Abstract
Achalasia is a rare esophageal motility disorder characterized by the incomplete relaxation of the lower esophageal sphincter (LES) and impaired peristaltic activity. The advent of high-resolution manometry (HRM) and the rapidly evolving role of therapeutic endoscopy have revolutionized the approach to the diagnosis and management of achalasia patients in the last decade. With advances in HRM technology and methodology, fluoroscopy and EndoFlip, achalasia can be differentiated into therapeutically meaningful phenotypes with a high degree of accuracy. Further, the newest treatment option, per-oral endoscopic myotomy (POEM), has become a staple therapy following the last 10 years of experience, and recent randomized trials appear to show no difference between POEM, graded pneumatic dilatation and surgical Heller myotomy in terms of short- and long-term efficacy or complication rate. On the other hand, how treatment outcomes are measured as well as the risk of reflux following therapy remain areas of contention. This review aims to summarize the recent advancements in achalasia testing and therapy, describes the recent randomized clinical trials as well as their potential setbacks, and touches on the future of personalizing achalasia treatment.
Keywords: esophageal achalasia, graded pneumatic dilatation, Laparoscopic Heller myotomy, Peroral endoscopic myotomy, esophago-gastric junction Outflow Obstruction, timed barium swallow, dysphagia, high resolution manometry
Introduction
Achalasia is a rare esophageal motor disorder that owes its name (from the Greek, a–, “not” + khálasis, “relaxation”) to its main pathophysiological feature, the incomplete relaxation of the lower esophageal sphincter (LES).1 In achalasia, a non-relaxing LES is, however, only part of the clinical picture. Diagnostic tools have evolved, especially with the advent of high-resolution manometry (HRM); esophageal pressure topography has led to the advent of the Chicago classification of motor disorders, which has revolutionized the approach to achalasia patients. This technology can differentiate achalasia into three different therapeutically-meaningful phenotypes, leading to unique treatment considerations for each phenotype, based on the observed pattern of esophageal function according to the latest Chicago classification: Type I has absent peristalsis, Type II is characterized by pan-esophageal pressurizations and Type III by premature and/or spastic esophageal contractions.2,3 Further, it can define a relatively novel entity of motility disorder, the esophago-gastric junction outflow obstruction (EGJOO), previously known as ‘variant achalasia’, whereby the EGJ does not relax, but motility remains intact.2–4 The lack of relaxation of the LES conjunctly with the impaired peristaltic reflex is therefore responsible for the most common clinical symptoms of achalasia: dysphagia, regurgitation of undigested food, chest pain and weight loss.5 Although not its original intention, in most clinical trials, the severity of the disorder and the efficacy of achalasia and EGJOO treatment is defined according to the Eckardt symptom score, which attributes points (ranging from 0 to 3) to each of the four aforementioned cardinal symptoms of the disease, with an overall score ranging from 0 to 12.6,7 (Figure 1).
Figure 1.
Eckardt symptom score. The score attributes points, ranging from 0 to 3, according to the reported frequency of the four cardinal achalasia symptoms. The overall score ranges from 0 to 12.6,7
Although rarely life-threatening, achalasia represents a life-long condition that seriously impacts on patients’ morbidity and quality of life.8 Left untreated, achalasia appears to have a natural history whereby the esophageal lumen dilates, which, over time, can progress and decompensate to a mega- or sigmoid esophagus, eventually sometimes even compromising nutrition and ability to feed orally. To date, no treatment is able to address the underlying etiology of achalasia, nor recover function. Rather, all efforts are aimed at disrupting the integrity of the LES, thus enabling LES relaxation, and in turn, permitting bolus clearance by means of the pharyngeal pump and gravity. Where treatment is successful, quality of life can commonly recover to normal/near normal for prolonged periods of time.5,8
Treatment options
Ever since the first attempt with a whale bone in 1674, dilatation of the LES has always been an appealing therapeutic option for any cause of esophageal obstruction, especially achalasia. After all, dilatation is an economic and time-efficient procedure with excellent short- and long-term response rates.9–11 Endoscopic dilatation has come a long way, with a variety of protocols having been introduced over the years, sometimes yielding to conflicting, and at times confusing, results with regard to efficacy, safety and reproducibility. It has become clear that efficacy of dilatation is dependent upon the diameter of the balloon, and so for adequate response, pneumatic dilatation (PD) is required with a non-compliant balloon, with a wide enough diameter to “disrupt” the EGJ muscle, commonly starting at 30-mm diameter. In the recent past, studies have shown that graded PD (dilating with incrementally wider balloons over two or three sittings with a pre-defined time interval) offers the best risk-to-benefit ratio and long-term outcomes.11,12 Laparoscopic Heller myotomy (LHM) is the traditional surgical alternative to PD. Until recently it was considered to be the gold standard and continues to provide adequate, long-term symptomatic relief. Further, LHM also allows for the operator to fashion an anti-reflux barrier in order to minimize subsequent reflux.13,14 Recently, and almost coincidentally with the widespread advances of HRM, per-oral endoscopic myotomy (POEM) has been accepted as a safe and effective alternative therapeutic strategy.15,16 Undertaken with a standard endoscope, under general anesthesia but commonly within the endoscopy unit, a submucosal tunnel is created from the mid-distal esophagus, and myotomy of the circular muscles is performed through to 2–3 cm beyond the LES, into the cardia. The myotomy can be performed using either an anterior or posterior approach with authors advocating the benefits for either: on one hand, the posterior approach avoids the left gastric artery within the anterior submucosa, thus limiting the risk of intraoperative bleeding; whilst on the other, the anterior approach preserves the oblique fibers, thus theoretically minimizing the risk of post-operative (Gastro-Esophageal Reflux Disease) GERD.17 Nonetheless, a prospective randomized controlled study showed that at a 2-year follow up, neither showed superiority in terms of efficacy or post-operative GERD.18 Regardless of the initial technique employed, if POEM needs to be repeated, the alternative approach should be considered. Advantages of POEM over LHM include the avoidance of the abdominal cavity structures as well as the opportunity to tailor the length of the myotomy along the esophageal axis in order to target the proximal end of a spastic contraction.19
Although there are a number of randomized controlled trials (RCTs) and comparative studies defining the utility and response to botulinum toxin injection,20,21 this procedure is short acting with some evidence that it might interfere with other more definitive therapies.22,23 To that end, recent guidelines recommend that botulinum toxin should be reserved primarily for treatment of those who are not able to tolerate or might be at risk of undergoing one of the other more definitive, yet invasive, treatment options of PD, endoscopic or surgical myotomy.5,24 Finally, esophagectomy can be considered as a final measure in patients with associated malignancy or “end-stage” achalasia. The latter is described in 2–5% of all achalasia at presentation and is characterized by a lumen diameter of more than 6 cm, not uncommonly with distortion of the esophageal lumen (sigmoid or megaesophagus).25 It is found in patients with longstanding, untreated or undertreated achalasia. Although Heller myotomy can still be successful in some cases,26 esophagectomy might be the last resort to relieve symptoms and prevent the nutritional complications of decompensated megaesophagus. As esophagectomy is burdened by a high surgical risk (up to 10% pneumonia and 7% post-operative leakage) and mortality risk (up to 2%),27 it is commonly considered when all other treatment modalities have been proven ineffective.5
Comparative therapeutic efficacy of achalasia treatments
All forms of achalasia therapy aim to disrupt the EGJ. Although to date studies have not found any of the three primary definitive treatment options (PD, LHM or POEM) to be superior, there are a number of caveats that need to be taken into account.
