Abstract
Background
Paraquat and diquat are widely used in agricultural production in many countries, which are very toxic to human beings. Paraquat can be detected in some diquat solution sold in the market. The blood concentration of paraquat or diquat is an important indicator for clinical diagnosis of paraquat or diquat poisoning. So, it is very meaningful to develop a method for simultaneous determination of paraquat and diquat in human plasma.
Objective
To develop and validate a HPLC‐DAD method for simultaneous determination of paraquat and diquat in human plasma and to apply it in the acute poisoning patients by these two herbicides.
Methods
Paraquat and diquat were simultaneously determined by HPLC‐DAD. The plasma was treated using Waters OASIS® Column and then separated on a Thermo Hypersil GOLD (250 × 4.6 mm, 5 μm) Column with the mobile phase consisted of 75 mmol/L sodium heptane sulfonate (containing 0.1 mol/L phosphoric acid, pH 3.0) and acetonitrile (87:13, v:v) at a flow rate of 1.0 mL/min. The full‐wavelength scanning was 200‐400 nm, and the detection wavelength of paraquat and diquat was 257nm and 310nm, respectively. 120 and 30 plasma samples from patients with paraquat and diquat poisoning were collected and analyzed by the established method.
Results
The standard curve for paraquat and diquat ranged from 0.05 to 20 μg/mL, and the precision of LLOQ for paraquat was 16.49%, which was required to be less than 20%. The precision of other concentrations was less than 14.14%. The recovery of paraquat and diquat was 95.38%‐103.97% and 94.79%‐98.40%, respectively. The results showed that paraquat and diquat were stable under various storage conditions. 120 plasma samples of paraquat poisoning patients and 30 plasma samples of diquat poisoning patients were determined by the established method. The blood concentration of paraquat ranged from 0.10 to 20.62 μg/mL, with an average of 3.61 μg/mL, while for diquat, the concentration ranged from 0 to 26.59 μg/mL, with an average of 2.00 μg/mL. Among the diquat suspected poisoning samples, 5 samples were detected not only diquat but also paraquat, and 2 samples were detected only paraquat, no diquat.
Conclusion
The HPLC‐DAD method established in this study was high throughput, high sensitivity, simple operation, and wide linear ranges. It can be used for the screening analysis and quantitative detection of paraquat and diquat in acute poisoning patients, which can provide basis for the treatment and prognosis of these two herbicides poisoning patients.
Keywords: acute poisoning patients, Diquat, high‐performance liquid chromatography, Paraquat, simultaneous determination
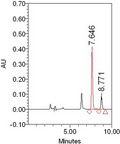
Both paraquat and diquat were determined in a suspected diquat poisoning patient plasma by the established method. The retention time of paraquat and diquat was 7.646 min and 8.771 min.
1. INTRODUCTION
Quaternary ammonium compounds are often used as herbicides and anticholinergics, which are often abused and closely related to many poisoning events because of their toxicity. 1 , 2
Paraquat (1‐1‐dimethyl‐4‐4‐bipyridine cation salt) and diquat (1,1'‐ethylidene‐2,2'‐bipyridine cation or dibromide salt) are classified as quaternary ammonium herbicides, both of which have quick and non‐selective effects. They can inhibit plant photosynthesis and then make it wither and die. So, they are widely used in agricultural production. 3 Paraquat is very toxic to human beings. It can be fatal for adults to take 1‐2 g orally. 4 Therefore, it has been banned in many countries in the late 1980s. Since July 1, 2016, paraquat aqueous solution was banned for sale and use in China, but can be used for export production. Glyphosate, glyphosate, diquat, and other pesticides were recommended for alternative use. However, paraquat can be detected in some of diquat samples on the market, and patients with paraquat poisoning or symptoms close to paraquat poisoning are often visited clinically. Compared with paraquat, diquat has higher cost, narrower killing spectrum, and worse effect. Therefore, paraquat is often used to pretend diquat.
