Abstract
Background
Objective measures used for the differential diagnosis and severity assessment of allergic rhinitis (AR) are still lacking. The involvement of hydrogen sulfide (H2S) in the development of AR indicates that nasal exhaled H2S (NeH2S) has potential as a biomarker to be used in AR patients. This study aimed to evaluate the application value of NeH2S measurement in the diagnosis and assessment of AR.
Methods
This study was a multi‐center cross‐sectional survey conducted in Northwestern China. Demographic information collection and rhinitis assessment were completed through questionnaires. The level of NeH2S and serum immunoglobulin E were measured.
Results
The level of NeH2S in general population ranged from 0 to 35 ppb, with a median value of 2 ppb. The NeH2S levels in seasonal allergic rhinitis (SAR) patients were significantly lower than those in general population (2 [1, 2.75] vs. 2 [2, 3] ppb; p = .023), and the NeH2S value of the SAR group tended to be lower than that of the non‐allergic rhinitis (NAR) group (2 [1, 2.75] vs. 2 [2, 3] ppb; p = .094). The subgroup of AR patients with symptoms lasting longer than 2 weeks per month had a lower NeH2S level compared with the subgroup of patients with symptoms lasting less than 2 weeks per month (2 [1, 2] vs. 2 [2, 3] ppb; p = .015).
Conclusion
This study described the distribution range of NeH2S levels in the general population. Further study with larger sample size was needed to clarify the relationship between NeH2S level and AR.
Keywords: allergic rhinitis, biomarker, diagnosis, gas signal messenger, nasal exhaled hydrogen sulfide, persistence, severity
This multi‐center cross‐sectional study surveyed the residents in Northwestern China and described for the first time the distribution range of NeH2S levels in the general population. Our results showed that NeH2S level of seasonal allergic rhinitis (SAR) patients was significantly lower than that of general population, and the NeH2S level of the SAR group tended to be lower than that of the non‐allergic rhinitis (NAR) group.

1. INTRODUCTION
Allergic rhinitis (AR) is a common allergic airway disease, which has become a global health problem. A retrospective research study conducted in the United States found that the prevalence of AR was as high as 19.9%. 1 In China, 47,216 telephone interviews were conducted and the standardized prevalence of adult AR in 18 major cities was 9.8%–23% in 2011. 2 In the grasslands of northern China, a prevalence as high as 32.4% was reported for epidemiologic AR, with the most common causative allergen being weed pollens. 3
The diagnosis of AR is based on coordination between a typical history of allergic symptoms and the detection of allergen‐specific immunoglobulin E (sIgE) by diagnostic tests. 4 The differential diagnosis between AR and non‐allergic rhinitis (NAR) also depends mainly on allergen sIgE tests. 5 In China, a large proportion of hospitals are not equipped to perform skin tests or even serum sIgE measurements. Under these conditions, an accurate differential diagnosis is challenging because AR and NAR often have similar symptoms; therefore, developing new objective methods to assist in the diagnosis of AR is necessary.
Presently, objective measures for the assessment of AR severity are still lacking. The extent of nasal obstruction and olfactory impairment can be measured objectively, but the evaluation of other typical symptoms, including itchy nose/eyes, rhinorrhea, and sneezing, can be assessed only through questionnaires and visual analogue scales. In recent years, research on the application of gas signal messengers, like nitric oxide (NO) and carbon monoxide (CO), in allergy medicine is rapidly gaining more attention because they are easy and fast to measure and they have a close relationship with disease activity. 6 , 7 Hydrogen sulfide (H2S) is increasingly recognized as another important gasotransmitter. 8
Endogenous H2S has been proved to contribute to the pathophysiology of various airway diseases, including chronic obstructive pulmonary disease (COPD), asthma, and pulmonary fibrosis. 9 Also, Yu et al reported that plasma H2S concentrations in AR guinea pig models were significantly lower than those in control animals and that intraperitoneally administered NaHS, which increased the level of H2S in the guinea pigs, could alleviate AR symptoms, suggesting that the endogenous H2S pathway is downregulated in AR. 10 The involvement of H2S in the development of AR indicates that nasal exhaled H2S (NeH2S) has potential as a biomarker to be used in AR diagnosis and severity assessments.
