Abstract
Objective
To determine AF prevalence and gaps in atrial fibrillation (AF) awareness and management in China.
Methods
We conducted a community-based survey of 47 841 adults (age ≥45 years) in seven geographic regions of China between 2014 and 2016. Participants underwent a structured questionnaire, a standard 12-lead ECG, physical examination and blood sampling. AF prevalence, defined by either ECG detection or self-report, was estimated according to sampling weights, non-response and age and sex distribution of the population. We used multivariable logistic regression to estimate associations among sociodemographic, clinical and geographic factors with the AF prevalence, awareness and treatment.
Results
The weighted AF prevalence was 1.8% (95% CI 1.7% to 1.9%), but varied from 0.9% to 2.4% across geographical regions and equates to being present in an estimated 7.9 (95% CI 7.4 to 8.4) million people in China. Among men and women, the AF prevalence increased from 0.8% and 0.6% in the age group 45–54 years to 5.4% and 4.9% in the age group ≥75 years, respectively. Proportions of people who were aware of having AF decreased overall from 65.3% in 45–54 year-olds to 53.9% in ≥75 year-olds and varied between sex (men 58.5%, women 68.8%) and residency status (urban 78.3%, rural 35.3%). Only 6.0% of patients with high-risk AF received anticoagulation therapy.
Conclusions
AF prevalence is higher than previously reported in China, with low awareness and large treatment gaps. Large-scale efforts are urgently needed to reduce AF adverse consequences.
Keywords: atrial fibrillation, epidemiology
Introduction
Atrial fibrillation (AF) is the most commonly sustained arrhythmia and a significant public health concern worldwide. A systematic review of population-based studies estimated that 33.5 million individuals had AF worldwide in 2010,1 but the figures vary according to population demographic characteristics and study design.2
Previous studies suggest that the age-standard AF prevalence among Chinese adults (age >35 years) is low, 0.6%–2.3%.1 3–6 However, because these estimates were derived from restricted populations of varying sample size, they are unlikely to be reliable when there is ongoing rapid ageing, urbanisation and lifestyle changes, with corresponding increases in obesity and diabetes mellitus, in the massive population of China.
Because AF is often asymptomatic and goes undiagnosed without screening, many people only have AF recognised when they present with a serious vascular event.7 8 There are no data regarding gaps in awareness and management of AF in Chinese adults, although it is well acknowledged that anticoagulants are underused due to concerns of bleeding, complexities over monitoring and high costs.9 10 To address these knowledge gaps in knowledge, we undertook the China Atrial Fibrillation Epidemiologic Study, a cross-sectional study designed to provide reliable contemporary data on the prevalence, awareness and anticoagulant use for AF in China.
Methods
Design and study population
The study design has been previously described.11 Briefly, a sample of representative 47 841 adults (age ≥45 years) was obtained through a two-stage, stratified cluster sampling design in the general population. First, regions of Jilin, the Xinjiang Uyghur Autonomous Region, Beijing, Henan, Jiangxi and Zhejiang, Guangdong and Yunnan were randomly selected to represent the seven geographic areas of China (Northeast, Northwest, North, Central, East, South and Southwest China). From each city or province, 4000 rural (1 to 13 representative villages) residents and 4000 urban (one to three representative of communities in a capital city) residents (living in the area for >6 months) were surveyed. Door-to-door visits of selected communities and villages were conducted, up to three times on different days, to invite eligible participants. There was no patient or public involvement in the design or analysis of this study. Written informed consent was obtained from each participant.
Data collection and measurement
All eligible individuals were invited to a survey site to undergo a face-to-face structured interview administered by trained study staff using a standard questionnaire and a physical examination and laboratory tests. Data were obtained regarding sociodemography (age, sex, marital status, education level, household income and medical insurance coverage); medical history of any symptoms, diagnosis and treatment of hypertension, diabetes mellitus, dyslipidaemia, coronary heart disease (CHD), stroke/transient ischaemic attack (TIA) and AF and lifestyle factors including diet, smoking, alcohol consumption (days per week) and physical activity according to the modified Global Physical Activity Questionnaire.12 For those who agreed to receive a 12-lead digital ECG, one standard supine ECG was also performed (GE MAC 800/5500), with results interpreted by automatic software (Marquette 12SL ECG Analysis Programs). A cardiologist visually rechecked all the automatically detected AF ECGs and 500 randomly selected other ECGs without AF, with 100% agreement. A 5 mL blood sample was drawn from the participant’s antecubital vein in the morning after an overnight fast (≥8 hours) for central measurement of blood glucose, creatinine, total cholesterol, low-density lipoprotein cholesterol (LDL-C) and triglycerides at GuangZhou Kingmed Testing Science & Technology, a laboratory certified by the College of American Pathologists.