With regard to PD, it is established that the wider the balloon diameter the better the outcome; however, this is offset by a higher the risk of esophageal perforation. Vela et al.28 originally found that the success of a single dilatation (defined as freedom from requiring additional PDs) was 62% at 6 months and 28% at 6 years, whilst the rate of success of graded PD and LHM was similar overtime: 90% versus 89% at 6 months and 44% versus 57% at 6 years. The European achalasia study, a RCT, underscored this concept and found that graded dilatation to 30 mm and then 35 mm (and if required, 40 mm) improves and equates outcomes (based on Eckardt score) when compared with LHM; at 5 years, the success rates were 82% for PD and 84% for LHM (p = 0.92).28,29 If the first dilatation was performed at 35 mm, the perforation rate was as high as 31%;24 however, when starting at 30 mm before progressing to wider balloons diameters, perforation rate dropped to 2.1% per procedure and up to 5% overall.30 As this equivalence in treatment outcomes and complication rate is predicated upon stepwise increase in dilatation, graded dilatation has become the standard where treatment with PD is required and is the basis of all modern guidelines.28,29
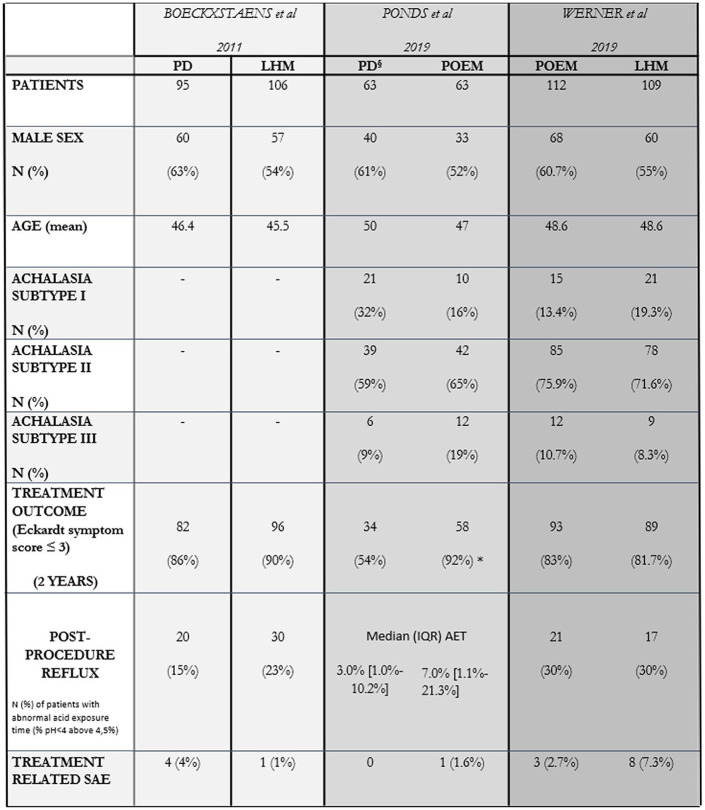
As the most recent treatment option for achalasia, comparative studies have shown POEM to be on par with both graded PD and LHM in terms of outcomes and complication rates.15,16–32 POEM also is durable over time, but there is a clear learning curve associated with success and reduction in complication rate.33,34 A pooled analysis of three cohort studies comparing POEM and LHM showed similar outcomes.35,36 In particular, the total adverse events, perforation rate and operative time were similar. Recently, two RCTs assessed outcomes following therapy in treatment naïve patients with achalasia; one comparing single or double pneumatic dilatation with POEM37 and another comparing Heller myotomy with POEM.38 Ponds et al. showed subjective response of POEM (based on the Eckardt score) to be superior to PD at 3 months (98% versus 80%; p < 0.01) and 2 years (92% versus 54%; p < 0.01) and therefore concluded that POEM was more effective.37 This study highlights the efficacy and safety of POEM with the setback of an increased risk of reflux (see below). On the other hand, it should be noted that outcomes from PD were reduced compared with other comparative and previous randomized studies, likely because the protocol was less aggressive; a second, larger dilatation was not routinely undertaken without persistence of symptoms at 3 weeks or an integrated relaxation pressure of more than 10 mmHg on repeat HRM. The protocol differed even from that which current guidelines recommend, that is, to dilate patients 30 mm and 35 mm routinely and to 40 mm if symptoms persist within 2–4 week intervals.28–30 Werner et al. conversely showed that at 2 years there was no difference in the intention to treat subjective outcome following either POEM or LHM (83% versus 81.7%)38 (Figure 2).
Figure 2.
Two-year results from three pivotal trials comparing LHM versus PD (Boeckxstaens et al.),29 POEM versus PD (Ponds et al.)37 and LHM versus POEM (Werner et al.),38 respectively.
All three different trials used the same primary end-point, that is, the number of patients with an Eckardt symptom score ⩽3 after 24 months from treatment to define treatment success rates. Ponds et al. found that the treatment success rate for POEM was significantly higher than PD at 2 years (*p < 0.001). The response rate to PD was 54% compared with 86% of the Boeckxstaens study, likely reflecting the differences in the used protocol (§). The achalasia subtypes according to the Chicago classification were not available at the time of the Boeckxstaens study, but were subsequently included in the long-term (5 years) results from the European Achalasia Trial (not shown in figure). Post-procedure reflux was evaluated by means of number of patients with abnormal acid exposure time (% time pH <4 greater than 4.5%) at 12 months in the Boeckxstaens and at 24 months in the Werner study. On the contrary, in the paper by Ponds et al. 7% of patients in the PD group were found to have esophagitis (all grade A) compared with 41% of patients in the POEM group (35% grade A–B and 6% grade C), while pH-impedance monitoring results at 1-year follow-up [expressed as median (IQR) percentage of time with esophageal pH < 4] were not significantly different among the two groups.
