Abstract
Glucagon-like peptide-1 (GLP-1) is produced in gut endocrine cells and in the brain, and acts through hormonal and neural pathways to regulate islet function, satiety, and gut motility, supporting development of GLP-1 receptor (GLP-1R) agonists for the treatment of diabetes and obesity. Classic notions of GLP-1 acting as a meal-stimulated hormone from the distal gut are challenged by data supporting production of GLP-1 in the endocrine pancreas, and by the importance of brain-derived GLP-1 in the control of neural activity. Moreover, attribution of direct vs indirect actions of GLP-1 is difficult, as many tissue and cellular targets of GLP-1 action do not exhibit robust or detectable GLP-1R expression. Furthermore, reliable detection of the GLP-1R is technically challenging, highly method dependent, and subject to misinterpretation. Here we revisit the actions of GLP-1, scrutinizing key concepts supporting gut vs extra-intestinal GLP-1 synthesis and secretion. We discuss new insights refining cellular localization of GLP-1R expression and integrate recent data to refine our understanding of how and where GLP-1 acts to control inflammation, cardiovascular function, islet hormone secretion, gastric emptying, appetite, and body weight. These findings update our knowledge of cell types and mechanisms linking endogenous vs pharmacological GLP-1 action to activation of the canonical GLP-1R, and the control of metabolic activity in multiple organs.
Keywords: diabetes, obesity, receptor, islets, brain, gastrointestinal tract, cardiovascular
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS
Plasticity of the endocrine pancreas enables the capacity for local GLP-1 production
The β-cell GLP-1R receives signals from both GLP-1 and glucagon to regulate glucose-stimulated insulin secretion
Detection of the GLP-1R in multiple cell types is challenging due to low levels of expression and technical validation of reagents
Brain-derived GLP-1 is synthesized in the brainstem and widely distributed to multiple GLP-1Rs throughout the central nervous system
GLP-1Rs within the central nervous system may be engaged by both CNS-derived and systemically circulating GLP-1 and GLP-1R agonists
The intestinal L cell network is the predominant contributor to circulating GLP-1
GLP-1 receptor agonists exert nonmetabolic actions in multiple cells and tissues that do not express the canonical GLP-1R
The gut endocrine system contains dozens of specialized plurihormonal endocrine cells whose peptide hormone products regulate a wide range of biological functions, ranging from control of ingestive behavior to the digestion, absorption, and assimilation of nutrients (1). Among the best studied of the gut hormones is glucagon-like peptide-1 (GLP-1), a product of the proglucagon gene (GCG). The cloning of the proglucagon cDNAs encoding GLP-1 within the GCG gene in the early 1980s (2, 3), enabled the study of the physiology of the glucagon-like peptides. Originally described as an incretin hormone that potentiates meal-stimulated insulin secretion, several decades of research have broadened the actions of GLP-1 beyond the endocrine pancreas to encompass activity within the nervous system, gut, kidney, pancreas, heart, and immune system (4-6). Moreover, the clinical development of GLP-1R agonists (GLP-1RAs) for the treatment of type 2 diabetes (T2D) and obesity has heightened interest in how physiological and pharmacological levels of GLP-1 act on key tissues relevant to the pathophysiology of diabetes and its complications.
A major advance in understanding GLP-1 action stemmed from the cloning and characterization of the GLP-1 receptor (GLP-1R) (7). GLP1R mRNA transcripts were found to be widely distributed in peripheral tissues and the central nervous system (CNS), providing a logical link between the demonstrated actions of GLP-1 and the expression of the canonical GLP-1R in cellular targets of GLP-1 action (8-10). Nevertheless, the precise cellular localization of GLP-1R has proved to be challenging, reflecting low levels of mRNA expression, and multiple technical challenges, including the inadvertent application of incompletely characterized nonspecific antisera for detection of GLP-1R protein expression (11).
The concept of GLP-1 as a gut-derived hormone acting on peripheral organs, has been refined through enhanced understanding of the importance of pancreatic- and brain-derived GLP-1. GLP-1 circulates at very low (picomolar) levels, is immediately inactivated by local gut and systemic expression of dipeptidyl peptidase-4 (DPP-4) (12, 13), and is rapidly cleared from the circulation by the kidney. Contemporaneously, evidence supporting the pancreatic production of GLP-1 has fostered the competing notion that the incretin activity of GLP-1 reflects local GLP-1 synthesis in α-cells, coupled to paracrine actions on adjacent GLP-1R+ β-cells (6). Moreover, notwithstanding the reduction of food intake and weight loss observed with pharmacological administration of GLP-1RAs, inactivation of gut GLP-1 production does not perturb control of food intake or body weight (14), consistent with considerable evidence implicating local brainstem-derived GLP-1, and not gut-derived GLP-1, as a key contributor to physiological GLP-1R-dependent signals regulating appetite and body weight.
Similarly enigmatic is the challenge of understanding the actions of GLP-1 and degradation-resistant GLP-1RA on organs and cell types that do not express the GLP-1R. For example, GLP-1RA reduce the rates and extent of myocardial infarction in humans and in preclinical studies (15-17), yet it remains challenging to detect GLP-1R protein expression in the left ventricle of the heart, the most common site of infarction (18). Moreover, GLP-1RAs reduce hepatic fat accumulation and are under clinical investigation for the treatment of nonalcoholic steatohepatitis (NASH), yet hepatocytes do not appear to express a functional canonical GLP-1R (19-21). Moreover, GLP-1RA rapidly reduce the levels of postprandial triglycerides in animals and humans, yet enterocytes do not exhibit GLP-1R expression or activity. These and related examples highlight how multiple pharmacological actions of GLP-1 and GLP-1RA are highly conserved across species, yet difficult to explain mechanistically based on the known expression patterns of the GLP-1R.
Several technological advances have enabled re-examination of these questions, refining our understanding of where and how GLP-1 is synthesized and acts to originate GLP-1R-dependent signals in multiple organs. Similarly, new tools have enabled more sensitive and reliable detection of GLP-1R expression. These include single cell RNA sequencing (scRNA-seq), reporter mice illuminating cellular sites of Glp1r transcriptional activity, more sensitive and specific validated GLP-1R antisera, advanced microscopy, and new chemical probes for enhanced detection of GLP-1R binding sites at single cell resolution.
Here we re-evaluate existing concepts of GLP-1 action, highlighting insights into established and emerging concepts illuminated by recent progress in the evaluation of GLP-1 production and in the expression and activity of the canonical GLP-1R. The sites of GLP-1 synthesis within the gastrointestinal tract, the brain, and the pancreas are described, and linked where possible, to actions of GLP-1 within these organs, which are transduced by the canonical GLP-1R. We focus on discussing established and emerging concepts of how circulating GLP-1 produces multiple actions, both directly and indirectly, on multiple organs and cell types with variable GLP-1R expression. Collectively, these findings update established and emerging concepts of GLP-1 action, and highlight mechanistic controversies surrounding GLP-1 action on cell types that do not express the GLP-1R.
The Pancreas
GLP-1 production, GLP-1R expression, and GLP-1R signaling in the pancreas
Is pancreas-derived GLP-1 relevant for islet function?
An ongoing debate centers on whether proglucagon processing in α-cells is capable of producing meaningful amounts of bioactive GLP-1 to influence β-cell function and glucose tolerance. Early reports demonstrated increased α-cell expression of Pcsk1, the prohormone processing enzyme required for GLP-1 production, in response to pancreatic injury or induction of experimental pancreatic inflammation (22-26), raising the hypothesis that α-cells could adapt to metabolic stress by increasing GLP-1 production. Active GLP-1 was detected, albeit at low levels relative to corresponding levels of glucagon in the same extracts, from both mouse and human pancreas (14). However, induction of metabolic stress in high-fat diet (HFD)–fed mice did not alter the levels of GLP-1 in the pancreas (14). Genetic deletion of Pcsk1 to eliminate prohormone convertase 1 (PC1) reduced islet content and secretion of GLP-1 by ~40%, leading to modest impairment of glucose intolerance in obese mice (25), reinforcing the key role of PC1 in α-cell GLP-1 production. However, analyses using the mouse perfused pancreas and a specific assay to detect active, fully processed GLP-1 reported mostly undetectable levels of active GLP-1 in the perfusate, arguing that α-cell GLP-1 production in the normal murine pancreas is negligible (27). On the other hand, the GLP-1R antagonist exendin(9-39) (Ex9) did not impair glycemic excursion in Gcg–/– mice with preserved intestinal GLP-1 production, yet selective rescue of pancreatic Gcg expression in Gcg–/– mice, together with Ex9 administration, implicated pancreatic GLP-1 in the control of glucose homeostasis in this mouse model (28). Complicating this interpretation are findings that glucagon, produced at much higher levels in the islet, is also capable of stimulating insulin secretion and controlling glucose homeostasis through the GLP-1R (27, 29-31). Consequently, paracrine interactions between α- and β-cells mediated by GCG peptides and the GLP-1R could be completely accounted for by glucagon secretion. Interestingly, while the levels of glucagon secreted from isolated mouse islets are ~300-fold greater than GLP-1, the potency of GLP-1 for insulin secretion is ~300-fold more than glucagon (31). Finally, if α-cells produce active GLP-1, this source does not contribute to the circulating concentrations in the plasma as mice with intestine-specific inactivation of the Gcg gene exhibit almost undetectable levels of active circulating GLP-1 (14). Figure 1A summarizes paracrine GLP1 signaling in the islet.
Figure 1.
GLP-1 source, GLP-1R labeling and GLP-1R signaling in pancreatic islets. (A) Potential sources of GLP1 in the islets, as well as paracrine signaling pathways. Proglucagon products from α-cells that activate islet GLP-1Rs include glucagon and potentially GLP-1. The biological relevance of GLP-1 produced by α-cells remains an unresolved line of investigation. (B) GLP1R labelling is observed at the membrane in β-cells. Predominantly cytoplasmic labelling is nonspecific unless the GLP1R is agonist-bound in which case some surface and punctate intracellular staining is detected due to GLP1R internalization. (C) Major GLP1R signaling pathways reported in primary rodent and human beta cells or islets. It is well established that GLP-1R signals through cAMP pathways, including PKA and EPAC2. β-arrestin 1 has been proposed to regulate GLP-1R signaling; however, the mechanisms of this pathway remain unresolved. (D) Actions mediated by the GLP-1R in pancreatic islets. ADCY, adenylate cyclase, GLP1R, Glucagon-like peptide-1 receptor; PKA, protein kinase A; EPAC2, exchange protein directly activated by cAMP 2; INS, insulin SST, somatostatin.
GLP1R/Glp1r mRNA expression in different islet cell types
Prior to the development of validated antibodies for the GLP-1R, the expression patterns of Glp1r/GLP1R in islet cells were derived from analysis of ligand binding and RNA levels. The GLP-1R cDNAs were originally cloned from rat (7) and human (32) pancreas, and later shown to be expressed in the mouse pancreas by reverse transcription-polymerase chain reaction (RT-PCR) (10). Currently, islets/pancreas samples are often used as a positive control when exploring Glp1r/GLP1R expression in other tissues (18). However, it is continuously debated which endocrine cell population within the islet express the GLP-1R. Multiple strategies have been deployed to identify Glp1r/GLP1R+ cell populations, including transcriptional analysis of sorted islet populations. Using genetic reporter lines to enrich for populations of α-, β-, and δ-cells, Glp1r was shown to be robustly expressed in mouse β-cells, moderately expressed in δ-cells, and expressed at low to undetectable levels in α-cells (33). These data align with a study employing bulk RNA-seq to analyze enriched cell populations obtained from reporter mice, showing Glp1r to be highly expressed in β-cells, less so in δ-cells, but absent in α-cells (34).
An alternative approach to identifying islet Glp1r localization utilized mice expressing Cre recombinase under control of the Glp1r promoter. Using Glp1r-Cre mice crossed with a fluorescent reporter line, cells are indelibly marked in response to Glp1r transcription (35). Such approaches are of high fidelity, since only a few molecules of Cre are required to excise the stop cassette and drive reporter protein expression. A downside of this approach is the potential disconnect between transcription and translation, although this is less likely in light of newer data (see below). Moreover, relative levels of GLP-1R cannot be determined between and within cell types, since reporter expression is generally driven using a Rosa26 promoter.
The first study to utilize this approach to investigate Glp1r promoter activity in islets reported strong activity in both β- and δ-cells, although Glp1r levels were ~10-fold higher in the β-cell population (35). Subsequent analysis of α-cell populations with double reporter mice (Glp1r-Cre:ROSA26-tdRFP:GLU-Venus) produced an estimate that 9.5% of α-cells are Glp1r+. Interestingly, the fraction of Glp1r+ α-cells also contained significantly more Ins1 expression than Glp1r– α-cells. Could these represent a subpopulation of unique α-cells that are GLP-1 responsive? Do they arise from dedifferentiated β-cells, explaining the presence of both Glp1r and Ins transcripts? Or do these results arise from contamination of a non–α-cell population? The importance of this subpopulation of α-cells remains unclear. A second study examined Glp1r activity in islet cell populations using an independent Glp1r-Cre mouse crossed with the mTmG reporter mouse line (36). The expression of Ins and Sst were found in the Glp1r+ population (GFP+), while Gcg expression was exclusive to the Glp1r– population (TdTomato+). This data set indicates little to no Glp1r expression in α-cells, conflicting with previous reports, but this approach cannot rule out a small population of α-cells being Glp1r positive.
scRNA-seq has been utilized for interrogation of the Glp1r expressing cell population within islets, and to assess for potential heterogeneity in expression within a particular cell population. For instance, in samples from human islets, GLP1R mRNA was robustly detectable in β- and δ-cell populations, with very few GLP1R+ α-cells (37), largely aligning with the results of cell population analysis from transgenic mice described above (Fig. 2). However, it was interesting to note the considerable range in expression levels between individual β-cells (and δ-cells), particularly since a significant portion of individual cells in either population were reported to be GLP1R–. Heterogeneity in β-cell GLP1R/Glp1r expression was common among all human and mouse scRNA-seq data analyzed (38), indicating this outcome is consistent despite the varied platforms used to obtain these data. It is exciting to imagine heterogeneity in the relative expression of GLP1R/Glp1r within specific cell populations, as this could reconcile the reports that only a fraction of α-cells are GLP-1R+ and potentially contribute to the ongoing discussion of β-cell heterogeneity (38, 39). However, the varying levels of GLP1R/Glp1r transcripts in different cells was subsequently proposed to reflect technological limitations associated with scRNA-seq, due to the significant drop out of low-expressing genes during the process of generating the cDNA library (39).
Figure 2.
Expression of GLP1R in 9940 human pancreatic islet endocrine cells. The UMAP (Uniform Manifold Approximation and Projection) plots were created to visualize gene expression in each cell. The x-axis and y-axis indicate the overall transcriptional difference of each cells arbitrated as “distance” in a 2-dimensional space; the closer the 2 cells on the plot, the more similar their transcriptomes are, and in turn they are more likely to share a lineage. The expression scales are continual and log2 normalized, ranging from light blue (low expression) to dark red (high expression) in color, Cell types were classified based on markers genes INS and MAFA (mature β cells), GCG (α cells), SST (δ cells), and PPY (PP cells). Count matrices were accessed on September 19, 2020, and aggregated from the scRNA-seq datasets reported by Segerstolpe et al. (37) (GEO accession number GSE73727), Enge et al. (220) (GEO accession number GSE81547), and Camunas-Soler et al. (38) (GEO accession number GSE124742). Human gene nomenclature: INS, insulin; GCG, glucagon; SST, somatostatin; PPY, pancreatic polypeptide Y; MAFA, V-maf musculoaponeurotic fibrosarcoma oncogene homologue A.
GLP-1R protein expression and localization in the rodent islet
Multiple approaches have localized GLP-1R in pancreatic islets. Early studies using immunohistochemistry (IHC) to examine rat pancreas showed immunoreactivity for GLP-1R in the majority of β-cells and δ-cells, with 1 in 5 α-cells also expressing the receptor, in line with single cell RT-PCR findings (40). Later studies using the same rat antibody showed that GLP-1R was predominantly expressed in the β-cell compartment, with no detectable GLP-1R protein or mRNA in α-cells and δ-cells (41, 42). Comparable results were obtained by an independent group using 2 commercial antibodies (42) although one of the reagents was subsequently shown to lack specificity for the GLP-1R (43). All of these early studies detected GLP-1R in the β-cell membrane as well as in the cytoplasm. While this could reflect the imaging technique used, cytoplasmic localization could also reflect nonspecific signal, since GLP-1R should be present predominantly at the plasma membrane in its unstimulated state (Fig. 1B). While a validated monoclonal antibody (Mab 7F38) has been reported which displays a clean membrane signal (44), it has not yet been used to extensively re-examine GLP-1R cellular localization in mouse islets. Recent studies using a novel antibody raised against the GLP-1R N-terminal region, and validated using a new floxed GLP-1R-null reporter mouse line, detected GLP-1R throughout the β-cell and α-cell cytoplasm (45). The reason for this discrepancy in the expression of the α-cell GLP-1R across studies is unclear, but might reflect the use of antibodies raised against different GLP-1R epitopes (eg, N-terminus or the wider extracellular domain).
