Abstract
Binge eating is characterized by the consumption of a large amount of palatable food in a short period of time and is a core feature of many eating disorders. Patients with eating disorders are also known to display impairments in inhibitory control, cognition and decision-making, which may promote and maintain binge eating symptomology. In the current study, we examined whether rats that were subsequently characterized as displaying a higher propensity to binge eat would show pre-existing deficits in reinforcer devaluation—a paradigm used to examine decision-making following reductions in the value of a food reinforcer. Female rats were first trained to respond on two levers for the delivery of two food reinforcers (sucrose and maltodextrin solutions). At the test stage, rats were provided 1 hr access to one of the two reinforcers to allow for devaluation via sensory specific satiety, immediately followed by an extinction test with both levers. Normal rats typically show reductions in responding on the lever associated with the devalued reinforcer (i.e., intact goal-directed responding). Subsequently, we used intermittent access to palatable food to identify high (BE prone [BEP]; n=14), intermediate (BE neutral [BEN]; n=48), and low (BE resistant [BER]; n=13) phenotypes of binge eating. Prior reinforcer devaluation performance showed BEN and BER rats suppressed responding on the lever associated with the devalued reinforcer while BEP rats did not. This insensitivity to instrumental reinforcer devaluation in BEP rats did not reflect impaired sensory-specific satiety as during a food choice test, BEP rats showed a more robust alteration in food preferences following devaluation. Additionally, across all rats sensory specific satiety was correlated with subsequent intake of palatable food. Collectively, these findings suggest dissociable effects of devaluation procedures on instrumental actions and consummatory behaviors in BEP rats, and may indicate that pre-existing differences in goal-directed behavior and sensory-specific satiety contribute to the propensity to overeat palatable food.
Keywords: inhibitory control, operant conditioning, habit learning, medial prefrontal cortex, binge eating disorder
1. Introduction
Eating disorders (EDs) are a devastating class of mental illnesses and display both the highest mortality rates and the largest sex difference (up to 8:1 female to male ratio) among psychiatric conditions [1–5]. Binge eating is a core feature that cuts across most sub-types of EDs, including: anorexia nervosa, bulimia nervosa and binge eating disorder. Binge eating is defined as the consumption of a large amount of food than would be considered normal in the same discrete period, combined with a feeling of a lack of control over the binge episode [1,6]. Traditionally, empirical studies have focused on psychosocial variables such as pubertal changes in body weight and the emphasis of dieting and thinness in females [7,8]; however, more recent studies include the neurobehavioral factors underlying EDs [9], with the hope that they may ultimately highlight novel pharmacotherapeutic targets.
Animal models have been utilized to examine mechanisms underlying EDs independent of the psychosocial variables that contribute to eating pathology. The binge eating resistant (BER)/binge eating prone (BEP) rat model of binge eating is an individual differences model that identifies a spectrum of persistent binge eating ranging from low (i.e., BER) to high (i.e., BEP). In this model, BEP rats consistently overconsume intermittently presented palatable food and mirror many of the key features observed with EDs in humans [10–12]. Robust sex differences have been noted with this model, with an increased prevalence of BEP in female rats as compared to males (6:1 female to male ratio) [13]. Moreover, the onset of binge eating phenotypes in rats emerges in mid-to-late puberty [14], similar to the increased prevalence of EDs during adolescence in humans [15]. On the other hand, as observed in girls [16], perinatal testosterone in female rats protects against the development of binge eating proneness in adolescence [17]. BEP rats also binge only on palatable food (not standard laboratory chow) and are willing to maintain high rates of intake under aversive conditions, such as footshock [18] or gastric discomfort [19], features that may mimic the loss of control seen with binge eating in human EDs.
We sought to examine whether BEP rats display pre-existing behavioral differences that may relate to their propensity to overconsume intermittently presented palatable food. To this end, we investigated whether food-seeking responses would be sensitive to changes in the value of food following devaluation [20–23]. Thus, when performing actions (e.g., responding on a lever) to gain access to palatable food reinforcers, rats are capable of performing in a manner that is driven by the current value of the reinforcer. Accordingly, when the reinforcer is devalued (e.g., via sensory-specific satiety), rats will display a spontaneous reduction in responding when tested under extinction conditions [21]. Studies suggest a critical role for mesolimbocortical reward circuity in reinforcer devaluation, including medial prefrontal cortex [24,25] and nucleus accumbens (ACB) [26]—areas that also show increased immunoreactivity in response to palatable food in BEP rats [12]. In the current study, female rats were first trained to respond on two separate levers for the delivery of distinct food reinforcers; one of the reinforcers was then devalued by sensory-specific satiety, and the capacity of this devaluation manipulation to separately alter responding for and consumption of the reinforcers was examined. On completion, all rats were phenotyped for their propensity to binge eat during intermittent access to palatable food. When the performance of BEP rats was examined, evidence of pre-existing deficits in the sensitivity of these rats to devaluation was revealed by their failure to previously suppress responding on the devalued lever. Conversely, sensory-specific satiety was more effective at altering the preference for the reinforcers in BEP rats during consumption tests. These findings point to dissociable effects of devaluation procedures on instrumental actions and consummatory behaviors in BEP rats.
2. Methods
2.1. Subjects
Eighty adult female Sprague Dawley rats (Envigo, Madison, WI) arrived at the colony at Michigan State University at postnatal day 90. Upon arrival, all rats were individually housed in rooms maintained at 70 ± 2°F with enrichment and ad libitum access to water and standard laboratory chow (Teklad 22/5 Rodent Diet, Envigo). Rats were food deprived throughout instrumental lever training and reinforcer devaluation tests, which began 5–7 d before the start of instrumental training. For phenotyping, rats were placed back onto ad libitum access to laboratory chow. All animals were handled in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with all protocols receiving approval from the Michigan State University Institutional Animal Care and Use Committee.
2.2. Apparatus
The behavioral training apparatus consisted of eight individual chambers (29.5 × 24.8 18.7 cm) (MedAssociates, Fairfax, VT) with aluminum front and back walls, clear polycarbonate sides and ceiling, and floor comprised of parallel, 0.48 cm stainless steel rods spaced 1.9 cm apart. A square front wall contained an opening that held an illuminated, clear acrylic food cup housed in an aluminum box. Housed within each food cup, was a food well into which solutions could be administered directly via tubing attached to a syringe that was placed on a syringe pump; a computer system allowed for precise dispensing of solutions when needed. Within the food cup was a photocell that tracked entries and time spent in the food cup, and behind the wall of the food cup was a relay switch that was used early in training to signal availability of the solutions. On the left and right sides of the food cup were retractable levers. Each chamber was illuminated by an infrared LED light. All stimuli and responses were controlled and recorded using Med Associates’ MED-PC® IV software. A video camera was located under the clear food cup to record consummatory responses. The camera images were digitized, recorded, and shown in real time on video monitors, displaying images of eight liquid wells concurrently.
2.3. Procedure
2.3.1. Instrumental training
After reaching 90% of their baseline weights, all rats were first habituated to the reinforcers: 20% sucrose and 20% maltodextrin with 1% NaCl. Over two days, rats received 50 ml of the two reinforcers in their home cage for 15 mins. Following pre-exposure, rats received a magazine training session for two consecutive days, with each session lasting ≈ 60 min. During these two sessions, rats received access to either 0.1 ml sucrose or maltodextrin delivery according to a random time delivery schedule of 240 s, and a total of 16 reinforcer deliveries. Rats subsequently received instrumental lever training for a total of ten sessions. At this stage, each rat was assigned a specific response-outcome contingency, where for half the rats’, responses on the left lever resulted in 0.1 ml sucrose delivery, whereas responses on the right lever led to 0.1 ml maltodextrin delivery. The remaining rats received the opposite response-outcome contingencies. For the first three sessions of instrumental training, rats received a single instrumental training session each day on one of the levers, where the reinforcer was available on a fixed-interval 20 s schedule, with each session lasting 30 min. On each day, half the rats were trained on the left lever and the remaining rats on the right lever; with the left and right lever training sessions alternating each day. For the remaining seven sessions of instrumental training, rats received two sessions per day; one with each lever, and the order of which was trained first alternating on a daily basis. During these sessions, reinforcer delivery was switched to a random ratio (RR) schedule, and the session duration was reduced to 20 min. For sessions four through six, the reinforcer delivery schedule was RR 5, thus on average every 5 responses resulted in reinforcer delivery. During sessions seven and eight the delivery schedule was increased to RR 10, and for sessions nine and ten, the delivery schedule was increased to RR 15.