AET, acid exposure time; IQR, interquartile range; LHM, laparoscopic Heller myotomy; PD, pneumatic dilatation; POEM, per-oral endoscopic myotomy; SAE, serious adverse event.
The burden of reflux disease following achalasia treatments
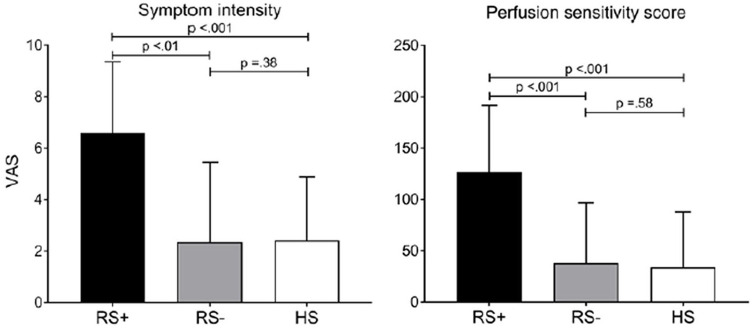
To reduce the likelihood and severity of reflux, a partial Dor (anterior) or Toupet (posteri-or) fundoplication almost invariably follows LHM.16,39 In so doing, the overall reflux risk, defined as abnormal esophageal acid exposure on ambulatory pH-impedance testing, was observed in 15% and 23% of the patients following PD and LHM respectively (p = 0.28).29 Although it is proposed that there is an increased predilection to reflux disease following POEM, on closer scrutiny of the data, in the vast majority of cases mucosal inflammation was associated with grade A esophagitis,31,40–42 which according to the recent Lyon consensus of reflux disease has an overlap with healthy, asymptomatic individuals.43 Furthermore, most patients with reflux symptoms following POEM commonly respond very well with acid reducing therapy.44 Ponds et al. found that following endoscopy at 1 year, reflux esophagitis was more common in the POEM than in the PD group (41% versus 7% respectively; p < 0.001) after cessation of acid reducing medication for 1 week; however, the majority were grade A esophagitis while only 6% had more severe grade C or D esophagitis. On the other hand, following ambulatory pH-impedance monitoring, the median acid exposure time (AET) was not different between the POEM (7%) and PD (3%) groups (p = 0.95). Furthermore, based on reflux symptoms questionnaires, there was no difference in the GERDQ score between the two at 1 year (p = 0.36) nor in the likelihood of proton pump inhibitor use (p = 0.98).37 In a comparative trial between POEM and LHM, Werner et al. showed that at 2 year follow-up, reflux esophagitis was evident in 44% following POEM and 29% following LHM (odds ratio, 2.00; 95% confidence interval, 1.03–3.85); however, the majority were grade A esophagitis with grade C/D esophagitis described in only 5% following POEM and 6% following LHM. Similarly, abnormal AET measurements were found in 30% following POEM as well as the LHM group at 24 months; however, post hoc analysis found that a higher proportion of patients were receiving low dose acid reducing medications following POEM than LHM (52.8% versus 27.2% at 2 years respectively).38 Recently, a study by the same group as the aforementioned RCT comparing POEM with PD has outlined how, following achalasia therapy, in many cases reflux symptoms do not accurately reflect the presence of esophagitis or pathological esophageal acid exposure.45 Out of 40 patients with treated achalasia, ambulatory pH-impedance monitoring found no difference in the degree of AET between patients with or without symptoms of reflux post therapy. On the other hand, patients with increased reflux-like symptoms (RS+) were much more likely to be sensitive to administered acid perfusion than those without symptoms (RS–); symptom intensity RS+:7(4.8–9) versus RS–:0.5(0–4.5) visual analogue scale, p < .001. It was therefore proposed that following achalasia therapy, reflux-like symptoms are rarely the consequence of acid reflux; rather, symptoms could be related to heightened esophageal sensitivity to acid or acid fermentation (Figure 3).45 In conclusion, the authors suggest that current evidence demonstrates a propensity to overemphasize the risk of post-operative GERD following achalasia therapy. Esophagitis of various degrees is commonly encountered in patients with treated achalasia;46 however, pH-impedance monitoring can reveal that pathological esophageal acid exposure can be due to mechanisms other than prototypical reflux episodes. These include acid fermentation and stasis of ingested acidic food due to poor clearance.45 In other words, reflux symptoms following any of the achalasia therapies does not always equate to reflux disease; rather, objective testing is required to define the mechanism. As such, in future clinical trials, clarity with regard to what is defined as conclusive “reflux disease” should be pre-determined in conjunction with the Lyon consensus.43 Although longevity studies beyond 2 years regarding post-POEM reflux sequelae are lacking, it appears that even where reflux disease is confirmed, in the majority acid reducing therapy tends to adequately control symptoms.44 Nevertheless, active monitoring should still be advised and possible long-term consequences of any reflux disorder, such as Barrett’s esophagus, should be identified.
Figure 3.
Results from the paper by Ponds et al. showing the maximum symptom intensity score expressed by visual analogue scale (VAS) in treated achalasia patients with (RS+) and without reflux symptoms (RS–). Symptom intensity in RS– patients was comparable to that of healthy subjects (HS)45 (Figure 3 of Ponds et al.45 – requested permission).
