Abstract
Background
Metabolic syndrome (MetS) prevalence in rheumatoid arthritis (RA) patients is known to vary considerably across the world. This study aimed to determine the prevalence of MetS in RA patients from western Mexico and to analyze the interrelation of the MetS components with the clinical variables of RA.
Methods
This case‐control study included 216 RA patients and 260 control subjects (CS). MetS prevalence was determined according to the NCEP/ATP III and the Latin American Consensus of the Latin American Diabetes Association (ALAD) criteria.
Results
MetS was observed in 30.6% RA patients and 33.3% of controls (p > 0.05) according to NCEP/ATP III and 28.7% in RA patients and 31.1% for controls using ALAD criteria. Total cholesterol, LDL‐C, and Castelli's I‐II indexes were lower in RA (p < 0.001) than in CS. The RA patients with MetS had more swollen joints than those without MetS (p = 0.018). In RA patients with MetS, DAS‐28 score correlated with smoking index (rho = 0.4601, p = 0.0004) and VLDL‐C (rho = 0.3108, p = 0.0056); similarly, rheumatoid factor (RF) correlated with age (rho = 0.2031, p = 0.0027), smoking index (rho = 0.3404, p < 0.0001), triglycerides (rho = 0.1958, p = 0.0039), and VLDL‐C (rho = 0.1761, p = 0.0162).
Conclusions
The MetS prevalence in RA patients from western Mexico is not higher than controls; however, in RA patients with MetS, some inflammatory markers are associated with MetS components; thus, the control of MetS in RA could be beneficial to regulate disease activity.
Keywords: Castelli's index, disease activity, lipid profiles, metabolic syndrome, rheumatoid arthritis, rheumatoid factor
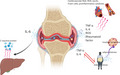
Relationship between rheumatoid arthritis (RA) and metabolic syndrome (MetS). The figure shows how the inflammatory markers of RA (IL‐6, TNF‐alpha, rheumatoid factor, etc,) can favor a response in different organs and tissues associated with the pathogenesis of MetS, such as the liver, pancreas, and the cardiovascular system, thus favoring the development of insulin resistance, atherosclerosis, and chronic inflammation. Likewise, the components of the Mets can favor the perpetuation of inflammation in RA.
1. INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune inflammatory rheumatic disease associated with high levels of inflammatory markers and chronic comorbidities such as cardiovascular disease (CVD). 1 , 2 , 3 , 4 It has been demonstrated that RA life expectancy is reduced compared to that of the general population. Currently, CVD is the leading cause of mortality in patients with RA, being approximately 50% of the total of RA‐associated deaths. 1 , 3 , 5
The RA is also associated with insulin resistance, dyslipidemia, and altered adipokines profile, which are included as metabolic syndrome (MetS) components. 6 MetS is defined as a set of manifestations that are contemplated as cardiovascular risk factors (obesity, glucose intolerance, dyslipidemia, and hypertension), that along with systemic inflammation, contributes to CVD, 7 , 8 , 9 and the prevalence of MetS has been associated with disease activity in RA. 8 , 9
In several populations, the overall risk of developing MetS seems to be significantly higher among patients with RA than in healthy controls; however, this differs considerably, based upon the diagnostic criteria used and the population ancestry. 10 On the other hand, the relationship between RA and MetS showed a significantly negative correlation in other countries such as Korea. 11 In Latin American countries, including Mexico, 12 , 13 , 14 there have been few solid studies regarding this topic 10 ; therefore, more research is needed to understand the metabolic changes of MetS in RA and its critical role in the development of CVD in RA patients from different geographic regions.
The biochemical profiles in RA occasionally does not reflect what observed in the general population, for example, low total cholesterol levels in RA patients have been associated with an increased cardiovascular risk, as well as, a high body mass index (BMI) has a protective effect on the amount of joint destruction in small joints in early RA. 15 , 16 , 17
The RA association with MetS also differs between chronic and early RA, 3 , 9 and the role of different characteristics of the disease, such as disease duration, activity, and the frequency of treatments, is not well defined yet. 17 , 18 , 19 , 20
Identifying MetS components in RA patients could provide a crucial opportunity for a preventive intervention; however, the controversy is evident about which factors are the most important to drive RA‐associated MetS. 18 , 19 , 20 This study aimed to assess the prevalence of MetS in RA patients from western Mexico and analyze the interrelation of the MetS components with the clinical variables of RA.
2. MATERIALS AND METHOD
2.1. Subjects
From February 2017 to May 2019, 216 RA patients and 270 control subjects (CS) were enrolled. The sample size was calculated with OPENEPI calculator 21 to detect an 80% statistical power with a confidence degree of 95% and an expected prevalence of MetS in RA patients of 17.5% reported in a previous study. 12 The Ethics Committee approved the protocol of the Hospital “Fray Antonio Alcalde” (HCG/CEI‐0153/18); written informed consent was obtained from all individuals.
