Abstract
Purpose:
The goal of this study is to construct nomograms to effectively predict the distant metastatic sites and overall survival (OS) of soft tissue sarcoma (STS) patients.
Methods:
STS case data between 2010 and 2015 for retrospective study were gathered from public databases. According to the chi-square and multivariate logistic regression analysis determined independent predictive factors of specific metastatic sites, the nomograms based on these factors were consturced. Subsequently, combined metastatic information a nomogram to predict 1-, 2-, and 3-year OS of STS patients was developed. The performance of models was validated by the area under the curve (AUC), calibration plots, and decision curve analyses (DCA).
Results:
A total of 7001 STS patients were included in this retrospective study, including 4901 cases in the training group and the remaining 2,100 patients in the validation group. Three nomograms were established to predict lung, liver and bone metastasis, and satisfactory results have been obtained by internal and external validation. The AUCs for predicting lung, liver, and bone metastases in the training cohort were 0.796, 0.799, and 0.766, respectively, and in the validation cohort were 0.807, 0.787, and 0.775, respectively, which means that the nomograms have good discrimination. The calibration curves showed that the models have high precision, and the DCA manifested that the nomograms have great clinical application prospects. Through univariate and multivariate COX regression analyses, 8 independent prognosis factors of age, grade, histological type, tumor size, surgery, chemotherapy, radiatiotherapy and lung metastasis were determined. A nomogram was then constructed to predict the 1-, 2-, and 3-years OS, which has a good performance in both internal and external validations.
Conclusion:
The nomograms for predicting specific metastatic sites and OS have good discrimination, accuracy and clinical applicability. The models could accurately predict the metastatic risk and survival information, and help clinical decision-making.
Keywords: soft tissue sarcoma, metastasis, overall survival, nomogram
Background
Soft tissue sarcoma (STS) is a heterogeneous solid tumor that originates from the mesenchyme of the mesoderm; it is relatively rare and accounts for 1% of all adult malignant tumors.1 In 2019, more than 12,000 new cases and more than 5,000 deaths from STSs were reported.2,3 A unique feature of STS is that the outcome is strongly influenced not only by the histological grade of the tumor of origin but also by its histological subtype, which is an important variable affecting metastasis and outcome. STSs are a complex, heterogeneous tumor family, and more than 50 different subtypes have been identified.4 The common subtypes include fibrosarcoma, leiomyosarcoma, liposarcoma, malignant fibrohistiocytoma, malignant peripheral nerve sheath tumor (MPNST) and others. STSs have a tendency to metastasize in the early stage, as approximately one-third of patients who had no signs of metastasis at diagnosis developed metastasis during the treatment period.5 Nearly half of STS patients will eventually develop distant metastatic disease.6 Once metastatic disease is detected, STS is almost incurable, and the treatment plan tends to consist only of palliative chemotherapy.7,8 In this case, the survival rate of STS patients drops sharply, and the median survival is only approximately 1 year.6 Achieving the early prediction of distant metastasis or even specific metastatic sites would be of great importance for optimal treatment selection and improved prognosis.
The prognosis of patients with advanced STS is not ideal, and the median overall survival (OS) is less than 2 years.9 And due to the heterogeneity of STSs, 2 patients who differ only in histological subtype may have very different prognoses. In addition, although the widely used American Joint Committee on Cancer (AJCC) TNM staging system represents the gold standard for classification system of STSs, criticisms of its limitations are still emerging.10 Hence, a highly accurate and widely applicable tool for predicting the metastatic site and survival rate is urgently needed. As a personalized and intuitive mathematical graphic score for prognosis, the nomogram has been widely used in medical prediction analyses. However, most of the current nomograms quantify only the overall survival or cancer-specific survival (CSS) of patients and do not predict the probability and location of tumor metastasis or include treatment and demographic information, which is not conducive to their clinical application.
Therefore, we aim to predict potential STS metastatic sites through 3 convenience nomograms based on the information from the Surveillance, Epidemiology, and End Results (SEER) database and provide a more accurate model for predicting OS that would help clinicians involved in clinical treatment and patient care.
Methods
Patients Selection
From 2010 to 2016, demographic and clinicopathological data of the 22247 STS cases were extracted from the SEER database (https://seer.cancer.gov/). This retrospective study was free from ethical review because the unidentified data were obtained from the publicly available SEER database. The inclusion criteria of STS patients were as follows: (1) The histological diagnosis of STS was the primary tumor (2) Patients’ tumor grading and staging information was complete. (3) Patients’ survival information was clear or follow-up was completed. The exclusion criteria were as follows: (1) Only diagnosed as sarcoma with no further histological subtype classification. (2) Cancer staging not based on AJCC seventh edition. (3) Incomplete or unknown treatment information, (4) Missing of specific metastatic sites, such as lung, liver, bone and brain. (5) Absence of personal information, such as insurance and marital status.
Variables Declaration
Combined with the size, category and characteristics of the data, drawing on the experience of previous study, information was analyzed and some variables were appropriately adjusted. Some continuous variables were cast to categorical variables, such as age (>60years, ≤60 years) and tumor size (<50 mm, 50-100 mm, >100 mm). For some categorical variables, we made the following adjustments due to their more subdivided categories. Primary site of the tumor was divided into 4 categories: head and neck, upper extremities, lower extremities and trunk. We defined other races out of white and black as others. Histologic subtypes were grouped as follows: fibrosarcoma, leiomyosarcoma, liposarcoma, malignant fibrohistiocytoma, MPNST, synovial sarcoma and others. Marital status was subdivided as married, unmarried, and divorced (widows included).
