu A. Role of Faecal Microbiota Transplantation for Maintenance of Remission in Patients With Ulcerative Colitis: A Pilot Study. J. Crohn’s. Colitis. 2019;13:1311–1317. doi: 10.1093/ecco-jcc/jjz060. [DOI] [PubMed] [Google Scholar]
- 37.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 38.Higgins J.P.T. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Cochrane Collaboration; London, UK: 2011. [(accessed on 19 February 2021)]. Available online: https://ci.nii.ac.jp/naid/20000796633/ [Google Scholar]
- 39.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;10:1–10. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Chapter 10: Analysing Data and Undertaking Meta-Analyses. Cochrane; London, UK: 2019. [Google Scholar]
- 41.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;3:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen M., Liu X.L., Zhang Y.J., Nie Y.Z., Wu K.C., Shi Y.Q. Efficacy and safety of fecal microbiota transplantation by washed preparation in patients with moderate to severely active ulcerative colitis. J. Dig. Dis. 2020;21:621–628. doi: 10.1111/1751-2980.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H.T., Huang H.L., Xu H.M., Luo Q.L., He J., Li Y.Q., Zhou Y.L., Nie Y.Q., Zhou Y.J. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp. Ther. Med. 2020;19:2650–2660. doi: 10.3892/etm.2020.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cold F., Browne P.D., Gunther S., Halkjaer S.I., Petersen A.M., Al-Gibouri Z., Hansen L.H., Christensen A.H. Multidonor FMT capsules improve symptoms and decrease fecal calprotectin in ulcerative colitis patients while treated—An open-label pilot study. Scand. J. Gastroenterol. 2019;54:289–296. doi: 10.1080/00365521.2019.1585939. [DOI] [PubMed] [Google Scholar]
- 45.Cui B., Feng Q., Wang H., Wang M., Peng Z., Li P., Huang G., Liu Z., Wu P., Fan Z., et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: Safety, feasibility, and efficacy trial results. J. Gastroenterol. Hepatol. 2015;30:51–58. doi: 10.1111/jgh.12727. [DOI] [PubMed] [Google Scholar]
- 46.Dang X.F., Wang Q.X., Yin Z., Sun L., Yang W.H. Recurrence of moderate to severe ulcerative colitis after fecal microbiota transplantation treatment and the efficacy of re-FMT: A case series. BMC Gastroenterol. 2020;20:401. doi: 10.1186/s12876-020-01548-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damman C.J., Brittnacher M.J., Westerhoff M., Hayden H.S., Radey M., Hager K.R., Marquis S.R., Miller S.I., Zisman T.L. Low Level Engraftment and Improvement following a Single Colonoscopic Administration of Fecal Microbiota to Patients with Ulcerative Colitis. PLoS ONE. 2015;10:e0133925. doi: 10.1371/journal.pone.0133925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishikawa D., Sasaki T., Osada T., Kuwahara-Arai K., Haga K., Shibuya T., Hiramatsu K., Watanabe S. Changes in Intestinal Microbiota Following Combination Therapy with Fecal Microbial Transplantation and Antibiotics for Ulcerative Colitis. Inflamm. Bowel Dis. 2017;23:116–125. doi: 10.1097/MIB.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 49.Jacob V., Crawford C., Cohen-Mekelburg S., Viladomiu M., Putzel G.G., Schneider Y., Chabouni F., O’Neil S., Bosworth B., Woo V., et al. Single Delivery of High-Diversity Fecal Microbiota Preparation by Colonoscopy Is Safe and Effective in Increasing Microbial Diversity in Active Ulcerative Colitis. Inflamm. Bowel Dis. 2017;23:903–911. doi: 10.1097/MIB.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kump P., Wurm P., Grochenig H.P., Wenzl H., Petritsch W., Halwachs B., Wagner M., Stadlbauer V., Eherer A., Hoffmann K.M., et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment. Pharmacol. Ther. 2018;47:67–77. doi: 10.1111/apt.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mizuno S., Nanki K., Matsuoka K., Saigusa K., Ono K., Arai M., Sugimoto S., Kiyohara H., Nakashima M., Takeshita K., et al. Single fecal microbiota transplantation failed to change intestinal microbiota and had limited effectiveness against ulcerative colitis in Japanese patients. Intest. Res. 2017;15:68–74. doi: 10.5217/ir.2017.15.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moayyedi P., Surette M.G., Kim P.T., Libertucci J., Wolfe M., Onischi C., Armstrong D., Marshall J.K., Kassam Z., Reinisch W., et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149:102–109. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Nishida A., Imaeda H., Ohno M., Inatomi O., Bamba S., Sugimoto M., Andoh A. Efficacy and safety of single fecal microbiota transplantation for Japanese patients with mild to moderately active ulcerative colitis. J. Gastroenterol. 2017;52:476–482. doi: 10.1007/s00535-016-1271-4. [DOI] [PubMed] [Google Scholar]
