Abstract
The calcium Sensing Receptor (CaSR) is a cell surface receptor belonging to the family of G-protein coupled receptors. CaSR is mainly expressed by parathyroid glands, kidneys, bone, skin, adipose tissue, the gut, the nervous system, and the cardiovascular system. The receptor, as its name implies is involved in sensing calcium fluctuations in the extracellular matrix of cells, thereby having a major impact on the mineral homeostasis in humans. Besides calcium ions, the receptor is also activated by other di- and tri-valent cations, polypeptides, polyamines, antibiotics, calcilytics and calcimimetics, which upon binding induce intracellular signaling pathways. Recent studies have demonstrated that CaSR influences a wide variety of cells and processes that are involved in inflammation, the cardiovascular system, such as vascular calcification, atherosclerosis, myocardial infarction, hypertension, and obesity. Therefore, in this review, the current understanding of the role that CaSR plays in inflammation and its consequences on the cardiovascular system will be highlighted.
Keywords: calcium-sensing receptor, CaSR, G-protein coupled receptors, inflammation, cardiovascular disorders, atherosclerosis, vascular calcification, myocardial ischemia, adipose tissue, hypertension
1. Introduction
The calcium sensing receptor or CaSR, as the name suggests, is a receptor that senses calcium in the extracellular environment of a cell. The human CASR gene is located on chromosome 3 and is comprised of 8 exons [1]. Exons 2 to 7 encode for a 1078 amino acid G protein coupled receptor, present on the surface of cells. The receptor was first cloned a bit more than 25 years ago [2] and since then it has been elaborately studied in various pathologies. CaSR is present in various organs and its exact function can differ based on its location. CaSR is not only activated by calcium ions, but by numerous ligands that including various cations, polyamines, polypeptides and antibiotics [3]. The combination of this wide variety of ligands triggers intricate intracellular signaling networks, which eventually influence many physiological and pathological processes.
The role of CaSR in mineral homeostasis has been widely studied due to its presence in calcitropic tissues such as kidneys, bone, and parathyroid glands. However, more recently the role that CaSR plays in the (patho)physiology of other metabolic processes is also being intensively investigated, while the role it plays in cardiovascular health is gaining scientific interest. This is especially fueled by the observation that CaSR is expressed on the surface of vascular and hematopoietic cells, which play a vital role in inflammatory processes and thereby has subsequent repercussions on cardiovascular diseases (CVDs).
A variety of studies have already been performed to investigate the effect of CaSR on inflammation and cardiovascular biology. This review will therefore highlight the major findings to give a comprehensive overview of different signaling pathways activated by CaSR and the pathological consequences of this activation on inflammation and especially in tissues relevant to CVD.
2. CaSR Structure and Regulation
The CaSR belongs to the family of G-protein coupled receptors, also known as the seven transmembrane domain receptors. First discovered in 1993, CaSR was the first membrane protein observed to have ion sensing capacities. It can sense even miniscule fluctuations of extracellular calcium in the human body [4]. The receptor is known for its ability to activate different intracellular signaling pathways based on its location and the type of ligand to which it binds to. CaSR is present in a multitude of organs, like parathyroid glands, kidney, bone, skin, the gut, pancreas, lungs, and heart. Concerning the ligands that bind to the receptor, extracellular calcium ions are not the only potent agonist of this receptor. There are a wide range of ions that can act as a ligand for the CaSR and these can act together to activate the receptor and induce intracellular signaling. The CaSR responds to di- and tri-valent cations including Sr2+, Ba2+, Mg2+, Ba2+, Mn2+, Ni2+, Gd3+, La3+ and Al3+ [3,5]. While ions such as Ca2+ and Mg2+ act as activators, sulphates act as negative allosteric modulators as well as stabilizers for the active confirmation of the receptor [6]. In addition, serum phosphate [7] binding to the receptor is considered necessary to maintain its structural integrity in its inactive state as well as regulate its activity. Polycations also tend to be agonists for which the effectiveness is dependent on the positive charge. These cations also often act together with amino acids to activate the receptor. Apart from ions, CaSR is also activated by polyamines such as spermines and spermidines, polypeptides such as polylysine and polyarginine, amyloid B peptides, glutathione and aminoglycoside antibiotics [3]. Upon binding of a ligand to the receptor, CaSR can induce various cellular processes, such as secretion of hormones, chemotaxis, apoptosis, proliferation and differentiation [8]. These intracellular signaling processes are believed to be dependent on each other, as well as having the ability to influence each other.
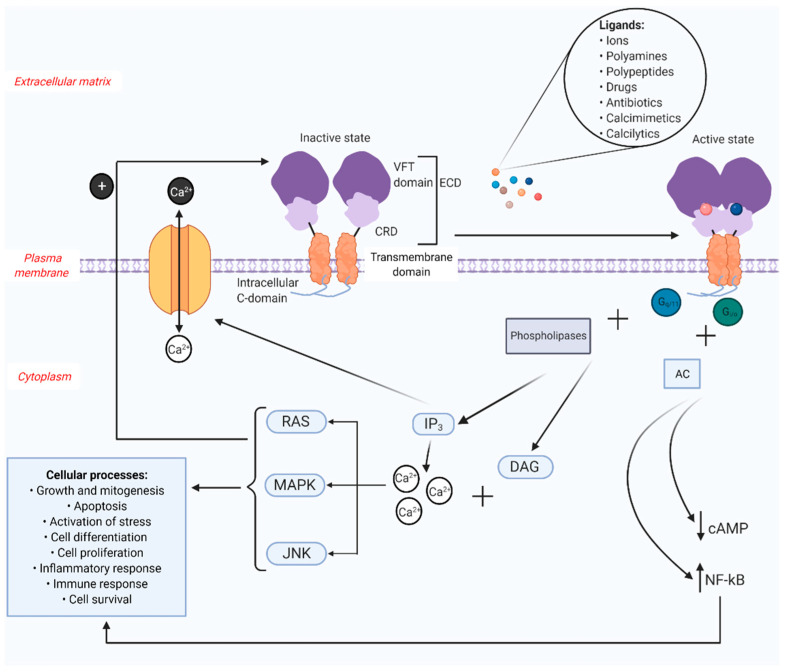
The structures of the inactive and active receptor have been well established [6,9] (Figure 1). The functional receptor exists as a disulfide-linked homodimer that is expressed on the cell surface [10]. It consists of three structural domains and has 1078 amino acids: a large N-terminal extracellular domain (ECD) with a cysteine rich domain (CRD) that links the ECD to a seven transmembrane domain, as well as an intracellular C-terminal domain [11]. The ECD, essential for the binding of ligands, is the biggest and has a characteristic shape resembling the Venus flytrap (VFT) and therefore is named accordingly [12]. This domain has two protomers that each contain a bi-lobed VFT that are separated from each other when the receptor is inactive [6]. When the receptor turns active, usually after an L-amino acid binds to the receptor and closes the groove between the lobes, conformational changes to the receptor occur bringing both the protomers in close proximity of each other [13]. In the C-terminal domain, protein kinase A (PKA) and protein kinase C (PKC) phosphorylation sites are present, which when bound to protein kinases, trigger downstream signaling pathways [14,15]. The intracellular domain is made up of different amino acids that help with lodging the receptor on the cell surface and intracellular signaling.
Figure 1.
