Abstract
Objective
There is a lack of robust data on significant gastrointestinal bleeding in older people using aspirin. We calculated the incidence, risk factors and absolute risk using data from a large randomised, controlled trial.
Design
Data were extracted from an aspirin versus placebo primary prevention trial conducted throughout 2010–2017 (‘ASPirin in Reducing Events in the Elderly (ASPREE)’, n=19 114) in community-dwelling persons aged ≥70 years. Clinical characteristics were collected at baseline and annually. The endpoint was major GI bleeding that resulted in transfusion, hospitalisation, surgery or death, adjudicated independently by two physicians blinded to trial arm.
Results
Over a median follow-up of 4.7 years (88 389 person years), there were 137 upper GI bleeds (89 in aspirin arm and 48 in placebo arm, HR 1.87, 95% CI 1.32 to 2.66, p<0.01) and 127 lower GI bleeds (73 in aspirin and 54 in placebo arm, HR 1.36, 95% CI 0.96 to 1.94, p=0.08) reflecting a 60% increase in bleeding overall. There were two fatal bleeds in the placebo arm. Multivariable analyses indicated age, smoking, hypertension, chronic kidney disease and obesity increased bleeding risk. The absolute 5-year risk of bleeding was 0.25% (95% CI 0.16% to 0.37%) for a 70 year old not on aspirin and up to 5.03% (2.56% to 8.73%) for an 80 year old taking aspirin with additional risk factors.
Conclusion
Aspirin increases overall GI bleeding risk by 60%; however, the 5-year absolute risk of serious bleeding is modest in younger, well individuals. These data may assist patients and their clinicians to make informed decisions about prophylactic use of aspirin.
Trial registration number
ASPREE. NCT01038583.
INTRODUCTION
Aspirin is widely used for the prevention of vascular events but is associated with a sustained increase in the risk of significant bleeding. Many guidelines now caution against its use among those at increased bleeding risk.1 However, data on gastrointestinal (GI) bleeding incidence and the additional risk with aspirin in general populations are lacking, particularly in older people in whom bleeding events are associated with greater morbidity. In addition, knowledge of the epidemiology of bleeding is limited by inconsistent definitions, a lack of exploration of other risk factors outside traditional vascular risk factors2 and selective reporting of bleeding site and severity.3 Hence, knowledge of the benefits and harms of aspirin use in older populations is incomplete, and to this end, aspirin-related bleeding is highlighted by the United States Preventive Services Task Force as an area of priority for research.4
The ASPirin in Reducing Events in the Elderly (ASPREE) trial5 provides an ideal opportunity to study the impact of aspirin on bleeding in older persons. The trial enrolled exclusively older people living in the community and had a large study size (n=19 114). Bleeding events were a prespecified outcome of the trial that were adjudicated using standardised criteria, and extensive information on comorbidities and medications use were recorded at randomisation and updated annually. This allows expanded identification of potential risk factors, and calculation of relative and absolute risks of bleeding that are generalisable to an older population.
In this study, we aim to assess the incidence of serious GI bleeding in an older population, the impact of aspirin, identify risk factors and calculate absolute bleeding risk according to age and presence of risk factors.
METHODS
Study design
The ASPREE trial was a randomised, placebo-controlled, double-blind clinical trial evaluating the effect of enteric-coated 100 mg aspirin on disability-free survival in community-dwelling people aged 70 years and older (or ≥65 years for US minorities of African Americans and Hispanics). Detailed trial methods have been reported elsewhere.6 Briefly, 19 114 participants were enrolled from March 2010 to December 2014, recruited via clinical trial centres in the USA or their primary care provider in Australia.7 Inclusion criteria were age 70 years or older (or ≥65 for US minorities), willing to participate in an aspirin trial and able to provide informed consent. Participants were required to be free of cardiovascular disease, dementia, significant physical disability or any illness expected to limit their life expectancy to 5 years. Key exclusion criteria included a high risk of bleeding, anaemia (defined as haemoglobin <12 g/dL in men and <11 g/dL in women), use of aspirin for secondary prevention or a contraindication to aspirin, high risk of bleeding (defined in our protocol as a bleeding diathesis, cerebral or aortic aneurysm, liver disease or known oesophageal varices, recent peptic ulcer or GI malignancy) or concurrent use of anticoagulants or other antiplatelet agents. Previous regular use of aspirin for primary prevention was permitted, provided the participant was willing to cease aspirin and be randomised to aspirin or placebo, while the use of non-steroidal anti-inflammatory drugs (NSAIDs) at the lowest effective dose was allowed, with advice provided to use these for the shortest time possible. Randomisation was performed remotely using a computer-generated schedule in a 1:1 ratio and stratified for general practice in Australia, country (Australia or the USA) and age (65–69, 70–79 and 80+ years). After enrolment, participants had a visit to their general practitioner for confirmation of inclusion/exclusion criteria and another visit with trial staff for final baseline data collection and randomisation. Following automated randomisation, participants were mailed a year’s supply of their allocated study medication (identical bottles of 400 unscored white pills which were 100 mg aspirin or placebo). Participants, medical practitioners and study staff were blinded to study arm. Loss to follow-up was approximately 1.5% in both arms. Participants underwent annual in-person interviews supplemented by telephone calls every 3 months. The trial was stopped early in June 2017 after a median follow-up of 4.7 years due to lack of efficacy for the primary endpoint. The study has continued in a follow-on observational design phase. We report here the results from the randomised controlled trial component. The ASPREE trial was approved by local ethics committees and each participant provided written, informed consent.