To evaluate the application value of NeH2S measurement in AR, this study measured the levels of NeH2S in the general population and in AR and NAR patients and then analyzed the correlation between the NeH2S level and the severity of AR symptoms.
2. MATERIALS AND METHODS
2.1. Study design
This study was a sub‐project of a multi‐center cross‐sectional epidemiological survey conducted in Shenmu city of Shaanxi province from August 8 to December 7, 2019. The whole survey targeted 5000 subjects in three rural towns and two urban communities, and this study planned to measure NeH2S levels in 2000 persons of them. Shenmu city is located on the edge of MuUs Desert in Northwestern China and is rich in coal resources. The inclusion criteria of the survey were as follows: (a) aged 18–65 years old, (b) living in Shenmu for more than 1 year, and (c) lacking significant disability or psychiatric disorders. All subjects were investigated face to face through the use of an interviewer‐administered questionnaire that included questions about demographic information, rhinitis diagnosis, rhinitis symptom duration, disease impact on quality of life, smoking history, family history, and comorbidities (eg, physician‐diagnosed asthma, rhinosinusitis). The level of NeH2S was measured, and venous blood was collected for the measurement of serum IgE levels. Using the ImmunoCAP system, the Phadiatop sIgE test, which covers more than 90% of common aeroallergens, 11 was performed, and sIgE antibodies against three representative inhaled allergens (ie, w6 for Artemisia pollens, d2 for dust mites, and m6 for Alternaria) were also measured. Artemisia pollen was included because it is the most common pollen allergen in Northwestern China. 3 The ethics approval of the Peking Union Medical College Hospital Ethics Committee (ZS‐2198) and the ethics approval of the Shenmu Hospital Ethics Committee (sm004) had been obtained, and all the subjects had given written informed consents.
2.2. AR and NAR classification criteria
The classification criteria used in this study were based on the description in the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines. 4 Individuals classified as AR patients had typical rhinitis symptoms and positive detection of serum sIgE against inhaled allergens (sIgE ≥ 0.35 KUA/L), and their medical history was concordant with the results of the allergen tests. Individuals classified as NAR patients had typical rhinitis symptoms but negative serum sIgE results for the common inhaled allergens tested.
Subjects with AR were further subdivided according to their time of exposure into seasonal AR (SAR) and perennial AR (PAR) groups. PAR is usually caused by dust mites, mold, or animal dander, whereas SAR is often induced by pollens, especially weed pollens with pollen season from August to September, in Northwestern China.
2.3. Measurement of nasal exhaled hydrogen sulfide
Measurement of NeH2S was performed using a nanocoulomb breathalyzer (Sunvou Medical Electronics, Jiangsu, China) in accordance with exhaled nitric oxide measurements as suggested by the American Thoracic Society/European Respiratory Society. 12 Food and intense exercise were withheld for 2 h before the examination to reduce disturbance. For NeH2S measurement, a nasal olive was placed firmly against one nostril and the other nostril was open. After a deep inhalation, air was expired through the olive at the flow rate of 10 ml/s into the analyzer for 10 s. During expiration, patients were instructed to exhale against a resistance of 10 cmH2O to obtain velum closure. After analysis, the instrument then displayed the NeH2S level measurement in the unit parts per billion (ppb). Breath analyzer was calibrated before research started using two calibration gases with H2S concentration of 60 ppb and 250 ppb. For the repeatability test, the standard gas with a concentration of 60 ppb was measured for 10 times, and the coefficient of variation was within 10%.
2.4. Statistical analyses
Statistical analyses were performed using SPSS software (ver. 23; SPSS Inc, Chicago, IL, USA). The normality of indexes was examined using a Kolmogorov‐Smirnov test. As the distribution of NeH2S level was proven to be abnormal, correlations between the NeH2S concentration and demographic parameters or rhinitis severity scores as evaluated by the questionnaire were determined via a Spearman's rank correlation, and the comparison of NeH2S levels between different subgroups was accomplished with a Mann‐Whitney U test. A p value of <0.05 was considered to indicate statistical significance.