According to the ACR 1987 classification criteria, 22 RA patients were diagnosed by a rheumatologist. They were enrolled in the study when they came to their clinical control visit at the Department of Internal Medicine/Rheumatology of the OPD Hospital Civil de Guadalajara "Fray Antonio Alcalde" in the state of Jalisco, Mexico. The rheumatologist conducted a medical record and 28 joints evaluation to estimate the clinical activity using the Disease Activity Score‐28 (DAS‐28). 23
The CS group included subjects without either known medical condition or treatment, with similar age, sex, and geographic regions as the RA group. They were randomly selected from the clinical laboratory of the OPD Hospital Civil de Guadalajara "Fray Antonio Alcalde" when they went for a regular health check‐up. The evaluation of physical activity, food consumption, and smoking index (number of cigarettes smoked per day × years of tobacco use) was realized during a personal interview in both study groups. Subjects were excluded from the study if they suffered from congestive heart disease, renal disease, endocrinological abnormalities, or were under medications that altered blood pressure, glucose, or lipid metabolism.
2.2. Anthropometric measurements
Anthropometric measurements were determined for all individuals with absolute reliability. Weight was measured with the subjects wearing lightweight clothes without shoes, and after an overnight fasting, using a standard scale (TANITA BC‐568 INNERSCAN). Height was measured and approximate to the nearest 0.1 cm using a Seca 213 mobile stadiometer, with the participant standing in a vertical plane with head in the Frankfort horizontal plane. Waist circumference was measured at the minimum circumference between the iliac crest and the lowest rib. Blood pressure was measured twice, while the patients were seating and resting for 5 min, using a digital sphygmomanometer. BMI was calculated as weight (kg) divided by height squared (m2).
2.3. Biochemical analysis
A blood sample was obtained from all individuals after an overnight fast (12 h). A full fasting lipid profile (triglycerides, total serum cholesterol, high‐density lipoprotein cholesterol [HDL‐C], low‐density lipoprotein cholesterol [LDL‐C]) and fasting blood glucose (FBS) concentrations were obtained using automated equipment (The Beckman Coulter AU5800 (Beckman Coulter Inc, Brea CA, USA). Serum C‐reactive protein (CRP) levels were determined by immunoturbidimetry (OSR6147). Castelli's Risk Index I was calculated as total serum cholesterol (mg/dl) divided by HDL‐C (mg/dl), and Castelli's Risk II was calculated as LDL‐C (mg/dl) divided by HDL‐C (mg/dl).
2.4. MetS definition
Subjects were diagnosed with MetS based on NCEP ATP III 24 and The Latin American Consensus of the Latin American Diabetes Association (ALAD) 25 criteria. Demographic data were collected by questionnaire.
2.5. Statistical analysis
Statistical analysis was performed using GraphPad Prism v6.0. The Shapiro‐Wilk normality test was applied to verify the normal distribution of the data. As it is appropriate, parametric or non‐parametric tests were used for the analysis. The nominal discontinuous variables were expressed as frequencies (number and percentages); the continuous variables with parametric distribution were expressed as means ± standard deviation (SD) and the non‐parametric variables as medians and interquartile ranges. The chi‐square (χ2) test was used to compare proportions. Student's t test was applied for two groups parametric quantitative analysis, and the Mann‐Whitney U test was used for non‐parametric quantitative determinations. Spearman's correlation analysis was used to discover the strength of a link between two sets of data. A multivariate logistic regression model was used to examine the independence of the predictors of RA parameters and MetS. A probability (p) value of less than 0.05 (p < 0.05) was considered significant.
3. RESULTS
3.1. Clinical and demographic characteristics
The demographic, clinical, and laboratory characteristics of RA patients and control subjects (CS) are described in Table 1. RA patients had lower weight and size than CS (p < 0.05). Also, RA patients reported less food intake per day compared to controls. No significant differences were observed between the two groups for age, sex, waist circumference, BMI, systolic blood pressure, physical activity, and smoking index. Most of the patients (75%) were diagnosed with RA 15 years before their inclusion in the study (median 7.8 years), and 50% had a DAS‐28 score < 3.1, which indicates a low activity disease. 70% of patients were treated with at least one disease‐modifying antirheumatic drug and 70% with nonsteroidal anti‐inflammatory drugs. Another minority of individuals was being prescribed <5 mg/day of prednisone (27%).
Table 1.
Sociodemographic, anthropometric, and clinical parameters in CS and RA patients
| CS (n = 270) | RA (n = 216) | p‐value | |
|---|---|---|---|
| Demographics and anthropometric components | |||
| Sex (Female/Male) | (240/30) | (194/22) | 0.769 |
| Age (years)† | 44 (33–55) | 46 (37−55) | 0.074 |
| Weight (kg)† | 69 (62−81) | 65 (55−73) | 0.0001 |
| Height (cm)† | 1.61 (1.56−1.65) | 1.56 (1.52−1.62) | <0.0001 |
| Waist circumference (cm)† | 98 (88−105) | 96 (87−103) | 0.0877 |
| BMI (kg/m2) ‡ | 27 (23−30) | 26 (23−30) | 0.0703 |
| Systolic pressure (mmHg)† | 116 (105−125) | 120 (108−126) | 0.7302 |
| Diastolic pressure (mmHg)† | 80 (70−82) | 78 (80−82) | 0.4601 |
| Meals per day‡ | 3.53 ± 1.236 | 2.91 ± 0.70 | <0.0001 |
| Physical activity n (%) | |||
| Never | 100 (37) | 68 (32) | 0.343 |
| Occasionally | 59 (22) | 57 (26) | |
| Frequently | 111 (41) | 91 (42) | |
| Smoking index ‡ | 1.46 ± 4.6 | 1.8 ± 4.6 | 0.7580 |
| Characteristics of RA | |||
| DAS‐28 score† | 3.1 (2.1−4.8) | − | |
| Evolution of RA (years)† | − | 7.82 (2−15) | − |
| Rheumatoid factor (UI/ml)† | 137 (20‐712) | − | |
| Drug treatment n (%) | |||
| NSAIDs | − | 189 (70) | − |
| Prednisone | − | 58 (27) | − |
| DMARDs | |||
| Sulfasalazine | − | 73 (34) | − |
| Chloroquine | − | 60 (28) | − |
| Methotrexate | − | 189 (70) | − |
According to the normal distribution, results expressed as median and interquartile ranges, Mann‐Whitney test † or mean ± standard deviation, Student's t test‡. Bold letters indicate statistically significant results.
