Abstract
Background and aims:
To investigate whether stroke aetiology affects outcome in patients with acute ischaemic stroke who undergo endovascular therapy.
Methods:
We retrospectively analysed patients from the Bernese Stroke Centre Registry (January 2010–September 2018), with acute large vessel occlusion in the anterior circulation due to cardioembolism or large-artery atherosclerosis, treated with endovascular therapy (±intravenous thrombolysis).
Results:
The study included 850 patients (median age 77.4 years, 49.3% female, 80.1% with cardioembolism). Compared with those with large-artery atherosclerosis, patients with cardioembolism were older, more often female, and more likely to have a history of hypercholesterolaemia, atrial fibrillation, current smoking (each p < 0.0001) and higher median National Institutes of Health Stroke Scale (NIHSS) scores on admission (p = 0.030). They were more frequently treated with stent retrievers (p = 0.007), but the median number of stent retriever attempts was lower (p = 0.016) and fewer had permanent stent placements (p ⩽ 0.004). Univariable analysis showed that patients with cardioembolism had worse 3-month survival [72.7% versus 84%, odds ratio (OR) = 0.51; p = 0.004] and modified Rankin scale (mRS) score shift (p = 0.043) and higher rates of post-interventional heart failure (33.5% versus 18.5%, OR = 2.22; p < 0.0001), but better modified thrombolysis in cerebral infarction (mTICI) score shift (p = 0.025). Excellent (mRS = 0–1) 3-month outcome, successful reperfusion (mTICI = 2b–3), symptomatic intracranial haemorrhage and Updated Charlson Comorbidity Index were similar between groups. Propensity-matched analysis found no statistically significant difference in outcome between stroke aetiology groups. Stroke aetiology was not an independent predictor of favourable mRS score shift, but lower admission NIHSS score, younger age and independence pre-stroke were (each p < 0.0001). Stroke aetiology was not an independent predictor of heart failure, but older age, admission antithrombotics and dependence pre-stroke were (each ⩽0.027). Stroke aetiology was not an independent predictor of favourable mTICI score shift, but application of stent retriever and no permanent intracranial stent placement were (each ⩽0.044).
Conclusion:
We suggest prospective studies to further elucidate differences in reperfusion and outcome between patients with cardioembolism and large-artery atherosclerosis.
Keywords: all cerebrovascular disease/stroke, CT, MR
Introduction
In patients with acute ischaemic stroke, the immediate aim is to restore blood flow to salvageable brain tissue. The long-term aim is to improve outcome by reducing disability and mortality. Effective options for reperfusion therapy are intravenous alteplase, intravenous tenecteplase and endovascular therapy (±intravenous thrombolysis). Endovascular therapy (±intravenous thrombolysis) has the potential to improve 3-month outcome in patients with acute ischaemic stroke and large vessel occlusion in the anterior circulation if they have no contraindications and can be treated within a few hours of stroke onset.1,2
Two of the most common stroke aetiologies involve cardioembolism and large-artery atherosclerosis.3,4 Large-artery atherosclerosis is typified by a significant narrowing of extra- or intracranial brain-supplying arteries, potentially leading to local vessel occlusion, embolism and/or, less frequently, to haemodynamic impairment.3,4 Investigation of stroke aetiology is requisite for targeted secondary prevention to reduce recurrence and may support improved outcome in dedicated stroke units.5,6 Vascular risk factor profiles and other data have been shown to differ between the two stroke aetiologies mentioned above, with acute ischaemic stroke of cardioembolic origin historically being associated with worse outcome.7–9 Stroke aetiology might influence the outcome of reperfusion.10,11 However, data on endovascular therapy (±intravenous thrombolysis) that include comparison of stroke aetiology are scarce.9,11–13
The aim of this study was to compare patients with cardioembolism versus large-artery atherosclerosis as determined stroke aetiology and to investigate whether stroke aetiology has an impact on reperfusion and outcome in patients with acute ischaemic stroke and large vessel occlusion in anterior circulation treated with endovascular therapy, including intra-arterial thrombolysis and/or mechanical thrombectomy (±intravenous thrombolysis).
Methods
Patients
The study patients were from the Bernese Stroke Registry, a prospectively collected database, which has been described previously.14,15 Patients were included in this analysis if they had suffered an acute ischaemic stroke in the anterior circulation [with a vessel occlusion location in at least one of the following segments: extra- or intracranial internal carotid artery (ICA), ICA-T or M1- or M2-segment of the middle cerebral artery (MCA)] between January 2010 and September 2018. Only patients who had endovascular therapy, including intra-arterial thrombolysis and/or mechanical thrombectomy (±intravenous thrombolysis) were eligible. Baseline characteristics and demographic data were recorded. Clinical assessment was performed by a certified stroke neurologist on admission using the 15-item version of the National Institutes of Health Stroke Scale (NIHSS) score.16 Patients underwent computed tomography angiography (CTA) and/or magnetic resonance angiography (MRA) to confirm acute ischaemic lesions and the site of acute vessel occlusion. All images were reviewed retrospectively by a board-certified stroke neurologist (MRH) and an interventional neuroradiologist (EIP), blinded to clinical findings. CTAs were acquired with a 64- or 128-slice multidetector-row CT scanner (Somatom Definition AS and Somatom Definition Edge; Siemens, Erlangen, Germany) with the patient in a head-first position, 1.5-s gantry rotation time, 0.6-mm collimation and a pitch of 1.2, 512 × 512 matrix size automatic exposure control (CARE Dose 4D; Siemens Healthcare, Forchheim, Germany) and automatically adjusted radiation output according to patient morphology. Forty millilitres of Iomeron® 400 (Bracco, Cadempino, Switzerland) was administered intravenously. Contrast-enhanced MRA of the neck and intracranial vessels and time-of-flight MRA of the intracranial arteries were acquired on a 1.5T or 3T MR scanner (Magnetom Avanto and Magnetom Verio; Siemens, Erlangen, Germany). The MR protocol included whole brain diffusion-weighted imaging [b = 1000t, 24 slices, thickness 5 mm, repetition time (TR) 3200 ms, echo time (TE) 87 ms, number of averages 2, matrix 256 × 256] yielding isotropic b0 and b1000 and time-of-flight angiography with the following parameters: 1.5 T: TR, 23 ms; TE, 7 ms; number of averages, 1; field of view (FOV) read, 180 mm; FOV phase, 100%; voxel size, 0.9 × 0.7 × 1.2 mm; flip angle, 25°; acquisition time, 3 min 28 s/3T: TR, 22 ms; TE, 3.6 ms; number of averages, 1; FOV read, 180 mm; FOV phase, 100%; voxel size, 0.6 mm × 0.4 mm × 1.2 mm; flip angle, 20°; acquisition time, 3 min 45 s. After CT and/or MR imaging, digital subtraction angiography (DSA) was performed on a biplane, high-resolution angiographic system (Axiom Artis zee and Axiom Artis Q; Siemens, Erlangen, Germany) using Iopamiro® 300 (Iopamidol, Bracco, Switzerland) as contrast agent.