Treatment choice and predictors of outcome
Both comparative and randomized studies thus far imply that there is no preference in treatment modality between graded PD, LHM and POEM; the decision for therapy should be based on local/operator expertise and patient choice. On the other hand, the achalasia subtype, defined according to the Chicago classification can provide insight into the prognosis following therapy.47,48 Subsequently, post-hoc analysis of the European achalasia trial confirmed that achalasia subtype can impact on treatment effectiveness; PD efficacy was as high as 100% in Type II achalasia, while its success rates in Type III achalasia dropped dramatically to 40% compared with 86% in those who received LHM. Nonetheless, this difference was not statistically significant owing to the small numbers of Type III achalasia patients: 10 PD and eight LHM (p = 0.12).49 On the other hand, POEM appears to be superior to LHM for treating Type III achalasia; in a comparative study of 75 patients with Type III achalasia, Kumbhari et al. showed a treatment success rate as high as 98% following POEM and 80% following LHM at a mean follow-up of 8.6 months and 21.5 months respectively (p < 0.01), along with lower complications rates and a reduced operative time for POEM.50 This advantage of POEM over LHM could reflect the proximal extension of the myotomy that can be achieved through the endoscopic approach; however, a randomized controlled trial confirming this benefit in Type III achalasia therapy with POEM is lacking. A recent meta-analysis of 75 studies that investigated up to 34 patient-specific possible predictors of outcomes of achalasia therapy found that only age and manometric subtype were identified as the most relevant predictors of clinical response; older patients appeared to respond better to PD compared with younger ones (<45 years) as it was considered to be “less invasive” than POEM or LHM whilst the latter were more effective in the younger patient cohorts or those with Type III achalasia.49 On the other hand, a dilated and sigmoid esophagus is associated with the least favorable treatment response.5
Caveats in achalasia diagnosis: pseudo-achalasia, opiates-induced achalasia and EGJOO
A clinical picture that is similar to achalasia can be present in patients with local or distant cancer (pseudoachalasia) as a result of the direct infiltration of the myenteric plexus or by its immune-mediated disruption induced by tumor-derived circulating autoantibodies.51 A clinical suspicion of pseudoachalasia should arise in patients who are more than 55 years of age with sudden onset of dysphagia and rapid weight loss. To that end, current guidelines recommend that an upper gastrointestinal endoscopy with biopsies is required in all those with a new suspicion of achalasia in order to exclude esophageal squamous cell carcinoma (ESCC) and/or esophageal adenocarcinoma (EA) that could mimic achalasia presentation.5 In this regard, it must be noted that achalasia itself, even when successfully treated, is a well-established risk factor for both ESCC and EA, with an estimated 50-fold increased risk of ESCC than in the general population, after at least 10 years from the initial treatment of the disease.52 Although there is no formal recommendation on routine endoscopic surveillance in current guidelines,5 3-yearly-endoscopy is considered good practice in long-standing achalasia.
Opioids can lead to esophageal motility abnormalities associated with increased contractile vigor, premature contraction and impaired EGJ relaxation, thus mimicking diffuse esophageal spasm and Type III achalasia.53,54 Such patients tend to endure a poorer response to conventional achalasia therapies and these motor abnormalities might be reduced or reversed after opioid withdrawal, although cessation is often challenging due to intractable pain or dependence.53,55
EGJOO can be mistaken for achalasia unless normal esophageal body motility is confirmed.2,3 In EGJOO, obstruction can be classified as primary (mechanical; e.g. stricture, malignancy, post-surgical) or secondary (functional; e.g. opioid induced, idiopathic, artifact).56,57 In the former, treatment targets the source of the structural obstruction; dilating strictures, managing eosinophilic esophagitis, surgically correcting anatomical anomalies. On the other hand, functional EGJOO can be either spurious (e.g. artifact from catheter angulation or patient position) or due to a true idiopathic, non-relaxing sphincter, in which case achalasia-like therapies can be employed.58 However, it is crucial, yet often challenging, to differentiate between the two before a decision is made to provide invasive therapy. In a recent study of 97 patients with functional EGJOO identified with standard HRM testing, eight responded to opioid reduction, 48 did not require therapy, while 29 underwent treatment akin to that for achalasia (Botox, pneumatic dilation, POEM), of whom 26 responded clinically (Eckardt score <3). This study found that the best test to discriminate between clinically relevant functional EGJOO and spurious or spontaneously resolving EGJOO was to include adjunctive testing (free-drinking or solid swallows) during HRM, with an 85% sensitivity and 84% specificity to defining clinically responsive EGJOO. On the other hand, barium swallow or standard small volume water swallows during HRM had nearly a 50% chance of missing the diagnosis (54% sensitivity for both).59
New tools in the investigation and follow-up of achalasia patients
Barium esophagography has traditionally been used in the preoperative staging of achalasia patients, as it can provide valuable information regarding esophageal morphology that can impact on therapy such as the presence of a sigmoid or dilated esophagus commonly seen in end stage achalasia or an underlying lesion missed at endoscopy due to food stasis. More recently, barium studies have been used not only in establishing a diagnosis, but to aid in objectively assessing post-therapeutic outcome; the timed barium swallow (TBS) can objectively evaluate the severity of obstruction prior to and efficiency of esophageal emptying after treatment.60 This technique involves taking multip-le sequential films at fixed intervals after a single swallow of 150–200 ml of a low-density barium suspension. The barium column height and width at 1, 2 and 5 min have been used as a de-facto measure of the degree of LES obstruction, and the post-treatment barium emptying has been shown to be a good objective predictor of treatment response,61 whilst the lack of improvement has been associated with symptoms recurrence, even in asymptomatic patients.62
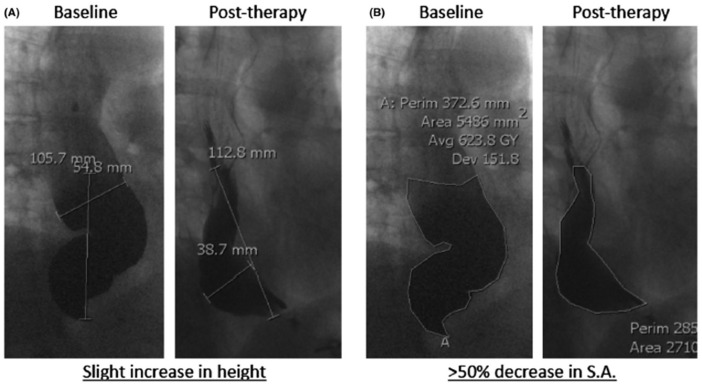
Although there is often good correlation between emptying on TBS and symptom response, it has been observed that measurement of the height of the barium column at 5 min might not accurately reflect improvement in esophageal emptying nor correlate with symptomatic response with a discordance of up to 31–50%.63,64 A recent study by our group found that, regardless of treatment modality, a change in barium surface area compared with prior to therapy better correlates with treatment response than the conventional post-therapy barium column height at 5 min; out of 24 patients who had achalasia therapy, the percent change in surface area between pre- and post-therapy was best at discriminating between responders and non-responders (sensitivity 100%, specificity 80%) compared with the stand alone standard 5 min post-barium column (sensitivity 75%, specificity 45%)65 (Figure 4).
Figure 4.
Results from the paper by Sanagapalli et al.65 showing that the height of the residual barium column after pneumatic dilatation can worsen due to change in the morphology of the esophagus following achalasia therapy (A), whilst the measurement of the surface area more accurately reflects the reduction of the barium retention following therapy (B) (Figure 2 of Ponds et al.66 – requested permission).
