Abstract
Background
Despite the recent improvement in colorectal cancer (CRC) treatment, it still has a poor prognosis with a low survival rate. Genetic and epigenetic mechanisms have proved to play a substantial role in CRC tumorigenesis and progression. According to Gene Ontology and TargetScan analyses, the B‐Raf proto‐oncogene (BRAF) gene is one of the microRNA‐17 (miR‐17) targets. We aimed to explore the prognostic value of B‐Raf protein and BRAF/microRNA‐17 (MIR‐17) gene expression signature in CRC archived samples.
Methods
B‐Raf protein expression was identified by immunohistochemistry, while gene expression studies were quantified by real‐time qPCR in 53 paired archived CRC specimens.
Results
The BRAF showed higher expressions in CRC specimens relative to non‐cancer tissues (p = 0.006). MIR17 expression was inversely and significantly correlated with both B‐Raf protein (r = −0.79, p < 0.001) and gene expression (r = −0.35, p = 0.010) and showed a significant direct correlation with a high rate of relapse (p = 0.020). BRAF/miR‐17 expression in CRC was associated inversely with tumor size, high grade of colonic carcinoma, lymph node metastasis, and carcinoma subtype. Spearman correlation and Kaplan‐Meier survival curve analyses revealed that disease‐free survival and overall survival were inversely and significantly correlated with positive B‐Raf protein expression (r = −0.31 and −0.35, p = 0.023 and 0.011, respectively) and directly correlated with log BRAF/MIR17 ratio (r = 0.50 and 0.41, p < 0.001 and = 0.003, respectively). Cox hazard regression analysis revealed the BRAF/MIR17 ratio could predict both types of patients' survival, among other variables.
Conclusion
BRAF/MIR17 ratio could have prognostic utility in patients with CRC. Further larger‐scale studies are warranted to confirm this utility.
Keywords: BRAF, colorectal cancer, gene expression, IHC, miR‐17, prognosis, qPCR
The workflow of explored BRAF/MIR17 prognostic utility

1. INTRODUCTION
Colorectal cancer (CRC) represents the third most common malignancy and the second leading cause of cancer‐related mortality worldwide. 1 It is characterized by a heterogeneous etiology derived from the constellation of potentially modifiable risk factors and multiple genetic/epigenetic alterations. 2 , 3 Although surgical resection in the earlier stage of the disease is associated with a relatively high 5‐year survival rate, the prognosis of late‐stage CRC patients remains poor. 4
Accumulating evidence revealed the implication of coding and non‐coding genes in every CRC tumorigenesis. 5 In this regard, a substantial number of genetic biomarkers, including the serine/threonine kinase B‐Raf proto‐oncogene (BRAF), DNA mismatch repair (dMMR) defects, the tumor protein p53 (P53), and Kirsten rat sarcoma viral oncogene homolog (KRAS), displayed prognostic impact and survival‐predictive potentials for CRC. 6 , 7 , 8 , 9 , 10 However, "the clinical use of these markers requires further research," proposed by Xia et al. 11
Given the central role of the protein kinase B‐Raf in the "RAS/RAF/MEK/ERK," the "mitogen‐activated protein kinase (MAPK)" signaling pathway, 12 its deregulation has been implicated in several aspects of malignant transformation and progression, including cell proliferation and survival, neoangiogenesis, invasion, and metastasis. 13 Several BRAF mutations confer important predictive value in the treatment of patients with colorectal carcinomas. 14 However, although BRAF gene mutation has been extensively studied (Figure S1A), there were scant (if any) reports exploring BRAF gene expression profile and B‐Raf protein as diagnostic and/or prognostic biomarker in CRC.
In addition to the genetic markers, the last few decades have witnessed remarkable developments in the field of epigenetic regulators based on the recent advances in high‐throughput techniques. 5 As an emerging family of non‐coding RNAs, microRNAs (miRNAs) have proved to be implied in several biological and pathological processes. 15 Their dysregulation can contribute to carcinogenesis and metastasis in several cancers, including CRC. 16 Our in silico analysis by searching the Diana pathway database (http://diana.imis.athena‐innovation.gr/) has revealed that microRNA‐17 (miR‐17‐5p) transcript ranked the second most important microRNA enriched in colorectal cancer pathway [KEGG ID: 05210] and it has 25 gene targets, including BRAF (Figure S2). Interestingly, Kishore et al also have reported complementarity between the BRAF gene and miR‐17 in the kidney cell line (HEK293). 17 Also, the Cancer Hallmarks Analytics tool has revealed the important role of B‐Raf in invasion and metastasis, replicative immortality, and genomic instability and mutation. At the same time, the miR‐17 gene (MIR‐17) is highly involved in inducing angiogenesis, resisting cell death, and sustaining proliferative signaling (Figure 1). 18
Figure 1.
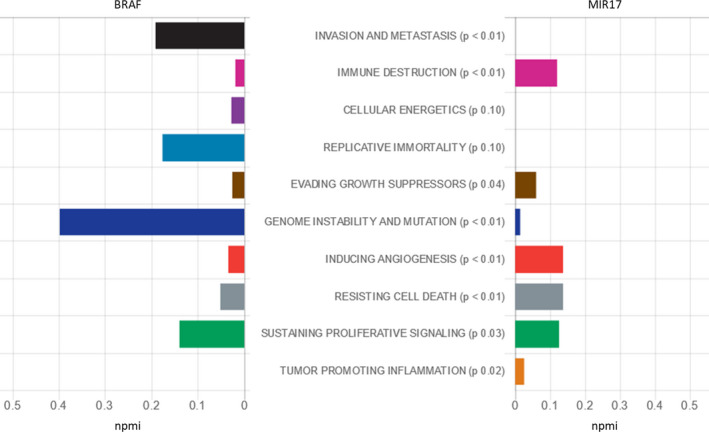
Role of BRAF and MIR‐17 genes in cancer hallmarks. Hallmark annotations were derived from The Cancer Hallmarks Analytics Tool (CHAT) web browser, which analyzes the strength of association between genes of interest and the hallmarks of cancer in the literature based on a text‐mining analysis of 26 million PubMed abstracts. npmi: normalized pointwise mutual information of h and q: npmi (h,q) = pmi (h,q)/‐log2 p(h,q). The "npmi" metric normalizes "pmi" by dividing by the negative log probability of co‐occurrence ‐log2 p(h,q), which constrains its values to the range [−1, 1]. Zero is a frequency of co‐occurrence matching random (no association, as for "pmi") and 1 for complete co‐occurrence (perfect association). [Data source: http://chat.lionproject.net]
Considering that both BRAF and miR‐17‐5p are closely correlated with cancer, and the multigene prognostic predictors have the theoretical advantages of optimal use of information from continuous variables, according to Győrffy et al, 19 we speculated that the transcriptional pattern of both markers might participate in disease progression and open up a new era for combined targeted therapy. In this sense, the current study aimed to explore the association of B‐Raf protein and BRAF/miR‐17 expression signatures with pathological features and outcomes of CRC patients to assist in the prognostic stratification of CRC patients and, subsequently, the individualized therapeutic management of such lethal disease.
