Abstract
Aims
Trial evidence indicates that glucagon-like peptide-1 receptor agonists (GLP-1 RAs) may reduce the risk of cardiovascular (CV) events in patients with diabetes and myocardial infarction (MI). We aimed to expand this observation to routine care settings.
Methods and results
Prospective observational study including all patients with diabetes surviving an MI and registered in the nationwide SWEDEHEART registry during 2010–17. Multivariable Cox regression analyses were used to estimate the association between GLP-1 RAs use and the study outcome, which was a composite of stroke, heart failure, Re-infarction, or CV death. Covariates included demographics, comorbidities, presentation at admission, and use of secondary CV prevention therapies. In total, 17 868 patients with diabetes were discharged alive after a first event of MI. Their median age was 71 years, 36% were women and their median estimated glomerular filtration rate was 75 mL/min/1.73m2. Of those, 365 (2%) were using GLP-1 RAs. During median 3 years of follow-up, 7005 patients experienced the primary composite outcome. Compared with standard of diabetes care, use of GLP-1 RAs was associated with a lower event risk [adjusted hazard ratio (HR) 0.72; 95% confidence interval (CI): 0.56–0.92], mainly attributed to a lower rate of re-infarction and stroke. Results were similar after propensity score matching or when compared with users of sulfonylurea. There was no suggestion of heterogeneity across subgroups of age, sex, chronic kidney disease, and STEMI.
Conclusion
GLP-1 RAs use, compared with standard of diabetes care, was associated with lower risk for major CV events in healthcare-managed survivors of an MI.
Keywords: GLP-1 receptor agonist, SWEDEHEART, Myocardial infarction, Diabetes, Cardiovascular events
Introduction
About 20–25% of patients admitted with a myocardial infarction (MI) in Europe have established diabetes mellitus.1 Patients with diabetes have long been known to be at high risk for morbidity and mortality after an MI,2,3 in part, because of more extensive coronary artery disease, additional cardiovascular (CV) risk factors, and higher burden of comorbidities.4,5 This increased CV burden is still present in diabetes patients with acute coronary syndrome (ACS), despite extensive use of modern evidence-based therapies.5 There is a need to improve secondary CV prevention strategies in these high-risk individuals.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are novel glucose-lowering treatments for type 2 diabetes with low risk for hypoglycaemia.6 Modest reductions in systolic blood pressure (BP), inflammation, and lipid concentrations as well as significant reduction in body weight have been observed in patients treated with GLP-1 RAs, and they have, therefore, been suggested as candidates for use in patients with diabetes at high risk of CV disease (CVD).7 Since the FDA requirements in 2008 of CV-safety data for novel diabetes agents, various trials have evaluated the efficacy and safety of GLP-1 RAs against placebo in diabetes patients with established CVD.8–14 While all of the trials showed CV safety (i.e. non-inferiority) over standard of care, some8,9,12,13 but not all10,11,14 observed efficacy in reducing their primary major adverse CV events (MACE) outcome (CV mortality, non-fatal MI, and non-fatal stroke). Differences across trials may be attributed not only to differences across GLP-1 RA agents, but also to differences in CV-risk profiles of included patients, including temporality of the event (prevalent vs. incident/acute CVD cases).
While trials assess drug safety and efficacy to gain regulatory approval, the rigours of trials can at times limit their generalizability to the larger population. Expanding trial evidence to observational studies from routine healthcare may offer complementary evidence to inform clinical decisions on the management of these patients. Against this background, we aimed to investigate, in a nationwide setting, the CV effectiveness associated to use of GLP-1 RAs at the time of an acute MI.
Methods
Data sources
We used data from SWEDEHEART (Swedish Web‐system for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies), a nationwide registry of patients hospitalized for suspected ACS or undergoing coronary or valve intervention.15 All Swedish hospitals (n = 72) contribute data to SWEDEHEART, covering around 90% of patients with MI treated in hospitals in Sweden. The registry is monitored on a regular basis, with 95–96% agreement with regards to key variables between the registry and electronic health records. Rich patient information is collected prospectively, including patient demographics, past medical history, medical treatment before admission, electrocardiographic changes, clinical investigations, medical treatment in hospital, interventions, hospital outcome, diagnoses, and medication at discharge. Patients receive information about their participation in SWEDEHEART at admission and are allowed to opt out, but individual consent is not required. For this study, and via each citizen’s unique personal identification number, SWEDEHEART was enriched with data linkages with the Swedish Registry of Dispensed Drugs, which contains all pharmacy‐drug dispensations in the country since 2005, and the National Patient Registry, which includes all ICD-10 diagnoses issues in connection with an outpatient-specialist or inpatient consultation in Sweden since 1997. The study protocol was approved by the regional Institutional Review Boards and adhered to the Declaration of Helsinki.