Avg, average; Dev, deviation; Perim, perimeter; S.A., surface area.
TBS has multiple attractive advantages, being simple to perform, reproducible, economic, is not invasive and does not requiring special radiological expertise. Furthermore, TBS is normally preferred by patients as an objective measure following therapy compared with the nasal catheter of HRM.5,67
Another addition to the diagnostic tools in stratifying achalasia patients has been the commercialization of the EndoFLIP device (Crospon Medical Devices, Galway, Ireland). By computing the luminal cross-sectional area and evaluating its relationship to the change in pressure within an inflatable bag surrounding a catheter with impedance sensors, the EndoFLIP device allows the quantification of EGJ distensibility and can be used to identify achalasia subtypes with a high degree of sensitivity and specificity, especially with the recent integration of manometry sensors.66,68 Further, EndoFLIP can be used intraoperatively to adjust the adequacy of the LES disruption during surgical myotomy or POEM.69 Also it can be used to define functionally relevant EGJOO that could benefit from achalasia therapy.70
As described under EGJOO above, the introduction of adjunctive testing has improved the diagnostic accuracy of identifying clinically relevant motility disorders. In achalasia, the majority are easily defined according to standard, small volume water swallows. However, occasionally, the non-relaxing sphincter requires more than just a small volume of water to identify a measurable obstruction, particularly when the esophagus is dilated or the LES is not tight. On the other hand, filling the esophagus with either fluid or food are simple, reproducible adjunctive tests that can facilitate the detection of the obstruction and can add important information to motility assessment.71,72 The rapid drink challenge (RDC) entails drinking up to 200 ml of water freely, commonly through a straw. Results are highly reproducible and normal values have been established that can define functional obstruction and achalasia.71 Furthermore, the presence of an obstructive pressure pattern during the RDC was significantly related to persistence of clinical symptoms (Eckardt score >3) in treated achalasia patients, thus suggesting RDC as a new tool in objectively assessing achalasia treatment outcomes.73 In a recent study, inclusion of RDC in patients with dysphagia and suspected achalasia, but who exhibited absent motility and a normal integrated relaxation pressure on single water swallows, identified the diagnosis in 79%, all of whom responded on subsequent treatment to the same degree as those with standard achalasia.74 The same applies to solid swallows, where the obstruction and pan-esophageal pressurization can be exhibited by filling of the esophagus with food. Crucially, this technique can also enhance the likelihood of inducing clinically relevant symptoms.75
Limitations of current trials and future directions: toward individualized therapy
Exciting recent developments have markedly improved the diagnosis and therapy of achalasia. Nonetheless, many questions remain unanswered and current trials are often heterogeneous in terms of patient selection (achalasia subtypes, age, preoperative esophageal anatomy, exposure to previous therapy) and treatment protocols. Furthermore, most published trials define treatment outcomes based on the subjective symptoms of patients, defined through the Eckardt score, which until now has not been validated for this purpose. Also, in most trials, an Eckardt score of >3 or a reduction of symptoms of <50% is considered to be equivalent to treatment failure; however, there is no clear explanation of how to interpret symptom persistence or recurrence at a later stage, which may reflect different underlying pathophysiological mechanisms (ineffective treatment versus other mechanisms). And, since every item that makes up the Eckardt score “weighs” the same in the final symptomatic score, this method does not allow to distinguish between specific symptoms. For example, residual dysphagia or chest pain might be associated with aperistalsis or be due to reflux from the stomach rather than from incomplete EGJ disruption, and weight does not change as dynamically from one clinic visit to the next, as might the other subjective symptoms. Therefore, it is clear that the evaluation of treatment efficacy based solely on subjective symptoms which can be perceived differently from one patient to the other can be misleading.76 Although objective measurements should be included, the most appropriate test has yet to be agreed upon nor have any entered into routine clinical practice in the evaluation of therapy and clinical outcome.
Importantly, although there are several options, not all treatments or expertise are widely available so the careful weighing of the risk to benefit ratio for the individual patients is not always possible and is based on availability rather than need. Still, many of these decisions are not based on evidence but on patient/operator “preference”.
Conclusion
In summary, statements regarding efficacy of achalasia therapy must take into account important caveats in the evidence. (i) To achieve the reported equivalence in success rates between the three treatment options, PD should be undertaken in a graded manner as routine, to 30 mm then 35 mm diameters several weeks apart. Furthermore, PD to 40 mm within the first sequence and the option of repeat dilatation in subsequent years should then be permitted based upon symptoms. If the measure of symptom response is made following only 30 mm and/or if future dilatation requirement is considered to be a failure of therapy, PD will always be inferior to the other forms of therapy, as was seen in the recent RCT comparing PD with POEM.37 (ii) Acid reducing therapy should be permitted following any achalasia therapy to treat reflux-like symptoms, should they arise; however, symptoms of reflux might not be due to true gastro-esophageal reflux, and should not be considered a failure of therapy.45 Furthermore, reflux symptoms should be differentiated from others that might be due to persistent obstruction, but present in a very similar manner. This can be achieved only by measuring objectively with TBS, EndoFlip or repeat HRM. There should also be a low threshold for repeating endoscopy whilst on therapy in order to confirm adequate mucosal healing where esophagitis has been observed.76 (iii) In Type III achalasia, there appears to be a preference for POEM, closely followed by LHM, with the least (but not absent) response being to PD;77 however, opiate use should always be ascertained as opiates can mimic Type III achalasia and dose reduction or cessation might be a safer option.53 As there is no clear evidence for one treatment option over another, it is advisable when defining the therapeutic strategy to take into account individual factors such as age and manometric subtype, patient preference, local expertise and local experience, so that every decision is made without bias and with full disclosure to the patient regarding the risks, benefits and merits of each procedure, with enough time provided for the patient to reach an informed decision5 (see Figure 5).