Acute stroke treatment with endovascular therapy including intra-arterial thrombolysis and/or mechanical thrombectomy (±intravenous thrombolysis) was performed according to international and institutional guidelines.1 Our institutional protocol included intra-arterial thrombolysis with urokinase (Medac GmbH and Opopharma Vertriebs AG), injected manually before or next to and distal to the thrombus, in dosages of up to 1,000,000 IU, in lower dosages in the case of pre-interventional intravenous thrombolysis.17 Our institutional protocol did not include tenecteplase for intravenous thrombolysis.
The decision to perform endovascular therapy was made on an individual basis, by a consensus between the treating neurologist and neuroradiologist and was independent of this study. Possible contraindications were considered in accordance with current institutional and international guidelines. Eligibility for intravenous thrombolysis was based on the time lapse from symptom onset (<4.5 h) or in recent years also based on last time seen well [inclusion if diffusion-weighted MR imaging and T2-weighted and/or fluid-attenuated inversion recovery (FLAIR) imaging mismatch and no clear and/or extensive hyperintensity, or if no area of hypoattenuation in CT imaging]. If feasible, endovascular therapy proceeded as follows: approaching/passing the thrombi with a microwire, application of angioplasty balloon catheters, clot aspiration and/or application of retrievable stents and, if needed, permanent stent placements.
After treatment for acute stroke, patients were intensively monitored in the stroke unit, intermediate or intensive care unit if deemed necessary. Besides neuroimaging, a standard investigation of stroke aetiology was performed according to international and local guidelines. This included laboratory analysis, 12-lead electrocardiogram, 7-day electrocardiogram (repeated twice more in the following weeks), transoesophageal and/or transthoracic echocardiography and neurovascular sonography.1 Stroke aetiology was determined using a slightly modified TOAST classification.1 Only patients whose stroke aetiology was determined to be cardioembolism or large-artery atherosclerosis were included in this study.3,4 Presence, location and degree of large-artery atherosclerosis were diagnosed in serial digital subtraction angiography images and confirmed in CTA and/or MRA. Patients with atrial fibrillation were included in the large-artery atherosclerosis group, if neuroimaging revealed large-artery atherosclerosis and multiple/multi-stage ischaemic lesions in a single ICA territory and plaque neuroimaging, history and other investigations further supported this classification.
Follow-up CTA/MRA were performed 12–24 h after acute stroke treatment and/or earlier in cases of secondary neurological deterioration. Clinical follow-up was done face-to-face by a certified neurologist or in a telephone interview by a trained study nurse at 3 months. Clinical outcome was measured with the post-interventional Updated Charlson Comorbidity Index, NIHSS score at discharge, change in NIHSS score (score at discharge minus score at admission), 3-month modified Rankin scale (mRS) score (excellent outcome = mRS 0–1; favourable outcome = mRS 0–2; survival = mRS 0–5), scored by a certified stroke neurologist.18,19 Imaging outcome measures were: successful reperfusion [modified thrombolysis in cerebral infarction (mTICI) 2b–3] immediately after DSA, final in-hospital infarct volume (measured semi-quantitatively by manual outlining in T2 or FLAIR MR and/or CT imaging, by multiplication of total area in single slices multiplied by slice thickness) and symptomatic intracranial haemorrhage (sICH) during follow-up, defined according to the ECASS III study.20,21
Statistical analysis
Statistical analysis was performed using SPSS 25.0 (SPSS Inc., Chicago, Illinois, USA). Baseline characteristics, demographic data, vascular risk factors, baseline imaging findings, treatment details and outcome variables were compared between patients with cardioembolism versus large-artery atherosclerosis using the χ2-test for categorical variables and Fisher’s exact test if appropriate, and the non-parametric median test for continuous and ordinal variables. Outcome parameters were dichotomized into excellent (mRS 0–1 versus 2–6) and favourable (mRS 0–2 versus 3–6) outcome; survival (mRS 0–5 versus 6) at 3 months; successful reperfusion (mTICI 2b–3 versus 0–2a), heart failure (yes versus no) and sICH (yes versus no). These binary outcome variables, stratified according to both stroke aetiology and successful reperfusion, were subjected to univariable logistic regression analysis. mRS, mTICI, Updated Charlson Comorbidity Index and final in-hospital infarct volume were subjected to ordinal regression analysis (shift analysis). Ordinal and multivariable logistic regression analysis was applied to identify independent predictors of a mRS and mTICI shift and of heart failure and sICH (variables entered included baseline characteristics, demographic data and treatment details with a p < 0.100 in univariable analysis). We also did a sensitivity analysis of patients who had a high-grade stenosis (according to NASCET and SAMMPRIS criteria) prior to large vessel occlusion.22,23
A propensity-matched analysis (in one analysis the variables entered included baseline characteristics and demographic data and in the other treatment details as well, if a p < 0.100 had been obtained in univariable analysis) was performed comparing patients with cardioembolism versus large-artery atherosclerosis.