A second approach to detect GLP-1R in islets relies upon orthosteric agonists or antagonists conjugated to various fluorophores (Cyanine 3 and 5, tetramethylrhodamine, silicon rhodamine) (46-48). Inherent advantages of this approach include visualization of GLP-1R capable of ligand binding and activation and live tissue imaging, which reduces background signal introduced by fixation. However, because peptides and some fluorophores are cell impermeable, GLP-1R already within the cell will not be readily detectable. Studies using fluorescent agonists reported similar findings to those with monoclonal antibody: GLP-1R expression largely confined to the β-cell compartment, with expression in a small subpopulation of α-cells (~1%) (48). Along these lines, well characterized fluorescent antagonists revealed predominant GLP-1R expression in β-cells (~85%), with slightly higher expression in α-cells (~5%) than shown with fluorescent agonist or antibody (49) but in agreement with Glp1r-Cre:ROSA26-tdRFP:GLU-Venus double reporter mouse approaches (35). Notably, for all fluorescent ligand localization approaches, staining overlapped with data obtained using IHC with antibodies validated in Glp1r–/– mouse tissue (48, 49).
GLP-1R protein expression and localization in the primate and human islet
Most of the studies investigating GLP-1R distribution in primate and human islets have used IHC. Using a validated monoclonal antibody (Mab 3F52), GLP-1R colocalized with insulin-positive cells in the monkey islet (44). Conversely, less colocalization was detected in glucagon-positive cells, with the observed overlap attributed to cytoplasmic extensions from β-cells (44). However, given the axial resolutions needed to accurately separate cell membranes, further studies will be required to exclude GLP-1R expression in monkey α-cells.
Early studies looking at human islets showed strong colocalization of GLP-1R with β-cells, with no staining seen in α-cells (41). Similarly, studies using a commercial antibody confirmed these results, showing GLP-1R immunopositivity in 0.25% of α-cells (50). It is worth noting however that the antibodies used in both these studies gave rise to predominantly cytoplasmic staining, at odds with the known localization of nonstimulated GLP-1R. Studies using a validated antibody with membrane-localized staining again showed GLP-1R expression largely confined to β-cells, but staining was not performed for glucagon, making α-cell quantification impossible (44). Consistent with these observations, Waser and colleagues utilized the Mab 3F52 antibody and detected membrane-localized GLP-1R immunopositivity in β-cells and δ-cells, but not in α-cells from human pancreata resected from individuals with insulinomas, chronic pancreatitis, or pancreatic cancer (51). Most recently, studies using the same antibody combined with confocal imaging showed that GLP-1R is expressed in most β-cells and very few α-cells (52). Of interest, GLP-1R was shown to be moderately expressed in somatostatin+ cells (ie, δ-cells) (52). Thus, based upon IHC, GLP-1R expression patterns in human reflect those seen in the mouse, but with some immunoreactivity also detected in δ-cells (the majority of mouse studies did not quantify somatostatin+ cells).
Tying together GLP-1R transcript and protein levels in the same cell
While the measurement of Glp1r mRNA transcripts is not burdened with the limitations present when assessing protein levels based on current approaches, extending Glp1r transcript abundance to protein is a necessary step to identify cells that permit GLP-1 activity. However, there appears to be significant discordance between detection of gene and protein expression of the GLP-1R in mouse islets (36). These observations stemmed from sequential efforts to first validate a novel GLP-1R antibody (53) in Glp1r–/– mice to demonstrate specificity for the GLP-1R in dispersed islets cells during flow cytometry. Next, this antibody was applied to Glp1r-Cre:mTmG reporter mice to compare Glp1r promoter activity with GLP-1R protein levels. The antibody failed to stain any TdTomato+ cells (Glp1r–), enhancing the validity of the 2 reagents (36). This population of Glp1r– cells were enriched for Gcg, marking this population as α-cells. Remarkably, 2 populations emerged with respect to GLP-1R staining when examining the GFP+ cells (Glp1r+). The population of GFP+ cells that stained positive with the antibody were enriched for Ins2 expression, while the population of cells that stained significantly lower with the antibody were enriched for Sst (36). This suggests that β-cells have robust GLP-1R protein levels, and that δ-cells have much less GLP-1R. These findings were corroborated in experiments in wild-type (WT) islets, where the GLP-1R antibody robustly stained β-cells, but failed to detect protein expression in either α- or δ-cells. The potential discrepancy between RNA and protein expression in δ-cells warrants further investigation to eliminate the potential contribution of technical limitations, especially given the relative paucity of this cell type. Finally, the majority of β-cells express the GLP-1R and this percentage was not changed by various stressors reported to modulate Glp1r levels, indicating little plasticity in expression of the β-cell GLP-1R.
Consensus view of islet GLP-1R expression
Given the number of studies, what is the consensus view of GLP-1R expression in the islet? It is clear that β-cells abundantly express GLP-1R (80-95%), while the receptor is absent or present in a very small proportion in α-cells (0-20%) (35, 36, 40, 41, 48, 49). δ-Cells are less well characterized and although some analyses reveal expression of the Glp1r transcript in these cells (33, 34), analyses at the protein level are less firm showing little to no GLP-1R expression (36, 41, 46, 49). These figures should however be interpreted with some limitations. Quantification based upon IHC is notoriously challenging. Indeed, it can be difficult to assign GLP-1R expression to adjacent cells whose membranes overlap (as is often the case for α-cells, β-cells, and δ-cells) (49). Nonspecific background signal in cells expressing low levels of GLP-1R is problematic, and differences in sensitivity/settings between different microscopes means that results are difficult if not impossible to calibrate across studies (compared with RNA-seq and fluorescence-activated cell sorting [FACS] which are more standardized). On the other hand, visualizing GLP-1R expression in the intact tissue avoids artefacts introduced by cell dissociation as well as removal of cells from the tissue setting. Moreover, utilizing FACS to isolate endocrine cell populations based on primary hormone expression can mask heterogeneous Glp1r expression in subpopulations of cells. For instance, if less than 10% of α-cells are Glp1r+, the remaining 90+% of α-cells in the binned sample may dilute this signal to yield the interpretation that α-cells are Glp1r–. Thus, combining multiple approaches to interrogate GLP-1R expression remains necessary to account for the limitations of a single approach. Table 1 summarizes GLP-1R transcript and protein levels based on the studies outlined above.
Table 1.
Relative GLP1R transcript and protein levels in the islet endocrine cell types based upon(33-36, 40, 41, 46, 48, 49)
| Cell type | Glp1r/GLP1R | GLP1R |
|---|---|---|
| β-cell | +++; robust and ubiquitous expression | +++; robust and ubiquitous expression, established direct GLP-1 activity |
| α-cell | +/–; little to no expression | +/–; little to no expression, direct GLP-1 activity is debated |
| δ-cell | ++; ubiquitous, but lower expression relative to β-cells | +/–; little or no expression, evidence for direct GLP-1 activity is minimal |
Where and how does GLP-1 act in the islet?
β-Cells
The molecular pharmacology of GLP-1R signaling and trafficking has been extensively studied in heterologous expression systems and is reviewed in detail elsewhere (54, 55). Here we briefly cover facets of GLP-1R signaling pertinent to insulin release from primary β-cells where the investigation of the sites and mechanisms of GLP-1R signaling remain less well characterized. Activation of the GLP-1R strongly induces adenylate cyclase and cyclic adenosine 3′,5′-monophosphate (cAMP) accumulation following ligand activation, with nanomolar concentrations of GLP-1/exendin-4 (Ex4) acting through cAMP and exchange protein directly activated by cAMP (EPAC) pathways in β-cells (56). cAMP acts as a potent stimulus of insulin secretory granule exocytosis by increasing voltage-dependent Ca2+ channel activity, Ca2+ influx, as well as directly sensitizing vesicles themselves for release (57, 58).
While cAMP constitutes the major mediator of GLP-1R signaling, contributions from β-arrestin are more debated. On one hand, both β-arrestin 1 (59) and β-arrestin 2 (60, 61) have been shown to be dispensable for acute stimulation of insulin secretion by GLP-1/GLP-1RA. However, other studies have shown that β-cell knockout of Arrb1 enhances insulin secretion in response to GLP-1, suggesting β-arrestin 1 limits GLP-1R signaling in β-cells (62). The differing concentrations of GLP-1 used in these experiments could reconcile the discrepant results in the β-arrestin 1 knockout models, as high concentrations of GLP-1 (100 nM) (59) may elicit different signaling pathways compared with GLP-1R activation with lower concentrations (0.3 nM) (62). Moreover, the absence of a phenotype in the single β-arrestin knockout models (59-61) could equally reflect the presence of redundancy in the GLP-1R signaling network and as such studies in double β-arrestin 1/2 knockout mice are needed. The major GLP-1R signaling pathways in rodents and human β-cells are shown in Fig. 1C.
Activation of GLP-1R by picomolar concentrations of GLP-1 has been demonstrated in mouse and human β-cells (63, 64). Rather than engaging cAMP synthesis, low concentrations of GLP-1 instead activate a phospholipase C (PLC)-protein kinase C (PKC)-Transient Receptor Potential Cation Channel Subfamily M Member 4/5-signaling cascade through the Gqα subunit, stimulating insulin secretion (63). Low concentrations of GLP-1 also increase Ca2+ fluxes in human β-cells, which fits with a role for inositol 1,4,5-trisphosphate 3–dependent mobilization of endoplasmic reticulum (ER) Ca2+ stores (inositol 1,4,5-trisphosphate 3 being downstream of PKC) (63, 64). Most recently, GLP-1R has been shown to signal predominantly via Gqα in mouse and human β-cells subject to chronic depolarizing stimuli (KATP channel mutations, hyperglycemia) (65). Since several of these experiments used chemical inhibitors with known off-target effects (eg, on store-operated Ca2+ entry, phosphodiesterases), it will be important to examine whether similar phenotypes exist in β-cells specifically deleted or knocked down for Gqα or Gsα. Lastly, GLP-1 also engages a network of β-cells in human islets, leading to coordinated Ca2+ rises (64). Such signaling is dependent on intercellular gap junction communication, which Ex4 has been shown to facilitate in a cAMP protein kinase (PK) A-EPAC–dependent manner (66).
α-Cells
The hypothesis that GLP-1 directly affects α-cell function remains controversial. Despite evidence supporting GLP-1R expression in only a small subpopulation of α-cells, studies have shown that GLP-1 influences α-cell signaling and glucagon secretion by inhibiting N and P/Q voltage-dependent Ca2+ channels (42, 45, 50). α-cells could plausibly express low levels of GLP1-R, which would be difficult to detect using immunostaining, yet sufficient to evoke a response in a highly amplified signaling cascade. However, low abundance Glp1r mRNA transcripts should still lead to recombination in the various GLP-1R-reporter mice discussed above, where somewhere between 0% (36) and 10% (35) of α-cells were labelled with reporter. Moreover, the most recent fluorophore antagonist approaches have single-molecule resolution and detected GLP-1R in only 1% to 5% of α-cells, with either complete absence or strong signal, suggesting that graded expression in the population is unlikely (49).
Another explanation for the actions of GLP-1 on α-cells could be through GLP-1 degradation products. Several reports suggest that GLP-1 (7-36) and GLP-1 (9-36) are glucagonostatic in WT and Glp1r–/– islets (67). Effects of GLP-1 (7-36) but not GLP-1 (9-36) were abolished by pretreatment with a DPP-4 inhibitor, suggesting that GLP-1 (7-36) is a propeptide for GLP-1 (9-36) in this setting (67). Notably, GLP-1 (9-36) was found to signal at the human glucagon receptor with a median effective concentration of 30 nM. While such concentrations are pharmacological, GLP-1 (9-36) is the major circulating form of GLP-1 and DPP-4 is present in islets, which could plausibly generate high local levels of cleavage products. These findings are however difficult to reconcile with previous studies showing that the GLP-1R-specific antagonist Ex9 (27) blocks the effects of GLP-1 on α-cell electrical activity and glucagon secretion (42). Although interesting, these data are unlikely to be relevant for degradation-resistant GLP-1RA and DPP-4 inhibitors, which suppress glucagon secretion without generating GLP-1 (9-36) through mechanisms requiring the known GLP-1R.
δ-Cells
Relatively few reports demonstrate direct actions of GLP-1 on somatostatin secretion. Studies using the perfused mouse pancreas preparation showed that GLP-1 lowers glucagon secretion in a paracrine manner via an increase in somatostatin output (which negatively regulates α-cell function) (68). Notably, the action of GLP-1 to inhibit glucagon secretion was prevented by blocking somatostatin receptor 2, which is expressed on human α-cells and β-cells (69). Similar results have been reported using the perfused rat pancreas (70). Such paracrine regulation of glucagon secretion by GLP-1 likely requires an intact microvasculature, since the somatostatin receptor antagonist CYN154806 did not block the glucagonostatic effects of GLP-1 in isolated mouse islets (42). It remains unclear how GLP1 increases somatostatin secretion in mouse δ-cells given the low levels of GLP-1R protein detected following FACS to isolate islet cells (see above). The expression and functional importance of the GLP-1R in δ-cells in the intact pancreas, and the consequences arising from conditional deletion of the GLP-1R in the δ-cell compartment requires further analysis.
GLP-1R expression and function in the exocrine pancreas
Interest in the roles of GLP-1R expression and activity in pancreatic acinar cells was heightened by the hypothesis that the clinical use of GLP-1RA might be linked to the development of pancreatitis or pancreatic cancer (11). Indeed, GLP-1RAs such as exenatide or liraglutide increase pancreatic mass and protein synthesis independent of changes in cell proliferation in mice (71). Moreover, native GLP-1 directly increases pancreatic enzyme secretion from and cAMP formation in mouse pancreatic acinar cells (72), actions requiring the canonical GLP-1R. The Glp1r mRNA transcript is detectable in RNA isolated from mouse pancreatic acini (72), and weak variable GLP-1R immunopositivity was detected in pancreatic acinar cells, but not in ductal cells, with the Mab 3F52 antibody in sections from pancreata obtained from monkeys with or without diabetes (44). Similar patterns of acinar cell GLP-1R-immunopositivity were detected in pancreatic specimens obtained at surgery from 10 nondiabetic individuals, as well as in 12 pancreatic specimens from individuals with T2D. In situ ligand binding detected GLP-1 binding sites in the pancreatic acinar cells, with radiolabeled ligand displaced in the presence of excess unlabeled GLP-1 (44). Waser and colleagues examined pancreatic GLP-R expression in the human pancreas by IHC using the Mab 3F52 antibody, as well as by in situ ligand binding autoradiography using 125I-GLP1(7-36)-amide (51). A combination of both diffuse and heterogeneous weak GLP-1R expression was observed in some but not all acini from pancreatic samples resected from insulinoma tumors, chronic pancreatitis, and a few cases of pancreatic cancer. No immunoreactive GLP-1R expression was detected in pancreatic ducts (51). Similar patterns of acinar cell GLP-1R localization were reported in the mouse pancreas, using the 125I-labeled GLP-1R antagonist Ex9 to detect in situ binding, as well as the Mab 7F38 antibody to localize GLP-1R immunopositivity in the exocrine pancreas of WT but not Glp1r–/– mice (73). Hence, the available data support low level GLP-1R expression in the pancreatic acinar but not ductal cells in mice and humans.
