Abstract
Humankind is facing its worst pandemic of the twenty-first century, due to infection of a novel coronavirus named as SARS-CoV2, started from Wuhan in China. Till now, 15 million people are infected, causing more than 600,000 deaths. The disease, commonly known as, COVID-19, was initially thought to be associated with ARDS only, but later on revealed to have many unexplained and atypical clinical features like coagulopathy and cytokinemia, leading to multi-organ involvements. The patients also suffer from ‘Silent Hypoxemia’, where there is no immediate respiratory signs and symptoms even though alarmingly low SpO2 level. We hypothesize that this covert hypoxemia may lead to molecular changes exacerbating coagulopathy and cytokine storm in COVID19 patients, which again, in turn, causes a vicious cycle of more hypoxemia/hypoxia and progression of the infection to more severe stages through HIF-1α dependent pathway. Although molecular mechanisms are yet to be substantiated by scientific evidence, hypoxemia remains an independent worsening factor in serious COVID 19 patients. Keeping all in mind, we propose that even in the early and asymptomatic cases, prophylactic oxygen therapy to be initiated to break the vicious cycle and to reduce the mortality in COVID 19 to save precious human lives.
Background
The world is facing one of its deadly pandemics caused by a novel Corona Virus SARS-CoV2. Starting from Wuhan in China in November 2019 it has spread to more than 200 countries throughout the globe. Till date, almost 115 million people have been infected and more than 2.54 million human lives have been lost due to this dreaded disease. COVID-19, the disease process caused by infection SARS-CoV2, is a complex disease presenting with a range of clinical symptoms, ranging from asymptomatic to severe and rapid multiple organ dysfunction syndrome (MODS) and ultimately death. During the initial stage of the pandemic, the primary symptom of COVID-19 was reported to be related to Severe Acute Respiratory Illness (SARI) but subsequently, the reports have been published which state that COVID-19 not only affects the lungs but also various other organs including the circulatory and immune system. Most patients infected with SARS-CoV-2 initially present clinically with mild symptoms, while some of these patients progressively experience severe infection and eventually died of acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS) [1]. The proposed mechanism for these complications is multifactorial.
COVID-19 and “Silent Hypoxemia”
COVID-19 patients are developing hypoxemia without signs of breathlessness aptly being termed as “Silent Hypoxemia” because it is insidious in onset and remains undetected until an advanced stage [2, 3]. Some physicians have also reported such low levels of blood oxygen that would normally cause a person to be unconscious or severely incapacitated [4]. These patients don’t show any symptoms of breathlessness, even though their chest X-rays show diffuse pneumonia with their oxygen saturation reaching as low as 50%. The sensation of breathlessness happens only when pneumonia causes the collapse of the air sacs leading to a fall in oxygen levels. But in the case of COVID-19, it is postulated that the lungs remain compliant initially and patients still can exhale carbon dioxide and hence not experiencing shortness of breath [5]. These extremely low oxygen levels most probably lead to cascades of molecular changes in various tissues which in turn can activate a series of events leading to the damage in various organs which can be lethal. In fact, a retrospective study on 140 COVID-19 patients demonstrated that hypoxemia is independently associated with mortality [6].
Hypoxic Conditions Leads to Increased Viral Infection
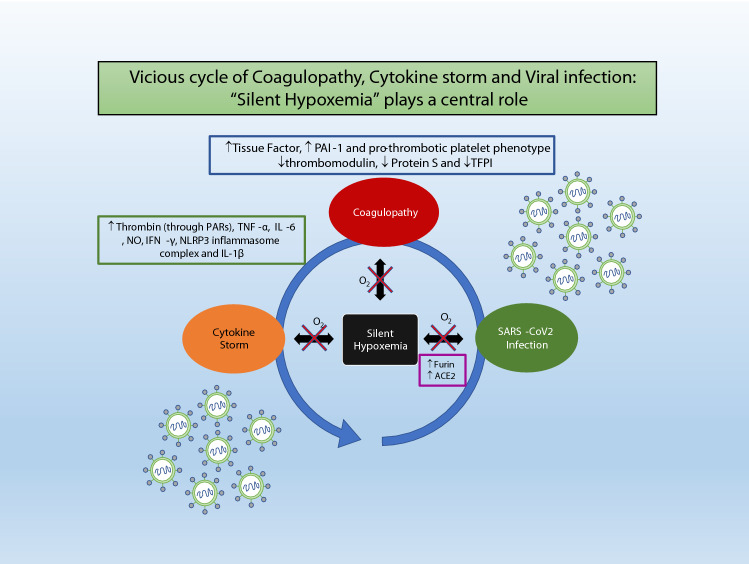
Furin, which is a very important host enzyme required for cleaving of S protein of the SARS-CoV2, leading to the binding of the virus with membrane-bound Angiotensin Convertase Enzyme 2 (ACE2), is upregulated along with ACE2 by a key hypoxia-responsive molecule HIF-1α [7–12]. Once SARS-COV2 infection takes place, it leads to silent hypoxemia which causes upregulation of Furin and ACE2, ultimately more severe infection. This suggests that silent hypoxemia is playing an important role in the progression of mild infection to more severe infection (Fig. 1).