Definition of outcome: clinically significant bleeding
There is no consensus-based, widely accepted definition for clinically significant bleeding associated with aspirin in primary prevention trials. While standardised criteria have been developed for use in cardiovascular trials to capture bleeding associated with revascularisation procedures,8 these are not directly transferable to the primary prevention setting. We therefore developed a bespoke definition using criteria reported in past aspirin trials (such as bleeding requiring hospitalisation or transfusion). Full details of our definition development have been reported previously.9 Briefly, a clinically significant GI bleeding event had to fulfil both of the following criteria: (1) bleeding substantiated by medical documentation (such as a reasonable description of haematemesis or melaena by patients or medical personnel, bleeding seen at imaging such as CT scan or visualisation of bleeding at endoscopy) and (2) bleeding that required admission to hospital, transfusion, prolongation of hospitalisation, surgery or resulted in death (see online supplementary table S1). GI bleeding events were further differentiated into upper or lower bleeding using clinical records, blood urea nitrogen, imaging including angiography, endoscopy reports and expert gastroenterologist opinion. This definition resulted in the inclusion of more severe bleeding events, while bleeding events such as epistaxis or per rectal bleeding that did not require hospitalisation were excluded.
Ascertainment of outcome
We undertook prospective collection of bleeding events from trial commencement to trial cessation in June 2017. Potential bleeding events were detected by notification from trial participants, reported at scheduled interviews, gathered from hospital records or notified by primary care practitioners or other personnel. On notification, the medical records were requested by our data team, and the case summaries were sent to the adjudication committee. Adjudication decisions were undertaken by two physicians blinded to trial arm and were entered into a customised database. All discordant adjudications were reviewed at meetings and resolved by a third physician adjudicator.
Definition and measurement of variables
Trained interviewers collected baseline demographic, clinical and other trial data including age, sex and smoking status (current, former or never smoker). Hypertension was defined as a blood pressure equal to or greater than 140/90 mm Hg at trial entry or use of an antihypertensive medication. Type 2 diabetes was defined as participant report of diabetes, fasting glucose of ≥126 mg/dL (≥7 mmol/L) or drug therapy for diabetes. Alcohol intake was self-reported as current, former or never. Chronic kidney disease (CKD) of stage 3 or greater was defined as either a calculated estimated glomerular filtration rate of <60 mL/min/1.73 m2 or urinary albumin:creatinine ratio of ≥3 mg/mmol at baseline.10 Waist circumference was defined as ‘at risk’ if above standard sex-specific cut-offs (≥88 cm for women and ≥102 cm for men). Regular use of aspirin prior to trial entry was collected for all except two participants.
Risk factors for GI bleeding
We included known and potential risk factors for bleeding in a Cox proportional hazards model. Potential risk factors for bleeding were identified from previous reports including individual patient data meta-analyses, that reported age, sex, smoking and diabetes,2,4 those included in risk prediction scores for anticoagulant-related upper GI bleeding11,12 and use of medications considered to increase bleeding risk, including NSAIDs, selective serotonin reuptake inhibitors (SSRIs) and proton pump inhibitors (PPIs). Data on all medication use were recorded at baseline and updated at annual interviews.
Statistical analysis
All analyses were undertaken on an intention-to-treat basis. We undertook analyses with overall GI bleeding events to allow comparisons to previous studies, followed by analyses for upper or lower GI bleeding separately given the differences in pathophysiology. We used Cox proportional hazards models to estimate the effect of aspirin on first overall bleeding event, and first upper or lower bleeding event separately. Cumulative incidence plots were used to show event risks. Additionally, for each bleeding type (overall, upper or lower GI bleeding), we conducted subgroup analysis for the following prespecified subgroups: age, sex, smoking, alcohol use, hypertension, type 2 diabetes, waist circumference, CKD and use of NSAIDs, PPIs or SSRIs. Heterogeneity of treatment effect across subgroups was tested based on subgroup-treatment interaction term in a Cox proportional hazards model using a significance level of p≤0.01 to account for the number of statistical analyses. To identify risk factors associated with increased bleeding risk, we fit a multivariable Cox proportional hazards regression model with the treatment allocation and risk factors as covariates, with the choice of covariates guided by those identified in previous studies and known to modify bleeding risk such as NSAIDs, as described above. An additional sensitivity analysis was undertaken to explore the effect of long-term PPI use on upper GI bleeding events. In this, we tested time-dependent variables for baseline and/or continuous use of PPI (updated use to the most recent calendar year), in multivariable analyses. The proportional hazards assumption for all models was checked using Schoenfeld residuals.
The absolute risk of an incident GI bleed for different clinical scenarios was quantified from the multivariable models inclusive of statistically significant risk factors for overall bleeds, upper GI (age, smoking, CKD and NSAID use) and lower GI (age, smoking, hypertension and included CKD) bleeding. The scenarios were: baseline risk (adults not on aspirin and without risk factors for bleeding) and baseline risk plus aspirin. We then calculated the increment in absolute risk when other significant risk factors were present. Models were derived for people aged 70 and 80 years to assess for increase in bleeding risk with age. In all analyses (excluding interaction testing), a p value ≤0.05 was considered significant. Statistical analyses were performed using R (R Core Team, 2014) with the survival package (Therneau, 2015). Patients or the public were not involved in the study design or reporting of this research.