2. SUBJECTS AND METHODS
2.1. Study participants and tissue specimens
The present retrospective study included the available 53 eligible archived primary colorectal carcinoma specimens staged according to the International Union Against Cancer TNM staging system (eighth edition) 20 and their paired non‐cancerous tissue, which have complete clinical and survival data. Paraffin sections of resected specimens of colorectal carcinoma and the clinical data were retrieved from archives of the Pathology lab, Mansoura University Hospital, in the last five years. None of the patients have received any neoadjuvant chemotherapy or radiotherapy before sample collection. The clinicopathological data, including patients' age, sex, tumor site, and size, were obtained from patients' medical records. Sections of cancer‐free tissues adjacent to the tumor were cut, examined, and collected to serve as controls during the genetic profiling and immunohistochemistry studies. Samples that were not homogeneous or histologically well‐characterized primary CRC determined by an experienced pathologist and those with incomplete clinical and/or survival data were excluded. The study was conducted following the guidelines in the Declaration of Helsinki and was approved by the authors' institutions. Informed consent of the patients was not applicable in the present study as achieved samples were retrieved for the study analysis.
2.2. Immunohistochemistry (IHC) methodology
IHC was performed on formalin‐fixed, paraffin‐embedded (FFPE) whole tissue sections of colorectal carcinomas for B‐Raf using rabbit monoclonal antibody anti‐BRAF V (Catalog No. IHC‐00607, GeneID 673, Isotype IgG, dilution 1:50, BETHYL laboratory, USA). First, for all cases, the original hematoxylin‐and‐eosin‐stained sections of the lesions were re‐examined for histopathological confirmation of the diagnosis and recording of histopathological parameters as histological type, grading, the staging of cancer, depth of tumor invasion in the wall, circumferential margin, lymph node metastasis, lymphovascular, and perineural invasion. Sections of 4 μm thickness were cut and spread on poly‐L‐lysine–coated slides. The paraffin sections were immersed in three xylene changes and hydrated using a graded series of alcohol solutions. Antigen retrieval was routinely performed by immersing the sections in 0.01 M Tris‐EDTA buffer (pH 9.0) (20 minutes). The sections were counterstained for 3 minutes with Meyer's hematoxylin, and then, they were mounted. Sections from breast carcinoma were used as a positive control. Negative controls were obtained by omitting the primary antibodies.
2.3. Interpretation of the immunohistochemical stain
All immune‐stained slides were evaluated two times by the pathologist, blinded to all clinical, histopathological, and genetic data. Scoring of B‐Raf cytoplasmic staining was according to the following scoring system: 0: negative; 1: weak staining if weak staining <20% of tumor cells, 2: moderate to strong staining in <20% of tumor cells, 3: weak staining in 20% to 70% of tumor cells, 4: moderate to strong staining in 20% to 70% of tumor cells; 5: weak staining in >70% of tumor cells, and 6: moderate to strong staining in >70% of tumor cells. 21 Examples of immunohistochemical staining for B‐Raf protein in CRC specimens and its intensity evaluation in the overall analysis are illustrated in Figure 2.
Figure 2.
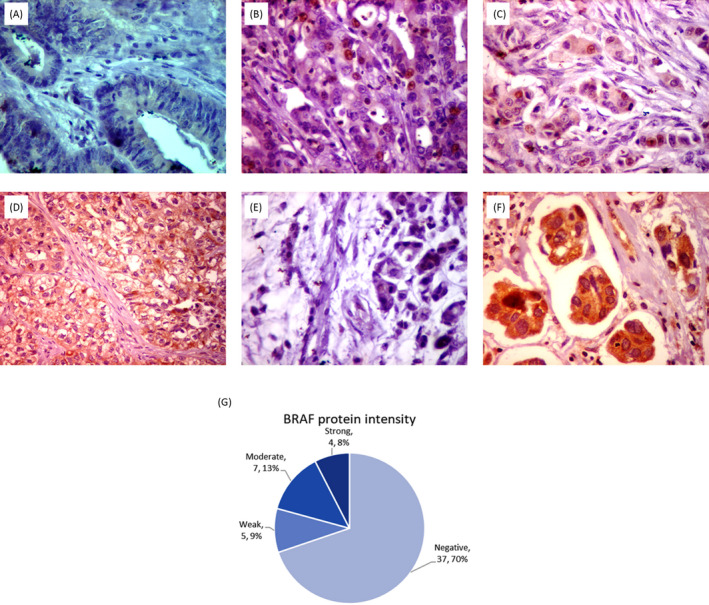
Immunohistochemistry of BRAF protein in colorectal cancer specimens. Immunohistochemical staining for B‐Raf protein (x400) showed (A) negative staining in colonic adenoma, (B) another colonic adenoma showed weak cytoplasmic staining with non‐specific nuclear staining, (C) grade 2 colonic adenocarcinoma showed moderate cytoplasmic staining, (D) grade 3 colonic adenocarcinoma showed strong cytoplasmic staining, (E) mucinous adenocarcinoma showed negative staining, (F) moderately differentiated adenocarcinoma with lymphovascular emboli showed strong cytoplasmic staining, and (G) protein intensity in the overall analysis of CRC samples
2.4. BRAF/MIR‐17 gene expression analysis
Total tissue ribonucleic acid, including small RNA, was extracted from the FFPE tissue sections using Qiagen miRNeasy FFPE Kit (217 504, Qiagen, Hilden, Germany) following the protocol supplied by the manufacturer. Treatment of samples with RNase‐free DNase I for two hours at 37°C was applied. RNA concentration and purity were determined using the "NanoDrop ND‐1000 spectrophotometer (NanoDrop Tech., Inc Wilmington, DE, USA)" by adjustment of the wavelength‐dependent extinction coefficient of the software setting on “33” to measure small RNA(s) in the solution. The integrity of the extracted RNA was checked by running 5 μL of the isolated RNA on agarose gel electrophoresis. The complementary DNA (cDNA) was prepared from total RNA using TaqManTM MicroRNA Reverse Transcription (RT) Kit (P/N 4366596; Applied Biosystems, Foster City, CA, USA), and either the miRNA‐17–specific stem‐loop primers (assay ID 002308) or the endogenous control RNU6B primers (assay ID 001093) were used for quantification of miR‐17. In BRAF expression quantification, the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, P/N 4368814) was used to convert the total RNA into cDNA. For each sample, a 15 μL reaction mixture contains 5μL of RNA extract, 0.15 μL of 100 mM of each deoxynucleotide triphosphate, 1 μL of MultiScribe® reverse transcriptase (50 U/μL), 1.5 μL of 10 × RT buffer, 0.19 μL of RNase inhibitor (20 U/ml), 3 μL of gene‐specific TaqMan® primer, and 4.16 μL of nuclease‐free water. RT was carried out in a T‐Professional Basic, Biometra PCR System (Biometra, Göttingen, Germany) at 16°C for 30 minutes, 42°C for 30 minutes, and 85°C for 5 minutes, and then hold at 4°C. Negative controls (ie, non‐template and non‐reverse transcriptase) were included in each experiment to ensure that PCR products were not due to contamination by genomic DNA.