Statistical Analysis
R software (www.r-project.org, version 4.0.2) and the SPSS 25.0 software were applied for the all statistical analyses, with P-values < 0.05 considered statistically significant. Demographics and clinicopathological information were summarized with statistics. By using R software, all patients involved in the study were randomly divided into training and validation sets. Analyze the relationship between the variable and the specific distant metastatic sites with the chi-square test. Significantly related variables confirmed in the chi-square test were included in the further multivariate logistic regression analysis. Hazard ratio (HR) was calculated to quantify the effect of each predictor on lung, liver and bone metastasis, and variables with statistically significant were identified as independent predictors. Then, by integrating the predictors of specific metastasis sites separately, the nomograms for predicting the possibility of lung, liver and bone metastasis in STS patients were developed. Next, including the lung, liver and bone and brain metastasis and survival information, univariate and multivariate COX regression analyses were devoted to ascertain the independent prognostic factors, and then the nomogram for predicting 1-, 2-, and 3-year OS of STS patients was constructed.
The training dataset was used to develop nomograms and internal validation, and the validation dataset was used to validate the performance of the model from the external. 1000 bootstrap samples for internal validation to prevent over-fitting. In order to evaluate the prediction performance of the nomograms, testing was carried out from 2 aspects of discrimination and calibration. The receiver operating characteristic (ROC) curves for predicting lung, liver, and bone metastasis were produced to test the discrimination ability over different sites, and the ROC curves at 1, 2 and 3 year were made to examine the discrimination ability over time. The area under curve (AUC) was calculated, which is equivalent to the concordance index (C-index) to quantify the discrimination of nomograms. The predictive accuracy was visually evaluated through calibration curves to compare the consistency between model predictions and observed results. In addition, decision curve analysis (DCA) was performed to assess the clinical utility of nomograms in the training and validation groups. A higher net benefit within a wide threshold range indicates that the model has more potential in clinical applications.
Result
Patient Characteristics
After screening through the application of inclusion and exclusion criteria, 7,001 STS patients from the SEER database were included retrospectively. The patients were randomly divided into a training group and validation group in a ratio of 7:3 by R software; a total of 4,901 patients were included in the training group, and the remaining 2,100 patients were in the validation group. In the whole cohort, the mean follow-up time (survival months) was 34.87 ± 22.17 months. More than 55% of patients were younger than 60 years old. The majority of patients were white (80.03%, n = 5603), and 56.86% were male. Regarding the statistics on treatment information, the numbers of patients receiving surgery, radiotherapy and chemotherapy were 6,472 (92.44%), 3,555 (50.78%) and 1,622 (23.17%), respectively.
Metastasis Pattern
A total of 643 patients with specific distant metastatic disease accounted for 9.1% of the entire collection. The specific metastatic site distribution was as follows: lung metastasis (5.8%, n = 407); liver metastasis (1.2%, n = 90); bone metastasis (1.9%, n = 138); and brain metastasis (0.2%, n = 16). The characteristics of the STS patients are shown in Table 1.
Table 1.
Characteristics of Soft Tissue Sarcoma Patients.
| Characteristics | Number(%) |
|---|---|
| Age (yrs) | |
| ≤60 | 3886 (55.5%) |
| >60 | 3115 (44.4%) |
| Race | |