Fig. 1.
Vicious cycle of Coagulopathy, Cytokine storm and Viral infection: “Silent Hypoxemia” plays a central role. Early detecting and treating “Silent Hypoxemia” with non-invasive oxygen therapy might stop this vicious cycle in an inexpensive way, ultimately reducing COVID-19-related morbidity and mortality.
Increased Coagulopathy Due to “Silent Hypoxemia”
Clinicians treating COVID-19 have reported a novel and alarming finding of development of microvascular clots in the lungs and other vital organs [13]. These clots are proposed to be responsible for systemic involvement of different organs and various complications including stroke, heart failure, renal failure etc. apart from respiratory failure. It has been revealed that there is a sevenfold increase in the incidence of a sudden stroke in young COVID-19 patients who don’t have any past medical history and have either mild symptoms (or in two cases, no symptoms) of COVID-19 [14]. 71.4% of non-survivors of COVID-19 have been classified as having overt disseminated intravascular coagulation (≥ 5 points) according to the International Society of Thrombosis and Haemostasis (ISTH) criteria [13]. Increased concentrations of D-dimer and other fibrin degradation products in these patients have been reported and are significantly associated with poor prognosis [13] (Fig. 1).
Persistent hypoxemia, silent or overt leads to tissue hypoxia of various degrees depending on the severity and duration of hypoxemia. It is reported that both systemic and local hypoxia is also associated with higher incidences of thrombosis [15, 16].
The Wessler stasis model of venous thrombosis has demonstrated that hypoxemia is necessary at least acutely, to trigger clot formation [17]. HIF-α which is a major player induces the expression of Tissue Factor and Plasminogen activator inhibitor-1(PAI-1) and downregulates the expression of tissue factor pathway inhibitor (TPFI), thrombomodulin and protein S, ultimately leading to the upregulation of the coagulation pathway while inhibiting anticoagulation and fibrinolysis pathways [18–24]. Hypoxia also converts platelets into a pro-thrombotic phenotype [25, 26]. In addition to the role of hypoxia in venous thrombus formation, vascular wall hypoxia and inflammation also plays a role in arterial thrombogenesis [27].
Additionally, activation of coagulation pathways also results in overproduction of various pro-inflammatory cytokines leading to multiorgan injury. For example, Thrombin is mainly involved in clot formation, but it also augments inflammation via proteinase-activated receptors (PARs), principally PAR1 [28].
In reverse, the hypercoagulable state prevents blood from flowing normally through the lung to pick up oxygen again leading to low oxygen in blood or “hypoxemia”- creating a vicious cycle.
Hypoxemia Causes Cytokine Storm in COVID-19
One of the major factors contributing to COVID-19 mortality is the massive overproduction of early response proinflammatory cytokines (tumour necrosis factor [TNF], IL-6, and IL-1β), described as a cytokine storm [1, 29]. Cytokine storm is leading to an increased risk of vascular hyperpermeability, multiorgan failure, and eventually death when the high cytokine concentrations remain persistent over time [30].
Hypoxemia induces synthesis and release of proinflammatory cytokines by different macrophage populations leading to an immediate cytokinemia with highly elevated levels of circulating TNF-α and IL-6 [31]. Hypoxemia also induces IFN-γ production in macrophages through NO-dependent pathway [32]. All these cytokines contribute to the severe inflammation or cytokine storm seen in COVID-19 patients. Gupta et al. have demonstrated that HIF-1α induces the expression of Leucine-rich-containing family, pyrin domain containing 3 (NLRP3) inflammasome complex and Interleukin-1β secretion, which are the key determinants of acute thrombotic events during hypoxic conditions [33]. HIFα independent signalling pathways can also regulate thrombogenesis by inducing various pro-inflammatory mediators such as Tumor Necrosis Factor (TNF) α and Interleukin (IL)-1 [33].
Inversely, this hyper-inflammatory environment causes damage to lung epithelial and endothelial cell, leading to vascular leakage, alveolar oedema, coagulopathy (due to endothelial cell inflammation) and again hypoxia (due to damage to lung epithelial and endothelial cells)- another component of a vicious cycle (Fig. 1).