The real‐time PCR amplification reactions were carried out following the MIQE (Minimum Information for Publication of Quantitative Real‐Time PCR Experiments) guidelines 22 in final volumes of 20 µL, including 1.33 µL RT products, 2 × TaqMan® Universal PCR Master Mix, 1 µL TaqMan® small RNA assay/RNU6B assay. For BRAF quantification, glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) expression was applied for data normalization. No‐template samples were included as negative controls in each rum. The PCR was performed on StepOne™ Real‐Time PCR System (Applied Biosystems) and incubated as follows: 95°C for 10 minutes followed by 40 cycles of 92°C for 15 seconds and 60°C for 1 minute. 23 Duplicate PCR runs were performed, and average values were estimated.
2.5. Gene expression data analysis
Consistent expression values were obtained with a nearly close cycle of quantification (Cq) values of the same specimen. Fold changes in miR‐17 and BRAF expression in each patient cancer tissue relative to the corresponding non‐cancer adjacent tissues (NAT) were estimated via the Livak method based on the quantification (threshold) cycle (Cq or CT) value as follows: relative expression = 2−ΔΔ Cq, where ΔΔCq = (Cq miR‐17/BRAF – Cq RNU6B/GAPDH)cancer − (Cq miR‐17/BRAF – Cq RNU6B/GAPDH)NAT. 24
2.6. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) for Windows, version 26.0 (Armonk, NY: IBM Corp.). The sample size and study power were calculated by G*Power version 3.0.10. The estimated power for the study design (gene expression) was 80% at total sample size = 106, calculated effect size = 0.55, and alpha error probability = 0.05. Means ± standard deviations were generated for continuous variables, and frequencies and percentages were estimated for categorical variables. Chi‐square and Fisher's exact, Student t, and Mann‐Whitney tests, when appropriate, were used to compare between cases and controls. The correlation coefficient was determined using the Spearman correlation test. Two‐sided p‐values of <0.05 were considered significant. Multiple Cox proportionate hazard regression analysis was performed to calculate the hazard ratio (HR) and 95% confidence interval (CI) for each putative independent variable. Kaplan‐Meier curves were plotted for overall (OS) and disease‐free survival (DFS).
3. RESULTS
3.1. Baseline characteristics of the study samples
The study included 53 patients with colorectal carcinoma; the patients' characteristics are summarized in Table 1. About 54.7% of tumors were on the right side (cecum and ascending colon), while 39.6% were on the left side (descending and sigmoid colon). The histological type of tumors was 38 (71.7%) adenocarcinoma, signet ring carcinoma 8 (15%), mucinous carcinoma 7 (13.2%). Most of the adenocarcinoma cases were grade 2 (81.6%). According to pTNM 8 staging, 4 cases (7.5%) were pT1 invaded only the mucosa and submucosa, 25 (47.2%) cases were pT2 invaded muscularis propria, 16 (30%) were pT3 invaded through muscularis propria to subserosa, and 8(15.1%) were pT4 invaded the serosa and peritoneum. Tumor deposited was detected in 33 cases of colonic carcinoma, with 22(41.5%) showed tumor deposits in up to 3 of dissected LNs (pN1) and 11 (20.8%) showed tumor deposits in 4 or more dissected LNs (pN2). Distant metastasis was detected clinically and radiologically in 10 (18.9%). Detection of the tumor within well‐defined vascular and lymphatic spaces (lymphovascular invasion) was detected in 18 (34.0%). On follow‐up of patients for three years after surgical removal and post adjuvant therapy, 16 (30.2%) cases showed relapse of the tumor, and 14 (26.4%) died.
Table 1.
Clinicopathologic criteria of 53 cases of colonic carcinoma
| Variables | Subtypes | N | % |
|---|---|---|---|
| Age | ≥60 | 32 | 60.4 |
| <60 | 21 | 39.6 | |
| Sex | Male | 35 | 66.0 |
| Female | 18 | 34.0 | |
| Grade | G1 | 5 | 13.2 |
| G2 | 31 | 81.6 | |
| G3 | 2 | 5.2 | |
| Signet ring | 8 | ||
| Mucinous | 7 | ||
| Site | Cecum and Ascending | 29 | 54.7 |
| Transverse | 3 | 5.7 | |
| Descending (descending and sigmoid colon) | 21 | 39.6 | |
| pT | T1 | 4 | 7.5 |
| T2 | 25 | 47.2 | |
| T3 | 16 | 30.2 | |
| T4 | 8 | 15.1 | |
| pN | N0 | 18 | 34.0 |
| N1 | 22 | 41.5 | |
| N2 | 11 | 20.8 | |
| M | M0 | 43 | 81.1 |
| M1 | 10 | 18.9 | |
| Stage | 1 | 10 | 18.9 |
| 2 | 8 | 15.1 | |
| 3 | 24 | 45.3 | |
| 4 | 10 | 18.9 | |
| Lymphovascular invasion | No | 35 | 66.0 |
| Yes | 18 | 34.0 | |
| Histopathological type | Adenocarcinoma | 38 | 71.7. |
| Mucinous | 7 | 13.2 | |
| Signet cell | 8 | 15.1 | |
| Relapse | No | 37 | 69.8 |
| Yes | 16 | 30.2 | |
| Mortality | Survived | 39 | 73.6 |
| Died | 14 | 26.4 |
Data are presented as number (N) and frequency (%).
Abbreviations: M, metastasis; N, lymph node; P, pathologic; T, tumor thickness.