RESULTS
From March 2010 to December 2014, 19 114 participants were enrolled in the trial, of whom 9525 were assigned to aspirin and 9589 were assigned to placebo (see online supplementary figure S1). Randomisation resulted in equal distribution of participant characteristics5 (see online supplementary table S2). The median age of participants was 74 years, 44% were men, 4% were current smokers and 25% were using PPIs at baseline. The median follow-up per person was 4.7 years (IQR 3.5–5.6 years) resulting in 88 389 person-year follow-up, with trial withdrawals and loss to follow-up of 1.2% and 1.6%, respectively, in each group.5
Incidence of GI bleeding in aspirin and placebo groups
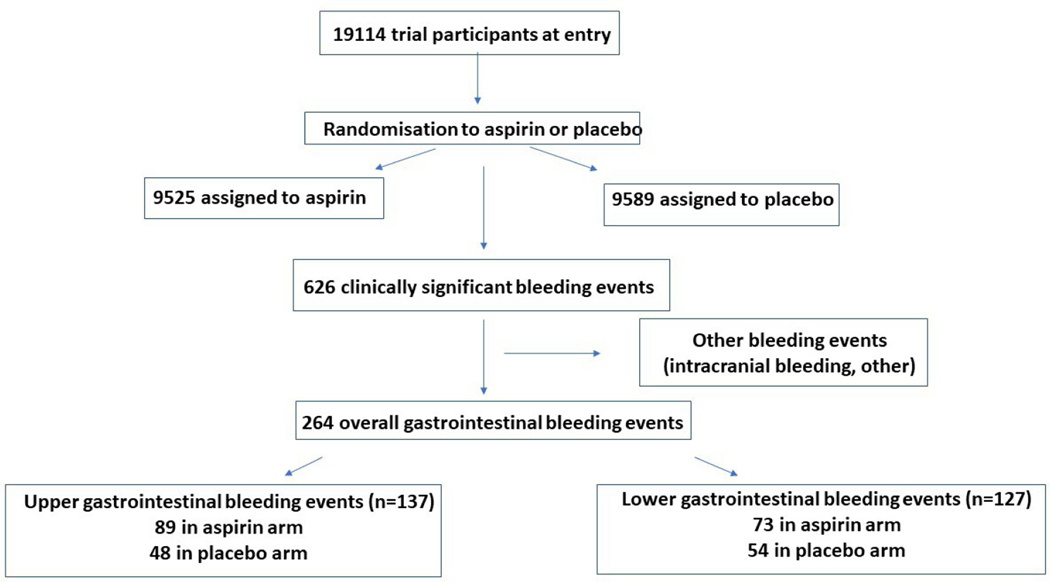
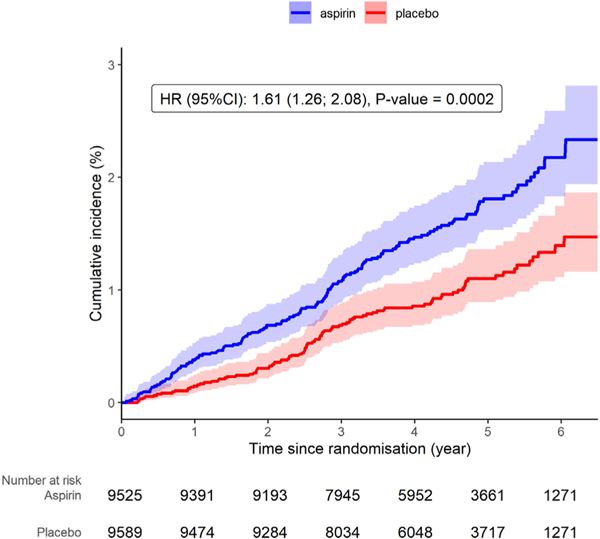
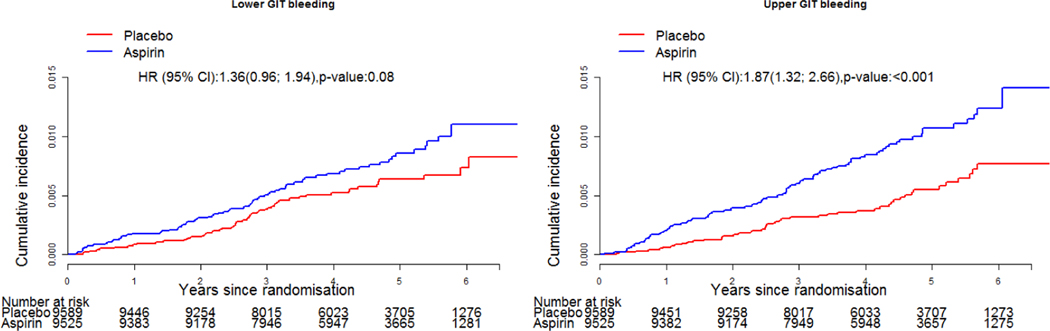
In total, there were 264 clinically significant GI bleeds, of which 137 were upper and 127 were lower GI bleeding in 257 participants (7 participants had 2 events and continued to be enrolled in the study but were withdrawn from treatment arm) (figure 1). There were two deaths attributed to GI bleeding, both of which occurred in the placebo arm. For the overall GI bleeding events, 162 occurred in the aspirin group, and 102 in the placebo group (HR 1.61, 95% CI 1.26 to 2.08, p=0.002). There was no evidence of attenuation in bleeding risk with time (figure 2). For upper GI bleeding events, 89 occurred in people assigned to aspirin (89/9525, 0.9%) and 48 occurred in people assigned to placebo (48/9589, 0.5%), HR 1.87 (95% CI 1.32 to 2.66, p<0.001; figure 3). The event rate for upper GI bleeding was 2.1/1000 py in the aspirin group, compared with 1.1/1000 py in the placebo group. For lower GI bleeding events, 73 occurred in the aspirin group (73/9525, 0.8%) and 54 in the placebo group (54/9589, 0.6%) (HR 1.36, 95% CI 0.96 to 1.94, p=0.08), with event rates of 1.7/1000 and 1.3/1000 py for aspirin and placebo groups, respectively (figure 3).
Figure 1.
Flow diagram of study participants with bleeding events.
Figure 2.
Cumulative incidence of GI bleeding events according to trial arm.
Figure 3.
Cumulative incidence of upper and lower GI bleeding events according to trial arm.