Concluding Remarks for GLP-1R Expression and Function in the Pancreas
Actions mediated by GLP-1 in the islet are shown in Fig. 1D. The major source of GLP-1 that binds GLP-1R in the healthy islet is still unresolved, although GLP-1 produced in α-cells may play a relatively more important role in the injured pancreas. Insulinotropic effects of GLP-1 and GLP1-RA are mediated by GLP-1Rs expressed predominantly (if not completely) in the β-cell compartment in both mouse and human islets, assessed using validated antibodies, probes and reporter mice across a range of techniques. Direct effects of GLP-1 on α-cell function and glucagon secretion remain debated and are unlikely to be mediated by the GLP-1R. Instead, degradation of GLP-1 by DPP-4 may yield truncation products that act upon GCGR expressed in α-cells. These findings are however unlikely to be pertinent for the glucagonostatic actions of most GLP-1RA, which are largely completely DPP-4-resistant. While GLP-1 increases somatostatin secretion, δ-cells likely express GLP-1R at low abundance and indirect effects cannot be excluded. Finally, GLP-1R is present in the exocrine pancreas, localized to the acinar cells, where GLP-1 acts to increase digestive enzyme release. Table 2 lists validated antibodies and probes for specific GLP1R detection.
Table 2.
List of antibodies and fluorescent ligands validated for GLP1R detection specificity using GLP1R-/- tissue or transfected cells (with and without human GLP1R).
| Reagent type | Catalogue number/name | Source | Reported cross-reactivity | Reference |
|---|---|---|---|---|
| Antibody | Mab 3F52 | Iowa DSHB | Human, primate | (44) |
| Antibody | Mab 7F38 | Iowa DSHB | Human, Mouse, Rabbit, Rat | (119) |
| Antibody | Glp1R0017/ GLP1R-APC | Cambridge University | Mouse | (36, 53) |
| GLP1R agonist | Ex4-Cy3 and Ex4-Cy5 | Novo Nordisk | Mouse | (48) |
| GLP1R agonist | Liraglutide750 | Novo Nordisk | Mouse, rat | (118) |
| GLP1R antagonist | Ex9-39750 | Novo Nordisk | Mouse, rat | (118) |
| GLP1R antagonist | LUXendin555, LUXendin645, and LUXendin651 | University of Birmingham | Mouse/hESC | (49) |
The Brain GLP-1 System
Where does brain GLP-1 originate from?
Proglucagon (GCG) mRNA transcripts are detected predominantly in the brainstem (74, 75), whereas the glucagon-like peptides (including GLP-1), and the GLP-1R are more widely distributed throughout the CNS (10, 75-80). Further characterization of the GLP-1 immunoreactive cells in the lower brainstem solidified the view that these cells are a specific population of neurons, rather than glial cells or endothelial cells (ECs), which are not catecholaminergic but primarily glutamatergic (79, 81). Additionally, the observation that GLP-1 immunoreactivity was localized to axon terminals in the hypothalamus further solidified the view that GLP-1 -producing cells in the brainstem are neurons and utilize GLP-1 as a neurotransmitter that is stored until release in the axon terminals. A first proof that this assumption is correct was provided by the observation that injection of a virus encoding shRNA against preproglucagon (PPG) into the nucleus tractus solitarius (NTS) causes a decrease in GLP-1 immunoreactivity in the paraventricular nucleus (PVN) of the hypothalamus (82). Functional studies in the rat combining immunohistochemical identification of these neurons with c-Fos immunodetection revealed their activation by malaise, stress, and gastric distension and linked them to the suppression of food intake (83-86).
The GLP-1-producing neurons are also called PPG neurons or GCG neurons. For the purpose of this review, the term PPG neurons will be used, independent of species, to describe the neurons that produce GLP-1 within the CNS. These are largely equivalent cell populations in higher mammals (87, 88). CNS GLP-1 expression has also been described in other vertebrates, such as fish and bird, but specific accounts of PPG neurons in their brainstem are lacking (89, 90).
The ability to interrogate the function of the CNS GLP-1 system was enabled by development of transgenic mice targeting the GLP-1-producing neurons using endogenous Gcg promoter sequences (91). The first mouse strain allowed fluorescent visualization of these cells in vitro and thus electrophysiological studies of these neurons in brain slices (92). Additionally, the strong expression of yellow fluorescent protein (YFP) in the cytoplasm of PPG neurons allowed visualization not only of the cell body and terminals of cells enabling Gcg transcriptional activity, but of the entire dendritic and axonal structure of these cells. These studies provided further characterization of the main population of PPG neurons in the NTS and in the intermediate reticular nucleus (IRT), and the population in the olfactory bulb that was previously detected by in situ hybridization (ISH) (93-95) (Fig. 2). Additional PPG neurons were located in the piriform cortex and in the lumbar–sacral spinal cord (93, 96). Widespread projections throughout the brain were confirmed in unprecedented detail by revealing not only terminal fields, that can be visualized with GLP-1 immunoreactivity, but also axons in passage. Importantly, these transgenic mice established that hippocampus and most areas of the cortex do not receive GLP-1 projections, findings with implications for understanding the mechanisms of neuroprotection that have been postulated for physiological GLP-1 action (97). Subsequent studies utilized mice with Cre-recombinase under the control of the Gcg promoter thereby facilitating functional manipulation of these neurons in vivo (98-102). Importantly, these mice allowed the selective ablation of NTS-PPG neurons and thereby the demonstration that loss of these neurons substantially decreased GLP-1 content in both hypothalamus and spinal cord (100) and thus corroborated the findings from rat, that interference with GLP-1 translation in the brainstem affects GLP-1 content in the forebrain (82). These Gcg-Cre transgenic mice enabled the use of Cre-dependent viral expression of Channelrhodopsin2-Green Fluorescent Protein (ChR2-GFP) to demonstrate that NTS-PPG neurons project to most (if not all) forebrain targets (103). These findings confirmed results of prior studies utilizing injection of retrograde tracers into various forebrain areas and colocalizing the tracer with GLP-1 immunoreactivity in the NTS and IRT (79, 104).
Manipulation of PPG neurons to interrogate their function in vivo
Studies in mouse and rat established that GLP-1 is produced by PPG neurons and is found in their cell bodies and axon terminals. Functionally, experiments using shRNA to knockdown GLP-1 expression produced a mild hyperphagic effect, mainly limited to rats on a HFD (82). Subsequent studies making use of transgenic mice with virally mediated Designer Receptors Exclusively Activated by Designer Drugs (DREADD) expression in the NTS PPG neurons, demonstrated that selective activation of these neurons produces a strong hypophagic effect (99-101).
This was followed by a study employing both inhibitory DREADD receptor expression as well as selective ablation of NTS PPG neurons by the expression of diphtheria toxin A subunit to examine effects on food intake and brain GLP-1 content (100). Both hypothalamic and spinal cord GLP-1 content was severely reduced upon ablation of these neurons, thus demonstrating that brainstem GLP-1 neurons are the principal source of GLP-1 within the CNS and that GLP-1 is stored in the axon terminals of the PPG neurons, all of which have their cell bodies in the lower brainstem. Interestingly, as observed before with shRNA knockdown in rat, there was little effect on basal food intake, but reduction of brainstem Gcg expression or ablation of PPG neurons increased post fast refeeding and impaired stress-induced feeding reduction (82, 100).
These findings, together with the phenotype of normal food intake and body weight observed in GcgGut–/– mice with intestine-specific deletion of the proglucagon-derived peptides (14), clarify the modest physiological importance of either GLP-1 derived from PPG neurons or the gut for control of food intake. Moreover, neither chemogenetic activation nor ablation of CNS PPG neurons or knockdown of brainstem Gcg mRNA affected glucose tolerance in regular chow–fed mice or rats (82, 100, 102). Interestingly, shRNA knockdown of GLP-1 expression from PPG neurons in HFD-fed rats resulted in moderately impaired glucose tolerance, whereas chemogenetic activation of PPG neurons in HFD-fed mice did not impact glucose tolerance. Intriguingly, correlation of NTS levels of Gcg mRNA with fat mass was observed in rats, implying signals linking the extent of adiposity to central PPG activity.
Intracerebroventricular (ICV) injection of the GLP-1R antagonist Ex9 in chow-fed rats and mice impaired glucose tolerance (82, 105), but ablation of PPG neurons in chow-fed mice did not impair glucose tolerance (100). The simplest explanation for this discrepancy would be that PPG neurons are not involved in the regulation of glucose tolerance, but that gut-derived GLP-1 is able to reach (parts of) the brain to affect glucose tolerance, or that ICV Ex9 actually accesses the general circulation and exerts direct effects, such as in the pancreas. Alternatively, basal constitutive GLP-1R signaling may exert tonic control of these processes, which may be disrupted by pharmacological administration of GLP-1R antagonists independent of the source of the GLP-1 ligand (106-108). Finally, chemogenetic activation of PPG neurons increases heart rate, but their inhibition or ablation has no effect on resting or active heart rate or blood pressure (109). All these results point at the central GLP-1 system as being capable of exerting significant effects on food intake and on autonomic nerve activity, but not producing these effects tonically in daily life.
What is the physiological role of brain-derived GLP-1?
These findings raise multiple questions, namely how important is brain-produced GLP-1 for physiology, how is it regulated and how and where does it act in the CNS? Is its regulation linked to circulating GLP-1? Interpretation of these findings requires careful scrutiny of the specific diets, age and sex of the animal species, housing conditions, and experimental manipulations utilized to infer conclusions about CNS GLP-1 action. For example, the actions of Ex9 to increase food intake were observed in satiated but not in hungry rats (110). Similarly, multiple studies have employed scheduled meals to observe CNS effects of GLP-1 on food intake (107, 111, 112). GLP-1 and GLP-1Rs within the brain may not be critical for feeding under ad libitum access to food, but only under conditions where a large meal is consumed, such as after a prolonged fast, or in the case of sporadic availability of highly palatable food to these rodents. A likely contributing signal here is the extent of gastric distension. Notably, c-Fos activation in NTS PPG neurons requires a higher degree of gastric distension, then for instance c-Fos activation in neighboring catecholaminergic neurons (83, 85).
PPG neurons may also have developed as a risk assessment system to balance predatory risk (manifested as stress leading to meal termination) with the need for food/energy intake. Progressive gastric distension may shift activity away from intake towards the avoidance of predation as the stomach becomes full and a higher percentage of PPG neurons becomes activated (84). Simultaneously, PPG neurons are also activated in situations of stress, be this psychological or restraint stress or consumption of a toxic substance, as mimicked experimentally by injection of lithium chloride LiCl or lipopolysaccharide (104, 113). Additionally, PPG neurons are activated by local (NTS) availability of leptin or cholecystokinin (CCK), as well as electrical stimulation of vagal terminals in the NTS (92, 114). Thus, PPG neurons are activated by signals linked to short- and long-term energy balance and can reduce food intake, but do not seem to play a major role in determining food intake and body weight in ad libitum fed rodents. Rather, their physiological role seems to be tied to the response to various forms to stress, but might lend themselves to be exploited clinically to achieve hypophagia and weight loss. A role for PPG neurons beyond the monitoring of short- and long-term energy balance was also suggested by a comprehensive study of their mono- and polysynaptic inputs within the central and peripheral nervous system (115). The localization of GLP-1Rs that serve to mediate the actions of GLP-1 released from PPG neurons is discussed below.
GLP-1 receptors within the nervous system
Various techniques, employing radiolabeled or fluorescent GLP-1R agonists or antagonists, or specific GLP-1R antisera, together with ISH, have localized GLP-1R expression within the nervous system (49, 116-120). Complementary studies have used mice with germline or conditional tissue-specific deletion of the GLP-1R to examine the importance of different GLP-1R populations in the CNS and periphery (121-126). Notably, the hypophagia caused by ICV injection of GLP-1 was fully abrogated in Glp1r–/– mice, consistent with the loss of binding sites for Ex4 in the Glp1r–/– mouse brain (121). These findings align with the demonstration that PPG neuron activation produces hypophagia, but that the loss or attenuation of PPG neuronal activity or genetic deletion of gut GLP-1 does not cause overeating or obesity (14, 100-102, 127). Thus, although GLP-1 has an essential role in the control of blood glucose, it is dispensable for the suppression of food intake at least under standard animal house holding conditions.
GLP-1Rs are expressed throughout the CNS, from the olfactory bulb down to the spinal cord (35, 75, 128, 129) (Fig. 3). The use of genetic targeting approaches has revealed specific cell types producing various GLP-1 responses. Adams et al. deleted the GLP-1R from either vesicular glutamate transporter 2 or vesicular GABA transporter–expressing cells, thus removing the receptor from glutamatergic and GABAergic neurons, respectively. Only those mice lacking GLP-1R in glutamatergic cells lost the hypophagic and long-term body weight–reducing effect of liraglutide, suggesting that liraglutide affects food intake and body weight via GLP-1Rs located on glutamatergic, but not GABAergic cells (125). In contrast, Fortin et al. used Glutamic Acid Decarboxylase (GAD)-Cre rats to target GABAergic neurons (130). Their strategy was to virally express an inhibitory DREADD receptor in GAD-expressing cells in the NTS and to activate this receptor in the presence of liraglutide. Inhibition of NTS GABAergic neurons prevented the anorectic effect of liraglutide. They concluded that GABAergic neurons in the NTS mediate the hypophagic effects of liraglutide. Reconciling these studies in mice and rats, Adams et al. demonstrated key roles for GLP-1Rs on glutamatergic but not GABAergic neurons yet they did not show that GABAergic neurons per se are not required for the feeding response (125). Similarly, Fortin et al. demonstrated that the activity of GABAergic neurons in the NTS is required for the hypophagic response to liraglutide, but they did not prove that it is actually the GLP-1R expressed on these neurons that is required for the response (130). Thus, one might hypothesize that liraglutide binds to GLP-1Rs on glutamatergic neurons, and these in turn signal to GABAergic neurons and their release of inhibitory transmitter is required for the suppression of food intake. Hence, caution is needed when using chemogenetic approaches to extrapolate the precise physiological pathways employed by GLP-1 within the CNS.
Figure 3.
GLP-1 receptors (GLP-1Rs) and brain-derived GLP-1 in the central nervous system. Within the CNS GLP-1 is produced by PPG neurons (light yellow) and distributed to their axon terminals ready for synaptic release (dark green). Names of brain areas that receive PPG innervation and express GLP-1 receptors are given as abbreviations in blue. Systemically distributed GLP-1 receptor agonists (GLP-1RAs) are depicted as red circles and access the circumventricular organs with a leaky blood–brain barrier, and the ventricles, but not the brain parenchyma. The olfactory bulb and the piriform cortex (Pir) form areas that express GLP-1Rs and harbor PPG neurons that project locally only. Abbreviations: AH, anterior hypothalamus; AP, area postrema; ARC, arcuate nucleus; Barr, Barrington’s nucleus; BNST, bed nucleus of the stria terminalis; CAA, central autonomic area (lamina X); caud Hipp, caudal ventral hippocampus; CeA, central nucleus of the amygdala; DMH, dorsomedial hypothalamus; DMNX, dorsal vagal motornucleus; GrO, granule cell layer of the olfactory bulb; IML, intermediolateral nucleus; IRT, intermediate reticular nucleus; LC; locus coeruleus; NAc, nucleus accumbens; NTS, nucleus tractus solitarius; OVLT, organum vasculosum of the lamina terminalis; PAG, periaqueductal grey; PBN, parabrachial nucleus; Pir, piriform cortex; PVN, paraventricular nucleus; PVT, paraventricular thalamus; Sep, lateral septum; SFO, subfornical organ; RPa, raphe pallidus; VLM, ventrolateral medulla; VMPO, ventromedial posterior nucleus.
Neuronal GLP-1Rs Outside the Blood–Brain Barrier
GLP-1Rs on vagal afferent neurons
GLP-1Rs are expressed by sets of vagal afferent neurons (35, 131, 132), which are mechanoreceptors (133). It has been tempting to assume that these GLP-1R expressing neurons are those stretch-activated vagal afferents that activate PPG neurons (85). However, a study examining this hypothesis found that GLP-1R expressing vagal afferent neurons represent only a very small fraction of the vagal afferent inputs to PPG neurons (135). GLP-1R-expressing vagal afferent neurons have their sensory inputs within the gastrointestinal tract, their cell bodies in the nodose ganglion, and their axon terminals in the NTS; where exactly their GLP-1Rs are located, remains unclear. Thus, the source of GLP-1 activating these neurons might be the gut-derived GLP-1 for the peripheral nerve endings, or the PPG neuron-derived GLP-1 if their central terminals express GLP-1R. The physiological role of GLP-1Rs expressed in vagal afferent neurons was examined by viral delivery of shRNA targeting the nodose ganglion (NG) GLP-1R in male Sprague Dawley rats (134). GLP-1R knockdown in vagal afferent neurons did not affect long term energy balance but did accelerate gastric emptying, findings consistent with the phenotype of mice with genetic reduction of Glp1r expression using Phox2b-Cre to target autonomic neurons including the NG (126). Reduction of NG Glp1r expression also blocked the effects of intraperitoneal, but not intraventricular, Ex4 on food intake and gastric emptying in rats (134), whereas the sustained actions of large GLP-1RA such as dulaglutide to generate weight loss were also modestly attenuated in mice with genetic reduction of NG Glp1r expression (126). Collectively, these observations imply that the GLP-1Rs expressed by vagal afferent neurons are located in the periphery, outside the blood-brain barrier, rather than on the nerve terminals inside the NTS. Thus, GLP-1Rs expressed by vagal neurons seem to be a physiological target for gut-derived or pharmacologically administered GLP-1.