3.2. Expression of the BRAF and MIR17 in CRC samples
As depicted in Figure 3, although miR‐17 expression in cancer tissues was comparable to that in non‐cancer ones (median (interquartile); 3.0 (0.06‐4.37), p = 0.13), the BRAF gene showed higher expressions in CRC specimens relative to NAT; 20.3 (1.6‐113.8), p = 0.006).
Figure 3.
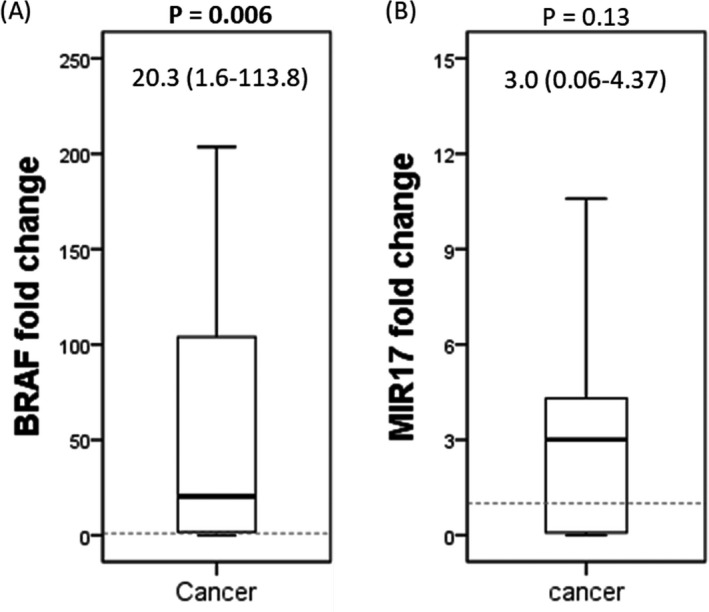
The relative expression level of BRAF and MIR17 genes in colorectal cancer specimens compared to control tissues. Values are presented as median (Q1‐Q3). The Mann‐Whitney U test was applied for p‐value calculation
3.3. Correlation analysis
Although there was a non‐significant correlation between BRAF gene and protein expressions, MIR17 expression was inversely and significantly correlated with both BRAF protein (r = −0.79, p < 0.001) and gene expression (r = −0.35, p = 0.010) (Figure 4).
Figure 4.
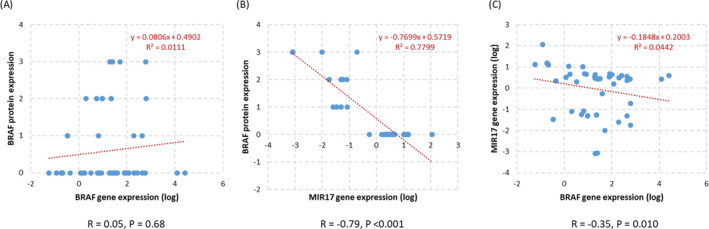
Correlation analysis for gene and protein expression in colorectal cancer. Spearman's correlation coefficient is shown
Correlation of BRAF gene/protein expressions and log BRAF/MIR17 ratio with the CRC specimens' clinicopathological features is shown in Table 2. High grade of colonic carcinoma showed significant association (p < 0.001) and correlation with B‐Raf protein positivity (p < 0.001, r = 0.72), but showed an inverse correlation of MIR17 expression and the log BRAF/MIR17 ratio (r = −0.49, p < 0.001, and r = −0.52, p < 0.001, respectively). Simultaneously, there was no significant correlation to BRAF gene expression (p = 0.09).
Table 2.
Correlation of BRAF protein and gene expressions with the clinicopathological features in the colorectal cancer specimens
| Variables | Braf protein intensity | BRAF gene expression | MIR17 expression | Log BRAF/MIR17 ratio | ||||
|---|---|---|---|---|---|---|---|---|
| PAss value | r (PCorr) | PAss value | r (PCorr) | PAss value | r (PCorr) | PAss value | r (PCorr) | |
| Age | 0.84 | 0.03 (0.82) | 0.13 | ‐0.09 (0.48) | 0.36 | ‐0.02 (0.85) | 0.51 | 0.14 (0.31) |
| Sex | 0.14 | ‐0.20 (0.14) | 0.91 | ‐0.016 (0.91) | 0.28 | 0.18 (0.18) | 0.91 | 0.016 (0.91) |
| Grade | <0.001 | 0.72 (<0.001) | 0.22 | ‐0.23 (0.09) | 0.030 | ‐0.49 (<0.001) | 0.001 | ‐0.52 (<0.001) |
| Site | 0.74 | 0.10 (0.45) | 0.39 | 0.09 (0.49) | 0.49 | ‐0.19 (0.15) | 0.95 | 0.03 (0.78) |
| T | 0.003 | 0.41 (0.002) | 0.09 | ‐0.23 (0.17) | 0.31 | ‐0.15 (0.28) | <0.001 | ‐0.55 (<0.010) |
| N | 0.22 | 0.24 (0.08) | 0.21 | 0.03 (0.81) | 0.29 | ‐0.28 (0.045) | 0.011 | ‐0.39 (0.004) |
| M | 0.44 | 0.10 (0.44) | 0.85 | ‐0.02 (0.85) | 0.52 | ‐0.08 (0.54) | 0.07 | ‐0.24 (0.07) |
| Stage | 0.43 | 0.15 (0.27) | 0.28 | 0.05 (0.71) | 0.69 | ‐0.20 (0.14) | 0.16 | ‐0.32 (0.019) |
| LVI | 0.58 | 0.07 (0.59) | 0.042 | 0.28 (0.041) | 0.051 | ‐0.08 (0.52) | 0.19 | 0.18 (0.19) |
| Subtype | <0.001 | 0.61 (<0.001) | 0.57 | ‐0.15 (0.25) | 0.76 | ‐0.44 (0.001) | 0.001 | ‐0.53 (<0.001) |
| Relapse | 0.38 | ‐0.11 (0.39) | 0.66 | ‐0.25 (0.06) | 0.020 | 0.33 (0.015) | 0.07 | ‐0.24 (0.07) |
| Died | 0.27 | 0.15 (0.28) | 0.77 | ‐0.03 (0.78) | 0.54 | ‐0.05 (0.69) | 0.06 | ‐0.25 (0.06) |
| DFS | 0.027 | ‐0.31 (0.023) | 0.75 | ‐0.03 (0.81) | 0.17 | 0.06 (0.63) | <0.001 | 0.50 (<0.001) |
| OS | 0.001 | ‐0.35 (0.011) | 1.0 | ‐0.11 (0.42) | 0.016 | 0.24 (0.08) | <0.001 | 0.41 (0.003) |
PAss (p‐values of the association analysis) were calculated by Mann‐Whitney U or Kruskal‐Wallis tests. Spearman's correlation analysis is expressed as a correlation coefficient (r) and its p‐values (PCorr). Bold values are statistically significant at p < 0.05.