| White | 5603 (80.0%) |
| Black | 766 (10.9%) |
| Other | 632 (9.0%) |
| Sex | |
| Female | 3020 (43.1%) |
| Male | 3981 (56.8%) |
| Grade | |
| I | 2155 (30.7%) |
| II | 1885 (26.9%) |
| III | 2318 (33.1%) |
| IV | 643 (9.1%) |
| Primary site | |
| head, face, neck | 414 (5.9%) |
| upr limb, shoulder | 944 (13.4%) |
| lower limb, hip | 3214 (45.9%) |
| heart, thorax abdomen pelvis trunk, NOS | 2429 (34.6%) |
| Histological type | |
| fibrosarcoma | 721 (9.6%) |
| leiomyosarcoma | 892 (11.9%) |
| liposarcoma | 1566 (21.0%) |
| malignant fibrohistiocytoma | 422 (5.6%) |
| malignant peripheral nerve sheath tumor | 237 (3.1%) |
| synovial sarcoma | 382 (5.1%) |
| Other | 2781 (37.3%) |
| T stage | |
| T1 | 2097 (29.9%) |
| T2 | 4904 (70.0%) |
| N stage | |
| N0 | 6718 (95.9%) |
| N1 | 283 (4.0%) |
| M stage | |
| M0 | 6358 (90.8%) |
| M1 | 643 (9.1%) |
| Tumor size (mm) | |
| <50 | 1849 (26.4%) |
| [50-100] | 2369 (33.8%) |
| >100 | 2783 (39.7%) |
| lymph nodes | |
| 0 | 6718 (95.9%) |
| 100 | 244 (3.4%) |
| 120 | 2 (<0.1%) |
| 150 | 1 (<0.1%) |
| 800 | 36 (0.5%) |
| Surgery | |
| Yes | 6472 (92.4%) |
| No | 529 (7.5%) |
| Radiotherapy | |
| Yes | 3555 (50.7%) |
| No | 3446 (49.2%) |
| Chemotherapy | |
| Yes | 1622 (23.1%) |
| No | 5379 (76.8%) |
| Lung metastasis | |
| Yes | 407 (5.8%) |
| No | 6594 (94.1%) |
| Liver metastasis | |
| Yes | 90 (1.2%) |
| No | 6911 (98.7%) |
| Bone metastasis | |
| Yes | 138 (1.9%) |
| No | 6863 (98.0%) |
| Brain metastasis | |
| Yes | 16 (0.2%) |
| No | 6985 (99.7%) |
| Insurance status | |
| Yes | 6771 (96.7%) |
| No | 230 (3.2%) |
| Marital status | |
| Married | 3928 (56.1%) |
| Unmarried | 1887 (26.9%) |
| Divorced | 1186 (16.9%) |
Development of Nomograms for Specific Distant Metastatic Sites
The chi-square analysis revealed some variables associated with the specific metastatic sites, including race, tumor grade, tumor primary site, histological type, T stage, N stage, tumor size and patient marital status, as shown in Table 2. After multivariate logistic regression analysis was performed on these important variables, independent predictors of different specific distant metastatic sites were identified, and the detailed results of the analysis are listed in Table 3. Finally, 5 independent risk factors were confirmed to be significantly related to lung metastasis of STS, including tumor size, T stage, N stage, tumor grade and histological type. Similarly, with the exception of tumor size, the other 4 independent risk factors mentioned above were significantly associated with liver metastasis. Moreover, N stage, tumor grade and tumor primary site were determined as independent risk predictors of STS bone metastasis. By integrating the above independent predictive indicators, nomograms for predicting specific metastatic sites (liver, lung and bone) were constructed and are shown in Figure 1.
Table 2.
Chi-Square Analysis of the Presence of Different Metastatic Sites of Soft Tissue Sarcoma.
| Lung metastasis | Liver metastasis | Bone metastasis | ||||
|---|---|---|---|---|---|---|
| Characteristics | χ2 | P-value | χ2 | P-value | χ2 | P-value |
| Age | 3.736 | 0.053 | 0.005 | 0.946 | 3.707 | 0.054 |
| Race | 6.405 | 0.041 | 5.381 | 0.068 | 1.797 | 0.407 |
| Sex | 2.848 | 0.091 | 1.005 | 0.316 | 2.486 | 0.115 |
| Grade | 99.749 | <.001 | 8.843 | 0.003 | 21.52 | <.001 |
| Primary site | 4.825 | 0.185 | 30.233 | <.001 | 31.658 | <.001 |
| Histological type | 97.464 | <.002 | 43.973 | <.001 | 17.733 | 0.007 |
| T stage | 62.11 | <.003 | 8.736 | 0.003 | 7.373 | 0.007 |
| N stage | 194.096 | <.004 | 39.267 | <.001 | 112.359 | <.001 |
| Tumor size | 59.474 | <.005 | 7.305 | 0.026 | 5.801 | 0.055 |
| Insurance status | 0.01 | 0.921 | 0.361 | 0.548 | 1.39 | 0.238 |
| Marital status | 8.915 | 0.012 | 1.226 | 0.542 | 9.531 | 0.009 |
Table 3.
Multivariate Logistic Regression of the Presence of Different Metastatic Sites of Soft Tissue Sarcoma.
