Abstract
Background
Efforts to minimize medication risks among older adults include avoidance of potentially inappropriate medications. Contemporary analysis of medication use in community-dwelling older people compared with the general population is lacking.
Participants
A total of 19,114 community-dwelling adults in Australia and the United States aged 70 years or older (65 years or older for U.S. minorities) without histories of major cardiovascular disease, cognitive impairment, or disability participated in a randomized, placebo-controlled trial of aspirin: ASPirin in Reducing Events in the Elderly study.
Measurements
Prescribed baseline medications obtained by self-report and medical record review were grouped by World Health Organization Anatomic and Therapeutic Chemical category. Potentially inappropriate medications were defined using a modified American Geriatrics Society Beers Criteria. Polypharmacy was defined as 5 or more medications, and hyperpolypharmacy defined as 10 or more medications. Cross-sectional descriptive statistics and adjusted odds ratios were computed.
Results
The median number of prescription medications per participant was three, regardless of age. Women had a higher medication prevalence. Cardiovascular drugs (primarily antihypertensives) were the most commonly reported (64%). Overall, 39% of the cohort reported taking at least one potentially inappropriate medication, with proton-pump inhibitors being the most commonly reported (21.2% of cohort). Of the cohort, 27% had polypharmacy, and 2% hyperpolypharmacy. Age 75 years or older, less than 12 years of education, hypertension, diabetes mellitus, chronic kidney disease, frailty, gastrointestinal complaint, and depressive symptoms were associated with an increased likelihood of potentially inappropriate medications and polypharmacy. For almost all medication classes, prevalence was equivalent or lower than the general older population.
Conclusion
Overall medication burden and polypharmacy are low in older adults free of major cardiovascular disease, disability, and cognitive impairment. The prevalence of potentially inappropriate medications is higher than previously reported and similar to more vulnerable populations as a result of the introduction of proton-pump inhibitors to the American Geriatrics Society Beers Criteria. Longitudinal follow-up is required to further understand the balance of benefits and risks for potentially inappropriate medications and polypharmacy in community-dwelling older people.
Keywords: healthy aging, pharmacoepidemiology, polypharmacy, potentially inappropriate medications
Prescription drugs are important for preserving health but are major expenditures in the United States and Australia. In the United States, prescription medications cost approximately $328 billion annually1; in Australia, medications cost $18.8 billion and contributed 13% of total health expenditure in 2014.2 People aged 65 years and older use approximately one third of all medications,3, 4 but make up a relatively small proportion of the population (~15–16%).5, 6 The older population is increasing in number. In the last decade, life expectancy for older people has increased,7 the prevalence of heart disease and uncontrolled hypertension has decreased,8, 9 and self-reported functional limitation has decreased.10 These changes have been observed concomitantly with higher usage of health services11 and increases in prescription medication use,12 suggesting that improved health care and pharmaceutical interventions are enabling more people to reach advanced ages in good health relative to their historical peers.
However, in reducing the burden of age-related chronic disease, overall greater medication exposure can increase adverse events resulting from complex dosing regimens, drug–drug interactions, and prescribing cascades. The pharmacokinetic and pharmacodynamic changes associated with aging make older adults particularly vulnerable to adverse effects of medication.13 A study14 estimated that 1 in 10 hospital admissions for people 60 years or older are attributed to adverse drug reactions, whereas another study15 estimated the annual cost in the United States to be $30.1 billion. Irrespective of the exact number, the cost of morbidity and mortality associated with adverse drug reactions is substantial.16
Medications associated with excess morbidity relative to their potential benefit are considered to be potentially inappropriate for older people and should be used with caution or avoided completely. Polypharmacy (use of 5 or more prescription medications) and hyperpolypharmacy (use of 10 or more medications) have also been linked with a high incidence of adverse reactions in older people.17 Analysis of the prevalence and impact of these medications has been the focus of many previous analyses, including studies of vulnerable older people admitted to hospital or who were in nursing home care18 and community cohorts with high prevalence of diabetes mellitus and cardiovascular disease.19 However, contemporary analysis of medication use in large cohorts of healthier community-dwelling older people using the latest criteria is lacking. Understanding how the epidemiology of medication use in community-dwelling older people differs from their more vulnerable peers can provide insight into medication risk and its avoidance in the community.
The objective of this article is to describe the baseline medication profile and correlates of community-dwelling older people using data from the Aspirin in Reducing Events in the Elderly (ASPREE) Study. Comparisons are made with published results for the general older population.
Methods
ASPREE Clinical Trial
Briefly, ASPREE was a randomized, placebo-controlled, double-blinded trial of low-dose (100 mg) aspirin’s ability to prolong disability-free life in 19,114 community-dwelling people aged 70 years or older (65 years or older for U.S. minorities) conducted in Australia (n=16,703) and the United States (n=2,411).20 Participants were required to be in good health, free of preexisting major cardiovascular disease, cognitively intact, and able to independently perform basic activities of daily living. The study commenced in March 2010 and concluded enrollment and baseline data collection in December 2014. Detailed methods and results of ASPREE are described elsewhere.20–22
Collection of Medication From Participants
Participants in ASPREE were asked to bring their medications to their baseline data collection visit. Research staff reviewed each medication and confirmed with the participant whether the medication was prescribed by his or her doctor. In addition to prescription medications, other medications of relevance to the aspirin intervention and main outcomes in ASPREE were recorded if participants reported taking them at least once per week on a regular basis, regardless of whether they were prescribed by a doctor or obtained without prescription. These included nonsteroidal antiinflammatory drugs (NSAIDs), acetaminophen, vitamin D, and open-label aspirin. When available, staff used clinic medical records to prompt participants about medications that they may have forgotten, with the aim of producing a comprehensive list. Medication data were transcribed onto a case report form, entered into the ASPREE data system, and subsequently coded according to the World Health Organization Anatomical and Therapeutic Chemical coding system (https://whocc.no/atc_ddd_index/).
Definitions
Potentially inappropriate medications (PIMs) were defined as listed under table 2 of the 2019 American Geriatrics Society (AGS) Beers Criteria for PIM use in older adults.23 This subgroup of medications within the AGS Beers Criteria was chosen because of the strong recommendation to avoid, as opposed to other subgroups of medications recommended for use with caution or avoided only for certain disease states. Where ASPREE collected insufficient data about the indication for the prescribed medication to determine if it met the AGS Beers Criteria (e.g., lack of dose, dosing regimen, or indication), the medication was not considered to be PIM (e.g., insulin sliding scale). Proton pump inhibitors (PPIs) were considered PIMs if they were not coprescribed with an NSAID and vice versa. A full list of PIMs used in this analysis and medications that were excluded is shown in Table S1.