Abbreviations: DFS, disease‐free survival; LVI, lymphovascular invasion; OS, overall survival.
Large tumor size was significantly associated with positive B‐Raf protein expression (p = 0.003, r = 0.41). It showed a significant inverse correlation with the log BRAF/MIR17 ratio (p < 0.001, r = −0.39) with no significant correlation to BRAF gene expression or MIR17 expression.
The lymph node metastasis was inversely correlated with MIR17 expression (p = 0.045, r = −0.28) and the log BRAF/MIR17 ratio (p = 0.004, r = −0.39), with no significant correlation to B‐Raf protein or gene expressions. The lymphovascular invasion (LVI) showed only significant association and correlation to high BRAF gene expression (p = 0.041, r = 0.28).
Although, carcinoma subtype showed significant association and correlation with B‐Raf protein positivity (p < 0.001, r = 0.61), an inverse correlation with MIR17 expression (p = 0.001, r = −0.44), and the log BRAF/MIR17 ratio (p < 0.001, r = −0.53), it showed no significant correlation with BRAF gene expression (p = 0.25).
3.4. Clinical and survival parameters
A high rate of relapse was significantly correlated with MIR17 expression (p = 0.020). DFS and OS were inversely and significantly correlated with positive B‐Raf protein expression (r = −0.31 and −0.35, p = 0.023 and 0.011, respectively), as well as directly and significantly correlated with log BRAF/MIR17 ratio (r = 0.50 and 0.41, p < 0.001 and = 0.003, respectively). Otherwise, no significant correlations and/or associations were observed between age, sex, site, metastasis, and the number of deceased patients and BRAF gene and protein expressions, MIR17 expression, or log BRAF/MIR17 ratio in the present specimens (Table 2, and Figure 5). Otherwise, log BRAF/MIR17 ratio showed significant association with the histopathological type and the pathological grade of the samples (both p = 0.001), the tumor size (p < 0.001), and the lymph node infiltration (p = 0.011) (Figure 5).
Figure 5.
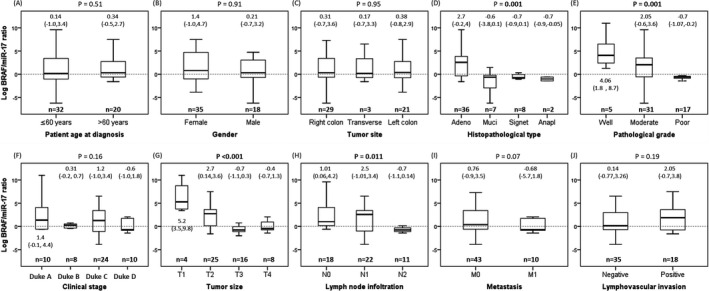
Association between BRAF/miR‐17 ratio and clinicopathological features. Kruskal‐Wallis and Mann‐Whitney U tests were applied. The significance level was set at p < 0.05
Analysis of the prognostic value of BRAF/miR‐17 ratio for detecting survival (OS and DFS) by Spearman correlation analysis test (Table 2) and Kaplan‐Meier survival curve (Figure 6) revealed OS was significantly and inversely correlated with positive B‐Raf protein (p < 0.001, r = −0.35), and directly and significantly correlated with log BRAF/MIR17 ratio (p < 0.001, r = 0.41), but no significant correlation to BRAF gene expression or MIR17 expression.
Figure 6.
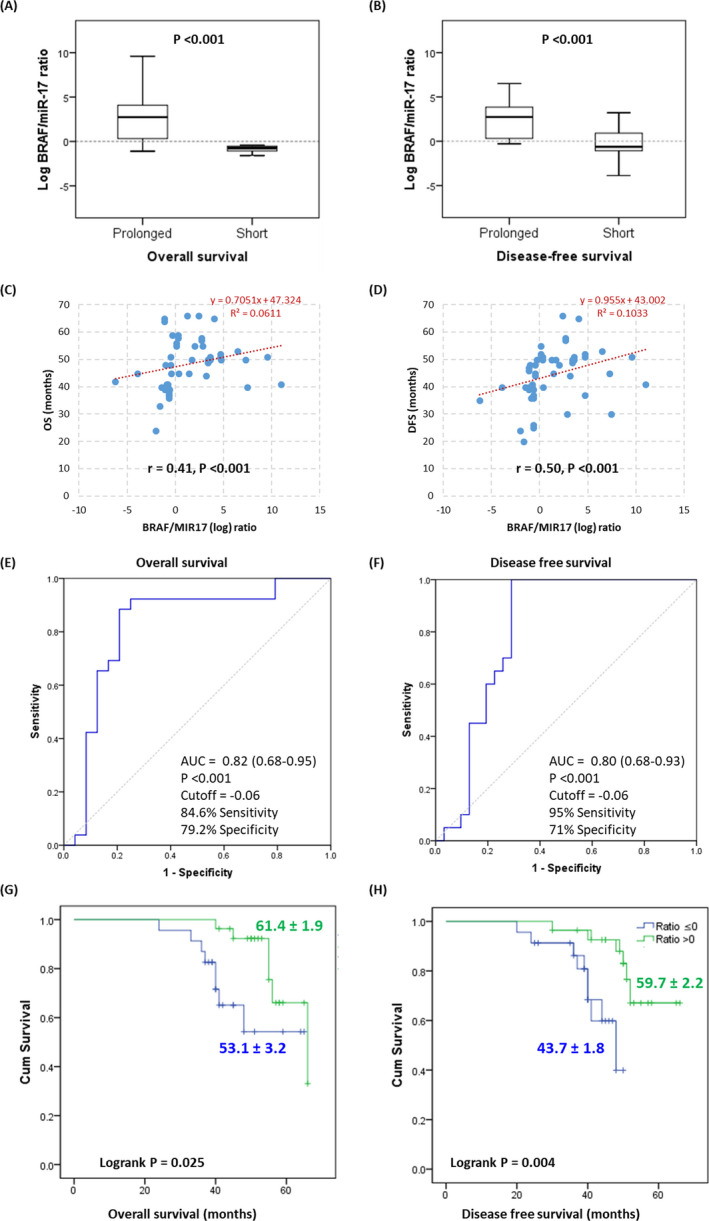
Prognostic value of the BRAF/miR‐17 ratio for detecting survival. (A‐B) Association of BRAF/MIR17 expression ratio with survival. (C‐D) Spearman correlation analysis between gene expression and survival. (E‐F) Receiver characteristic operator curve to discriminate between prolonged and short survivors. (G‐H) Kaplan‐Meier survival curve for overall and disease‐free survival. LogRank (Mantel‐Cox) test was used
Cox proportional hazard regression analysis of OS and DFS among CRC patients revealed that tumor grade, tumor size, and the presence of distant metastasis, as well as increased BRAF/MIR17 ratio, could predict both types of patients survival (Table 3).