| Lung metastasis | Liver metastasis | Bone metastasis | ||||
|---|---|---|---|---|---|---|
| Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | 0.235 | - | 0.493 | |||
| ≤60 | 1 | 1 | ||||
| >60 | 0.841 (0.633-1.119) | 0.235 | 0.843 (0.517-1.374) | 0.493 | ||
| Race | 0.134 | 0.126 | - | |||
| White | 1 | 1 | ||||
| Black | 1.268 (0.875-1.838) | 0.209 | 1.291 (0.897-1.860) | 0.169 | ||
| Other | 0.699 (0.427-1.146) | 0.156 | 0.714 (0.436-1.170) | 0.181 | ||
| Sex | 0.087 | - | - | |||
| Female | 1 | |||||
| Male | 1.260 (0.967-1.643) | 0.087 | ||||
| Grade | <.001 | <.001 | 0.002 | |||
| I-II | 1 | 1 | 1 | |||
| III-IV | 2.718 (1.820-4.057) | <.001 | 2.677 (1.792-4.000) | <.001 | 2.676 (1.421-5.038) | 0.002 |
| Primary site | - | 0.847 | <.001 | |||
| head, face, neck | 1 | 1 | ||||
| upr limb, shoulder | 1.215 (0.594-2.487) | 0.593 | 1.212 (0.278-5.282) | 0.797 | ||
| lower limb, hip | 1.322 (0.700-2.498) | 0.390 | 1.578 (0.451-5.513) | 0.475 | ||
| heart, thorax abdomen pelvis trunk | 1.287 (0.680-2.434) | 0.438 | 4.072 (1.210-13.702) | 0.023 | ||
| Histological type | <.001 | <.001 | 0.387 | |||
| fibrosarcoma | 1 | 1 | 1 | |||
| leiomyosarcoma | 1.920 (1.068-3.454) | 0.029 | 1.853 (1.028-3.342) | 0.040 | 2.108 (0.690-6.439) | 0.191 |
| liposarcoma | 0.153 (0.062-0.378) | <.001 | 0.158 (0.064-0.392) | <.001 | 0.996 (0.297-3.337) | 0.994 |
| malignant fibrohistiocytoma | 0.790 (0.367-1.700) | 0.547 | 0.772 (0.360-1.658) | 0.507 | 0.784 (0.169-3.637) | 0.756 |
| MPNST | 1.243 (0.576-2.686) | 0.579 | 1.431 (0.669-3.063) | 0.356 | 1.222 (0.286-5.208) | 0.787 |
| synovial sarcoma | 2.334 (1.226-4.443) | 0.010 | 2.555 (1.358-4.809) | 0.004 | 1.883 (0.541-6.549) | 0.320 |
| Other | 1.189 (0.694-2.036) | 0.529 | 1.213 (0.708-2.079) | 0.482 | 1.221 (0.422-3.533) | 0.712 |
| T stage | 0.027 | 0.031 | 0.270 | |||
| T1 | 1 | 1 | 1 | |||
| T2 | 3.164 (1.142-8.765) | 0.027 | 3.072 (1.107-8.519) | 0.031 | 3.102 (0.415-23.187) | 0.270 |
| N stage | <.001 | <.001 | <.001 | |||
| N0 | 1 | 1 | 1 | |||
| N1 | 5.234 (3.624-7.558) | <.001 | 5.675 (3.929-8.195) | <.001 | 6.574 (3.836-11.269) | <.001 |
| Tumor size (mm) | 0.003 | 0.007 | 0.610 | |||
| <50 | 1 | 1 | 1 | |||
| [50-100] | 1.319 (0.437-3.980) | 0.624 | 1.306 (0.433-3.938) | 0.635 | 0.484 (0.061-3.804) | 0.490 |
| >100 | 2.092 (0.684-6.398) | 0.195 | 2.007 (0.656-6.136) | 0.222 | 0.580 (0.071-4.712) | 0.610 |
| Marital status | 0.608 | - | 0.164 | |||
| Married | 1 | 1 | ||||
| Unmarried | 1.101 (0.813-1.490) | 0.536 | 1.494 (0.920-2.427) | 0.104 | ||
| Divorced | 0.893 (0.615-1.298) | 0.553 | 0.825 (0.414-1.645) | 0.586 |
Figure 1.
Three nomograms based on independent significant risk factors for predicting the probability of lung (A), liver (B) and bone (C) metastasis.
Validation of Nomograms for Specific Distant Metastatic Sites
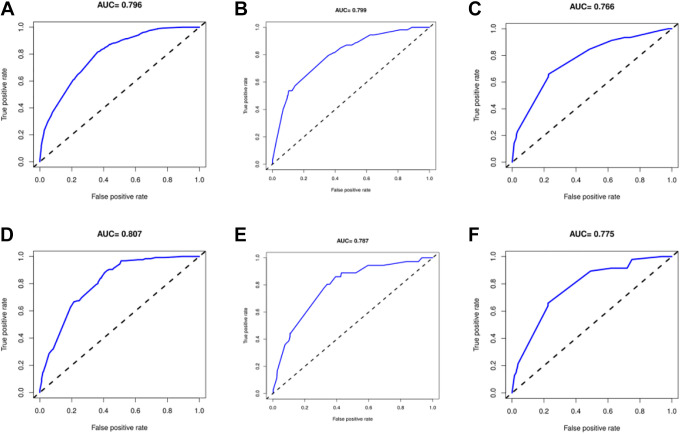
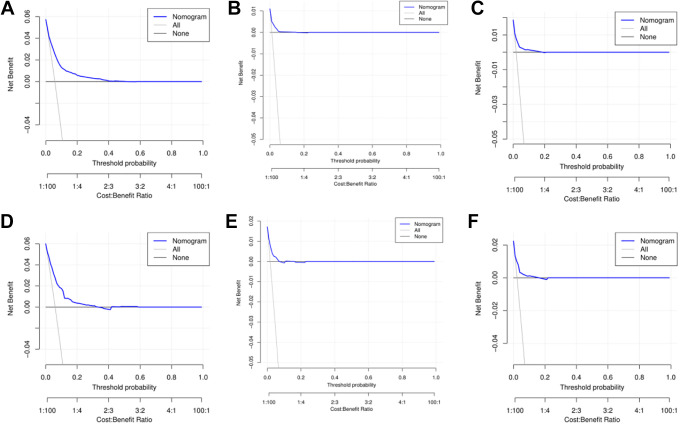
The internal validation of the model indicated that the C-index of the nomograms for predicting lung, liver and bone metastases was 0.796, 0.799, and 0.766, respectively. External validation through the validation cohort showed that the AUCs of the nomograms for lung, liver and bone metastasis prediction were 0.807, 0.787 and 0.775, respectively. The AUCs for lung, liver and bone metastases in the training and validation cohorts are illustrated in Figure 2. Moreover, the calibration curves of predictive lung, liver, and bone metastasis nomograms in the training and validation groups are listed in Figure 3 and show no obvious differences from the ideal line, which demonstrates that the models’ predictions correspond closely to reality. As shown in Figure 4A and 4B, DCAs showed that under a wide range of threshold probabilities, our nomograms yielded higher net benefits in predicting STS lung metastasis. Similarly, nomograms for predicting liver or bone metastatic sites also had high clinical utility (Figure 4 C-F).