3. RESULTS
3.1. The NeH2S level distribution in the general population
A total of 1886 persons underwent the test for NeH2S measurement, and all subjects were of Han nationality. Although the data came from an epidemiological survey, this article does not report the results of the incidences or risk factors of allergic disorders in Shenmu city, which will be discussed in other forthcoming papers, but instead is focused mainly on analyzing the NeH2S levels in the general population and in rhinitis patients, especially AR patients.
In the 1886 subjects completing NeH2S test, 1600 people did not suffer from either rhinitis or asthma, and they were considered to represent general population in this article. This group was composed of 908 women and 692 men, with an average age of 48 ± 10 years old. The level of NeH2S in this population ranged from 0 to 35 ppb, with a median value of 2 ppb. The distribution of NeH2S levels in this population was shown in Figure 1. The level of NeH2S was not affected by age, sex, or smoking history.
Figure 1.
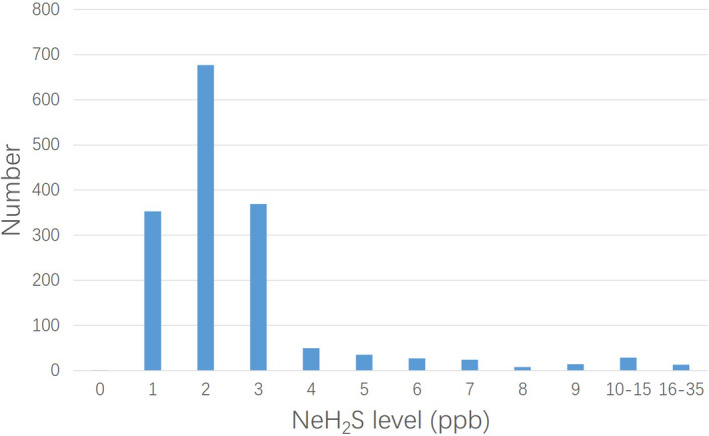
The distribution of NeH2S levels in the general population. NeH2S, nasal exhaled hydrogen sulfide
3.2. Comparison of NeH2S levels among different types of rhinitis patients and general population
In the 1,886 people who underwent NeH2S test, there were 85 AR patients (68 SAR and 17 PAR) and 201 NAR patients. As shown in Table 1, there were no significant differences in the male:female ratios or the percentages of smokers among the four groups (SAR, PAR, NAR, and general population). However, the age distribution was different among the four groups, with the SAR group having the youngest subjects and the general population group having the eldest. Notably, as mentioned above, the level of NeH2S was not correlated with age, so this difference in subject age among groups should not interfere with the comparisons of NeH2S levels.
Table 1.
Demographic characteristics and NeH2S levels of different types of rhinitis patients and general population
| Parameters | SAR (n = 68) | PAR (n = 17) | NAR (n = 201) | general population (n = 1600) | p value |
|---|---|---|---|---|---|
| Age (years) | 42 ± 10 | 46 ± 14 | 46 ± 10 | 48 ± 10 | <0.001*** |
| Females (No., %) | 37, 54.4% | 10, 58.8% | 123, 61.2% | 908, 56.8% | 0.643 |
| Nonsmokers (n) | 44 | 12 | 133 | 923 | 0.106 |
| Former smokers (n) | 7 | 1 | 14 | 108 | |
| Current smokers (n) | 17 | 4 | 54 | 569 |
Abbreviations: NAR, non‐allergic rhinitis; NeH2S, nasal exhaled hydrogen sulfide; PAR, perennial allergic rhinitis; SAR, seasonal allergic rhinitis.
p value < 0.001.
As shown in Table 2 and Figure 2, the NeH2S levels in SAR patients were significantly lower than those in general population (2 [1, 2.75] vs. 2 [2, 3] ppb; p = .023), and the NeH2S value of the SAR group tended to be lower than that of the NAR group (2 [1, 2.75] vs. 2 [2, 3] ppb; p = .094). No significant difference in the NeH2S level was found between the PAR and general population groups or between the NAR and general population groups.
Table 2.