Study population
We included all adult (>18 years) survivors of an MI during 2010–17 with a diagnosis of diabetes mellitus and with a dispended glucose-lowering drug at the time of their MI, which was considered the index date of the study (Supplementary material online, Figure S1). We selected this period because GLP-1 RAs were introduced in Sweden in 2009. Exclusion criteria included previous history of MI, in-hospital death, and concomitant use of sodium-glucose transport protein-2 (SGLT-2) inhibitors.
Study design, exposure, and covariates
The study exposure was GLP-1 RAs use vs. non-use (i.e. standard of diabetes care). Use of GLP-1 RAs was defined as a dispensation of this medication within 1 month after hospital discharge or 6 months prior to index event. Patients were considered on-treatment until death or end of follow-up (intention to treat analysis). Information on the use of other antidiabetic medications was also collected.
Study covariates included age, sex, smoking habits, and body mass index (BMI) as registered per SWEDEHEART protocol. Body mass index was considered a covariate because at the time of data collection GLP-1 RAs were subsidized in Sweden for type 2 diabetes patients with BMI >30 kg/m2. Estimated glomerular filtration rate (eGFR) was calculated with the 2009 CKD-EPI creatinine-based equation16 using plasma creatinine measured at hospital admission. Since information on race is not available in Sweden, we assumed all patients Caucasian.
Comorbidities included heart failure (HF), cancer, hypertension, stroke, peripheral vascular disease (PVD), and atrial fibrillation. Information on patient presentation and hospital course variables considered Killip class, ST-segment elevation MI (STEMI) and non-STEMI, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG). Information on secondary CV prevention medications dispensed at discharge [angiotensin-converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARBs), β-blockers, statins, aspirin, and P2Y12-receptor blockers] was also collected.
Study outcomes
The primary study outcome was the incidence of MACE, defined as the composite of non-fatal stroke, HF, or MI and death due to CV causes. Outcomes were ascertained by linkage with the Patient Register, which has complete coverage of hospitalization diagnoses and deaths in the country, with virtually no loss to follow-up. Hospitalizations occurring within the first 30 days post-discharge were attributed to the index event. Patients were followed until the occurrence of an event or censoring (i.e. non-CV death or end of follow-up), whichever occurred first.
Statistical analysis
Baseline patient characteristics were described as median and interquartile range or counts with proportion. Unadjusted and adjusted cumulative incidence curves were estimated. The adjusted curves are obtained with regression standardization, which uses the predicted cumulative function of each patient and then averaged them over the observed distribution of the confounders in the population.17,18 Multivariable Cox proportional hazard models were used to estimate the association between GLP-1 RAs use and the risk of CV outcomes. We adjusted for clinical relevant variables known to influence both exposure and outcome, and then added other treatment known to influence outcome. Thus, the models were adjusted for age, sex, smoking, BMI, comorbidities [HF, stroke, PVD, cancer, hypertension, CKD (eGFR <60 mL/min/1.73 m2), PCI, CABG, atrial fibrillation, Killip class, and STEMI], and medication use (aspirin, statins, ACEi/ARBs, beta-blockers, and P2Y12-receptor blockers). Effect modification was investigated in the following strata defined a priori: age (< or ≥70 years), sex, eGFR category (eGFR < or ≥60 mL/min/1.73 m2), and STEMI. There were no missing values, except for BMI (missing in 6% of patients), smoking (missing in 7% of patients), and eGFR (missing in 3% of patients). Complete cases were considered for the analysis. The assumptions of proportionality and linearity (on the log hazard scale) in the Cox model were tested using Schoenfeld and martingale residuals, respectively. No relevant violation of this assumption was observed.
We performed several additional analyses to test the robustness of our results. First, we tested the association of GLP-1 RAs use vs. non-use on outcomes in a 1:5 matched cohort, matched by age, sex, and eGFR category. The analysis was performed by comparing cumulative incidence survival curves using the log-rank test. Second, we matched users and non-users with a 1:5 matching ratio using a propensity score (PS) approach.19 We used nearest-neighbour matching without replacement, using a calliper width equal to 0.01 on the logit of the PS. All covariates included in the multivariable Cox regression were used in the PS model. The matching was considered acceptable if the standardized mean difference between treatment groups was <0.1 (Supplementary material online, Table S1). The association between GLP-1 RAs use and study outcomes in the PS matched cohort was evaluated both graphically and with an univariable Cox proportional hazard model. The matched analyses do not assume proportional hazards or linearity for the covariates that are being matched on. However, the matched analyses may have low power to detect a difference between GLP-1 RAs use vs. non-use if the hazard ratio (HR) between these groups is highly non-proportional. We assessed the proportional hazards assumption in the matched analyses by Schoenfeld residuals and did not detect any major deviation from proportionality in either of these. Third, the main analysis was performed comparing GLP-1 RAs users and sulfonylurea users, overall and after excluding users of glyburide. Finally, in order to evaluate robustness of our design to bias in misclassification of the exposure, we repeated our main analysis after excluding patients with events occurring within the first 30 days of follow-up. Statistical analysis and data derivation were performed using SAS (SAS Institute Inc., Cary, NC, USA) and R (version 3.4.1).