In many developing countries, deliberate self‐poisoning with paraquat continues to be a major public health concern. Most of paraquat poisoning is due to misuse or suicide with a very small lethal dose. 5 The clinical course of paraquat poisoning is rapid, and has very strong toxicity to all organs and tissues, not only leads to acute organ damage, but also leads to a variety of complications. Patients with severe paraquat‐induced poisoning may succumb to multiple organ failure involving the circulatory and respiratory systems with a fatality rate of up to 60%‐70%. 6 At present, there is no specific antidote to paraquat poisoning. Many studies indicate that the mortality of paraquat poisoning is closely related to its blood concentration. Therefore, the blood concentration of paraquat is an important indicator for clinical diagnosis of paraquat poisoning. 7 It was found that when paraquat concentration was higher than 2000, 600, 300, and 100 ng/ml at 4, 6, 10, and 24h after poisoning, the survival rate of patients would be very low. 8 Therefore, it is very important to early, quickly, and accurately determine the paraquat concentration in the blood of patients. In addition, the confusion between paraquat and diquat on sale in the market is not clear. So, it is very meaningful to develop and establish a method for simultaneous determination of paraquat and diquat in the blood of acute poisoning patients, which can provide basis for early and accurate diagnosis and treatment of paraquat and diquat poisoning.
At present, there are few reports about the simultaneous determination of paraquat and diquat in human body fluids, mainly LC‐MS/MS method. 9 , 10 This method is not very easy to popularize because of the expensive instrument. Heptafluorobutyric acid, an ion pair reagent, was usually used as the mobile phase, which is difficult to volatilize, can produce the ion suppression production, reduce the detection sensitivity, and also cause pollution to the instrument. However, HPLC is often used to determine paraquat alone in human blood. So, this study aimed to develop a simple and rapid HPLC method for simultaneous determination of paraquat and diquat in human plasma and apply it to clinical practice of paraquat and diquat poisoning patients. Because the maximum absorption wavelength of paraquat and diquat is different, a diode array detector with full‐wavelength scanning is selected in this method, which is convenient for qualitative and quantitative detection of compounds under different wavelengths.
2. MATERIALS AND METHODS
2.1. Reagents and Materials
Paraquat (99.2%, batch number 6320x) and diquat (99.8%, batch number 20 160 323) were purchased from Sigma‐Aldrich Labor Chemikalien GMBH (Milwaukee, USA) and Shanghai Pesticide Research Institute Co., Ltd., respectively (Shanghai, China). Acetonitrile (chromatographic pure, batch number 0 000 152 366) and methanol (chromatographic pure, batch number 0 000 189 995) were provided by JT Baker (Phillipsburg, USA). Sodium heptane sulfonate (batch number 20171012s1210) was provided by Agela Technologies (Tianjin, China). Ethyl acetate (chromatography pure, batch number 151 331 612) was provided by AppliChem (Darmstadt, Germany). Phosphoric acid (85%, chromatography pure) was provided by Fisher Scientific‐Janssen Physicalaan (Geel, Belgium). Ammonium formate (chemical pure, batch number 990 215) and potassium dihydrogen phosphate (>99.5%, analytical pure) were provided by China Pharmaceutical (Group) Shanghai Chemical Reagent Co., Ltd. (Shanghai, China) and Shandong Chemical Research Institute (Jinan, China), respectively. Purified water was provided by Hangzhou Wahaha Group Co., Ltd. (Hangzhou, China). Blank human plasma was obtained from healthy donors collected and stored immediately at −20°C until analysis, which was approved by the ethics committee of Qilu Hospital of Shandong University. The signed informed consent was obtained from each donor.
2.2. Instrumentation and analytical conditions
Sample analysis was performed on a Waters 2695‐2996 Series HPLC System equipped with a quaternary pump, a column oven, an automatic sampler, and a diode array detector (Waters Company, USA). The separation was achieved on a Hypersil Gold C18 Column (250 mm × 4.6 mm, 5 μm; Thermo) at 25°C. The mobile phase was a mixture of acetonitrile‐75 mmol/L sodium heptanesulfonate water solution (including 0.1 mol/L phosphoric acid) (13:87, v/v), in which pH value was adjusted to 3.0. The flow rate was 1.0 mL/min. The injection volume was 20 μl. Full‐wavelength scanning ranging from 200 nm to 400 nm was used. The detection wavelength of paraquat and diquat was 257 nm and 310 nm.
2.3. Preparation of standard and quality control (QC) samples
Stock solutions (2 mg/ml) were independently prepared by dissolving 0.02 g paraquat and diquat in 10 ml water. The mixed standard working solution of paraquat and diquat (1 mg/ml) was obtained by diluting the stock solution with water. Then, a series of mixed standard working solutions (500, 250, 100, 50, 25, 5, and 2.5 μg/ml) were prepared by appropriate dilution of the mixed standard solution (1 mg/ml) with water.