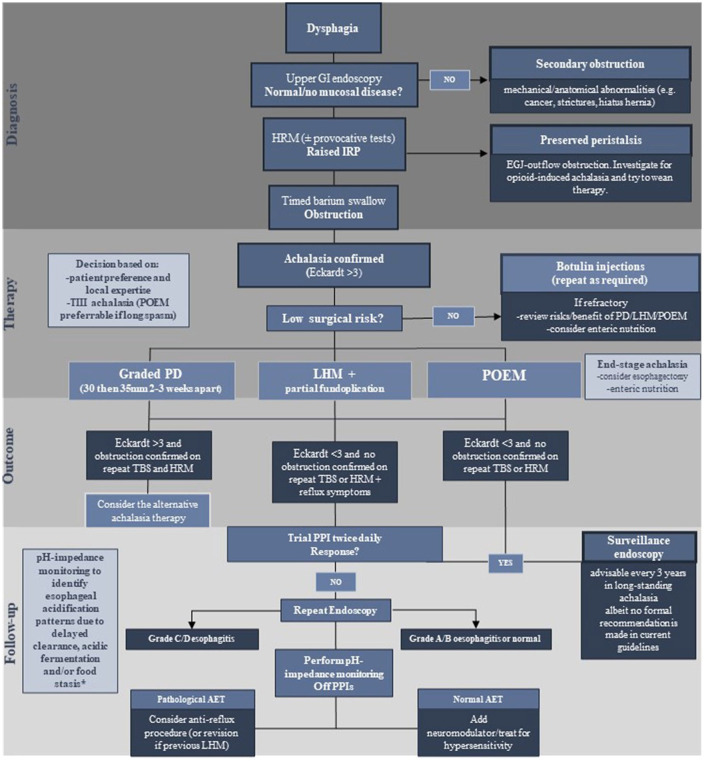
Figure 5.
Algorithm depicting current management of achalasia patients from a diagnostic, therapeutic and follow-up perspective.
*Esophageal acidification pattern at pH-impedance monitoring described in Ponds et al.45
AET, acid exposure time; EGJ, esophago-gastric junction; GI, gastrointestinal; HRM, high resolution manometry; IRP, Integrated Relaxation Pressure; LHM, laparoscopic Heller myotomy; PD, pneumatic dilatation; POEM, per-oral endoscopic myotomy; PPI, proton pump inhibitor; TBS, timed barium swallow; TIII, type III achalasia.
Achalasia is a life-long disorder that can severely impact on patients’ health and quality of life. Outstanding improvements in diagnostic and therapeutic approaches have been made in the last decade, and both comparative and randomized trials have reported excellent outcomes following all three of the most common treatment modalities: graded dilatation, and surgical and endoscopic myotomy. There remain questions regarding subsequent reflux risk and its long-term impact but it is becoming apparent that this risk might be similar across the board and that there might even be an element of hypersensitivity to symptoms of reflux following achalasia therapy. Trials are still lacking, however, with regard to treatment decision making according to achalasia morphology, but the rapid progress made in technology and interpretation appears to lead the way towards individualization of achalasia therapy.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Marcella Pesce  https://orcid.org/0000-0001-5996-4259
https://orcid.org/0000-0001-5996-4259
Contributor Information
Marcella Pesce, Department of Clinical Medicine and Surgery, University “Federico II” of Naples, Naples, Italy.
Rami Sweis, University College London Hospital, GI Services, 235 Euston Rd, London, NW1 2BU, UK.
References
- 1. Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet 2014; 383: 83–93. [DOI] [PubMed] [Google Scholar]
- 2. Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015; 27: 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: the Chicago classification. J Clin Gastroenterol 2008; 42: 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bredenoord AJ, Fox M, Kahrilas PJ, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil 2012; 24: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaninotto G, Bennett C, Boeckxstaens G, et al. The 2018 ISDE achalasia guidelines. Dis Esophagus 2018; 31: doy071. [DOI] [PubMed] [Google Scholar]
- 6. Eckardt VF, Gockel I, Bernhard G. Pneumatic dilation for achalasia: late results of a prospective follow up investigation. Gut 2004; 53: 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel DA, Sharda R, Hovis KL, et al. Patient-reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus 2017; 30: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eckhardt VF, Hoischen T, Bernhard G. Life expectancy, complications, and causes of death in patients with achalasia: results of a 33-year follow-up investigation. Eur J Gastroenterol Hepatol 2008; 20: 956–960. [DOI] [PubMed] [Google Scholar]
- 9. Zerbib F, Thetiot V, Richy F, et al. Repeated pneumatic dilations as long-term maintenance therapy for esophageal achalasia. Am J Gastroenterol 2006; 101: 692–697. [DOI] [PubMed] [Google Scholar]
- 10. Bravi I, Nicita MT, Duca P, et al. A pneumatic dilation strategy in achalasia: prospective outcome and effects on oesophageal motor function in the long term. Aliment Pharmacol Ther 2010; 31: 658–665. [DOI] [PubMed] [Google Scholar]
- 11. Hulselmans M, Vanuytsel T, Degreef T, et al. Long-term outcome of pneumatic dilation in the treatment of achalasia. Clin Gastroenterol Hepatol 2010; 8: 30–35. [DOI] [PubMed] [Google Scholar]
- 12. Katzka DA, Castell DO. Review article: an analysis of the efficacy, perforation rates and methods used in pneumatic dilation for achalasia. Aliment Pharmacol Ther 2011; 34: 832–839. [DOI] [PubMed] [Google Scholar]
- 13. Ortiz A, de Haro LF, Parrilla P, et al. Very long-term objective evaluation of heller myotomy plus posterior partial fundoplication in patients with achalasia of the cardia. Ann Surg 2008; 247: 258–264. [DOI] [PubMed] [Google Scholar]
- 14. Kurian AA, Bhayani N, Sharata A, et al. Partial anterior vs partial posterior fundoplication following transabdominal esophagocardiomyotomy for achalasia of the esophagus: meta-regression of objective postoperative gastroesophageal reflux and dysphagia. JAMA Surg 2013; 148: 85–90. [DOI] [PubMed] [Google Scholar]
- 15. Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010; 42: 265–271. [DOI] [PubMed] [Google Scholar]
- 16. Campos GM, Vittinghoff E, Rabl C, et al. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg 2009; 249: 45–57. [DOI] [PubMed] [Google Scholar]
- 17. Mohan BP, Ofosu A, Chandan S, et al. Anterior versus posterior approach in peroral endoscopic myotomy (POEM): a systematic review and meta-analysis. Endoscopy 2020; 52: 251–258. [DOI] [PubMed] [Google Scholar]
- 18. Ichkhanian Y, Abimansour JP, Pioche M, et al. Outcomes of anterior versus posterior peroral endoscopic myotomy 2 years post-procedure: prospective follow-up results from a randomized clinical trial. Endoscopy. Epub ahead of print 22 June 2020. DOI: 10.1055/a-1204-4242. [DOI] [PubMed] [Google Scholar]
- 19. Stavropoulos SN, Modayil RJ, Friedel D, et al. The international per oral endoscopic myotomy survey (IPOEMS): a snapshot of the global POEM experience. Surg Endosc 2013; 27: 3322–3338. [DOI] [PubMed] [Google Scholar]