Results
Baseline characteristics
Out of 17 868 patients with diabetes surviving their first MI, 365 (2%) patients were identified as users of GLP-1 RAs. Of these, 316 (86.6%) patients received liraglutide and the remaining 49 received exenatide, lixisenatide, or dulaglutide (7.7%, 4.9%, and 0.8%, respectively). Compared with non-use, patients using GLP-1 RAs were on average younger (median age 65 vs. 71 years), more frequently ex-smokers and more frequently obese (Table 1). Users of GLP-1 RAs more often had hypertension or CKD and had undergone PCI. The proportion of patients with Killip >1 was slightly lower among GLP-1 users (8.8% vs. 13.1%) as well as the proportions of users of ACEi/ARBs at discharge.
Table 1.
Baseline characteristics of diabetes patients at discharge of myocardial infarction
| Overall | No GLP-1 RA users | GLP-1 RA users | |
|---|---|---|---|
| Number of individuals | 17 868 | 17 503 | 365 |
| Age (years) | 71 [63, 79] | 71 [63, 79] | 65 [60, 71] |
| Age category (years) | |||
| <70 | 8152 (45.6) | 7903 (45.2) | 249 (68.2) |
| ≥70 | 9716 (54.4) | 9600 (54.8) | 116 (31.8) |
| Women | 6417 (35.9) | 6296 (36.0) | 121 (33.2) |
| Smoking | |||
| Never smoker | 6804 (38.1) | 6689 (38.2) | 115 (31.5) |
| Ex-smoker | 6370 (35.7) | 6195 (35.4) | 175 (47.9) |
| Current smoker | 3407 (19.1) | 3347 (19.1) | 60 (16.4) |
| Missing | 1287 (7.2) | 1272 (7.3) | 15 (4.1) |
| BMI (kg/m2) | 28.3 [25.3, 31.8] | 28.1 [25.3, 31.6] | 32.3 [29.4, 35.5] |
| BMI category (kg/m2) | |||
| <30 | 10 636 (59.5) | 10 531 (60.2) | 105 (28.8) |
| ≥30 | 6109 (34.2) | 5863 (33.5) | 246 (67.4) |
| Missing | 1123 (6.3) | 1109 (6.3) | 14 (3.8) |
| eGFR (mL/min/1.73 m2) | 75 [53, 91] | 75 [53, 91] | 80 [58, 96] |
| eGFR category (mL/min/1.73 m2) | |||
| <60 | 5544 (31.0) | 5445 (31.1) | 99 (27.1) |
| ≥60 | 11 722 (65.6) | 11 468 (65.5) | 254 (69.6) |
| Missing | 602 (3.4) | 590 (3.4) | 12 (3.3) |
| Comorbidities | |||
| STEMI | 5500 (30.8) | 5390 (30.8) | 110 (30.1) |
| Heart failure | 1613 (9.0) | 1583 (9.0) | 30 (8.2) |
| Cancer | 727 (4.1) | 715 (4.1) | 12 (3.3) |
| Hypertension | 13 570 (75.9) | 13 261 (75.8) | 309 (84.7) |
| PCI | 11 772 (65.9) | 11 503 (65.7) | 269 (73.7) |
| CABG | 2126 (11.9) | 2072 (11.8) | 54 (14.8) |
| Stroke | 2350 (13.2) | 2316 (13.2) | 34 (9.3) |
| PVD | 1148 (6.4) | 1131 (6.5) | 17 (4.7) |
| Atrial fibrillation | 2500 (14.0) | 2458 (14.0) | 42 (11.5) |
| Killip > 1 | 2321 (13.0) | 2289 (13.1) | 32 (8.8) |
| Medications | |||
| Aspirin | 16 216 (90.8) | 15 879 (90.7) | 337 (92.3) |
| Statins | 15 967 (89.4) | 15 625 (89.3) | 342 (93.7) |
| ACEi/ARBs | 10 331 (57.8) | 10 172 (58.1) | 159 (43.6) |
| Beta-blockers | 15 769 (88.3) | 15 445 (88.2) | 324 (88.8) |
| P2Y12 inhibitors | 13 239 (74.1) | 12 984 (74.2) | 255 (69.9) |
| Metformin | 9551 (53.5) | 9387 (53.6) | 164 (44.9) |
| Sulfonylurea | 2436 (13.6) | 2400 (13.7) | 36 (9.9) |
| DPP4i | 1056 (5.9) | 1041 (5.9) | 15 (4.1) |
| Insulin | 8350 (46.7) | 8173 (46.7) | 177 (48.5) |
ACEi, angiotensin-converting-enzyme inhibitor; ARBs, angiotensin receptor blockers; BMI, Body mass index; CABG, coronary artery bypass grafting; DPP4i, dipeptidyl peptidase 4 inhibitors; eGFR, estimated glomerular filtration rate; GLP-1 RA, GLP-1 receptor agonist; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; STEMI, ST-segment elevation myocardial infarction.