Table 3.
Cox proportional hazard regression analysis of overall and disease‐free survival among patients with colorectal cancer
| Variables | OS | p‐value | DFS | p‐value | ||
|---|---|---|---|---|---|---|
| HR | (95%CI) | HR | (95%CI) | |||
| Age (> 60 vs. ≤ 60) | 1.03 | 0.97‐1.09 | 0.40 | 1.00 | 0.94‐1.07 | 0.88 |
| Sex (female vs. male) | 2.00 | 0.42‐9.46 | 0.38 | 1.27 | 0.26‐6.21 | 0.77 |
| Location (left/transverse vs. right) | 1.14 | 0.27‐4.83 | 0.85 | 1.67 | 0.29‐9.54 | 0.56 |
| Type (Adeno vs. non‐adeno) | 0.36 | 0.06‐2.27 | 0.27 | 0.28 | 0.04‐2.04 | 0.20 |
| Grade (G3 vs G1/2) | 11.47 | 1.74‐75.61 | 0.011 | 9.13 | 1.20‐69.71 | 0.039 |
| T status (T3/4 vs. T1/2) | 0.02 | 0.00‐0.37 | 0.008 | 0.03 | 0.00‐0.42 | 0.009 |
| LN status (positive vs. negative) | 0.43 | 0.05‐3.56 | 0.43 | 0.34 | 0.04‐2.68 | 0.30 |
| Metastasis (positive vs. negative) | 28.95 | 1.42‐590.09 | 0.029 | 46.83 | 1.82‐1,205.20 | 0.020 |
| LVI (positive vs. negative) | 2.04 | 0.43‐9.57 | 0.36 | 3.04 | 0.55‐16.81 | 0.20 |
| Staging (duke C/D vs. A/B) | 1.38 | 0.18‐10.43 | 0.75 | 0.93 | 0.10‐8.85 | 0.94 |
| BRAF/MIR17 ratio (over‐ vs. under‐expressed) | 0.58 | 0.37‐0.90 | 0.015 | 0.52 | 0.31‐0.89 | 0.017 |
Bold values indicate statistically significant at p < 0.05.
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; LN, lymph node; Lt, left; LVI, lymphovascular invasion; OS, overall survival; Rt, right.
4. DISCUSSION
Colorectal cancer is a heterogeneous disease that manifests through a variable clinical course and with significant inconsistency in response to treatment, even within tumors with similar histopathological features. 25 Although the introduction of cytotoxic drugs has improved the treatment of advanced CRC, the individual response to chemoradiotherapy varies tremendously from one patient to another. However, the observed progress in CRC molecular therapies may provide new insight into treating this disease. 26
Here in the present study, we explored the role of B‐Raf protein and gene expression, in addition to the BRAF/MIR17 signature in CRC specimens. The findings indicated that B‐Raf protein and gene expression showed higher expression in CRC specimens relative to non‐cancerous tissue and significantly associated with different prognostic indices in terms of a higher grade of colonic carcinoma, large tumor size, and carcinoma subtype (in case of B‐Raf protein), and presence of lymphovascular invasion (in case of BRAF gene). Also, B‐Raf protein expression showed a significant association and inverse correlation with DFS and OS. These findings support the previous studies, which report the significant implication of B‐Raf in cancer. 27 , 28 As a member of the protein kinase family, "serine/threonine‐protein kinase," B‐Raf is involved in the mitogenic signal transduction from the cell membrane to the nucleus. It phosphorylates the MAP2K1 and thereby activates the highly oncogenic "MAP kinase signal transduction" pathway (Figure S1). 27 , 28 B‐Raf protein is mainly one of the cytoplasmic proteins involved in cell survival, proliferation, and motility while inhibiting differentiation, 29 , 30 which supports its association with the unfavorable prognostic indices mentioned above. In colorectal cancer, B‐Raf plays multifaceted roles in tumor progression, diagnosis, and prognosis and may act as a predictor of response to combined targeted therapies. 31 It was also found to be overexpressed in other types of cancers such as melanoma, thyroid papillary carcinoma, hairy cell leukemia, Langerhans neoplasms, pediatric astrocytoma, and glioma (http://www.cancerindex.org/geneweb/BRAF.htm).
In the present study, the BRAF gene and protein expressions showed a negative correlation with the selected miR‐17 expression, which supports B‐Raf as one of the potential targets of miR‐17, as evidenced by our in silico analysis. To mitigate the non‐specific tissue expression of BRAF expression, the authors were interested in exploring its expression as part of the BRAF/MIR17A ratio, which showed a significant inverse correlation with tumor size, high‐grade colonic carcinoma, lymph node metastasis, and carcinoma subtype.
Generally, microRNAs are involved in various biological and cellular processes, such as cell proliferation, differentiation, angiogenesis, and apoptosis. 32 , 33 This cluster of non‐coding RNAs has been implicated in several chronic diseases and cancers, including CRC. 34 , 35 Various genomic data on microRNAs are currently available, including their interactions with related proteins or genes. The key challenge is how to apply these data from myriad sources to investigate its diagnostic/prognostic utility in the clinic.
MiR‐17, as a member of the miR‐17‐92a family, was involved in the deregulation of several players in tumorigenesis. 36 , 37 It was implicated in regulating epithelial‐mesenchymal transition and proliferation in CRC via targeting cytochrome 7 B family, member 1, and salt‐inducible kinase‐1 genes, respectively. 37 , 38 The present overall analysis found no significant difference between cancerous and non‐cancerous specimens in terms of MIR‐17 expression, which contrasts with other studies that showed significant upregulation of this microRNA in CRC tissues. 37 , 39 , 40 This difference could be due to the relatively small number of samples that were available in this work, the different clinicopathological characteristics of the patients, or due to different genetic background of the included samples from others as this is the first time to explore the MIR17 relative expression in CRC samples collected from the Middle Eastern population. Further work on larger samples in the same population will be required to confirm this finding.