Individuals with a history of AF (489 self-reported) or detected on ECG acquisition (443 ECG-detected) were asked to answer additional AF-related questions on any history of thromboembolism, rheumatic heart disease, heart failure, major bleeding, thyroid dysfunction, respiratory disease and use of antiarrhythmic drugs, cardioversion, rate control agents and anticoagulation (online supplemental figure S2).
heartjnl-2020-317915supp001.pdf (444KB, pdf)
Definitions
Smoking status was defined as non-smoker, former smoker (≥1 year) or current smoker according to self-report. Habitual drinker was defined as self-reported alcohol use ≥5 days per week in the previous year. Manual labour included farmers, fisheries, factory workers and salespeople. Hypertension was defined as measured systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg, taking antihypertensive medication or self-report of a diagnosis of hypertension given by a physician. Diabetes mellitus was defined as a fasting blood glucose ≥7.0 mmol/L, self-report of a previous test with postprandial blood glucose ≥11.1 mmol/L or HbA1c≥7%, taking antidiabetic medicines or self-report of a diagnosis given by a physician. Dyslipidaemia was defined as total cholesterol ≥240 mg/dL, LDL-C ≥160 mg/dL, taking a statin or other lipid-lowering agent or self-report of the diagnosis given by a physician. CHD was defined as any history of myocardial infarction (MI), percutaneous coronary intervention or coronary artery bypass grafting. Stroke was defined as a diagnosis given by a physician or self-report of any acute-onset, TIA or permanent neurological deficit. Sedentary lifestyle was defined according to the definition used in the global surveillance of physical activity levels of adult:13 30 min of moderate-intensity leisure-time physical activity on ≥5 days per week or 20 min of vigorous-intensity leisure-time physical activity on ≥3 days every week. The risk of thromboembolism was assessed according to the CHA2DS2-VASc system.
All research staff received training covering questionnaire administration, BP and other anthropometric measurements and ECG acquisition. An electronic data capture system was created on Pad (Samsung T310/T330 tablet, Korea), with range and logic check functions and ability to control for missing data. Quality assurance staff reviewed all submitted data.
AF was diagnosed according to a positive ECG trace in the study or self-report of AF made by a physician. Awareness of AF was defined as knowledge of the diagnosis of AF. Valvular AF was defined in relation to self-report of rheumatic heart disease according to any mitral stenosis or mechanical valve replacement.
Statistical analysis
We estimated that including 48 000 participants in this study would allow a reliable estimate of 1% prevalence of AF, with a 95% CI of 0.75% to 1.25% (assuming an intracluster correlation of 0.01 and a design effect of 8).14 15 The characteristics of participants are described with continuous data presented as means (SD) if normally distributed and categorical variables as proportions. AF prevalence and risk factors were estimated with three-step weightings. Synthesised weights were multiplied by a sampling weight, a non-response weight and a population weight (age and sex). Sampling weight was used to adjust for different selection probabilities. Non-response weight was used to adjust for difference of responder and non-responder. Population weight was adjusted for deviations in the sample compared with the total adult population in China, according to the Population Census in 2010, particularly for sex and age.16 in the total adult population in China, according to the Population Census in 2010. Prevalence of AF was stratified by age group, sex, residence (urban versus rural) and geographic region. Cochran-Armitage test was used to evaluate the trend of AF prevalence in different age groups.
Logistic regression models were constructed using SAS PROC Surveylogistic to determine associations of prevalent AF and clinically relevant covariates of age-group, sex, area of residence (urban versus rural), history of hypertension, diabetes mellitus, CHD and stroke, sedentary lifestyle and body mass index (BMI). Data are reported as crude OR and multivariable adjusted OR (aOR) with 95% CI. Proportions of AF awareness were similarly reported and associations with clinical variables analysed in logistic regression models with the inclusion of age-group, sex, area of residence (rural versus urban), level of education, insurance cover, occupation (manual/unstable labour versus mental labour) and comorbidities of hypertension, diabetes, CHD and stroke. All p values are two-sided with a standard significance level (<0.05). All analyses were performed using SAS V.9.1.
Results
A total of 64 893 people were invited to participate across 39 sites in the seven regions (online supplemental figure S1) and 47 841 (73.7%) completed the survey (response rates 66.4% and 79.3% in men and women, respectively; 80.3% and 69.0% for residents in rural and urban areas, respectively). In 42 031 (87.9%) who completed a 12-lead ECG recording, there were 932 cases of AF (422 men, 510 woman), which included 598 (64.2%) with a prior diagnosis and 334 (35.8%) with newly detected AF by ECG. This corresponded to a weighted AF prevalence of 1.8% (95% CI 1.7% to 1.9%) (further details are outlined in online supplemental figure S2). Table 1 shows that compared with adults without AF, those with AF were on average 6–7 years older and more likely to have comorbidities of hypertension, diabetes mellitus, dyslipidaemia, CHD and history of stroke/TIA. Proportions of AF were lower in individuals with manual labour occupations or rural residence. Based on weighted prevalence and size of population in the 2010 census, the estimated number of patients with AF was 7.9 (95% CI 7.4 to 8.4) million in China.
Table 1.