Structure and function of CaSR. The CaSR is composed of three structural domains: the extracellular domain (ECD) consisting of a Venus flytrap (VFT) domain and a cysteine rich domain (CRD); the transmembrane domain connected by the CRD to the VFT; and an intracellular C-domain. The extracellular ligands of CaSR include various ions, polyamines, polypeptides, drugs, antibiotics, calcimimetics and calcilytics. These ligands bind to the inactive receptor to initiate conformational changes to the receptor resulting in receptor activation. The active receptor differs from the inactive receptor in such a way that the protomers in the VFT are in close proximity to each other. The surface receptor, when activated by its ligands, can trigger numerous intracellular signaling pathways, which in turn activate different cellular processes. The activation of CaSR couples Gi/o and Gq/11 families of heterotrimeric G proteins. Along with phospholipases, Gq/11 prompts inositol triphosphate (IP3) and diacylglycerol (DAG) pathways. IP3 controls calcium channels on the surface of the cell, thereby regulating the efflux and influx of calcium ions. DAG, along with the cytosolic calcium will activate rat sarcoma (RAS)/mitogen-activated protein kinase (MAPK)/c-Jun n-terminal kinases (JNK) pathways which are involved in the initiation of several cellular processes. These pathways also engage in a feedback mechanism required for the proper functioning of the receptor. Gi/o interacts with adenylate cyclase (AC) to curb the production of cyclic adenosine monophosphate (cAMP). The receptor also upregulates the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), which is again associated with various cellular processes (Created with BioRender.com).
The intracellular signaling by CaSR is expected to occur in different stages (Figure 1). The first stage after receptor activation is the coupling of CaSR to Gi/o and Gq/11 families of heterotrimeric G proteins that consequently activate signaling pathways [16]. The phospholipases are also activated, which in turn activate inositol triphosphate (IP3) and diacylglcerol (DAG) pathways. Gi/o along and adenylate cyclase (AC) inhibits cyclic adenosine monophosphate (cAMP) production. The inositol phosphate pathway oversees the control of the intracellular calcium levels, especially in the endoplasmic reticulum (ER). The second stage is when the activated DAG and the cytosolic calcium activates rat sarcoma (RAS), mitogen-activated protein kinase (MAPK) and c-Jun n-terminal kinases (JNK) pathways [17], resulting in a wide range of cellular processes. These pathways are also known to influence the receptor function using a positive feedback mechanism. Finally, different ionic and pH conditions and calcimimetics usually tend to activate transcription factors complexes such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) to control various cellular processes, which have been mentioned earlier and are visualized in Figure 1.
3. CaSR in Cardiovascular System-Related Cells
3.1. CaSR in Non-Hematopoietic Cardiovascular Cells
Several studies have identified the presence of CaSR on the membrane of vascular cells and indicated that the receptor is an active player regarding the physiology of the cardiovascular system. For the first time, a study by Wang et al. demonstrated the presence of CaSR in cardiac myocytes. It was observed that an increase in the calcium content of the supernatant of the cell culture medium in which rat cardiac myocytes were grown in-vitro also triggered an increase in the intracellular calcium levels and increased cardiac activity [18]. The consistent activation and reactivation of the receptor with the changing calcium levels due to the feedback mechanism helps with the normal contractility of the muscle cells. Therefore, CaSR is not just responsible for regulating the ion channels but also for regulating the membrane potential. Calcium ions enter the cardiac myocytes via L type ion transport channels, sepcially the L type Ca2+ channels (LTCC). This leads to increased secretion of Ca2+ from the endoplasmic and sarcoplasmic reticulum which in turn leads to an increased contractility of the cells. When cultured cardiomyocytes were stimulated with oxLDL in-vitro to imitate hyperlipidemia, it was seen that the expression of CaSR significantly increases. Additionally, an increased rate of apoptosis and angiogenesis through mitochondrial pathways was observed [19].
Perhaps the most important non-hematopoietic cell type in vascular tissues is the smooth muscle cell. This is the cell type that influences and plays a decisive role in the contraction and relaxation of the vasculature. CaSR is expressed in vascular smooth muscle cells (vSMCs) [20]. Activation of CaSR in aortic SMCs allows calcium to enter the cells via receptor-operated channels (ROC). These channels signal intracellularly through the phospholipase C (PLC)/PKCε pathway resulting in a CaSR mediated rise of the intracellular calcium levels [21]. Mice that lack CaSR in SMCs were observed to have various compromised vasculature functions, such as vessel tone and blood pressure [22]. Impaired vascular contractility was also found to be independent of the endothelium and mainly reliant on vSMC CaSR. Mice with impaired vascular contractility were also observed to be hypotensive [22], further confirming an important role of CaSR in the vascular tone.
Besides SMCs and the cardiomyocytes, endothelial cells (ECs) also play an important role in the cardiovascular system as they line every vessel. For example, EC dysfunction is the start of an inflammatory process in the arteries. CaSR is also expressed in aortic ECs, and it is mainly localized to the cytoplasm rather than the cell membrane [23]. This is believed to be caused by the receptor recycling or very high biosynthesis due to different post-translational modifications. Both mRNA and CaSR proteins were discovered in vascular ECs in rat and rabbit mesenteric arteries, porcine coronary arteries [24] and human aorta [25]. Studies indicate that extracellular calcium induces nitric oxide (●NO) production in ECs through the CaSR [26,27].
3.2. CaSR in Hematopoietic Cardiovascular Cells
CaSR is expressed on various immune cells, such as monocytes, macrophages, proerythroblasts, erythroblasts and megakaryocytes [28]. Myeloid precursors also express CaSR but to a lesser extent as compared to other bone marrow cells such as proerythroblasts. Furthermore, it has been demonstrated that CaSR is expressed by T lymphocytes [29], although not by B lymphocytes [30].
The presence of CaSR on the surface of hematopoietic cells contributes majorly to cardiovascular biology as for example the expression of CaSR on monocytes has been implicated in chemotaxis, a key process in inflammatory diseases [31]. CaSR present in macrophages also plays a vital role in micropinocytosis, which facilitates pathogen or tissue damage sensing through pattern recognition [32].
The presence of CaSR in peripheral blood monocytic cells was demonstrated by Olszak et al., who confirmed that the receptor was also present on the cell surface. The expression of CaSR is necessary for the chemotactic response by the cells [33].
4. Pathological Role of CaSR in Inflammation and Cardiovascular Diseases
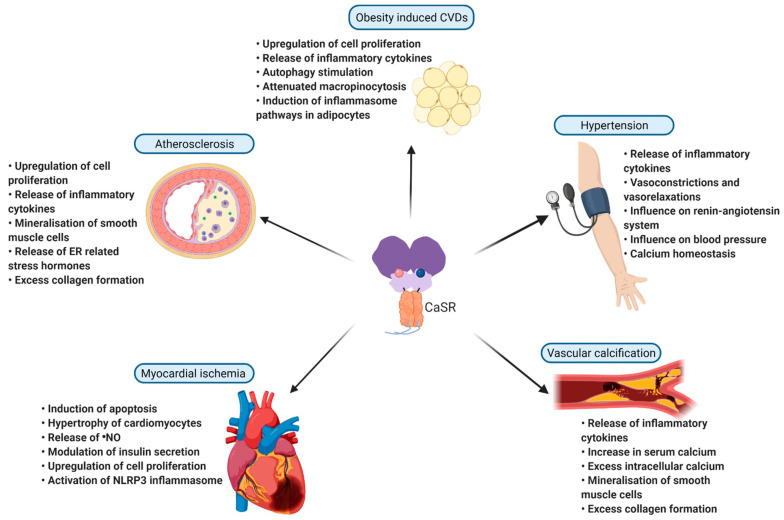
Abnormal expression and function of the CaSR gene has been studied intensively suggesting that the receptor plays an important role in not just calcium homeostasis related disorders but also in various other non-calcium related diseases in humans. It is now well established that almost all components of the cardiovascular system functionally express CaSR, and that this receptor seems to be quite important for maintaining vascular integrity. As of now, there have been more than 230 mutations found within the CASR gene and more than half of which cause the receptor to become dysfunctional [34]. Moreover, CaSR polymorphisms are known to be associated with coronary artery diseases (CADs) such as myocardial infarction (MI) and atherosclerosis [35]. These abnormalities of CaSR in hematopoietic cells and vascular cells contribute to a variety of conditions, especially the promotion of local and systemic inflammation, which are destructive to vascular tissue and eventually cause various morbidities. The pathological role of CaSR will be discussed in more detail below (Figure 2).