Results
Patients overall
Between January 2010 and September 2018, 2704 patients with acute stroke were treated with endovascular therapy or intravenous thrombolysis at the Bernese stroke centre. Of these patients, 1705 underwent endovascular therapy including intra-arterial thrombolysis and/or mechanical thrombectomy (±intravenous thrombolysis). In 681 patients with acute ischaemic stroke in the anterior circulation, cardioembolism was determined to be the stroke aetiology whereas large-artery atherosclerosis was diagnosed in 169 patients (in 13% of patients in intracranial location). This resulted in a total of 850 patients (median age 77.4 years, 49.3% female) eligible for inclusion in this study (Figure 1). Differences in baseline characteristics, demographic data and in treatment details were noted between groups. Patients with cardioembolism versus large-artery atherosclerosis were older (p < 0.0001), more frequently female (p < 0.0001), more likely to have a history of hypercholesterolaemia (p < 0.0001), atrial fibrillation (p < 0.0001), current smoking (p < 0.0001) and a higher median NIHSS score on admission (p = 0.030). Also, they were more likely to have suffered a previous acute ischaemic stroke (p = 0.056) and to have been less independent (p = 0.063) than patients with large-artery atherosclerosis pre-stroke. Time delays from symptom onset to admission (p = 0.688) as well as to endovascular therapy (±intravenous thrombolysis) (p = 0.375), location of acute large vessel occlusion (p = 0.163) and therapy modality applied (p = 0.274) were similar in both stroke aetiology groups. A stent retriever was more often used in patients with cardioembolism (p = 0.007), but the median number of stent retriever attempts was lower (1 versus 2, p = 0.016) and they had fewer permanent extra- (p < 0.0001) as well as fewer intracranial (p = 0.004) stent placements than patients with large-artery atherosclerosis (Table 1).
Figure 1.
Flow chart of patient selection.
Table 1.
Demographic data, baseline characteristics and treatment details according to stroke aetiology.
| Cardioembolic
group n = 681 |
Large-artery atherosclerosis
group n = 169 |
p-value* | |
|---|---|---|---|
| Age, median years (range) | 78.8 (18–98) | 71.2 (48–94) | 0.0001 |
| Women | 365 (53.6%) | 54 (32%) | 0.0001 |
| Vascular risk factors | |||
| Arterial hypertension | 519 (76.4%) | 124 (73.4%) | 0.405 |
| Diabetes mellitus | 116 (17.2%) | 37 (22%) | 0.143 |
| Hypercholesterolaemia | 338 (50.4%) | 121 (73.3%) | 0.0001 |
| Atrial fibrillation | 503 (75%) | 14 (8.9%) | 0.0001 |
| Current smoking | 87 (12.9%) | 56 (33.3%) | 0.0001 |
| Coronary heart disease | 175 (26%) | 34 (20.1%) | 0.111 |
| Previous stroke | 98 (14.5%) | 15 (8.9%) | 0.056 |
| Independent (mRS 0–2) pre-stroke | 587 (87.7%) | 155 (92.8%) | 0.063 |
| Time delays, median minutes (range) | |||
| From symptom onset to admission | 144 (0–1413) | 161 (0–1314) | 0.688 |
| From symptom onset to EVT | 233 (13–1436) | 243 (37–1409) | 0.375 |
| Admission NIHSS score, median (range) | 15 (0–38) | 13 (1–28) | 0.030 |
| Admission antithrombotics | 384 (56.7%) | 65 (38.5%) | 0.0001 |
| Location of acute large vessel occlusion | 0.163 | ||
| Extracranial ICA | 5 (0.7%) | 6 (3.6%) | |
| Intracranial ICA | 33 (4.8%) | 8 (4.7%) | |
| ICA-T (±extracranial ICA) | 35 (5.1%) | 10 (5.9%) | |
| ICA and A1 tandem | 4 (0.6%) | 1 (0.6%) | |
| M1 segment (±ICA) | 436 (64%) | 107 (63.3%) | |
| M2 segment (±ICA) | 144 (21.1%) | 33 (19.5%) | |
| M1 or M2 segment and A1 segment | 4 (0.6%) | 4 (2.4%) | |
| Therapy modality | 0.274 | ||
| IA-urokinase | 33 (4.8%) | 5 (3%) | |
| Mechanical thrombectomy† | 347 (51%) | 86 (50.9%) | |
| IA-urokinase and mechanical thrombectomy† | 49 (7.2%) | 7 (4.1%) | |
| IVT and EVT | 252 (37%) | 71 (42%) | |
| Anaesthesia | 0.0001 | ||
| Local | 37 (5.4%) | 7 (4.1%) | |
| Conscious sedation | 203 (29.8%) | 25 (14.8%) | |
| General | 441 (64.8%) | 137 (81.1%) | |
| Time from groin puncture to end of EVT, median minutes (range) | 54 (9–360) | 90 (18–239) | 0.0001 |
| Application of stent retriever | 579 (85%) | 129 (76.3%) | 0.007 |
| Number of stent retriever attempts, median (range) | 1 (1–8) | 2 (1–6) | 0.016 |
| Permanent stenting | |||
| Extracranial | 19 (2.8%) | 107 (63.3%) | 0.0001 |
| Intracranial | 13 (1.9%) | 10 (5.9%) | 0.004 |
Values are presented as number (%) unless otherwise specified. Percentages indicate available data. Missing data were considered as missing, as they were incidentally missing.
p-values compare both groups.
Either clot aspiration and/or application of retrievable stents.
EVT, endovascular therapy; ICA, internal carotid artery; IVT, intravenous thrombolysis; mRS, modified Rankin scale; NIHSS, National Institutes of Health Stroke Scale.
Univariable analysis showed that likelihood of excellent outcome at 3 months was comparable between the two stroke aetiology groups (p = 0.642). Patients with cardioembolism versus large-artery atherosclerosis showed a tendency towards less favourable 3-month outcome [40.6% versus 48.8%, odds ratio (OR) = 0.72, p = 0.059] and poorer 3-month survival (72.7% versus 84%, OR 0.51, p = 0.004) and more post-interventional heart failure (33% versus 18.5%, OR 2.17, p < 0.0001). There was a smaller shift towards lower 3-month mRS scores for patients with cardioembolism (p = 0.043), but a larger shift towards better mTICI scores after endovascular therapy (p = 0.025; Figure 2). Successful reperfusion (p = 0.168) and sICH (p = 0.683) did not differ between the two stroke aetiology groups (Table 2).