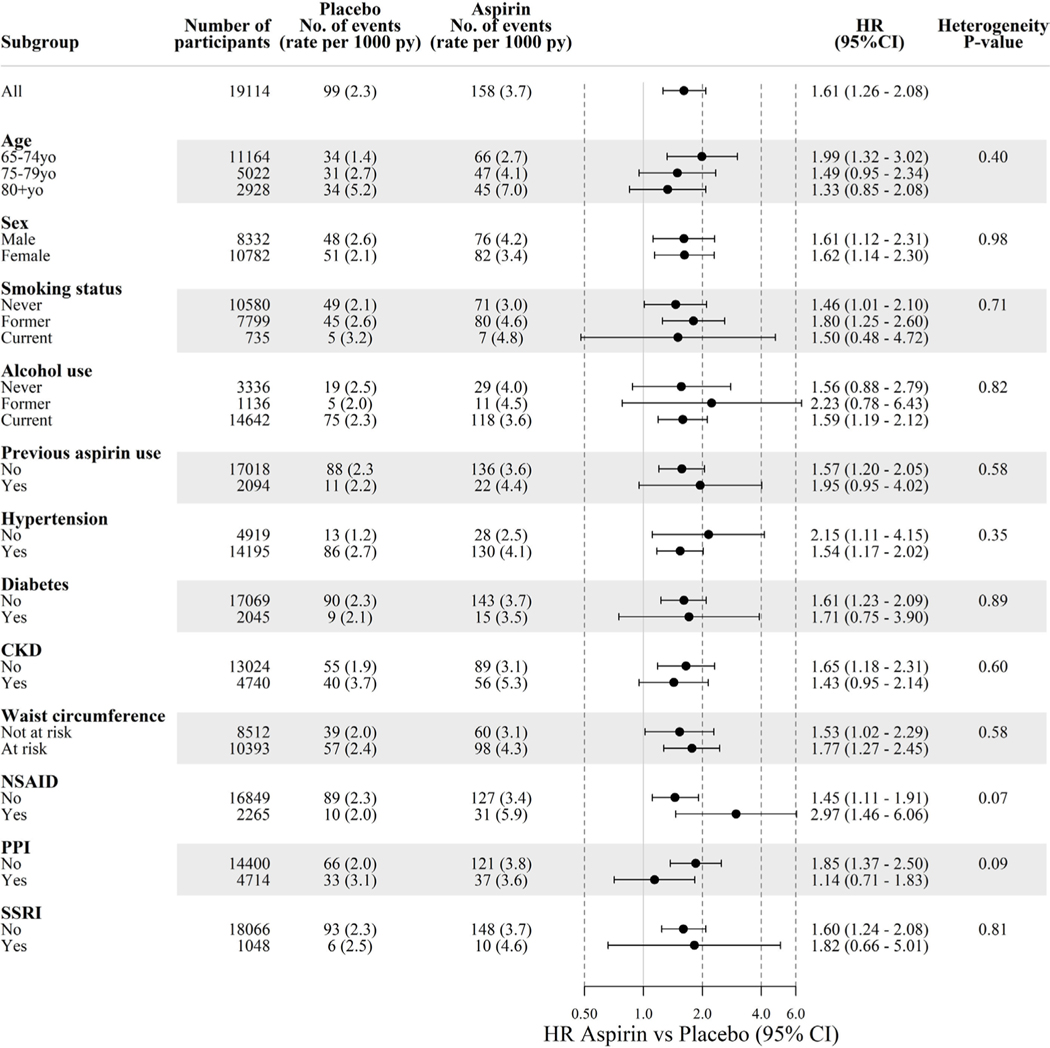
Subgroup analyses
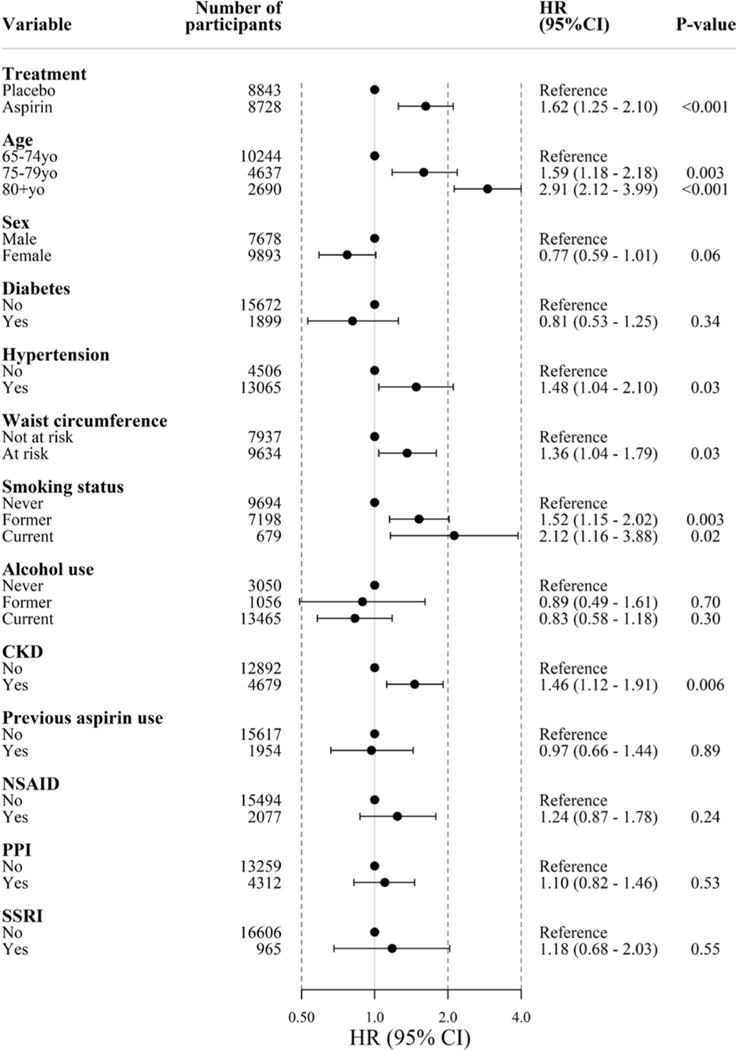
To explore the impact of aspirin on bleeding risk and the presence of effect modification, we analysed bleeding risk according to prespecified subgroups. These indicate that the effect of aspirin on overall (figure 4), upper and lower GI bleeding (see online supplementary figure S2) was not significantly modified in any subgroup.
Figure 4.