Figure 2.
CaSR in CVDs. CaSR is implicated in various cardiovascular disorders, where it exerts its role by influencing various factors involved in the development and progression of the disease. CaSR is involved in myocardial ischemia, vascular calcification, hypertension, atherosclerosis, and obesity induced CVDs. This figure gives a brief summary of the involvement of CaSR in the above-mentioned CVDs and the ways in which CaSR influences each one of them (created with BioRender.com). ER: endoplasmic reticulum; NLRP3: NLR family pyrin domain containing 3; ●NO: nitric oxide.
4.1. Imbalance in Mineral Homeostasis and Cardiovascular Disease
To support an array of cellular functions, the concentration of mineral ions in the blood must be kept within a certain physiological range [36]. When the concentrations increase or decrease outside this range the equilibrium in the mineral homeostasis is disturbed leading to disruption of several metabolomic processes. In this way abnormal mineral homeostasis accelerates the development of cardiovascular disorders, which is supported by the fact that several studies have associated circulating calcium levels with vascular diseases and death. For example, changes in serum calcium levels can independently cause pathological disturbances in vascular tissue such as vascular calcification [37], coronary artery disease and myocardial infarction [38] and it can aggravate blood pressure and blood lipid levels, which are known risk factors of CVD. Studies have calculated an increase in the odds ratio of coronary heart disease and cardiovascular mortality when CaSR genetically varies due to single nucleotide polymorphisms and causes an increase in the circulating serum calcium [35]. Activation of CaSR by gadolinium in vitro in rat ventricular myocytes resulted in the phosphorylation of ERK1/2, JNK and p38 MAPK, the activation of the caspase 9 pathway and induction of apoptosis [39]. This observation was further supported by a study by Lu et al. showing that the disturbed extracellular calcium homeostasis also altered the calcium homeostasis in the Endoplasmic Reticulum (ER) and mitochondria eventually causing apoptosis and release of stress hormones which in turn can induce heart failure [40]. Several heterozygous inactivating mutations of CaSR have been shown to contribute to hypercalcemia, with high PTH values [5]. Nonetheless, familial hypocalciuric hypercalcemia (FHH) patients are found to have a unique variant of the CaSR gene even though they do not have hyperparathyroidism. This novel variant highlighted that serine 147 is an important site on the gene that is essential for sensing calcium as substitution of this site results in a disturbance in the calcium homeostasis [41]. On the other hand, gain-of-function mutations of CaSR have been shown to result in hypocalcaemia as well as hypomagnesemia [42]. Elevated levels of calcium in urine or hypercalciuria are also related to a variant of the CaSR gene, which decreases the expression of the receptor in renal tubules [43,44]. Everything considered, it has become clear that the sensing of calcium, being the main objective of the CaSR, is vital for the protection against cardiovascular disorders.
4.2. Heart Failure
Apoptosis is a type of cell death that occurs in cases of undesirable changes in the cell environment, such inflammation. Cardiomyocyte apoptosis, in response to a variety of stressors, contributes majorly to heart failure. This happens in a manner that enables cells to undergo apoptosis causing increased cardiac fibrosis and an increased number of hypertrophic cardiomyocytes [45]. Apoptosis and hypertrophy have been linked to the calcium homeostasis and CaSR in the vasculature. For example, CaSR activation leads to the activation of pathways that are calcium ion dependent, such as Ca2+/calmodulin-dependent protein kinase II (CaMKII) and calcineurin pathways [46], which are known to induce apoptosis and hypertrophy of cardiac myocytes. Furthermore, angiotensin II (AngII) has been observed to be involved in CaSR induced cardiac fibrosis in-vitro [47]. The presence of AngII increases the expression of CaSR in myocardial tissue which consecutively increases the proliferation of the fibroblasts, as well as their phenotypic transformation. This increased expression of CaSR additionally increases the intracellular calcium release, stimulates autophagy, and results in excess collagen formation and MEK pathway phosphorylation. When cardiomyocytes were injured with excess lipopolysaccharide (LPS) in-vitro, it was observed that CaSR is activated and that the activated CaSR acts via ER stress factors to induce autophagy and further alleviate apoptosis in these cells [48]. Furthermore, neutrophils that are recruited to sites of inflammation and in which CaSR is activated were observed to exacerbate MI by stimulating the apoptosis and myocardial fibrosis. The CaSR-activated neutrophils were seen to upregulate levels of interleukin-1 receptor (IL-1R), matrix metalloproteinase-2 (MMP2), alpha smooth muscle actin (α-SMA), collagen I and collagen III in cardiac fibroblasts via excessive release of IL-1β [49].
Cardiac fibrosis can result in excess matrix mineralization and collagen secretion which also stimulates proliferation and activation of these fibroblasts. There has been concrete proof that CaSR in expressed in cardiac fibroblasts [50]. Cardiac fibrosis is a very important aftermath in cardiomyopathy, along with cardiac remodeling and activation of inflammatory pathways [51]. One specific type of cardiomyopathy is diabetic cardiomyopathy. This is caused by diabetes mellitus, which is characterized by hyperglycemia due to a deficiency of insulin. Recently, it was shown that CaSR is one of the factors that modulates insulin secretion [52]. The constant hyperglycemic condition upregulates the expression of CaSR in cardiac fibroblasts which eventually leads to an increase in the intracellular calcium levels and activation of autophagy and the Smurf2 ubiquitin proteasome which is highly involved in protein degradation [52]. The increased levels of intracellular calcium in cardiac fibroblast also activate the TGF-β1/Smads pathway, which in turn stimulates the proliferation and myocardial fibrosis following activation of cardiac fibroblasts [27]. The above described studies demonstrate that CaSR can play important roles in the various pathological phases of heart failure.