GLP-1Rs in circumventricular organs
Similar uncertainty arises in the context of interrogating the importance of GLP-1Rs expressed by neurons in the circumventricular organs that lack a functional blood brain barrier. These regions, such as the area postrema (AP), median eminence and part of the neighboring arcuate nucleus (ARC), subfornical organ, and organum vasculosum of the lamina terminalis, are accessible from the circulation, and thus are potential targets for postprandially released gut-derived GLP-1 as well as confirmed targets for systemically administered GLP-1RAs (Fig. 3). However, some of these regions are also innervated by PPG neurons (93). The two circumventricular regions that have been investigated in some detail are the median eminence/ARC and the AP. While systemic administration of GLP-1RA leads to activation visualized by c-Fos immunoreactivity, in many areas of the brain, it is particularly these areas where clear binding of fluorescent GLP-1RA is observed (116-118).
Most likely, engagement of GLP-1Rs in these areas, as indirectly visualized by c-Fos staining in GLP-1R+ cells (125), leads to additional secondary c-Fos activation along downstream pathways in the CNS (116-118). Experiments employing physical lesions to destroy the AP, the ARC, or the PVN, as well as subdiaphragmatic vagal afferent deafferentation indicated that the majority of the hypophagic effect of the GLP-1RA liraglutide resides within the ARC (118). Chronic infusion of Ex9 into the PVN, but not the ARC, increased body weight (118); however, Ex9 reduced the body weight–lowering effect of liraglutide when administered into the ARC, but not the PVN. These results indicate that while the ARC may be a key target for the anorectic actions of liraglutide there is no tonic activation of the ARC GLP-1Rs by endogenous GLP-1 released either by the gut or the PPG neurons.
The available evidence distinguishes between the weight lowering effects of systemically administered GLP-1RA from those of PPG neuronal activation. This notion is supported by studies dissociating the hypophagic effects of PPG neuron activation from the food intake suppressing actions of subcutaneous semaglutide (135). It seems possible that GLP-1Rs expressed in the ARC may be activated by high circulating levels of GLP-1 under conditions of severe intestinal malaise or following bariatric surgery. This distinction may explain why GLP-1RA therapy is associated with an increased sensation of nausea (136), while chemogenetic activation of brainstem PPG neurons does not elicit adverse responses in mice (102, 135).
GLP-1Rs inside the blood–brain barrier
Most other GLP-1R-expressing brain regions receive axonal inputs from PPG neurons, and are likely activated by GLP-1 originating from those cells. However, brainstem PPG neurons do not project to the hippocampus or to the cerebral cortex (93). Thus, it is currently unclear how GLP-1Rs located in these areas are activated. A small population of PPG neurons has been reported in the piriform cortex (93), but these do not give rise to axons in other parts of the cortex or the hippocampus. Activation of GLP-1Rs in the ventral hippocampus also results in hypophagia, and it has been suggested that volume transmission from the lateral ventricles, which had detectable levels of GLP-1, might account for their physiological activation (137). These observations are consistent with the possibility that PPG neurons might be the source of GLP-1 within the ventricle, as PPG axons are found along the ependymal cell layer covering the surface of the ventricles and intraventricular injection of a retrograde tracer labelled a fraction of NTS PPG neurons (137). While this is plausible, it should be noted, that both GLP-1RA and GLP-1R antagonists given subcutaneously access the ventricular space (49, 116, 118). Hence, there is no discernable barrier for pharmacological or gut-derived GLP-1 to reach the brain ventricles, but it does not provide evidence that post-prandially released GLP-1 would meaningfully access the ventricles. Additionally, the observation that there is very little entry of systemically administered GLP-1R agonists into the brain parenchyma beyond the circumventricular organs, and particularly into the ventral hippocampus, suggests that there is no free diffusion between the ventricular space and the brain parenchyma (116, 118). This would then argue against PPG neuron-derived GLP-1 entering the ventricles and exiting at the level of the ventral hippocampus. A final area that is not innervated by brainstem PPG neurons is the olfactory bulb. However, the olfactory bulb contains both GLP-1- and GLP-1R-expressing cells (75, 95, 138), and could thus constitute an independent local GLP-1 system. Whether or not the olfactory bulb is accessible to GLP-1 from the general circulation is not clear; no studies utilizing GLP-1R agonists or antagonists to date have visualized binding in the region of the olfactory bulb.
When interrogating the function of GLP-1R populations within the brain, modulation of food intake is often the primary measured output, but the physiological mechanisms underlying modulation of this output varied substantially between studies and brain regions. For example, food intake might also be reduced due to a reduction in the reward value of the specific substance tested, findings potentially relevant for the actions of substance abuse, the sensation of nausea or other malaise, or the presence of acute or chronic stress (139-142). Additionally, neural GLP-1R activation has been linked to an increased cardiovascular output, or to changes in body temperature, or increased peripheral brown fat activity, all likely consequences of activation of the sympathetic nervous system (143-146) and therefore in line with the proposed functional role of PPG neurons in stress responses as discussed above.
Concluding Remarks about GLP-1R Expression and GLP-1 Action in the Nervous System
The emergence of fluorescently labeled GLP-1RA and transgenic mouse models combined with the use of viral vectors to dissect the brain GLP-1 system over the past decade has highlighted our lack of deep understanding of GLP-1 action in the brain. It is now emerging that there is no direct link between the gut and brain GLP-1 system, and that GLP-1RAs exert their appetite-suppressing effects primarily, if not exclusively, by interacting with GLP-1Rs on cells in the circumventricular organs outside the blood–brain barrier, that then signal deeper into the brain but do not involve activation of PPG neurons, the brain’s own source of GLP-1. Understanding the physiology of brain-derived GLP-1 is even more nascent and unravelling the details of its regulation and various actions including, but not limited to, the reduction in food intake, promises to be an exciting journey of discovery.
GLP-1R expression in the cardiovascular system
Cardiovascular safety trials with GLP-1RA have demonstrated reduction in all-cause and cardiovascular mortality, and lower rates of major cardiovascular events including stroke and myocardial infarction (15, 16). GLP-1RA also increase heart rate in humans and animals, findings attributed in part to activation of the sympathetic nervous system, inhibition of the parasympathetic nervous system, and activation of the sinoatrial (SA) GLP-1R (143, 144, 147, 148). GLP-1R signaling also attenuates the extent of experimental stroke in preclinical studies (97), and GLP-1RA reduce the rates of stroke in humans with risk factors for or established cardiovascular disease (149). Furthermore, GLP-1RA also reduce albumin excretion, and trials are underway to determine whether these agents may reduce rates of dialysis, decline of eGFR, and renal replacement in people with diabetic kidney disease. Collectively, these observations have created great interest in understanding how GLP-1RA exert their mechanisms of action in the cardiorenal system. Nevertheless, there is still relative uncertainty and some controversy about the precise cell types and tissues that express the GLP-1R in the heart, peripheral vasculature, and kidney (Fig. 4).
Figure 4.
GLP-1 receptor expression in heart and blood vessels within select organs and actions of GLP-1 associated with these organ and cell types. The arrow depicts the relative levels of GLP-1R expression deduced from human gene expression databases such as the Genotype Tissue Expression portal.
Localization of GLP-1R in the heart
Despite considerable effort, the precise identity of GLP-1R+ cells in the heart remains elusive (44). Analysis of hearts from 3 cynomolgus monkeys, including sections from males and females, revealed GLP-1R-immunopositive cells using Mab 3F52 only in SA myocytes, which were identified by costaining sections for hyperpolarization activated cyclic nucleotide gated potassium channel 4. In a single frozen sample taken adjacent to the SA node confirmed to be GLP-1R immunopositive by IHC, 125I-GLP-1 binding was detected and diminished following addition of excess unlabeled ligand. GLP-1R-immunopositive cells were also visualized in sections from the SA node from a single normal human heart (44).
Wallner et al. reported GLP1R expression in right atrium (RA) tissue and right ventricle (RV) tissue from 7 human heart samples that were not suitable for transplantation. Relative GLP1R expression by qPCR was 3.4-fold higher in the RA than in the RV (150). GLP1R mRNA transcripts were also amplified by conventional PCR generating a 103 base pair cDNA product from RNA isolated from the RA and RV, and from 3 samples of isolated cardiomyocytes from the left ventricle (LV). Clarke and colleagues analyzed GLP-1R protein expression in the human heart by IHC using Mab 3F52 to analyze cardiac tissue obtained from anonymous donors from 2 tissue biobanks (151). Extensive GLP-1R-immunopositivity was reported in atrial and ventricular cardiomyocytes, but not within ECs or vascular smooth muscle cells (VSMCs). The proportion of GLP-1R+ cells, and the number of hearts or sections examined was not described (151). Giblett and colleagues from the same research group also analyzed GLP-1R protein expression in human heart tissue obtained from nondiabetic human subjects with ischemic heart disease using Mab 3F52 (152). Patchy GLP-1R immunoreactivity was observed in cardiac myocytes from the RV and LV; however, the precise location of the GLP-1R-immunopositive cardiomyocytes was not described, and the proportion of GLP-1R-immunopositive cells detected within each analyzed heart was not reported (152).
GLP1R expression was examined in RNA samples from all 4 chambers of 15 healthy and diseased human hearts by qPCR, and RT-PCR for the full-length mRNA transcript (18). GLP1R mRNA transcripts were detected in all of the hearts examined, with similar levels in RA vs RV but lower levels in the LV vs the LA. The relative abundance of the GLP1R in heart tissue was similar to that detected in human islet RNA samples. Nevertheless, the cellular localization of GLP-1R expression in the heart, assessed using both ISH and IHC with Mab 3F52, was not identified in the majority of sections analyzed, except in the RA, where GLP1R RNA was detected by ISH (18). Moreover, GLP-1R protein was not detectable in human cardiac tissue by Western blot analysis using Mab 3F52, although this antibody did recognize the GLP-1R protein in extracts subjected to immunoprecipitation and immunoblotting from cells transfected with a cDNA encoding the GLP1R. The discrepant results for GLP-1R localization in the human heart using the same antibody in various reports likely reflect different technical conditions and controls employed for IHC in various laboratories.
Analysis of Glp1r expression in the mouse heart reveals levels of Glp1r mRNA transcripts are higher in the atria than ventricles. PCR amplification of Glp1r transcript in 3 samples from normotensive or hypertensive angiotensin II-infused mice detected Glp1r mRNA transcripts in atria however much lower levels were detected in ventricular tissue (153). Whether this discrepancy reflects true-species differences or region-specific differences in RNA sampling of human ventricular biopsies is unclear as comparable levels of GLP1R mRNA transcripts were detected in RNA extracted from biopsies obtained from all 4 chambers of the human heart (18). Consistent with Glp1r expression in an atrial myocyte-like cell type, Glp1r expression was reduced in mouse atria using the cardiomyocyte specific αMyosin Heavy Chain promoter to drive tamoxifen inducible Cre-ER recombinase in cardiomyocytes (147). To further determine the identity of mouse atrial GLP-1R+ cells, Baggio et al. used the Hcn4 promoter, classically expressed in SA node cells, to drive Cre recombinase expression and specifically target SA gene expression (148). Although Hcn4-Cre reduced levels of Gcgr mRNA transcripts in control experiments using floxed Gcgr mice, no reduction of atrial Glp1r levels was achieved using Hcn4-Cre and floxed Glp1r mice. Hence, the precise atrial cell type(s) that express the murine cardiac GLP-1R remain uncertain.
Assessment of GLP-1R expression in blood vessels
Despite the multiplicity of studies describing actions of GLP-1RA on blood vessels, there is surprisingly little rigorous information that describes GLP-1R expression in normal or diseased VSMCs, ECs, or other vascular cell types (Fig. 4). Much of the data in this area rely on ECs and VSMCs analyzed after cell culture ex vivo, which may not be representative of GLP-1R expression in the same cell types in vivo. Indeed GLP1R mRNA transcripts were not detected by qPCR in RNA isolated from human coronary artery VSMCs or ECs (18). However, Richards et al. reported Glp1r promoter-directed fluorescent cells in the aorta, and arteries and arterioles of multiple tissues, and Glp1r mRNA transcripts were detected by qPCR in mouse aorta, albeit at levels 90% lower than corresponding expression of Glp1r in RNA isolated from mouse atrial tissue (35). Fluorescent cells within the intestinal vasculature costained with α-smooth muscle actin and neural/glial antigen 2, a marker for pericytes, and were thought to represent arterial, rather than venous blood vessels. Nevertheless, Glp1r expression was not examined in the majority of cells+ for the fluorescent reporter.
GLP1R mRNA transcripts were detected by qPCR in the aortic intima and media obtained from subjects, with and without obesity, scheduled for aortic surgery (154). Extensive diffuse GLP-1R-immunopositivity was detected in the aorta using Mab 3F52. Notably, GLP1R/GLP-1R expression was not quantified relative to other GLP1R+ tissues, nor assessed using the same reagents and conditions for comparative purposes of specificity in organs known not to express the GLP-1R.
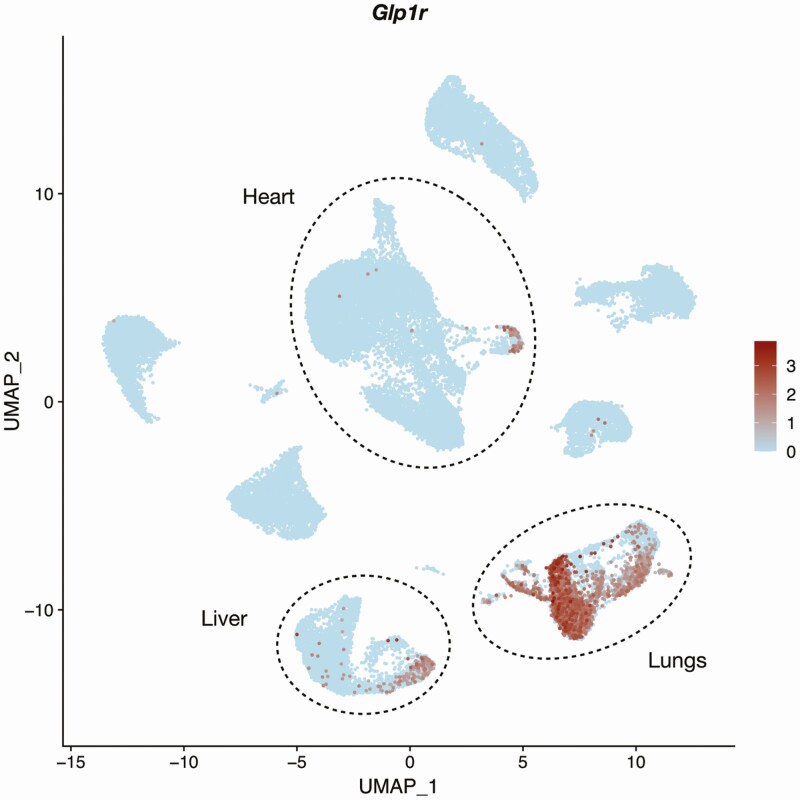
Single cell analysis of murine endothelial cells from 20 organs showed Glp1r+ cells most prominently in the lung (Fig. 5), with some Glp1r+ positive ECs also detected in cells from the liver and heart (155). Given the limitations of read depth needed to identify low abundance transcripts with scRNA-seq, this is likely a conservative snapshot of EC Glp1r expression. Consistent with Glp1r expression in ECs, mice expressing EC-specific Cdh5-dependent Cre recombinase exhibited reduced Glp1r expression in a lung EC preparation isolated using CD31 and CD102 coated beads (156). However, no markers were used to assess the extent of EC enrichment, and knockdown of Glp1r was not examined in resistance arteries, kidneys, heart, aorta, or other vascular sites that might contain EC GLP-1R expression. Using Glp1r-Cre mice crossed with a ROSA26-tdRFP reporter, Richards and colleagues also detected Glp1r-directed reporter gene expression in a few blood vessels within the cardiac ventricles, with a morphology consistent with VSMCs (35). Overall, the data suggest that Glp1r/GLP1R expression in the vascular system is highly heterogeneous, with high expression in mouse lung ECs and restricted expression in subsets of VSMCs in the kidney (discussed next), rather than broadly expressed throughout the vasculature.