Abbreviations: CS, control subject; DAS‐28, disease activity score 28; DMARDs, disease‐modifying antirheumatic drugs; NSAIDs, nonsteroidal anti‐inflammatory drugs; RA, rheumatoid arthritis.
3.2. Biochemical and paraclinical parameters of CS and RA patients
Glucose, lipid profile, and the Castelli's risk index‐I (CRI‐I) and (CRI‐II) were determined in both study groups. Table 2 shows that the levels of glucose, HDL‐C, triglycerides, and VLDL‐C were similar (p > 0.05) between CS and RA patients. The total serum cholesterol and LDL‐C were higher in CS than in RA patients (p = 0.0045 and p = 0.0006, respectively); similarly, CRI‐I (CT/ HDL‐C: CS 4.27 [3.5–5] vs. RA 3.89 [3.2–4.5], p = 0.0002) and CRI‐II (LDL‐C/HDL‐C: CS 2.65 [2.1–3–3] vs. RA 2.3 [1.8–2.8], p < 0.0001) were higher in CS than in RA patients.
Table 2.
Biochemical and Paraclinical Parameters of Patients with RA and CS
|
CS (n = 270) Median (P25−P75) |
RA (n = 216) Median (P25−P75) |
p‐value | |
|---|---|---|---|
| Glucose (mg/dl) | 93 (88−101) | 95 (89−100) | 0.2539 |
| Triglycerides (mg/dl) | 109 (78−164) | 99 (78−135) | 0.1434 |
| Total cholesterol (mg/dl) | 183 (150−213) | 168 (143−196) | 0.0045 |
| HDL‐C (mg/dl) | 42 (35−48) | 43 (37−53) | 0.0681 |
| LDL‐C (mg/dl) | 116 (90−134) | 102 (82−121) | 0.0006 |
| VLDL‐C (mg/dl) | 22 (16−33) | 20 (16−27) | 0.2975 |
| CRI‐I | 4.27 (3.5−5) | 3.89 (3.2−4.5) | 0.0002 |
| CRI‐II | 2.65 (2.1−3.3) | 2.3 (1.8–2.8) | <0.0001 |
Data expressed as median and interquartile ranges (25th percentile [P25] and 75th percentile [P75], Mann‐Whitney test. Bold letters indicate statistically significant results.
Abbreviations: CRI‐I, Castelli's Risk Index I; CRI‐II, Castelli's Risk Index II; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; milligrams (mg), deciliters (dl); VLDL‐C, very‐low‐density lipoprotein cholesterol.
3.3. Frequency of MetS in CS and RA patients
According to NCEP/ATP‐III criteria, the overall frequency of MetS was 33.3% in CS and 30.6% in RA patients (p = 0.514, Table 3). This result did not suffer significant variations after adjusting for height and weight (p = 0.325). The waist circumference criterion was more frequent (p = 0.0150) in patients with RA (68.5%) than in CS (57.7%). Based on ALAD criteria, 31.1% CS and 28.7% RA patients had MetS (p = 0.5218).
Table 3.
Frequency of MetS according to who different criteria in CS and RA patients
| CS (n = 270) | RA (n = 216) | p‐value | |
|---|---|---|---|
| n (%) | n (%) | ||
| NCEP/ATP‐III criteria | |||
| Waist circumference > 102 cm in men and >88 cm in women | 156 (57.7) | 148 (68.5) | 0.0150 |
| Hypertriglyceridemia (TG ≥ 150 mg/dl) | 75 (27.7) | 42 (19.4) | 0.6605 |
| Hypertension (BP ≥ 130/85 mmHg) | 54 (20) | 41 (19) | 0.7784 |
| Hyperglycemia ≥ 110 mg/dl | 63 (23.3) | 52 (24) | 0.8485 |
| Low HDL‐C < 40 mg/dl in men and <50 mg/dl in women | 163 (60.3) | 136 (62.9) | 0.5594 |
| MetS | 90 (33.3) | 66 (30.6) | 0.5145 |
| ALAD criteria | |||
| Waist circumference > 94 cm in men and > 88 cm in women | 182 (67.4) | 149 (68.9) | 0.7114 |
| MetS | 84 (31.1) | 62 (28.7) | 0.5218 |
Results expressed as number (n) and percentage (%). Bold letters indicate statistically significant results.
Abbreviations: ALAD, Latin American Diabetes Association; BP, blood pressure; NCEP/ATP‐III, The National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III).