2.3.2. Sensory-specific satiety and extinction testing
On the day following the final session of instrumental training, rats received sensory-specific satiety to devalue one of the two food reinforcers during home cage prefeeding. A drinking bottle containing 50 ml of either 20% Sucrose or 20% Maltodextrin + 1% NaCl was placed in each rat’s cage for 1 hr, following which consumption was measured and recorded. Devalued reinforcer assignments were counterbalanced according to the previous response-outcome contingencies, as well as the average responses per minute during instrumental training sessions 9 and 10. Immediately following home cage sensory-specific satiety, rats were given a 10 min extinction test in the behavioral training apparatus. The extinction test was the first-time rats experienced both levers simultaneously, however lever presses did not result in reinforcer delivery. Testing in the absence of reinforcement tests whether responding is driven by response-outcome associations as it requires a rat to spontaneously suppress actions on the lever associated with the devalued reinforcer without receiving any feedback from the reinforcers themselves.
2.3.3. Sensory-specific satiety and reinforcer choice test
On the day following extinction testing, rats again experienced sensory-specific satiety using the same reinforcer and parameters from the previous day. Immediately following home cage prefeeding, rats were given a 15-minute choice test in the experimental apparatus. During this choice test, the rats were given free access to palate cups separately containing 15 ml of each reinforcer and attached to the chamber floors. The cups were placed each side of the food cup and their placement was counterbalanced such that a given reinforcer was placed on the opposite side to where in training the extended lever had led to its delivery. This 15 min test was used to evaluate whether sensory specific satiety was successful in reducing the value of the reinforcers and whether this devaluation would transfer to the conditioning chambers.
2.3.4. Phenotyping
At the conclusion of testing, all rats were placed back on ad libitum chow and water for one week to elevate their body weights back to baseline levels. During this period, the 12-hour light: 12-hour dark cycle was altered by 1 hour daily until lights on was at 11:00 PM and lights off was at 11:00 AM. This transition was necessary as it more feasibly permitted testing the rats during the dark cycle [11, 12]. Phenotyping took place over the course of two weeks and during this period pre-measured lab chow (≈60–70 g) was available to each rat in its homecage and replenished daily. On Mondays, Wednesdays, and Fridays, the amount of lab chow consumption during the previous 24 hr period along with the rat’s body weight, was measured immediately prior to lights off. Subsequently, following lights off, rats were given 4 hr home cage access to ≈ 25–27g of palatable food in the form of Betty Crocker® Extra Creamy Vanilla Frosting. This palatable food is composed of 18% fat and 82% carbohydrates, including sucrose, high maltose corn syrup and corn starch. The palatable food was placed into a small petri dish and was fixed into the rat’s homecage via a wire hook. At the end of this 4 hr period, palatable food was removed and both palatable food and chow consumption were measured. Between these feeding test days, both lab chow and body weights were recorded immediately prior to lights off.
2.4. Data and statistical analysis
To identify BEP and BER rats, a tertile approach based on 4 hr palatable food consumption from the six feeding test days was utilized. Mean palatable food consumption values were divided into top, middle, and bottom tertiles, with each rat being sorted into one of the three terties on each feeding test days. Rats were classified as BEP if they scored within the top tertile on at least 3 of 6 feeding test days (i.e., ≥ 50%) and never within the bottom tertile. Rats were classified as BER if they scored within the bottom tertile of palatable food consumption on at least 3 of 6 feeding tests and never within the top tertile. Rats who did not meet the criterion for BEP or BER classification were classified as binge eating neutral (BEN). These criteria for BEP/BER classification are based on previous studies conducted by this lab [9,12,13,27], and typically reveal BEP and BER classifications, each in the range of ≈ 20% [9,13,27]. To evaluate the effect of phenotype, the mean body weight throughout the 2 wk phenotyping period was calculated for each rat and analyzed using a between subjects (BER, BEN, BEP) one-way ANOVA. The mean 4 hr palatable food as well as lab chow consumption during feeding test days was also analyzed in a similar manner, along with mean 24 hr lab chow consumption across feeding test and non-feeding test days. These analyses were necessary to verify that BER, BEN, and BEP rats did not differ in their average body weight and chow consumption (4hr and 24hr).
In the instrumental training phase, we recorded the responses per minute (RPM) on the two levers and averaged these responses across two-session blocks. These responses were identified based upon whether their associated reinforcer would subsequently be devalued (i.e., to-be-devalued) or not (i.e., tobe-maintained) during sensory-specific satiety. Initially the data were analyzed using a two-way within-subject lever (to-be-devalued, to-be-maintained) X session block (1–5) ANOVA. Following phenotyping, the data were subjected to a three-way mixed phenotype (BEP, BEN, BER) X lever X session ANOVA. To confirm equal rates of responding across the two levers for each phenotype, a one-way repeated measures ANOVA was also applied.
For the test stage data, both effect sizes and p values were reported to provide a clear indication of detected effects. Lever response data from the test stage were analyzed using a one-way repeated measures ANOVA comparing response rates on each lever (devalued, maintained). Using the classification of binge eating status, a two-way mixed phenotype (BEP, BEN, BER) X lever ANOVA was also implemented. In addition, to assess the impact of reinforcer devaluation on responding for each phenotype, a one-way repeated measures ANOVA comparing responding on the devalued and maintained levers was used. Cohen’s d effect sizes for these within-subject measures were calculated (effect size interpretation: small, d =.20; medium, d =.50; large, d =.80). Furthermore, in order to correct for the dependence among the within-subject means, we also report a more conservative effect size analysis in which the correlation between responses on the maintained and devalued levers for each group was incorporated [28]. The choice test data (consumption in ml/15 min) were subjected to one-way repeated measures ANOVA to determine differences in consumption for the devalued and maintained reinforcers. These data were also analyzed using phenotype as a between-subject variable, and reinforcer type as a within-subject variable (devalued, maintained). One-way repeated measures ANOVA on the consumption data was also conducted for each phenotype separately. Reinforcer intake (in ml) during each 1 hr prefeeding sensory-specific satiety session was also analyzed using one-way ANOVA. When appropriate, Bonferroni post-hoc comparisons were conducted to examine significant main effects, whereas any significant interactions resulting from the ANOVAs were followed up by tests of simple main effects. Finally, Pearson correlation coefficients and linear regression model were conducted to determine correlations between test responding following reinforcer devaluation and mean palatable food intake during phenotyping.
3. Results
3.1. Phenotyping
One rat was euthanized at the start of the study as it developed Pica, whereas an additional four rats were excluded as they failed to acquire the instrumental lever responses—these rats were excluded from all analyses. Thus, from the remaining subjects, phenotyping resulted in the following sample sizes: BER (n =13), BEN (n = 48); BEP (n = 14). One-way ANOVA was conducted on the mean palatable food consumption across the six feeding test days. As this measure was used via the tertile approach to define binge eating status, expected significant differences in palatable food consumption across the phenotypes were revealed (F(1,72) = 52.05, p<0.001) (Table 1). Post-hoc comparisons confirmed that BEP rats consumed significantly more than BEN’s, which consumed significantly more than BER’s (p’s<0.01). This increased consumption of palatable food was not compensated for when we examined mean lab chow consumption at 4 hr or 24 hr following the feeding tests (F’s<1.86; p’s>0.16). Similarly, there were also no differences in lab chow intake between the phenotypes for mean 24 hr consumption on non-feeding test days (F<1). Finally, mean body weights averaged across the total feeding test period were also comparable across the phenotypes (F(1,72) = 1.87, p=0.16).
Table 1.