Figure 5.
Expression of mouse Glp1r in 40 449 murine endothelial cells. Count matrices were accessed on September 19, 2020, and reported by Kalucka et al. (221) (ArrayExpress ID E-MTAB-8077).
GLP-1R expression in renal blood vessels
The location of GLP-1R expression within the kidneys has been controversial, at times localized to glomerular or tubular epithelium in studies using GLP-1R antisera that do not detect the GLP-1R or lack specificity (11, 20, 43). In vivo autoradiography using 125I-labeled GLP-1 (7-36) amide, IHC using well-characterized GLP-1R antibodies, and ISH have detected GLP-1R in the rat, mouse and monkey renal vasculature, particularly in arterial VSMCs (44, 157-159). Pyke et al. (44) analyzed kidney from 7 normal rhesus monkeys using Mab 3F52. GLP-1R-immunopositivity was limited to a small number of VSMCs within the preglomerular vascular compartment. These findings were corroborated by analysis of matched sections using in situ radiolabeled ligand binding, which identified binding in a vessel that also stained positive with Mab 3F52 (44).
RT-PCR of RNA from isolated mouse kidney fractions, and ISH analysis of mouse kidney detected Glp1r mRNA transcripts in blood vessels and not in the tubular fraction (157). A few GLP-1R+ renin+ juxtaglomerular cells were reported in the afferent arterioles of the monkey and mouse kidney (44, 158). Detection of GLP-1R immunopositivity in several VSMCs within the preglomerular vascular compartment was corroborated by analysis of matched sections using in situ radiolabeled ligand binding, which identified binding in a blood vessel that also stained positive with Mab 3F52 (44). Moreover, the same antisera failed to identify GLP-1R immunopositive cells in the kidney from Glp1r–/– mice. Despite detection of GLP-1R+ cells within a subset of the renal vasculature, the majority of renal blood vessels and VSMCs examined by ICC or ISH do not express the GLP-1R, and studies using validated reagents have not detected the GLP-1R in glomerular epithelial or tubular cells (44, 157, 158). Hence, the mechanisms by which GLP-1 modulates renal function, including control of albumin, water and salt excretion, either directly through control of the renal vasculature, or via indirect mechanisms, remain uncertain.
GLP-1 production in enteroendocrine L cells along the gut
Enteroendocrine L cells have classically been defined as the GCG+ cell type giving rise to GLP-1 in the gut (1). In humans and mice, the majority of L cells reside in the ileum and colon, with fewer cells located in the duodenum and jejunum. L cells sense various nutrients from the luminal surface and in turn secrete GLP-1 into the local circulation (160). Numerous cognate receptors for non-nutrient GLP-1 secretagogues are expressed on the basolateral side of L cells, thus enabling L cells to simultaneously sense molecules in the circulation (161-163). L cells in the proximal gut are capable of responding briskly to nutritive stimuli (14, 164); the available data suggest that distal gut L cells exhibit secretory responses biased towards non-nutrient secretagogues (164). Analysis of mice with marked reduction of Gcg expression within the entire, or selectively in the distal gut, reveals essential contributions of intestinal Gcg expression to the pool of circulating GLP-1, and in the control of gastric emptying and glucose homeostasis (14).
scRNA-seq analyses have illuminated the transcriptomic heterogeneity evident across subsets of L cells (Fig. 6). One of the first scRNA-seq studies on the mouse small intestine recovered 310 out of 7216 (4.3%; which is higher than often achieved) quality control–passed Enteroendocrine cells (EECs) (165), and 10% of these were identified as Gcg+. Subsequently, several reports characterized individual L cells isolated from mouse reporter lines. Sequencing of 259 individual YFP+ cells isolated from the small intestine of GLU-Venus mice identified 3 main subgroups of L cells. These corresponded to (1) Gcg/Pyy double-positive cells, which account for 50% of L cells; (2) Gcg/Tph1/Pzp triple-positive cells, which account for 35% L cells; and (3) Gcg/Gip double-positive cells, which account for 15% L cells (166). Similarly, a comprehensive survey of 6906 small intestine EECs from Neurog3Chrono reporter mice identified more than 90% of L cells as multihormonal (167). The detection of Gcg in EECs correlated strongly with Cck, followed by Pyy, Ghrl, and Sct coexpression (Fig. 6). A scRNA-seq study on 1560 intestinal YFP+ cells isolated from Neurod1-Cre; Rosa26-YFP mice confirmed that colonic L cells are also mostly multihormonal (168). Although both small and large bowl L cells can produce neurotensin, L cells in the distal colon preferentially expressed Pyy and Insl5. Studies of human EECs have relied primarily on bulk RNA-seq for transcriptome profiling (169-171). To circumvent the difficulty of isolating L cells from human intestine with high purity, a recent study sequenced 2255 cells single cells from human small intestinal organoids transduced with doxycycline-inducible NEUROG3 transgenes to drive differentiation towards endocrine lineages (172). Three L cell subtypes among the 2255 EECs sequenced were PYYHigh, PYYLow, and NTSHigh cells, all of which were also detected in the mouse L cell scRNA-seq datasets. A discrepancy between the mouse and human data was the absence of TPH1+ and GIP+ L cells, although the number of organoid-derived L cells sequenced in the study was not high (<100), and the L cell subtypes in the organoid might not faithfully recapitulate the ones in the human intestine in situ. Nevertheless, the analyses and corroboration of mouse and human L cell scRNA-seq datasets support a conserved transcriptional program in L cells across the species. Analysis of human jejunal L cell transcriptomics, and L cell density identified dysregulation of enteroendocrine differentiation signatures associated with reduced L cell density in obese people with T2D (173), providing a possible explanation for reduced meal-stimulated levels of GLP-1 in some of these individuals. Collectively, these findings raise several important questions that warrant further interrogation; do different L cell subtypes produce and secrete GLP-1 in a secretagogue-selective manner with different secretory capacity? Can we attribute the functional heterogeneity of L cells predominantly to their transcriptional signatures or does their location within the gut, or along the crypt–villus axis, cellular neighborhood and coexisting disease states modify their phenotype? Are there new molecular targets that could be identified from these datasets for augmenting endogenous GLP-1 secretion?
Figure 6.
Coexpression of the proglucagon gene (Gcg) and enteroendocrine genes in 411 murine small intestine L cells. Count matrices were accessed on September 19, 2020, and retrieved from the scRNA-seq datasets reported by Gehart et al. (167) (GEO accession number GSE113561). Mouse gene nomenclature: Pyy, peptide YY; Gip, glucose-dependent insulinotropic polypeptide; Nts, neurotensin; Tph1, tryptophan hydroxylase 1; Sst, somatostatin; Ghrl, ghrelin; Sct, secretin; Cck, cholecystokinin.
GLP-1R expression in the gastrointestinal tract
GLP-1R expression is detected across the proximal and distal regions of gastrointestinal tract. The close proximity of intestinal GLP-1R-expressing immune cells and enteric neurons to L cells within the intestine may facilitate immediate paracrine actions of GLP-1. Remarkably, despite robust actions of GLP-1RA to reduce enterocyte chylomicron synthesis and secretion, the canonical GLP-1R is not detectable within the small or large bowel enterocytes. Below we highlight regional considerations and GLP-1R+ cell types within the gut.
GLP-1R expression and biology in gut intraepithelial lymphocytes
Intestinal intraepithelial lymphocytes (IELs) are a unique population of T cells with innate-like immune function. Percoll-purified IELs express high levels of Glp1r relative to the thymus, spleen, and lymph nodes as shown by qPCR (174). Furthermore, IELs from the small bowel express higher levels of Glp1r than colonic IELs. Whole body loss of the Glp1r does not perturb the number and composition of IELs (174). The IEL GLP-1R is functional, as Ex-4 directly augments cyclic adenosine monophosphate (cAMP) levels in murine gut IELs ex vivo (174). Within the intestinal epithelium, both TCRγδ + and TCRαβ + IELs express comparable levels of Glp1r as shown by qPCR on sorted IELs (175). The importance of gut IEL GLP-1Rs for regulating incretin biology and metabolism has been examined in the context of β7–/– mice, which lack gut IELs due to defects in lymphocyte gut homing (175). Reconstitution of irradiated Ldlr–/– mice with a chimera of β7–/–/Glp1r–/– bone marrow raised plasma GLP-1, improved intraperitoneal glucose tolerance, lowered plasma cholesterol, and reduced aortic plaque size after 14 weeks of high-fat high-cholesterol diet feeding, relative to irradiated WT mice with a chimera of β7–/–/WT bone marrow. How gut GLP-1R-expressing IELs control the bioavailability of local and systemic GLP-1 remains uncertain (175). The physiological importance of the enteroendocrine–IEL axis for local and systemic immunity remains incompletely defined.
Stomach and Brunner’s glands express the GLP1R
Human gastric mucosa and parietal cells express GLP1R mRNA transcripts as detected by RT-PCR (176) and multiple GLP-1R-immunopositive cells were detected within the gastric epithelium using a partially characterized monoclonal antibody Mab 28141, findings consistent with the detection of Glp1r transcripts in rat parietal cells (177). GLP-1 may act on the parietal cell GLP-1R to block gastric acid secretion (178, 179); however, few studies have explored the function of GLP-1R signaling within parietal cells in vivo.
Brunner’s glands, found within the proximal duodenum, are a set of submucosal GLP-1R+ exocrine glands specialized in secreting mucus. The glands were highly GLP-1R-immunoreactive using validated GLP-1R antibodies in both mice (using Mab 7F38) and in humans (using Mab 3F52) (44, 119). CD-1 mice injected with a single dose of Ex4 (1 mg/kg) showed upregulation of Il33 and Ccl20 in RNA isolated from Brunner’s glands (180); however, the physiological importance of the Brunner’s gland GLP-1R system remains poorly defined.
GLP-1R expression in the enteric nervous system
The enteric nervous system (ENS) is unique in its capability to transduce signals somewhat independently from the brain or the spinal cord (181). It comprises 3 main cell types: enteric neurons, enteric glial cells, and interstitial cells of Cajal. These cells cluster in the myenteric plexuses, found in the muscle layer of the gut, and in the submucosal plexuses, found in the submucosal layer of the gut, along the entire gastrointestinal tract (181).
Multiple lines of evidence demonstrate GLP-1R expression in subsets of enteric neurons. A study using mouse reporter lines expressing Rosa26-tdRFP or Rosa26-eYFP under the control of Glp1r-Cre found a small number of fluorescent-positive enteric neurons (35); treatment with 10 nmol/L GLP-1 increased their action potential firing frequency ex vivo. About 60% and 19% of these fluorescent-positive neurons from the small and large intestine, respectively, were positive for neuronal nitric oxide synthase, implying that these neurons are likely inhibitory motor neurons (35). Complementary analyses employed Wnt1-Cre to knock down Glp1r in the mouse central and enteric nervous system (126). Glp1r mRNA transcripts were more abundant in the muscle layer (including the myenteric plexus) than in the submucosal layer (including the submucosal plexus). Wnt1-Cre enabled knockdown of more than 95% of Glp1r mRNA in the ileal muscle layer, but only 80% in the jejunal and as low as 60% in the duodenal muscle layer (126). Human myenteric plexuses were reported to contain GLP-1R immunoreactivity; however, these experiments utilized an antibody, Ab39072 subsequently shown to lack sensitivity and specificity (20, 182). A single cell atlas of the murine (and human) ENS affirmed the presence of Glp1r/GLP1R mRNA transcripts in various neuronal subsets within the ENS (Fig. 7) (183). Beyond enteric neurons, it remains unclear whether enteric glial cells and interstitial cells of Cajal express the Glp1r.
Figure 7.
Expression of Glp1r in 4974 murine ileal and colonic enteric neurons. Enteric neuron subtypes types were classified based on neuropeptide genes Adcyap1 (PACAP neurons and Gal (galanin neurons). Count matrices were accessed on September 19, 2020, and aggregated from the scRNA-seq datasets reported by Drokhlyansky et al (183) (Broad Institute Single Cell Portal SCP1038). Mouse gene nomenclature: Adcyap1, pituitary adenylate cyclase activating polypeptide or PACAP; Gal, galanin.
The role(s) of GLP-1R signaling in enteric neurons have not been fully elucidated. Inference from the scRNA-seq data suggest that the GLP-1R-expressing enteric neurons are secretomotor/vasodilator neurons. Analysis of mice expressing a novel Glp1r-Cre; lox-tdTomato reporter demonstrated tdTomato-positive neurons lining the stomach and the intestine—these neurons were identified as mechanosensitive and were not involved in nutrient detection (133). Consistent with these findings, mice lacking Glp1r in their enteric neurons exhibited normal food intake, gastric emptying, body weight, and glucose control (126), suggesting that GLP-1R expression in enteric neurons is physiologically dispensable for the classical metabolic actions of GLP-1 and some aspects of nutrient sensing. Notably, adenosine triphosphate coreleased with GLP-1 from L cells, might also trigger vagal activation through P2Y2/3, adding further complexity to L cell–ENS communication (184)
Potential GLP1R expression in intestinal smooth muscle
Whether GLP-1R agonism inhibits gut motility indirectly through neural mechanisms, or in part through direct interaction with a smooth muscle GLP-1R remains unclear (185-187). GLP-1 may also reduce smooth muscle cell inflammation in the context of lipopolysaccharide-induced Tnf and Il1a expression in colonic smooth muscle cells isolated from BALB/c mice (188). Notably, ascertainment of definitive nonvascular smooth muscle cell GLP-1R expression remains challenging to date, as contamination from rare cell types within ex vivo preparations, including enteric neurons, cannot be excluded.
Thus, the local gut GLP-1 system is comprised of 3 main components: L cells as the main GLP-1 producer, and enteric neurons and IELs as the primary responders to GLP-1. Although GLP-1 secretion by L cells contributes to a majority of active GLP-1 levels in the circulation, it is worth mentioning that the local gut environment is frequently exposed to very high levels of GLP-1 (likely higher than the circulatory levels), especially when L cells are stimulated after meal ingestion or under various stress conditions. One can hypothesize that the presence of GLP-1R-expressing enteric neurons and IELs may present a feedback mechanism to modulate GLP-1 levels, as recently suggested (175). Moreover, local GLP-1 bioactivity is further modified by vascular and potentially enterocyte DPP-4 activity (189, 190). As enteric neurons and IEL subpopulations are difficult to isolate and study, the actions of GLP-1 on these cell types remains incompletely understood.
GLP-1 action in the immune system
GLP-1R agonists reduce systemic inflammation in preclinical and clinical studies; however, the mechanisms linking GLP-1R signaling to modulation of immune cell activity remain uncertain (191). Low levels of Glp1r transcripts have been detected in isolated thymocytes, splenocytes, and lymph node cells from C57BL/6J mice by RT-PCR (192).
Glp1r expression was also detected by RT-PCR in murine T cells, both in the basal state and after stimulation with aCD3/CD28, as well as in TH1 and TH17 polarized cells by RT-PCR; however, levels were not compared relative to Glp1r expression in other cell types or tissues (193). Whole body Glp1r–/– mice display normal cellularity and immunophenotypes in the thymus, spleen, lymph nodes, and bone marrow, suggesting that expression of Glp1r is dispensable for the normal development of these immune organs (192). Recent studies have begun to explore the biology of GLP-1R expression in tissue-resident immune cells.
Invariant natural killer T cells as targets of GLP-1 action
Invariant natural killer T (iNKT) cells exhibit properties of T cells and NKT cells; iNKT cells recognize the antigen-presenting CD1d that binds a variety of foreign lipids (194). A case report examined the putative direct effects of GLP-1 on human iNKT cells in the context of psoriasis. Two individuals with T2D and psoriasis treated with liraglutide exhibited reduced number of circulating iNKT cells after 6 weeks of treatment (195). Flow cytometric analyses revealed that 55% of CD3+ T cells and 96% of TCR-Vα24+ iNKT cells showed positive intracellular staining using an unspecified PE-GLP-1R antibody, accompanied by increased levels of cAMP levels in these cells following incubation with GLP-1. Exogenous liraglutide treatment of HFD-fed mice for 5 days reduced the number of iNKT cells in the blood and adipose tissues (196). Moreover, HFD-fed Cd1d–/– mice, which lack iNKT cells in the body, lost less body weight when treated with liraglutide. Nevertheless, the expression of the GLP-1R in mouse iNKT cells was not definitively established and there is little rigorous evidence that B cells, NK, NKT, or innate lymphoid cells express the canonical GLP-1R.