Otherwise, a high rate of relapse was significantly correlated with MIR17 expression. As a part of the BRAF/MIR17 ratio, MIR17 expression showed significant associations and correlations with unfavorable prognostic indices, including DFS and OS. These findings, in part, are consistent with the association of miR‐17 with poor cancer prognosis documented in a previous meta‐analysis. 41 Furthermore, the circulating miR‐17‐92a cluster extracted from CRC patient serum was correlated with a high recurrence rate and poor prognosis. 42 Diosdado and colleagues reported a continuous miR‐17 upregulation during the progression of adenoma to adenocarcinoma. 43 Other studies also identified miR‐17 as a predictor of cancer occurrence and poor prognosis 44 , 45 and correlated its deregulation with a metastatic hepatic spread in CRC. 39 , 46 Recently, Fiala et al reported miR‐17 deregulation as one of the promising predictive biomarkers associated with an unfavorable response to anti‐epidermal growth factor receptor (EGFR) monoclonal antibody therapy in metastatic CRC patients. 40 Additionally, Ast et al specified matrix metallopeptidase 2 (MMP2), fascin actin‐bundling protein 1 (FSCN1), laminin subunit gamma 1 (LAMC1), transforming growth factor‐beta 1 (TGFB1), dystonin (DST), ETS proto‐oncogene 1 (ETS1), fibrillin 1 (FBN1), fibroblast growth factor 2 (FGF2), fibronectin 1 (FN1), integrin subunit alpha V (ITGAV), ITGB1, peroxidasin (PXDN), and TIMP metallopeptidase inhibitor 2 (TIMP2) as potential targets in extracellular matrix remodeling that could mediate some of the essential roles miR‐17 play in cancer, including CRC. 47 Interestingly, a recent functional study by Han et al identified the primary miR‐17 processing could be regulated via the circular non‐coding RNA "circLONP2," another family of non‐coding RNAs, which correlated with CRC progression and invasion through modifying the "maturation and the intercellular exosomal dissemination" of miR‐17. 48
It is worth noting that this study is a preliminary one limited by the relatively small sample size, the retrospective nature of the study design, and the lack of in vitro functional studies that clarify the exact molecular mechanisms by which BRAF/MIR17 mediates its signature in CRC. Also, it will be interesting to explore the association of BRAF/MIR17 signature with the most common BRAF V600E mutation by the newly emerged PCR techniques to add to its clinical utility. 49 , 50 Based on these limitations, future large‐scale studies supported by functional in vitro and gene mutation exploring studies are warranted to confirm and validate the results.
5. CONCLUSION
Collectively, our findings support the prognostic utility of miR‐17 and/or BRAF/MIR17 ratio in CRC in terms of association with poor prognostic indices and survival. Future prospective large‐scale studies are recommended to confirm the study findings.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Fig S1‐S2
ACKNOWLEDGMENTS
The authors would like to acknowledge the approval and the support of this research study by grant no. (MED‐2017‐1‐8‐F‐7517) from the Deanship of Scientific Research, Northern Border University, Arar, KSA.
Ibrahiem AT, Fawzy MS, Abu AlSel BT, Toraih EA. Prognostic value of BRAF/MIR‐17 signature and B‐Raf protein expression in patients with colorectal cancer: A pilot study. J Clin Lab Anal.2021;35:e23679. 10.1002/jcla.23679
Contributor Information
Afaf T. Ibrahiem, Email: afaf.ibrahiem@yahoo.com.
Manal S. Fawzy, Email: manal2_khashana@ymail.com.
Baraah T. Abu AlSel, Email: braaseel@yahoo.com.
Eman A Toraih, Email: etoraih@tulane.edu.
DATA AVAILABILITY STATEMENT
All datasets presented in this study are included in the article/supplementary material.
REFERENCES
- 1. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors, and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713‐732. [DOI] [PubMed] [Google Scholar]
- 2. Zoratto F, Rossi L, Verrico M, et al. Focus on genetic and epigenetic events of colorectal cancer pathogenesis: implications for molecular diagnosis. Tumour Biol. 2014;35(7):6195‐6206. [DOI] [PubMed] [Google Scholar]
- 3. Murphy N, Ward HA, Jenab M, et al. Heterogeneity of Colorectal Cancer Risk Factors by Anatomical Subsite in 10 European Countries: A Multinational Cohort Study. Clin Gastroenterol Hepatol. 2019;17(7):1323‐31.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363‐385. [DOI] [PubMed] [Google Scholar]
- 5. Jung G, Hernández‐Illán E, Moreira L, Balaguer F, Goel A. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol. 2020;17(2):111‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch‐repair status. Nature. 2002;418(6901):934. [DOI] [PubMed] [Google Scholar]
- 7. Diep CB, Thorstensen L, Meling GI, Skovlund E, Rognum TO, Lothe RA. Genetic tumor markers with prognostic impact in Dukes' stages B and C colorectal cancer patients. J Clin Oncol. 2003;21(5):820‐829. [DOI] [PubMed] [Google Scholar]
- 8. French AJ, Sargent DJ, Burgart LJ, et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res. 2008;14(11):3408‐3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walther A, Johnstone E, Swanton C, Midgley R, Tomlinson I, Kerr D. Genetic prognostic and predictive markers in colorectal cancer. Nat Rev Cancer. 2009;9(7):489‐499. [DOI] [PubMed] [Google Scholar]
- 10. Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC‐3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol. 2010;28(3):466‐474. [DOI] [PubMed] [Google Scholar]
- 11. Xia X, Yang B, Zhai X, et al. Prognostic role of microRNA‐21 in colorectal cancer: a meta‐analysis. PLoS One. 2013;8(11):e80426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289‐305. [PMC free article] [PubMed] [Google Scholar]
- 13. Caputo F, Santini C, Bardasi C, et al. BRAF‐Mutated Colorectal Cancer: Clinical and Molecular Insights. Int J Mol Sci. 2019;20(21):5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite‐stable colon cancers. Cancer Res. 2005;65(14):6063‐6069. [DOI] [PubMed] [Google Scholar]
- 15. Zhang S, Han Q, Zhu K, Wang Q. The association of miR‐27a rs895819 polymorphism with colorectal cancer risk in Chinese population. J Clin Lab Anal. 2020;34:e23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moridikia A, Mirzaei H, Sahebkar A, Salimian J. MicroRNAs: Potential candidates for diagnosis and treatment of colorectal cancer. J Cell Physiol. 2018;233(2):901‐913. [DOI] [PubMed] [Google Scholar]
- 17. Kishore S, Jaskiewicz L, Burger L, Hausser J, Khorshid M, Zavolan M. A quantitative analysis of CLIP methods for identifying binding sites of RNA‐binding proteins. Nat Methods. 2011;8(7):559‐564. [DOI] [PubMed] [Google Scholar]