Characteristics of 47 841 participants by sex and prevalent AF*
| Characteristic | Men | Women | ||||
| With AF (n=422) | Without AF (n=18 073) | P value | With AF (n=510) | Without AF (n=28 836) | P value | |
| Age, years | 67.6 (9.8) | 61.3 (9.7) | <0.0001 | 66.6 (10.0) | 59.7 (9.6) | <0.0001 |
| Rural resident | 159 (37.7) | 8704 (48.2) | <0.0001 | 147 (28.8) | 12 800 (44.4) | <0.0001 |
| Han ethnicity | 409 (96.9) | 17 199 (95.2) | 0.10 | 4993 (96.7) | 27 357 (94.9) | 0.07 |
| Education, high school or above | 312 (73.9) | 13 188 (73.0) | 0.66 | 371 (72.8) | 20 866 (72.4) | 0.85 |
| Married | 377 (89.3) | 16 708 (92.5) | 0.02 | 369 (72.4) | 24 052 (83.4) | <0.0001 |
| Manual labour | 252 (59.7) | 12 138 (67.2) | 0.0002 | 310 (60.8) | 19 457 (67.5) | 0.0002 |
| Health insurance | 414 (98.1) | 17 681 (97.8) | 0.70 | 495 (97.1) | 28 239 (97.9) | 0.17 |
| Annual household income ≥¥30 000 (US$4470) | 239 (56.6) | 10 534 (58.3) | 0.75 | 295 (57.8) | 15 500 (53.8) | 0.02 |
| Hypertension | 288 (68.3) | 9642 (53.4) | <0.0001 | 342 (67.1) | 13 931 (48.3) | <0.0001 |
| Diabetes mellitus | 94 (22.3) | 2684 (14.9) | <0.0001 | 108 (21.2) | 3958 (13.7) | <0.0001 |
| Dyslipidaemia | 133 (31.5) | 4647 (25.7) | 0.01 | 219 (42.9) | 9170 (31.8) | <0.0001 |
| Coronary heart disease | 56 (13.3) | 601 (3.3) | <0.0001 | 57 (11.9) | 635 (2.2) | <0.0001 |
| Stroke/TIA | 73 (17.3) | 1757 (9.7) | <0.0001 | 96 (18.8) | 2506 (8.7) | <0.0001 |
| CHA2DS2-VASc | 2.13 (1.58) | 1.12 (1.27) | <0.0001 | 3.08 (1.64) | 1.99 (1.22) | <0.0001 |
| Current smoker | 127 (30.1) | 8158 (45.1) | <0.0001 | 24 (4.7) | 1377 (4.8) | <0.0001 |
| Habitual drinker | 72 (17.1) | 3529 (19.5) | 0.0013 | 19 (3.7) | 834 (2.9) | 0.37 |
| Sedentary lifestyle | 118 (28.0) | 4987 (27.6) | 0.87 | 80 (15.7) | 3714 (12.9) | 0.15 |
| BMI, kg/m² | 24.8 (6.6) | 23.6 (7.4) | 0.0003 | 25.0 (5.6) | 23.8 (8.0) | 0.06 |
| Waist circumference, cm | 89.6 (10.9) | 87.4 (10.4) | <0.0001 | 86.0 (11.0) | 83.9 (10.5) | <0.0001 |
| SBP, mm Hg | 134.4 (20.0) | 133.2 (19.2) | 0.21 | 135.8 (21.1) | 131.5 (20.3) | <0.0001 |
| Heart rate, beats/min | 78.1 (15.3) | 74.6 (11.6) | <0.0001 | 78.4 (15.1) | 75.7 (10.8) | <0.0001 |
| Total cholesterol, mmol/L | 4.6 (1.0) | 5.0 (1.0) | <0.0001 | 5.2 (1.2) | 5.3 (1.1) | <0.0001 |
| LDL cholesterol, mmol/L | 2.7 (0.9) | 3.0 (0.9) | <0.0001 | 3.1 (1.0) | 3.2 (0.9) | 0.21 |
| Serum creatinine, μmol/L | 80.5 (22.2) | 76.4 (16.3) | 0.01 | 62.4 (16.9) | 58.3 (13.1) | 0.41 |
| eGFR, mL/min per 1.73 m² | 98.8 (30.0) | 106.1 (25.6) | 0.001 | 108.8 (32.2) | 117.3 (29.1) | 0.0003 |
Data are expressed as number (%) or mean (SD).
*Prevalent AF defined as either previous diagnosis or on ECG detection.
AF, atrial fibrillation; BMI, body mass index; eGFR, estimated glomerular filtration rate (estimated as 175×Scr−1.234×age−0.179 (if women, ×0.79)); LDL, low-density lipoprotein; SBP, systolic blood pressure; TIA, transient ischaemic attack.