Incidence of GI bleeding according to subgroup. Diabetes=self-report of diabetes mellitus or fasting glucose ≥126 mg/dL (≥7 mmol/L) or on treatment for diabetes. Hypertension=systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or on treatment for high blood pressure. Chronic kidney disease (CKD)=estimated glomerular filtration rate < 60 mL/min/1.73 m2 or albumin-to-creatinine ratio ≥3 mg/mmol. NSAID, non-steroidal anti-inflammatory drugs; PPI, proton pump inhibitor; SSRI, selective serotonin reuptake inhibitor.
Multivariable models for overall GI bleeding
After adjusting for other covariates, aspirin significantly increased the risk of overall GI bleeding (multivariable HR 1.62, 95% CI 1.25 to 2.10, p<0.001, figure 5). Older age was associated with increasing bleeding risk, estimated at approximately 60% higher risk for those aged 75–79 years compared with the reference group of ≤74 years (HR 1.59, 95% 1.18 to 2.18), and a nearly threefold elevated risk for those aged ≥80 years (HR 2.91 (95% CI 2.12 to 3.99)). Hypertension and smoking (current or former) were also associated with higher bleeding risk (for current smoking, HR 2.12 95% CI 1.16 to 3.88), as was CKD (HR 1.46, 95% CI 1.12 to 1.91). Type 2 diabetes, alcohol intake and previous aspirin use were not identified as significant risk factors.
Figure 5.
Multivariable model of risk factors for GI bleeding (HR and 95% CI). Diabetes=self-report of diabetes mellitus or fasting glucose ≥126 mg/dL (≥7 mmol/L) or on treatment for diabetes. Hypertension=systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or on treatment for high blood pressure. Chronic kidney disease (CKD)=estimated glomerular filtration rate < 60 mL/min/1.73 m2 or albumin-to-creatinine ratio ≥3 mg/mmol. NSAID, non-steroidal anti-inflammatory drugs; PPI, proton pump inhibitor; SSRI, selective serotonin reuptake inhibitor.
Multivariable models for upper GI bleeding
After adjusting for significant covariates, the effect of aspirin on risk of upper GI bleeding was higher than for overall bleeding (multivariable HR 1.92, 95% CI 1.35 to 2.74, p<0.001; see online supplementary figure S3). Older age was associated with increasing upper GI bleeding risk, estimated at twice for those aged 75–79 years compared with the reference group of ≤74 years (HR 2.06, 95% 1.38 to 3.08), and a threefold risk for those aged ≥80 years (HR 3.00, 95% CI 1.95 to 4.61). Current smoking was associated with higher bleeding risk (HR 2.39, 95% CI 1.13 to 5.04), as were CKD and NSAID use (HR 1.52, 95% CI 1.07 to 2.17, and HR 1.62, 95% CI 1.08 to 2.42, respectively).
Additional sensitivity analyses for PPI use showed that neither baseline use of PPI (reported by 25% of the cohort (4714/19 114 persons)), nor continuous use of PPI therapy (reported as continuous use at each annual visit throughout the trial, 1925/19 114 persons, 10.1%) was associated with a reduced risk of upper tract bleeding. The adjusted risk of bleeding in those using PPI at baseline was HR 0.92 (95% CI 0.62 to 1.37), while the adjusted risk of bleeding for people reporting continuous PPI use was similarly equivocal (HR 0.98, 95% CI 0.68 to 1.42).
Multivariable models for lower GI bleeding
Aspirin was also associated with increased risk of lower GI bleeding, although the magnitude was less than for upper GI bleeding (multivariable HR 1.42, 95 CI 1.00 to 2.03, p=0.05; (see online supplementary figure S4). Older age, hypertension and former smoking were all significant risk factors, while the association with CKD did not reach statistical significance (HR 1.40, 95% CI 0.96 to 2.03, p=0.08). No associations of lower GI bleeding with diabetes, alcohol, PPI or NSAID use were identified.
Absolute risk of overall GI bleeding with aspirin and other risk factors
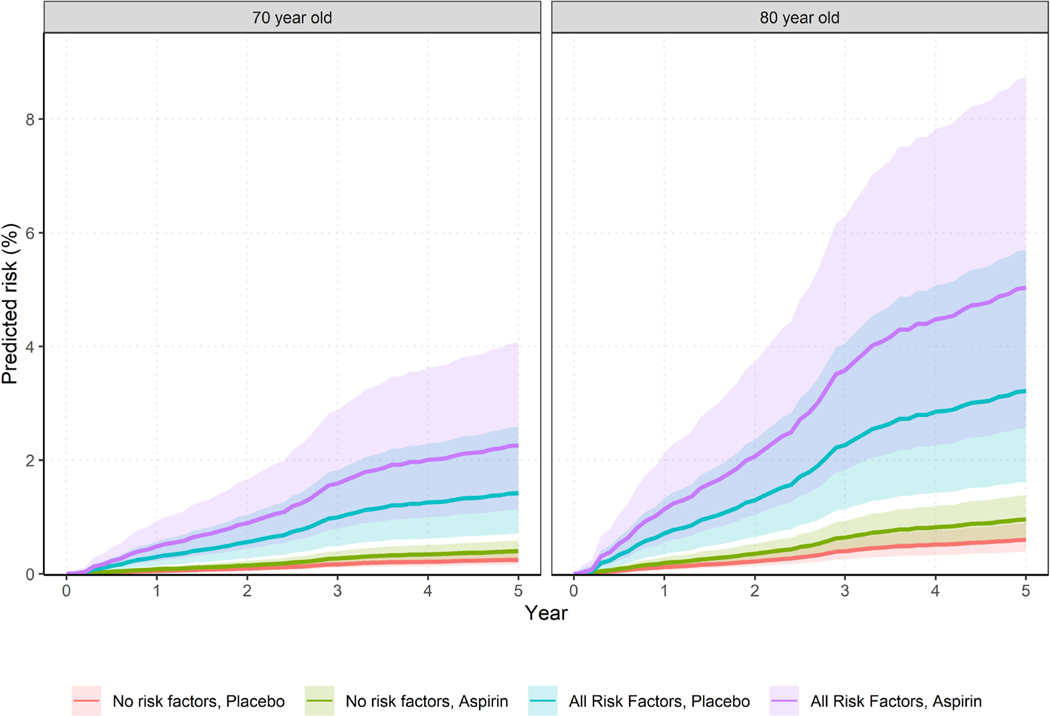
The absolute risk of overall GI bleeding was estimated according to age, aspirin use and risk factors, to assist individualised decision-making and risk prediction. The absolute 5-year risk of an incident GI bleed for a well 70 years old who is not on aspirin and has no identified risk factors was estimated at 0.25% (95% CI 0.16% to 0.37%). This risk is nearly doubled with aspirin use (0.40%, 95% CI 0.26% to 0.59%). The risk is higher at age 80 years without aspirin (0.60%, 95% CI 0.39% to 0.89%) and with aspirin (0.96%, 95% CI 0.64% to 1.38%; table 1, figure 6). When risk factors of smoking, hypertension, elevated waist circumference and CKD are present in addition to aspirin use, bleeding risk increased to 2.26% (95% CI 1.13% to 4.07%) and 5.03% (2.56% to 8.73%) for 70 and 80 years old, respectively. In figure 6, shading around the hazard curves provide information on the uncertainty (95% CIs) around the point estimates.