Risk of major adverse cardiovascular events
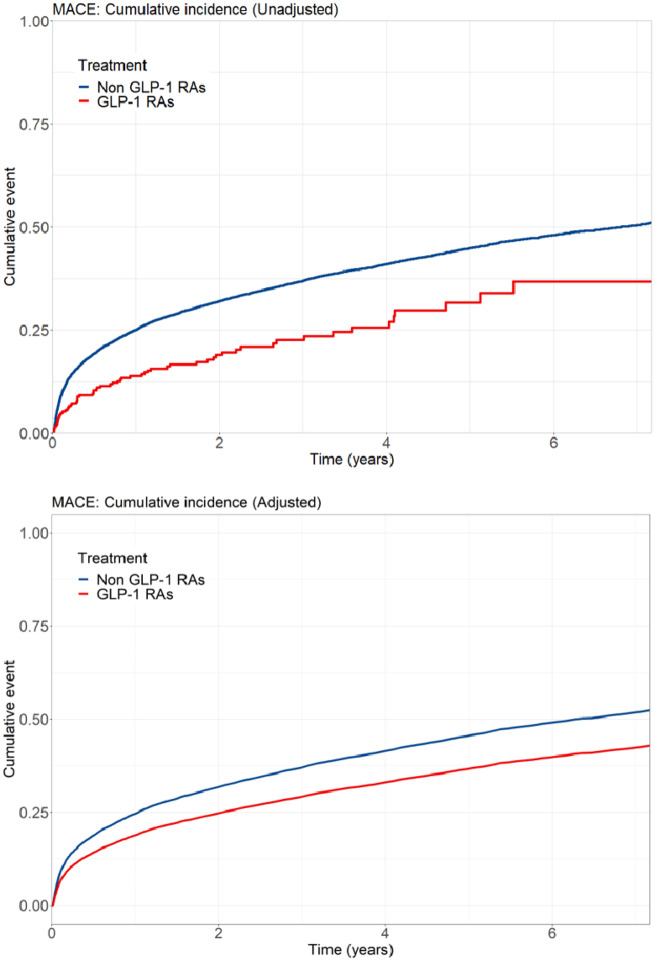
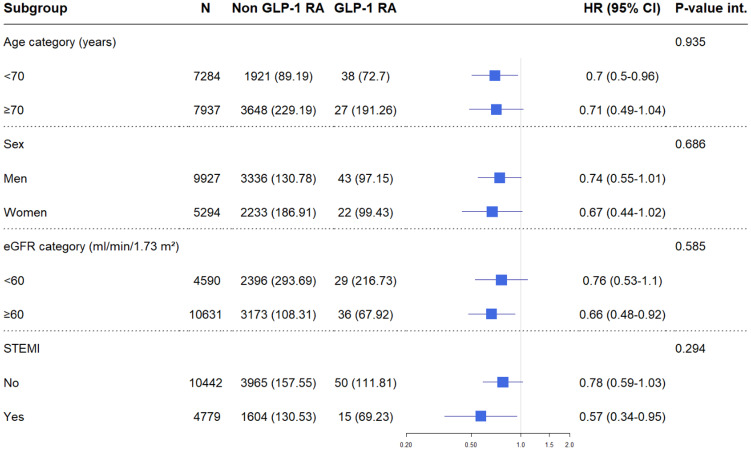
Among patients fulfilling inclusion and exclusion criteria, 15 221 were considered complete cases with no missing values in any of the covariates employed and included in subsequent analyses (Supplementary material online, Figure S1 and Table S1). During a median follow-up time of 2.98 years, 5634 patients experienced MACE. The cumulative incidence for MACE is shown in Figure 1, with lower observed event incidence among GLP-1 RAs users. The event rate among GLP-1 RAs users was lower compared with non-use (97.9 vs. 148.7 per 1000 person-years, respectively). The unadjusted HR associated with GLP-1 RAs use compared with non-use for the composite outcome of MACE was 0.57 [95% confidence interval (CI): 0.45–0.73]. After multivariable adjustment, this association attenuated but remained significantly lower, showing a 28% risk reduction among GLP-1 RAs users (HR: 0.72; 95% CI: 0.56–0.92) (Table 2). The direction of the associations for each single MACE component favoured GLP-1 RAs, in particular with stroke and myocardial re-infarction, but it was not statistically significant for CV death (Supplementary material online, Figure S2). There was no suggestion of heterogeneity with similar benefit of GLP-1 RAs use in patients with diverse age, sex, eGFR category, or STEMI/NSTEMI (Figure 2).