“Silent Hypoxemia” Causes Vicious Cycle of Coagulopathy, Cytokine Storm and Viral Infection
Keeping the above observations in mind, we postulate that at the molecular level, silent hypoxemia, coagulopathy and cytokine storm are major contributing factors to COVID-19 mortality and morbidity. We hypothesize that the silent hypoxemia which is occurring at initial stages of the infection and not getting addressed; persists, contributing to a very complex and vicious cycle of increased intensity of viral infection (due to increased expression of Furin and ACE2), coagulopathy, cytokine storm and ultimately more hypoxia/hypoxemia (Fig. 1).
Early Institution of Prophylactic Oxygen Can Save Precious Human Lives
In our opinion, “Silent Hypoxemia” should be recognised and treated in patients having mild symptoms of COVID-19 by non-invasive oxygen therapy through nasal prongs or a face mask, so that severe complications of blood coagulation and cytokine storm can be avoided in these patients in an inexpensive manner leading to a decrease in the morbidity and mortality.
Discussion
In the current scenario, it is becoming a challenge to understand the exact mechanism of these life-threatening complications in COVID-19 patients. Literature suggests that hypoxemia is one of the important contributing factors for coagulopathy [15–17, 27]. It is proposed that hypoxia induces Early Growth Response (EGR) 1, which in turn regulate thrombus formation. Moreover, HIFs or HIF targeted genes induces prothrombotic factors like PAI-1 and tissue factors, while antithrombotic factors like thrombomodulin, Protein S and TFPI. However, the exact role of these pathways is yet to be elucidated in COVID-19 pathology. A detailed study showing an association between tissue/ serum HIF levels with various prothrombotic and antithrombotic factors in COVID-19 patients is required to substantiate our theory.
While it has also been reported by various studies that increase in pro-inflammatory cytokines like thrombin, TNFα, IL6, IFN γ, IL-1β is also associated with hypoxia [31–33], the other hand, it is proposed that intense viral infection is caused by increased in Furin and ACE2 expression in the human cells, resulted by the relatively hypoxic environment in the lungs and other upper respiratory tracts tissue [11, 12]. Sudden rise in proinflammatory cytokines, otherwise known as ‘Cytokine Storm’ in context with SARS-CoV2 infection, has been reported in literature along with their clinical outcome[1, 29, 30]. However, the molecular mechanism detailing the pathophysiology of hypercytokinemia in COVID-19 patients is not much clear yet. Proposed interventions, directed to reduce the release of proinflammatory cytokines by using therapeutic agents like corticosteroids, are reported in the scientific literature, although the exact mechanism behind the cause of this cytokine storm is sparsely evaluated [34, 35]. Again it is reported that HIF mediated pathways are responsible for increased inflammatory responses and as well as hypercoagulable state, so we propose crosstalk between the two most important complications in COVID-19.
How hypoxia increases the viral load of SARS-CoV2 remains only in theory to date. Substantial proof in the form of scientific evidence is yet to be proved in a practical scenario. Mechanistic study in in-vitro and in-vivo models to substantiate the actual scenario remains to be conducted.
It is evident with clinical and radiological evidence that there is damage of lung tissue leads to the ARDS or SARI, with documented reduced SpO2 levels. However, many of the COVID-19 patients experience to ‘Happy Hypoxia’, the other synonym of ‘Silent Hypoxemia’, where unlike other diseases there very little discomfort to the patients even at a very low oxygen tension. WHO, in this context, recommended early oxygen therapy to patients even without signs of severe respiratory distress, hypoxemia (i.e. SpO2 < 90%) or shock [36]. They also recommended that oxygen should not be restricted because of concerns about a patient’s respiratory drive [36]. A preliminary report from India suggesting that early oxygen therapy is successful in treating corona infection in more than 300 patients [37], while reports from China suggested that higher oxygen saturation more than SpO2 90% is independently associated with reduced mortality in seriously ill patients [6].
Conclusion
Early Institution of Prophylactic Oxygen Can Save Precious Human Lives
As per routine clinical practice, for other diseases, a patient is put into oxygen, with either clinical featured of hypoxemia or documented SpO2 level below 95% even in critically ill patients [38]. In our opinion, “Silent Hypoxemia” should be recognised early and treated in patients having mild symptoms of COVID-19, by non-invasive oxygen therapy through nasal prongs or a face mask, so that severe complications of blood coagulation and cytokine storm can be avoided in these patients in an inexpensive manner leading to a decrease in the morbidity and mortality. Overall while it is accepted that the hypoxia results in poor outcome in terms of survival [6, 39], we are of the opinion that even in asymptomatic COVID 19 patients, an early institution of prophylactic oxygen therapy will lead to reduced overall morbidity and save precious human life. Moreover, the overall cost–benefit ratio in an institution of early O2 therapy vs managing the complications of COVID-19 clearly tilts the balance to the Oxygen therapy.