Table 2.
Medication Prevalence in Aspirin in Reducing Events in the Elderly Study Participants by Drug Class and Sex and Country
| Medication Class | Men | Women | Unadjusted Odds Ratioa (95% CI) | Australia | United States | Adjusted Odds Ratiob (95% CI) |
|---|---|---|---|---|---|---|
| Insulin | 1% 84 |
1% 73 |
0.67 (0.40–0.92) | 1% 105 |
2% 52 |
3.53 (2.49–4.94) |
| Oral glucose-lowering drugs | 7% 576 |
5% 526 |
0.69 (0.61–0.78) | 5% 825 |
11% 277 |
2.58 (2.22–2.98) |
| Proton pump inhibitors | 23% 1908 |
26% 2806 |
1.18 (1.11–1.27) | 26% 4422 |
12% 292 |
0.38 (0.34–0.43) |
| Diuretics | 15% 1212 |
22% 2346 |
1.63 (1.51–1.76) | 17% 2915 |
27% 643 |
1.68 (1.52–1.86) |
| β-blockers | 6% 479 |
10% 1075 |
1.82 (1.62–2.03) | 7% 1192 |
15% 362 |
2.27 (1.99–2.57) |
| Calcium channel antagonists | 17% 1395 |
18% 1907 |
1.07 (0.99–1.15) | 17% 2840 |
19% 462 |
1.2 (1.07–1.34) |
| Drugs acting on the RAA pathway | 40% 3319 |
43% 4656 |
1.15 (1.08–1.22) | 43% 7148 |
34% 827 |
0.7 (0.64–0.76) |
| Statins | 28% 2337 |
34% 3650 |
1.31 (1.23–1.4) | 31% 5158 |
34% 829 |
1.13 (1.03–1.24) |
| NSAIDs | 15% 1227 |
16% 1691 |
1.08 (0.99–1.17) | 15% 2586 |
14% 332 |
0.85 (0.75–0.96) |
| Opioids | 3% 258 |
5% 540 |
1.65 (1.42–1.92) | 4% 721 |
3% 77 |
0.71 (0.55–0.89) |
| Benzodiazepines | 4% 327 |
8% 878 |
2.17 (1.91–2.48) | 7% 1134 |
3% 71 |
0.41 (0.32–0.52) |
| Antidepressants | 7% 593 |
14% 1552 |
2.19 (1.99–2.43) | 12% 1924 |
9% 221 |
0.71 (0.61–0.82) |
CI = confidence interval; NSAIDs = nonsteroidal antiinflammatory drugs; RAA = renin-angiotensin-aldosterone.
World Health Organization Anatomical and Therapeutic Chemical codes used to define medication classes: insulins, A10A; oral glucose-lowering drugs, A10B; proton pump inhibitors, A02BC; diuretics, C03; β-blockers, C07; prevalence of calcium channel antagonists, C08; drugs acting on the RAA pathway, C09; statins, C10AA; NSAIDs, M01A; bisphosphonates, M05BA; opioids, N02A; benzodiazepines, N05CD and N05BA; and antidepressants, N06A.
Men (reference) versus women.
Adjusted for age and sex, Australia (reference) versus United States.
Polypharmacy and hyperpolypharmacy were defined as the concurrent use of 5 or more or 10 or more prescription medications, respectively.24 Hypertension was defined as systolic blood pressure of greater than 139 mm Hg or diastolic blood pressure greater than 89 mm Hg or receiving pharmaceutical treatment for high blood pressure. Diabetes mellitus was defined as self-report of diabetes mellitus or fasting blood glucose of greater than or equal to 126 mg/dl or on pharmaceutical treatment for diabetes mellitus. Frailty was categorized on the basis of the adapted Fried frailty criteria, which included body weight, strength, exhaustion, walking speed, and physical activity.25 The category of prefrail included participants who met one or two of these five criteria, and the category of frail included those who met three or more criteria. Gastrointestinal complaint was defined as a self-report of gastro-esophageal reflux disease, gastritis, or dyspepsia. Chronic kidney disease was defined as an estimated glomerular filtration rate of less than 60 ml/minute/1.73 m2 or a urine albumin creatinine ratio of three or more. Quality of life was measured using the 12-Item Short Form Survey (SF-12) reported as Mental Component Score or Physical Component Score.26 Depressive symptoms were assessed with the Center for Epidemiologic Studies Short Depression Scale.27 Gait speed was assessed as the average time of two trials to walk 3 m. Grip strength was the mean of three trials of the dominant hand using a Jamar hand dynamometer.
Statistical Analysis
Descriptive statistics were used to summarize medication prevalence data. Odds ratios (ORs) from logistic regression models were used to describe associations between sex and country with baseline medication prevalence (Table 1), baseline medication class (Table 2), and PIMs, polypharmacy, and hyperpolypharmacy (Table 3). In this study, the OR were also used to describe associations between age category, ethnicity, years of education, gastrointestinal complaint, hypertension, diabetes mellitus, chronic kidney disease, frailty status, quality of life, grip strength, gait speed, cognitive score on the Modified Mini-Mental State Examination,28 depressive symptoms and PIMs (Table 4), polypharmacy, and hyperpolypharmacy (Table 5). In Table 4, the OR were calculated from logistic regression models, whereas the OR in Table 5 were calculated from ordinal logistic regression models (as a result of more than two outcome levels of polypharmacy and hyperpolypharmacy, which also followed a natural order). Sex and country were adjusted for in the OR of Tables 4 and 5, whereas age and sex were adjusted for in the OR that compared Australia with the United States in Tables 2 and 3.
Table 1.