Table 1.
Absolute risk of GI bleeding over 5 years for people on placebo or aspirin, according to age and risk factors
| 70 years old |
80 years old |
|||
|---|---|---|---|---|
| Additional risk factors | Placebo | Aspirin | Placebo | Aspirin |
| None | 0.25% (0.16% to 0.37%) | 0.40% (0.26% to 0.59%) | 0.60% (0.39% to 0.89%) | 0.96% (0.64% to 1.38%) |
| Smoking | 0.52% (0.25% to 0.99%) | 0.58% (0.4% to 0.84%) | 1.21% (0.56% to 2.3%) | 1.91% (0.9% to 3.58%) |
| CKD | 0.35% (0.21% to 0.57%) | 0.57% (0.34% to 0.9%) | 0.85% (0.52% to 1.32%) | 1.36% (0.86% to 2.05%) |
| Hypertension (HT) | 0.36% (0.24% to 0.54%) | 0.58% (0.4% to 0.84%) | 0.88% (0.61% to 1.23%) | 1.41% (1.03% to 1.88%) |
| At-risk waist circumference (WC) | 0.32% (0.21% to 0.47%) | 0.52% (0.35% to 0.76%) | 0.78% (0.51% to 1.17%) | 1.25% (0.83% to 1.83%) |
| Smoking and CKD | 0.74% (0.33% to 1.48%) | 1.18% (0.52% to 2.34%) | 1.67% (0.73% to 3.3%) | 2.62% (1.16% to 5.09%) |
| Smoking, CKD and WC | 0.97% (0.43% to 1.94%) | 1.54% (0.68% to 3.06%) | 2.2% (0.95% to 4.37%) | 3.45% (1.49% to 6.75%) |
| Smoking, at-risk WC | 0.68% (0.33% to 1.29%) | 1.09% (0.52% to 2.05%) | 1.59% (0.73% to 3.04%) | 2.51% (1.16% to 4.75%) |
| Smoking, HT and WC | 1.0% (0.53% to 1.74%) | 1.60% (0.85% to 2.76%) | 2.33% (1.22% to 4.05%) | 3.67% (1.94% to 6.27%) |
| HT, at-risk WC | 0.48% (0.33% to 0.66%) | 0.76% (0.55% to 1.05%) | 1.15% (0.83% to 1.56%) | 1.84% (1.38% to 2.41%) |
| CKD and at-risk WC | 0.46% (0.28% to 0.73%) | 0.74% (0.45% to 1.17%) | 1.12% (0.69% to 1.73%) | 1.78% (1.11% to 2.71%) |
| CKD and HT | 0.52% (0.33% to 0.8%) | 0.83% (0.54% to 1.24%) | 1.25% (0.87% to 1.75%) | 1.99% (1.45% to 2.67%) |
| CKD, HT and WC | 0.68% (0.45% to 1%) | 1.09% (0.74% to 1.57%) | 1.64% (1.17% to 2.24%) | 2.61% (1.93% to 3.44%) |
| Smoking, CKD, at-risk WC and HT | 1.42% (0.71% to 2.59%) | 2.26% (1.13% to 4.07%) | 3.22% (1.62% to 5.7%) | 5.03% (2.56% to 8.73%) |
CKD, chronic kidney disease.
Figure 6.
Absolute bleeding risk over 5 years for GI bleeding according to age and risk factors (shading represents 95% CI around the estimates). Red line=no risk factors, on placebo, green line=no risk factors but on aspirin, blue line=all risk factors and on placebo, purple line=aspirin and all risk factors; modelled risk factors were those significant in multivariable analyses.
Absolute risk of upper and lower GI bleeding
For a 70 years old with no identified risk factors and not on aspirin, the estimated 5-year risk of upper GI bleeding was 0.22% (95% CI 0.14% to 0.33%), and nearly doubled with aspirin use (0.40%, 95% CI 0.26% to 0.59%; see online supplementary table S3 and figure S5). The risk is higher at age 80 years without aspirin (0.52%, 95% CI 0.36% to 0.75%)). The presence of aspirin and additional risk factors for an 80 years old of smoking, CKD and NSAID use substantially increased bleeding risk (5.76%, 95% CI 2.38% to 11.32%). For lower GI bleeding, the absolute 5-year risk was 0.16% (95% CI 0.09 to 0.28) for a 70 years old with no risk factors, and increased to 0.22% (95% CI 0.13 to 0.37) with aspirin use (see online supplementary table S4 and figure S5). The risk is higher at age 80 years without aspirin (0.37%, 95% CI 0.20% to 0.64%), and up to 2.09% (95% CI 1.45% to 2.91%) with aspirin and additional risk factors of smoking, CKD and hypertension. The absolute risk estimates for lower GI bleeding are less than that seen for upper GI bleeding, reflecting the smaller HRs for risk factors from the multivariable model. Additional data on the absolute risk at 1 year are provided in the supplementary data (see online supplementary table S5).