GLP-1R expression in myeloid lineages
Immunoreactive GLP-1R protein has been detected in mouse peritoneal macrophages by Western blotting using incompletely characterized antisera (197, 198), and in macrophage-like cell lines. However, full-length Glp1r transcripts have not been consistently detected in macrophages from mouse tissues or peritoneum, in the basal state or after induction of inflammation (20). Flow cytometry was used to investigate GLP-1R expression in peripheral blood from individuals with allergic asthma (199). About 10% and 5% of blood neutrophils and eosinophils, respectively, from healthy control subjects stained positive with an Alexa Fluor 700-conjugated GLP-1R antibody. The proportion of GLP-1R-immuno-positive eosinophils was reduced to 2% in subjects with asthma. The specificity of the GLP-1R antibody used in this study, AF700-GLP-1R, remains uncertain. Overall, the expression of GLP-1R has not yet been robustly confirmed in myeloid cells.
GLP-1R transcripts in miscellaneous tissues
Adipose tissue
GLP1R transcripts are reported in adipocyte cell lines ex vivo and in human visceral and subcutaneous adipose tissues by Taqman-based qPCR (200). Nevertheless, the precise GLP-1R+ cell type(s) within rodent or human adipose tissue, ranging from vascular ECs or VSMCs, immune cells, or adipocytes themselves, has not been defined with certainty.
Iacobellis et al. detected GLP1R and GLP2R RNA by RNA-seq and qPCR in 8 human epicardial adipose tissue (EAT) samples from individuals with coronary artery disease with and without T2D (201). GLP2R mRNA transcripts were ~5-fold more abundant than GLP1R mRNA; GLP1R expression was described as “relatively low” however precise quantification or comparison with GLP1R expression in other tissues was not provided. GLP-1R-immunopositivity was detected in EAT and subcutaneous adipose tissue (SAT) from a diabetic and nondiabetic subject, analyzed by fluorescent IHC (Mab 3F52). Remarkably, in contrast to relatively comparable levels of GLP1R mRNA in EAT vs SAT, GLP-1R immunopositive cells were much more abundant in EAT, compared to SAT (201). Tyramide signal amplification (which also has the potential to amplify signal from nonspecific primary antibody binding) was used to detect GLP-1R immunopositivity, due to the very low levels of GLP-1R expression, The precise identity of the GLP-1R+ cells in EAT was not determined. A follow-up study from the same group reported GLP1R expression in EAT by quantitative microarray analysis from 17 subjects undergoing coronary artery bypass grafting. Remarkably GLP2R mRNA transcripts were reported to be ~57-fold more abundant in EAT, relative to levels quantified for GLP1R (202).
Thyroid
GLP-1R agonists produce thyroid C-cell hyperplasia in rats and mice (203, 204), consistent with expression of the GLP-1R in calcitonin-producing C cells within the rat and mouse thyroid (203). The development of C cell proliferation and calcitonin secretion in response to GLP-1R agonism in mice and rats is mediated by the canonical GLP-1R (204, 205) prompting considerable regulatory concern, and more detailed investigation of GLP-1R expression in the human thyroid. Out of 59 normal, hyperplastic and neoplastic human thyroid glands analyzed by IHC with the Mab 3F52 antibody, no GLP-1R-immunopositive follicular or C cells were detected in normal or hyperplastic thyroid tissue. Two of 10 cases with sporadic medullary thyroid carcinoma exhibited scattered GLP-1R-immunopositive C cells (51). Receptor autoradiography using 125I-labeled GLP-1 (7-36) amide detected the presence of GLP-1Rs in a human case of medullary thyroid cancer. Several studies have reported more widespread GLP-1R expression in the normal and neoplastic thyroid (206, 207). However, these analyses used GLP-1R antibodies lacking specificity (18, 20, 43). The available data suggest that GLP-1R+ cells are generally rare or absent in the normal human thyroid, yet may be expressed in a subset of C cells in the context of C cell hyperplasia or medullary thyroid cancer.
Eyes
Diabetic complications such as retinopathy, neuropathy, and nephropathy heighten the interest in the presence of GLP-1R in the eyes, peripheral nerves, and kidney. Although GLP-1R was reported in human retinal pigment epithelia by both qPCR and by IHC, these analyses used the subsequently discontinued ab39072 antisera (208). Later studies using ISH and IHC with the GLP-1R antibody Mab 3F52 did not detect GLP-1R in the majority of cells within the human retina; however, <1% of the cells in the ganglion cell layer were found to be GLP-1R+ (209). Using the same antibody, and protocols, GLP-1R-immunopositive cells were detected in GLP-1R+ tissues including the pancreas, Brunner’s glands, and kidney.
Liver
There is considerable interest in the investigational assessment of GLP-1RA for the treatment of NASH, based on preclinical and human studies demonstrating these agents reduce hepatic fat, inflammation and progression of fibrosis (210, 211). Nevertheless, the mechanisms linking GLP-1R signaling to control of NASH remain elusive, as levels of hepatic Glp1r/GLP1R expression are relatively low and sometimes undetectable, relative to expression in pancreas, kidney, gut, lung, and heart (10, 212). Indeed, Boland and colleagues did not detect GLP1R mRNA transcripts in human liver, Kupffer or stellate cell fractions, prepared from 3 human liver biopsy samples analyzed by bulk RNA-seq (213). Although a substantial number of studies report hepatocyte GLP-1R expression in mouse and human liver by immunocytochemistry or Western blotting, the great majority of these analyses use antisera that have not been validated for sensitive and specific detection of the GLP-1R (191). Moreover, isolated murine hepatocytes do not exhibit canonical cAMP responses to GLP-1R agonists, fail to bind fluorescent GLP-1 in vitro, and do not express the Glp1r (19-21, 214).
GLP1R mRNA transcripts have been detected by RT-PCR in RNA isolated from human liver, and levels were reported to be reduced in livers from individuals with NASH (215). However, assignment of GLP-1R to hepatocytes was carried out using an antibody with unknown sensitivity and specificity. It seems likely that low level GLP-1R expression in liver reflects the presence of the GLP-1R in nonhepatocyte cells, such as immune cells or blood vessels. Indeed a fraction of ECs from mouse liver contained Glp1r mRNA transcripts detected by scRNA-seq (Fig. 5) (155). Hence, it seems reasonable to conclude that the actions of GLP-1R agonists on hepatocytes are likely indirect, through incompletely identified pathways.
Concluding Remarks for GLP-1 Action Outside the Pancreas and Nervous System
The available data highlight multiple actions of GLP-1 and GLP-1R agonists, deduced from both preclinical and human studies, on key target cell types and tissues that do not exhibit robust GLP-1R expression. For example, GLP-1R agonists reduce the rates of myocardial infarction, decrease hepatic lipid accumulation, and attenuate renal albumin excretion, yet the GLP-1R is not expressed within most cardiomyocytes, hepatocytes, or renal glomerular and tubular epithelium. Similarly, GLP-1R agonism leads to reduced atherosclerosis, attenuated chylomicron secretion and decreased inflammation, yet the majority of VSMCs, ECs, enterocytes, and immune cells do not express the canonical GLP-1R. Collectively, these findings highlight the importance of indirect mediators of GLP-1 action as key contributors to mechanisms ensuing from activation of the canonical GLP-1R.
Caveats and limitations
Although considerable progress has been made in detecting GLP-1R expression, including characterization of 2 monoclonal antibodies suitable for detection of the primate and mouse/rat GLP-1R (3F52 and 7F38, respectively), it is still possible to incorrectly assign GLP-1R expression using these 2 antibodies. Importantly, the presence or absence of GLP-1R-immunopositivity remains highly technique and tissue dependent. Ideally, GLP-1R antisera should be assessed for specificity simultaneously under the conditions deployed for histochemistry or Western blotting, using tissues from Glp1r–/– mice, or with human cells or organoids engineered to delete the GLP-1R. Although a predominantly membrane pattern of cellular staining is ideally observed when using chemical probes or validated antisera to detect the GLP-1R (18, 44, 49), several reports of diffuse cytoplasmic staining raise questions about whether the antisera were used under conditions optimized for specificity. For example, the 7F38 antisera has detected diffuse cytoplasmic and some membrane GLP-1R-immunopositivity throughout the entire islet (158), a pattern of staining which can be more difficult to interpret, relative to the more restrictive cellular localization of islet and membrane-localized GLP-1R expression. Indeed, in the context of establishing optimal technical conditions for the sensitive and specific detection of the human GLP-1R in human heart using IHC and the 3F52 antibody (18), we detected extensive cytoplasmic staining in tissues or cell types not known to express the GLP-1R in some of our pilot experiments. Hence, wherever possible, multiple complementary techniques should be used to interrogate the expression of the GLP-1R in specific cell types and tissues (18, 44, 49, 116, 118) including the use of negative controls and GLP-1R knockout tissues where applicable.
Many of the studies described herein utilize mice and rats as models for GLP-1 action and GLP-1R activation. The experimental conclusions may reflect the age, sex, species, housing conditions, diet, and health of the animals, as well as the specific experimental and technical approaches utilized (216). For example, the use of chemogenetic and optogenetic studies to interrogate PPG neurons informs the potential of broadly activating these neurons, but may not necessarily reflect the selective actions of GLP-1, acting through the GLP-1R, in the nervous system. Moreover, careful controls are required when using both these techniques, since optogenetic channels alter neuronal properties (217), and DREADD ligands such as clozapine-N-oxide can be converted to functional metabolites in the brain (218). Similarly, activation or deletion of the Gcg gene perturbs the expression of not only GLP-1, but multiple structurally related proglucagon-derived peptides (PGDPS), including oxyntomodulin and glucagon which may also signal through the GLP-1R. Hence, attribution of the results of these studies to the GLP-1/GLP-1R system should be done with caution. Furthermore, most studies localizing GLP-1 or the GLP-1R utilize postmortem tissue and do not reveal information on GLP-1/GLP-1R expression in the live animal, or in live tissue.
This review did not examine the potential actions of GLP-1 degradation products, generated by enzymatic cleavage of GLP-1, which may produce multiple biological actions independent of the known GLP-1R (219). This biology may be relevant to circumstances characterized by pharmacological infusion of GLP-1, bariatric surgery, or GLP-1-producing tumors, and has been discussed elsewhere (6, 191). Moreover, although human islet GLP-1 action and GLP-1R expression has been extensively studied in the pancreas, much less is known about GLP-1R localization in other human organs and their cell types (Fig. 8). As noted herein, the very low expression of the GLP-1R may preclude its reliable detection by RNA-seq, and more particularly in scRNA-seq, even in islet cells known to express the canonical GLP-1R and often used as a positive control (36). Hence, the old adage “absence of evidence is not evidence for absence” remains relevant for interpretation of these single cell or bulk data sets in tissues known to express low levels of the full length GLP1R mRNA. Along these lines, sensitive in situ transcript visualization techniques such as RNAScope and single molecular fluorescence ISH might prove transformative for understating GLP-1R mRNA localization and abundance in complex tissues.
Figure 8.
Schematic depiction of major targets of GLP-1 action, and the cell types within key organs that express the GLP-1 Receptor (GLP1R). The GLP-1 peptide is produced in and shown adjacent to the intestine, pancreas, and brain, the 3 major sites of GCG expression. EC, endothelial cells; IEL, intestinal intraepithelial lymphocytes; SA node, sinoatrial node; VSMC, vascular smooth muscle cell.
Recent technological advances coupled with reagent development have fostered a more accurate and complete understanding of how and where endogenous and pharmacologically administered GLP-1 and GLP-1RA acts to control metabolism. Rapid ongoing developments in this field are likely to further refine existing insights, answering important mechanistic questions, while raising new testable hypotheses designed to illuminate the expanding direct vs indirect cellular targets of GLP-1 action.
Acknowledgments
Financial Support: D.J.D. is supported by CIHR grant 154321, investigator-initiated studies of peptide hormone action sponsored by Novo Nordisk, a Banting and Best Diabetes Centre-Novo Nordisk Chair in Incretin Biology and the Novo Nordisk Foundation-Mt. Sinai Hospital fund for the study of gut peptides. B.A.M. is supported by a fellowship from the CIHR and C.K.W. is supported by a Banting and Best Diabetes Centre fellowship. D.J.H. was supported by Medical Research Council (MRC) (MR/N00275X/1 and MR/S025618/1) and Diabetes UK (17/0005681) Project Grants. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Starting Grant 715884 to D.J.H.). J.E.C. is supported by funding from the American Diabetes Association (1-18-JDF-017) and the National Institutes of Health (DK123075, DK125353). S.T. was supported by the Medical Research Council (MR/N02589X/1), British Heart Foundation (FS/14/43/30960), and NIH (R01 DK095757).
Glossary
Abbreviations
- AP
area postrema
- ARC
arcuate nucleus
- CCK
cholecystokinin
- cDNA
complementary deoxyribonucleic acid
- cAMP
cyclic adenosine
- 3′
5′-monophosphate
- CNS
central nervous system
- DPP-4
dipeptidyl peptidase-4
- DREADD
designer receptors activated by designer drugs
- EAT
epicardial adipose tissue
- EC
endothelial cell
- ENS
enteric nervous system
- EPAC
exchange protein directly activated by cAMP
- ER
endoplasmic reticulum
- Ex4
exendin-4
- Ex9
exendin(9-39)
- FACS
fluorescence-activated cell sorting
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- GCG
glucagon gene or protein
- GFP
green fluorescent protein
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- GLP-1RA
glucagon-like peptide-1 receptor agonist
- HFD
high-fat diet
- ICV
intracerebroventricular
- IEL
intestinal intraepithelial lymphocyte
- IHC
immunohistochemistry
- iNKT cells
invariant natural killer T cells
- ISH
in situ hybridization
- LA
left atrium
- LV
left ventricle
- Mab
monoclonal antibody
- NASH
nonalcoholic steatohepatitis
- NG
nodose ganglion
- NG2
neural/glial antigen 2
- NTS
nucleus tractus solitarius
- PK
protein kinase
- PLC
phospholipase C
- PPG
preproglucagon
- PVN
paraventricular nucleus
- qPCR
quantitative polymerase chain reaction
- RA
right atrium
- RV
right ventricle
- RNA-seq
ribonucleic acid sequencing
- scRNA-seq
single cell RNA sequencing
- RT-PCR
reverse transcription-polymerase chain reaction
- SA
sinoatrial
- SAT
subcutaneous adipose tissue
- TCR
T cell receptor
- T2D
type 2 diabetes
- VSMC
vascular smooth muscle cell
- YFP
yellow fluorescent protein
- WT
wild type
Additional Information
Disclosures: D.J.D. has been a consultant or speaker for Forkhead Therapeutics, Intarcia, Kallyope, Merck, and Novo Nordisk Inc. in the last 12 months. Mt. Sinai Hospital has received grants from Merck, Novo Nordisk, and Takeda Inc. for investigator-initiated preclinical studies of glucagon-like peptide action in the Drucker laboratory. D.J.D. does not hold stock or equity in any of these companies.