10 μl of the above mixed standard working solution was added to a 10‐ml plastic tube, and then, 490 μl of blank plasma was added to the tube to obtain the calibration standard samples, which concentration was 20, 10, 5, 2, 1, 0.5, 0.2, and 0.05 μg/ml. The quality control (QC) samples were similarly prepared at low, medium, and high concentrations of 0.1, 1.5, and 15 μg/ml. All stock and working solutions in water were kept at 4°C and brought to room temperature before use.
2.4. Sample extraction procedures
Waters Oasis® WCX solid‐phase extraction column (3 cc/60mg; Waters Corporation Milford, Massachusetts, USA) was used for the sample extraction, which was activated with 2 ml of methanol, 2 ml of water, and 2 ml of phosphate buffer (pH 7.0) before use. 500 μl of plasma was added to the column and eluted with 2ml of ammonium formate (20 mmol/L, pH 8.0) and 2ml of methanol, and then eluted with 1 ml of a mixture solution consisting of acetonitrile, ethyl acetate, and formic acid (4:4:2, v/v/v). The eluent was collected in a centrifuge tube and dried in a thermostatic water bath at 40°C under a gentle stream of nitrogen. The residue was reconstituted with 100 μL of mobile phase and transferred to the injection vials for analysis.
2.5. Method validation
The method was validated in terms of system suitability test, selectivity, extraction recovery, calibration curves, lower limit of quantitation (LLOQ), precision and accuracy, and stability according to the US Food and Drug Administration and Chinese State Food and Drug Administration guidelines for the validation of bioanalytical methods.
2.5.1. System suitability test
The standard solution of paraquat and diquat (5 μg/ml) was used to evaluate the system suitability, which included replicate injections (n = 5), the resolution of paraquat and diquat, the tailing factor, and the theoretical plate count. The acceptance criteria were as follows: the relative standard deviation (RSD) of the peak area and retention time of paraquat and diquat should be less than 2.0%, the tailing factor should be between the range of 0.95‐1.05, while the theoretical plate count should be more than 5000.
2.5.2. Selectivity
Six blank plasma and plasma spiked with paraquat and diquat were used to evaluate the selectivity in order to identify the potential interference of endogenous substances at retention times of the two analytes under the developed HPLC conditions.
2.5.3. Extraction recovery
The extraction recoveries were investigated at two concentration levels (0.1 and 15 μg/ml) in triplicate for each analyte. The recoveries were calculated by dividing the peak area for the pre‐spiked sample by the peak area for the post‐spiked sample and multiplying by 100%.
2.5.4. Calibration curves and lower limit of quantitation
The calibration curves were generated using eight none zero concentration levels (0.05, 0.2, 0.5, 1, 2, 5, 10, and 20μg/mL), typically described by the equation y = ax+b, where y corresponds to the peak area and x to the concentration. The calibration curves were constructed using quadratic least‐squares regression with the reciprocal of the concentration squared (1/x2) as the weighting factor. The back‐fit biases of each concentration were calculated and should be less than ± 15%. The LLOQ (0.05 μg/mL) was defined as the lowest concentration on the calibration curve with the precision of < 20%, expressed as relative standard deviation (RSD) and accuracy between 80% and 120%.
2.5.5. Precision and accuracy
QC samples at concentration of 0.05, 0.1, 1.5, and 15 μg/ml were freshly prepared and used to evaluate the precision and accuracy. The intra‐day precision was assessed on the same day of five replicates for each level. The inter‐day precision was studied at the same concentrations on three consecutive days. In addition, accuracy and precision of diluted samples were determined. Five high concentration samples (40 μg/ml for both paraquat and diquat) were performed and diluted two times with human blank plasma. The calculated concentrations were compared with the nominal concentrations. The accuracy was determined by comparing the mean measured concentration to its theoretical value and expressed as mean relative error (RE %). Precision was expressed as RSD. The RE and RSD should be less than ± 20% for concentration of 0.05 μg/ml and ± 15% for concentrations of 0.1, 1.5, and 15 μg/ml.
2.5.6. Stability
The stability was studied using two concentration levels (0.1 and 15 μg/ml) in triplicate stored or processed under different conditions, that is, storage at −20°C for 1 day and 7 days, freezing (−20°C) and thawing (room temperature) for two cycles, leaving plasma samples to stand on the benchtop for 5 h, and leaving the post‐extraction samples in the autosampler for 24 h at room temperature. The accuracy expressed as RE % should be less than ± 15%.