Figure 2.
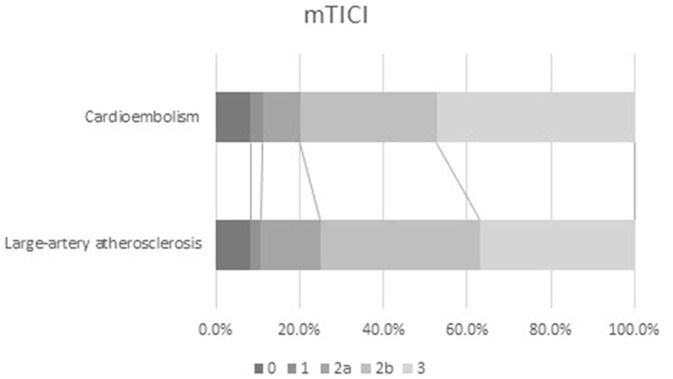
Successful reperfusion according to stroke aetiology.
mTICI, modified thrombolysis in cerebral infarction.
Table 2.
Outcome according to stroke aetiology.
| Cardioembolic group n = 673 |
Large-artery atherosclerosis group*
n = 168 |
p-value† | Odds ratio† (95% CI) | p-value shift analysis | |
|---|---|---|---|---|---|
| mRS at 3 months | 0.043‡ | ||||
| 0–1 | 127 (19.5%) | 29 (17.9%) | 0.642 | 1.11 (0.71–1.74) | |
| 0–2 | 264 (40.6%) | 79 (48.8%) | 0.059 | 0.72 (0.51–1.01) | |
| 0–5 (survival) | 474 (72.7%) | 136 (84%) | 0.004 | 0.51 (0.32–0.80) | |
| Median, range | 3, 0–6 | 3, 0–6 | 0.133 | ||
| Successful reperfusion (mTICI 2b–3) | 543 (79.9%) | 126 (75%) | 0.168 | 1.32 (0.89–1.96) | 0.025‡ |
| Updated Charlson Comorbidity index | 0.313‡ | ||||
| Median, range | 3, 0–16 | 2, 0–12 | 0.479 | ||
| Subitem heart failure | 215 (33%) | 30 (18.5%) | <0.0001 | 2.17 (1.41–3.43) | |
| Symptomatic intracranial haemorrhage | 31 (4.6%) | 9 (5.4%) | 0.683 | 0.85 (0.40–1.83) | NA |
Values are presented as number (%). Percentages indicate available data. Missing data were considered as missing, as they were incidentally missing.
Denominator.
Values compare both groups.
Shift over the whole spectrum of the score.
CI, confidence interval; mRS, modified Rankin scale; mTICI, modified thrombolysis in cerebral infarction.
Patients with cardioembolism
Univariable analysis also showed that excellent outcome at 3 months was similar in patients with cardioembolism and with successful versus unsuccessful reperfusion (p = 0.277). Favourable 3-month outcome (43.8% versus 27.7%, OR 2.04, p = 0.001) and 3-month survival (75.6% versus 61.1%, OR 1.97, p = 0.001) were more often seen in patients with successful versus unsuccessful reperfusion. The same patients also showed a larger shift towards lower 3-month mRS scores and post-interventional Updated Charlson Comorbidity Indexes (each p < 0.0001). sICH occurred in 4.5% patients with cardioembolism and successful reperfusion and in 5.2% patients with unsuccessful reperfusion (p = 0.707) (Table 3).
Table 3.
Outcome according to reperfusion.
| Cardioembolic group | Successful reperfusion
group n = 520 |
Unsuccessful reperfusion group*
n = 131 |
p-value† | Odds ratio† (95% CI) | p-value shift analysis |
|---|---|---|---|---|---|
| mRS at 3 months | <0.0001‡ | ||||
| 0–1 | 106 (20.4%) | 21 (16.2%) | 0.278 | 1.33 (0.80–2.22) | |
| 0–2 | 228 (43.8%) | 36 (27.7%) | 0.001 | 2.04 (1.34–3.11) | |
| 0–5 (survival) | 393 (75.6%) | 80 (61.1%) | 0.001 | 1.97 (1.32–2.96) | |
| Median, range | 3, 0–6 | 4, 0–6 | <0.0001 | ||
| Updated Charlson Comorbidity Index | <0.0001‡ | ||||
| Median, range | 2, 0–16 | 4, 0–13 | <0.0001 | ||
| Subitem heart failure | 162 (31.2%) | 52 (40%) | 0.056 | 0.70 (0.46–1.01) | |
| Symptomatic intracranial haemorrhage | 24 (4.5%) | 7 (5.2%) | 0.707 | 0.85 (0.36–2.01) | NA |
| Large-artery atherosclerosis group (all patients) | Successful reperfusion
group n = 126 |
Futile reperfusion group*
n = 41 |
p-value† | Odds ratio† (95% CI) | p-value shift analysis |
| mRS at 3 months | 0.083‡ | ||||
| 0–1 | 25 (20.8%) | 4 (9.8%) | 0.120 | 2.43 (0.79–7.47) | |
| 0–2 | 63 (52.5%) | 15 (36.6%) | 0.081 | 1.92 (0.92–3.97) | |
| 0–5 (survival) | 102 (85%) | 33 (80.5%) | 0.499 | 1.37 (0.55–3.45) | |
| Median, range | 2, 0–6 | 3, 0–6 | 0.093 | ||
| Updated Charlson Comorbidity Index | 0.503‡ | ||||
| Median, range | 2, 0–12 | 4, 0–9 | 0.534 | ||
| Subitem heart failure | 21 (17.5%) | 9 (22%) | 0.528 | 0.75 (0.31–1.81) | |
| Symptomatic intracranial haemorrhage | 7 (5.6%) | 2 (4.9%) | 0.868 | 1.15 (0.23–5.75) | NA |
| Large-artery atherosclerosis group (high-grade stenosis patients only) | Successful reperfusion
group n = 65 |
Futile reperfusion group*
n = 24 |
p-value† | Odds ratio† (95% CI) | p-value shift analysis |
| mRS at 3 months | 0.523‡ | ||||
| 0–1 | 14 (21.5%) | 3 (12.5%) | 0.342 | 1.92 (0.50–7.39) | |
| 0–2 | 29 (44.6%) | 11 (45.8%) | 0.918 | 0.95 (0.37–2.44) | |
| 0–5 (survival) | 56 (86.2%) | 19 (79.2%) | 0.422 | 1.64 (0.49–5.50) | |
| Median, range | 3, 0–6 | 3, 0–6 | 0.766 | ||
| Updated Charlson Comorbidity Index | 0.806‡ | ||||
| Median, range | 2, 0–12 | 2, 0–9 | 0.826 | ||
| Subitem heart failure | 10 (15.4%) | 5 (20.8%) | 0.544 | 0.69 (0.21–2.28) | |
| Symptomatic intracranial haemorrhage | 4 (5.8%) | 1 (4%) | 0.733 | 1.48 (0.16–13.88) | NA |
Values are presented as number (%). Percentages indicate available data. Missing data were considered as missing, as they were incidentally missing.