4.3. Atherosclerosis and Vascular Calcification
Atherosclerosis is a chronic inflammatory disease, characterized by the progressive build-up of lipids and immune cells in the artery wall [53]. This pathology is initiated when ECs of the vessel wall are irritated by various stress factors and the subsequent accumulation of low-density lipoproteins (LDL) particles, leading to the chemotactic attraction of monocytes. These monocytes, after infiltrating the arterial wall, differentiate into macrophages and ingest cholesterol and store it in their cytoplasm to become ‘foam cells’ [54] triggering plaque development. During later stages of plaque development, SMCs from the medial layer undergo phenotypical changes and migrate into the intimal layer of the arteries to provide structural support to the growing lesion [55]. The formation and growth of atherosclerotic plaques can impede the blood flow either through vessel occlusion or plaque rupture, thereby causing other cardiovascular morbidities such as MI. Vascular calcification is a comorbidity in patients with hypertension, diabetes and kidney disease and a distinguishing characteristic of atherosclerosis. Vascular calcification associated with atherosclerosis is an active process that resembles bone formation regarding cellular processes [56]. Calcification is mainly caused by the accumulation of minerals in the medial layer of the vasculature wherein the SMCs ingest enormous amounts of minerals, mainly calcium, and are thereby reprogrammed to exhibit an osteo- and/or chondrogenic phenotype [56]. Calcification of aortic valves has also been recognized as an important cardiovascular risk factor in older adults. The valves of the aorta are calcified when valvular interstitial cells, which are the most abundant cells in valves, start expressing an osteoblast-like phenotype. Considering the notion that extracellular calcium is the main trigger for vascular calcification, it is not surprising that the expression of CaSR in interstitial cells is elevated in calcified aortic valves compared to healthy valves [57]. Downregulating CaSR in these cells has also been shown to reduce the calcium induced valvular calcification in-vitro. Valvular calcification is a predictor for valvular stenosis and eventually vascular calcification and atherosclerosis [58,59]. Even though the presence of CaSR in valvular interstitial cells has been discovered and the upregulated expression of the receptor in cases of calcification has been seen in-vitro and in-vivo, there is a lack of information on the exact mechanism by which CaSR influences stenosis in the valves. Atherosclerotic lesions are sources of calcium ions, activating CaSR in miscellaneous vascular cells that consequently stimulate the migration of immune cells to promote further plaque growth. Mineralization of the SMCs is demonstrated to be influenced by CaSR to a greater extent than by the movement of calcium ions via cell surface ion channels [20]. For example, a study demonstrated that overexpression of CaSR in vSMCs reduces calcium deposition by the cells and obstructs their change into a calcifying phenotype [60]. The same study also found that calcimimetics can be used to keep a constant expression of CaSR which inhibits calcification suggesting that this could be a good therapeutic strategy to combat vascular calcification. Further supporting this notion is the observation that increased concentrations of extracellular calcium in case of vascular calcification also activates the NLR family pyrin domain containing 3 (NLRP3) inflammasome and caspase-1 and increases levels of IL-1β in cardiac tissue in a CaSR dependent manner [61]. Moreover, vSMCs in atherosclerotic lesions also express MMP-2 which causes the breakdown of the extracellular matrix and thereby reduces plaque stability [62]. CaSR mediates the production of MMP-2 in the presence of cholesterol via the p13K/Akt pathway and can thereby also modulate plaque stability [63]. Furthermore, activation of CaSR in SMCs activates the MEK1/ERK1/2 pathways and the PLC-IP3 pathway, thereby increasing cell proliferation and survival respectively [64]. All of the in vitro and in vivo studies indicate that CaSR has a protective effect against atherosclerosis and vascular calcification by directly influencing and preventing mineral depositions in atherosclerotic plaques by SMCs.
4.4. Myocardial Infarction
The formation of atherosclerotic plaques can obstruct the blood flow in arteries, for example causing the blood flow to the cardiac muscles to be reduced, thereby reducing the oxygen levels. This diminishes the ability of the cardiac muscles to pump blood and causes abnormal cardiac rhythms and disequilibrium in myocardial metabolism causing a condition called myocardial ischemia [65]. Long-lasting ischemia results in the death of myocardial tissue and leads to the onset of the pathological condition termed MI [66]. The involvement of CaSR in myocardial ischemia is a debatable topic as it promotes ischemia and protects the myocardium against it. For example, the activation of CaSR exerts a protective function against endothelial injury in ischemia or reperfusion injury by inhibiting platelet activation [67]. Addititonaly, it also offers protection to cardiac tissues against apoptosis and oxidative stress caused by this pathological condition [68]. On the other hand, CaSR phosphorylates PKCδ, which is known to regulate intracellular calcium levels [69] by translocating it to the ER resulting in ER related stress hormones to further inducing apoptosis in the vascular cells in-vivo [27]. specifically ECs, the dysfunction of which is a critical factor in myocardial ischemia. ECs secrete inflammatory factors in excess to induce autophagy, apoptosis and ER stress on themselves and other vascular cells to further alleviate the injury [70]. An in vitro study on human umbilical vein endothelial cells (HUVECs) revealed that CaSR regulates the response of these cells to ischemia reperfusion injury. The expression of CaSR influences autophagy and angiogenesis to defer the cell migration and apoptosis, and can thereby play an important role in myocardial ischemia [71].
During MI, a severe inflammatory response is triggered and various immune cells are attracted to the site of inflammation and injury. It has been shown that CaSR not only responds to inflammation but can also further promote it [1]. For example, activation of CaSR in T lymphocytes was shown to result in an increased release of IL-6 and TNFα as cellular responses to stress signals into circulation via MAPK-ERK, MAPK-JNK, and NF-κB [27,29]. This activation causes further deterioration and thereby aggravates the pathological consequences of MI. This is further supported by the observation that silencing of CaSR and thereby blocking of the NF-κB pathway in T lymphocytes reduces the apoptosis rate and the secretion of pro-inflammatory cytokines (Th1 cytokines) and anti-inflammatory cytokines (Th2 cytokines) [29]. Further linking CaSR with MI, is the notion that both CaSR and NLRP3 inflammasome are activated in the peripheral neutrophils upon MI occurrence [49]. Neutrophils play an important role in the onset of MI by releasing various pro-inflammatory cytokines and chemokines. Furthermore, it could be shown that the CaSR-activated neutrophils instigate cardiomyocyte apoptosis and thereby aggravate ischemia [49]. Besides neutrophils, macrophages also play an important role in MI. It has been observed that the activation of CaSR promotes the secretion of IL-1β and TNF-α in monocyte-derived macrophages [27,72]. Furthermore, blocking of CaSR in macrophages attenuates micropinocytosis, which interferes with the ability of the cells to sense nucleotide-binding oligomerization domain-containing protein 2 (NOD2) ligands and delivery of ligands to Toll-like receptors (TLRs) [32]. In summary, CaSR seems to play an important role in ischemia induced MI by influencing various cells and processes.
4.5. Hypertension
Persistent elevation of blood pressure in arteries is a medical condition called hypertension, an extremely important cause of heart failure. Hypertension causes organ failure leading to vascular remodeling, which is distinguished by apoptosis, inflammation and fibrosis [73]. The pathogenesis of hypertension is also promoted by vascular inflammation. Intake of appropriate amounts of dietary calcium is necessary to provide a protective effect on cardiovascular tissues against hypertension. Various studies have already demonstrated that the presence of CaSR in vSMCs is necessary to keep the blood pressure level within the physiological range and to reduce hypertension [22,74]. This influence on blood pressure by CaSR can be explained by its inhibitory effects on the renin-angiotensin-aldosterone system (RAAS) [75]. For example, systemic inhibition of CaSR using a known allosteric inhibitor NPS-2143 results in an elevated blood pressure in normotensive rats [76]. RAAS works through PLC-IP3 pathways, and the unwarranted activation of RAAS can pathologically cause excessive proliferation of vSMCs, vasoconstriction and myocardial hypertrophy [27]. Inhibition of CaSR therefore increases the proliferation of vSMCs and reduces apoptosis. A study conducted on rats with spontaneous hypertension indicated that CaSR is selectively activated in the myocardium triggering reverse cardiac remodeling [77]. Along with hypertension and increasing age, the rats in this study also showed a progressive increase of myocardial apoptosis, hypertrophy and a deteriorating cardiac function. However, the activation of CaSR in these rats led to a reduction in blood pressure, suggesting that CaSR plays a role in inhibiting the progression of myocardial injuries through the inhibition of RAAS in cardiac tissues [77]. In line with this, another study demonstrated that in the mesenteric arteries of hypertensive rats, the expression of CaSR is decreased [27]. This reduced expression of CaSR enhanced the vasodilation of the arteries and regulated the vascular tension and the blood pressure in the rats through the PLC-IP3/cAMP/RAAS pathways [27]. In rats with spontaneous hypertension, AngII also activates CaSR, as well as NLRP3 inflammasome in vSMCs [78].
Vasorelaxations and constrictions are the main regulators of blood pressure in the vascular system. The vascular tension is controlled by intracellular calcium in vascular tissue [79]. Increased levels of calcium stimulate CaSR in the endothelium which promotes endothelium dependent vasorelaxation through an increase in the release of ●NO via the eNOS pathway [26]. CaSR influences the ●NO system via heterodimeric TRPV4-TRPC1-mediated calcium influx [80]. In the vascular microenvironment, different pumps act together to influx calcium ions in excess to form what are called calcium clouds. These calcium clouds trigger the activation of CaSR in ECs. Vasorelaxations are aided by hyperpolarization of vSMCs that are caused by changes in ion channels. However, the activation of these channels is also supported by the stimulation of CaSR, suggesting the existence of a prominent feedback loop [81].