2.6. Application in acute poisoning patients
This method was used to determine the concentrations of paraquat and diquat in the plasma of acute poisoning patients in the emergency department of Qilu Hospital of Shandong University. 3 mL venous blood of each patient was collected into vacutainer tubes containing disodium ethylenediaminetetraacetic acid (EDTA‐Na) as the anticoagulant and then centrifuged at 5000 rpm for 5 min. The plasma was removed to an Eppendorf tube and stored at −20°C until analysis. 120 plasma samples of paraquat poisoning patients and 30 plasma samples of diquat poisoning were obtained. This study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). All protocols were reviewed and approved by the Scientific Research Ethics Committee of Qilu Hospital of Shandong University. Signed informed consent forms were obtained from all patients who participated in the study or their caregivers.
3. RESULTS
3.1. Method validation
3.1.1. System suitability test
The system suitability was evaluated using the standard solution of paraquat and diquat (5 μg/mL) by five replicate injections. The RSD of peak area and retention time of paraquat were 1.96% and 0.27%, while that of diquat was 1.95% and 0.26%. The mean tailing factors for paraquat and diquat were 1.026 and 1.027. The resolution of paraquat and diquat was 4.739. The mean theoretical plate counts for paraquat and diquat were 18 594 and 18 947. The results showed that the system suitability could meet the requirements.
3.1.2. Selectivity
Six blank plasma samples were used to investigate the selectivity of the method. Typical chromatograms obtained from blank plasma, spiked plasma sample with the paraquat and diquat, and blank plasma spiked with paraquat and diquat are shown in Figure 1. Paraquat and diquat were detected with excellent resolution and good peak shapes, and no interference from the endogenous substances was observed at the retention time of paraquat and diquat, which was 7.71 min and 8.86 min, respectively.
Figure 1.
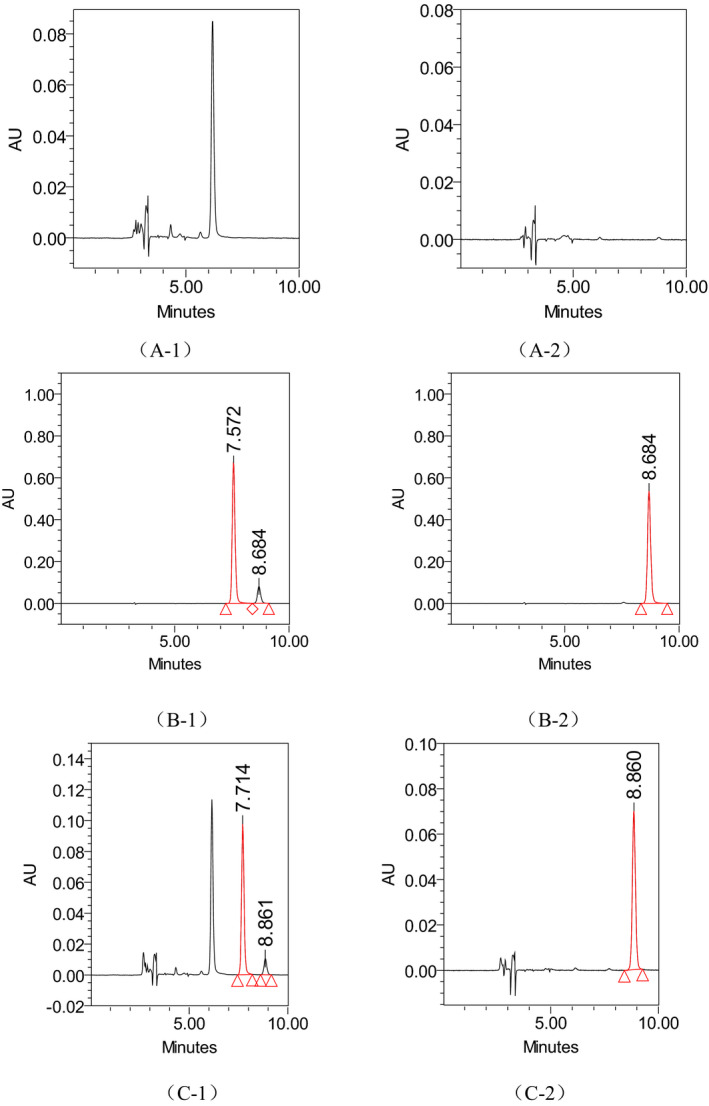
The typical chromatograms of blank plasma (A‐1, A‐2), standard solution of paraquat and diquat (B‐1, B‐2), and blank plasma spiked with paraquat and diquat (C‐1, C‐2). Note: (A‐1), (A‐2), and (A‐3) were specific chromatograms of paraquat at 257 nm, while (B‐1), (B‐2), and (B‐3) were specific chromatograms of diquat at 310 nm
3.1.3. Extraction recovery
The extraction recovery of paraquat was ranged from 95.38% to 103.97%, while the recovery of diquat was ranged from 94.79% to 98.40%, as shown in Table 1. The results were good and reproducible. It indicated that the analytical method could be kept free from endogenous substance in human plasma.