Figure 1.
Unadjusted and adjusted cumulative incidence of major adverse cardiovascular event among users and non-users of GLP-1 receptor agonist. GLP-1 RAs, GLP-1 receptor agonists; MACE, major adverse cardiovascular event. Cumulative incidence curve is adjusted for: age, sex, smoking, body mass index, estimated glomerular filtration rate category, comorbidities (heart failure, cancer, hypertension, percutaneous coronary intervention, coronary artery bypass grafting, stroke, peripheral vascular disease, atrial fibrillation, Killip, ST-segment elevation myocardial infarction), and cardiovascular medications (aspirin, statins, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, P2Y12 inhibitors).
Table 2.
Risk of cardiovascular events associated with use of GLP-1 RA use vs. non-use
| N events (IR per 1000 PY) | Non GLP-1 RA (IR per 1000 PY) | GLP-1 RA (IR per 1000 PY) | Adjusted HR (95% CI) | |
|---|---|---|---|---|
| MACE (composite) | 5634 (147.80) | 5569 (148.69) | 65 (97.91) | 0.72 (0.56–0.92) |
| Single components of MACE | ||||
| Stroke | 860 (17.45) | 855 (17.63) | 5 (6.39) | 0.42 (0.18–1.02) |
| Heart failure | 3577 (82.99) | 3535 (83.37) | 42 (60.25) | 0.81 (0.60–1.10) |
| Myocardial re-infarction | 2437 (54.53) | 2409 (54.8) | 28 (38.34) | 0.71 (0.49–1.04) |
| CV death | 1354 (26.56) | 1344 (26.78) | 10 (12.7) | 0.73 (0.39–1.36) |
Model adjusted for: age, sex, smoking, body mass index, eGFR category, comorbidities (heart failure, cancer, hypertension, percutaneous coronary intervention, coronary artery bypass grafting, stroke, peripheral vascular disease, atrial fibrillation, Killip, ST-segment elevation myocardial infarction), and cardiovascular medications (aspirin, statins, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, P2Y12 inhibitors).
CI, confidence interval; CV, cardiovascular; GLP-1 RA, GLP-1 receptor agonist; HR, hazard ratio; IR, Incidence rate; MACE, major adverse cardiovascular event; PY, person-years.
Figure 2.
Subgroup analyses: risk of major adverse cardiovascular events among users vs. non-users of GLP-1 receptor agonist by age, sex, ST-segment elevation myocardial infarction (STEMI), and estimated glomerular filtration rate category. CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; P-value int., P-value of interaction; STEMI, ST-segment elevation myocardial infarction. Model adjusted for (when relevant): age, sex, smoking, body mass index, estimated glomerular filtration rate category, comorbidities (heart failure, cancer, hypertension, percutaneous coronary intervention, coronary artery bypass grafting, stroke, peripheral vascular disease, atrial fibrillation, Killip, ST-segment elevation myocardial infarction), and cardiovascular medications (aspirin, statins, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, P2Y12 inhibitors).
As a sensitivity analysis, GLP-1 RAs users were matched with five non-users by age, sex, and eGFR and confirmed a lower cumulative incidence of MACE compared with standard of care (P-value of log-rank test = 0.007) (Supplementary material online, Figure S3). The 1:5 PS-matching analysis showed similar results than our main analysis, if any stronger in magnitude [HR for MACE 0.61 (0.46–0.80), Supplementary material online, Table S2]. Comparisons against users of sulfonylurea showed results in general consistent with our main analysis (Supplementary material online, Table S3 and Table S4 and Figure S4) for the outcomes of MACE, stroke, HF, and myocardial re-infarction. We note, however, a lack of protection against CV death in GLP1-RAs (HR 1.05; 95% CI: 0.54–2.04) accompanied by broad CIs that rendered the association non-significant. Similar results were also shown when we excluded glyburide users from the sulfonylurea group (Supplementary material online, Table S5). Finally, excluding patients with events occurring in the first 30 days of follow-up showed results consistent in direction and magnitude to your main analysis (Supplementary material online, Table S6). However, broader CIs yielded no statistically significant associations when evaluating the association with single components of MACE.