Comparison of the NeH2S level among different types of rhinitis patients and general population
| NeH2S level (ppb) | SAR (%, n = 68) | PAR (%, n = 17) | NAR (%, n = 201) | general population (%, n = 1600) |
|---|---|---|---|---|
| 1 | 29.4% | 5.9% | 22.4% | 22.1% |
| 2 | 45.6% | 64.7% | 45.8% | 42.3% |
| 3 | 23.5% | 23.5% | 18.9% | 23.1% |
| 4 | 0% | 5.9% | 4.5% | 3.1% |
| ≥5 | 1.5% | 0% | 8.5% | 9.4% |
| p value compared to general population | 0.023* | 0.791 | 0.506 |
Abbreviations: NAR, non‐allergic rhinitis; NeH2S, nasal exhaled hydrogen sulfide; PAR, perennial allergic rhinitis; SAR, seasonal allergic rhinitis.
p value < 0.05.
Figure 2.
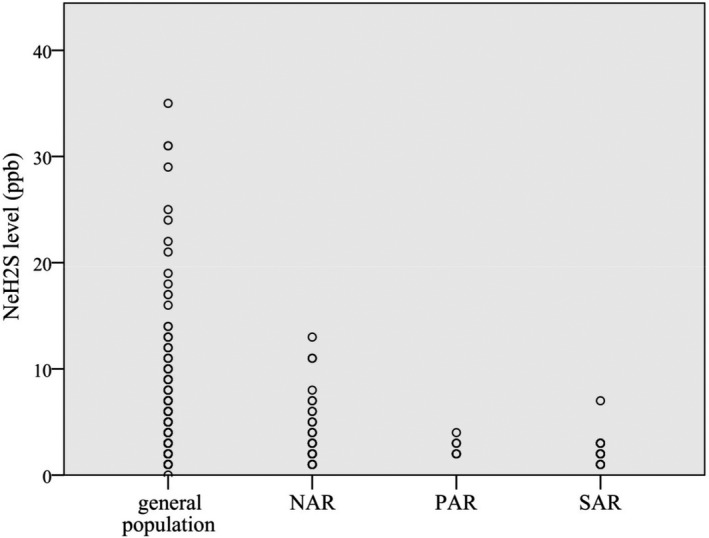
The distribution features of NeH2S levels in different types of rhinitis patients and general population. NAR, non‐allergic rhinitis; NeH2S, nasal exhaled hydrogen sulfide; SAR, seasonal allergic rhinitis; PAR, perennial allergic rhinitis
3.3. Relationship between the NeH2S level and AR persistence or severity
No significant correlation was found between the NeH2S level and the assessed parameters reflecting the persistence or severity of AR symptoms (Table 3). There was also no significant correlation found between the NeH2S level and total IgE or Phadiatop sIgE concentration. In the 94 AR patients included in this study, 21 had coexisting asthma. No significant difference was found in the NeH2S level between patients with asthma and those without asthma.
Table 3.
Correlations between the NeH2S level and the parameters reflecting the persistence or severity of AR symptoms
| Parameters | Correlation with NeH2S level | ||
|---|---|---|---|
| Correlation coefficient | p value | ||
| Course of AR (years) | 0.174 | 0.111 | |
| SAR or PAR | ~ | 0.135 | |
| Persistence of AR symptoms | Months with symptoms per year a | 0.144 | 0.189 |
| Weeks with symptoms per month b | −0.187 | 0.087 | |
| Days with symptoms per week c | −0.158 | 0.148 | |
| Severity of AR symptoms | Sleep disturbance d | −0.118 | 0.282 |
| Impairment of school or work d | −0.097 | 0.378 | |
| Impairment of leisure or sport d | −0.021 | 0.851 | |
| Impairment of other daily activities d | −0.013 | 0.903 | |
| Comorbidities | With or without asthma | ~ | 0.285 |
| With or without rhinosinusitis | ~ | 0.161 | |
| IgE level | Total IgE | −0.036 | 0.751 |
| Phadiatop sIgE | 0.008 | 0.943 | |
Abbreviations: AR, allergic rhinitis; NeH2S, nasal exhaled hydrogen sulfide; PAR, perennial allergic rhinitis; SAR, seasonal allergic rhinitis; sIgE, specific immunoglobulin E.
This parameter was divided into five levels to evaluate the correlation: 1‐less than one month, 2‐one to three months, 3‐four to six months, 4‐seven to nine months, 5‐more than ten months.
This parameter was divided into four levels to evaluate the correlation: 1‐less than one week, 2‐one to two weeks, 3‐three to four weeks, 4‐every day.