In conclusion, it is believed that CaSR responds to systemic and local calcium ion concentrations to modulate the vascular tone of the vessels [14]. The normal expression of CaSR thereby functions as a protective shield against hypertension and its associated cardiac pathologies.
4.6. Obesity’s Influence
In recent years, obesity has progressed into a disease that torments a large portion of the population worldwide. Obesity is a condition in which there is an excess accumulation of lipids in the body resulting in increased and enlarged adipose tissue. CVDs linked to obesity are mostly due to adipose tissue dysfunction, its enlargement, and the release of inflammatory factors from the adipose tissue [82]. Enlarged adipose tissues also show higher expression levels of CaSR [83]. It has been observed that the activation of CaSR in human preadipocytes stimulates autophagy, which is an essential mechanism for cellular homeostasis, because when damaged organelles are not degraded by lysosomes this leads to cardiometabolic comorbidities especially in conditions of obesity [84]. All of the studies on adipose tissue autophagy unfortunately do not stipulate the exact cell type is responsible for this process and therefore more research is needed to elucidate this further [85]. CaSR is believed to activate the NLRP3 inflammasome via the ERK pathway in preadipocyte, as in many other cell types [86]. In cases of obesity, the adipose tissue expands and starts accumulating immune cells to facilitate the already disturbed metabolism [87]. Activating CaSR in macrophages, which are prominent in adipose tissues, induces inflammation in preadipocytes via the NLRP3 inflammasome pathway suggesting that interfering with the expression of CaSR in obesity could work as a therapeutic mechanism. Looking at all the studies focusing on the roles of CaSR in adipose tissue and obesity, it could be inferred that CaSR does not contribute towards protection of the adipose tissue, but it rather promotes a dysfunctional state and could thereby contribute to CVDs [88].
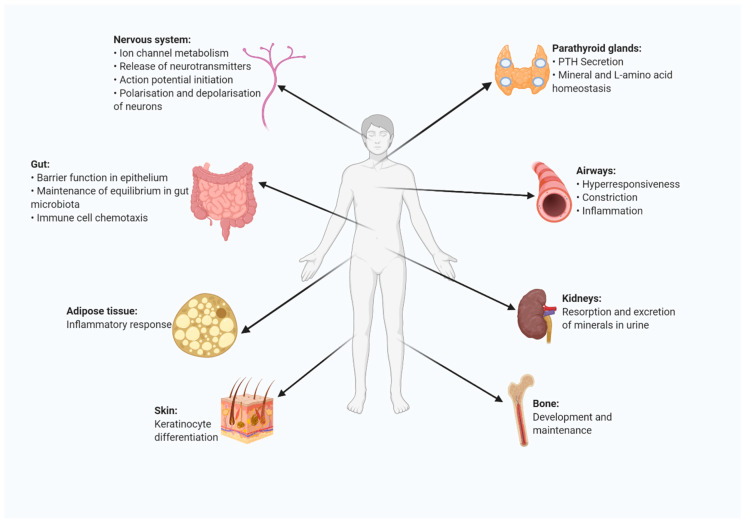
5. Presence and Function of CaSR in Other Organs and Organ Systems
Besides its important direct role in cardiovascular related cells, CaSR is also known to be present on the surface of different cells and to influence plasma mineral ion concentrations, especially calcium and phosphate [89] (Figure 3). The predominant expression of CaSR is in the parathyroid glands. Even though the well-known role of the receptor is the negative control of parathyroid function to influence the calcium homeostasis via the suppression of parathyroid hormone (PTH) secretion [4,90], the receptor has now been studied in various other contexts as well leading to an increased understanding of the role this receptor can play in various (patho)physiological conditions. To start with, the parathyroid CaSR is also involved in monitoring the plasma levels of L-amino acids [91]. Furthermore, CaSR is also expressed in thyroidal C-cells, where it regulates calcitonin secretion to again influence mineral homeostasis in the blood [92]. Another tissue in which a rather high expression of CaSR is seen is the kidney [93], wherein the receptor influences the mineral homeostasis by influencing the resorption and excretion of ions in urine. The expression of CaSR along the nephron influences the final compositions of urine and plasma that leaves the renal area [94]. The activated renal CaSR is also involved in inhibiting the tubular response to vasopressin thereby limiting water resorption by the kidneys [95]. CaSR is also expressed in osteoblasts, osteocytes, osteoclasts, and chondrocytes [96] and along with calcium ions the receptor is involved in bone development and maintenance [27,97]. In addition, CaSR is believed to be widely expressed in the nervous system [98]. Here, the receptor is part of processes including the ion channel metabolism, release of neurotransmitters, action potential initiation, and polarization and depolarization of neurons [99]. CaSR is similarly found to be expressed in the gut epithelium, aiding with the barrier function [100] and related cellular functions there, which include the maintenance of an equilibrium of the gut microbiota and immune cell chemotaxis. The importance of CaSR in this organ is further supported by the observation that the absence of CaSR in the gut resulted in increased local and systemic inflammation in mouse models [101]. The airways contain endothelial and smooth muscle cells that also express CaSR. In cases of severe asthma, the levels of cationic proteins and polyamines in the airway increases exponentially and it is believed that CaSR is implicated in this [102]. The receptor promotes disease development by aggravating the hyperresponsiveness, constriction and inflammation of the airway. Finally, around a decade ago CaSR expression was also demonstrated in adipocytes and pre-adipocytes [103]. The expression of the receptor in these cells is involved in inflammatory responses of the adipose tissue and has an anti-lipolysis function [104,105].
Figure 3.
CaSR expression and function in a range of organs and tissues in the human body. CaSR plays a (patho)physiological role in multiple organs and tissues in humans. CaSR is present in parathyroid glands, kidneys, bone, skin, the gut, airways, adipose tissues and in the nervous system, where it exerts various functions (created with BioRender.com).
6. Concluding Remarks
CaSR is expressed on the surfaces of cells of a wide variety of tissues, where it performs numerous functions by means of activating diverse signaling pathways depending on the bound ligand. CaSR in general plays an important role in various pathological processes, such as cardiovascular pathologies. The receptor influences and promotes acute and chronic inflammation and triggers various intracellular signaling pathways, thereby participating in atherosclerosis, vascular calcification, cardiomyopathy, cardiac fibrosis, and myocardial infarction. CaSR is implicated in various diseases, making it a very interesting pharmacological target to study. Although studies on targeting of this receptor to inhibit the progression of CVDs are scarce, targeting of this receptor in vascular tissues could be an interesting novel therapeutic mechanism to control and manipulate the cardiovascular biology. Therefore, future studies should focus on the therapeutic potential of the CaSR in inflammatory processes and cardiovascular pathologies to elucidate the exact potential.