3.4. Relationship of MetS parameters with RA characteristics
As it was expected, Table 4 shows that all lipid and paraclinical components of MetS were higher in RA patients with MetS (p < 0.05) than those without MetS. However, the inflammatory parameters (CRP, ESR, RF) and the activity of the disease (DAS‐28 index) did not show significant differences between both groups (p > 0.05). Multivariate logistic regression analysis did not show any variable with statistical significance (data not shown).
Table 4.
Characteristics of RA patients based on the presence or the absence of MetS
| RA with metabolic syndrome (n = 60) | RA without metabolic syndrome (n = 156) | p‐value | |
|---|---|---|---|
| Age (years)‡ | 53.23 ± 11.23 | 42.56 ± 12.34 | <0.0001 |
| Sex (Female/Male) | 8/52 (13.3/76.7) | 14/142 (8.9/91.1) | 0.3420 |
| BMI (kg/m2)‡ | 28.54 ± 3.727 | 25.59 ± 5.229 | <0.0001 |
| Weight (kg)‡ | 73.51 ± 12.22 | 62.42 ± 13.20 | <0.0001 |
| Waist circumference (cm)† | 101 (98–107.5) | 90.25 (83.50–100) | <0.0001 |
| Systolic pressure (mmHg)† | 129.5 (120–138) | 114 (105–122) | <0.0001 |
| Diastolic pressure (mmHg)† | 83 (78–86) | 75 (70–80) | <0.0001 |
| Evolution of RA (years)‡ | 7.75 ± 7.90 | 7.89 ± 7.43 | 0.7274 |
| DAS‐28† | 3 (2.1–5.1) | 3.15 (2–4) | 0.6890 |
| Swollen joints† | 1 (0–4) | 0 (0–1) | 0.0183 |
| Painful joints† | 3 (0–6) | 2 (0–6) | 0.6114 |
| ESR (mm/h)† | 25 (16–34) | 25 (18–35) | 0.8206 |
| CRP (mg/dl)† | 6.05 (3.7–9.3) | 5.950 (2.7–12.3) | 0.6599 |
| RF (UI/ml)† | 107.2 (40.4–147.4) | 86.2 (20.2–145.3) | 0.3157 |
| Glucose (mg/dl)‡ | 104.4 ± 13.6 | 92.97 ± 7.613 | <0.0001 |
| Total Cholesterol (mg/dl)† | 211 (189.5–271) | 161 (140–188) | <0.0001 |
| Triglycerides (mg/dl)† | 147 (99–174) | 94 (76–124) | <0.0001 |
| HDL‐C (mg/dl)† | 39 (34–48) | 45 (39–55) | 0.0010 |
| LDL‐C (mg/dl)† | 119 (97–130) | 92 (98–117) | <0.0001 |
| VLDL‐C (mg/dl)† | 29 (20–38) | 19 (15–25) | <0.0001 |
According to the normal distribution, results expressed as median and interquartile ranges, Mann‐Whitney test† or mean ± standard deviation, Student's t test‡. Bold letters indicate statistically significant results.
Abbreviations: BMI, Body mass index; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; milligrams (mg), deciliters (dl); VLDL‐C, very‐low‐density lipoprotein cholesterol.
A Spearman correlation analysis was performed between the activity variables of RA (RF, CRP, ESR, and disease activity) and the parameters of MetS. Table 5 shows positive correlation of DAS‐28 score with the smoking index (rho = 0.4601, p = 0.0004) and VLDL‐C (rho = 0.3108, p = 0.0056) levels; similarly, RF correlated with age (rho = 0.2031, p = 0.0027), smoking index (rho = 0.3404, p < 0.0001), triglycerides (rho = 0.1958, p = 0.0039), and VLDL‐C (rho = 0.1761, p = 0.0162). Monotherapy versus multi‐therapy in the treatment of RA patients showed no association with any biochemical variables (p > 0.05, data not shown).
Table 5.
Correlation between RF, CRP, ESR, and DAS‐28 with anthropometric and biochemical components of MetS in RA patients with MS
| DAS‐28‐score | ESR (mm/hr) | RF | CRP (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| rho | p‐value | rho | p‐value | rho | p‐value | rho | p‐value | |
| Age | 0.092 | 0.370 | 0.013 | 0.842 | 0.203 | 0.002 | −0.044 | 0.512 |
| Smoking index | 0.460 | <0.0001 | 0.107 | 0.163 | 0.340 | <0.0001 | 0.084 | 0.278 |
| Weight | −0.167 | 0.102 | −0.111 | 0.101 | −0.028 | 0.675 | 0.176 | 0.009 |
| Waist circumference | −0.162 | 0.113 | 0.044 | 0.520 | 0.040 | 0.551 | 0.198 | 0.003 |
| BMI | −0.162 | 0.113 | −0.108 | 0.112 | −0.030 | 0.654 | 0.173 | 0.010 |
| Triglycerides | 0.179 | 0.080 | 0.114 | 0.093 | 0.195 | 0.003 | 0.088 | 0.193 |
| Cholesterol | −0.028 | 0.781 | 0.121 | 0.073 | 0.028 | 0.680 | −0.046 | 0.497 |
| HDL‐C | −0.154 | 0.131 | 0.004 | 0.945 | 0.047 | 0.487 | −0.002 | 0.973 |
| Glucose | 0.069 | 0.501 | −0.104 | 0.126 | 0.034 | 0.618 | 0.049 | 0.472 |
| Systolic pressure | 0.024 | 0.811 | −0.140 | 0.309 | 0.020 | 0.770 | 0.067 | 0.322 |
| Diastolic pressure | −0.056 | 0.585 | −0.067 | 0.323 | 0.020 | 0.770 | 0.128 | 0.060 |
| LDL‐C | −0.113 | 0.270 | 0.124 | 0.067 | −0.050 | 0.464 | −0.050 | 0.461 |
| VLDL‐C | 0.310 | 0.005 | 0.103 | 0.158 | 0.176 | 0.016 | 0.046 | 0.527 |
| CRI‐I | 0.088 | 0.393 | 0.129 | 0.057 | 0.0001 | 0.999 | −0.029 | 0.668 |
| CRI‐II | −0.060 | 0.560 | 0.112 | 0.098 | −0.065 | 0.338 | −0.059 | 0.386 |
Data analyzed by Spearman's rank‐order correlation (rho). Bold letters indicate statistically significant results.