DISCUSSION
In this study using data from a large randomised controlled trial of aspirin in older people, significant GI bleeding is increased 60% by aspirin (87% for upper and 36% for lower GI bleeding). A recent meta-analysis of 13 trials of aspirin for primary prevention in mainly younger populations found a similar risk for combined serious GI bleeding (HR 1.56, 95% CI 1.38 to 1.78).3 We did not observe any increase in fatal GI bleeding with aspirin in our elderly population, with only two fatal bleeds in the placebo arm. This is consistent with the findings from a meta-analysis of aspirin primary prevention trials specifically addressing fatal bleeds, which found no increased risk.13
Analyses stratified by bleeding site suggest that the absolute risk of upper GI bleeding is higher than for lower GI bleeding at all ages, and the impact of aspirin is greater for upper GI bleeding. This may be explained by mechanistic differences, whereby in upper GI bleeding, aspirin induces local effects with mucosal injury with reduced mucus and bicarbonate secretion, and systemic effects of prostaglandin depletion and platelet inactivation, effects which may be synergistic. Lower GI bleeding is more commonly due to a range of causes such as bleeding from diverticula with arterial rupture, angiodysplasia or haemorrhoids. Of note, study methodology may also affect estimated risk, with large-scale observational data from national databases suggesting a much higher risk of lower GI bleeding (HR 2.75, 95% CI 2.06 to 3.65)14 than observed in our trial.
Other important risk factors for bleeding were age (highest in those ≥80 years), smoking, hypertension, CKD and an elevated waist circumference. The 5-year risk of GI bleeding ranged from 0.25% (95% CI 0.16% to 0.37%) in a 70 years old without other risk factors to over 5% (5.03% (95% CI 2.56% to 8.73%)) in an 80 years old with multiple risk factors. CKD is rarely noted as a risk factor in bleeding studies, potentially because data on kidney function are not routinely collected. Our analyses indicate that the presence of ≥stage 3 CKD was associated with a 46% higher overall bleeding risk, which is important in older populations since even in our well, community-based trial participants, the prevalence of ≥stage 3 CKD at study entry was 25%. A large observational study of a population database in Taiwan indicated that CKD defined by International Classification of Diseases codes was also associated with upper GI bleeding.15 CKD may contribute to bleeding risk via increased prevalence of small bowel angiodysplasia,16 and while uraemia-related platelet dysfunction17 is a well-recognised phenomenon, this would seem unlikely to be a major factor in our study as very few participants were found to have advanced kidney disease.
Our data build on current knowledge of bleeding risk factors studied in younger populations. In an individual patient data meta-analysis of six aspirin primary prevention trials (n=95 000), the identified risk factors for all extracranial bleeds (n=550) were age, male sex, smoking, hypertension, body mass index and diabetes.2 In addition, a systematic review for the United States Preventive Services Task Force inclusive of additional trials found age, male sex and diabetes were risk factors.4 We did not find that people with diabetes incurred increased bleeding risk in our study, and this may reflect a true finding, or reduced power to find an effect in a single trial compared with meta-analysis. Large trials in women only such as the Women’s Health Study found a slightly lower risk of significant GI bleeding (defined as that requiring transfusion) with a relative risk 1.40, 95% CI 1.07 to 1.83.18
Coprescription of PPI therapy with aspirin is commonly undertaken to reduce bleeding risk; however, this effect was not observed in this study. A lack of protective effect may be due to lack of power in our study, or because the analyses of PPI therapy were observational and affected by unknown confounders.
It may also be that prophylactic use does not greatly impact significant bleeds that present to hospital, which is the type of bleeding captured by our criteria. A recent, well-conducted trial of prophylactic PPI use in people commencing aspirin or novel anticoagulants also found PPIs did not reduce bleeding events, with a similar event rate to our study, although the authors note that PPIs may reduce bleeding from an identified gastroduodenal lesion.19 Other randomised trials20 and systematic reviews of trials of PPIs and other gastroprotectants with heterogeneous outcome definitions have shown conflicting results.21,22 Conversely, retrospective studies report a greater than 75% relative risk reduction.23 Of note, any potential benefit of long-term prophylactic use should be considered in the context of possible side effects of PPI therapy including increased risk of enteric infections such as Clostridium difficile infection.24
The major strengths of this study include a randomised trial design with careful collection and adjudication of bleeding events where it is unlikely many bleeding events were missed, and physician review of each event which supported a consistent approach to bleeding classification. In addition, the target population was exclusively older people whose bleeding risk has been understudied to date. The collection of a wide variety of characteristics such as CKD enabled us to incorporate risk factors pertinent to an older population. The trial was embedded in the general population, providing robust estimates on baseline risk of bleeding in well, older persons and the incremental impact of aspirin, advancing age and additional risk factors.
There are also limitations to highlight. The study design excluded individuals with a previous major bleeding episode or conditions associated with high bleeding risk; thus, our results cannot be extrapolated to these groups. Previous data suggest that bleeding estimates are higher in cohort-based studies. 4 In addition, we found uncertainty around the point estimates for absolute risk as illustrated by the widening CIs particularly as more risk factors are present. This reflects insufficient statistical power despite the large trial size, and future meta-analyses of bleeding events from trials may provide improved precision. We were unable to ascertain Helicobacter pylori status for our participants, which would have been useful for analyses of upper GI bleeds. Finally, our definition of serious bleeding events was rigorous and excluded other bleeding events that may still be of importance to patients but did not result in hospitalisation, such as epistaxis.