Abbreviations
| α-SMA | Alpha smooth muscle actin |
| AC | Adenylate cyclase |
| AngII | Angiotensin II |
| CAD | Coronary artery disease |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| cAMP | Cyclic adenosine monophosphate |
| CaSR | Calcium sensing receptor |
| CRD | Cysteine rich domain |
| CVD | Cardiovascular disease |
| DAG | Diacylglycerol |
| EC | Endothelial cell |
| ECD | Extracellular domain |
| ER | Endoplasmic reticulum |
| FHH | Familial hypocalciuric hypercalcemia |
| HUVEC | Human umbilical vein endothelial cell |
| IL-1R | Interleukin-1 receptor |
| IP3 | Inositol triphosphate |
| JNK | C-Jun n-terminal kinases |
| LDL | Low-density lipoproteins |
| LPS | Lipopolysaccharide |
| LTCC | L type Ca2+ channel |
| MAPK | Mitogen-activated protein kinase |
| MMP2 | Matrix metalloproteinase-2 |
| MI | Myocardial infarction |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| ●NO | Nitric oxide |
| NOD2 | Nucleotide-binding oligomerization domain-containing protein 2 |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PLC | Phospholipase C |
| PTH | Parathyroid hormone |
| RAAS | Renin-angiotensin-aldosterone system |
| RAS | Rat sarcoma |
| ROC | Receptor-operated channels |
| TLR | Toll-like receptor |
| VFT | Venus fly trap |
| vSMC | Vascular smooth muscle cell |
Author Contributions
Writing—original draft and figure preparation, S.S.S.; review and editing, E.P.C.v.d.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Interdisciplinary Center for Clinical Research within the faculty of Medicine at the RWTH Aachen University, the DZHK (German Centre for Cardiovascular Research) and the BMBF (German Ministry of Education and Research), and NWO-ZonMw Veni (91619053) to E.P.C.v.d.V.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hendy G.N., Canaff L. Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin. Cell Dev. Biol. 2016;49:37–43. doi: 10.1016/j.semcdb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Brown E. Cloning and functional characterization of extracellular Ca2+-sensing receptors from parathyroid and kidney. Bone. 1995;17:S7–S11. doi: 10.1016/8756-3282(95)00199-N. [DOI] [PubMed] [Google Scholar]
- 3.Gerbino A., Colella M. The different facets of extracellular calcium sensors: Old and new concepts in calcium-sensing receptor signalling and pharmacology. Int. J. Mol. Sci. 2018;19:999. doi: 10.3390/ijms19040999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollak M.R. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75:1297–1303. doi: 10.1016/0092-8674(93)90617-Y. [DOI] [PubMed] [Google Scholar]
- 5.Vahe C. Diseases associated with calcium-sensing receptor. Orphanet J. Rare Dis. 2017;12:1–9. doi: 10.1186/s13023-017-0570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geng Y. Structural mechanism of ligand activation in human calcium-sensing receptor. Elife. 2016;5:e13662. doi: 10.7554/eLife.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centeno P.P. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-019-12399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kallay E. Physiology and Pathophysiology of the Extracellular Calcium-Sensing Receptor. Am. J. Med. 2018;9:413. doi: 10.3389/fphys.2018.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C. Structural basis for regulation of human calcium-sensing receptor by magnesium ions and an unexpected tryptophan derivative co-agonist. Sci. Adv. 2016;2:e1600241. doi: 10.1126/sciadv.1600241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai M., Trivedi S., Brown E.M. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J. Biol. Chem. 1998;273:23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C., Miller C.L., Brown E.M., Yang J.J. The calcium sensing receptor: From calcium sensing to signaling. Sci. China Life Sci. 2015;58:14–27. doi: 10.1007/s11427-014-4779-y. [DOI] [PubMed] [Google Scholar]
- 12.Khan M.A., Conigrave A.D.J.B. Mechanisms of multimodal sensing by extracellular Ca2+-sensing receptors: A domain-based survey of requirements for binding and signalling. Br. J. Pharmacol. 2010;159:1039–1050. doi: 10.1111/j.1476-5381.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorvin C.M. Molecular and clinical insights from studies of calcium-sensing receptor mutations. J. Mol. Endocrinol. 2019;63:R1–R16. doi: 10.1530/JME-19-0104. [DOI] [PubMed] [Google Scholar]
- 14.Smajilovic S., Tfelt-Hansen J. Calcium acts as a first messenger through the calcium-sensing receptor in the cardiovascular system. Cardiovasc. Res. 2007;75:457–467. doi: 10.1016/j.cardiores.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Hu J. A region in the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+ J. Biol. Chem. 2005;280:5113–5120. doi: 10.1074/jbc.M413403200. [DOI] [PubMed] [Google Scholar]
- 16.Leach K. International Union of Basic and Clinical Pharmacology. CVIII. Calcium-Sensing Receptor Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2020;72:558–604. doi: 10.1124/pr.119.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofer A.M., Brown E.M. Extracellular calcium sensing and signalling. Nat. Rev. Mol. Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 18.Wang R. Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. Eur. J. Biochem. 2003;270:2680–2688. doi: 10.1046/j.1432-1033.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- 19.Guo J. Increased expression of calcium-sensing receptors induced by ox-LDL amplifies apoptosis of cardiomyocytes during simulated ischaemia–reperfusion. Clin. Exp. Pharmacol. Physiol. 2010;37:e128–e135. doi: 10.1111/j.1440-1681.2010.05345.x. [DOI] [PubMed] [Google Scholar]
- 20.Alam M.U. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc. Res. 2009;81:260–268. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- 21.Chow J.Y. Calcium-sensing receptor modulates extracellular Ca2+ entry via TRPC-encoded receptor-operated channels in human aortic smooth muscle cells. Am. J. Physiol. Cell Physiol. 2011;301:C461–C468. doi: 10.1152/ajpcell.00389.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schepelmann M. The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am. J. Physiol. Cell Physiol. 2016;310:C193–C204. doi: 10.1152/ajpcell.00248.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guha S. Dietary γ-Glutamyl Valine Ameliorates TNF-α-Induced Vascular Inflammation via Endothelial Calcium-Sensing Receptors. J. Agric. Food Chem. 2020;68:9139–9149. doi: 10.1021/acs.jafc.0c04526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weston A.H. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: Studies with calindol and Calhex 231. Circ. Res. 2005;97:391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- 25.Ziegelstein R.C., Xiong Y., He C., Hu Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 2006;342:153–163. doi: 10.1016/j.bbrc.2006.01.135. [DOI] [PubMed] [Google Scholar]
- 26.Horinouchi T., Mazaki Y., Terada K., Miwa S. Extracellular Ca2+ promotes nitric oxide production via Ca2+-sensing receptor-Gq/11 protein-endothelial nitric oxide synthase signaling in human vascular endothelial cells. J. Pharmacol. Sci. 2020;143:315–319. doi: 10.1016/j.jphs.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Goltzman D., Hendy G.N. The calcium-sensing receptor in bone—Mechanistic and therapeutic insights. Nat. Rev. Endocrinol. 2015;11:298. doi: 10.1038/nrendo.2015.30. [DOI] [PubMed] [Google Scholar]
- 28.House M.G. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J. Bone Miner. Res. 1997;12:1959–1970. doi: 10.1359/jbmr.1997.12.12.1959. [DOI] [PubMed] [Google Scholar]
- 29.Li T. Expression of the calcium sensing receptor in human peripheral blood T lymphocyte and its contribution to cytokine secretion through MAPKs or NF-κB pathways. Mol. Immunol. 2013;53:414–420. doi: 10.1016/j.molimm.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Hammond C.M., White D., Tomic J., Shi Y., Spaner D.E. Extracellular calcium sensing promotes human B-cell activation and function. J. Am. Soc. Hematol. 2007;110:3985–3995. doi: 10.1182/blood-2007-05-088468. [DOI] [PubMed] [Google Scholar]