References
- 1. Drucker DJ. Evolving concepts and translational relevance of enteroendocrine cell biology. J Clin Endocrinol Metab. 2016;101(3):778-786. [DOI] [PubMed] [Google Scholar]
- 2. Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304(5924):368-371. [DOI] [PubMed] [Google Scholar]
- 3. Heinrich G, Gros P, Lund PK, Bentley RC, Habener JF. Pre-proglucagon messenger ribonucleic acid: nucleotide and encoded amino acid sequences of the rat pancreatic complementary deoxyribonucleic acid. Endocrinology. 1984;115(6):2176-2181. [DOI] [PubMed] [Google Scholar]
- 4. Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127(12):4217-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95(2):513-548. [DOI] [PubMed] [Google Scholar]
- 6. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thorens B. Expression cloning of the pancreatic β cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA. 1992;89(18):8641-8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137(7):2968-2978. [DOI] [PubMed] [Google Scholar]
- 9. Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358(3):219-224. [DOI] [PubMed] [Google Scholar]
- 10. Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134(5):2156-2164. [DOI] [PubMed] [Google Scholar]
- 11. Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes. 2013;62(10):3316-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140(11):5356-5363. [DOI] [PubMed] [Google Scholar]
- 13. Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35(6):992-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song Y, Koehler JA, Baggio LL, Powers AC, Sandoval DA, Drucker DJ. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metab. 2019;30(5):976-986.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like Peptide-1 receptor agonists and dipeptidyl Peptidase-4 inhibitors. Circulation. 2017;136(9):849-870. [DOI] [PubMed] [Google Scholar]
- 16. Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776-785. [DOI] [PubMed] [Google Scholar]
- 17. Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58(4):975-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baggio LL, Yusta B, Mulvihill EE, et al. GLP-1 receptor expression within the human heart. Endocrinology. 2018;159(4):1570-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007;56(12):3006-3013. [DOI] [PubMed] [Google Scholar]
- 20. Panjwani N, Mulvihill EE, Longuet C, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology. 2013;154(1):127-139. [DOI] [PubMed] [Google Scholar]
- 21. Landgraf D, Tsang AH, Leliavski A, et al. Oxyntomodulin regulates resetting of the liver circadian clock by food. Elife. 2015;4:e06253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nie Y, Nakashima M, Brubaker PL, et al. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J Clin Invest. 2000;105(7):955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Z, Stanojevic V, Avadhani S, Yano T, Habener JF. Stromal cell-derived factor-1 (SDF-1)/chemokine (C-X-C motif) receptor 4 (CXCR4) axis activation induces intra-islet glucagon-like peptide-1 (GLP-1) production and enhances beta cell survival. Diabetologia. 2011;54(8):2067-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Traub S, Meier DT, Schulze F, et al. Pancreatic α cell-derived glucagon-related peptides are required for β cell adaptation and glucose homeostasis. Cell Rep. 2017;18(13):3192-3203. [DOI] [PubMed] [Google Scholar]
- 26. Marchetti P, Lupi R, Bugliani M, et al. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55(12):3262-3272. [DOI] [PubMed] [Google Scholar]
- 27. Svendsen B, Larsen O, Gabe MBN, et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep. 2018;25(5):1127-1134.e2. [DOI] [PubMed] [Google Scholar]
- 28. Chambers AP, Sorrell JE, Haller A, et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab. 2017;25(4):927-934.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moens K, Flamez D, Van Schravendijk C, Ling Z, Pipeleers D, Schuit F. Dual glucagon recognition by pancreatic beta-cells via glucagon and glucagon-like peptide 1 receptors. Diabetes. 1998;47(1):66-72. [DOI] [PubMed] [Google Scholar]
- 30. Capozzi ME, Wait JB, Koech J, et al. Glucagon lowers glycemia when beta-cells are active. JCI Insight 2019;5(16):e129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Capozzi ME, Svendsen B, Encisco SE, et al. beta Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dillon JS, Tanizawa Y, Wheeler MB, et al. Cloning and functional expression of the human glucagon-like peptide-1 (GLP-1) receptor. Endocrinology. 1993;133(4):1907-1910. [DOI] [PubMed] [Google Scholar]
- 33. Adriaenssens AE, Svendsen B, Lam BY, et al. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59(10):2156-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DiGruccio MR, Mawla AM, Donaldson CJ, et al. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab. 2016;5(7):449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richards P, Parker HE, Adriaenssens AE, et al. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gray SM, Xin Y, Ross EC, et al. Discordance between GLP-1R gene and protein expression in mouse pancreatic islet cells. J Biol Chem. 2020;295(33):11529-11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Segerstolpe Å, Palasantza A, Eliasson P, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24(4):593-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Camunas-Soler J, Dai XQ, Hang Y, et al. Patch-Seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab. 2020;31(5):1017-1031.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Benninger RKP, Hodson DJ. New understanding of β-cell heterogeneity and in situ islet function. Diabetes. 2018;67(4):537-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heller RS, Kieffer TJ, Habener JF. Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreas. Diabetes. 1997;46(5):785-791. [DOI] [PubMed] [Google Scholar]
- 41. Tornehave D, Kristensen P, Rømer J, Knudsen LB, Heller RS. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J Histochem Cytochem. 2008;56(9):841-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Marinis YZ, Salehi A, Ward CE, et al. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 2010;11(6):543-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor–or not? Endocrinology. 2013;154(1):4-8. [DOI] [PubMed] [Google Scholar]
- 44. Pyke C, Heller RS, Kirk RK, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280-1290. [DOI] [PubMed] [Google Scholar]
- 45. Zhang Y, Parajuli KR, Fava GE, et al. GLP-1 receptor in pancreatic α-cells regulates glucagon secretion in a glucose-dependent bidirectional manner. Diabetes. 2019;68(1):34-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clardy SM, Mohan JF, Vinegoni C, et al. Rapid, high efficiency isolation of pancreatic ß-cells. Sci Rep. 2015;5:13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clardy SM, Keliher EJ, Mohan JF, et al. Fluorescent exendin-4 derivatives for pancreatic β-cell analysis. Bioconjug Chem. 2014;25(1):171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lehtonen J, Schäffer L, Rasch MG, Hecksher-Sørensen J, Ahnfelt-Rønne J. Beta cell specific probing with fluorescent exendin-4 is progressively reduced in type 2 diabetic mouse models. Islets. 2015;7(6):e1137415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ast J, Arvaniti A, Fine NHF, et al. Super-resolution microscopy compatible fluorescent probes reveal endogenous glucagon-like peptide-1 receptor distribution and dynamics. Nat Commun. 2020;11(1):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ramracheya R, Chapman C, Chibalina M, et al. GLP-1 suppresses glucagon secretion in human pancreatic alpha-cells by inhibition of P/Q-type Ca2+ channels. Physiol Rep. 2018;6(17):e13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-like-peptide-1 receptor expression in normal and diseased human thyroid and pancreas. Mod Pathol. 2015;28(3):391-402. [DOI] [PubMed] [Google Scholar]
- 52. Saponaro C, Gmyr V, Thévenet J, et al. The GLP1R agonist liraglutide reduces hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin via somatostatin release. Cell Rep. 2019;28(6):1447-1454.e4. [DOI] [PubMed] [Google Scholar]
- 53. Biggs EK, Liang L, Naylor J, et al. Development and characterisation of a novel glucagon like peptide-1 receptor antibody. Diabetologia. 2018;61(3):711-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tomas A, Jones B, Leech C. New insights into beta-cell GLP-1 receptor and cAMP signaling. J Mol Biol. 2020;432(5):1347-1366. [DOI] [PubMed] [Google Scholar]
- 55. MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51 (Suppl 3):S434-S442. [DOI] [PubMed] [Google Scholar]
- 56. Kashima Y, Miki T, Shibasaki T, et al. Critical role of cAMP-GEFII–Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276(49):46046-46053. [DOI] [PubMed] [Google Scholar]
- 57. Ammälä C, Ashcroft FM, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993;363(6427):356-358. [DOI] [PubMed] [Google Scholar]
- 58. Seino S, Takahashi H, Fujimoto W, Shibasaki T. Roles of cAMP signalling in insulin granule exocytosis. Diabetes Obes Metab. 2009;11 (Suppl 4):180-188. [DOI] [PubMed] [Google Scholar]
- 59. Barella LF, Rossi M, Zhu L, et al. β-Cell-intrinsic β-arrestin 1 signaling enhances sulfonylurea-induced insulin secretion. J Clin Invest. 2019;129(9):3732-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ravier MA, Leduc M, Richard J, et al. β-Arrestin2 plays a key role in the modulation of the pancreatic beta cell mass in mice. Diabetologia. 2014;57(3):532-541. [DOI] [PubMed] [Google Scholar]
- 61. Zhu L, Almaça J, Dadi PK, et al. β-arrestin-2 is an essential regulator of pancreatic β-cell function under physiological and pathophysiological conditions. Nat Commun. 2017;8:14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Willard FS, Douros JD, Gabe MB, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5(17):e140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shigeto M, Ramracheya R, Tarasov AI, et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J Clin Invest. 2015;125(12):4714-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hodson DJ, Mitchell RK, Bellomo EA, et al. Lipotoxicity disrupts incretin-regulated human β cell connectivity. J Clin Invest. 2013;123(10):4182-4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oduori OS, Murao N, Shimomura K, et al. Gs/Gq signaling switch in β cells defines incretin effectiveness in diabetes. J Clin Invest. 2020;130(12):6639-6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Farnsworth NL, Walter R, Piscopio RA, Schleicher WE, Benninger RKP. Exendin-4 overcomes cytokine-induced decreases in gap junction coupling via protein kinase A and Epac2 in mouse and human islets. J Physiol. 2019;597(2):431-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guida C, Miranda C, Asterholm IW, et al. GLP-1(9–36) mediates the glucagonostatic effect of GLP-1 by promiscuous activation of the glucagon receptor. bioRxiv. 2020;785667. doi: 10.1101/785667 [DOI] [Google Scholar]
- 68. Ørgaard A, Holst JJ. The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia. 2017;60(9):1731-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kailey B, van de Bunt M, Cheley S, et al. SSTR2 is the functionally dominant somatostatin receptor in human pancreatic beta- and alpha-cells. Am J Physiol Endocrinol Metabol. 2012;303(9):E1107-E1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51(12):2263-2270. [DOI] [PubMed] [Google Scholar]
- 71. Koehler JA, Baggio LL, Cao X, et al. Glucagon-like peptide-1 receptor agonists increase pancreatic mass by induction of protein synthesis. Diabetes. 2015;64(3):1046-1056. [DOI] [PubMed] [Google Scholar]
- 72. Hou Y, Ernst SA, Heidenreich K, Williams JA. Glucagon-like peptide-1 receptor is present in pancreatic acinar cells and regulates amylase secretion through cAMP. Am J Physiol Gastrointest Liver Physiol. 2016;310(1):G26-G33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wewer Albrechtsen NJ, Albrechtsen R, Bremholm L, et al. Glucagon-like peptide 1 receptor signaling in acinar cells causes growth-dependent release of pancreatic enzymes. Cell Rep. 2016;17(11):2845-2856. [DOI] [PubMed] [Google Scholar]
- 74. Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem. 1988;263(27):13475-13478. [PubMed] [Google Scholar]
- 75. Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261-280. [DOI] [PubMed] [Google Scholar]
- 76. Conlon JM, Samson WK, Dobbs RE, Orci L, Unger RH. Glucagon-like polypeptides in canine brain. Diabetes. 1979;28(7):700-702. [DOI] [PubMed] [Google Scholar]
- 77. Tager H, Hohenboken M, Markese J, Dinerstein RJ. Identification and localization of glucagon-like peptides in rat brain. Proc Natl Acad Sci USA. 1980;77(10):6229-6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988;271(4):519-532. [DOI] [PubMed] [Google Scholar]
- 79. Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77(1):257-270. [DOI] [PubMed] [Google Scholar]
- 80. Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, Heilig JS. Glucagon-like peptide-1 in the rat brain: distribution of expression and functional implication. J Comp Neurol. 2013;521(10):2235-2261. [DOI] [PubMed] [Google Scholar]
- 81. Zheng H, Stornetta RL, Agassandian K, Rinaman L. Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Struct Funct. 2015;220(5):3011-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31(10):3904-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kreisler AD, Davis EA, Rinaman L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol Behav. 2014;136:47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kreisler AD, Rinaman L. Hindbrain glucagon-like peptide-1 neurons track intake volume and contribute to injection stress-induced hypophagia in meal-entrained rats. Am J Physiol Regul Integr Comp Physiol. 2016;310(10):R906-R916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R470-R478. [DOI] [PubMed] [Google Scholar]
- 86. Gaykema RP, Daniels TE, Shapiro NJ, Thacker GC, Park SM, Goehler LE. Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Res. 2009;1294:61-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zheng H, Cai L, Rinaman L. Distribution of glucagon-like peptide 1-immunopositive neurons in human caudal medulla. Brain Struct Funct. 2015;220(2):1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vrang N, Grove K. The brainstem preproglucagon system in a non-human primate (Macaca mulatta). Brain Res. 2011;1397:28-37. [DOI] [PubMed] [Google Scholar]
- 89. Honda K. Glucagon-related peptides and the regulation of food intake in chickens. Anim Sci J. 2016;87(9):1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rønnestad I, Gomes AS, Murashita K, Angotzi R, Jönsson E, Volkoff H. Appetite-controlling endocrine systems in teleosts. Front Endocrinol (Lausanne). 2017;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8(6):532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes. 2010;59(8):1890-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Llewellyn-Smith IJ, Gnanamanickam GJ, Reimann F, Gribble FM, Trapp S. Preproglucagon (PPG) neurons innervate neurochemically identified autonomic neurons in the mouse brainstem. Neuroscience. 2013;229:130-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Thiebaud N, Llewellyn-Smith IJ, Gribble F, Reimann F, Trapp S, Fadool DA. The incretin hormone glucagon-like peptide 1 increases mitral cell excitability by decreasing conductance of a voltage-dependent potassium channel. J Physiol. 2016;594(10):2607-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Llewellyn-Smith IJ, Marina N, Manton RN, Reimann F, Gribble FM, Trapp S. Spinally projecting preproglucagon axons preferentially innervate sympathetic preganglionic neurons. Neuroscience. 2015;284:872-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. During MJ, Cao L, Zuzga DS, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9(9):1173-1179. [DOI] [PubMed] [Google Scholar]
- 98. Parker HE, Adriaenssens A, Rogers G, et al. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55(9):2445-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shi X, Chacko S, Li F, et al. Acute activation of GLP-1-expressing neurons promotes glucose homeostasis and insulin sensitivity. Mol Metab. 2017;6(11):1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Holt MK, Richards JE, Cook DR, et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68(1):21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liu J, Conde K, Zhang P, et al. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron. 2017;96(4):897-909.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gaykema RP, Newmyer BA, Ottolini M, et al. Activation of murine pre-proglucagon-producing neurons reduces food intake and body weight. J Clin Invest. 2017;127(3):1031-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Williams DL, Lilly NA, Edwards IJ, Yao P, Richards JE, Trapp S. GLP-1 action in the mouse bed nucleus of the stria terminalis. Neuropharmacology. 2018;131:83-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277(2):R582-R590. [DOI] [PubMed] [Google Scholar]
- 105. Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest. 2012;122(1):388-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Serre V, Dolci W, Schaerer E, et al. Exendin-(9-39) is an inverse agonist of the murine glucagon-like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3’,5’-monophosphate levels and beta-cell glucose competence. Endocrinology. 1998;139(11):4448-4454. [DOI] [PubMed] [Google Scholar]
- 107. Meeran K, O’Shea D, Edwards CM, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7-36) amide or exendin-(9-39) alters body weight in the rat. Endocrinology. 1999;140(1):244-250. [DOI] [PubMed] [Google Scholar]
- 108. Patterson JT, Ottaway N, Gelfanov VM, et al. A novel human-based receptor antagonist of sustained action reveals body weight control by endogenous GLP-1. ACS Chem Biol. 2011;6(2):135-145. [DOI] [PubMed] [Google Scholar]
- 109. Holt MK, Cook DR, Brierley DI, et al. PPG neurons in the nucleus of the solitary tract modulate heart rate but do not mediate GLP-1 receptor agonist-induced tachycardia in mice. Mol Metab. 2020;39:101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69-72. [DOI] [PubMed] [Google Scholar]
- 111. Thiele TE, Seeley RJ, D’Alessio D, et al. Central infusion of glucagon-like peptide-1-(7-36) amide (GLP-1) receptor antagonist attenuates lithium chloride-induced c-Fos induction in rat brainstem. Brain Res. 1998;801(1-2):164-170. [DOI] [PubMed] [Google Scholar]
- 112. Grill HJ, Carmody JS, Amanda Sadacca L, Williams DL, Kaplan JM. Attenuation of lipopolysaccharide anorexia by antagonism of caudal brain stem but not forebrain GLP-1-R. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1190-R1193. [DOI] [PubMed] [Google Scholar]
- 113. Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol. 1999;277(5):R1537-R1540. [DOI] [PubMed] [Google Scholar]