Figure 2.
The receiver operating characteristic (ROC) curves of the nomograms of predicting STS patients’ lung (A), liver (B) and bone (C) metastasis in the training group; the ROC curves of the nomograms of predicting STS patients’ lung (D), liver (E) and bone (F) metastasis in the validation group.
Figure 3.
The calibration curves of the nomograms for predicting lung (A), liver (B) and bone (C) in the training group; the calibration curves of the nomograms for predicting lung (D), liver (E) and bone (F) in the validation group.
Figure 4.
Decision curve analysis (DCA) for the nomograms of predicting lung (A), liver (B) and bone (C) in the training group; DCA for the nomograms of predicting lung (D), liver (E) and bone (F) in the validation group.
Establishment and Validation of the Nomogram for Predicting OS
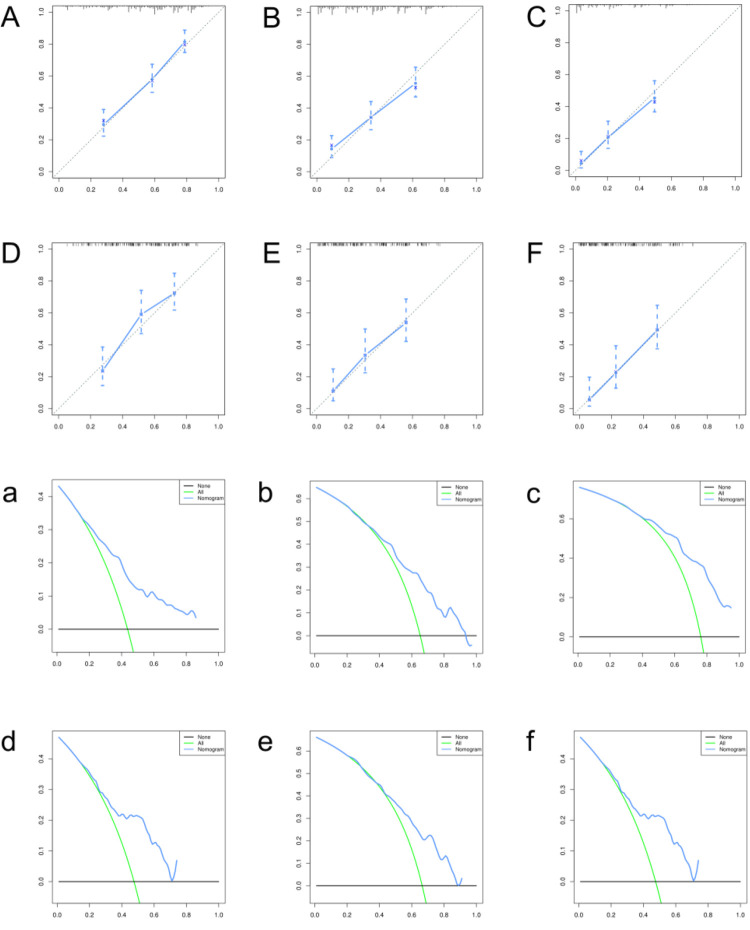
In the univariate Cox regression analysis, 9 variables with a P-value <0.05, including age, tumor grade, histological type, tumor size, patient marital status, surgery, chemotherapy, radiotherapy and lung metastasis, were regarded as important prognostic variables and were included in further analysis. Then, 8 independent prognostic factors related to OS of STS, including age, tumor grade, histological type, tumor size, surgery, chemotherapy, radiotherapy and lung metastasis, were identified by multivariate Cox regression analysis (Table 4). The nomogram of 1-, 2-, and 3-year OS for STS patients is depicted in Figure 5. In the training set, the C-index of the nomogram for OS was 0.710, and the calibration curves illustrated no obvious deviation between the prediction and the actual outcomes, based on which the developed nomogram was judged to have good discrimination and prediction abilities in the internal validation. Similarly, in the validation group, the c-index of the OS nomogram was 0.703, signifying that the predictivity of the nomogram was accurate. The OS predicted by the nomogram of 1-, 2-, and 3 years matched the actual observations, which suggests that the nomogram has satisfactory confidence in different time spans (Figure 6 A-F). DCA showed that if the threshold probability is more than 20% in the first year in the training group (20% from 70% in the validation group), the nomogram to predict the probability yields more net benefits. There are also good net benefits in the second and third year, which means that our nomogram for OS has good applicability.
Table 4.