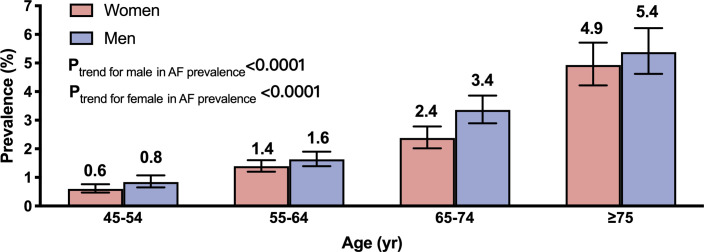
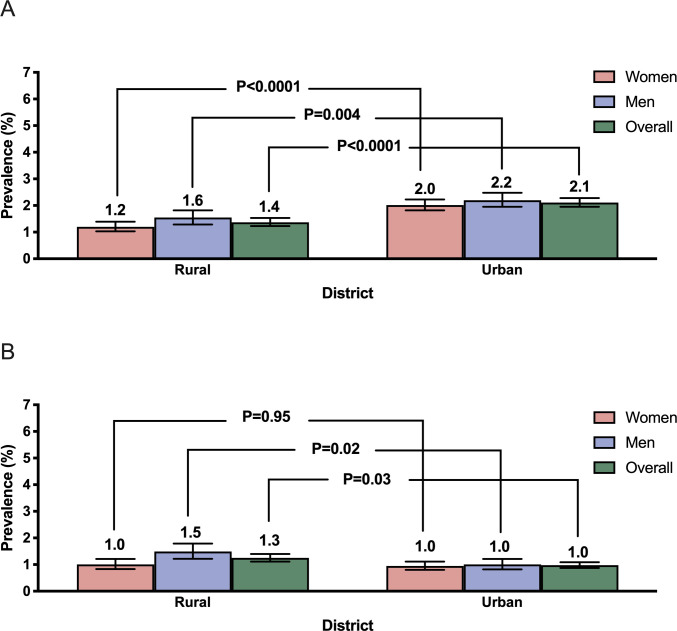
Weighted prevalence of AF, as shown in figure 1, was higher with older age-groups (p trend <0.0001), men compared with women (1.9%, 95% CI 1.7% to 2.1% vs 1.7%, 95% CI 1.5% to 1.8%; p<0.0001) and in urban compared with rural residents (2.1%, 95% CI 2.0% to 2.3% vs 1.4%, 95% CI 1.2% to 1.5%; p<0.0001) (figure 2A). The difference between urban and rural residents was driven by differences in self-reported AF, whereas the figures for ECG-detected AF were comparable between these groups (figure 2B). Prevalence of AF varied more than 2-fold across geographical regions, from 0.9% in Southwest China to 2.4% in North China (online supplemental table S1).
Figure 1.
Weighted prevalence of prevalent atrial fibrillation in men and women, stratified by age. Bars represent proportions and error bars represent 95% CI.
Figure 2.
Weighted prevalence of prevalent atrial fibrillation (A) and ECG-detected atrial fibrillation (B) in men and women, stratified by residence. Bars represent proportions and error bars represent 95% CI.
Table 2 shows that the odds of prevalent AF was nearly twice as high in older age-groups 45–54, 55–64, 65–74 and ≥75 years (aOR 2.07, 95% CI 1.53 to 2.80; 4.01, 2.95 to 5.44; 6.95, 4.96 to 9.74) and higher in men, urban areas, those with a history of hypertension, CHD, stroke/TIA, sedentary lifestyle and being overweight (BMI 25.0–29.9 kg/m2) or obese (BMI ≥30.0 kg/m2).
Table 2.
Factors associated with prevalent atrial fibrillation
| Variable | Prevalence | Univariate analysis | Multivariable analysis | ||
| n/N (%) | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age group, years | <0.0001 | <0.0001 | |||
| 45–54 | 92/14 579 (0.6) | 1 | 1 | ||
| 55–64 | 280/18 115 (1.6) | 2.29 (1.69 to 3.10) | 2.07 (1.53 to 2.80) | ||
| 65–74 | 297/10 583 (2.8) | 4.77 (3.54 to 6.43) | 4.01 (2.95 to 5.44) | ||
| ≥75 | 225/4528 (5.0) | 8.73 (6.34 to 12.04) | 6.95 (4.96 to 9.74) | ||
| Sex | 0.0002 | 0.05 | |||
| Men | 409/18 482 (2.2) | 1.37 (1.16 to 1.61) | 1.19 (1.00 to 1.40) | ||
| Residence | <0.0001 | <0.0001 | |||
| Urban | 602/26 007 (2.3) | 1.66 (1.41 to 1.95) | 1.57 (1.31 to 1.88) | ||
| History of hypertension | 605/24 178 (2.5) | 2.07 (1.74 to 2.48) | <0.0001 | 1.25 (1.04 to 1.50) | 0.02 |
| History of diabetes mellitus | 191/6833 (2.8) | 1.61 (1.30 to 2.00) | <0.0001 | 1.08 (0.87 to 1.35) | 0.49 |
| History of CHD | 107/1343 (8.0) | 4.54 (3.51 to 5.88) | <0.0001 | 2.60 (1.96 to 3.45) | <0.0001 |
| History of stroke/TIA | 163/4426 (3.7) | 2.14 (1.73 to 2.65) | <0.0001 | 1.50 (1.20 to 1.86) | 0.0003 |
| Sedentary lifestyle | 190/8891 (2.1) | 1.29 (1.05 to 1.57) | 0.01 | 1.40 (1.13 to 1.75) | 0.002 |
| BMI, kg/m² | <0.0001 | <0.0001 | |||
| <25 | 458/28 308 (1.6) | 1 | 1 | ||
| 25–29.9 | 336/15 890 (2.1) | 1.28 (1.07 to 1.54) | 1.23 (1.02 to 1.47) | ||
| ≥30 | 100/3607 (2.8) | 1.95 (1.49 to 2.55) | 1.80 (1.37 to 2.36) | ||
ORs and p values were adjusted for age group, sex, residence (urban versus rural), history of hypertension, CHD and stroke/TIA, sedentary lifestyle and BMI.