Mean comparisons between phenotypes on body weight and consumption measures taken during the Feeding Test period.
| BER n = 13 | BEN n = 48 | BEP n = 14 | Mean [S.E.M] | One-way ANOVA Results |
||
|---|---|---|---|---|---|---|
| Statistics | Effect size Eta Squared | |||||
| F(1, 72) | p | η2 | ||||
| Feeding Test Period (13 days) | ||||||
| Body Weight (g) | ||||||
| 24hr Measurement | ||||||
| BER | 241.45 [3.88] | 1.87 | .161 | .048 | ||
| BEN | 244.63 [2.23] | |||||
| BEP | 252.85 [1.75] | |||||
| Feeding Test Days (6 days) | ||||||
| PF (g) | ||||||
| 4hr Measurement | ||||||
| BER | 6.69 [0.18] | 52.047 | .000*** | .587 | ||
| BEN | 8.63 [0.20] | |||||
| BEP | 11.97 [0.59] | |||||
| Chow (g) | ||||||
| 4hr Measurement | ||||||
| BER | 4.36 [1.23] | .488 | .615 | .013 | ||
| BEN | 4.25 [0.68] | |||||
| BEP | 3.84 [0.98] | |||||
| 24hr Measurement | ||||||
| BER | 7.40 [2.21] | 1.86 | .162 | .048 | ||
| BEN | 6.67 [1.16] | |||||
| BEP | 6.71 [1.96] | |||||
| Non-Feeding Test Days (7 days) | ||||||
| Chow (g) | ||||||
| 24hr Measurement | ||||||
| BER | 14.29 [1.44] | 0.326 | .722 | .008 | ||
| BEN | 11.74 [1.60] | |||||
| BEP | 12.23 [1.31] | |||||
Note: Two-tailed p-values at α = .05 are presented for means calculated from feeding test period measures. Eta Squared values reflect effect sizes and represent the magnitude of between-group differences. S.E.M. = standard error of the mean;
p < .001
3.2. Instrumental training
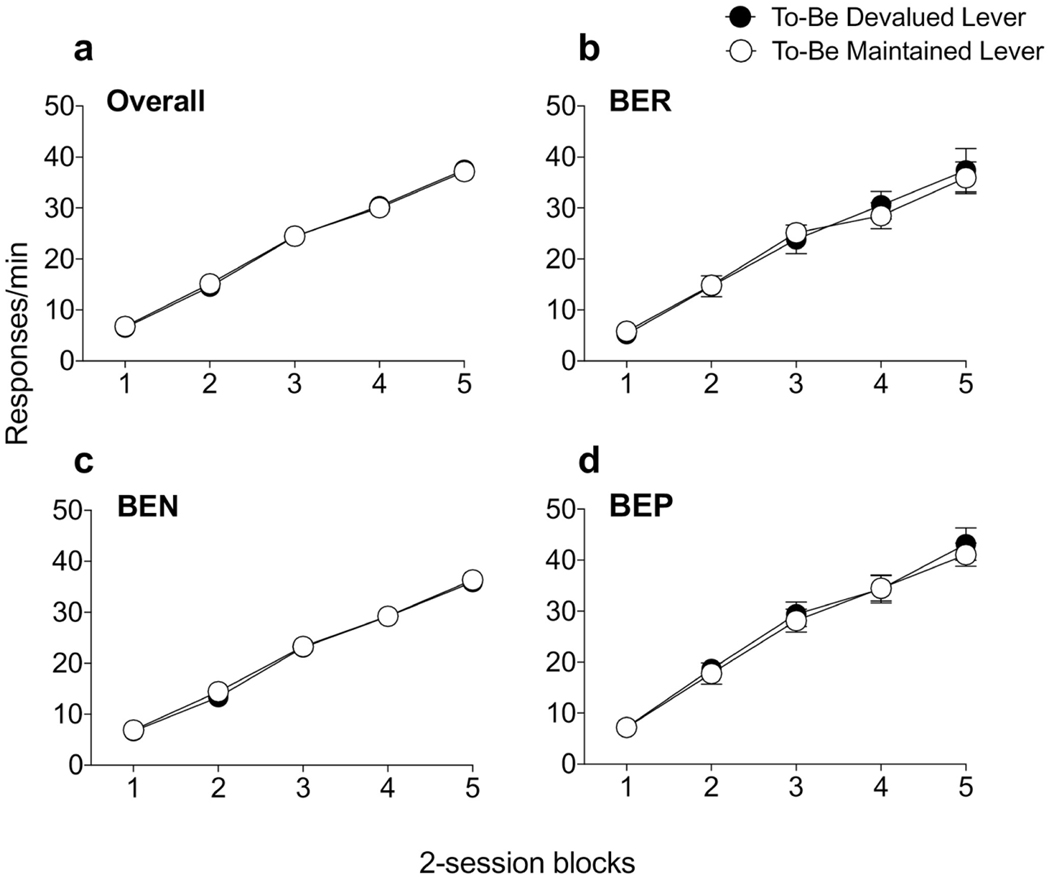
As training progressed, all rats acquired lever responding, increasing their response as the reinforcement schedule became stricter (Fig. 1a). Two-way ANOVA revealed a main effect of session only (F(4,296) = 515.5, p<0.0001), with post-hoc comparisons confirming significant increases in response rates across each session block (p’s<0.001). When we examined the pattern of responding following the subsequent completion of phenotyping, three-way ANOVA similarly revealed a main effect of session only (F(4,288) = 392.5, p<0.0001), with no effects of phenotype, nor interaction with any of the variables (Largest F-value; phenotype, F(2,72) = 2.50, p=0.09). Finally, within each phenotype (Fig. 1b–d), there was no difference in the acquisition of responding to the devalued or maintained levers (F’s<1; p’s>0.70); thus, prior to the extinction test all rats showed comparable instrumental responding.
Figure 1.
Instrumental training averaged across two-session blocks. (a) All rats showed evidence of instrumental responding, gradually increasing their responding following increases in the mean number of responses required to obtain the to-be-devalued and the to-be-maintained reinforcer. There were also no differences in responding to the two separate levers in either (b) BER, (c) BEN or (d) BEP rats. Error bars indicate SEM.