Author Contributions
Conception and design: AC, AP. Drafting of the article: AC, AP. Critical revision of the article for important intellectual content: PC. Revision Correction: AC, RK, AP. Final approval of the article: PC, AP.
Financial Support
Council of Scientific and Industrial Research, India for fellowship to Anshika Chauhan [Grant No: 09/141(0209)/2019] and Rajandeep Kaur [Grant No: 09/141(0210)/2019].
Declarations
Conflict of interest
Authors have disclosed no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/29/2021
A Correction to this paper has been published: 10.1007/s12291-021-00979-w
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie J, Tong Z, Guan X, Du B, Qiu H, Slutsky AS. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensive Care Med. 2020;46(5):837–840. doi: 10.1007/s00134-020-05979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 Pneumonia in Wuhan. China JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottestad W, Seim M, Mæhlen JO. COVID-19 with silent hypoxemia. Tidsskr Nor Laegeforen. 2020 doi: 10.4045/tidsskr.20.0299. [DOI] [PubMed] [Google Scholar]
- 5.Expert warns key symptom affecting coronavirus patients is a SILENT killer. Available at https://www.express.co.uk/life-style/health/1272667/coronavirus-latest-discovery-silent-hypoxia-pneumonia-expert-warning. Accessed 27 Apr 2020
- 6.Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, et al. Association between hypoxemia and mortality in patients With COVID-19. Mayo Clin Proc. 2020;95(6):1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M, Kleine-Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78(4):779–784.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7):e00127–e220. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu J, Zhang J, Gong Y, Testa CL, Klein-Szanto AJ. Regulation of HIF-1 alpha by the proprotein convertases furin and PC7 in human squamous carcinoma cells. Mol Carcinog. 2015;54(9):698–706. doi: 10.1002/mc.22131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi S, Wollenzien H, Leclerc E, Jarajapu YP. Hypoxic regulation of angiotensin-converting enzyme 2 and Mas receptor in human CD34+ cells. J Cell Physiol. 2019;234(11):20420–20431. doi: 10.1002/jcp.28643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covid-19 causes sudden strokes in young adults, doctors say. Available at https://edition.cnn.com/2020/04/22/health/strokes-coronavirus-young-adults/index.html. Accessed on 27 Apr 2020
- 15.Bovill EG, van der Vliet A. Venous valvular stasis-associated hypoxia and thrombosis: what is the link? Annu Rev Physiol. 2011;73:527–545. doi: 10.1146/annurev-physiol-012110-142305. [DOI] [PubMed] [Google Scholar]
- 16.Hamer JD, Malone PC, Silver IA. The PO2 in venous valve pockets: its possible bearing on thrombogenesis. Br J Surg. 1981;68(3):166–170. doi: 10.1002/bjs.1800680308. [DOI] [PubMed] [Google Scholar]
- 17.Millet J, Vaillot M, Theveniaux J, Brown NL. Experimental venous thrombosis induced by homologous serum in the rat. Thromb Res. 1996;81:497–502. doi: 10.1016/0049-3848(96)00023-0. [DOI] [PubMed] [Google Scholar]
- 18.Liao H, Hyman MC, Lawrence DA, Pinsky DJ. Molecular regulation of the PAI-1 gene by hypoxia: contributions of Egr-1, HIF-1alpha, and C/EBPalpha. FASEB J. 2007;21(3):935–949. doi: 10.1096/fj.06-6285com. [DOI] [PubMed] [Google Scholar]
- 19.Cui XY, Skretting G, Tinholt M, Stavik B, Dahm AEA, Sahlberg KK, et al. A novel hypoxia response element regulates oxygen-related repression of tissue factor pathway inhibitor in the breast cancer cell line MCF-7. Thromb Res. 2017;157:111–116. doi: 10.1016/j.thromres.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Yan SF, Zou YS, Gao Y, Zhai C, Mackman N, Lee SL, et al. Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia. Proc Natl Acad Sci USA. 1998;95(14):8298–8303. doi: 10.1073/pnas.95.14.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans CE, Bendahl PO, Belting M, Branco C, Johnson RS. Diverse roles of cell-specific hypoxia-inducible factor 1 in cancer-associated hypercoagulation. Blood. 2016;127(10):1355–1360. doi: 10.1182/blood-2015-09-671982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa S, Shreeniwas R, Butura C, Brett J, Stern DM. Modulation of endothelial function by hypoxia: perturbation of barrier and anticoagulant function, and induction of a novel factor X activator. Adv Exp Med Biol. 1990;281:303–312. doi: 10.1007/978-1-4615-3806-6_32. [DOI] [PubMed] [Google Scholar]