BMI, body mass index; CHD, coronary heart disease; TIA, transient ischaemic attack.
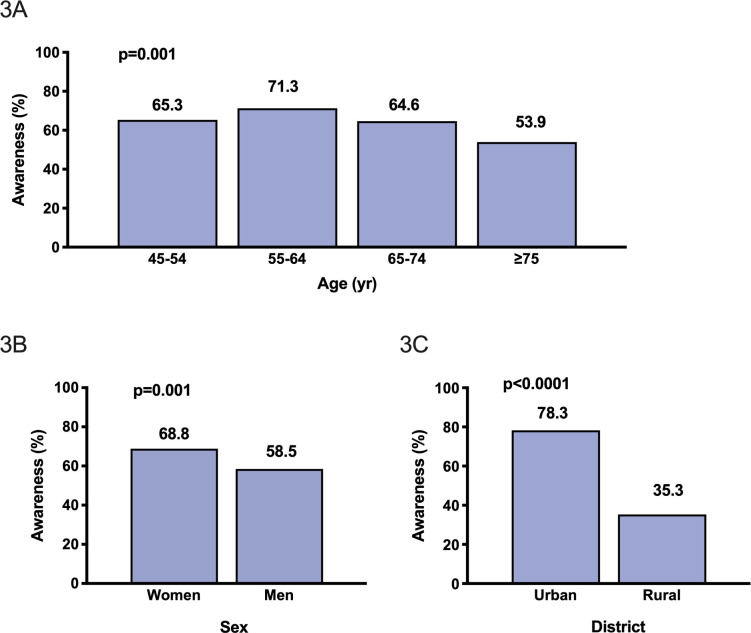
Among the 932 patients with a diagnosis of AF, 334 (35.8%) were unaware of its presence and only ECG-detected in the study. The proportion of awareness decreased with increasing age (p for trend 0.0007) and was lower in men (58.5%) than women (68.8%) (p=0.0011) and in rural (35.3%) compared with urban (78.3%) residents (p<0.0001; figure 3A–C). Independent predictors of AF unawareness were age ≥75 years, being men, rural residency, lower education level and no stable occupation, while AF awareness was associated with a history of stroke (table 3).
Figure 3.
Awareness of atrial fibrillation by age (A), sex (B) and residency (C). Bars represent proportions.
Table 3.
Factors associated with atrial fibrillation awareness
| Variables | Prevalence | Univariate analysis | Multivariable analysis | ||
| n/N (%) | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age group, years | 0.0007 | 0.01 | |||
| 45–54 | 34/98 (34.7) | 1 | 1 | ||
| 55–64 | 84/293 (28.7) | 1.32 (0.81 to 2.15) | 0.95 (0.55 to 1.65) | ||
| 65–74 | 110/311 (35.4) | 0.97 (0.60 to 1.56) | 0.7 (0.41 to 1.21) | ||
| ≥75 | 106/230 (46.1) | 0.62 (0.38 to 1.02) | 0.50 (0.29 to 0.88) | ||
| Sex | 0.001 | 0.004 | |||
| Women | 159/510 (31.2) | 1 | 1 | ||
| Men | 175/422 (41.5) | 0.64 (0.49 to 0.84) | 0.63 (0.46 to 0.86) | ||
| Residence | <0.0001 | <0.0001 | |||
| Rural | 198/306 (64.7) | 1 | 1 | ||
| Urban | 136/626 (21.7) | 6.60 (4.89 to 8.93) | 4.46 (2.87 to 6.94) | ||
| Education | <0.0001 | 0.005 | |||
| College or above | 38/191 (19.4) | 1 | 1 | ||
| Middle school | 198/492 (40.2) | 0.38 (0.25 to 0.59) | 0.59 (0.36 to 0.98) | ||
| Primary school or below | 98/249 (39.4) | 0.37 (0.25 to 0.55) | 0.52 (0.33 to 0.82) | ||
| Occupation | <0.0001 | 0.06 | |||
| Mental | 35/222 (15.8) | 1 | 1 | ||
| Manual labour | 224/562 (39.9) | 0.28 (0.19 to 0.42) | 0.64 (0.40 to 1.02) | ||
| Unstable occupation | 75/148 (50.7) | 0.18 (0.11 to 0.30) | 0.32 (0.18 to 0.55) | ||
| Insurance coverage | <0.0001 | 0.17 | |||
| Urban employees/residence insurance | 148/609 (24.3) | 1 | 1 | ||
| New Rural Cooperative Medical Scheme | 175/300 (58.3) | 0.23 (0.17 to 0.31) | 0.86 (0.55 to 1.35) | ||
| Other | 11/23 (47.8) | 0.35 (0.15 to 0.81) | 0.42 (0.16 to 1.08) | ||
| Comorbidities | |||||
| Hypertension | 209/630 (33.2) | 1.42 (1.07 to 1.89) | 0.01 | 1.23 (0.88 to 1.72) | 0.23 |
| Diabetes | 54/202 (26.7) | 1.71 (1.21 to 2.41) | 0.003 | 1.20 (0.81 to 1.78) | 0.38 |
| CHD | 24/113 (21.2) | 2.26 (1.41 to 3.62) | 0.0007 | 1.70 (0.99 to 2.90) | 0.05 |
| Stroke/TIA | 35/169 (20.7) | 2.47 (1.66 to 3.68) | <0.0001 | 2.09 (1.33 to 3.29) | 0.001 |
ORs and p values adjusted for age group, sex, urban or rural residency, education, insurance coverage, manual or mental labour and no stable occupation, history of hypertension, diabetes, CHD and stroke/TIA.