This parameter was divided into four levels to evaluate the correlation: 1‐less than one day, 2‐one to three days, 3‐four to six days, 4‐every day.
These parameters were divided into five levels to evaluate the correlation: 0‐none, 1‐mild, 2‐moderate, 3‐severe, 4‐very severe.
However, if dividing the AR patients based on whether their symptoms persisted for more than 2 weeks per month, the subgroup with symptoms lasting longer than 2 weeks (n = 51) had a significantly lower NeH2S level compared with the subgroup with a shorter symptom duration (n = 34), 2 [1, 2] vs. 2 [2, 3] ppb; p = .015 (Table 4).
Table 4.
Relationship between NeH2S level and persistency of AR symptoms
| NeH2S level (ppb) | AR persisting for more than 2 weeks per month (%, n = 51) | AR persisting for less than 2 weeks per month (%, n = 34) |
|---|---|---|
| 1 | 27.5% | 20.6% |
| 2 | 58.8% | 35.3% |
| 3 | 13.7% | 38.2% |
| 4 | 0% | 2.9% |
| ≥5 | 0% | 2.9% |
Abbreviations: AR, allergic rhinitis; NeH2S, nasal exhaled hydrogen sulfide.
4. DISCUSSION
The present study found that NeH2S level of SAR patients was significantly lower than that of general population, and the NeH2S level of the SAR group tended to be lower than that of the NAR group. No significant correlation was found between the NeH2S level and most of the assessed parameters that reflect the persistence or severity of AR symptoms. The only exception was that the subgroup of patients with symptoms lasting longer than 2 weeks per month had a lower NeH2S level compared with the subgroup of patients with symptoms lasting less than 2 weeks per month.
This study tested the NeH2S level in more than 1000 individuals who did not suffer from rhinitis or asthma, revealing the distribution characteristics of normal NeH2S levels in general population for the first time in China. These levels had a skewed distribution, with most results in the range of 1–4 ppb. A previous study reported that the H2S concentration in mouth‐exhaled air in healthy adults was about 8–16 ppb, 13 which is higher than the NeH2S level found in our study. The reason for this difference may stem from the tissue‐specific expressions of the enzymes responsible for the generation of endogenous H2S. 14 Our data analysis also indicated that the level of NeH2S does not differ with variations of age, sex, or smoking habit, which means NeH2S is a relatively stable gas messenger suitable for use as a signaling biomarker. According to a previous study, the serum H2S concentration was similar between the 50‐ to 60‐year‐old group and the 70‐ to 80‐year‐old group, 15 which is consistent with our finding.
Accumulating research data indicate that endogenous H2S has anti‐inflammatory and antioxidant effects 14 , 16 and that it has a close relationship with allergic airway disorders. The endogenous H2S level in pulmonary tissue was lower than normal in a rat model of asthma. 17 Similarly, Yu et al found that the plasma H2S concentrations were lower in the AR model animals compared with the control group animals, 10 and supplementation with NaHS could relieve AR symptoms. 18 Our study observed that the NeH2S levels of SAR patients were significantly lower than those of general population, which is concordant with the literature reports. However, this result was not enough to justify the role of NeH2S in the diagnosis of AR, because the sample size of AR patients was small in this study, with only 68 SAR patients and 17 PAR patients, and the sensitivity of the device was limited with the deviation of 1ppb. Notably, the NeH2S levels in PAR patients were not significantly different from those in control subjects. In order to further investigate the characteristics of NeH2S in different subtypes of AR patients and confirm the difference between AR patients and normal controls, we need to conduct research with larger sample size of AR patients and improved sensitivity of the test in the future.