Abbreviations: BMI, Body mass index; CRI‐I, Castelli's Risk Index I; CRI‐II, Castelli's Risk Index II; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; milligrams (mg), deciliters (dl); VLDL‐C, very‐low‐density lipoprotein cholesterol.
4. DISCUSSION
Ethnical, nutritional, and sociodemographic status are some factors determining the prevalence of MetS. 10 In RA, insulin resistance may be produced secondary to the inflammatory component of the disease, and it may also increase the risk of MetS and cardiovascular complications. 26 Thus, understanding the pathophysiology underlying this syndrome may help us explain these risk factors and their prevalence in this type of patient. 27
Research about MetS prevalence in RA patients has shown a wide range of estimates globally; therefore, more research is needed to understand the interrelationship between MetS and RA in different geographic regions. A recent meta‐analysis evaluated the prevalence and risk of MetS in RA patients, and it showed a general prevalence of MetS of 30.65%, but the results varied from 14.32% to 37.83%, based upon the diagnostic criteria used. The overall pooled odds ratio of MetS in RA patients was of 1.44 compared to controls, but this ranged from 0.70 to 4.09, depending on the criteria used and ethnicity. 10
In healthy Mexicans, there has been reported a high prevalence of MetS among adults, in comparison with reports from other countries, including the United States and Latin America; the estimated Prevalence of MetS according to the ATP III and WHO criteria is 36% and 31%, respectively, 28 similar to that observed in this study (ATP‐III criteria = 33.3%, and ALAD criteria = 31.1%). There are also a few studies regarding MetS in RA patients in the Mexican population from northern, southern, and central, 12 , 13 , 14 but not from western Mexico.
Although many studies have reported a higher prevalence of MetS among RA patients than controls, 8 , 10 others performed in central Mexico, Argentina, and Iran have reported otherwise, 12 , 19 , 29 being the reason of why the results are controversial. In the present work, no differences were observed in the MetS prevalence among RA and CS (p > 0.05), but there was a tendency of the low prevalence of MetS in RA patients in comparison with CS according to NCEP/ATP‐III and ALAD criteria, similar to that reported in the population of central Mexico (RA = 26.3% vs CS = 30%, p < 0.01) (RA = 26.3% vs CS = 30%, p < 0.01). 12 In Iranian 30 and Greek populations, 31 similar to us, these differences have not been observed either. In the Iran Population, the only marked difference observed was the significantly longer duration of the disease in RA patients with MetS, compared to those without MetS. 30
Discrepancies regarding the higher or lower frequency of MetS in RA in comparison with CS could be due to age, sex, ancestry, treatment, and geographic residency. In this study, no differences in the presence of these variables were identified between both study groups. Nevertheless, a potential weakness of this study could be the differences observed between weight, size, and meals per day between groups (p < 0.001), being RA patients lighter and less tall than controls, but not BMI changes were observed. RA patients also reported a lower number of meals per day, even though multivariate analysis models did not show any changes according to these variables (data not shown).
Considering the previous findings among the Mexican population, 12 , 13 , 14 we suggest that ethnicity could be a significant component of the differences between MetS prevalence in RA patients from Mexico and other countries. However, as nutritional habits are major risk factors for MetS, a more detailed and validated survey evaluation of food consumption is also further required to clear this hypothesis, including the amount of food, energy, and nutrients consumed, or patterns of food consumption and physical activity.
The differences regarding weight (p = 0.0001) and the number of meals eaten per day (p < 0.0001) between RA and CS could be due to a "rheumatoid cachexia" status in RA patients, which includes a loss of cell mass (skeletal muscle) and the increase in fat mass, resulting in an apparently stable body composition throughout life. 32 It has been studied that the development of depressive factors due to RA pathology's disabling effects 33 could be one of the likely causes answers to minor food intake throughout the day, and also to the low weight among RA patients.