Medication Prevalence by Total Number of Medications, Age, and Sex
| All Total | Men | Women | Unadjusted Odds Ratio (95% CI), Men (Reference) vs Women | |
|---|---|---|---|---|
| N | 19,114 | 8332 (44) | 10,782 (56) | – |
| Age | 74 (71.62–77.68) | 73.82 (71.55–77.31) | 74.12 (71.68–77.95) | – |
| Hypertensiona | 14,195 (74) | 6274 (75) | 7921 (74) | – |
| Diabetes mellitusb | 2045 (11) | 1046 (13) | 999 (9) | – |
| Dyslipidemiac | 12,467 (65) | 4591 (55) | 7876 (73) | – |
| Chronic kidney diseased | 4740 (25) | 2010 (24) | 2730 (25) | – |
| Osteoarthritise | 4633 (54) | 1531 (43) | 3102 (62) | – |
| 1–2 morbidities | 12,070 (63) | 5495 (66) | 6575 (61) | – |
| 3 or more morbidities | 5781 (30) | 2122 (25) | 3659 (34) | – |
| Number of medications | ||||
| 0 | 2475 (13) | 1411 (17) | 1064 (10) | Reference |
| 1–2 | 6003 (31) | 2932 (35) | 3071 (28) | 1.39 (1.26–1.53) |
| 3–4 | 5548 (29) | 2292 (28) | 3256 (30) | 1.88 (1.71–2.07) |
| 5–6 | 3109 (16) | 1086 (13) | 2023 (19) | 2.47 (2.22–2.75) |
| 7 or more | 1979 (10) | 611 (7) | 1368 (13) | 2.97 (2.62–3.36) |
| Number of medications by age group | ||||
| 65–69 | 3 (1–5) | 3 (1–5) | 3 (1–5) | 1.07 (1.01–1.13) |
| 70–74 | 3 (1–4) | 2 (1–4) | 3 (1–5) | 1.13 (1.11–1.15) |
| 75–79 | 3 (1–5) | 2 (1–4) | 3 (2–5) | 1.15 (1.12–1.17) |
| 80–84 | 3 (2–5) | 3 (1–4) | 4 (2–5) | 1.18 (1.14–1.23) |
| 85 or older | 3 (2–5) | 3 (1–4) | 3 (2–5) | 1.18 (1.11–1.26) |
CI = confidence interval.
Data are presented as n (% of column total) or median (interquartile range).
Hypertension = systolic blood pressure greater than or equal to 140 mm Hg or diastolic blood pressure greater than or equal to 90 mm Hg or on treatment for high blood pressure.
Diabetes mellitus was defined as self-report of diabetes mellitus or fasting blood glucose of greater than or equal to 126 mg/dl or on pharmaceutical treatment for diabetes mellitus.
Dyslipidemia includes those taking cholesterol-lowering medications or serum cholesterol greater than or equal to 212 mg/dl (Australia) and greater than or equal to 240 mg/dl (United States).
Chronic kidney disease was defined as an estimated glomerular filtration rate of less than 60 ml/minute/1.73 m2 or a urine albumin creatinine ratio of three or more.
Self-reported osteoarthritis. A specific question about osteoarthritis was asked after June 2013, hence the percentage is of the total asked about osteoarthritis (n=5677).
Table 3.
Potentially Inappropriate Medications, Polypharmacy, and Hyperpolypharmacy by Total Cohort, Sex, and Country
| All Total | Men | Women | Unadjusted Odds Ratioa (95% CI) | Australia | United States | Adjusted Odds Ratiob (95% CI) | |
|---|---|---|---|---|---|---|---|
| Potentially inappropriate medications | 39% 7396 |
33% 2738 |
43% 4658 |
1.55 (1.46–1.65) | 40% 6664 |
30% 732 |
0.63 (0.57–0.69) |
| Polypharmacy | 27% 5088 |
20% 1697 |
31% 3391 |
1.79 (1.68–1.92) | 26% 4351 |
31% 737 |
1.23 (1.12–1.35) |
| Hyperpolypharmacy | 2% 423 |
1% 119 |
3% 304 |
2 (1.62–2.49) | 2% 336 |
4% 87 |
1.72 (1.34–2.19) |
Men (reference) versus women.
Adjusted for age and sex, Australia (reference) versus United States.
Table 4.
Presence of PIMs by Baseline Characteristics of the Aspirin in Reducing Events in the Elderly Study Cohort at Enrollment
| No PIMs n=11,718 | PIMs n=7396 | Adjusted Odds Ratio (95% CI)a | |
|---|---|---|---|
| Age | 73.8 (71.6–77.5) | 74.2 (71.7–78.0) | 1.01 (1.00–1.02) |
| 65–74 | 59 (6969) | 57 (4195) | Reference |
| 75–84 | 37 (4302) | 39 (2916) | 1.10 (1.04–1.17) |
| 85 or older | 4 (447) | 4 (285) | 1.04 (0.89–1.21) |
| Race | |||
| White | 90 (10,583) | 93 (6867) | Reference |
| Non-White | 10 (1135) | 7 (529) | 0.99 (0.86–1.13) |
| Years of education | |||
| ≥ 12 years | 57 (6717) | 51 (3760) | Reference |
| < 12 years | 43 (5000) | 49 (3636) | 1.2 (1.13–1.28) |
| GI complaintb | 7 (807) | 42 (3114) | 10.23 (9.24–11.34) |
| Hypertension | 72 (8478) | 77 (5717) | 1.3 (1.22–1.39) |
| Diabetes mellitus | 10 (1121) | 12 (924) | 1.47 (1.34–1.62) |
| CKD | 24 (2772) | 27 (1968) | 1.18 (1.1–1.26) |
| Not frail | 61 (7196) | 55 (4050) | Reference |
| Prefrail | 37 (4329) | 42 (3118) | 1.33 (1.26–1.42) |
| Frail | 2 (193) | 3 (228) | 2.25 (1.84–2.74) |
| MCSc | 56.01± 6.74 | 55.15 ± 7.67 | 1.02 (1.01–1.02) |
| PCSc | 49.53 ± 8.13 | 46.45 ± 9.38 | 1.04 (1.04–1.04) |
| 3MSc | 93.5 ± 4.68 | 93.3 ± 4.53 | 1.01 (1.01–1.02) |
| Grip strengthc | 26.3 (20.3–35.3) | 24.0 (18.7–31.3) | 1.02 (1.01–1.02) |
| Gait speed | 2.94 (2.58–3.4) | 3.03 (2.64–3.53) | 1.18 (1.14–1.22) |
| CES-D-10 < 8 | 92 (10,725) | 88 (6506) | Reference |
| CES-D-10 8 or more | 8 (991) | 12 (888) | 1.43 (1.3–1.58) |
3MS = Modified Mini-Mental Examination score; CES-D-10 = Center of Epidemiology Studies Short Depression Scale score; CI = confidence interval; CKD = chronic kidney disease; GI = gastrointestinal; MCS = Mental Component Score from the 12-Item Short Form Survey; PCS = Physical Component Score from the 12-Item Short Form Survey; PIMs = potentially inappropriate medications.
Data are presented as percentage of column (n), median (interquartile range), or mean ± SD unless otherwise indicated. CKD is defined as defined as an estimated glomerular filtration rate of less than 60 ml/minute/1.73 m2 or a urine albumin creatinine ratio of three or more. Grip strength is for dominant hand.