3.3. Sensory-specific satiety and instrumental reinforcer devaluation
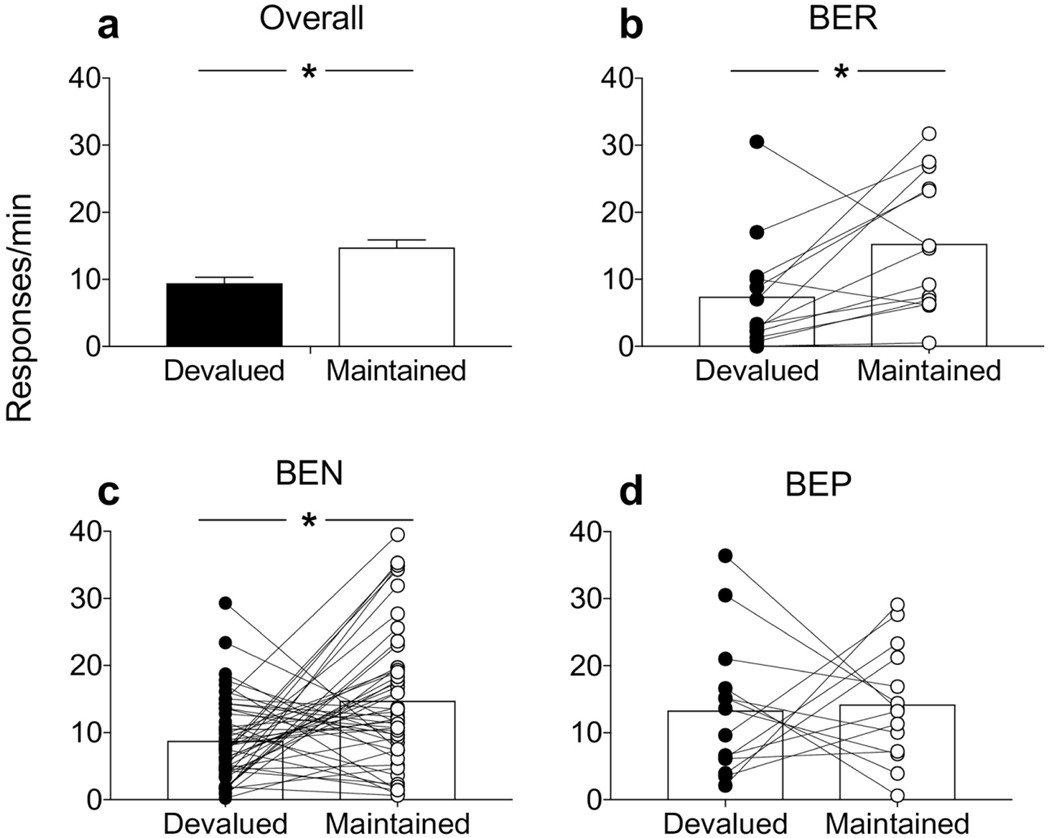
During sensory-specific satiety, all rats consumed similar amounts of the prefed (devalued) reinforcer (consumption ml/hour ± SEM; BER = 16.65 ± 2.26, BEN = 18.54 ± 1.26, BEP = 17.57 ± 1.97); one-way ANOVA revealed no effect of phenotype (F<1). The data of primary interest are depicted in Figure 2. In general, rats showed reinforcer devaluation, as evidenced by their suppression of responding on the lever associated with the devalued reinforcer during the extinction test (Fig. 2a.). Repeated measures ANOVA revealed a significant main effect of response type (i.e., devalued, maintained) (F(1,74) = 11.97, p<0.0001). When these data were re-analyzed based on the subsequent results from phenotyping, two-way phenotype X response ANOVA also revealed a main effect of response (F(1,72) = 7.09, p<0.01) but no interaction between the variables (F(2,72) = 1.44, p=0.35). Despite the lack of phenotype X response interaction, when we examined the performance of individual rats within each phenotype, 84% of BEP rats showed greater responses on the maintained compared to the devalued lever (Fig. 2b). By comparison, 64% of rats from the BEN group showed a numerical preference for the maintained lever (Fig. 2c), whereas only 50% of BEP rats displayed this pattern of responding (Fig. 2d). ANOVA for each phenotype were consistent with these impressions as it revealed for both BER (F(1,12) = 6.26, p<0.05) and BEN rats (F(1,47) = 9.66, p<0.01) significantly suppressed performance on the devalued action, whereas BEP rats showed similarly high rates of responding on both levers (F<1). Large effect sizes were also revealed in BER (d = 1.32) and BEN (d = 1.05), but not BEP rats (d = 0.15). In addition, when we incorporated into the analysis the correlation between these measures, moderate effect sizes were also reported for BER (d = 0.59) and BEN (d = 0.60) groups, however no evidence of a devaluation effect was revealed in BEP rats (d = 0.05). Thus, BEP rats appear to display a pre-existing reduction in their sensitivity to instrumental reinforcer devaluation.
Figure 2.
Instrumental reinforcer devaluation following sensory-specific satiety. (a) Overall rats showed clear evidence of instrumental reinforcer devaluation, suppressing their performance on the lever associated with the devalued reinforcer. However, while (b) BER and (c) BEN rats displayed intact devaluation performance, (d) BEP rats showed a reduced sensitivity to devaluation as revealed by similar responding on both levers. Symbols connected by lines reflect response data from individual rats. *p’s<0.05, error bars indicate SEM.
3.4. Sensory-specific satiety and reinforcer choice test
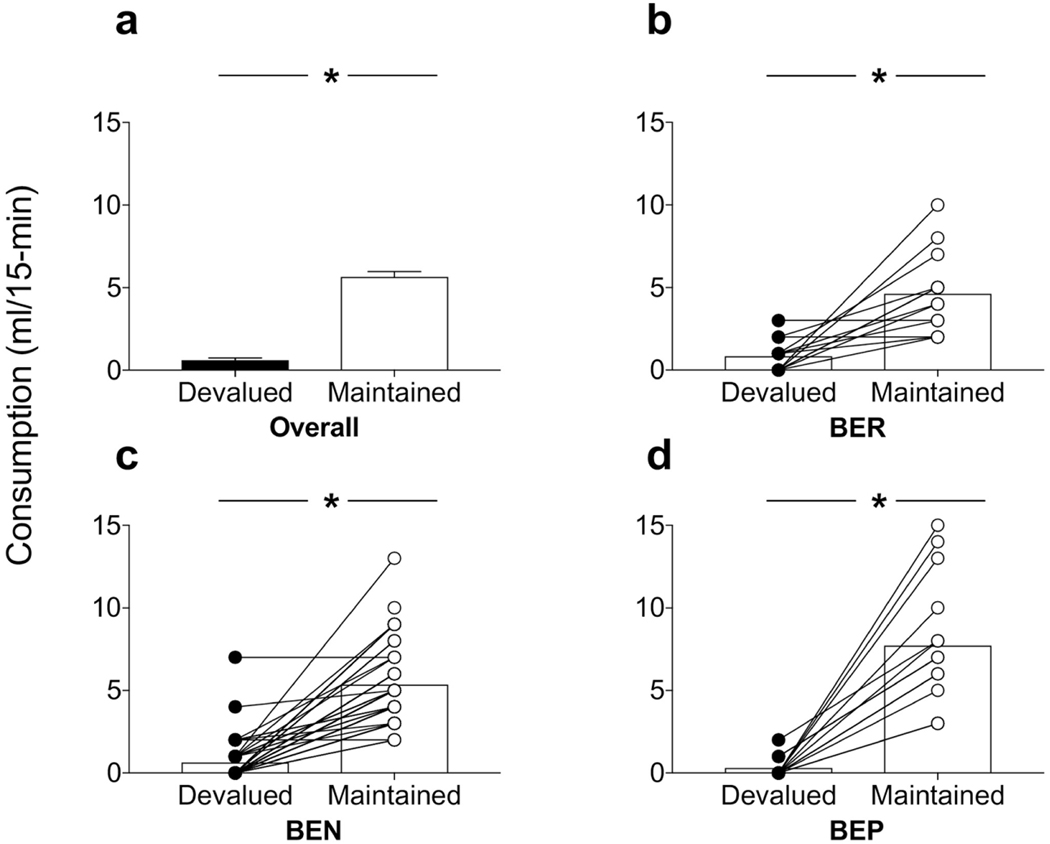
There were no differences between the phenotypes in prefeeding during sensory-specific satiety (consumption ml/hour ± SEM; BER = 20.84 ± 0.977, BEN = 19.82 ± 1.23, BEP = 21.23 ± 2.05). One-way ANOVA revealed no effect of phenotype (F<1). During the choice test, one BEP and BEN rat knocked over their palate cup, thus their data are excluded from the analysis. The choice test results from the remaining rats are presented in Figure 3. All rats showed reduced preference toward the prefed devalued reinforcer and consumed significantly more of the maintained reinforcer (Fig. 3a). Repeated measures ANOVA on the intake data revealed a significant effect of reinforcer (F(1,72) = 182.50, p<0.0001). When these data were re-examined following phenotyping, while all rats showed similar reduction in consumption of the devalued food, BEP rats showed increased intake of the maintained reinforcer relative to the other two phenotypes. Two-way phenotype X reinforcer ANOVA revealed a main effect of phenotype (F(2,70) = 4.49, p=0.01), reinforcer (F(1,70) = 922.52, p<0.001), and a significant interaction between the two variables (F(2,70) = 7.34, p<0.001). To evaluate the nature of the interaction, tests of simple main effect revealed comparable devalued reinforcer intake between the phenotypes (F’s<1.4, p’s>0.23), whereas BEP rats (Fig. 3d) consumed significantly more of the maintained reinforcer relative to both BER (F(1,70) = 11.01, p=0.001) (Fig. 3b) and BEN (F(1,70) = 11.46, p=0.001) (Fig. 3c) rats. One-way ANOVA for each phenotype also confirmed strong preferences in intake for the maintained relative to the devalued reinforcer (Smallest F-value; BER, (F(1,12) = 20.46, p<0.0001). The Cohen’s d analyses also revealed large effect sizes for all phenotypes (d’s > 0.8). Finally, BEP rats displayed greater overall intake during the choice test (consumption ml/15 min ± SEM; 8.38 ml ± 1.08) compared to both BER (5.46 ml ± 0.62) and BEN (5.89 ± 0.39) groups. One-way ANOVA revealed a main effect of phenotype (F(2,70) = 4.49, p=0.01), due to significant increased intake in BEP rats relative to both BER and BEN groups (p’s<0.05).