- 23.Pilli VS, Datta A, Afreen S, Catalano D, Szabo G, Majumder R. Hypoxia downregulates protein S expression. Blood. 2018;132(4):452–455. doi: 10.1182/blood-2018-04-841585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinsky DJ, Liao H, Lawson CA, Yan SF, Chen J, Carmeliet P, et al. Coordinated induction of plasminogen activator inhibitor-1 (PAI-1) and inhibition of plasminogen activator gene expression by hypoxia promotes pulmonary vascular fibrin deposition. J Clin Invest. 1998;102(5):919–928. doi: 10.1172/JCI307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron SJ, Mix DS, Ture SK, Schmidt RA, Mohan A, Pariser D, et al. Hypoxia and Ischemia Promote a Maladaptive Platelet Phenotype. Arterioscler Thromb Vasc Biol. 2018;38(7):1594–1606. doi: 10.1161/ATVBAHA.118.311186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyagi T, Ahmad S, Gupta N, Sahu A, Ahmad Y, Nair V, et al. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced prothrombotic phenotype. Blood. 2014;123(8):1250–1260. doi: 10.1182/blood-2013-05-501924. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura Y, Yamashita A, Iwakiri T, Sugita C, Okuyama N, Kitamura K, et al. Vascular wall hypoxia promotes arterial thrombus formation via augmentation of vascular thrombogenicity. Thromb Haemost. 2015;114(1):158–172. doi: 10.1160/TH14-09-0794. [DOI] [PubMed] [Google Scholar]
- 28.José RJ, Williams AE, Chambers RC. Proteinase-activated receptors in fibroproliferative lung disease. Thorax. 2014;69(2):190–192. doi: 10.1136/thoraxjnl-2013-204367. [DOI] [PubMed] [Google Scholar]
- 29.Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–e47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108(5):1303–14. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- 31.Ertel W, Morrison MH, Ayala A, Chaudry IH. Hypoxemia in the absence of blood loss or significant hypotension causes inflammatory cytokine release. Am J Physiol. 1995;269(1 Pt 2):R160–R166. doi: 10.1152/ajpregu.1995.269.1.R160. [DOI] [PubMed] [Google Scholar]
- 32.Angele MK, Schwacha MG, Smail N, Catania RA, Ayala A, Cioffi WG, et al. Hypoxemia in the absence of blood loss upregulates iNOS expression and activity in macrophages. Am J Physiol. 1999;276(2):C285–C290. doi: 10.1152/ajpcell.1999.276.2.C285. [DOI] [PubMed] [Google Scholar]
- 33.Gupta N, Sahu A, Prabhakar A, Chatterjee T, Tyagi T, Kumari B, et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A. 2017;114(18):4763–4768. doi: 10.1073/pnas.1620458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang SS, Lipes J. Corticosteroids for critically ill COVID-19 patients with cytokine release syndrome: a limited case series. Can J Anaesth. 2020;67(10):1462–1464. doi: 10.1007/s12630-020-01700-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolilekas L, Loverdos K, Giannakaki S, Vlassi L, Levounets A, Zervas E, et al. Can steroids reverse the severe COVID-19 induced “cytokine storm”? J Med Virol. 2020;92(11):2866–2869. doi: 10.1002/jmv.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.https://www.who.int/csr/disease/coronavirus_infections/InterimGuidance_ClinicalManagement_NovelCoronavirus_11Feb13u.pdf. Accessed on 27 May 05 2020.
- 37.People Recover From COVID-19 With Early Oxygen Therapy In Bhopal. Available at https://www.ndtv.com/india-news/coronavirus-india-396-people-recover-from-covid-19-with-early-oxygen-therapy-in-madhya-pradeshs-bhopal-2225770. Accessed on 27 May 05 2020
- 38.Yang X, Shang Y, Yuan S. Low versus high pulse oxygen saturation directed oxygen therapy in critically ill patients: a randomized controlled pilot study. J Thorac Dis. 2019;11(10):4234–4240. doi: 10.21037/jtd.2019.09.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kashani KB. Hypoxia in COVID-19: Sign of Severity or Cause for Poor Outcomes. Mayo Clin Proc. 2020;95(6):1094–1096. doi: 10.1016/j.mayocp.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]