No PIMs (reference) versus PIMs, adjusted for country and sex.
Data collection about GI complaints was introduced part way through recruitment. Percentage is of the total number of participants who were asked about GI complaints, which included self-report of gastro-esophageal reflux disease, gastritis, or dyspepsia.
Odds ratios for MCS, PCS, grip strength, and 3MS is per one-unit decrease.
Table 5.
Polypharmacy and Hyperpolypharmacy by Baseline Characteristics of the Aspirin in Reducing Events in the Elderly Study Cohort at Enrollment
| 0–4 Medications, n=14,026 | 5–9 Medications, n=4665 | 10 or More Medications, n=423 | Adjusted Odds Ratioa(95% CI) | |
|---|---|---|---|---|
| Age | 73.8 (71.6–77.4) | 74.6 (71.8–78.5) | 74.5 (71.8–78.2) | 1.03 (1.02–1.03) |
| 65–74 | 60 (8442) | 53 (2488) | 55% (234) | Reference |
| 75–84 | 36 (5072) | 42 (1970) | 42% (176) | 1.29 (1.21–1.38) |
| 85 or older | 4 (512) | 4 (207) | 3% (13) | 1.29 (1.09–1.52) |
| Race | ||||
| White | 92 (12,891) | 90 (4192) | 87 (367) | Reference |
| Non-White | 8 (1135) | 10 (473) | 13 (56) | 1.24 (1.08–1.43) |
| Years of education | ||||
| ≥ 12 years | 57 (7953) | 49 (2298) | 53 (226) | Reference |
| < 12 years | 43 (6072) | 51 (2367) | 47 (197) | 1.39 (1.3–1.49) |
| GI complaintb | 16 (2213) | 32 (1516) | 45 (192) | 2.63 (2.4–2.9) |
| Hypertension | 69 (9662) | 89 (4130) | 95 (403) | 3.88 (3.53–4.27) |
| Diabetes mellitus | 7 (980) | 20 (938) | 30 (127) | 3.84 (3.49–4.21) |
| CKD | 22 (3086) | 32 (1483) | 40 (171) | 1.73 (1.61–1.86) |
| Not frail | 63 (8854) | 48 (2255) | 32 (137) | Reference |
| Prefrail | 36 (4983) | 47 (2213) | 59 (251) | 1.83 (1.72–1.96) |
| Frail | 1 (189) | 4 (197) | 8 (35) | 4.54 (3.74–5.52) |
| MCSc | 55.9 ± 6.74 | 55.2 ± 7.96 | 54.2 ± 9.2 | 1.01 (1.01–1.02) |
| PCSc | 49.9 ± 7.73 | 44.4 ± 9.72 | 38.9 ± 10.27 | 1.08 (1.07–1.08) |
| 3MSc | 93.6 ± 4.61 | 93.0 ± 4.66 | 93.1 ± 4.48 | 1.03 (1.03–1.04) |
| Grip strengthc | 26.3 (20.3–35.0) | 22.7 (18.0–30.0) | 21.3 (16.7–26.8) | 1.03 (1.03–1.04) |
| Gait speed | 2.91 (2.55–3.34) | 3.17 (2.75–3.74) | 3.44 (2.96–4.24) | 1.51 (1.46–1.56) |
| CES-D-10 < 8 | 92 (12,852) | 87 (4050) | 78 (329) | Reference |
| CES-D-10 8 or more | 8 (1171) | 13 (614) | 22 (94) | 1.72 (1.55–1.9) |
3MS = Modified Mini-Mental Examination score; CES-D-10 = Center of Epidemiology Studies Short Depression Scale score; CI = confidence interval; CKD = chronic kidney disease; GI = gastrointestinal; MCS = Mental Component Score from the 12-Item Short Form Survey; PCS = Physical Component Score from the 12-Item Short Form Survey; PIMs = potentially inappropriate medications.
Data are presented as percentage of column (n), median (interquartile range), or mean ± SD unless otherwise indicated. CKD is defined as defined as an estimated glomerular filtration rate of less than 60 ml/minute/1.73 m2 or a urine albumin creatinine ratio of three or more. Grip strength is for dominant hand.
Ordinal log, 0–4 medications (reference) adjusted for country and sex.
Data collection about GI complaints was introduced part way through recruitment. Percentage is of the total number of participants who were asked about GI complaints, which included self-report of gastro-esophageal reflux disease, gastritis, or dyspepsia.
Odds ratios for MCS, PCS, grip strength, and 3MS is per one-unit decrease.
Results
At study entry, the median age of ASPREE participants was 74 years, 56% of participants were women, 91% were White, and 28% lived alone. Overall, 74% had hypertension, and 11% had diabetes mellitus.21 As expected based on the eligibility criteria, these results demonstrate that ASPREE participants were relatively healthier than the general population of a similar age in both countries.21 Overall, 13% of participants reported not taking any prescription medication (n=2475), 31% reported taking one or two prescribed medications (n=6003), 29% took three or four medications (n=5548), 16% took five or six medications (n=3106), and the remaining 10% reported taking seven or more prescription medications (n=1979; Table 1). The median number of prescription medications per participant was three, regardless of age. Women were more likely to be on a higher number of medications than were men, with 90% of women taking at least one medication compared with 83% of men.
Prevalence of medications by class, sex, and country within ASPREE is shown in Table 2. Overall, cardiovascular drugs were the most prevalent class in the cohort (64%; see Table S2). Sex differences were observed across all medication classes, with women being more likely to report being on each medication class, except dermatologicals and antiinfective and sensory medications, in which there were no differences by sex (Table S2). The prevalence of oral glucose-lowering drugs was higher in the United States compared with Australia (adjusted OR 2.58, 95% confidence interval [CI] 2.22–2.98) as was the prevalence of diuretics (United States; adjusted OR 1.68, 95% CI 1.52–1.86), and β-blockers (adjusted OR 2.27, 95% CI 1.99–2.57; Table 2). The prevalence of PPIs (adjusted OR 0.38, 95% CI 0.34–0.43), drugs acting on renin-angiotensin-aldosterone pathway (adjusted OR 0.70, 95% CI 0.64–0.76), benzodiazepines (adjusted OR 0.41, 95% CI 0.32–0.52), and antidepressants (adjusted OR 0.71, 95% CI 0.61–0.82) was lower in the United States. Prevalence of other medication classes was broadly similar between the two countries.