An interesting finding involves the low total cholesterol and LDL‐C levels and Castelli's I‐II atherogenic indices in RA patients compared to CS (p < 0.001), which indicates lower cardiovascular risk in these patients. In a previous study performed in the Mexican population, the average of both indices in patients with RA was 4.36 and 2.59, similar to the present study; authors attribute this finding to the treatment with hydroxychloroquine, since patients treated with this drug had a lower frequency of atherogenic dyslipidemia. 34 Also, methotrexate seems to exert other cardioprotective properties on lipids and endothelium in RA patients, as serum of patients treated with methotrexate showed an increased cholesterol efflux capacity mediated by ABCG‐1 and scavenger receptor class B type I. In vitro, methotrexate inhibits foam cell formation by promoting reverse cholesterol transport through activation of adenosine A2 receptor, and it increases in cholesterol 27‐hydroxylase and ABCA‐1. 35 , 36 In our study, only 70% of patients were taking pharmacological therapy combined, including methotrexate and chloroquine, so we were unable to identify the effect of drugs on lipid profiles; therefore, it is pertinent that future studies may contemplate this variable to clarify the relationship that is projected to lower atherogenic risk in patients with RA.
Controversially, there are also reports showing decreases in total cholesterol and LDL‐C prior and during RA development, but even so, the risk of CVD is latent in these patients 37 ; this paradoxical effect is not well understood yet, and its interactions with inflammation and CVD in RA are known to be complex. 38
It is not clear whether the components of MetS precede the appearance of inflammatory diseases or if they are a consequence of its complications. Chung et al (2008) reported that the frequency of MetS is higher in patients with chronic RA (42%) than those with early RA (30%). 39 In this study, the RA evolution time was not concluded to be a determining factor for MetS, as RA patients with MetS had a similar time of evolution than those without it. However, we must consider that RA patients with MetS were older than those without MetS (years, 53.23 vs 42.56, respectively, p < 0.0001), which is a well‐understood risk factor for MetS. 40
The inflammatory markers or disease activity of RA did not show any significant differences according to the presence or absence of MetS, except for the number of swollen joints, as these were more prevalent in the patients with MetS (Median [IQR] = RA with MetS 1 (0–4) vs RA without MetS 0 (0–1), p = 0.018). This association could be due to the overweight in the MetS groups since it has been reported a positive correlation between BMI and the number of swelling joints in RA patients. 41 Based on these findings, we suggest that MetS is not a subsequent RA pathophysiology event, but it could be a promoter of some of components that influence the disease activity or disability.
On the other hand, there was a positive correlation between disease activity (DAS‐28 score) in RA patients with MetS with smoking index and VLDL‐C levels. This finding could be explained by the effect of tobacco smoking on tissue protein citrullination, and also detonate of autoantibodies synthesis, which show to induce the disease progression and disease activity in patients with rheumatoid arthritis. 42 , 43 , 44 On the other hand, the association of DAS‐28 and VLDL‐C levels is not clear yet, but it has been reported that RA patients with high disease activity had alterations of the lipid profile. 38 , 45
Rheumatoid factor (RF) correlated with age and smoking index, which agrees with other reports 46 , 47 , 48 ; likewise, RF correlated with triglycerides, which could be explained by this autoantibody´s positive correlation with a high RA disease activity, 49 a status associated with lipids alteration.
Regarding the correlation of CRP with some MetS components such as weight, waist circumference, and BMI, it could be explained by the fact that because these factors are the primary determinant of chronic inflammation in subjects with the MetS and are strongly related with proinflammatory cytokines such as IL‐6, which induces the synthesis of CRP. 50
Some limitations of this study are that most patients were being treated on a combined drug therapy, since some drugs like chloroquine could alter metabolism, lipid profile, and inflammation markers in RA. Also, patients with a low disease activity (DAS‐28 ranges of 2.6–3.9) were overrepresented because most of the patients (75%) had DAS‐28 < 3.9.
In conclusion, the present study's findings suggest that there is no sufficient evidence of a higher prevalence of metabolic syndrome in RA patients from western Mexico compared with controls. RA patients' age and female sex were associated with MetS, just as reported in the general population, implicating that MetS could be an isolated event of RA course in our population. However, in RA patients with MetS, CRP, RF levels, and disease activity score (DAS‐28) are associated with MetS components, so in this clinical context, the control of MetS in RA could effectively control disease activity outcomes. Further research around the topic might explore the basis of the lower lipid profiles and Castelli's indexes in RA patients. Also, the control study should have a better handle of the pharmacologic effects on RA patient´s therapy to establish the pathophysiology of lipid abnormalities in RA.
CONFLICT OF INTEREST
There is no conflict of interest in this work.
ACKNOWLEDGMENTS
The authors thank all the contributors of this work.
García‐Chagollán M, Hernández‐Martínez SE, Rojas‐Romero AE, et al. Metabolic syndrome in rheumatoid arthritis patients: Relationship among its clinical components. J Clin Lab Anal.2021;35:e23666. 10.1002/jcla.23666
Mariel García‐Chagollán and Susana Elizabeth Hernández‐Martínez authors contributed equally to this work.
Funding informationThis work was supported by funding from the PRODEP‐SEP Mexico under Grant (number UDG‐PTC‐1433) assigned to HBJ.
DATA AVAILABILITY STATEMENT
All the data related to this work are available at the corresponding author. The data used to support the findings of this study are included in the article.