CHD, coronary heart disease; TIA, transient ischaemic attack.
AF was detected by ECG in 443 individuals; prevalence of ECG-detected AF was also higher in older ages (p trend <0.0001, online supplemental figure S3), and in men residents of rural (1.5%, 95% CI 1.2% to 1.8%) compared with urban (1.0%, 95% CI 0.8% to 1.2%) areas (p=0.02, figure 2B), but there was no difference for women by residential area (figure 2B). Of the 932 patients with prevalent AF, 442 patients (47.4%) provided further medical information that included 5.9% (13/220) men and 10.4% (23/222) women with rheumatic heart disease. Among the 896 adults with non-valvular AF, 532 patients had high CHA2DS2-VASc scores (≥2 men or ≥3 women), and of these, anticoagulation therapy was used in only 32 patients (6.0%).
Discussion
In this large nationally representative, community-based survey, we estimated that nearly 8 million adults aged 45 years or more in China (or 1.8% of the population) have a diagnosis of AF based on either a medical history or a single time-point ECG screen. AF was associated with older age, obesity, sedentary lifestyle and history of hypertension and CHD. Overall, more than one-third of those with AF were unaware of their condition, which was more common in the extremes of age (45–54 and ≥75 years), men, rural residents and in those with low education and free of major vascular comorbidities. Only 6% of individuals with AF and high predicted stroke risk were prescribed warfarin anticoagulation.
Lower age-standardised and sex-standardised AF prevalence (0.6%) were reported in adults (age ≥30 years) in a study conducted across 13 provinces of China 16 years ago,3 and another survey (0.7%) in adults (age ≥35 years) in 2018.17 Our study used both self-report and ECG screening and took account of a wide range of sociodemographic and risk factor variables that are known to influence the pattern of AF in populations. We recognise, however, that our figures may still be an underestimate, as a 2015 community-based study of adults aged 75 and 76 years in Sweden found that less than one fifth (17%) of those with AF detected on two times a day recording over a 2-week period were identified on the first ECG.18 If these data were applied to the older Chinese population, where almost half of the cases of AF occur, the true prevalence of AF in our older participants may be at least three times higher than in our study. Other factors that influence AF prevalence are the approaches to screening and access to the healthcare system.
There is a wide variation in the reported AF prevalence across the globe, with the lowest and highest burdens being in Asia and North America, respectively.1 In the global population, the reported AF prevalence was 2.1% in Germany from 2007 to 200819 and 3.0% in Sweden from 2004 to 2010.20 Among the US Medicare beneficiaries, AF prevalence was 3.1%, 5.0% and 8.1% among those aged 66–69, 70–74 and 75–79 years, respectively, in 2007,21 which are modestly higher than in our study. Conversely, AF prevalence is apparently lower in Asia compared with Western populations: 0.7% in Korean adults (>20 years),22 0.9% in Japanese (age ≥40 years),23 and 1.1% in Taiwanese (age ≥20 years).24 However, as the great majority of these studies used facility-based claims or health-check data, where AF was ancillary to other study objectives, the low prevalence figures could be explained on the basis of differences in sampling frames, methods of case ascertainment, random error and demographic structures. Moreover, differing patterns of climate, diet, lifestyle and methods of AF detection also contribute to variation in AF prevalence across regions. In our study, AF prevalence varied more than twofold across geographical regions.
The low AF awareness in our study highlights the importance of education about AF in Chinese people and especially in rural locations, the elderly and groups of low socioeconomic, education and health insurance status. Yet, our results are consistent with a study of African-Americans where only one-third were aware they had AF, either because of it not being recognised or from poor understanding of its health significance.25 This phenomenon is commonly explained on the basis of social determinants of health (SDOH), with poor access and health literacy in disadvantaged populations, which highlight the importance of ongoing actions to mitigate the adverse consequences of SDOH to improve population health.