Denominator.
Values compare both groups.
Shift over the whole spectrum of the score.
CI, confidence interval; mRS, Modified Rankin scale; NA, not applicable.
Patients with large-artery atherosclerosis
Excellent 3-month outcome was similar in patients with large-artery atherosclerosis whether or not reperfusion was successful (p = 0.120). There was a tendency towards more favourable 3-month outcome (mRS 0–2) (p = 0.081) in patients with large-artery atherosclerosis who had successful reperfusion. Survival at 3 months (p = 0.499) did not differ between the two reperfusion groups. Patients with successful reperfusion showed a tendency towards a better shift of lower 3-months mRS scores (p = 0.083). sICH occurred in 5.6% patients with large-artery atherosclerosis who had successful reperfusion and in 4.9% patients in whom reperfusion was unsuccessful (p = 0.868) (Table 3). In the subgroup analysis of patients who had a high-grade stenosis prior to large vessel occlusion, outcomes were similar in both reperfusion groups (Table 3).
Patients in propensity-matched analysis
In propensity-matched analysis there was no difference in group comparisons, only, there was a tendency towards a lower median NIHSS score at discharge in patients with cardioembolism (4 versus 7, p = 0.076) not matched to the patients with large-artery atherosclerosis for therapy details (Tables 4 and 5).
Table 4.
Outcome according to stroke aetiology in propensity-matched analysis (matched according to demographic data, baseline characteristics and therapy details).
| Cardioembolic
group n = 61 |
Large-artery atherosclerosis group*
n = 61 |
p-value† | Odds ratio† (95% CI) | p-value shift analysis | |
|---|---|---|---|---|---|
| mRS at 3 months | 0.570‡ | ||||
| 0–1 | 16 (26.2%) | 11 (18%) | 0.278 | 1.62 (0.68–3.85) | |
| 0–2 | 32 (52.5%) | 28 (45.9%) | 0.469 | 1.30 (0.64–2.65) | |
| 0–5 (survival) | 47 (77%) | 48 (78.7%) | 0.827 | 0.91 (0.39–2.14) | |
| Median, range | 2, 0–6 | 3, 0–6 | 0.463 | ||
| NIHSS score at discharge from acute care | |||||
| Median change, range | −5, −21 to 34 | −5, −21 to 37 | 0.925 | ||
| Median, range | 4, 0–42 | 7, 0–42 | 0.142 | ||
| Final in-hospital infarct volume | 0.535 | 0.336‡ | |||
| <30 ml | 30 (50%) | 24 (40%) | |||
| 30–90 ml | 21 (35%) | 26 (43.3%) | |||
| >90 ml | 9 (15%) | 10 (16.7%) | |||
| Successful reperfusion (mTICI 2b/3) | 46 (75.4%) | 40 (65.6%) | 0.235 | 1.61 (0.73–3.54) | 0.200‡ |
| Updated Charlson Comorbidity Index | 0.215‡ | ||||
| Median, range | 2, 0–12 | 4, 0–9 | 0.393 | ||
| Subitem heart failure | 20 (32.8%) | 15 (24.6%) | 0.318 | 1.50 (0.68–3.30) | |
| Symptomatic intracranial haemorrhage | 5 (8.2%) | 4 (6.6%) | 0.730 | 1.27 (0.33–4.99) | NA |
Values are presented as number (%). Percentages indicate available data. Missing data were considered as missing, as they were incidentally missing.
Denominator.
Values compare both groups.
Shift over the whole spectrum of the score.
CI, confidence interval; mRS, modified Rankin scale; mTICI, modified thrombolysis in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale; NA, not applicable.
Table 5.
Outcome according to stroke aetiology in propensity-matched analysis (matched according to demographic data and baseline characteristics).
| Cardioembolic
group n = 118 |
Large-artery atherosclerosis group*
n = 118 |
p-value† | Odds ratio† (95% CI) | p-value shift analysis | |
|---|---|---|---|---|---|
| mRS at 3 months | 0.580‡ | ||||
| 0–1 | 29 (24.6%) | 24 (20.3%) | 0.436 | 1.28 (0.69–2.36) | |
| 0–2 | 63 (53.4%) | 57 (48.3%) | 0.435 | 1.23 (0.74–2.04) | |
| 0–5 (survival) | 94 (79.7%) | 102 (86.4%) | 0.168 | 0.61 (0.31–1.23) | |
| Median, range | 2, 0–6 | 3, 0–6 | 0.362 | ||
| NIHSS score at discharge from acute care | |||||
| Median change, range | −7, −20 to 34 | −6, −25 to 37 | 0.640 | ||
| Median, range | 4, 0–42 | 7, 0–42 | 0.076 | ||
| Final in-hospital infarct volume | 0.286 | 0.519‡ | |||
| <30 ml | 56 (48.7%) | 49 (41.5%) | |||
| 30–90 ml | 40 (34.8%) | 53 (44.9%) | |||
| >90 ml | 19 (16.5%) | 16 (13.6%) | |||
| Successful reperfusion (mTICI 2b/3) | 97 (82.2%) | 88 (74.6%) | 0.156 | 1.58 (0.84–2.95) | 0.418‡ |
| Updated Charlson Comorbidity Index | 0.899‡ | ||||
| Median, range | 2, 0–16 | 2, 0–10 | 0.907 | ||
| Subitem heart failure | 31 (26.3%) | 21 (17.8%) | 0.118 | 1.65 (0.88–3.08) | |
| Symptomatic intracranial haemorrhage | 3 (2.6%) | 5 (4.2%) | 0.491 | 0.60 (0.14–2.57) | NA |
Values are presented as number (%). Percentages indicate available data. Missing data were considered as missing, as they were incidentally missing.