Table 1.
The precision and accuracy for paraquat and diquat in human plasma
| Analyte |
Concentration (μg/ml) |
Intra‐day | Inter‐day | Extraction recovery | |||||
|---|---|---|---|---|---|---|---|---|---|
|
Mean ± SD (μg/ml) |
Accuracy RE% |
Precision RSD% |
Mean ± SD (μg/ml) |
Accuracy RE% |
Precision RSD% |
Mean ± SD (%) |
RSD % |
||
| Paraquat | 0.05 | 0.052 ± 0.004 | 4.0 | 7.07 | 0.056 ± 0.009 | 12.0 | 16.49 | – | – |
| 0.1 | 0.098 ± 0.010 | −2.0 | 9.76 | 0.101 ± 0.011 | 1.0 | 11.06 | 95.38 ± 7.01 | 7.35 | |
| 1.5 | 1.560 ± 0.066 | 4.0 | 4.20 | 1.484 ± 0.088 | −1.1 | 5.90 | – | – | |
| 15 | 14.634 ± 0.454 | −2.4 | 3.10 | 14.580 ± 0.961 | −2.8 | 6.60 | 103.97 ± 0.89 | 0.85 | |
| Diquat | 0.05 | 0.052 ± 0.006 | 4.0 | 12.39 | 0.050 ± 0.007 | 0.0 | 14.14 | – | – |
| 0.1 | 0.097 ± 0.011 | −3.0 | 11.30 | 0.099 ± 0.012 | −1.0 | 12.60 | 94.79 ± 3.57 | 3.76 | |
| 1.5 | 1.590 ± 0.057 | 6.0 | 3.60 | 1.504 ± 0.095 | 0.3 | 6.28 | – | – | |
| 15 | 14.868 ± 0.478 | −0.9 | 3.22 | 14.928 ± 1.074 | −0.5 | 7.19 | 98.40 ± 0.79 | 0.81 | |
3.1.4. Calibration curves and lower limit of quantitation
The calibration curves were established ranging from 0.05 to 20 μg/mL for paraquat and diquat. A good linearity was found for the two analytes with correlation coefficients (r2) more than 0.99. The equations of the calibration curves were as follows: y = 3.76e + 005 x + 1.33e + 002 (r2 = 0.9968) for paraquat and y = 3.16e + 005 x + 2.82e + 004 (r2 = 0.9988) for diquat. The back‐fit biases of each concentration level for paraquat and diquat were less than 11.39% and 6.10%. The LLOQ was 0.05 μg/mL with RSD of 13.48% for paraquat and 8.07% for diquat.
3.1.5. Precision and accuracy
The results of the precision and accuracy at four different QC levels are also shown in Table 1. The intra‐day precision of paraquat and diquat, expressed as RSD %, was varied from 3.1% to 9.76% and 3.22% to 12.39, while the inter‐day precision of paraquat and diquat was varied from 5.9% to 16.49% (LLOQ) and 6.28% to 14.14%. The intra‐day accuracy of paraquat and diquat, expressed as RE %, was ranged from −2.0% to 4.0%, and −3.0% to 4.0%, while the inter‐day accuracy of paraquat and diquat was ranged from −2.8% to 12.0%, and −1.0% to 0.3%. In addition, the precision and accuracy of diluted samples were 3.48% and −3.31% for paraquat and 3.19% and −3.39% for diquat, respectively. Overall, all the results indicated that the method was accurate and precise for each compound.
3.1.6. Stability
All the stability results of paraquat and diquat in human plasma are summarized in Table 2. The data indicated that paraquat and diquat in human plasma were stable after storage at −20°C for 7 days and two freeze‐thaw cycles. It was also stable when left on benchtop for 5 h before processed and in autosampler for 24 h after processed.
Table 2.