The proportion of participants reporting use of medication that may be a PIM, polypharmacy, and hyperpolypharmacy is shown in Table 3. Overall, 39% of the cohort reported taking at least one PIM. Polypharmacy was present in 27% of the cohort and hyperpolypharmacy in 2%. Women were more likely to report a PIM (OR 1.55, 95% CI 1.46–1.65), have polypharmacy (OR 1.79, 95% CI 1.68–1.92), or have hyperpolypharmacy (OR 2.00, 95% CI 1.62–2.49). Australians were more likely to report a PIM (adjusted OR 0.63, 95% CI 0.57–0.69), but less likely to have polypharmacy (adjusted OR 1.23, 95% CI 1.12–1.35) or hyperpolypharmacy (adjusted OR 1.72, 95% CI 1.34–2.19). Of the participants reporting a PIM, 54.8% reported a PPI without concurrent use of an NSAID (21.2% of total cohort), 17.3% reported an NSAID without concurrent use of a PPI (6.7% of total cohort), 15.8% reported benzodiazepines (6.1% of total), 11.8% reported taking androgens or estrogens (4.6% of total), and 11.6% reported taking medications with anticholinergic properties (4.5% of total; see Figure 1 and Table S1). Of those taking a PPI, when specifically asked, 89% reported a history of a gastrointestinal complaint (i.e., self-report of gastro-esophageal reflux disease, gastritis, or dyspepsia).
Figure 1.
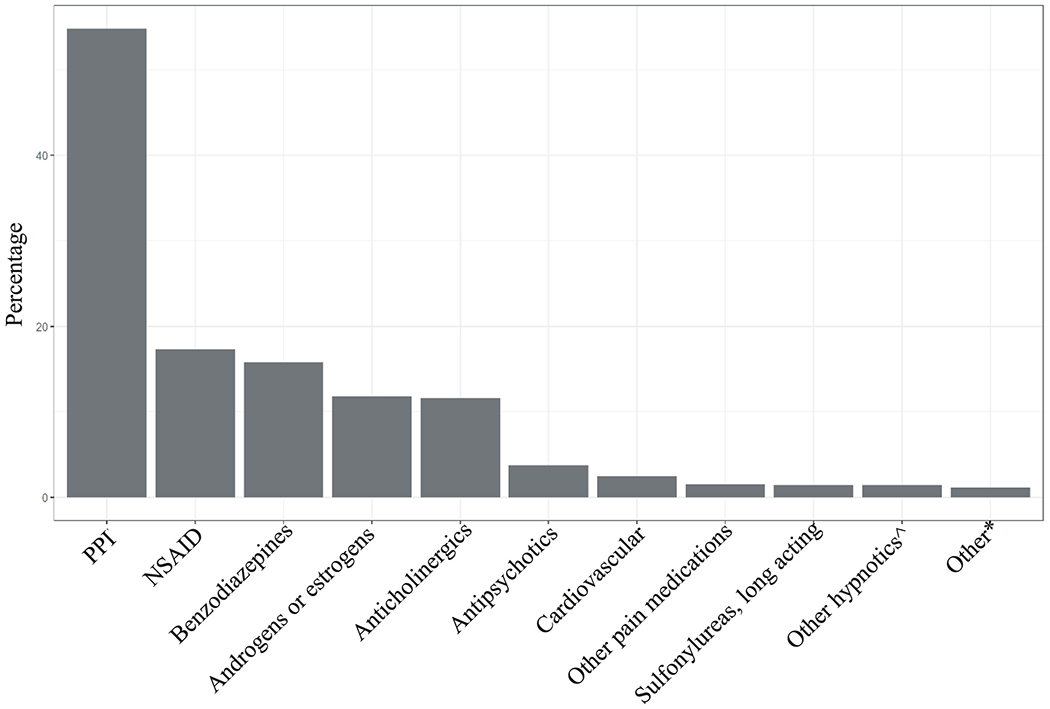
Common potentially inappropriate medications by medication class. PPI indicates a proton pump inhibitor without coprescription of a nonsteroidal antiinflammatory drug; NSAID indicates a nonsteroidal antiinflammatory drug without coprescription of a proton pump inhibitor. ^Includes nonbenzodiazepine and benzodiazepine receptor agonist hypnotics. *“Other” includes the following: gastrointestinal medications, 0.5%; skeletal muscle relaxants, 0.5%; barbiturates, 0.1%; genitourinary, 0.05%; other endocrine such as thyroid, growth hormone, and megestrol, 0.04%; and meprobamate, 0.01%.
Baseline characteristics of participants grouped by the presence of PIMs are shown in Table 4. Participants were more likely to report taking a PIM if aged 75–84 years compared with participants aged 65–74 years (adjusted OR 1.10, 95% CI 1.04–1.17); they received less than 12 years of education (adjusted OR 1.20, 95% CI 1.13–1.28); they reported a gastrointestinal complaint (adjusted OR 10.23, 95% CI 9.24–11.34), hypertension (adjusted OR 1.30, 95% CI 1.22–1.39), diabetes mellitus (adjusted OR 1.47, 95% CI 1.34–1.62), or chronic kidney disease (adjusted OR 1.18, 95% CI 1.10–1.26); or had depressive symptoms as evidenced by a score of eight or more on the Center for Epidemiologic Studies Short Depression Scale questionnaire (adjusted OR 1.43, 95% CI 1.30–1.58). Participants in the categories of prefrail and frail were more likely to be on PIMs (prefrail adjusted OR 1.33, 95% CI 1.26–1.42; frail adjusted OR 2.25, 95% CI 1.84–2.74), as were participants with a poorer Physical Component Score for related quality of life (adjusted OR 1.04, 95% CI 1.04–1.04); however, no clinically relevant difference in grip strength was observed based on prevalence of PIMs. Participants reporting a PIM were more likely to have a slightly slower gait speed test (2.94 seconds vs 3.03 seconds; adjusted OR 1.18, 95% CI 1.14–1.22). Although a statistically significant effect was identified, no clinically relevant difference was observed in baseline Modified Mini-Mental Examination scores or Mental Component Scores of the SF-12 quality-of-life questionnaire based on the presence of a PIM.