Survival Analysis of Patients With Soft Tissue Sarcoma.
| Characteristics | Univariate analyses HR (95% CI) |
P-value | Multivariate analyses HR (95% CI) |
P-value |
|---|---|---|---|---|
| Age | ||||
| ≤60 | 1 | 1 | ||
| >60 | 1.792 (1.405-2.286) | <.001 | 1.488 (1.094-2.024) | 0.011 |
| Race | ||||
| White | 1 | |||
| Black | 1.273 (0.918-1.765) | 0.149 | ||
| Other | 0.727 (0.441-1.196) | 0.209 | ||
| Sex | ||||
| Female | 1 | |||
| Male | 1.117 (0.873-1.427) | 0.379 | ||
| Grade | ||||
| I-II | 1 | 1 | ||
| III-IV | 1.025 (1.007-1.044) | 0.006 | 1.029 (1.010-1.049) | 0.003 |
| Primary site | ||||
| head, face, neck | 1 | |||
| upr limb, shoulder | 1.064 (0.532-2.128) | 0.861 | ||
| lower limb, hip | 0.982 (0.540-1.784) | 0.952 | ||
| heart, thorax abdomen pelvis trunk | 0.977 (0.540-1.767) | 0.939 | ||
| Histological type | ||||
| fibrosarcoma | 1 | 1 | ||
| leiomyosarcoma | 1.158 (0.650-2.064) | 0.618 | 0.860 (0.473-1.564) | 0.621 |
| liposarcoma | 0.991 (0.455-2.158) | 0.981 | 0.994 (0.435-2.272) | 0.989 |
| malignant fibrohistiocytoma | 3.029 (1.472-6.234) | 0.003 | 2.712 (1.288-5.709) | 0.009 |
| MPNST | 1.494 (0.710-3.142) | 0.290 | 1.712 (0.786-3.733) | 0.176 |
| synovial sarcoma | 0.871 (0.461-1.645) | 0.670 | 1.075 (0.543-2.129) | 0.835 |
| Other | 1.053 (0.618-1.796) | 0.848 | 0.993 (0.567-1.740) | 0.980 |
| T stage | ||||
| T1 | 1 | |||
| T2 | 1.379 (0.899-2.116) | 0.141 | ||
| N stage | ||||
| N0 | 1 | |||
| N1 | 1.120 (0.829-1.512) | 0.460 | ||
| Tumor size (mm) | ||||
| <50 | 1 | 1 | ||
| [50-100] | 1.099 (0.677-1.785) | 0.701 | 1.138 (0.690-1.877) | 0.611 |
| >100 | 1.639 (1.029-2.613) | 0.038 | 1.768 (1.085-2.880) | 0.022 |
| Insurance status | ||||
| No | 1 | |||
| Yes | 0.936 (0.481-1.820) | 0.845 | ||
| Marital status | ||||
| Married | 1 | 1 | ||
| Unmarried | 0.790 (0.604-1.033) | 0.085 | 0.937 (0.694-1.265) | 0.672 |
| Divorced | 1.441 (1.019-2.038) | 0.039 | 1.041 (0.718-1.511) | 0.830 |
| Surgery | ||||
| No | 1 | 1 | ||
| Yes | 0.494 (0.387-0.632) | <.001 | 0.442 (0.340-0.574) | <.001 |
| Radiatiotherapy | ||||
| No | 1 | 1 | ||
| Yes | 0.588 (0.459-0.752) | <.001 | 0.574 (0.443-0.743) | <.001 |
| Chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 0.665 (0.516-0.855) | 0.002 | 0.620 (0.455-0.844) | 0.002 |
| Lung metastasis | ||||
| No | 1 | 1 | ||
| Yes | 1.622 (1.186-2.218) | 0.002 | 1.562 (1.115-2.186) | 0.009 |
| Liver metastasis | ||||
| No | 1 | |||
| Yes | 1.069 (0.769-1.487) | 0.690 | ||
| Bone metastasis | ||||
| No | 1 | |||
| Yes | 0.833 (0.627-1.106) | 0.207 | ||
| Brain metastasis | ||||
| No | 1 | |||
| Yes | 1.912 (0.982-3.723) | 0.056 |
Figure 5.
The nomogram for predicting the overall survival of patients with soft tissue sarcoma.
Figure 6.
The calibration curves of the nomogram for predicting overall survival at 1-(A), 2-(B) and 3- years(C) in the training group; the calibration curves of the nomogram at 1-(D), 2-(E) and 3 years(F) in the validation group; decision curve analysis (DCA) of the nomogram at 1-(a), 2-(b) and 3 years(c) in the training group; DCA of the nomogram at 1-(d), 2-(e) and 3-years(f) in the validation group.
Discussion
Despite the increasing understanding of STS, treatment is still an insurmountable challenge due to the overall rarity and complexity of STS. Currently, the direction of cancer treatment has changed to improve prognosis. If the OS of patients or even the specific metastatic site can be accurately predicted, clinicians will be better able to develop appropriate treatment plans for STS patients.
In this study, we developed not only 3 nomograms that can predict specific distal metastatic sites but also a nomogram for the 1-, 2-, and 3-year survival probability of STS patients. These nomograms were proven to have satisfactory discrimination power and accuracy in both internal and external validations and can be regarded as a simple and reliable forecasting tool. DCA shows that these nomograms have greater net benefits under a broader threshold, which indicates that the models can be routinely used in clinics to assist physicians in predicting the metastatic sites and prognoses of STS patients.