We found here that the NeH2S level of SAR patients tended to be lower than that of NAR patients, although this difference was not statistically significant. This result suggests that an NeH2S measurement has the possibility to become an accessory examination, assisting in the differential diagnosis of AR, especially in cases where the similar symptoms of AR and NAR obscure their distinction and the medical institution assessing the patient is unable to perform the diagnostic test of detecting allergen sIgE. The difference in NeH2S levels between these two types of rhinitis is probably related to their different pathogeneses. AR is classically considered to result from an IgE‐mediated allergy associated with eosinophilic inflammation in the nose. 4 Pollen‐induced SAR is the most characteristic IgE‐mediated allergic disease, with eosinophils always being found in the mucosa and submucosa and in nasal secretions, 19 whereas neurogenic inflammation, including inflammation of the trigeminal nerve reflex, autonomous neurologic disorders, and abnormal neurologic responses, plays the major role in the development of NAR, 20 with the exception of NAR with eosinophilic syndrome (NARES), which also involves nasal eosinophilic inflammation. 21 It was reported that mouth‐exhaled H2S levels were negatively correlated with sputum eosinophil counts in asthma patients 22 ; this finding may explain our observation in the present study that SAR patients tended to have lower NeH2S levels compared with NAR patients. The existence of NARES in the NAR group might be the reason why the difference in NeH2S levels between the SAR and NAR groups was not statistically significant.
In a rat model of asthma, the H2S concentrations of serum and lung tissue were positively correlated with peak expiratory flow (PEF), and mouth‐exhaled H2S levels in asthma patients were positively correlated with the percent of predicted forced expiratory volume in 1 s and the Asthma Control Test (ACT) score. 22 These results from prior work indicate that endogenous H2S plays a critical role in airway inflammation and can be used to assess disease severity and activity. Based on these ideas, our study evaluated the correlations between NeH2S levels and various parameters that reflect the severity and persistence of AR symptoms. However, our analysis produced mostly negative results. Only the number of weeks with symptoms per month showed some relationship with the measured NeH2S levels, and AR patients who reported having symptoms for more than 2 weeks per month had lower NeH2S levels compared with patients who reported a shorter duration of symptoms. This trend is consistent with previous findings in asthma patients. However, the correlation in our study of the nasal H2S level with disease severity was not evident, possibly because the integral NeH2S levels of the AR patients were very low and the data dispersion was small.
Our study described for the first time the distribution range of NeH2S levels in the general population and provided reference data for future studies about NeH2S. We also observed that SAR patients had lower NeH2S levels compared with general population. However, due to the limitation of small sample size of AR patients and restricted sensitivity of the device, the results of this study is not sufficient to support the application of NeH2S in the diagnosis of AR. Further study with larger sample size is needed to clarify the relationship between NeH2S level and the diagnosis and severity assessment of AR.
CONFLICT OF INTEREST
The authors report no competing interests.
AUTHOR CONTRIBUTIONS
Kai Guan and Qiang Wang designed the project and guided the survey. Lisha Li, Yonglin Liu, Zixi Wang, Le Cui, and Yingyang Xu conducted the survey. Kai Guan and Qiang Wang contributed to the NeH2S measurement. Lisha Li and Yonglin Liu contributed to the serum immunoglobulin E tests. Zixi Wang, Le Cui, and Yingyang Xu contributed to data entry. Lisha Li and Yonglin Liu analyzed the data and wrote the manuscript. Kai Guan and Qiang Wang took part in data statistics and revised the manuscript.
ACKNOWLEDGEMENT
We thank the support from Municipal Fund of Shenmu (2019‐5) and Natural Sciences Foundation of Beijing (7172179).
Li L, Liu Y, Wang Q, et al. Levels of nasal exhaled hydrogen sulfide in the general population and allergic rhinitis patients. J Clin Lab Anal.2021;35:e23678. 10.1002/jcla.23678
Lisha Li, Yonglin Liu and Qiang Wang are the first authors.
Funding informationThis work was supported by grants from Municipal Fund of Shenmu (2019‐5) and Natural Sciences Foundation of Beijing (7172179).
DATA AVAILABILITY STATEMENT
The raw data required to produce these findings cannot be shared at this time as the data also forms part of an ongoing study.