Figure 3.
Food choice test following sensory-specific satiety. (a) Overall the devaluation was effective in biasing consumption away from the devalued reinforcer and toward the non-prefed maintained reinforcer. While (b) BER and (c) BEN rats displayed comparable intake during this test, (d) BEP rats showed an increase in consumption directed towards the maintained reinforcer. Symbols connected by lines reflect response data from individual rats. *p’s<0.001, error bars indicate SEM.
3.5. Pearson correlations
3.5.1. Correlation of responding during instrumental reinforcer devaluation and mean palatable food consumption
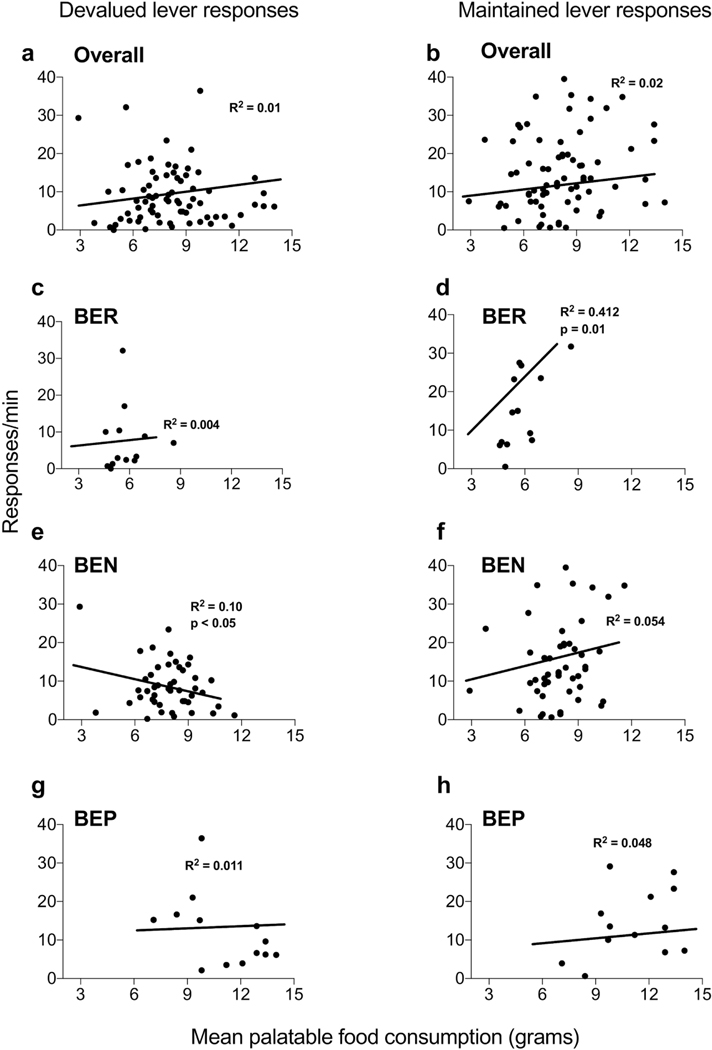
When we examined all rats, neither the devalued (r = 0.12, R2 = 0.01, p = 0.28) or maintained responses (r = 0.15, R2 = 0.02, p = 0.18) served as a significant predictor for future consumption of palatable food (Fig. 4a & 4b). However, when the linear regression models were conducted for each phenotype separately, several significant correlations were revealed. In BEN rats, responding on the devalued lever negatively correlated (r = 0.28, R2 = 0.10, p<0.05) with palatable food consumption during phenotyping (Fig. 4e). This was not the case for either BER (r = 0.06, R2 = 0.004, p=0.84) (Fig. 4c) or BEP (r = 0.13, R2 = 0.01, p=0.65) rats (Fig. 4g). A similar analysis for responses on the maintained lever revealed a positive correlation in BER rats (r = 0.64, R2 = 0.41, p=0.01) (Fig. 4d), but not BEN (r = 0.23, R2 = 0.05, p=0.11) (Fig. 4f) or BEP (r = 0.22, R2 = 0.04, p=0.44) (Fig. 4h) rats.
Figure 4.
Correlations between instrumental reinforcer devaluation responding and palatable food intake during phenotyping. Across all rats, responding on the (a) devalued lever did not correlate with palatable food intake. (c) BER and (g) BEP rats also showed no correlation between devalued responding and palatable food intake. However, a negative correlation was revealed in (e) BEN rats between devalued responding and palatable food intake. (b) For maintained responses, across all rats the rate of responding did not correlate with palatable food intake; this was also the case for (f) BEN and (h) BEP rats. However, (d) in BER rats a positive correlation was revealed in responding on the maintained lever and mean palatable food intake during phenotyping.
3.5.2. Correlation of consumption during reinforcer choice and mean palatable food consumption
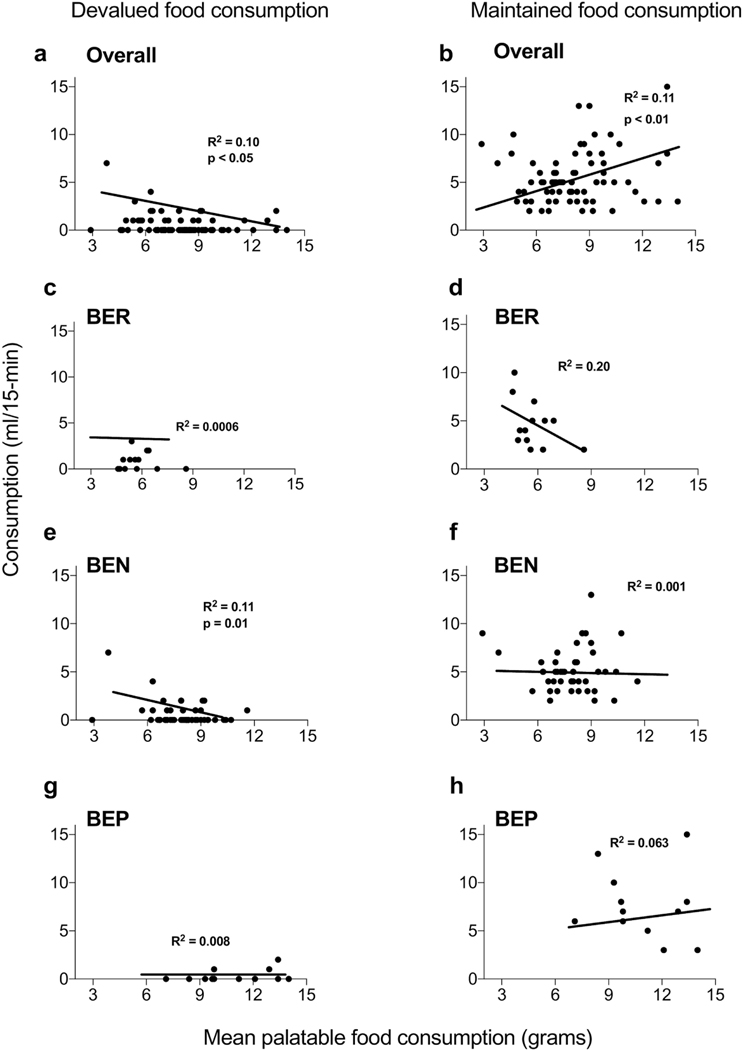
Simple linear regression for all rats revealed that consumption of the devalued reinforcer during the choice test was negatively correlated with palatable food intake during phenotyping (r = 0.23, R2 = 0.10, p = <0.05) (Fig. 5a), whereas maintained reinforcer consumption was positively correlated (r = 0.30, R2 = 0.11, p = <0.01) (Fig. 5b). When we examined each group separately, devalued food consumption was not correlated with palatable food consumption in BER (r = 0.02, R2 = 0.0006, p=0.93) (Fig. 5c) or BEP (r = 0.09, R2 = 0.008, p=0.76) (Fig. 5g) rats, however in BEN rats (r = 0.34, R2 = 0.11, p=0.01) (Fig. 5e) a significant negative correlation was noted. When the same analysis was adopted for maintained food consumption, neither BER (r = 0.44, R2 = 0.20, p=0.12) (Fig. 5d), BEN (r = 0.03, R2 = 0.001, p=0.78) (Fig. 5f) or BEP (r = 0.25, R2 = 0.063, p = 0.38) (Fig. 5h) rats showed a significant correlation with this measure and subsequent palatable food consumption.