Discussion
In this nationwide cohort study of healthcare-managed patients with diabetes surviving an MI, we observe that GLP-1 RAs use in incident MI patients may offer additional cardioprotective benefits over standard of diabetes care. We, thus, expand evidence from clinical trials to routine clinical practice and to patients with recent MI.
Most of the patients in our study used liraglutide as their GLP-1 agent, which represents the market penetration of this drug in Sweden. In this regard, our results are, thus, in line with the analyses from the LEADER trial which showed a CV benefit of liraglutide use compared with placebo, also in patients with history of CVD at inclusion (HR: 0.83; 95% CI: 0.74–0.93).8 It also accords with the observational analysis of administrative databases by Svanström et al.20 showing a lower MACE risk in new users of liraglutide with a history of CVD (HR: 0.81; 95% CI: 0.71–0.92). Both these studies evaluated prevalent CVD patients at baseline, lacking information on the severity of the CV event per se or on the temporality of the association. We, thus, expand current evidence to a homogeneous and well-characterized cohort of survivors of an MI also taking into account relevant information on event severity and underlying kidney function. Our results, however, disagree with the observational analysis of Patorno et al.,21 who reported no significant difference in the risk of a composite CV outcome (MI, unstable angina, stroke, or coronary revascularization) between users of GLP-1 RAs and DPP-4 inhibitors. We note, however, that 70% of the patients in that study were using exenatide and follow-up was limited to 1 year. In our study, we find interesting the early separation of the survival curves, something that contrasts with the late effect observed in pivotal trials. Although we do not have a clear explanation for this, we speculate that it could be attributed to both the inclusion of prevalent GLP-1 RA users and the possibility of stronger effectiveness during the acute phases of myocardial injury. However, interventional studies to confirm or refute these speculations are so far lacking.
While cardioprotection has also been observed in clinical trials of semaglutide, albiglutide, and dulaglutide,9,12,13 no CV risk differences were reported in the pivotal trial of exenatide and lixisenatide,10,11 and the associations were less consistent in semaglutide participants from PIONEER-6 trial.14 Differences in the temporality of the MI event (i.e. inclusion of patients with a long-term history of MI,8–10 or within 90 days from their MI event13,14) or problems with compliance10 may explain the differences observed. Nonetheless, meta-analyses of most of these trials suggest collectively that the CV risk reduction is rather consistent, with a meta-estimated 10–13% CV risk reduction overall.22–24 Furthermore, we observed no differences in subsequent HF hospitalization among GLP-1 RA users, something that agrees with recent meta-analysis on liraglutide pivotal trials25 and in a post hoc analysis of the EXSCEL-trial.26 Given the predominance of liraglutide use in our setting, we were unable to compare single GLP-1 agents.
Subgroup analyses show similar results across age strata, sex, and severity of the MI event. Furthermore, across the kidney function spectrum enrolled, there was no consistent effect modification by the presence or absence of chronic kidney disease (eGFR < 60 mL/min/1.73 m2). This again is in agreement with post hoc analyses from LEADER showing consistent MACE reduction regardless presence of chronic kidney disease27 and may represent an interesting alternative for patients with diabetic kidney disease, especially in view of plausible effects of GLP-1 use in retarding chronic kidney disease progression.28,29 Diabetic kidney disease develops frequently among patients with diabetes, but the majority of them die from CV causes and infections before needing kidney replacement therapy.30 We were unable to study kidney replacement therapy as an outcome, because although we identified about 200 events in our cohort, only two events occurred among GLP-1 RA users.
Although the mechanism through which some of the GLP-1 RAs reduce MACE is not clear, the cardio-protective effects seem to go beyond glucose control,31 possibly involving weight loss, as well as improved insulin resistance and lipid levels.32–36 Pre-clinical studies have shown a reduction of atherosclerosis formation by GLP-1 RAs through inhibition of plaque progression and promoting plague stabilization37; GLP-1 RAs may reduce systemic and vascular inflammation by lowering the expression of inflammatory proteins involved in the development and progression of atherosclerosis38; and finally, studies have suggested a dose-dependent effect between GLP-1 RAs and changes in BP by increasing electrolytes excretion39,40 and reducing the circulating levels of RAS system components.41,42 However, not all of these mechanisms have been confirmed in clinical trials and warrant further characterization.