- 20. Annese V, Bassotti G, Coccia G, et al. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia study group. Gut 2000; 46: 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaezi MF, Richter JE, Wilcox CM, et al. Botulinum toxin versus pneumatic dilatation in the treatment of achalasia: a randomised trial. Gut 1999; 44: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zaninotto G, Annese V, Costantini M, et al. Randomized controlled trial of botulinum toxin versus laparoscopic heller myotomy for esophageal achalasia. Ann Surg 2004; 239: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Z-Q, Li Q-L, Chen W-F, et al. The effect of prior treatment on clinical outcomes in patients with achalasia undergoing peroral endoscopic myotomy. Endoscopy 2019; 51: 307–316. [DOI] [PubMed] [Google Scholar]
- 24. Weusten BLAM, Barret M, Bredenoord AJ, et al. Endoscopic management of gastrointestinal motility disorders – part 1: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2020; 52: 498–515. [DOI] [PubMed] [Google Scholar]
- 25. Duranceau A, Liberman M, Martin J, et al. End-stage achalasia. Dis Esophagus 2012; 25: 319–330. [DOI] [PubMed] [Google Scholar]
- 26. Sweet MP, Nipomnick I, Gasper WJ, et al. The outcome of laparoscopic heller myotomy for achalasia is not influenced by the degree of esophageal dilatation. J Gastrointest Surg 2008; 12: 159–165. [DOI] [PubMed] [Google Scholar]
- 27. Aiolfi A, Asti E, Bonitta G, et al. Esophagectomy for end-stage achalasia: systematic review and meta-analysis. World J Surg 2018; 42: 1469–1476. [DOI] [PubMed] [Google Scholar]
- 28. Vela MF, Richter JE, Khandwala F, et al. The long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol 2006; 4: 580–587. [DOI] [PubMed] [Google Scholar]
- 29. Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic heller’s myotomy for idiopathic achalasia. N Engl J Med 2011; 364: 1807–1816. [DOI] [PubMed] [Google Scholar]
- 30. Moonen A, Annese V, Belmans A, et al. Long-term results of the European achalasia trial: a multicentre randomised controlled trial comparing pneumatic dilation versus laparoscopic heller myotomy. Gut 2016; 65: 732–739. [DOI] [PubMed] [Google Scholar]
- 31. Repici A, Fuccio L, Maselli R, et al. GERD after per-oral endoscopic myotomy as compared with heller’s myotomy with fundoplication: a systematic review with meta-analysis. Gastrointest Endosc 2018; 87: 934–943.e18. [DOI] [PubMed] [Google Scholar]
- 32. Schlottmann F, Luckett DJ, Fine J, et al. Laparoscopic heller myotomy versus Peroral Endoscopic Myotomy (POEM) for achalasia: a systematic review and meta-analysis. Ann Surg 2018; 267: 451–460. [DOI] [PubMed] [Google Scholar]
- 33. Hungness ES, Sternbach JM, Teitelbaum EN, et al. Per-oral Endoscopic Myotomy (POEM) after the learning curve: durable long-term results with a low complication rate. Ann Surg 2016; 264: 508–517. [DOI] [PubMed] [Google Scholar]
- 34. Zhang X-C, Li Q-L, Xu M-D, et al. Major perioperative adverse events of peroral endoscopic myotomy: a systematic 5-year analysis. Endoscopy 2016; 48: 967–978. [DOI] [PubMed] [Google Scholar]
- 35. Bhayani NH, Kurian AA, Dunst CM, et al. A comparative study on comprehensive, objective outcomes of laparoscopic heller myotomy with per-oral endoscopic myotomy (POEM) for achalasia. Ann Surg 2014; 259: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 36. Ujiki MB, Yetasook AK, Zapf M, et al. Peroral endoscopic myotomy: a short-term comparison with the standard laparoscopic approach. Surgery 2013; 154: 893–897; discussion 897–900. [DOI] [PubMed] [Google Scholar]
- 37. Ponds FA, Fockens P, Lei A, et al. Effect of peroral endoscopic myotomy vs pneumatic dilation on symptom severity and treatment outcomes among treatment-naive patients with achalasia: a randomized clinical trial. JAMA 2019; 322: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Werner YB, Hakanson B, Martinek J, et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med 2019; 381: 2219–2229. [DOI] [PubMed] [Google Scholar]
- 39. Rebecchi F, Giaccone C, Farinella E, et al. Randomized controlled trial of laparoscopic heller myotomy plus Dor fundoplication versus Nissen fundoplication for achalasia: long-term results. Ann Surg 2008; 248: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 40. Inoue H, Sato H, Ikeda H, et al. Per-oral endoscopic myotomy: a series of 500 patients. J Am Coll Surg 2015; 221: 256–264. [DOI] [PubMed] [Google Scholar]
- 41. Von Renteln D, Fuchs K-H, Fockens P, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology 2013; 145: 309–311.e1–3. [DOI] [PubMed] [Google Scholar]
- 42. Barbieri LA, Hassan C, Rosati R, et al. Systematic review and meta-analysis: efficacy and safety of POEM for achalasia. United European Gastroenterol J 2015; 3: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon consensus. Gut 2018; 67: 1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Familiari P, Greco S, Gigante G, et al. Gastroesophageal reflux disease after peroral endoscopic myotomy: analysis of clinical, procedural and functional factors, associated with gastroesophageal reflux disease and esophagitis. Dig Endosc 2016; 28: 33–41. [DOI] [PubMed] [Google Scholar]
- 45. Ponds FA, Oors JM, Smout AJPM, et al. Reflux symptoms and oesophageal acidification in treated achalasia patients are often not reflux related. Gut 2021; 70: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leeuwenburgh I, Van Dekken H, Scholten P, et al. Oesophagitis is common in patients with achalasia after pneumatic dilatation. Aliment Pharmacol Ther 2006; 23: 1197–1203. [DOI] [PubMed] [Google Scholar]
- 47. Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology 2008; 135: 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boeckxstaens G, Zaninotto G. Achalasia and esophago-gastric junction outflow obstruction: focus on the subtypes. Neurogastroenterol Motil 2012; 24(Suppl. 1): 27–31. [DOI] [PubMed] [Google Scholar]
- 49. Rohof WO, Salvador R, Annese V, et al. Outcomes of treatment for achalasia depend on manometric subtype. Gastroenterology 2013; 144: 718–725; quiz e13–e14. [DOI] [PubMed] [Google Scholar]