Baseline characteristics of ASPREE participants grouped by the presence of polypharmacy and hyperpolypharmacy are shown in Table 5. Participants aged 75 years or older were more likely to have polypharmacy and hyperpolypharmacy compared with those younger than the age of 74 (75–84 adjusted OR 1.29, 95% CI 1.21–1.38; 85 or older adjusted OR 1.29, 95% CI 1.09–1.52). So too were minorities (adjusted OR 1.24, 95% CI 1.08–1.43), those with a gastrointestinal complaint (adjusted OR 2.63, 95% CI 2.40–2.90), hypertension (adjusted OR 3.88, 95% CI 3.53–4.27), diabetes mellitus (adjusted OR 3.84, 95% CI 3.49–4.21), chronic kidney disease (adjusted OR 1.73, 95% CI 1.61–1.86), less than 12 years of education (adjusted OR 1.39, 95% CI 1.30–1.49), depressive symptoms (adjusted OR 1.72, 95% CI 1.55–1.90), or were in the prefrail and frail categories (prefrail adjusted OR 1.83, 95% CI 1.72–1.96; frail adjusted OR 4.54, 95% CI 3.74–5.52). Polypharmacy and hyperpolypharmacy were also associated with poorer results for the Physical Component Score (adjusted OR 1.08, 95% CI 1.07–1.08). Overall, no clinically relevant difference in Mental Component Score of the SF-12 questionnaire, grip strength, or Modified Mini-Mental Examination score was observed for polypharmacy or hyperpolypharmacy.
Discussion
This study found that community-dwelling older people free of cardiovascular disease, major physical disability, and cognitive impairment take fewer prescription medications compared with the general older U.S. population. The median number of medications (three per participant) was lower than recent reports from a large population-based study in a slightly younger cohort (60 years or older) that reported a median of five medications per person.29 Although cardiovascular medications were the most commonly reported group, the highest frequency medication classes were for primary prevention (i.e., antihypertensives and statins), which is consistent with a community-dwelling population with low comorbidity burden.
When compared with the U.S. health 2017 report of prescription medication use in people aged 65 years or older, the overall ASPREE cohort had a lower prevalence of oral glucose-lowering drugs, β-blockers, statins, and antidepressants and a similar prevalence of diuretics, calcium channel antagonists, and drugs acting on the renin-angiotensin-aldosterone pathway.30 Australian participants had a lower prevalence of statins and NSAIDs compared with survey data of prescription and over-the-counter medication use from a random sample of Australians aged 50 years or older.31 Similarly, the prevalence of NSAID use in this study cohort (15% of total) was lower than the 21% reported by another study32 based on an analysis of National Health and Nutrition Examination Survey data, and the prevalence of opioids was equivalent to published results of a national U.S. survey (3.7% prevalence for people aged older than 70 years).33 Although country differences were observed with regard to the prevalence of PPIs, the overall prevalence was similar to that of the general U.S. population aged 65 years or older (i.e., 20% for men and 26% for women).30
Despite being a relatively healthy older cohort, PIMs were still notably prevalent (39%), primarily driven by use of PPIs, NSAIDs, and benzodiazepines. Previous reports of the prevalence of PIMs vary based on the study setting, most often in adults who were more sick. For example, from hospital-based studies (including inpatient and outpatient studies) with a sample size of at least 1000 participants, the reported prevalence of PIMs was between 48.5% and 57% using the AGS Beers Criteria.34, 35 In the general older U.S. population, a national study using Medicare Part D administrative data and the 2003 AGS Beers Criteria reported the prevalence of PIMs to be 32%.36 Recently, a community-based study of older drivers reported at least one PIM in only 18.5% of the cohort.37 Although the current findings show a higher prevalence, the 2012 AGS Beers Criteria did not include PPIs, and they were excluded from the second study for other reasons. Given that PPIs were the most commonly reported PIM in this cohort (21% of total), it is not surprising that the overall prevalence of PIMs was higher than these previous reports in community-based people. Likewise, the higher prevalence of PIMs in Australians is likely explained by the higher prevalence of PPIs. At a population level, the prevalence of PPIs has increased dramatically in the past 20 years (from 7% in 1994 to 20.7% in 2014) and, as stated previously, the prevalence in this cohort is similar to the contemporary general older population.30 With regard to other common PIMs, the prevalence of benzodiazepines (4% for men and 8% for women) was slightly lower than in the general older U.S. (6% and 11%, respectively)38 and Australian populations (16% prevalence in those aged 65 years or older).39
Although there is a recommendation to avoid PIMs in older individuals because the risks may outweigh the benefits, it is important to note that this is a general recommendation, and in practice the details of the clinical situation determine whether the medication is potentially inappropriate. Others have demonstrated an association between the presence of comorbidity and the prevalence of PIMs,35 and clinical factors not available to ASPREE may mean that certain medications were indicated as a result of the failure of preferred first-line options or lack of viable alternatives. For example, according to the AGS Beers Criteria, PPIs should be avoided because of the risk of Clostridium difficile infection, bone loss, and fractures. However, PPIs are not considered PIMs in the presence of high-risk conditions such as chronic NSAID exposure, erosive esophagitis, Barret’s esophagitis, a pathological hypersecretory condition, or if they are prescribed because of a failure of a drug discontinuation trial or treatment with an H2 antagonist.23 ASPREE did not collect data on previous treatment failures, and given that 89% of participants on PPIs also reported a gastrointestinal complaint, it is likely that the overall prevalence of PPIs considered to be PIMs is overestimated in this cohort. In Australia, PPIs are only available by prescription, whereas they can be accessed over the counter in the United States. This means that use of PPIs may be underestimated for U.S. participants who make up 12.6% of the cohort. The AGS Beers Criteria has been criticized for not assessing clinical outcomes and thus not establishing an association between prescription of the medications on the list and adverse events.40 However, a recent meta-analysis that used both the AGS Beers Criteria and the Screening Tool of Older Person’s Prescriptions showed an association between PIMs and adverse drug reactions and hospitalizations.41 Still, the long-term impact of PIMs on clinical outcomes (e.g., bleeding, cognitive decline) in adults who have reached older ages free of significant life-limiting illness or disability requires further study, as these individuals may be resilient to the potential adverse effects.
Regarding correlates of PIMs use, those considered to be prefrail or frail were more likely to be prescribed a PIM, which is consistent with previous studies.42 No difference was observed for the Modified Mini-Mental Examination or Mental Component Score of the SF-12 quality-of-life questionnaire, which is not surprising given the eligibility criteria requiring participants to be free from cognitive impairment and the types of PIMs reported. However, specific examination of medications known to impact cognitive function in the general older population (e.g., drugs with anticholinergic properties) and longitudinal follow-up of cognitive outcomes will provide more meaningful insight.