Although anticoagulation is an effective treatment to reduce the risk of AF-related stroke, we found very low use in eligible individuals, consistent with other studies.10 Although data are emerging that suggest the uptake of anticoagulation has increased recently in China,10 these observations are from hospitals located in large cities and are unlikely to apply to rural areas. Our national study with broader coverage indicates considerable widespread under-treatment of AF in China, highlighting a critical unmet health need that needs to be addressed to avoid considerable premature loss of productive life.
AF is also a marker of high risk of cardiovascular events, including heart failure, MI and cardiovascular death,26 and our study highlights the high proportion of comorbidities in these patients, with a higher proportion of hypertension, diabetes mellitus, dyslipidaemia, CHD and stroke. Integrated and aggressive management of comorbid risk factors should also contribute to reduce adverse outcomes in this patient group.
Study limitations
While cities and provinces were selected randomly in the seven regions of China, the communities and villages within each were selected on feasibility, with subsequent units further selected according to sampling weights of population census data. As such, our study may have been limited by some degree of sampling bias, further complicated by participation bias from a response rate of 75%; participants were likely healthier and differed in other ways to those who failed to respond or refused participation. Second, self-reported AF history may not be reliable, although AF can still be underestimated on the basis of a single ECG because of the high rate of paroxysmal AF.27 Moreover, studies have shown that self-reported AF is an equivalent stroke risk factor to ECG-detected AF. Thus, self-reported AF in combination with ECG-detected AF are commonly used to detect AF in population-based studies.28 Finally, as AF detection and management may have changed in the several years since the completion of the study, our data may not be current.
Conclusion
Our large national survey shows a low 1.8% AF prevalence among adults (aged ≥45 years) China, although this is still more than twofold higher than that noted in previous reports. Given the large and rapidly changing demographic profile and lifestyles of the Chinese people, our findings of a high degree of undiagnosed and undertreated cases of AF raise concerns over health policy and practice in this country. National strategies that focused on prevention, detection, awareness and treatment of AF are urgently needed in China.
Key messages.
What is already known on this subject?
Atrial fibrillation (AF) is the most commonly sustained arrhythmia and a significant public health concern worldwide. Previous studies suggest that the age-standard prevalence of AF among Chinese adults (age >35 years) is low (0.6%–2.3%).
What might this study add?
In a representative sample of community-based 47 841 adults (age ≥45 years), the weighted prevalence of AF was 1.8% (95% CI 1.7% to 1.9%), but which equates to 7.9 (95% CI 7.4 to 8.4) million people in China. Awareness of AF is low, and treatment gaps are large.
How might this impact on clinical practice?
AF prevalence is higher than previously reported in China, but awareness is low, and there are large treatment gaps. Large-scale efforts are urgently needed to reduce the adverse consequences of increasing AF in China.
Acknowledgments
For continuous consultant in study design and implementation, we thank Wei Zheng (Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Centre, Vanderbilt-Ingram Cancer Centre, Vanderbilt University School of Medicine).
Footnotes
Contributors: XD, YY, YZ, SW, XG, XZ, HL, XC, JDong and CM had designed the study and were responsible for data collection. XD, JDu and SX did the statistical analysis. XD, CA, HA, MH and SX prepared the manuscript. All authors contributed to reviewing or revising the manuscript and approved the final version.
Funding: This work was supported by the National Key Research and Development Program of China (grant number. 2016YFC0900901, 2016YFC1301002, 2017YFC0908803 and 2018YFC1312501), the National Science Foundation of China (grant number. 81530016) and Biosense Webster, Inc.
Disclaimer: The sponsor of the study had no role in study design, data collection, data analyses, data interpretation, nor writing of the report.
Competing interests: CM has received honoraria for presentations from AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson and Pfizer. JDo has received honoraria for presentations from Johnson & Johnson. CA received grants from the National Health and Medical Research Council of Australia and Takeda and advisory board feeds from Amgen and Boehringer Ingelheim. HA received honoraria for presentations from Bayer, Daiichi Sankyo, MSD, Takeda, Teijin, and fees for consultancy from Kyowa Kirin. MH received grant from the World Heart Federation for the Emerging Leaders program, supported by grants from Boehringer Ingelheim and Novartis with previous support from AstraZeneca and Bupa. MH also received support from the American Heart Association, Verily and AstraZeneca and the American Medical Association for work unrelated to this project.
Patient consent for publication: Not required.