Denominator.
Values compare both groups.
Shift over the whole spectrum of the score.
CI, confidence interval; mRS, modified Rankin scale; mTICI, modified thrombolysis in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale.
Stroke aetiology as predictor of outcome
Stroke aetiology was neither an independent predictor of favourable mRS score shift, but lower admission NIHSS score, younger age and independence pre-stroke (each p < 0.0001), nor of heart failure, but older age, admission antithrombotics and dependence pre-stroke (each ⩽0.027), nor of favourable mTICI score shift, but application of stent retriever and no permanent intracranial stent placement (each ⩽0.044). Neither stroke aetiology nor the other variables entered into the model were independent predictors of sICH (Table 6).
Table 6.
Predictors of a favourable mRS and mTICI score shift and of heart failure.
| Favourable mRS shift | p-value |
|---|---|
| Stroke aetiology | 0.922 |
| Admission NIHSS score | <0.0001 |
| Age | <0.0001 |
| Independence (mRS 0–2) pre-stroke | <0.0001 |
| Permanent intracranial stent placement | 0.057 |
| Atrial fibrillation | 0.310 |
| Previous stroke | 0.412 |
| Current smoking | 0.441 |
| Anaesthesia | 0.511 |
| Permanent extracranial stent placement | 0.580 |
| Hypercholesterolaemia | 0.680 |
| Admission antithrombotics | 0.755 |
| Application of stent retriever | 0.839 |
| Women | 0.995 |
| Favourable mTICI score shift | p-value |
| Stroke aetiology | 0.395 |
| Application of stent retriever | <0.0001 |
| Permanent intracranial stent placement | 0.044 |
| Anaesthesia | 0.197 |
| Admission antithrombotics | 0.219 |
| Atrial fibrillation | 0.282 |
| Current smoking | 0.419 |
| Women | 0.493 |
| Permanent extracranial stent placement | 0.509 |
| Independence (mRS 0–2) pre-stroke | 0.694 |
| Previous stroke | 0.831 |
| Hypercholesterolaemia | 0.936 |
| Age | 0.968 |
| Admission NIHSS score | 0.984 |
| Heart failure | p-value |
| Stroke aetiology | 0.295 |
| Age | 0.001 |
| Admission antithrombotics | 0.013 |
| Independence (mRS 0–2) pre-stroke | 0.027 |
| Previous stroke | 0.315 |
| Women | 0.395 |
| Application of stent retriever | 0.433 |
| Permanent intracranial stent placement | 0.571 |
| Current smoking | 0.616 |
| Permanent extracranial stent placement | 0.633 |
| Atrial fibrillation | 0.800 |
| Hypercholesterolaemia | 0.843 |
| Anaesthesia | 0.863 |
| Admission NIHSS score | 0.912 |
mRS, modified Rankin scale; mTICI, modified thrombolysis in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale.
Discussion
The main findings of our study were as follows: univariable analysis showed that 3-month outcome was similar between patients with cardioembolism and those with large-artery atherosclerosis, despite a better reperfusion shift in patients with cardioembolism. However, in patients within the cardioembolism-group, successful reperfusion was more important for better outcome than it was for patients in the large-artery atherosclerosis-group. We did not find stroke aetiology to be an independent predictor of a better 3-month mRS and mTICI score shift. In propensity-matched analysis, clinical and imaging outcome was not different between groups.
At present, data on whether stroke aetiology impacts reperfusion and outcome in endovascularly treated patients with acute ischaemic stroke are scarce.
Guglielmi and co-workers also investigated endovascular therapy (±intravenous thrombolysis) in 666 patients with cardioembolism versus large-artery atherosclerosis.9 Patients were selected from the MR-CLEAN Registry. They found similar rates of favourable 3-month (mRS 0–2) outcome (35% versus 46%), mortality (33% versus 23%), successful reperfusion (56% versus 53%) and sICH (7% versus 5%) between patients with cardioembolism versus large-artery atherosclerosis. But, the median 3-month mRS was lower [(3 versus 4, adjustedOR 1.45 (95% confidence interval (CI) 1.03–2.05)] in patients with large-artery atherosclerosis. They also noted that patients with cardioembolism versus large-artery atherosclerosis were older, more frequently female, less independent pre-stroke and showed more favourable treatment details. So, the main findings of this study are in line with those of our study although patients more often had lower 3-month mRS scores in our analysis, probably because of higher rates of successful reperfusion. Successful reperfusion is crucial for a favourable outcome, particularly in patients with cardioembolism.24,25 Also, in our analysis, successful reperfusion turned out to be more important for better outcome for patients within the cardioembolism group than for patients within the large-artery atherosclerosis group. A possible explanation for this finding might be that better collaterals had developed over the course of time in a proportion of patients with large-artery atherosclerosis causing a high-grade stenosis.9
Another study investigating endovascular therapy (±intravenous thrombolysis) in 649 Chinese patients with cardioembolism or large-artery atherosclerosis was reported by Sun and co-workers.11 They found higher rates of favourable 3-month outcome (50.2% versus 36.5%; p < 0.001) and survival (81.2% versus 68.2%; p < 0.001), and lower rates of sICH (11.7% versus 20%; p = 0.004) in patients with large-artery atherosclerosis than in those with cardioembolism and no significant difference in rates of successful reperfusion (83.2% versus 84.5%; p = 0.671). Large-artery atherosclerosis in Asians is more often in an intracranial location. This is in contrast to Caucasians, who more frequently suffer from large-artery atherosclerosis of extracranial brain-supplying arteries, especially if patients are matched for age. Therefore, direct comparability of this study with our analysis is limited. This is also true of the study by Yang and co-workers, who compared 45 patients in a propensity-matched analysis.12 They too found comparable rates of favourable 3-month outcome, successful reperfusion (mTICI 2b–3) and sICH. However, they included only patients with occlusions of the M1 segment of the MCA in their analysis.