A prospective study of 951 STS patients showed that histological subtype is an independent predictor of metastatic disease.11 Similarly, a study based on patients in the sarcoma group of the French Cancer Center found that synovial sarcoma and leiomyosarcoma had a high risk of developing into metastatic disease.12 Similarly, we determined that histological type is an independent predictor of lung metastasis and liver metastasis and that synovial sarcoma and leiomyosarcoma are the subtypes with the highest scores for predicting lung metastasis and liver metastasis, respectively. The reason for this outcome may be that histological differentiation can create different biological characteristics of the sarcomas, and metastasis is mainly related to the biological characteristics of the lesion. Komdeur et al.13 proposed that 70% of patients with high-grade STS will develop metastases, which was much higher than for low-grade STS. According to our nomograms for predicting lung, liver and bone metastasis, patients with high-grade STS suffered a higher risk of metastasis and thus a worse prognosis. A previous study asserted that large tumor size was an adverse predictor of distant spread.14 According to the seventh edition of the AJCC staging criteria, the tumors are divided into stages T1 and T2 based on the maximum tumor diameters with a cutoff of 5 cm, and they are also divided into stages N1 and N0 according to the presence or absence of regional lymph node metastasis. A high-grade T stage indicates a larger tumor and deeper infiltration, which may increase the risk of sarcoma cells entering vessels. Tumor size and T stage were embedded in the nomograms in the present study because they have a significant influence in predicting the lung and liver metastases of STSs. Interestingly, we found that tumor size was indeed significantly correlated with lung and liver metastasis of soft tissue sarcoma, but no similar correlation was found for bone metastasis. Similar results were reported by Younis et al.15 Moreover, the nomogram of bone metastasis shows that these predictive factors, histology grade, N stage and primary site, were related to the development of bone metastasis, which is in line with their conclusions. Patients with lymph node metastasis but not synchronized distant metastases are rare, accounting for only approximately 2% of all STS patients.16 David et al.17 supported the notion that lymph node positivity is a predictive sign of biological aggressiveness and distant metastasis. In the nomograms for predicting specific metastatic sites, especially lung metastasis and bone metastasis, higher N stage scores are associated with metastasis, indicating that patients with positive lymph nodes suffer a higher risk of distant metastasis.
Lung metastasis is a predictive sign of poor prognosis in terms of OS. This conclusion has also been verified by Nabeel et al. They reported a remarkable drop in the cure rate of STS patients with pulmonary metastasis, and the long-term survival rates are even less optimistic.18 Ferguson et al. reported similar findings in previous studies, and they found that the 5-year survival rate of patients with lung metastasis is less than 10%.19 In the last stages of life, as the average life expectancy increases, the risk of emerging cancers grows exponentially.20 It is worth noting that in cancer patients, advancing age is a poor prognostic signal for OS because approximately 70% of cancer deaths occur in individuals over 65 years old. In this investigation, age was an independent prognostic factor, and older patients had a worse prognosis. This may be attributable to the fact that old-age-related complications affect their quality of life or even threaten their lives. Another reason may be that they are at greater risk of deleterious effects when receiving treatments such as systemic chemotherapy and surgery. To date, apart from age, many studies have also found that histological type, tumor grade and tumor size have important effects on the prognosis of STSs,21-25 which is concordant with our conclusion. Based on our Cox regression analysis, we found that these indicators were significant independent predictors of OS.
Common clinical treatment methods for soft tissue sarcoma, including surgery, radiotherapy and chemotherapy, are all confirmed by our OS nomogram as independent prognostic indicators. For diseases without metastasis, the widely accepted strategy is surgery combined with or without adjuvant radiotherapy.26,27 Some studies have shown that even for patients with metastases (such as lung and liver metastases), active surgical treatment can still achieve a good survival rate.28-31 As either a supplement to surgery or a type of palliative treatment, radiotherapy can effectively relieve pain in STS patients.19 In a study of 9068 retroperitoneal STS patients, Nussbaum et al.32 indicated that surgery combined with adjuvant radiotherapy can improve patient survival in comparison to surgery alone. A multicenter retrospective study involving 8249 patients concluded that radiotherapy and surgery as independent parameters have significant predictive value for the prognosis of STS patients,33 consistent with our experience. Chemotherapy is an important treatment for patients who cannot tolerate the conditions of surgery.34 However, the effect of chemotherapy on improving OS in primary STS is a long-standing controversy. The most impressive experiments by Frustaci et al.35 proved that the OS of patients receiving adjuvant chemotherapy was significantly improved. Grobmyer et al. suggest that neoadjuvant chemotherapy can improve cancer-specific survival by slowing the progression of metastasis.36 Moreover, a considerable number of patients with advanced STS can survive in the long term after receiving anthracycline chemotherapy and may even be completely cured.37 Overall, our nomogram indicated that these 3 conventional treatments are beneficial to the survival of STS patients.
In recent years, many studies on the prognosis of STS have been launched. Some researchers reported that their nomograms can provide patients with more individualized and accurate treatment strategies than traditional methods. However, some studies had not taken into consideration the influence of the treatment-related factors or the combining of metastasis and clinicopathological information, and some was unable to predict specific metastasis, which may greatly affect the survival of STS patients. Moreover, as far as we know, we have constructed the first nomogram to predict the specific metastasis sites of soft tissue sarcoma, which take a step further than only predicting the occurrence of distant metastasis. For example, clinicians can easily evaluate the risk of lung metastasis by calculating the scores of 5 variables: tumor size, T and N stages, grade and histological type. With the nomogram, the prognosis of patients with high risk of lung metastasis can get effectively improved, to whom examinations of specific sites are supposed to be enhanced and more targeted treatment can be provided. Similarly, for patients with low risk metastasis, certain preventive measures can be reduced to lessen the the risk of side effects and economic burden.