REFERENCES
- 1. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider‐diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang XD, Zheng M, Lou HF, et al. An increased prevalence of self‐reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016;71(8):1170‐1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X‐Y, Ma T‐T, Wang X‐Y, et al. Prevalence of pollen‐induced allergic rhinitis with high pollen exposure in grasslands of northern China. Allergy. 2018;73(6):1232‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines‐2016 revision. J Allergy Clin Immunol. 2017;140(4):950‐958. [DOI] [PubMed] [Google Scholar]
- 5. Hellings PW, Klimek L, Cingi C, et al. Non‐allergic rhinitis: position paper of the European academy of allergy and clinical immunology. Allergy. 2017;72(11):1657‐1665. [DOI] [PubMed] [Google Scholar]
- 6. Liu C, Zheng K, Liu X, et al. Use of nasal nitric oxide in the diagnosis of allergic rhinitis and nonallergic rhinitis in patients with and without sinus inflammation. J Allergy Clin Immunol Pract. 2020;8(5):1574–1581. [DOI] [PubMed] [Google Scholar]
- 7. Pereira AA, Pollard SL, Locke R, et al. Association between exhaled carbon monoxide and asthma outcomes in Peruvian children. Respir Med. 2018;145:212‐216. [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Wang X, Chen Y, Yao W. Exhaled hydrogen sulfide predicts airway inflammation phenotype in COPD. Respir Care. 2015;60(2):251‐258. [DOI] [PubMed] [Google Scholar]
- 9. Bazhanov N, Ansar M, Ivanciuc T, Garofalo RP, Casola A. Hydrogen sulfide: a novel player in airway development, pathophysiology of respiratory diseases, and antiviral defenses. Am J Respir Cell Mol Biol. 2017;57(4):403‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yu S, Yan Z, Che N, Zhang X, Ge R. Impact of carbon monoxide/heme oxygenase on hydrogen sulfide/cystathionine‐gamma‐lyase pathway in the pathogenesis of allergic rhinitis in guinea pigs. Otolaryngol Head Neck Surg. 2015;152(3):470‐476. [DOI] [PubMed] [Google Scholar]
- 11. Zeng G, Hu H, Zheng P, et al. The practical benefit of Phadiatop test as the first‐line in vitro allergen‐specific immunoglobulin E (sIgE) screening of aeroallergens among Chinese asthmatics: a validation study. Ann Transl Med. 2018;6(8):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Thoracic S, European RS. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912‐930. [DOI] [PubMed] [Google Scholar]
- 13. Sun Y, Wang XM, Chen YH, Zhu RX, Liao CC. Exhaled hydrogen sulfide in patients with chronic obstructive pulmonary disease and its correlation with exhaled nitric oxide. Chin Med J (Engl). 2013;126(17):3240‐3244. [PubMed] [Google Scholar]
- 14. Xiao Q, Ying J, Xiang L, Zhang C. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine (Baltimore). 2018;97(44):e13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y‐H, Yao W‐Z, Geng B, et al. Endogenous hydrogen sulfide in patients with COPD. Chest. 2005;128(5):3205‐3211. [DOI] [PubMed] [Google Scholar]
- 16. Liu Z, Wang X, Li L, Wei G, Zhao M. Hydrogen sulfide protects against paraquat‐induced acute liver injury in rats by regulating oxidative stress, mitochondrial function, and inflammation. Oxid Med Cell Longev. 2020;2020:6325378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y‐H, Wu R, Geng B, et al. Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine. 2009;45(2):117‐123. [DOI] [PubMed] [Google Scholar]
- 18. Shaoqing Y, Ruxin Z, Yinjian C, Jianqiu C, Zhiqiang Y, Genhong L. Down‐regulation of endogenous hydrogen sulphide pathway in nasal mucosa of allergic rhinitis in guinea pigs. Allergol Immunopathol (Madr). 2009;37(4):180‐187. [DOI] [PubMed] [Google Scholar]
- 19. Wallace DV, Dykewicz MS. Seasonal Allergic Rhinitis: a focused systematic review and practice parameter update. Curr Opin Allergy Clin Immunol. 2017;17(4):286‐294. [DOI] [PubMed] [Google Scholar]
- 20. Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46(9):1139‐1151. [DOI] [PubMed] [Google Scholar]
- 21. Becker S, Rasp J, Eder K, Berghaus A, Kramer MF, Groger M. Non‐allergic rhinitis with eosinophilia syndrome is not associated with local production of specific IgE in nasal mucosa. Eur Arch Otorhinolaryngol. 2016;273(6):1469‐1475. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Wang X, Chen Y, Yao W. Correlation between levels of exhaled hydrogen sulfide and airway inflammatory phenotype in patients with chronic persistent asthma. Respirology. 2014;19(8):1165‐1169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data required to produce these findings cannot be shared at this time as the data also forms part of an ongoing study.