Giray and co-workers also investigated endovascular therapy (±intravenous thrombolysis) in 52 patients with cardioembolism or large-artery atherosclerosis.13 They found higher rates of favourable 3-month outcome (73.7% versus 39.4%; p = 0.036) in patients with large-artery atherosclerosis versus cardioembolism despite similar rates of successful reperfusion (78% versus 60.6%; p = 0.293) and sICH (10.5% versus 3%; p = 0.546). The authors suggested that longer delay from symptom onset to reperfusion, higher NIHSS score on admission, lower Alberta Stroke Programme Early Computed Tomography Score and longer procedure times with a higher median number of stent retriever attempts (2 versus 1; p = 0.02) in patients with cardioembolism probably contributed to their poorer mRS.
In our study, age and NIHSS score on admission were among the baseline characteristics and demographic data likely to worsen the outcome for patients with cardioembolism, which helps explain our findings in the univariable analysis. These findings are in line with historical findings that ischaemic stroke of cardioembolic origin is associated with worse outcome.7–9 However, in our study, most treatment details were more favourable in patients with cardioembolism, which helps explain our findings in the propensity-matched analysis. Patients with cardioembolism had more frequently a shorter median delay from groin-puncture to end of endovascular therapy, less frequently received general anaesthesia, had a higher number of stent retriever applications, fewer stent retriever attempts and fewer permanent stent placements. In their study Guglielmi and co-workers also reported longer procedure times and fewer first-pass effects in patients with large-artery atherosclerosis.9 There are several reasons why endovascular therapy might be more challenging in patients with large-artery atherosclerosis. First, vessel access is more likely to be difficult. Although a stent retriever improves outcome, optimal contact with the thrombus surface is crucial for its successful application. However, this contact can be limited in patients with large-artery atherosclerosis because atherosclerotic lesions can have an irregular shape.26 Furthermore, since acute large vessel occlusion in large-artery atherosclerosis is typically caused by an underlying unstable plaque, there is a high risk of reocclusion by subsequent platelet activation and recurrent thrombus formation, which might be addressed by permanent extra- or intracranial stenting and administration of blood thinners.26 Moreover, cardioembolic thrombi are less likely to adhere to the site of vessel occlusion than in-situ thrombi, facilitating reperfusion.27 However, this might be true for arterio-arterial embolic thrombi as well. Also, cardioembolic thrombi tend to be more organized and to consist of a higher white platelet-fibrin fraction in comparison with the red erythrocyte-fibrin thrombi of large-artery atherosclerosis. Less organized and red thrombi have been reported to be easier to resolve and/or retrieve.28,29
Last but not least, in our study, heart failure was likelier in patients with cardioembolism versus large-artery atherosclerosis and impacting outcome, but not independently. Patients with heart failure are mostly elderly. These patients suffer from several diseases and experience various symptoms limiting daily activities and quality of life.30
Strengths and limitations
Strengths of our study are the considerable sample size and the setting of a tertiary stroke centre with a high level of expertise in endovascular therapy.
Limitations are the retrospective design, imbalances of baseline variables and the inclusion of patients over a period of time where treatment has evolved. Also, it is likely that a few patients who did have cardioembolism or large-artery atherosclerosis were excluded because of undetermined stroke aetiology. Moreover, we did not differentiate between intracranial and extracranial location of large-artery atherosclerosis. Then, follow-up data on a few patients were missing. Missing data were considered as missing, as they were incidentally missing. Finally, it might have been even more meaningful to compare cardioembolism with large-artery atherosclerosis at the same vessel occlusion site due to the different pathogenesis of atherosclerotic occlusion and the heterogeneity of interventional protocols.
Conclusion
We suggest prospective studies to further elucidate differences in reperfusion and outcome in patients with cardioembolism versus large-artery atherosclerosis.
Acknowledgments
We are grateful to the whole Bernese stroke team, which contributed to data acquisition for this study, and to Susan E. Kaplan for proofreading of this manuscript. The funders of the study had no role in the study design, data collection, data analysis, data interpretation or in the writing of the report. The first and last authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Availability of data: Raw data of all patients included in this study can be made available upon request to the corresponding author and after clearance by the local ethics committee.
Conflict of interest statement: MA: Personal fees (speaker honoraria) from Bayer, Medtronic and Covidien, and scientific advisory board honoraria from Amgen, Bayer, Boehringer Ingelheim, BMS, Pfizer, Covidien, Daichy Sankyo and Nestlé Health Science, not directly relevant to the submitted work.
JG: Global PI of STAR/SWIFT DIRECT (Medtronic). CEC-member of the Promise Study (Penumbra). Swiss National Foundation grant (MRI in stroke), not directly relevant to the submitted work.
SJ: Scientific advisory board honoraria from Bayer/Boehringer Ingelheim/Pfizer, not directly relevant to the submitted work.
UF: Consultant for Medtronic/Stryker/CSL Behring, Co-PI of SWIFT DIRECT and BEYOND SWIFT (Medtronic), PI of SWITCH/ELAN, research support of the Swiss National Foundation/Swiss Heart Foundation and Medtronic, not directly relevant to the submitted work.
MRH: Scientific advisory board honoraria from Amgen and a grant from the Bangerter foundation, not directly relevant to the submitted work. Research grant from the Inselspital, University Hospital Bern, for research on the topic of stroke aetiology.
The other authors have nothing to disclose.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research grant by the Inselspital, University Hospital Bern.
Ethics statement: The study was approved by the local ethics committee of the canton of Bern (KEK 231/14) and therefore conforms to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Data analyses followed STROBE reporting guidelines.
Informed consent: Patients or their relatives gave informed consent for treatment and study participation.