Our study has some strengths and limitations. Strengths are the rich quality of information collected from a nationwide register in a country with universal healthcare and medication costs subsidize by the government. This may reduce biases due to socio-economic differences or access to healthcare. Limitations include our inability to establish causality in the observed associations and to adequately separate prior use vs. post-MI initiation of GLP-1 RAs. Drug exposure status was identified from filled prescriptions; non-use of dispensed drugs would lead to exposure misclassification. The primary intention-to-treat exposure definition allowed for complete follow-up and estimation of the overall impact of starting treatment with GLP-1 RA, but might also have resulted in exposure misclassification. Given the drug indication, we can safely assume that all GLP1-RAs had type 2 diabetes. However, we could not differentiate type 1 from type 2 diabetes reliably, and some non-users may have type 1. This being said, previous validation studies in SWEDEHEART evidence that <5% of participants had type 1 diabetes,43 which argues against relevant misclassification bias. We lack information on important covariates such as HbA1c and diabetes duration. As in any observational study, and despite the application of several key measures to reduce the potential for bias—including use of an active comparator, and PS matching—confounding from unmeasured risk factors cannot be ruled out. We have a relatively low number of GLP-1 RAs users in our study. However, these are representative of the Swedish population during 2010–17. In any case, extrapolation to other periods and countries should be done with caution. Nonetheless, considering the very limited evidence to date on GLP-1 RA use in a near MI-event, our data are of value in reporting at least no adverse events in this high-risk patient group. From a patient selection point of view, it may be argued that if GLP-1 RA is effective in primary CV prevention,8,9 our incident MI cases represent a selective patient population in which the GLP-1 RA treatment was less effective. If true, this would have brought our estimates towards the null.
We conclude that compared with the standard of diabetes care, the use of GLP-1 RA by routinely cared survivors of an acute MI was associated with a lower risk of subsequent major CV adverse events.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Pharmacotherapy online.
Conflict of interest: J.J.C. reports receiving funding from AstraZeneca, Viforpharma, Astellas, and Novartis for projects unrelated to this study. T.J. reports research grants from Novartis and Merck and fees for lecturing and consulting from Astra-Zeneca, Aspen, Bayer, Merck, Novartis, and Sanofi. A.N. reports receiving consulting and lectures fees from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and Merck in Sweden all unrelated to this study. P.L. reports receiving consulting and lectures fees from AstraZeneca, Amgen, Boehringer Ingelheim, and Sanofi in Sweden all unrelated to this study. The rest of the co-authors have no conflicts of interest to report.
Supplementary Material
References
- 1. Alabas OA, Jernberg T, Pujades-Rodriguez M, Rutherford MJ, West RM, Hall M, Timmis A, Lindahl B, Fox KAA, Hemingway H, Gale CP. Statistics on mortality following acute myocardial infarction in 842,897 Europeans. Cardiovasc Res 2020;116:149–157. [DOI] [PubMed] [Google Scholar]
- 2. Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA 2007;298:765–775. [DOI] [PubMed] [Google Scholar]
- 3. Norhammar A, Lindback J, Ryden L, Wallentin L, Stenestrand U; on behalf of the Register of Information and Knowledge about Swedish Heart Intensive Care Admission (RIKS-HIA). Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admission. Heart 2006;93:1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodfield SL, Lundergan CF, Reiner JS, Greenhouse SW, Thompson MA, Rohrbeck SC, Deychak Y, Simoons ML, Califf RM, Topol EJ, Ross AM. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol 1996;28:1661–1669. [DOI] [PubMed] [Google Scholar]
- 5. Ritsinger V, Saleh N, Lagerqvist B, Norhammar A. High event rate after a first percutaneous coronary intervention in patients with diabetes mellitus: results from the Swedish coronary angiography and angioplasty registry. Circ Cardiovasc Interv 2015;8:e002328. [DOI] [PubMed] [Google Scholar]
- 6. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 2016;18:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DCESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 8. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 10. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Ohman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJV, Probstfield JL, Riddle MC, Solomon SD, Tardif J-C. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 12. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes Committees and Investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529. [DOI] [PubMed] [Google Scholar]
- 13. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T ; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130. [DOI] [PubMed] [Google Scholar]
- 14. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsboll T, Warren ML, Bain SC. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–851. [DOI] [PubMed] [Google Scholar]
- 15. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish Web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rothman KG, Lash T. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 18. Sjölander A. Regression standardization with the R package stdReg. Eur J Epidemiol 2016;31:563–574. [DOI] [PubMed] [Google Scholar]