- 31.Klein G.L., Castro S.M., Garofalo R.P. The calcium-sensing receptor as a mediator of inflammation. Semin. Cell Dev. Biol. 2016;49:52–56. doi: 10.1016/j.semcdb.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canton J. Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages. Nat. Commun. 2016;7:1–12. doi: 10.1038/ncomms11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olszak I.T. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J. Clin. Investig. 2000;105:1299–1305. doi: 10.1172/JCI9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hannan F.M., Babinsky V.N., Thakker R.V. Disorders of the calcium-sensing receptor and partner proteins: Insights into the molecular basis of calcium homeostasis. J. Mol. Endocrinol. 2016;57:R127. doi: 10.1530/JME-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.März W. Alanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortality. J. Clin. Endocrinol. Metab. 2007;92:2363–2369. doi: 10.1210/jc.2006-0071. [DOI] [PubMed] [Google Scholar]
- 36.Shingu T. Significance of intracellular free calcium and magnesium and calcium-regulating hormones with sodium chloride loading in patients with essential hypertension. J. Hypertens. 1991;9:1021–1028. doi: 10.1097/00004872-199111000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Bolland M.J. Abdominal aortic calcification on vertebral morphometry images predicts incident myocardial infarction. J. Bone Miner. Res. 2010;25:505–512. doi: 10.1359/jbmr.091005. [DOI] [PubMed] [Google Scholar]
- 38.Larsson S.C., Burgess S., Michaelsson K. Association of Genetic Variants Related to Serum Calcium Levels with Coronary Artery Disease and Myocardial Infarction. JAMA. 2017;318:371–380. doi: 10.1001/jama.2017.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y.-H. Calcium-sensing receptor induces rat neonatal ventricular cardiomyocyte apoptosis. Biochem. Biophys. Res. Commun. 2006;350:942–948. doi: 10.1016/j.bbrc.2006.09.142. [DOI] [PubMed] [Google Scholar]
- 40.Lu F.-H. Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell. Physiol. Biochem. 2013;31:728–743. doi: 10.1159/000350091. [DOI] [PubMed] [Google Scholar]
- 41.Majumdar S.K. A Novel Variant in the Calcium-Sensing Receptor Associated with Familial Hypocalciuric Hypercalcemia and Low-to-Normal PTH. Case Rep. Endocrinol. 2020 doi: 10.1155/2020/8752610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roszko K.L., Bi R.D., Mannstadt M. Autosomal dominant hypocalcemia (hypoparathyroidism) types 1 and 2. Front. Physiol. 2016;7:458. doi: 10.3389/fphys.2016.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu K. The G allele of CaSR R990G polymorphism increases susceptibility to urolithiasis and hypercalciuria: Evidences from a comprehensive meta-analysis. Biomed Res. Int. 2015 doi: 10.1155/2015/958207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vezzoli G. Risk of nephrolithiasis in primary hyperparathyroidism is associated with two polymorphisms of the calcium-sensing receptor gene. J. Nephrol. 2015;28:67–72. doi: 10.1007/s40620-014-0106-8. [DOI] [PubMed] [Google Scholar]
- 45.Li X.M. Downregulation of survival signalling pathways and increased apoptosis in the transition of pressure overload-induced cardiac hypertrophy to heart failure. Clin. Exp. Pharmacol. Physiol. 2009;36:1054–1061. doi: 10.1111/j.1440-1681.2009.05243.x. [DOI] [PubMed] [Google Scholar]
- 46.Lu M. Calcium sensing receptor-related pathway contributes to cardiac injury and the mechanism of Astragaloside IV on cardioprotection. Front. Pharmacol. 2018;9:1163. doi: 10.3389/fphar.2018.01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chi J. Activation of calcium-sensing receptor-mediated autophagy in angiotensinII-induced cardiac fibrosis in vitro. Biochem. Biophys. Res. Commun. 2018;497:571–576. doi: 10.1016/j.bbrc.2018.02.098. [DOI] [PubMed] [Google Scholar]
- 48.Han G. Relationship between CaSRs and LPS-injured cardiomyocytes. Int. J. Clin. Exp. Pathol. 2018;11:1965. [PMC free article] [PubMed] [Google Scholar]
- 49.Ren Z. Calcium-sensing receptor on neutrophil promotes myocardial apoptosis and fibrosis after acute myocardial infarction via NLRP3 inflammasome activation. Can. J. Cardiol. 2020;36:893–905. doi: 10.1016/j.cjca.2019.09.026. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y. Calcium sensing receptor protects high glucose-induced energy metabolism disorder via blocking gp78-ubiquitin proteasome pathway. Cell Death Dis. 2017;8:e2799. doi: 10.1038/cddis.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cavalera M., Wang J., Frangogiannis N.G. Obesity, metabolic dysfunction, and cardiac fibrosis: Pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl. Res. 2014;164:323–335. doi: 10.1016/j.trsl.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rybczyńska A. Activity of the calcium-sensing receptor influences blood glucose and insulin levels in rats. Pharmacol. Rep. 2017;69:709–713. doi: 10.1016/j.pharep.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 53.Tedgui A., Mallat Z. Cytokines in atherosclerosis: Pathogenic and regulatory pathways. Physiol. Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 54.Schaftenaar F., Frodermann V., Kuiper J., Lutgens E. Atherosclerosis: The interplay between lipids and immune cells. Curr. Opin. Lipidolgy. 2016;27:209–215. doi: 10.1097/MOL.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 55.Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:1–13. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durham A.L., Speer M.Y., Scatena M., Giachelli C.M., Shanahan C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018;114:590–600. doi: 10.1093/cvr/cvy010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee S.H., Choi J.-H. Involvement of immune cell network in aortic valve stenosis: Communication between valvular interstitial cells and immune cells. Immune Netw. 2016;16:26–32. doi: 10.4110/in.2016.16.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otto C.M., O’Brien K. Why is there discordance between calcific aortic stenosis and coronary artery disease? Heart. 2001;85:601–602. doi: 10.1136/heart.85.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carabello B.A., Paulus W.J. Aortic stenosis. Lancet. 2009;373:956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 60.Molostvov G., Hiemstra T.F., Fletcher S., Bland R., Zehnder D. Arterial Expression of the Calcium-Sensing Receptor Is Maintained by Physiological Pulsation and Protects against Calcification. PLoS ONE. 2015;10:e0138833. doi: 10.1371/journal.pone.0138833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rossol M. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat. Commun. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Risinger G.M., Jr., Hunt T.S., Updike D.L., Bullen E.C., Howard E.W. Matrix metalloproteinase-2 expression by vascular smooth muscle cells is mediated by both stimulatory and inhibitory signals in response to growth factors. J. Biol. Chem. 2006;281:25915–25925. doi: 10.1074/jbc.M513513200. [DOI] [PubMed] [Google Scholar]
- 63.Li H.X. Involvement of calcium-sensing receptor in oxLDL-induced MMP-2 production in vascular smooth muscle cells via PI3K/Akt pathway. Mol. Cell Biochem. 2012;362:115–122. doi: 10.1007/s11010-011-1133-6. [DOI] [PubMed] [Google Scholar]
- 64.Molostvov G., Fletcher S., Bland R., Zehnder D. Extracellular calcium-sensing receptor mediated signalling is involved in human vascular smooth muscle cell proliferation and apoptosis. Cell. Physiol. Biochem. 2008;22:413–422. doi: 10.1159/000185484. [DOI] [PubMed] [Google Scholar]
- 65.Lu L. Myocardial infarction: Symptoms and treatments. Cell Biochem. Biophys. 2015;72:865–867. doi: 10.1007/s12013-015-0553-4. [DOI] [PubMed] [Google Scholar]
- 66.Thygesen K. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007;116:2634–2653. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y. Calcium sensing receptor initiating cystathionine-gamma-lyase/hydrogen sulfide pathway to inhibit platelet activation in hyperhomocysteinemia rat. Exp. Cell Res. 2017;358:171–181. doi: 10.1016/j.yexcr.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 68.Luo Y. Activation of the CaR-CSE/H2S pathway confers cardioprotection against ischemia-reperfusion injury. Exp. Cell Res. 2020;398:112389. doi: 10.1016/j.yexcr.2020.112389. [DOI] [PubMed] [Google Scholar]