- 50. Kumbhari V, Tieu AH, Onimaru M, et al. Peroral endoscopic myotomy (POEM) vs laparoscopic heller myotomy (LHM) for the treatment of type III achalasia in 75 patients: a multicenter comparative study. Endosc Int Open 2015; 3: E195–E201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Woodfield CA, Levine MS, Rubesin SE, et al. Diagnosis of primary versus secondary achalasia: reassessment of clinical and radiographic criteria. Am J Roentgenol 2000; 175: 727–731. [DOI] [PubMed] [Google Scholar]
- 52. Torres-Aguilera M, Remes Troche JM. Achalasia and esophageal cancer: risks and links. Clin Exp Gastroenterol 2018; 11: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ratuapli SK, Crowell MD, DiBaise JK, et al. Opioid-Induced Esophageal Dysfunction (OIED) in patients on chronic opioids. Am J Gastroenterol 2015; 110: 979–984. [DOI] [PubMed] [Google Scholar]
- 54. Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 2010; 31: 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ravi K, Murray JA, Geno DM, et al. Achalasia and chronic opiate use: innocent bystanders or associated conditions? Dis Esophagus 2016; 29: 15–21. [DOI] [PubMed] [Google Scholar]
- 56. Richter JE, Clayton SB. Diagnosis and management of esophagogastric junction outflow obstruction. Am J Gastroenterol 2019; 114: 544–547. [DOI] [PubMed] [Google Scholar]
- 57. Babaei A, Szabo A, Yorio SD, et al. Pressure exposure and catheter impingement affect the recorded pressure in the Manoscan 360 system. Neurogastroenterol Motil 2018; 30: e13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lynch KL, Yang Y-X, Metz DC, et al. Clinical presentation and disease course of patients with esophagogastric junction outflow obstruction. Dis Esophagus 2017; 30: 1–6. [DOI] [PubMed] [Google Scholar]
- 59. Sanagapalli S, McGuire J, Leong RW, et al. The clinical relevance of manometric esophagogastric junction outflow obstruction can be determined using rapid drink challenge and solid swallows. Am J Gastroenterol. Epub ahead of print 28 October 2020. DOI: 10.14309/ajg.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 60. de Oliveira JM, Birgisson S, Doinoff C, et al. Timed barium swallow: a simple technique for evaluating esophageal emptying in patients with achalasia. Am J Roentgenol 1997; 169: 473–479. [DOI] [PubMed] [Google Scholar]
- 61. Blonski W, Kumar A, Feldman J, et al. Timed barium swallow: diagnostic role and predictive value in untreated achalasia, esophagogastric junction outflow obstruction, and non-achalasia dysphagia. Am J Gastroenterol 2018; 113: 196–203. [DOI] [PubMed] [Google Scholar]
- 62. Vaezi MF, Baker ME, Achkar E, et al. Timed barium oesophagram: better predictor of long-term success after pneumatic dilation in achalasia than symptom assessment. Gut 2002; 50: 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vaezi MF, Baker ME, Richter JE. Assessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagram. Am J Gastroenterol 1999; 94: 1802–1807. [DOI] [PubMed] [Google Scholar]
- 64. Rohof WO, Lei A, Boeckxstaens GE. Esophageal stasis on a timed barium esophagogram predicts recurrent symptoms in patients with long-standing achalasia. Am J Gastroenterol 2012; 108: 49–55. [DOI] [PubMed] [Google Scholar]
- 65. Sanagapalli S, Plumb A, Maynard J, et al. The timed barium swallow and its relationship to symptoms in achalasia: analysis of surface area and emptying rate. Neurogastroenterol Motil 2020; 32: e13928. [DOI] [PubMed] [Google Scholar]
- 66. Ponds FA, Bredenoord AJ, Kessing BF, et al. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil. Epub ahead of print 25 July 2016. DOI: 10.1111/nmo.12908. [DOI] [PubMed] [Google Scholar]
- 67. Kostic S, Andersson M, Hellström M, et al. Timed barium esophagogram in the assessment of patients with achalasia: reproducibility and observer variation. Dis Esophagus 2005; 18: 96–103. [DOI] [PubMed] [Google Scholar]
- 68. Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol 2016; 111: 1726–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Teitelbaum EN, Boris L, Arafat FO, et al. Comparison of esophagogastric junction distensibility changes during POEM and heller myotomy using intraoperative FLIP. Surg Endosc 2013; 27: 4547–4555. [DOI] [PubMed] [Google Scholar]
- 70. Triggs JR, Carlson DA, Beveridge C, et al. Functional luminal imaging probe panometry identifies achalasia-type esophagogastric junction outflow obstruction. Clin Gastroenterol Hepatol 2020; 18: 2209–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ang D, Hollenstein M, Misselwitz B, et al. Rapid drink challenge in high-resolution manometry: an adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil. Epub ahead of print 15 July 2016. DOI: 10.1111/nmo.12902. [DOI] [PubMed] [Google Scholar]
- 72. Ang D, Misselwitz B, Hollenstein M, et al. Diagnostic yield of high-resolution manometry with a solid test meal for clinically relevant, symptomatic oesophageal motility disorders: serial diagnostic study. Lancet Gastroenterol Hepatol 2017; 2: 654–661. [DOI] [PubMed] [Google Scholar]
- 73. Marin I, Caballero N, Guarner-Argente C, et al. Rapid drink challenge test for the clinical evaluation of patients with Achalasia. Neurogastroenterol Motil 2018; 30: e13438. [DOI] [PubMed] [Google Scholar]
- 74. Sanagapalli S, Roman S, Hastier A, et al. Achalasia diagnosed despite normal integrated relaxation pressure responds favorably to therapy. Neurogastroenterol Motil 2019; 31: e13586. [DOI] [PubMed] [Google Scholar]
- 75. Hollenstein M, Thwaites P, Butikofer S, et al. Pharyngeal swallowing and oesophageal motility during a solid meal test: a prospective study in healthy volunteers and patients with major motility disorders. Lancet Gastroenterol Hepatol 2017; 2: 644–653. [DOI] [PubMed] [Google Scholar]
- 76. Taft TH, Carlson DA, Triggs J, et al. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterol Motil 2018; 30: e13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oude Nijhuis RAB, Prins LI, Mostafavi N, et al. Factors associated with achalasia treatment outcomes: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020; 18: 1442–1453. [DOI] [PubMed] [Google Scholar]