Figure 5.
Correlations between consumption during reinforcer choice test and palatable food intake during phenotyping. Across all rats (a) consumption of the devalued reinforcer was negatively correlated with mean palatable food consumption, whereas (b) consumption of the maintained reinforcer was positively correlated. No significant correlations were revealed for maintained reinforcer consumption in (c) BER and (g) BEP rats. Similarly, devalued reinforcer consumption was not significantly correlated with palatable food intake in either (d) BER, (f) BEN or (h) BEP rats. However, (e) a significant negative correlation was observed between consumption of the devalued reinforcer and palatable food intake during phenotyping in BEN rats.
4. Discussion
Instrumental reinforcer devaluation was used to examine the extent to which female rats were capable of using reductions in outcome value to guide their behavior in a goal-directed manner [20,21,23,29]. Subsequently, all rats were phenotyped using a model that identifies natural differences in binge eating proneness during intermittent access to palatable food [10,12,27]. When reinforcer devaluation performance was reexamined, both BER and BEN rats previously showed intact and comparable test performance; however, BEP rats displayed reduced sensitivity to instrumental reinforcer devaluation. This impairment in BEP rats occurred despite evidence that the devaluation procedure itself (i.e., sensory-specific satiety) was actually more effective at altering reinforcer preferences in BEP compared to either BER or BEN rats.
Our findings suggest that BEP rats display a pre-existing insensitivity to flexibly adjust their responding in a goal-directed manner following reductions in reinforcer value. It should be noted while a significant phenotype X response interaction was not revealed, during the extinction test BEP rats failed to show a devaluation effect as evidenced by the weak effect size and indifference in responding to the devalued and maintained levers. Conversely, both BER and BEN rats displayed intact goal-directed responding. This reduced sensitivity to instrumental reinforcer devaluation in BEP rats may reflect impairments in encoding detailed response-outcome associations for each lever during training. On the other hand, BEP rats may have been unable to retrieve these associations during the test stage and/or integrate the reduction in reinforcer value into the appropriate response-outcome association. Despite this, BEP rats remained sensitive to some of the reinforcing impact of reinforcer delivery. Thus, BEP rats showed comparable acquisition of responding to the levers, which suggests that unlike BER and BEN rats, performance may have be driven by a more general reinforcement mechanism. Alternatively, the behavior of BEP rats might reflect an increased tendency to establish habits [20]. This latter form of responding is more typically seen when rats are overtrained to respond on a lever [20,30] and results in performance that is driven by a stimulus-response reinforcement mechanism. Under these conditions, subjects’ responding is insensitive to reductions in the value of the reinforcer [20], similar to the pattern of responding observed in BEP animals.
These findings advance our current understanding of behavioral and cognitive impairments associated with binge eating proneness. Using a somewhat similar phenotyping procedure, male BEP rats were shown to display impairment in reversal learning in the Barnes maze [31]. As performance in this task required spatial navigation, this may have been achieved by either encoding detailed cognitive representations of the environment (i.e., “place strategy”), or via associations between specific cues from the environment and the animals’ response in a particular direction (i.e., “response strategy”) [32–34]. This dichotomy between place and response driven-behaviors may be analogous to the distinction between goals and habits in instrumental conditioning [35], as response-based strategies in the plus maze are impervious to outcome devaluation [36]. It is tempting to consider that the lack of behavioral flexibility displayed by BEP rats in reversal learning [27] may reflect an enhanced tendency to perform in a rigid manner guided by previous stimulus-response associations. Irrespective of the merits of this account, our findings add to the growing evidence that intermittent access to palatable food is associated with disruptions in goal-directed behavior [37–40]. However, we report for the first time pre-existing behavioral impairments in BEP rats, which may predispose animals to maintain persistent high rates of consumption to palatable foods when exposed to an intermittent schedule.
An additional notable and related feature of our findings reflects the observation that despite the failure of BEP rats in guiding their instrumental performance in a manner dependent on the motivational status of the reinforcer, the devaluation itself was especially efficacious in altering consummatory behavior. That is, following sensory-specific satiety, rats subsequently characterized as BEP showed augmented consumption towards the reinforcer that had not been prefed. Sensory-specific satiety reflects a reduction in the hedonic value for a specific food eaten during a meal, which typically occurs without an influence in the attraction towards other foods [41–43]. Accordingly, palatable foods not recently eaten will maintain their hedonic properties and be readily consumed, which is consistent with observations that increases in food intake result from access to a variety of foods—the so called ‘buffet effect’, seen in both humans and animals [44–48]. It appears as though BEP rats may be idiosyncratically influenced by this phenomenon, as during the choice test they consumed more of the maintained (i.e., not recently eaten) reinforcer, despite all rats displaying comparable intake during sensory-specific satiety. This predisposition in BEP rats may also contribute to their subsequent propensity to overconsume intermittently provided highly palatable food, as during each feeding test they were also confronted with a choice between sated (i.e., lab chow) and a non-sated palatable food (i.e., Betty Crocker® Extra Creamy Vanilla Frosting).
Noteworthy relationships between test stage performance and palatable food intake during phenotyping were also revealed. In BER rats, the rate of responding on the maintained lever predicted future palatable food intake. It is tempting to speculate that the more resistant nature of BER rats to binge eat may be reflective of a greater tendency for appetitive behaviors in these rats to be under the control of goal-directed mechanisms. This is also consistent with a greater proportion of BER rats displaying a response preference for the maintained relative to the devalued lever, which declined between the groups as a function of binge eating status (i.e., BER = 84%; BEN = 64%; BEP = 50%). On the other hand, in BEN rats the rate of responding on the devalued lever was negatively correlated with the mean intake of palatable food intake during phenotyping. Interestingly, a negative correlation between devalued food consumption during the choice test was also revealed for these rats. This suggests that the degree of suppression both in responding to the devalued action and consuming the devalued food, was curiously associated with an increased susceptibility for eating of palatable food during the feeding tests in BEN rats. Finally, while the simple regression analysis for all rats revealed no overall relationships with respect to lever responding and future palatable food intake, consumption during the choice test was predictive of palatable food consumption during phenotyping. Specifically, consumption of the devalued food was negatively correlated with palatable food intake, whereas a positive correlation was revealed when maintained food consumption during the choice test was examined. While this suggests that the sensitivity to sensory-specific satiety may be a useful predictor of future binge eating status in female rats, this interpretation should be considered cautiously given the overall large sample size and that these correlations were generally not observed at the group level.
Successful performance in instrumental reinforcer devaluation requires control by cortico-limbo-striatal circuits, including prelimbic cortices, the nucleus accumbens core, the basolateral amygdala and the medial lateral striatum [23,24,26]. Conversely, the transition to habitual control reflects guidance from the infralimbic cortex and the dorsolateral straitum [24,49] (see,[50]). We have previously shown that BEP rats display enhanced c-fos expression (a commonly used marker for neuronal activity) in the infralimbic and prelimbic cortex along with the ventral striatum, in response to palatable food consumption [12,27]. It has been shown that prefrontal manipulation of dopamine, serotonin or BDNF in rats leads to reinforcer devaluation deficits [51,63,64], which is consistent with the altered prefrontal gene expression for a number of dopaminergic, serotonergic and neurotropic markers in BEP rats [31]. Thus, collectively, given the reliance of these systems and molecular circuits in modulating the transition between goal-directed and habitual behavior, it is possible that dysfunctional prefrontal-striatal activity may underlie the BEP impairments in reinforcer devaluation reported here.