The stability of paraquat and diquat in human plasma at various conditions (expressed as RE %)
| Analyte |
Concentration (μg/ml) |
Benchtop (5h) |
In autosampler (24h) | Two freeze‐thaw cycles | 7 days at‐20°C |
|---|---|---|---|---|---|
| Paraquat | 0.1 | −0.67 | −5.00 | 8.33 | 6.00 |
| 15 | 1.66 | −1.42 | 0.42 | 2.93 | |
| Diquat | 0.1 | 8.33 | −8.67 | −2.33 | 7.33 |
| 15 | 2.93 | −1.82 | 2.32 | 5.13 |
3.2. Application in acute poisoning patients
The established method was successfully used for the determination of paraquat and diquat in acute poisoning patients. 120 plasma samples of paraquat poisoning patients and 30 plasma samples of suspected diquat poisoning patients were analyzed. The chromatograms of paraquat and diquat poisoning patient plasma are shown in Figure 2. The measured concentrations ranged from 0.1 to 20.62 μg/ml with a mean of 3.61 (standard error = 4.56) μg/mL for paraquat and 0 to 26.59 μg/ml with a mean of 2.00 (standard error = 6.20) μg/ml for diquat. The 95% confidence intervals for mean of paraquat and diquat were 2.79‐4.44 μg/ml and −0.36‐5.62 μg/ml. Paraquat and diquat were both determined in five suspected diquat poisoning samples with concentration of 0.16, 0.14, 0.15, 17.29, and 26.59 μg/ml for diquat and 0.13, 0.19, 0.18, 9.14, and 12.87 μg/ml for paraquat. No diquat but paraquat was detected in another two suspected diquat poisoning samples with concentration of 0.51 and 0.38 μg/ml. The results indicated that diquat sold on the market may be paraquat or containing paraquat.
Figure 2.
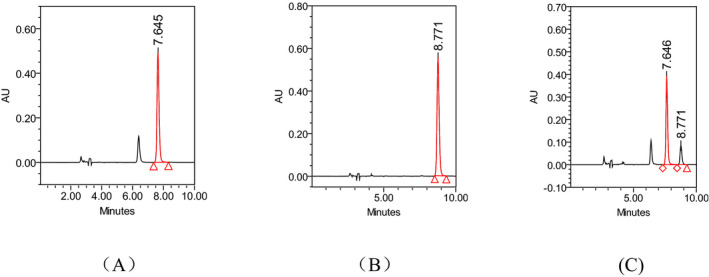
The chromatograms of paraquat and diquat in paraquat (A) or diquat (B) poisoning samples, and both paraquat and diquat in the same suspected diquat poisoning sample (C)
4. DISCUSSIONS
There are several reports published for the determination of paraquat in human plasma, mainly including chromatography and chromatography‐tandem mass spectrometry. However, there are few reports about the detection methods of diquat in human plasma due to its less use and less poisoning events before. But since paraquat aqueous solution was banned for sale and use in China, diquat poisoning events were more and more. So, it is very necessary to establish a method for simultaneous determination of paraquat and diquat in plasma obtained from acute poisoning patients. In this study, a HPLC‐DAD method with LLOQ of 0.05 μg/ml was established and validated for simultaneous determination of paraquat and diquat in human plasma. This method only required 0.5 ml plasma and was successfully applied in the determination of paraquat and diquat poisoning patients. Although the LLOQ of this study was higher than the reported LC‐MS/MS methods 9 , 10 with LOQ of 0.005 μg/ml, it could meet the detection requirements of paraquat and diquat in clinic. Because survival is likely if the paraquat concentration in the plasma does not exceed 2.0, 0.6, 0.3, 0.16, and 0.1 μg/ml at 4, 6, 10, 16, and 24, respectively, after ingestion. Moreover, the above two LC‐MS/MS methods were only applied to determine one or two paraquat poisoning patients who were dead with concentration of 0.64, 2.5, and 2.8 μg/ml. The established HPLC method in this study was successfully applied to determine 120 plasma samples of paraquat poisoning patients and 30 plasma samples of suspected diquat poisoning patients.