REFERENCES
- 1. Dessein PH, Joffe BI, Veller MG, et al. Traditional and nontraditional cardiovascular risk factors are associated with atherosclerosis in rheumatoid arthritis. J Rheumatol. 2005;32(3):435‐442. [PubMed] [Google Scholar]
- 2. Nurmohamed MT. Cardiovascular risk in rheumatoid arthritis. Autoimmun Rev. 2009;8(8):663‐667. [DOI] [PubMed] [Google Scholar]
- 3. Ferraz‐Amaro I, González‐Juanatey C, López‐Mejias R, Riancho‐Zarrabeitia L, González‐Gay MA. Metabolic syndrome in rheumatoid arthritis. Mediators Inflamm. 2013;2013:710928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García‐Chagollán M, Ledezma‐Lozano IY, Hernández‐Bello J, Sánchez‐Hernández PE, Gutiérrez‐Ureña SR, Muñoz‐Valle JF. Expression patterns of CD28 and CTLA‐4 in early, chronic, and untreated rheumatoid arthritis. J Clin Lab Anal. 2020;34(5):e23188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108(13):1546‐1551. [DOI] [PubMed] [Google Scholar]
- 6. Kerekes G, Nurmohamed MT, González‐Gay MA, et al. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol. 2014;10(11):691‐696. [DOI] [PubMed] [Google Scholar]
- 7. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415‐1428. [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Fu L, Shi J, et al. The risk of metabolic syndrome in patients with rheumatoid arthritis: a meta‐analysis of observational studies. PLoS One. 2013;8(10):e78151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. da Cunha VR, Brenol CV, Brenol JCT, et al. Metabolic syndrome prevalence is increased in rheumatoid arthritis patients and is associated with disease activity. Scand J Rheumatol. 2012;41(3):186‐191. [DOI] [PubMed] [Google Scholar]
- 10. Hallajzadeh J, Safiri S, Mansournia MA, et al. Metabolic syndrome and its components among rheumatoid arthritis patients: A comprehensive updated systematic review and meta‐analysis. PLoS One. 2017;12(3):e0170361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee S‐H, Choi H, Cho B‐L, et al. Relationship between Metabolic Syndrome and Rheumatoid Arthritis. Korean J Fam Med. 2016;37(1):44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parra‐Salcedo F, Contreras‐Yáñez I, Elías‐López D, Aguilar‐Salinas CA, Pascual‐Ramos V. Prevalence, incidence and characteristics of the metabolic syndrome (MetS) in a cohort of Mexican Mestizo early rheumatoid arthritis patients treated with conventional disease modifying anti‐rheumatic drugs: the complex relationship between MetS and disease activity. Arthritis Res Ther. 2015;17(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zaragoza‐García O, Navarro‐Zarza JE, Maldonado‐Anicacio JY, Castro‐Alarcón N, Rojas IP, Guzmán‐Guzmán IP. Hypertriglyceridaemic waist is associated with hyperuricaemia and metabolic syndrome in rheumatoid arthritis patients. Diabetes Metab Syndr. 2019;13(1):722‐729. [DOI] [PubMed] [Google Scholar]
- 14. Zonana‐Nacach A, Santana‐Sahagún E, Jiménez‐Balderas FJ, Camargo‐Coronel A. Prevalence and factors associated with metabolic syndrome in patients with rheumatoid arthritis and systemic lupus erythematosus. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2008;14(2):74‐77. [DOI] [PubMed] [Google Scholar]
- 15. Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res. 2012;64(10):1471‐1479. [DOI] [PubMed] [Google Scholar]
- 16. van der Helm‐van Mil AHM, van der Kooij SM, Allaart CF, Toes REM, Huizinga TWJ. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67(6):769‐774. [DOI] [PubMed] [Google Scholar]
- 17. Jin Z, Cai G, Zhang P, et al. The value of the neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio as complementary diagnostic tools in the diagnosis of rheumatoid arthritis: a multicenter retrospective study. J Clin Lab Anal. 2020;e23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toms TE, Panoulas VF, John H, Douglas KMJ, Kitas GD. Methotrexate therapy associates with reduced prevalence of the metabolic syndrome in rheumatoid arthritis patients over the age of 60‐ more than just an anti‐inflammatory effect? A cross sectional study. Arthritis Res Ther. 2009;11(4):R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sahebari M, Goshayeshi L, Mirfeizi Z, et al. Investigation of the association between metabolic syndrome and disease activity in rheumatoid arthritis. ScientificWorldJournal. 2011;11:1195‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karakoc M, Batmaz I, Sariyildiz MA, et al. The relationship of metabolic syndrome with disease activity and the functional status in patients with rheumatoid arthritis. J Clin Med Res. 2012;4(4):279‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sullivan KM, Dean A, Soe MM. OpenEpi: a web‐based epidemiologic and statistical calculator for public health. Public Health Rep. 2009;124(3):471‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315‐324. [DOI] [PubMed] [Google Scholar]
- 23. Prevoo MLL, Hof MAV, Kuper HH, Leeuwen MAV, Putte LBAVD, Riel PLCMV. Modified disease activity scores that include twenty‐eight‐joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44‐48. [DOI] [PubMed] [Google Scholar]
- 24. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735‐2752. [DOI] [PubMed] [Google Scholar]