Our findings may also reflect susceptibility to the establishment of habits via sex differentiation of the mesocoritcolimbic reward system, which underlies the formation of goal- and habitual behaviors. Male rats show increased mesocorticolimbic expression of D1 and D2 receptors relative to females, whereas ovarian hormones can modulate dopamine activity in striatal regions [52,53]. Moreover, relative to males, female rats display an enhanced propensity to self-administer psychoactive drugs [52] along with a reduced sensitivity to reinforcer devaluation [54], and an enhanced tendency to develop habitual behavior following instrumental training [55]. Although the neurobiological and hormonal factors underlying these differences remain to be elucidated, we have recently shown that perinatal testosterone exposure contributes to the propensity for binge eating proneness after the onset of puberty [17]. Thus, future studies should examine whether testosterone perinatal organizational effects of central circuitry may protect against the insensitivity to devaluation in females and relatedly contribute to BEP status.
5. Conclusion
Traditional ideas regarding the etiology of EDs focused on sociocultural and psychosocial variables, however more recent characterizations also include the genetic and neurobehavioral determinants underlying pathology [4,57–59]. Our findings may be consistent with the loss of control over palatable food consumption in EDs, which hampers an individual’s ability to utilize dietary restraint and limit maladaptive overconsumption [1,60]. In bulimic syndromes, this manifests more broadly as difficulties withholding actions (i.e., impaired inhibitory control) [61,62], which may be consistent with the current observed failure of BEP rats to suppress performance on a lever associated with a devalued reinforcer. It should be noted that while rats in this study experienced weight loss during food deprivation for instrumental training, it is unlikely that this significantly altered the spectrum of individual differences in binge eating proneness, as the rates of BEP and BER rats were generally comparable to previous studies [9,13,27]. In sum, our findings suggest that BEP rats display pre-existing differences that could ultimately enhance vulnerability to the eating of palatable food on an intermittent schedule. This includes a reduced sensitivity to instrumental devaluation and enhancement in consummatory behavior following sensory-specific satiety.
Highlights.
Female rats were trained in an instrumental reinforcer devaluation task
Intermittent access to palatable food was used to characterize binge eating proneness
Binge eating prone (BEP) rats displayed pre-existing instrumental reinforcer devaluation deficits
In BEP rats sensory-specific satiety was more effective at altering the preference for the reinforcers
Devaluation procedures have a dissociable influence over consummatory and instrumental behaviors in BEP rats
Acknowledgements
This work was in part supported by NIH grant R01 DK111475 to AWJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fifth ed., Washington, DC, 2013. [Google Scholar]
- [2].Arcelus J, Mitchell AJ, Wales J, Nielsen S, Mortality rates in patients with anorexia nervosa and other eating disorders: A meta-analysis of 36 studies, Arch. Gen. Psychiatry 68 (2011) 724–731. [DOI] [PubMed] [Google Scholar]
- [3].Fichter MM, Quadflieg N, Hedlund S, Long-term course of binge eating disorder and bulimia nervosa: Relevance for nosology and diagnostic criteria, Int. J. Eat. Disord 41 (2008) 577–586. [DOI] [PubMed] [Google Scholar]
- [4].Klump KL, Culbert KM, Sisk CL, Sex differences in binge eating: Gonadal hormone effects across development, Annu. Rev. Clin. Psychol 13 (2017) 183–210. [DOI] [PubMed] [Google Scholar]
- [5].Smink FRE, van Hoeken D, Hoek HW, Epidemiology of eating disorders: Incidence, prevalence and mortality rates, Curr. Psychiatry Rep 14 (2012) 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hudson JI, Hiripi E, Pope HG Jr, Kessler RC, The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication, Biol. Psychiatry 61 (2007) 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Andersen A, DiDomenico L, Andersen AE, & DiDomenico L.Diet vs. shape content of popular male and female magazines: A dose‐response relationship to the incidence of eating disorders?. International Journal of Eating Disorders, 11(3) (1992), 283–287. [Google Scholar]
- [8].Fornari V, Dancyger I, Psychosexual development and eating disorders, Adolescent Medicine Clinics 14, no. 1 (2003): 61. [PubMed] [Google Scholar]
- [9].Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL, The effects of ovariectomy on binge eating proneness in adult female rats, Horm. Behav 59 (2011) 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ, High intake of palatable food predicts binge-eating independent of susceptibility to obesity: An animal model of lean vs obese binge-eating and obesity with and without binge-eating, Int. J. Obes 31 (2007) 1357–1367. [DOI] [PubMed] [Google Scholar]
- [11].Klump KL, Suisman JL, Culbert KM, Kashy DA, Sisk CL, Binge eating proneness emerges during puberty in female rats: A longitudinal study, J. Abnorm. Psychol 120 (2011) 948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sinclair EB, Culbert KM, Gradl DR, Richardson KA, Klump KL, Sisk CL, Differential mesocorticolimbic responses to palatable food in binge eating prone and binge eating resistant female rats, Physiol. Behav 152 (2015) 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Klump KL, Racine S, Hildebrandt B, Sisk CL, Sex differences in binge eating patterns in male and female adult rats, Int. J. Eat. Disord 46 (2013) 729–736. [DOI] [PubMed] [Google Scholar]
- [14].Klump KL, Puberty as a critical risk period for eating disorders: A review of human and animal studies, Horm. Behav 64 (2013) 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patton GC, Selzer R, Coffey C, Carlin JB, Wolfe R, Onset of adolescent eating disorders: Population based cohort study over 3 years, BMJ. 318 (1999) 765–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Culbert KM, Burt SA, McGue M, Iacono WG, Klump KL, Puberty and the genetic diathesis of disordered eating attitudes and behaviors, J. Abnorm. Psychol 118 (2009) 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Culbert KM, Sinclair EB, Hildebrandt BA, Klump KL, and Sisk CL Perinatal testosterone contributes to mid-to-post pubertal sex differences in risk for binge eating in male and female rats. Journal of abnormal psychology 127, no. 2 (2018): 239. [DOI] [PubMed] [Google Scholar]
- [18].Oswald KD, Murdaugh DL, King VL, Boggiano MM, Motivation for palatable food despite consequences in an animal model of binge eating, Int. J. Eat. Disord 44 (2011) 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moshe L, Bekker L, Weller A, A potential animal model of maladaptive palatable food consumption followed by delayed discomfort, Front. Neurosci 11 (2017) 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Adams CD, Variations in the sensitivity of instrumental responding to reinforcer devaluation. The Quarterly Journal of Experimental Psychology Section B 34, no. 2b (1982): 77–98. [Google Scholar]
- [21].Holland PC, Relations between pavlovian-instrumental transfer and reinforcer devaluation., J. Exp. Psychol. Anim. Behav. Process 30 (2004) 104–117. [DOI] [PubMed] [Google Scholar]