- 18. Baker S, Ali I, Silins I, et al. Cancer Hallmarks Analytics Tool (CHAT): a text mining approach to organize and evaluate scientific literature on cancer. Bioinformatics. 2017;33(24):3973‐3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Győrffy B, Hatzis C, Sanft T, Hofstatter E, Aktas B, Pusztai L. Multigene prognostic tests in breast cancer: past, present, future. Breast Cancer Res. 2015;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population‐based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67(2):93‐99. [DOI] [PubMed] [Google Scholar]
- 21. Estrella JS, Tetzlaff MT, Bassett RL, et al. Assessment of BRAF V600E Status in Colorectal Carcinoma: Tissue‐Specific Discordances between Immunohistochemistry and Sequencing. Mol Cancer Ther. 2015;14(12):2887‐2895. [DOI] [PubMed] [Google Scholar]
- 22. Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real‐time PCR experiments. Clin Chem. 2009;55(4):611‐622. [DOI] [PubMed] [Google Scholar]
- 23. Toraih EA, Aly NM, Abdallah HY, et al. MicroRNA‐target cross‐talks: Key players in glioblastoma multiforme. Tumour Biol. 2017;39(11):1010428317726842. [DOI] [PubMed] [Google Scholar]
- 24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods. 2001;25(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 25. Blanco‐Calvo M, Concha Á, Figueroa A, Garrido F, Valladares‐Ayerbes M. Colorectal cancer classification and cell heterogeneity: a systems oncology approach. Int J Mol Sci. 2015;16(6):13610‐13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weng W, Feng J, Qin H, Ma Y. Molecular therapy of colorectal cancer: progress and future directions. Int J Cancer. 2015;136(3):493‐502. [DOI] [PubMed] [Google Scholar]
- 27. Brennan DF, Dar AC, Hertz NT, et al. A Raf‐induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature. 2011;472(7343):366‐369. [DOI] [PubMed] [Google Scholar]
- 28. Lavoie H, Sahmi M, Maisonneuve P, et al. MEK drives BRAF activation through allosteric control of KSR proteins. Nature. 2018;554(7693):549‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flockhart RJ, Webster DE, Qu K, et al. BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res. 2012;22(6):1006‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vitiello M, Tuccoli A, D'Aurizio R, et al. Context‐dependent miR‐204 and miR‐211 affect the biological properties of amelanotic and melanotic melanoma cells. Oncotarget. 2017;8(15):25395‐25417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bettington M, Walker N, Clouston A, Brown I, Leggett B, Whitehall V. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62(3):367‐386. [DOI] [PubMed] [Google Scholar]
- 32. Sun Z, Shi K, Yang S, et al. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dexheimer PJ, Cochella L. MicroRNAs: From Mechanism to Organism. Front Cell Dev Biol. 2020;8:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schetter AJ, Okayama H, Harris CC. The role of microRNAs in colorectal cancer. Cancer J. 2012;18(3):244‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fadaka AO, Pretorius A, Klein A. Biomarkers for Stratification in Colorectal Cancer: MicroRNAs. Cancer Control. 2019;26(1):1073274819862784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fang LL, Wang XH, Sun BF, et al. Expression, regulation and mechanism of action of the miR‐17‐92 cluster in tumor cells (Review). Int J Mol Med. 2017;40(6):1624‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang C, Liu J, Xu L, et al. MicroRNA‐17 promotes cell proliferation and migration in human colorectal cancer by downregulating SIK1. Cancer Manag Res. 2019;11:3521‐3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xi XP, Zhuang J, Teng MJ, et al. MicroRNA‐17 induces epithelial‐mesenchymal transition consistent with the cancer stem cell phenotype by regulating CYP7B1 expression in colon cancer. Int J Mol Med. 2016;38(2):499‐506. [DOI] [PubMed] [Google Scholar]
- 39. Lai H, Zhang J, Zuo H, et al. Overexpression of miR‐17 is correlated with liver metastasis in colorectal cancer. Medicine (Baltimore). 2020;99(9):e19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fiala O, Sorejs O, Hosek P, et al. Association of miR‐125b, miR‐17 and let‐7c Dysregulations With Response to Anti‐epidermal Growth Factor Receptor Monoclonal Antibodies in Patients With Metastatic Colorectal Cancer. Cancer Genom Proteom. 2020;17(5):605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang C, Yu M, Yao X. MicroRNA‐17 and the prognosis of human carcinomas: a systematic review and meta‐analysis. BMJ Open. 2018;8(5):e018070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kawahara H, Watanabe K, Toyama Y, Yanagisawa S, Kobayashi S, Yanaga K. Determination of circulating tumor cells for prediction of recurrent colorectal cancer progression. Hepatogastroenterology. 2012;59(119):2115‐2118. [DOI] [PubMed] [Google Scholar]
- 43. Diosdado B, van de Wiel MA, Terhaar Sive Droste JS, et al. MiR‐17‐92 cluster is associated with 13q gain and c‐myc expression during colorectal adenoma to adenocarcinoma progression. Br J Cancer. 2009;101(4):707‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Masuda T, Hayashi N, Kuroda Y, Ito S, Eguchi H, Mimori K. MicroRNAs as Biomarkers in Colorectal Cancer. Cancers (Basel). 2017;9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gmerek L, Martyniak K, Horbacka K, et al. MicroRNA regulation in colorectal cancer tissue and serum. PLoS One. 2019;14(8):e0222013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang X, Chen L, Jin H, et al. Screening miRNAs for early diagnosis of colorectal cancer by small RNA deep sequencing and evaluation in a Chinese patient population. Onco Targets Ther. 2016;9:1159‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ast V, Kordaß T, Oswald M, et al. MiR‐192, miR‐200c and miR‐17 are fibroblast‐mediated inhibitors of colorectal cancer invasion. Oncotarget. 2018;9(85):35559‐35580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Han K, Wang FW, Cao CH, et al. CircLONP2 enhances colorectal carcinoma invasion and metastasis through modulating the maturation and exosomal dissemination of microRNA‐17. Mol Cancer. 2020;19(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Z, Sun K, Jing C, Cao H, Ma R, Wu J. Comparison of droplet digital PCR and direct Sanger sequencing for the detection of the BRAFV600E mutation in papillary thyroid carcinoma. J Clin Lab Anal. 2019;33(6):e22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li X, Du H, Luo J, et al. Comparison of the Clinical Validity of Droplet Digital PCR to ARMS‐PCR for BRAF V600E Mutation Detection in Thyroid Nodules. J Clin Lab Anal. 2020;15:e23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.