This study identifies areas for future research. Currently, there is limited understanding on how bleeding impacts patients in the long term. Future studies examining how serious bleeding influences survival, disability-free survival and quality of life are needed. An understanding of how patients value bleeding events compared with thrombotic events avoided would be very helpful to inform benefit and risk discussions on aspirin use. The mechanism of how CKD influences bleeding risk is also important for care of people with CKD. In conclusion, this study provides population-based data on GI bleeding in older populations and the impact of aspirin, providing robust and clinically meaningful estimates for use in clinical practice and future epidemiological studies.
Supplementary Material
Significance of this study.
What is already known on this subject?
-
►
Older people are at higher risk of serious GI bleeding than younger people.
-
►
There is little trial-based data to inform baseline risk and the additional risk conferred by aspirin use.
-
►
Current data in older populations are largely observational, with heterogeneous cohorts and variable definitions of significant bleeding.
What are the new findings?
-
►
The absolute risk of serious GI bleeding (requiring hospitalisation or transfusion) is modest in a well 70-year-old person (5-year risk of around 0.25%).
-
►
The addition of aspirin increases an older persons baseline risk of bleeding by approximately 60%.
-
►
Other risk factors that further increase bleeding risk are advancing age, smoking, hypertension, truncal obesity, chronic kidney disease and non-steroidal anti-inflammatory drugs use.
How might it impact on clinical practice in the foreseeable future?
-
►
Clinicians may use these data to assess bleeding risk, review the indication for aspirin and target modifiable risk factors to reduce harm.
Acknowledgements
The authors acknowledge the dedicated and skilled staff in Australia and the USA for the conduct of the trial. They are also most grateful to the ASPREE participants who so willingly volunteered for this study, and the general practitioners and medical clinics who supported the participants.
Funding SEM is funded by the Vincent Fairfax Family Foundation Establishment Fellowship & Hugh Rogers Fellowship. ASPREE was supported by the National Institute on Aging and the National Cancer Institute at the National Institutes of Health (grant number U01AG029824); the National Health and Medical Research Council of Australia (grant numbers 334047, 1127060); Monash University (Australia); and the Victorian Cancer Agency (Australia).
Footnotes
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request. Requests for data access will be via the ASPREE Principal Investigators with details for applications provided through the website, www.ASPREE.org, and in accord with the NIH policy on data sharing, details available at https://grants.nih.gov/grants/policy/data_sharing/.
REFERENCES
- 1.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Circulation 2019;140:e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA 2019;321:277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitlock EP, Burda BU, Williams SB, et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. preventive services Task force. Ann Intern Med 2016;164:826–35. [DOI] [PubMed] [Google Scholar]
- 5.McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med 2018;379:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ASPREE Investigator Group. Study design of aspirin in reducing events in the elderly (ASPREE): a randomized, controlled trial. Contemp Clin Trials 2013;36:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockery JE, Collyer TA, Abhayaratna WP, et al. Recruiting general practice patients for large clinical trials: lessons from the aspirin in reducing events in the elderly (ASPREE) study. Med J Aust 2019;210:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research Consortium. Circulation 2011;123:2736–47. [DOI] [PubMed] [Google Scholar]
- 9.Margolis KL, Mahady SE, Nelson MR, et al. Development of a standardized definition for clinically significant bleeding in the aspirin in reducing events in the elderly (ASPREE) trial. Contemp Clin Trials Commun 2018;11:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polkinghorne KR, Wolfe R, Jachno KM, et al. Prevalence of chronic kidney disease in the elderly using the aspirin in reducing events in the elderly study cohort. Nephrology 2019;24:1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 12.Hippisley-Cox J, Coupland C. Predicting risk of upper gastrointestinal bleed and intracranial bleed with anticoagulants: cohort study to derive and validate the QBleed scores. BMJ 2014;349:g4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elwood PC, Morgan G, Galante J, et al. Systematic review and meta-analysis of randomised trials to ascertain fatal gastrointestinal bleeding events attributable to preventive low-dose aspirin: no evidence of increased risk. PLoS One 2016;11:e0166166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen W-C, Lin K-H, Huang Y-T, et al. The risk of lower gastrointestinal bleeding in low-dose aspirin users. Aliment Pharmacol Ther 2017;45:1542–50. [DOI] [PubMed] [Google Scholar]
- 15.Luo P-J, Lin X-H, Lin C-C, et al. Risk factors for upper gastrointestinal bleeding among aspirin users: an old issue with new findings from a population-based cohort study. J Formos Med Assoc 2019;118:939–44. [DOI] [PubMed] [Google Scholar]
- 16.Karagiannis S, Goulas S, Kosmadakis G, et al. Wireless capsule endoscopy in the investigation of patients with chronic renal failure and obscure gastrointestinal bleeding (preliminary data). World J Gastroenterol 2006;12:5182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost 2004;30:579–89. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Cook NR, Lee I-M, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–304. [DOI] [PubMed] [Google Scholar]
- 19.Moayyedi P, Eikelboom JW, Bosch J, et al. Pantoprazole to prevent gastroduodenal events in patients receiving rivaroxaban and/or aspirin in a randomized, double-blind, placebo-controlled trial. Gastroenterology 2019;157:403–12. [DOI] [PubMed] [Google Scholar]
- 20.Yeomans N, Lanas A, Labenz J, et al. Efficacy of esomeprazole (20 mg once daily) for reducing the risk of gastroduodenal ulcers associated with continuous use of low-dose aspirin. Am J Gastroenterol 2008;103:2465–73. [DOI] [PubMed] [Google Scholar]
- 21.Scally B, Emberson JR, Spata E, et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials. Lancet Gastroenterol Hepatol 2018;3:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran-Duy A, Vanmolkot FH, Joore MA, et al. Should patients prescribed long-term low-dose aspirin receive proton pump inhibitors? A systematic review and meta-analysis. Int J Clin Pract 2015;69:1088–111. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Geraghty OC, Mehta Z, et al. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017;390:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaezi MF, Yang Y-X, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology 2017;153:35–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.