- [22].Johnson AW, Bannerman DM, Rawlins NP, Sprengel R, Good MA, Impaired outcome-specific devaluation of instrumental responding in mice with a targeted deletion of the AMPA receptor glutamate receptor 1 subunit, J. Neurosci 25 (2005) 2359 LP–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johnson AW, Gallagher M, Holland PC, The basolateral amygdala is critical to the expression of pavlovian and instrumental outcome-specific reinforcer devaluation effects, J. Neurosci 29 (2009) 696 LP–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proceedings of the National Academy of Sciences 109, no. 46 (2012): 18932–18937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Johnson AW, Han S, Blouin AM, Saini J, Worley PF, During MJ, Holland PC, Baraban JM, Reti IM, Localized disruption of Narp in medial prefrontal cortex blocks reinforcer devaluation performance, Learn. Mem 17 (2010) 620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].West EA, Carelli RM, Nucleus accumbens core and shell differentially encode reward-associated cues after reinforcer devaluation, J. Neurosci 36 (2016) 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hildebrandt BA, Sinclair EB, Sisk CL, Klump KL. Exploring reward system responsivity in the nucleus accumbens across chronicity of binge eating in female rats. International Journal of Eating Disorders 51, no. 8 (2018): 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lakens D, Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs, Front. Psychol 4 (2013) 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Balleine B, Dickinson A, Instrumental performance following reinforcer devaluation depends upon incentive learning, Q. J. Exp. Psychol. Sect. B 43 (1991) 279–296. [Google Scholar]
- [30].Yin HH, Knowlton BJ, Balleine BW, Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning, Eur. J. Neurosci 19 (2004) 181–189. [DOI] [PubMed] [Google Scholar]
- [31].Chawla A, Cordner ZA, Boersma G, Moran TH, Cognitive impairment and gene expression alterations in a rodent model of binge eating disorder, Physiol. Behav 180 (2017) 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dudchenko PA, How do animals actually solve the T maze?, Behav. Neurosci 115 (2001) 850–860. [PubMed] [Google Scholar]
- [33].Hull CL, Principles of behavior: An introduction to behavior theory, Appleton-Century, Oxford, England, 1943. [Google Scholar]
- [34].Packard MG, McGaugh JL, Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning, Neurobiol. Learn. Mem 65 (1996) 65–72. [DOI] [PubMed] [Google Scholar]
- [35].Balleine B, Dickinson A, Signalling and incentive processes in instrumental reinforcer devaluation, Q. J. Exp. Psychol. Sect. B 45 (1992) 285–301. [PubMed] [Google Scholar]
- [36].Kosaki Y, Pearce JM, McGregor A, The response strategy and the place strategy in a plus-maze have different sensitivities to devaluation of expected outcome, Hippocampus. 28 (2018) 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Furlong T, Jayaweera H, Balleine B, Corbit L, Binge-like consumption of a palatable food accelerates habitual control of behavior and is dependent on activation of the dorsolateral striatum, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kendig MD, Boakes RA, Rooney KB, Corbit LH, Chronic restricted access to 10% sucrose solution in adolescent and young adult rats impairs spatial memory and alters sensitivity to outcome devaluation, Physiol. Behav 120 (2013) 164–172. [DOI] [PubMed] [Google Scholar]
- [39].Kosheleff AR, Araki J, Tsan L, Chen G, Murphy NP, Maidment NT, Ostlund SB, Junk food exposure disrupts selection of food-seeking actions in rats, Front. Psychiatry 9 (2018) 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Tantot F, Parkes SL, Marchand AR, Boitard C, Naneix F, Layé S, Trifilieff P, Coutureau E, Ferreira G, The effect of high-fat diet consumption on appetitive instrumental behavior in rats, Appetite. 108 (2017) 203–211. [DOI] [PubMed] [Google Scholar]
- [41].Rolls BJ, Sensory-specific satiety, Nutr. Rev 44 (1986) 93–101. [DOI] [PubMed] [Google Scholar]
- [42].Rolls ET, Taste, olfactory, and food reward value processing in the brain, Prog. Neurobiol 127–128 (2015) 64–90. [DOI] [PubMed] [Google Scholar]
- [43].Hetherington MM, Rolls BJ, Sensory-specific satiety: Theoretical frameworks and central characteristics, in: Why We Eat What We Eat Psychol. Eating, American Psychological Association, Washington DC, US, 1996: pp. 267–290. [Google Scholar]
- [44].Ahn S, Phillips AG, Repeated cycles of restricted food intake and binge feeding disrupt sensory-specific satiety in the rat, Behav. Brain Res 231 (2012) 279–285. [DOI] [PubMed] [Google Scholar]
- [45].González A, Recio SA, Sánchez J, Gil M, de Brugada I, Effect of exposure to similar flavours in sensory specific satiety: Implications for eating behaviour, Appetite. 127 (2018) 289–295. [DOI] [PubMed] [Google Scholar]
- [46].Reichelt A, Morris M, Westbrook R, Cafeteria diet impairs expression of sensory-specific satiety and stimulus-outcome learning, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rolls BJ, Rolls ET, Rowe EA, Sweeney K, Sensory specific satiety in man, Physiol. Behav 27 (1981) 137–142. [DOI] [PubMed] [Google Scholar]
- [48].Brondel L, Romer M, Van Wymelbeke V, Walla P, Jiang T, Deecke L, Rigaud D, Sensory-specific satiety with simple foods in humans: No influence of BMI?, Int. J. Obes 31 (2006) 987. [DOI] [PubMed] [Google Scholar]
- [49].Yin HH, Knowlton BJ, Balleine BW, Inactivation of dorsolateral striatum enhances sensitivity to changes in the action–outcome contingency in instrumental conditioning, Behav. Brain Res 166 (2006) 189–196. [DOI] [PubMed] [Google Scholar]
- [50].Shipman ML, Trask S, Bouton ME, Green JT, Inactivation of prelimbic and infralimbic cortex respectively affects minimally-trained and extensively-trained goal-directed actions, Neurobiol. Learn. Mem 155 (2018) 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].West EA, Forcelli PA, McCue DL, Malkova L, Differential effects of serotonin-specific and excitotoxic lesions of OFC on conditioned reinforcer devaluation and extinction in rats, Behav. Brain Res 246 (2013) 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Becker JB, Perry AN, Westenbroek C, Sex differences in the neural mechanisms mediating addiction: A new synthesis and hypothesis, Biol. Sex Differ 3 (2012) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Becker JB, Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis, Neurosci. Lett 118 (1990) 169–171. [DOI] [PubMed] [Google Scholar]
- [54].Hammerslag LR, Gulley JM, Age and sex differences in reward behavior in adolescent and adult rats, Dev. Psychobiol 56 (2014) 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schoenberg HL, Sola EX, Seyller E, Kelberman M, Toufexis DJ, Female rats express habitual behavior earlier in operant training than males., Behav. Neurosci 133 (2019) 110–120. [DOI] [PubMed] [Google Scholar]
- [56].Bulik CM, Kleiman SC, Yilmaz Z, Genetic epidemiology of eating disorders, Curr. Opin. Psychiatry 29 (2016) 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A, Nothing tastes as good as skinny feels: The neurobiology of anorexia nervosa, Trends Neurosci. 36 (2013) 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Klump KL, Holly A, Iacono WG, McGue M, Willson LE, Physical Similarity and Twin Resemblance for Eating Attitudes and Behaviors: A test of the equal environments assumption, Behav. Genet 30 (2000) 51–58. [DOI] [PubMed] [Google Scholar]
- [59].Griffin JB, Loss of Control, in: Walker JW, Hall HK, Hurst WD (Ed.), Clinical Methods: The History, Physical, and Laboratory Examinations, thrid ed., Butterworths, Boston, MA, 1990: pp. 918–919. [PubMed] [Google Scholar]
- [60].Brooks SJ, Rask-Andersen M, Benedict C, Schiöth HB, A debate on current eating disorder diagnoses in light of neurobiological findings: Is it time for a spectrum model?, BMC Psychiatry. 12 (2012) 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bartholdy S, Dalton B, O’Daly OG, Campbell IC, Schmidt U, A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task, Neurosci. Biobehav. Rev 64 (2016) 35–62. [DOI] [PubMed] [Google Scholar]
- [62].Wu M, Hartmann M, Skunde M, Herzog W, Friederich H-C, Inhibitory control in bulimic-type eating disorders: A systematic review and meta-analysis, PLoS One. 8 (2013) e83412–e83412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zimmermann KS, Yamin JA, Rainnie DG, Ressler KJ, Gourley SL. Connections of the Mouse Orbitofrontal Cortex and Regulation of Goal-Directed Action Selection by Brain-Derived Neurotrophic Factor. Biological Psychiatry, 81(4), (2017) 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hitchcott PK, Jennifer J, Quinn J J.R. Taylor. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cerebral cortex 17, no. 12 (2007): 2820–282 [DOI] [PubMed] [Google Scholar]