- 54.Okahara K., Ishikawa D., Nomura K., Ito S., Haga K., Takahashi M., Shibuya T., Osada T., Nagahara A. Matching between Donors and Ulcerative Colitis Patients Is Important for Long-Term Maintenance after Fecal Microbiota Transplantation. J. Clin. Med. 2020;9:1650. doi: 10.3390/jcm9061650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paramsothy S., Kamm M.A., Kaakoush N.O., Walsh A.J., van den Bogaerde J., Samuel D., Leong R.W.L., Connor S., Ng W., Paramsothy R., et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet. 2017;389:1218–1228. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 56.Rossen N.G., MacDonald J.K., de Vries E.M., D’Haens G.R., de Vos W.M., Zoetendal E.G., Ponsioen C.Y. Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J. Gastroenterol. 2015;21:5359–5371. doi: 10.3748/wjg.v21.i17.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schierova D., Brezina J., Mrazek J., Fliegerova K.O., Kvasnova S., Bajer L., Drastich P. Gut Microbiome Changes in Patients with Active Left-Sided Ulcerative Colitis after Fecal Microbiome Transplantation and Topical 5-aminosalicylic Acid Therapy. Cells. 2020;9:2283. doi: 10.3390/cells9102283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokol H., Landman C., Seksik P., Berard L., Montil M., Nion-Larmurier I., Bourrier A., Le Gall G., Lalande V., De Rougemont A., et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome. 2020;8:12. doi: 10.1186/s40168-020-0792-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sood A., Mahajan R., Juyal G., Midha V., Grewal C.S., Mehta V., Singh A., Joshi M.C., Narang V., Kaur K., et al. Efficacy of fecal microbiota therapy in steroid dependent ulcerative colitis: A real world intention-to-treat analysis. Intest. Res. 2019;17:78–86. doi: 10.5217/ir.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sood A., Singh A., Mahajan R., Midha V., Kaur K., Singh D., Bansal N., Dharni K. Clinical Predictors of response to Faecal Microbiota Transplantation in patients with active ulcerative colitis. J. Crohn’s. Colitis. 2020:jjaa163. doi: 10.1093/ecco-jcc/jjaa163. [DOI] [PubMed] [Google Scholar]
- 61.Uygun A., Ozturk K., Demirci H., Oger C., Avci I.Y., Turker T., Gulsen M. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine. 2017;96:e6479. doi: 10.1097/MD.0000000000006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaughn B.P., Vatanen T., Allegretti J.R., Bai A., Xavier R.J., Korzenik J., Gevers D., Ting A., Robson S.C., Moss A.C. Increased Intestinal Microbial Diversity Following Fecal Microbiota Transplant for Active Crohn’s Disease. Inflamm. Bowel Dis. 2016;22:2182–2190. doi: 10.1097/MIB.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vermeire S., Joossens M., Verbeke K., Wang J., Machiels K., Sabino J., Ferrante M., Van Assche G., Rutgeerts P., Raes J. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J. Crohn’s. Colitis. 2016;10:387–394. doi: 10.1093/ecco-jcc/jjv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y., Ren R., Sun G., Peng L., Tian Y., Yang Y. Pilot study of cytokine changes evaluation after fecal microbiota transplantation in patients with ulcerative colitis. Int. Immunopharmacol. 2020;85:106661. doi: 10.1016/j.intimp.2020.106661. [DOI] [PubMed] [Google Scholar]
- 65.Yang Z., Bu C., Yuan W., Shen Z., Quan Y., Wu S., Zhu C. Fecal Microbiota Transplant via Endoscopic Delivering Through Small Intestine and Colon: No Difference for Crohn’s Disease. Dig. Dis. Sci. 2020;65:150–157. doi: 10.1007/s10620-019-05751-y. [DOI] [PubMed] [Google Scholar]
- 66.Zhang T., Cui B., Li P., Zhang F. Short-term surveillance of cytokines and CRP cannot predict efficacy of fecal microbiota transplantation for ulcerative colitis. Gastroenterology. 2016;150:S380–S381. doi: 10.1016/S0016-5085(16)31339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu E.W., Gao L., Stastka P., Cheney M.C., Mahabamunuge J., Torres Soto M., Ford C.B., Bryant J.A., Henn M.R., Hohmann E.L. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLOS Med. 2020;17:e1003051. doi: 10.1371/journal.pmed.1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Salhy M., Mazzawi T. Fecal microbiota transplantation for managing irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2018;12:439–445. doi: 10.1080/17474124.2018.1447380. [DOI] [PubMed] [Google Scholar]
- 69.Baunwall S.M., Lee M.M., Eriksen M.K., Mullish B.H., Marchesi J.R., Dahlerup J.F., Hvas C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine. 2020;29:100642. doi: 10.1016/j.eclinm.2020.100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim K.O., Gluck M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019;52:137–143. doi: 10.5946/ce.2019.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freitag T.L., Hartikainen A., Jouhten H., Sahl C., Meri S., Anttila V.-J., Mattila E., Arkkila P., Jalanka J., Satokari R. Minor Effect of Antibiotic Pre-treatment on the Engraftment of Donor Microbiota in Fecal Transplantation in Mice. Front. Microbiol. 2019;10:2685. doi: 10.3389/fmicb.2019.02685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oliphant K., Cochrane K., Schroeter K., Daigneault M.C., Yen S., Verdu E.F., Allen-Vercoe E. Effects of Antibiotic Pretreatment of an Ulcerative Colitis-Derived Fecal Microbial Community on the Integration of Therapeutic Bacteria In Vitro. mSystems. 2020;5:e00404-19. doi: 10.1128/mSystems.00404-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji S.K., Yan H., Jiang T., Guo C.Y., Liu J.J., Dong S.Z., Yang K.L., Wang Y.J., Cao Z.J., Li S.L. Preparing the Gut with Antibiotics Enhances Gut Microbiota Reprogramming Efficiency by Promoting Xenomicrobiota Colonization. Front. Microbiol. 2017;8:1208. doi: 10.3389/fmicb.2017.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.