LC‐MS method has great advantages in the simultaneous determination of multiple compounds. So, LC‐MS method was first tried to determine paraquat and diquat. C18 column was used for separating the analytes, and the mixture of 10 mmol/L ammonium formate and acetonitrile was used as mobile phase. Because paraquat and diquat were high water‐soluble compounds, the proportion of water phase in mobile was relatively large. However, paraquat and diquat could not be well‐retained. Gradient elution with different proportion of water and organic phase was tried, but the results were still unsatisfactory. Several LC‐MS/MS methods were reported for simultaneous determination of paraquat and diquat in human body fluids. But the mobile phase of these methods contained heptafluorobutyric acid 9 , 10 or trichloroacetic acid, 11 which was not volatile and could cause the ion inhibition, even damaged the instrument. Finally, HPLC method was used. Because the maximum absorption wavelength of paraquat (257 nm) and diquat (310 nm) was different, diode array detector (DAD), which can scan all wavelengths, was used for simultaneous determination of paraquat and diquat.
Solid‐phase extraction (SPE) and protein precipitation were usually used to extract paraquat. Protein precipitation is easy to operate. Trichloroacetic acid or perchloric acid is commonly used as precipitants, which can cause damage to the column and reduce its efficiency due to the low pH value. Therefore, SPE method with Waters OASIS® WCX was selected to extract paraquat and diquat in this study. The column was activated with methanol, water, and phosphate before use, which could produce high extraction efficiency. The plasma was first eluted with ammonium formate buffer solution to remove the soluble impurities, then eluted with methanol to remove the liposoluble impurities, and finally eluted with the mixture of acetonitrile, ethyl acetate, and formic acid (4:4:2), which was collected for further analysis. The above elution solution was selected based on the physical and chemical properties and the extraction recoveries of paraquat and diquat. The mixed solution of acetonitrile‐formic acid with proportion of 4:1 and 4:2 was firstly tried to use as the elution solution, and the extraction recoveries of paraquat were about 25% and 56%, and could not meet the detection requirement. It indicated that the more the formic acid, the higher the recoveries. Then, the mixed solution of acetonitrile‐ethyl acetate‐formic acid with proportion of 4:2:2 and 4:4:2 was tried. The extraction recoveries of paraquat and diquat increased and could be achieved about 100% (4:4:2). So acetonitrile‐ethyl acetate‐formic acid (4:4:2) was finally selected to use as the elution solution. The eluent was tired to be directly injected for HPLC analysis. However, the retention time of paraquat and diquat was different with the standard solution prepared with the mobile phase, which is advanced about 0.5 min due to the large difference between the eluent composition and the mobile‐phase composition. The separation of paraquat and diquat was not good either. Finally, the eluent was dried in water bath with nitrogen, which can not only solve the problem of earlier retention time, and make paraquat and diquat separated completely, but also improve the detection sensitivity. Based on this, the amount of plasma was reduced to 0.5 ml, which was 1 ml in the published reference.
Water‐soluble compounds are suitable for internal standard due to the high water solubility of paraquat and diquat. Bergenin and vancomycin were tried to use as internal standard. However, the maximum absorption wavelength of bergenin was 275 nm. Its absorption was less at 257 nm and 310 nm. The maximum absorption of vancomycin was 280 nm. It was suitable for internal standard of paraquat due to the good absorption at 257 nm, but not suitable for diquat because of its no absorption at 310 nm. It is difficult to find an internal standard with high water solubility and good absorption at the wavelength of paraquat and diquat. Therefore, the external standard method, reported in other HPLC methods for the determination of paraquat, was used to quantify paraquat and diquat in this study, which could produce accurate results after fully methodology validation. It can be used for analysis of clinical samples.
It has been reported 12 that the concentration of paraquat in dead group was 1.95‐22.2 μg/ml, while in survival group it was 0‐2.99 μg/ml. The best critical concentration to judge the clinical outcome of paraquat poisoning is 2.43 μg/ml. In this study, the concentration obtained from the 120 samples of paraquat poisoning patients was ranged from 0.10 to 20.62 μg/ml with a mean of 3.61 μg/ml. The concentration of 70 samples was less than 1.9 μg/ml. The concentration of 35 samples was in the range of 1.9‐10 μg/ml. The concentration of 15 samples was higher than 10 μg/ml. If the concentration of paraquat was higher than 2.9 μg/ml, the cure rate is low according to the clinical feedback.
The concentration range of death and survival of diquat poisoning has not been summarized because the cases of diquat poisoning in clinic are less. 30 samples obtained from suspected diquat poisoning patients were analyzed in this study. The determined concentration range of diquat was 0‐26.59 μg/ml with a mean of 2.00 μg/ml. Both diquat and paraquat were detected in five samples obtained from suspected diquat poisoning patients. No diquat but paraquat was detected in two samples obtained from suspected diquat poisoning patients. This phenomenon indicates that diquat sold on the market may be paraquat or containing paraquat. The paraquat poisoning is still inevitable. Therefore, the method of simultaneous determination of paraquat and diquat in human plasma established in this study plays an important role in determining the toxic species of acute poisoning patients and can provide valuable reference for clinicians to make accurate diagnosis as soon as possible and take appropriate treatment measures.