- 19. Fu EL, Groenwold RHH, Zoccali C, Jager KJ, van Diepen M, Dekker FW. Merits and caveats of propensity scores to adjust for confounding. Nephrol Dial Transplant 2019;34:1629–1635. [DOI] [PubMed] [Google Scholar]
- 20. Svanström H, Ueda P, Melbye M, Eliasson B, Svensson AM, Franzen S, Gudbjornsdottir S, Hveem K, Jonasson C, Pasternak B. Use of liraglutide and risk of major cardiovascular events: a register-based cohort study in Denmark and Sweden. Lancet Diabetes Endocrinol 2019;7:106–114. [DOI] [PubMed] [Google Scholar]
- 21. Patorno E, Everett BM, Goldfine AB, Glynn RJ, Liu J, Gopalakrishnan C, Kim SC. Comparative cardiovascular safety of glucagon-like peptide-1 receptor agonists versus other antidiabetic drugs in routine care: a cohort study. Diabetes Obes Metab 2016;18:755–765. [DOI] [PubMed] [Google Scholar]
- 22. Hussein H, Zaccardi F, Khunti K, Seidu S, Davies MJ, Gray LJ. Cardiovascular efficacy and safety of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a systematic review and network meta-analysis. Diabet Med 2019;36:444–452. [DOI] [PubMed] [Google Scholar]
- 23. Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, Pagidipati NJ, Chan JC, Gustavson SM, Iqbal N, Maggioni AP, Ohman P, Poulter NR, Ramachandran A, Zinman B, Hernandez AF, Holman RR. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018;6:105–113. [DOI] [PubMed] [Google Scholar]
- 24. Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP-1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: an updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes Obes Metab 2019;21:2576–2580. [DOI] [PubMed] [Google Scholar]
- 25. Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Kober L, Petrie MC, McMurray J. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 2019;7:776–785. [DOI] [PubMed] [Google Scholar]
- 26. Fudim M, White J, Pagidipati NJ, Lokhnygina Y, Wainstein J, Murin J, Iqbal N, Ohman P, Lopes RD, Reicher B, Holman RR, Hernandez AF, Mentz RJ. Effect of once-weekly exenatide in patients with type 2 diabetes mellitus with and without heart failure and heart failure-related outcomes: insights from the EXSCEL Trial. Circulation 2019;140:1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mann JFE, Fonseca V, Mosenzon O, Raz I, Goldman B, Idorn T, von Scholten BJ, Poulter NR; The LEADER Publication Committee on behalf of the LEADER Trial Investigators. Effects of liraglutide versus placebo on cardiovascular events in patients with type 2 diabetes mellitus and chronic kidney disease. Circulation 2018;138:2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 2017;377:839–848. [DOI] [PubMed] [Google Scholar]
- 29. Bethel MA, Mentz RJ, Merrill P, Buse JB, Chan JC, Goodman SG, Iqbal N, Jakuboniene N, Katona BG, Lokhnygina Y, Lopes RD, Maggioni AP, Ohman PK, Poulter NR, Ramachandran A, Tankova T, Zinman B, Hernandez AF, Holman RR. Renal outcomes in the EXenatide Study of Cardiovascular Event Lowering (EXSCEL). Diabetes 2018;67:522-P. [Google Scholar]
- 30. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017;12:2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mannucci E, Dicembrini I, Lauria A, Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care 2013;36(Suppl. 2):S259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu SC, Tu YK, Chien MN, Chien KL. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta-analysis. Diabetes Obes Metab 2012;14:810–820. [DOI] [PubMed] [Google Scholar]
- 33. Newman JD, Vani AK, Aleman JO, Weintraub HS, Berger JS, Schwartzbard AZ. The changing landscape of diabetes therapy for cardiovascular risk reduction: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1856–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond ) 2014;38:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DCW, Le Roux CW, Violante Ortiz R, Jensen CB, Wilding J. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 2015;373:11–22. [DOI] [PubMed] [Google Scholar]
- 36. Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract 2017;3:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sudo M, Li Y, Hiro T, Takayama T, Mitsumata M, Shiomi M, Sugitani M, Matsumoto T, Hao H, Hirayama A. Inhibition of plaque progression and promotion of plaque stability by glucagon-like peptide-1 receptor agonist: serial in vivo findings from iMap-IVUS in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis 2017;265:283–291. [DOI] [PubMed] [Google Scholar]
- 38. Krasner NM, Ido Y, Ruderman NB, Cacicedo JM. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One 2014;9:e97554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 2004;89:3055–3061. [DOI] [PubMed] [Google Scholar]
- 40. Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 2011;301:F355–F363. [DOI] [PubMed] [Google Scholar]
- 41. Skov J, Dejgaard A, Frøkiær J, Holst JJ, Jonassen T, Rittig S, Christiansen JS. Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab 2013;98:E664–E671. [DOI] [PubMed] [Google Scholar]
- 42. Yang J, Jose PA, Zeng C. Gastrointestinal-renal axis: role in the regulation of blood pressure. J Am Heart Assoc 2017;6:e005536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ritsinger V, Hero C, Svensson AM, Saleh N, Lagerqvist B, Eeg-Olofsson K, Norhammar A. Mortality and extent of coronary artery disease in 2776 patients with type 1 diabetes undergoing coronary angiography: a nationwide study. Eur J Prev Cardiol 2017;24:848–857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.