- 25. Robles L, Carlos J. Síndrome metabólico: concepto y aplicación práctica. An Fac Med. 2013;74(4):315‐320. [Google Scholar]
- 26. Medina G, Vera‐Lastra O, Peralta‐Amaro AL, et al. Metabolic syndrome, autoimmunity and rheumatic diseases. Pharmacol Res. 2018;133:277‐288. [DOI] [PubMed] [Google Scholar]
- 27. Razzouk L, Muntner P. Ethnic, gender, and age‐related differences in patients with the metabolic syndrome. Curr Hypertens Rep. 2009;11(2):127‐132. [DOI] [PubMed] [Google Scholar]
- 28. Gutiérrez‐Solis AL, Datta Banik S, Méndez‐González RM. Prevalence of metabolic syndrome in Mexico: a systematic review and meta‐analysis. Metab Syndr Relat Disord. 2018;16(8):395‐405. [DOI] [PubMed] [Google Scholar]
- 29. Salinas MJH, Bertoli AM, Lema L, et al. Prevalence and correlates of metabolic syndrome in patients with rheumatoid arthritis in Argentina. J Clin Rheumatol. 2013;19(8):439‐443. [DOI] [PubMed] [Google Scholar]
- 30. Karimi M, Mazloomzadeh S, Kafan S, Amirmoghadami H. The frequency of metabolic syndrome in women with rheumatoid arthritis and in controls. Int J Rheum Dis. 2011;14(3):248‐254. [DOI] [PubMed] [Google Scholar]
- 31. Karvounaris SA, Sidiropoulos PI, Papadakis JA, et al. Metabolic syndrome is common among middle‐to‐older aged Mediterranean patients with rheumatoid arthritis and correlates with disease activity: a retrospective, cross‐sectional, controlled, study. Ann Rheum Dis. 2007;66(1):28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85(1):89‐99. [DOI] [PubMed] [Google Scholar]
- 33. Margaretten M, Julian L, Katz P, Yelin E. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int J Clin Rheumatol. 2011;6(6):617‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Batún Garrido JA, Olán F, Hernández Núñez É. Dyslipidemia and atherogenic risk in patients with rheumatoid arthritis. Clin Investig Arterioscler. 2016;28(3):123‐131. [DOI] [PubMed] [Google Scholar]
- 35. England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018;361:k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reiss AB, Carsons SE, Anwar K, et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP‐1 monocyte/macrophages. Arthritis Rheum. 2008;58(12):3675‐3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amezaga Urruela M, Suarez‐Almazor ME. Lipid paradox in rheumatoid arthritis: changes with rheumatoid arthritis therapies. Curr Rheumatol Rep. 2012;14(5):428‐437. [DOI] [PubMed] [Google Scholar]
- 38. Erum U, Ahsan T, Khowaja D. Lipid abnormalities in patients with Rheumatoid Arthritis. Pak J Med Sci. 2017;33(1):227‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chung CP, Oeser A, Solus JF, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196(2):756‐763. [DOI] [PubMed] [Google Scholar]
- 40. Jiang B, Zheng Y, Chen Y, et al. Age and gender‐specific distribution of metabolic syndrome components in East China: role of hypertriglyceridemia in the SPECT‐China study. Lipids Health Dis. 2018;17(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ranganath VK, Duffy EL, Garg VK, et al. Obesity impacts swelling of ankle and foot joints in early rheumatoid arthritis patients. J Clin Rheumatol. 2019;25(3):e8‐e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Papadopoulos NG, Alamanos Y, Voulgari PV, Epagelis EK, Tsifetaki N, Drosos AA. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients? Clin Exp Rheumatol. 2005;23(6):861‐866. [PubMed] [Google Scholar]
- 43. Alsalahy MM, Nasser HS, Hashem MM, Elsayed SM. Effect of tobacco smoking on tissue protein citrullination and disease progression in patients with rheumatoid arthritis. Saudi Pharm J. 2010;18(2):75‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Melayah S, Changuel M, Mankaï A, Ghedira I. IgA is the predominant isotype of anti‐β2 glycoprotein I antibodies in rheumatoid arthritis. J Clin Lab Anal. 2020;34(6):e23217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cacciapaglia F, Anelli MG, Rinaldi A, et al. Lipids and atherogenic indices fluctuation in rheumatoid arthritis patients on long‐term tocilizumab treatment. Mediators Inflamm. 2018;2018:e2453265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dequeker J, Van Noyen R, Vandepitte J. Age‐related rheumatoid factors. Incidence and characteristics. Ann Rheum Dis. 1969;28(4):431‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heliövaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J Rheumatol. 1993;20(11):1830‐1835. [PubMed] [Google Scholar]
- 48. Beduleva L, Sidorov A, Semenova K, et al. Comparison of the specificity of rheumatoid factor detected by latex fixation with that of regulatory rheumatoid factor. J Clin Lab Anal. 2020;e23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aletaha D, Alasti F, Smolen JS. Rheumatoid factor, not antibodies against citrullinated proteins, is associated with baseline disease activity in rheumatoid arthritis clinical trials. Arthritis Res Ther. 2015;17(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Devaraj S, Singh U, Jialal I. Human C‐reactive protein and the metabolic syndrome. Curr Opin Lipidol. 2009;20(3):182‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data related to this work are available at the corresponding author. The data used to support the findings of this study are included in the article.