Ethics approval: The ethics committee of Beijing Anzhen Hospital approved the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available due to participant privacy.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 2014;129:837–47. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kornej J, Börschel CS, Benjamin EJ, et al. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res 2020;127:4–20. 10.1161/CIRCRESAHA.120.316340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou Z, Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol 2008;18:209–16. 10.2188/jea.JE2008021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coppens M, Eikelboom JW, Hart RG, et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J 2013;34:170–6. 10.1093/eurheartj/ehs314 [DOI] [PubMed] [Google Scholar]
- 5. Deng H, Guo P, Zheng M, et al. Epidemiological characteristics of atrial fibrillation in southern China: results from the Guangzhou heart study. Sci Rep 2018;8:17829. 10.1038/s41598-018-35928-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X, Fu Q, Song F, et al. Prevalence of atrial fibrillation in different socioeconomic regions of China and its association with stroke: results from a national stroke screening survey. Int J Cardiol 2018;271:92–7. 10.1016/j.ijcard.2018.05.131 [DOI] [PubMed] [Google Scholar]
- 7. Soliman EZ, Howard G, Meschia JF, et al. Self-reported atrial fibrillation and risk of stroke in the reasons for geographic and racial differences in stroke (regards) study. Stroke 2011;42:2950–3. 10.1161/STROKEAHA.111.621367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reading SR, Go AS, Fang MC, et al. Health literacy and awareness of atrial fibrillation. J Am Heart Assoc 2017;6:e005128. 10.1161/JAHA.116.005128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X, Li Z, Zhao X, et al. Use of warfarin at discharge among acute ischemic stroke patients with nonvalvular atrial fibrillation in China. Stroke 2016;47:464–70. 10.1161/STROKEAHA.115.011833 [DOI] [PubMed] [Google Scholar]
- 10. Chang S-S, Dong J-Z, Ma C-S, et al. Current status and time trends of oral anticoagulation use among Chinese patients with nonvalvular atrial fibrillation: the Chinese atrial fibrillation registry study. Stroke 2016;47:1803–10. 10.1161/STROKEAHA.116.012988 [DOI] [PubMed] [Google Scholar]
- 11. Xia S, Du X, Guo L, et al. Sex differences in primary and secondary prevention of cardiovascular disease in China. Circulation 2020;141:530–9. 10.1161/CIRCULATIONAHA.119.043731 [DOI] [PubMed] [Google Scholar]
- 12. The World Health Organization . Global physical activity surveillance (GPAQ), 2004. Available: https://www.who.int/ncds/surveillance/steps/GPAQ/en/ [Accessed 28 Jan 2019].
- 13. Hallal PC, Andersen LB, Bull FC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 2012;380:247–57. 10.1016/S0140-6736(12)60646-1 [DOI] [PubMed] [Google Scholar]
- 14. Carlin JB, Hocking J. Design of cross-sectional surveys using cluster sampling: an overview with Australian case studies. Aust N Z J Public Health 1999;23:546–51. 10.1111/j.1467-842X.1999.tb01317.x [DOI] [PubMed] [Google Scholar]
- 15. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998;17:857–72. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–22. 10.1016/S0140-6736(12)60033-6 [DOI] [PubMed] [Google Scholar]
- 17. Wang Z, Chen Z, Wang X, et al. The disease burden of atrial fibrillation in China from a national cross-sectional survey. Am J Cardiol 2018;122:793–8. 10.1016/j.amjcard.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 18. Svennberg E, Engdahl J, Al-Khalili F, et al. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–84. 10.1161/CIRCULATIONAHA.114.014343 [DOI] [PubMed] [Google Scholar]
- 19. Wilke T, Groth A, Mueller S, et al. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace 2013;15:486–93. 10.1093/europace/eus333 [DOI] [PubMed] [Google Scholar]
- 20. Norberg J, Bäckström S, Jansson J-H, et al. Estimating the prevalence of atrial fibrillation in a general population using validated electronic health data. Clin Epidemiol 2013;5:475–81. 10.2147/CLEP.S53420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes 2012;5:85–93. 10.1161/CIRCOUTCOMES.111.962688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee S-R, Choi E-K, Han K-D, et al. Trends in the incidence and prevalence of atrial fibrillation and estimated thromboembolic risk using the CHA2DS2-VASc score in the entire Korean population. Int J Cardiol 2017;236:226–31. 10.1016/j.ijcard.2017.02.039 [DOI] [PubMed] [Google Scholar]
- 23. Inoue H, Fujiki A, Origasa H, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol 2009;137:102–7. 10.1016/j.ijcard.2008.06.029 [DOI] [PubMed] [Google Scholar]
- 24. Chao T-F, Liu C-J, Tuan T-C, et al. Lifetime risks, projected numbers, and adverse outcomes in Asian patients with atrial fibrillation: a report from the Taiwan nationwide AF cohort study. Chest 2018;153:453–66. 10.1016/j.chest.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 25. Meschia JF, Merrill P, Soliman EZ, et al. Racial disparities in awareness and treatment of atrial fibrillation: the reasons for geographic and racial differences in stroke (regards) study. Stroke 2010;41:581–7. 10.1161/STROKEAHA.109.573907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Du X, Ninomiya T, de Galan B, et al. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: results of the advance study. Eur Heart J 2009;30:1128–35. 10.1093/eurheartj/ehp055 [DOI] [PubMed] [Google Scholar]
- 27. Aboaf AP, Wolf PS. Paroxysmal atrial fibrillation. A common but neglected entity. Arch Intern Med 1996;156:362–7. [PubMed] [Google Scholar]
- 28. Howard G, McClure LA, Moy CS, et al. Self-reported stroke risk stratification: reasons for geographic and racial differences in stroke study. Stroke 2017;48:1737–43. 10.1161/STROKEAHA.117.016757 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2020-317915supp001.pdf (444KB, pdf)