As expected, this study observed a low prevalence of polypharmacy (27%) compared with other studies, reflecting a relatively healthy volunteer cohort. A study43 reported 67.4% prevalence of polypharmacy in a cross-sectional study of people aged 70 years or older. In another population study,44 it was found that 45% of older people reported polypharmacy. Given the prevalence of conditions in this age group that require pharmaceutical management, often with multiple medications, such as hypertension (71%), dyslipidemia (57%), osteoarthritis (50%), diabetes mellitus (26%), and chronic kidney disease (23%),45–47 it is not surprising that one in four ASPREE participants reported taking five or more medications. Regardless, the prevalence of polypharmacy was low compared with the general older population, where the estimated prevalence of polypharmacy is 42.6% in the United States30 and 36.1% in Australia.48
Limitations
A key limitation of this analysis is the lack of information regarding the clinical indication surrounding the PIMs, which limits the detail to which the AGS Beers Criteria could be definitively applied. Previous research has shown that use of explicit criteria to evaluate prescribing may account for only a small portion of drugs deemed inappropriate by implicit review.49 This study also did not collect information on who initially prescribed the medication and hence cannot trace whether medications were initially prescribed during an acute hospital admission, by a private medical specialist, or in primary care. A previous study of community-dwelling older people showed that hospital admission is independently associated with increased use of PIMs.50 Some medications with anticholinergic properties, such as diphenhydramine and PPIs in the United States, are available over the counter, and their use may be underestimated in this cohort. Assessment of the associations of PIMs and polypharmacy on cognition was limited by the eligibility criteria, which resulted in the exclusion of older people with cognitive impairments or histories of dementia.
Despite these limitations, this study found that even healthy older adults with relatively low overall medication burden still use PIMs. Because it is not known whether community-dwelling older people experience the same adverse effects from PIMs and polypharmacy as the general older population, forthcoming analysis of longitudinal data will help ascertain whether these adults suffer adverse consequences of PIMs at similar rates to those less healthy, vulnerable older adults.
Conclusion
Overall medication exposure is relatively low in community-dwelling older adults free of cardiovascular disease, physical disability, and cognitive impairment. However, the prevalence of medications where the risks may potentially outweigh the benefits was consistent with previous studies of more vulnerable populations with higher medication burden. Longitudinal follow-up is required to further understand the risk profile of PIMs and polypharmacy in community-dwelling older people.
Supplementary Material
Acknowledgments
A. G. Bayer provided aspirin and matching placebo. The authors acknowledge the dedicated and skilled staff in Australia and the United States for the conduct of the trial. The authors also are most grateful to the Aspirin in Reducing Events in the Elderly Study participants, who so willingly volunteered for this study, and the general practitioners and medical clinics who support the participants in the Aspirin in Reducing Events in the Elderly Study. Trial registration: International Standard Randomized Controlled Trial Number Register (ISRCTN83772183) and clinicaltrials.gov (NCT01038583). The funding sources were not involved in the design or conduct of the study; collection, management, or analysis of the data; interpretation of the results; preparation, review, or approval of the manuscript; or decision to submit for publication.
Footnotes
ASPREE Investigator Group listed on www.aspree.org.
Conflict of interest: The authors have declared no conflicts of interest for this article.
Supporting Information
The following supporting information is available in the online version of this paper:
Table S1. List of Potentially Inappropriate Medications (PIM).
Table S2. Medication Prevalence by Anatomical and Therapeutic Chemical Medication Class.
References
- 1.Table 94. National health expenditures, average annual percent change, and percent distribution, by type of expenditure: United States, selected years 1960–2016. 1970;2. [Google Scholar]
- 2.Australia’s Health 2014, Table of contents [Internet]. Australian Institute of Health and Welfare. Available from https://www.aihw.gov.au/reports/australias-health/australias-health-2014/contents/table-of-contents. Accessed July 1, 2019. [Google Scholar]
- 3.Gerberding JL, Barry PP, Wetle TF. The state of aging and health in America 2004. 48. [Google Scholar]
- 4.Commission corporateName:Productivity. Economic Implications of an Ageing Australia – Productivity Commission Research Report [Internet], 2005. Available from https://www.pc.gov.au/inquiries/completed/ageing/report. Accessed August 11, 2019.
- 5.Fact Sheet: Aging in the United States – Population Reference Bureau [Internet]. Available from https://www.prb.org/aging-unitedstates-fact-sheet/. Accessed August 3, 2019.
- 6.Older Australia at a glance, Australia’s changing age & gender profile [Internet]. Australian Institute of Health and Welfare. Available from https://www.aihw.gov.au/reports/older-people/older-australia-at-a-glance/contents/demographics-of-older-australians/australia-s-changing-age-and-gender-profile. Accessed August 3, 2019. [Google Scholar]
- 7.Statistics (US) NC for H. Table 14, Life expectancy at birth and at age 65, by sex: Organisation for Economic Co-operation and Development (OECD) countries, selected years 1980–2015 [Internet], 2018. Available from https://www.ncbi.nlm.nih.gov/books/NBK532684/table/ch4.tab14/. Accessed December 22, 2019.
- 8.Statistics (US) NC for H. Trend Tables [Internet], National Center for Health Statistics (US), 2018. Available from http://www.ncbi.nlm.nih.gov/books/NBK532684/. Accessed December 22, 2019. [Google Scholar]
- 9.Table 54. Hypertension among adults aged 20 and over, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. 2017;2. [Google Scholar]
- 10.Statistics (US) NC for H. Figure 11, Functional limitation among adults aged 18 and over, by age and level of difficulty: United States, 2010–2016 [Internet], 2018. Available from https://www.ncbi.nlm.nih.gov/books/NBK532688/figure/ch3.fig11/. Accessed December 22, 2019.
- 11.Statistics (US) NC for H. Table 65, Health care visits to doctor offices, emergency departments, and home visits within the past 12 months, by selected characteristics: United States, selected years 1997–2016 [Internet], 2018. Available from https://www.ncbi.nlm.nih.gov/books/NBK532684/table/ch4.tab65/. Accessed December 22, 2019.
- 12.Statistics (US) NC for H. Table 79, Prescription drug use in the past 30 days, by sex, race and Hispanic origin, and age: United States, selected years 1988–1994 through 2011–2014 [Internet], 2018. Available from https://www.ncbi.nlm.nih.gov/books/NBK532684/table/ch4.tab79/. Accessed December 22, 2019.