5. CONCLUSION
In this study, a HPLC‐DAD method was developed to simultaneously quantify the concentrations of paraquat and diquat in human plasma. The method is both sensitive and selective, and shows good validation performance. The method is easy to popularize due to the cheaper instrument. The LLOQ and the amount of plasma required were lower than the method reported in the references. This method was successfully applied to determine paraquat and diquat in acute poisoning patients, which provided valuable reference for clinicians to make accurate diagnosis and treatment.
ACKNOWLEDGMENTS
This work was supported by Medical and Health Technology Development projects in Shandong Province (2017WS002) and the National Natural Science Foundation of China (No. 81803381).
Yuan G, Li R, Zhao Q, et al. Simultaneous determination of paraquat and diquat in human plasma by HPLC‐DAD: Its application in acute poisoning patients induced by these two herbicides. J Clin Lab Anal.2021;35:e23669. 10.1002/jcla.23669
Guiyan Yuan and Rong Li are contributed equally to this work.
Contributor Information
Xiaojing Wang, Email: wxjjinan@163.com, Email: grc7636@126.com.
Ruichen Guo, Email: wxjjinan@163.com, Email: grc7636@126.com.
DATA AVAILABILITY STATEMENT
All relevant data are included in the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author upon request.
REFERENCES
- 1. Saeed SA, Wilks MF, Coupe M. Acute diquat poisoning with intracerebral bleeding. Postgrad Med J. 2001;77:329‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ballard KD, Vickery WE, Nguyen LT, et al. An analytical strategy for quaternary ammonium neuromuscular blocking agents in a forensic setting using LC‐MS/MS on a tandem quadrupole/time‐of‐flight instrument. J Am Soc Mass Spectrom. 2006;17:1457‐1468. [DOI] [PubMed] [Google Scholar]
- 3. Pateiro‐Moure M, Arias‐Estévez M, Simal‐Gándara J. Critical review on the environmental fate of quaternary ammonium herbicides in soils devoted to vineyards. Environ Sci Technol. 2013;47:4984‐4998. [DOI] [PubMed] [Google Scholar]
- 4. Baselt RC. Disposition of Toxic Drugs and Chemicals in Man, 5th edn. California: Chemical Toxicology Institute; 2000. [Google Scholar]
- 5. Dinis‐Oliveira RJ, Sarmento A, Reis P, et al. Acute paraquat poisoning: report of a survival case following intake of a potential lethal dose. Pediatr Emerg Care. 2006;22:537‐540. [DOI] [PubMed] [Google Scholar]
- 6. Tan JT, Letchuman Ramanathan G, Choy MP, et al. Paraquat poisoning: experience in hospital taiping (year 2008‐october 2011). Med J Malaysia. 2013;68:384‐388. [PubMed] [Google Scholar]
- 7. Sun L, Li GQ, Yan PB, et al. Prediction of outcome following paraquat poisoning by arterial lactate concentration‐time data. Exp Ther Med. 2014;8:652‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seok SJ, Kim YH, Gil HW, et al. The time between paraquat ingestion and a negative dithionite urine test in an independent risk factor for death and organ failure in acute paraquat intoxication. J Korean Med Sci. 2012;27:993‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang KC, Chen SM, Hsu JF, et al. Simultaneous detection and quantitation of highly water‐soluble herbicides in serum using ion‐pair liquid chromatography‐tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:211‐218. [DOI] [PubMed] [Google Scholar]
- 10. Ariffin MM, Anderson RA. LC/MS/MS analysis of quaternary ammonium drugs and herbicides in whole blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;842:91‐97. [DOI] [PubMed] [Google Scholar]
- 11. Ruan XL, Qiu JJ, Wu C, et al. Magnetic single‐walled carbon nanotubes‐dispersive solid‐phase extraction method combined with liquid chromatography‐tandem mass spectrometry for the determination of paraquat in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;965:85‐90. [DOI] [PubMed] [Google Scholar]
- 12. Yue XJ, Li W, Li PF. Determination of paraquat in plasma by HPLC‐MS/MS. Lab Med. 2018;33:132‐138. (Article in Chinese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author upon request.