ORCID iD: Mirjam R Heldner  https://orcid.org/0000-0002-3594-2159
https://orcid.org/0000-0002-3594-2159
Contributor Information
Meredeth Zotter, Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Eike I. Piechowiak, Institute of Diagnostic and Interventional Neuroradiology, Inselspital, University Hospital and University of Bern, Bern, Switzerland
Rupashani Balasubramaniam, Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Rascha Von Martial, Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Kotryna Genceviciute, Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Marisa Blanquet, Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Nedelina Slavova, Institute of Diagnostic and Interventional Neuroradiology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Hakan Sarikaya, Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Marcel Arnold, Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Jan Gralla, Institute of Diagnostic and Interventional Neuroradiology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Simon Jung, Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Urs Fischer, Department of Neurology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Marwan El-Koussy, Institute of Diagnostic and Interventional Neuroradiology, Inselspital, University Hospital and University of Bern, Bern, Switzerland.
Mirjam R. Heldner, Department of Neurology, Inselspital, University Hospital and University of Bern, Freiburgstrasse, Bern, 3010, Switzerland.
References
- 1. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 2. Rodrigues FB, Neves JB, Caldeira D, et al. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ 2016; 353: i1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993; 24: 34–41. [DOI] [PubMed] [Google Scholar]
- 4. Ay H, Furie KL, Singhal A, et al. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 2005; 58: 688–697. [DOI] [PubMed] [Google Scholar]
- 5. Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2014; 45: 2160–2236. [DOI] [PubMed] [Google Scholar]
- 6. D’Anna L, Gigli GL, Gregoraci G, et al. Identification of stroke etiology may contribute to improve the outcome in dedicated units. J Stroke Cerebrovasc Dis 2015; 24: 802–810. [DOI] [PubMed] [Google Scholar]
- 7. Schulz UG, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke: importance of population-based studies. Stroke 2003; 34: 2050–2059. [DOI] [PubMed] [Google Scholar]
- 8. Song YM, Kwon SU, Sung J, et al. Different risk factor profiles between subtypes of ischemic stroke. A case-control study in Korean men. Eur J Epidemiol 2005; 20: 605–612. [DOI] [PubMed] [Google Scholar]
- 9. Guglielmi V, LeCouffre NE, Zinkstok SM, et al. ; MR-CLEAN Registry Investigators. Collateral circulation and outcome in atherosclerotic versus cardioembolic cerebral large vessel occlusion. Stroke 2019; 50: 3360–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Yiin GS, Geraghty OC, et al. ; Oxford Vascular Study. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol 2015; 14: 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun B, Shi Z, Pu J, et al. Effects of mechanical thrombectomy for acute stroke patients with etiology of large artery atherosclerosis. J Neurol Sci 2019; 396: 178–183. [DOI] [PubMed] [Google Scholar]
- 12. Yang W, Zhang Y, Li Z, et al. Differences in safety and efficacy of endovascular treatment for acute ischemic stroke: a propensity score analysis of intracranial atherosclerosis-related occlusion versus embolism. Clin Neuroradiol. Epub ahead of print 1 April 2020. DOI: 10.1007/s00062-020-00899-x [DOI] [PubMed] [Google Scholar]
- 13. Giray S, Ozdemir O, Bas DF, et al. Does stroke etiology play a role in predicting outcome of acute stroke patients who underwent endovascular treatment with stent retrievers? J Neurol Sci 2017; 372: 104–109. [DOI] [PubMed] [Google Scholar]
- 14. Heldner MR, Hsieh K, Broeg-Morvay A, et al. Clinical prediction of large vessel occlusion in anterior circulation stroke: mission impossible? J Neurol 2016; 263: 1633–1640. [DOI] [PubMed] [Google Scholar]
- 15. Heldner MR, Chaloulos-Iakovidis P, Panos L, et al. Outcome of patients with large vessel occlusion in the anterior circulation and low NIHSS score. J Neurol 2020; 267: 1651–1662. [DOI] [PubMed] [Google Scholar]
- 16. Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIHSS score using video training. NINDS TPA Stroke Study Group. Stroke 1994; 25: 2220–2226. [DOI] [PubMed] [Google Scholar]
- 17. Kaesmacher J, Bellwald S, Dobrocky T, et al. Safety and efficacy of intra-arterial urokinase after failed, unsuccessful, or incomplete mechanical thrombectomy in anterior circulation large-vessel occlusion stroke. JAMA Neurol 2020; 77: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 19. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
- 20. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 21. Suh SH, Cloft HJ, Fugate JE, et al. Clarifying differences among thrombolysis in cerebral infarction scale variants: is the artery half open or half closed? Stroke 2013; 44: 1166–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eliasziw M, Smith RF, Singh N, et al. Further comments on the measurement of carotid stenosis from angiograms. North American Symptomatic Carotid Endarterectomy Trial (NASCET) group. Stroke 1994; 25: 2445–2449. [DOI] [PubMed] [Google Scholar]
- 23. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manning NW, Warne CD, Meyers PM. Reperfusion and clinical outcomes in acute ischemic stroke: systematic review and meta-analysis of the stent-retriever-based, early window endovascular stroke trials. Front Neurol 2018; 9: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007; 38: 967–973. [DOI] [PubMed] [Google Scholar]
- 26. Krasteva MP, Lau KK, Mordasini P, et al. Intracranial atherosclerotic stenoses: pathophysiology, epidemiology, risk factors and current therapy options. Adv Ther 2020; 37: 1829–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molina CA, Montaner J, Arenillas JF, et al. Differential pattern of tissue plasminogen activator-induced proximal middle cerebral artery recanalization among stroke subtypes. Stroke 2004; 35: 486–490. [DOI] [PubMed] [Google Scholar]
- 28. Boeckh-Behrens T, Schubert M, Förschler A, et al. The impact of histological clot composition in embolic stroke. Clin Neuroradiol 2014; 26: 189–197. [DOI] [PubMed] [Google Scholar]
- 29. Ogata H, Yutani R, Otsuba H, et al. Heart and vessel pathology underlying brain infarction in 142 stroke patients. Ann Neurol 2008; 63: 770–781. [DOI] [PubMed] [Google Scholar]
- 30. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017; 3: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]