- 13.Turnheim K When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol 2003;38 (8):843–53. [DOI] [PubMed] [Google Scholar]
- 14.Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol 2017;73(6):759–70. [DOI] [PubMed] [Google Scholar]
- 15.Sultana J, Cutroneo P, Trifirò G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother 2013;4(Suppl1):S73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batel Marques F, Penedones A, Mendes D, Alves C. A systematic review of observational studies evaluating costs of adverse drug reactions. Clin Outcomes Res CEOR 2016;8:413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed B, Nanji K, Mujeeb R, Patel MJ. Effects of polypharmacy on adverse drug reactions among geriatric outpatients at a tertiary care hospital in Karachi: a prospective cohort study. PLoS One 2014;9(11):e112133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redston MR, Hilmer SN, McLachlan AJ, Clough AJ, Gnjidic D. Prevalence of potentially inappropriate medication use in older inpatients with and without cognitive impairment: a systematic review. J Alzheimers Dis JAD 2018;61(4):1639–52. [DOI] [PubMed] [Google Scholar]
- 19.Jirón M, Pate V, Hanson LC, Lund JL, Jonsson Funk M, Sturmer T. Trends in prevalence and determinants of potentially inappropriate prescribing in the United States: 2007 to 2012. J Am Geriatr Soc 2016;64(4):788–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ASPREE Investigator Group. Study design of ASPirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials 2013;36(2):555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil JJ, Woods RL, Nelson MR, et al. Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) study. J Gerontol A Biol Sci Med Sci 2017;72 (11):1586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med 2018;379:1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2019 American Geriatrics Society Beers Criteria® Update Expert Panel, Fick DM, Semla TP, et al. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019;67(4):674–94. [DOI] [PubMed] [Google Scholar]
- 24.Morin L, Johnell K, Laroche M-L, Fastbom J, Wastesson JW. The epidemiology of polypharmacy in older adults: register-based prospective cohort study. Clin Epidemiol 2018;12 (10):289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe R, Murray AM, Woods RL, et al. The aspirin in reducing events in the elderly trial: Statistical analysis plan. Int J Stroke Off J Int Stroke Soc 2018;13(3):335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1(3):385–401. [Google Scholar]
- 28.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48(8):314–8. [PubMed] [Google Scholar]
- 29.Christensen LD, Reilev M, Juul-Larsen HG, et al. Use of prescription drugs in the older adult population-a nationwide pharmacoepidemiological study. Eur J Clin Pharmacol 2019;75(8):1125–33. [DOI] [PubMed] [Google Scholar]
- 30.Health, United States, 2017 [Internet], 2019. Available from /nchs/hus.htm. Accessed August 11, 2019. [Google Scholar]
- 31.Morgan TK, Williamson M, Pirotta M, Stewart K, Myers SP, Barnes J. A national census of medicines use: a 24-hour snap-shot of Australians aged 50 years and older. Med J Aust 2012;196(1):50–3. [DOI] [PubMed] [Google Scholar]
- 32.Davis JS, Lee HY, Kim J, et al. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart 2017;4(1):e000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain 2008;138(3):507–13. [DOI] [PubMed] [Google Scholar]
- 34.Kondo N, Nakamura F, Yamazaki S, et al. Prescription of potentially inappropriate medications to elderly hemodialysis patients: prevalence and predictors. Nephrol Dial Transplant 2015;30(3):498–505. [DOI] [PubMed] [Google Scholar]
- 35.Di Giorgio C, Provenzani A, Polidori P. Potentially inappropriate drug prescribing in elderly hospitalized patients: an analysis and comparison of explicit criteria. Int J Clin Pharm 2016;38(2):462–8. [DOI] [PubMed] [Google Scholar]
- 36.Holmes HM, Luo R, Kuo Y-F, Baillargeon J, Goodwin JS. Association of potentially inappropriate medication use with patient and prescriber characteristics in Medicare Part D. Pharmacoepidemiol Drug Saf 2013;22(7):728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li G, Andrews HF, Chihuri S, et al. Prevalence of potentially inappropriate medication use in older drivers. BMC Geriatr 2019;19(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry 2015;72(2):136–42. [DOI] [PubMed] [Google Scholar]
- 39.Windle A, Elliot E, Duszynski K, Moore V. Benzodiazepine prescribing in elderly Australian general practice patients. Aust N Z J Public Health 2007;31(4):379–81. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton H, Gallagher P, Ryan C, Byrne S, O’Mahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med 2011;171(11):1013–9. [DOI] [PubMed] [Google Scholar]
- 41.Xing XX, Zhu C, Liang HY, et al. Associations between potentially inappropriate medications and adverse health outcomes in the elderly: a systematic review and meta-analysis. Ann Pharmacother 2019;53(10):1005–19. [DOI] [PubMed] [Google Scholar]
- 42.Maclagan LC, Maxwell CJ, Gandhi S, et al. Frailty and potentially inappropriate medication use at nursing home transition. J Am Geriatr Soc 2017;65(10):2205–12. [DOI] [PubMed] [Google Scholar]
- 43.Herr M, Robine J-M, Pinot J, Arvieu J-J, Ankri J. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf 2015;24(6):637–46. [DOI] [PubMed] [Google Scholar]
- 44.Lechevallier-Michel N, Gautier-Bertrand M, Alpérovitch A, et al. Frequency and risk factors of potentially inappropriate medication use in a community-dwelling elderly population: results from the 3C Study. Eur J Clin Pharmacol 2005;60(11):813–9. [DOI] [PubMed] [Google Scholar]
- 45.National Center for Health Statistics (US). Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities [Internet]. Hyattsville, MD: National Center for Health Statistics; 2016. Available from http://www.ncbi.nlm.nih.gov/books/NBK367640/. Accessed August 31, 2019. [PubMed] [Google Scholar]
- 46.Barbour KE, Helmick CG, Theis KA, et al. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation – United States, 2010–2012. MMWR Morb Mortal Wkly Rep 2013;62(44):869–73. [PMC free article] [PubMed] [Google Scholar]
- 47.USRDS [Internet]. Available from https://www.usrds.org/2015/view/. Accessed August 31, 2019.
- 48.Page AT, Falster MO, Litchfield M, Pearson S-A, Etherton-Beer C. Polypharmacy among older Australians, 2006–2017: a population-based study. Med J Aust 2019;211(2):71–5. [DOI] [PubMed] [Google Scholar]
- 49.Steinman MA, Rosenthal GE, Landefeld CS, Bertenthal D, Kaboli PJ. Agreement between drugs-to-avoid criteria and expert assessments of problematic prescribing. Arch Intern Med 2009;169(14):1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez T, Moriarty F, Wallace E, McDowell R, Redmond P, Fahey T. Prevalence of potentially inappropriate prescribeing in older people in primary care and its association with hospital admission: longitudinal study. BMJ 2018;363:k4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