- 114. Hisadome K, Reimann F, Gribble FM, Trapp S. CCK stimulation of GLP-1 neurons involves α1-adrenoceptor-mediated increase in glutamatergic synaptic inputs. Diabetes. 2011;60(11):2701-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Holt MK, Pomeranz LE, Beier KT, Reimann F, Gribble FM, Rinaman L. Synaptic inputs to the mouse dorsal vagal complex and its resident preproglucagon neurons. J Neurosci. 2019;39(49):9767-9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gabery S, Salinas CG, Paulsen SJ, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6):e133429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Salinas CBG, Lu TT, Gabery S, et al. Integrated brain atlas for unbiased mapping of nervous system effects following liraglutide treatment. Sci Rep. 2018;8(1):10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jensen CB, Pyke C, Rasch MG, Dahl AB, Knudsen LB, Secher A. Characterization of the glucagonlike peptide-1 receptor in male mouse brain using a novel antibody and in situ hybridization. Endocrinology. 2018;159(2):665-675. [DOI] [PubMed] [Google Scholar]
- 120. Reiner DJ, Leon RM, McGrath LE, et al. Glucagon-like peptide-1 receptor signaling in the lateral dorsal tegmental nucleus regulates energy balance. Neuropsychopharmacology. 2018;43(3):627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Scrocchi LA, Brown TJ, MaClusky N, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med. 1996;2(11):1254-1258. [DOI] [PubMed] [Google Scholar]
- 122. Sisley S, Gutierrez-Aguilar R, Scott M, D’Alessio DA, Sandoval DA, Seeley RJ. Neuronal GLP1R mediates liraglutide’s anorectic but not glucose-lowering effect. J Clin Invest. 2014;124(6):2456-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Smith EP, An Z, Wagner C, et al. The role of β cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metab. 2014;19(6):1050-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Burmeister MA, Ayala JE, Smouse H, et al. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes. 2017;66(2):372-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Adams JM, Pei H, Sandoval DA, et al. Liraglutide modulates appetite and body weight through glucagon-like peptide 1 receptor-expressing glutamatergic neurons. Diabetes. 2018;67(8):1538-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Varin EM, Mulvihill EE, Baggio LL, et al. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Rep. 2019;27(11):3371-3384.e3. [DOI] [PubMed] [Google Scholar]
- 127. Cheng W, Ndoka E, Hutch C, et al. Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice. JCI Insight. 2020;5(7):e134359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996;137(11):5159-5162. [DOI] [PubMed] [Google Scholar]
- 129. Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab. 2015;4(10):718-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fortin SM, Lipsky RK, Lhamo R, et al. GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Sci Transl Med. 2020;12(533):eaay8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Vahl TP, Tauchi M, Durler TS, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148(10):4965-4973. [DOI] [PubMed] [Google Scholar]
- 132. Bai L, Mesgarzadeh S, Ramesh KS, et al. Genetic identification of vagal sensory neurons that control feeding. Cell. 2019;179(5):1129-1143.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166(1):209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes. 2016;65(1):34-43. [DOI] [PubMed] [Google Scholar]
- 135. Brierley DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems independently and additively suppress eating. bioRxiv. 2020. doi: 10.1101/2020.08.03.234427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696-1705. [DOI] [PubMed] [Google Scholar]
- 137. Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology. 2015;40(2):327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Thiebaud N, Gribble F, Reimann F, Trapp S, Fadool DA. A unique olfactory bulb microcircuit driven by neurons expressing the precursor to glucagon-like peptide 1. Sci Rep. 2019;9(1):15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kinzig KP, D’Alessio DA, Herman JP, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23(15):6163-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Williams DL. Neural integration of satiation and food reward: role of GLP-1 and orexin pathways. Physiol Behav. 2014;136:194-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Harasta AE, Power JM, von Jonquieres G, et al. Septal glucagon-like peptide 1 receptor expression determines suppression of cocaine-induced behavior. Neuropsychopharmacology. 2015;40(8):1969-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hsu TM, Noble EE, Liu CM, et al. A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Mol Psychiatry. 2018;23(7):1555-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23(7):2939-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110(1):43-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Lee SJ, Sanchez-Watts G, Krieger JP, et al. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Mol Metab. 2018;11:33-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lockie SH, Heppner KM, Chaudhary N, et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes. 2012;61(11):2753-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ussher JR, Baggio LL, Campbell JE, et al. Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol Metab. 2014;3(5):507-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Baggio LL, Ussher JR, McLean BA, et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol Metab. 2017;6(11):1339-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bellastella G, Maiorino MI, Longo M, et al. Glucagon-like peptide-1 receptor agonists and prevention of stroke systematic review of cardiovascular outcome trials with meta-analysis. Stroke. 2020;51(2):666-669. [DOI] [PubMed] [Google Scholar]
- 150. Wallner M, Kolesnik E, Ablasser K, et al. Exenatide exerts a PKA-dependent positive inotropic effect in human atrial myocardium: GLP-1R mediated effects in human myocardium. J Mol Cell Cardiol. 2015;89(Pt B):365-375. [DOI] [PubMed] [Google Scholar]
- 151. Clarke SJ, Giblett JP, Yang LL, et al. GLP-1 is a coronary artery vasodilator in humans. J Am Heart Assoc. 2018;7(22):e010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Giblett JP, Axell RG, White PA, et al. Glucagon-like peptide-1-mediated cardioprotection does not reduce right ventricular stunning and cumulative ischemic dysfunction after coronary balloon occlusion. JACC Basic Transl Sci. 2019;4(2):222-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kim M, Platt MJ, Shibasaki T, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19(5):567-575. [DOI] [PubMed] [Google Scholar]
- 154. Kimura T, Obata A, Shimoda M, et al. Down-regulation of vascular GLP-1 receptor expression in human subjects with obesity. Sci Rep. 2018;8(1):10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Feng W, Chen L, Nguyen PK, Wu SM, Li G. Single cell analysis of endothelial cells identified organ-specific molecular signatures and heart-specific cell populations and molecular features. Front Cardiovasc Med. 2019;6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Helmstädter J, Frenis K, Filippou K, et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arterioscler Thromb Vasc Biol. 2020;40(1):145-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Fujita H, Morii T, Fujishima H, et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85(3):579-589. [DOI] [PubMed] [Google Scholar]
- 158. Jensen EP, Poulsen SS, Kissow H, et al. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am J Physiol Renal Physiol. 2015;308(8):F867-F877. [DOI] [PubMed] [Google Scholar]
- 159. Ronn J, Jensen EP, Wewer Albrechtsen NJ, Holst JJ, Sorensen CM. Glucagon-like peptide-1 acutely affects renal blood flow and urinary flow rate in spontaneously hypertensive rats despite significantly reduced renal expression of GLP-1 receptors. Physiol Rep. 2017;5(23):e13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav. 2012;106(3):387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Brighton CA, Rievaj J, Kuhre RE, et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology. 2015;156(11):3961-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Christensen LW, Kuhre RE, Janus C, Svendsen B, Holst JJ. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol Rep. 2015;3(9):e12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lebrun LJ, Lenaerts K, Kiers D, et al. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep. 2017;21(5):1160-1168. [DOI] [PubMed] [Google Scholar]
- 164. Panaro BL, Yusta B, Matthews D, et al. Intestine-selective reduction of Gcg expression reveals the importance of the distal gut for GLP-1 secretion. Mol Metab. 2020;37:100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Haber AL, Biton M, Rogel N, et al. A single-cell survey of the small intestinal epithelium. Nature. 2017;551(7680):333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Glass LL, Calero-Nieto FJ, Jawaid W, et al. Single-cell RNA-sequencing reveals a distinct population of proglucagon-expressing cells specific to the mouse upper small intestine. Mol Metab. 2017;6(10):1296-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gehart H, van Es JH, Hamer K, et al. Identification of enteroendocrine regulators by real-time single-cell differentiation mapping. Cell. 2019;176(5):1158-1173.e16. [DOI] [PubMed] [Google Scholar]
- 168. Billing LJ, Larraufie P, Lewis J, et al. Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice - identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol Metab. 2019;29:158-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wang Y, Song W, Wang J, et al. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J Exp Med. 2020;217(2):e20191130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Roberts GP, Larraufie P, Richards P, et al. Comparison of human and murine enteroendocrine cells by transcriptomic and peptidomic profiling. Diabetes. 2019;68(5):1062-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Goldspink DA, Lu VB, Miedzybrodzka EL, et al. Labeling and characterization of human GLP-1-secreting L-cells in primary ileal organoid culture. Cell Rep. 2020;31(13):107833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Beumer J, Puschhof J, Bauzá-Martinez J, et al. High-resolution mRNA and secretome atlas of human enteroendocrine cells. Cell. 2020;181(6):1291-1306.e19. [DOI] [PubMed] [Google Scholar]
- 173. Osinski C, Le Gleau L, Poitou C, et al. Type 2 diabetes is associated with impaired jejunal enteroendocrine GLP-1 cell lineage in human obesity. Int J Obes. 2021;45(1):170-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Yusta B, Baggio LL, Koehler J, et al. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64(7):2537-2549. [DOI] [PubMed] [Google Scholar]
- 175. He S, Kahles F, Rattik S, et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature. 2019;566(7742):115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Broide E, Bloch O, Ben-Yehudah G, Cantrell D, Shirin H, Rapoport MJ. GLP-1 receptor is expressed in human stomach mucosa: analysis of its cellular association and distribution within gastric glands. J Histochem Cytochem. 2013;61(9):649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Schmidtler J, Dehne K, Allescher HD, et al. Rat parietal cell receptors for GLP-1-(7-36) amide: northern blot, cross-linking, and radioligand binding. Am J Physiol. 1994;267(3 Pt 1):G423-G432. [DOI] [PubMed] [Google Scholar]
- 178. O’Halloran DJ, Nikou GC, Kreymann B, Ghatei MA, Bloom SR. Glucagon-like peptide-1 (7-36)-NH2: a physiological inhibitor of gastric acid secretion in man. J Endocrinol. 1990;126(1):169-173. [DOI] [PubMed] [Google Scholar]
- 179. Schjoldager BT, Mortensen PE, Christiansen J, Orskov C, Holst JJ. GLP-1 (glucagon-like peptide 1) and truncated GLP-1, fragments of human proglucagon, inhibit gastric acid secretion in humans. Dig Dis Sci. 1989;34(5):703-708. [DOI] [PubMed] [Google Scholar]
- 180. Bang-Berthelsen CH, Holm TL, Pyke C, et al. GLP-1 induces barrier protective expression in Brunner’s glands and regulates colonic inflammation. Inflamm Bowel Dis. 2016;22(9):2078-2097. [DOI] [PubMed] [Google Scholar]
- 181. Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9(5):286-294. [DOI] [PubMed] [Google Scholar]
- 182. Anand U, Yiangou Y, Akbar A, et al. Glucagon-like peptide 1 receptor (GLP-1R) expression by nerve fibres in inflammatory bowel disease and functional effects in cultured neurons. PLoS One. 2018;13(5):e0198024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Drokhlyansky E, Smillie CS, Van Wittenberghe N, et al. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182(6):1606-1622.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lu VB, Rievaj J, O’Flaherty EA, et al. Adenosine triphosphate is co-secreted with glucagon-like peptide-1 to modulate intestinal enterocytes and afferent neurons. Nat Commun. 2019;10(1):1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. May AT, Crowe MS, Blakeney BA, et al. Identification of expression and function of the glucagon-like peptide-1 receptor in colonic smooth muscle. Peptides. 2019;112:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Amato A, Baldassano S, Liotta R, Serio R, Mulè F. Exogenous glucagon-like peptide 1 reduces contractions in human colon circular muscle. J Endocrinol. 2014;221(1):29-37. [DOI] [PubMed] [Google Scholar]
- 187. Halim MA, Degerblad M, Sundbom M, et al. Glucagon-like peptide-1 inhibits prandial gastrointestinal motility through myenteric neuronal mechanisms in humans. J Clin Endocrinol Metab. 2018;103(2):575-585. [DOI] [PubMed] [Google Scholar]
- 188. Al-Dwairi A, Alqudah TE, Al-Shboul O, Alqudah M, Mustafa AG, Alfaqih MA. Glucagon-like peptide-1 exerts anti-inflammatory effects on mouse colon smooth muscle cells through the cyclic adenosine monophosphate/nuclear factor-κB pathway in vitro. J Inflamm Res. 2018;11:95-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Mulvihill EE, Varin EM, Gladanac B, et al. Cellular sites and mechanisms linking reduction of dipeptidyl peptidase-4 activity to control of incretin hormone action and glucose homeostasis. Cell Metab. 2017;25(1):152-165. [DOI] [PubMed] [Google Scholar]
- 190. Varin EM, Mulvihill EE, Beaudry JL, et al. Circulating levels of soluble dipeptidyl peptidase-4 are dissociated from inflammation and induced by enzymatic DPP4 inhibition. Cell Metab. 2019;29(2):320-334.e5. [DOI] [PubMed] [Google Scholar]
- 191. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740-756. [DOI] [PubMed] [Google Scholar]
- 192. Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia. 2010;53(4):730-740. [DOI] [PubMed] [Google Scholar]
- 193. Moschovaki Filippidou F, Kirsch AH, Thelen M, et al. Glucagon-like peptide-1 receptor agonism improves nephrotoxic serum nephritis by inhibiting T-cell proliferation. Am J Pathol. 2020;190(2):400-411. [DOI] [PubMed] [Google Scholar]
- 194. Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4(3):231-237. [DOI] [PubMed] [Google Scholar]
- 195. Hogan AE, Tobin AM, Ahern T, et al. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54(11):2745-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lynch L, Hogan AE, Duquette D, et al. iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab. 2016;24(3):510-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Arakawa M, Mita T, Azuma K, et al. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59(4):1030-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. 2012;425(2):304-308. [DOI] [PubMed] [Google Scholar]
- 199. Mitchell PD, Salter BM, Oliveria JP, et al. Glucagon-like peptide-1 receptor expression on human eosinophils and its regulation of eosinophil activation. Clin Exp Allergy. 2017;47(3):331-338. [DOI] [PubMed] [Google Scholar]
- 200. Ejarque M, Guerrero-Pérez F, de la Morena N, et al. Role of adipose tissue GLP-1R expression in metabolic improvement after bariatric surgery in patients with type 2 diabetes. Sci Rep. 2019;9(1):6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Iacobellis G, Camarena V, Sant DW, Wang G. Human epicardial fat expresses glucagon-like peptide 1 and 2 receptors genes. Horm Metab Res. 2017;49(8):625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Dozio E, Vianello E, Malavazos AE, et al. Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: a target to modulate cardiovascular risk? Int J Cardiol. 2019;292:218-224. [DOI] [PubMed] [Google Scholar]
- 203. Bjerre Knudsen L, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151(4):1473-1486. [DOI] [PubMed] [Google Scholar]
- 204. Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153(3):1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Yamada C, Yamada Y, Tsukiyama K, et al. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149(2):574-579. [DOI] [PubMed] [Google Scholar]
- 206. Gier B, Butler PC, Lai CK, Kirakossian D, DeNicola MM, Yeh MW. Glucagon like peptide-1 receptor expression in the human thyroid gland. J Clin Endocrinol Metab. 2012;97(1):121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Song Y, Zhou M, Cao Y, Qi J, Geng J, Liu X. Expression of GLP-1 receptor and CD26 in human thyroid C-cells: the association of thyroid C-cell tumorigenesis with incretin-based medicine. Oncol Lett. 2017;13(4):2684-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hernández C, Bogdanov P, Corraliza L, et al. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2016;65(1):172-187. [DOI] [PubMed] [Google Scholar]
- 209. Hebsgaard JB, Pyke C, Yildirim E, Knudsen LB, Heegaard S, Kvist PH. Glucagon-like peptide-1 receptor expression in the human eye. Diabetes Obes Metab. 2018;20(9):2304-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Armstrong MJ, Gaunt P, Aithal GP, et al. ; LEAN trial team . Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679-690. [DOI] [PubMed] [Google Scholar]
- 211. Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2020. doi: 10.1056/NEJMoa2028395 [DOI] [PubMed] [Google Scholar]
- 212. Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48(5):736-743. [DOI] [PubMed] [Google Scholar]
- 213. Boland ML, Laker RC, Mather K, et al. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist cotadutide via modulating mitochondrial function and lipogenesis. Nat Metab. 2020;2(5):413-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Tomas E, Stanojevic V, Habener JF. GLP-1-derived nonapeptide GLP-1(28-36)amide targets to mitochondria and suppresses glucose production and oxidative stress in isolated mouse hepatocytes. Regul Pept. 2011;167(2-3):177-184. [DOI] [PubMed] [Google Scholar]
- 215. Svegliati-Baroni G, Saccomanno S, Rychlicki C, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31(9):1285-1297. [DOI] [PubMed] [Google Scholar]
- 216. Drucker DJ. Never waste a good crisis: confronting reproducibility in translational research. Cell Metab. 2016;24(3):348-360. [DOI] [PubMed] [Google Scholar]
- 217. Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neurosci. 2012;15(8):1102-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Gomez JL, Bonaventura J, Lesniak W, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357(6350):503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. Clinical review: the extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab. 2009;94(6):1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Enge M, Arda HE, Mignardi M, et al. Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell. 2017;171(2):321-330.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Kalucka J, de Rooij LPMH, Goveia J, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180(4):764-779.e20. [DOI] [PubMed] [Google Scholar]