- 69.Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu T. The role of MCPIP1 in ischemia/reperfusion injury-induced HUVEC migration and apoptosis. Cell. Physiol. Biochem. 2015;37:577–591. doi: 10.1159/000430378. [DOI] [PubMed] [Google Scholar]
- 71.Xie X. MCPIP1-induced autophagy mediates ischemia/reperfusion injury in endothelial cells via HMGB1 and CaSR. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-20195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xi Y.-H. The functional expression of calcium-sensing receptor in the differentiated THP-1 cells. Mol. Cell. Biochem. 2010;342:233–240. doi: 10.1007/s11010-010-0489-3. [DOI] [PubMed] [Google Scholar]
- 73.Touyz R.M. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018;114:529–539. doi: 10.1093/cvr/cvy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X. NLRP3 inflammasome is involved in calcium-sensing receptor-induced aortic remodeling in SHRs. Mediat. Inflamm. 2019;2019 doi: 10.1155/2019/6847087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward B.K., Magno A.L., Walsh J.P., Ratajczak T. The role of the calcium-sensing receptor in human disease. Clin. Biochem. 2012;45:943–953. doi: 10.1016/j.clinbiochem.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 76.Rybczynska A. Blockade of calcium channels and AT1 receptors prevents the hypertensive effect of calcilytic NPS 2143 in rats. J. Physiol. Pharmacol. 2010;61:163. [PubMed] [Google Scholar]
- 77.Zhang T. Calcimimetic R568 improved cardiac remodeling by classic and novel renin-angiotensin system in spontaneously hypertensive rats. Exp. Biol. Med. 2019;244:789–801. doi: 10.1177/1535370219854325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu C. CaSR activates PKCdelta to induce cardiomyocyte apoptosis via ER stressassociated apoptotic pathways during ischemia/reperfusion. Int. J. Mol. Med. 2019;44:1117–1126. doi: 10.3892/ijmm.2019.4255. [DOI] [PubMed] [Google Scholar]
- 79.Hatton D.C., McCarron D.A. Dietary calcium and blood pressure in experimental models of hypertension. A review. Hypertension. 1994;23:513–530. doi: 10.1161/01.HYP.23.4.513. [DOI] [PubMed] [Google Scholar]
- 80.Greenberg H.Z. Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vasc. Pharmacol. 2017;96:53–62. doi: 10.1016/j.vph.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Greenberg H.Z. Stimulation of calcium-sensing receptors induces endothelium-dependent vasorelaxations via nitric oxide production and activation of IKCa channels. Vasc. Pharmacol. 2016;80:75–84. doi: 10.1016/j.vph.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vecchié A. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018;48:6–17. doi: 10.1016/j.ejim.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 83.Mattar P. Calcium-Sensing Receptor in Adipose Tissue: Possible Association with Obesity-Related Elevated Autophagy. Int. J. Mol. Sci. 2020;21:7617. doi: 10.3390/ijms21207617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mattar P. Autophagy mediates calcium-sensing receptor-induced TNFα production in human preadipocytes. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3585–3594. doi: 10.1016/j.bbadis.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 85.Clemente-Postigo M., Tinahones A., Bekay R.E., Malagón M.M., Tinahones F.J. The Role of Autophagy in White Adipose Tissue Function: Implications for Metabolic Health. Metabolites. 2020;10:179. doi: 10.3390/metabo10050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Espessailles A., Mora Y.A., Fuentes C., Cifuentes M. Calcium-sensing receptor activates the NLRP3 inflammasome in LS14 preadipocytes mediated by ERK1/2 signaling. J. Cell. Physiol. 2018;233:6232–6240. doi: 10.1002/jcp.26490. [DOI] [PubMed] [Google Scholar]
- 87.Russo L., Lumeng C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology. 2018;155:407–417. doi: 10.1111/imm.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bravo-Sagua R., Mattar P., Díaz X., Lavandero S., Cifuentes M. Calcium sensing receptor as a novel mediator of adipose tissue dysfunction: Mechanisms and potential clinical implications. Front. Physiol. 2016;7:395. doi: 10.3389/fphys.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hannan F.M. The calcilytic agent NPS 2143 rectifies hypocalcemia in a mouse model with an activating calcium-sensing receptor (CaSR) mutation: Relevance to autosomal dominant hypocalcemia type 1 (ADH1) Endocrinology. 2015;156:3114–3121. doi: 10.1210/en.2015-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conigrave A.D. The calcium-sensing receptor and the parathyroid: Past, present, future. Front. Physiol. 2016;7:563. doi: 10.3389/fphys.2016.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Conigrave A.D. L-amino acids regulate parathyroid hormone secretion. J. Biol. Chem. 2004;279:38151–38159. doi: 10.1074/jbc.M406373200. [DOI] [PubMed] [Google Scholar]
- 92.Brown E.M., MacLeod R.J. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 93.Riccardi D. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am. J. Physiol. Ren. Physiol. 1998;274:F611–F622. doi: 10.1152/ajprenal.1998.274.3.F611. [DOI] [PubMed] [Google Scholar]
- 94.Graca J. Comparative expression of the extracellular calcium-sensing receptor in the mouse, rat, and human kidney. Am. J. Physiol. Ren. Physiol. 2016;310:F518–F533. doi: 10.1152/ajprenal.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Procino G. Calcium-sensing receptor and aquaporin 2 interplay in hypercalciuria-associated renal concentrating defect in humans. An in vivo and in vitro study. PLoS ONE. 2012;7:e33145. doi: 10.1371/journal.pone.0033145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Santa Maria C. Seminars in Cell & Developmental Biology. Elsevier; Amsterdam, The Netherlands: 2016. pp. 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang W., Tu C., Chen T.-H., Bikle D., Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci. Signal. 2008;1:ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang W. Complex Formation with the Type B γ-Aminobutyric Acid Receptor Affects the Expression and Signal Transduction of the Extracellular Calcium-sensing Receptor: Studies with HEK-293 Cells and Neurons. J. Biol. Chem. 2007;282:25030–25040. doi: 10.1074/jbc.M700924200. [DOI] [PubMed] [Google Scholar]
- 99.Washburn D.L., Anderson J.W., Ferguson A.V. A subthreshold persistent sodium current mediates bursting in rat subfornical organ neurones. J. Physiol. 2000;529:359. doi: 10.1111/j.1469-7793.2000.00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakhoul N.L. Calcium-sensing receptor deletion in the mouse esophagus alters barrier function. J. Physiol. Gastrointest. Liver Physiol. 2020;318:G144–G161. doi: 10.1152/ajpgi.00021.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Owen J.L., Cheng S.X., Ge Y., Sahay B., Mohamadzadeh M. Seminars in Cell & Developmental Biology. Elsevier; Amsterdam, The Netherlands: 2016. pp. 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.North M.L. Increased ornithine-derived polyamines cause airway hyperresponsiveness in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 2013;48:694–702. doi: 10.1165/rcmb.2012-0323OC. [DOI] [PubMed] [Google Scholar]
- 103.Cifuentes M., Albala C., Rojas C. Calcium-sensing receptor expression in human adipocytes. Endocrinology. 2005;146:2176–2179. doi: 10.1210/en.2004-1281. [DOI] [PubMed] [Google Scholar]
- 104.He Y. Involvement of calcium-sensing receptor in inhibition of lipolysis through intracellular cAMP and calcium pathways in human adipocytes. Biochem. Biophys. Res. Commun. 2011;404:393–399. doi: 10.1016/j.bbrc.2010.11.129. [DOI] [PubMed] [Google Scholar]
- 105.Cifuentes M. Obesity-associated proinflammatory cytokines increase calcium sensing receptor (CaSR) protein expression in primary human adipocytes and LS14 human adipose cell line. Arch. Biochem. Biophys. 2010;500:151–156. doi: 10.1016/j.abb.2010.05.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.