Inevitably, there are several limitations in the present study that should be mentioned. First, although as much relevant information as possible is included, since this is a retrospective study, there are still some selection biases that cannot be avoided. Second, some key information in the SEER database is missing, such as the specific surgery performed, radiotherapy methods used, surgical margin status, chemotherapy drugs administered, biological marker status, etc. Finally, there were unfortunately too few samples with brain metastases to develop a nomogram. Therefore, future data collection should be more detailed and comprehensive to reduce biases, and more data should be collected in a wider range of patients and over a longer time period to establish a nomogram of brain metastasis. In addition, to further validate our nomograms, prospective studies should be performed.
Conclusions
The nomograms constructed based on a retrospective study of more than 7000 cases is used to predict specific metastasis sites and OS, with good discrimination, accuracy and clinical applicability. The models could accurately predict the metastatic risk and survival information and help clinical decision-making.
Abbreviations
- STS
Soft tissue sarcoma
- OS
Overall survival
- SEER
Surveillance, Epidemiology, and End Results
- HR
Hazard ratio
- ROC
Receiver operating characteristic
- AUC
area under curve
- C-index
Concordance index
- DCA
Decision curve analysis.
Footnotes
Authors’ Contributions: QH T and C H conceived and designed the study. QH T collected data. QH T, C H and H Z analyzed data and provided statistical recommendations. PC, M K conducted literature search. C H and XM S generated the tables and figures. QH T and YJ W wrote the manuscript. C H, YJ W, and C Z helped to critically review and comprehensive revise the manuscript. XX M supervised the research. All authors contributed to the research and approved the final version of this article.
Availability of Data and Materials: The data analyzed during the current study are available from the SEER data set repository, or the corresponding author upon reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: The study protocol was approved by the SEER program from the National Cancer Institute, US (reference number 15260-Nov2018). There’s no need for informed consent in our study since the unidentified data were free from medical ethics review.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Natural Science Foundation of China (81871804 and 81672200); the National Key Research and Development Program of China (2019YFC0121400).
ORCID iD: QiHao Tu  https://orcid.org/0000-0002-3289-9618
https://orcid.org/0000-0002-3289-9618
References
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA A Cancer J Clin. 2010, 60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 2. Li L, Bai Z, Zhang L, Zhang Y, et al. Meta-analysis of hematological biomarkers as reliable indicators of soft tissue sarcoma prognosis. Front Oncol. 2020;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Mehren M, Randall R, Benjamin R, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Nati Compr Canc Net. 2018, 16(5):536–563. [DOI] [PubMed] [Google Scholar]
- 4. Callegaro D, Miceli R, Mariani L, Raut C, Gronchi A. Soft tissue sarcoma nomograms and their incorporation into practice. Cancer. 2017;123(15):2802–2820. [DOI] [PubMed] [Google Scholar]
- 5. Krishnan C, Kim H, Park J, Han I. Outcome after surgery for extremity soft tissue sarcoma in patients presenting with metastasis at diagnosis. Am J Clin Oncol. 2018;41(7):681–686. [DOI] [PubMed] [Google Scholar]
- 6. Italiano A, Mathoulin-Pelissier S, Cesne A, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117(5):1049–1054. [DOI] [PubMed] [Google Scholar]
- 7. Karavasilis V, Seddon B, Ashley S, Al-Muderis O, Fisher C, Judson I. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112(7):1585–1591. [DOI] [PubMed] [Google Scholar]
- 8. Van Glabbeke M, van Oosterom A, Oosterhuis J, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol. 1999;17(1):150–157. [DOI] [PubMed] [Google Scholar]
- 9. Du X, Wei H, Zhang P, Yao W, Cai Q. Heterogeneity of soft tissue sarcomas and its implications in targeted therapy. Front Oncol. 2020;10:564852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tseng W, Pasquali S, Hu J, Menendez L, Gronchi A. Staging systems and nomograms in soft tissue sarcoma: outcome prediction by categorization or personalization? Chin Clin Oncol. 2019;8(S1):S12. [DOI] [PubMed] [Google Scholar]
- 11. Koea J, Leung D, Lewis J, Brennan M. Histopathologic type: an independent prognostic factor in primary soft tissue sarcoma of the extremity? Ann Surg Oncol. 2003;10(4):432–440. [DOI] [PubMed] [Google Scholar]
- 12. Coindre J, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French federation of cancer centers sarcoma group. Cancer. 2001;91(10):1914–1926. [DOI] [PubMed] [Google Scholar]
- 13. Komdeur R, Hoekstra H, van den Berg E, et al. Metastasis in soft tissue sarcomas: prognostic criteria and treatment perspectives. Cancer Metastasis Rev. 2002;21(2):167–183. [DOI] [PubMed] [Google Scholar]
- 14. Torosian M, Friedrich C, Godbold J, Hajdu S, Brennan M. Soft-tissue sarcoma: initial characteristics and prognostic factors in patients with and without metastatic disease. Semin Surg Oncol. 1988;4(1):13–19. [DOI] [PubMed] [Google Scholar]
- 15. Younis M, Summers S, Pretell-Mazzini J. Bone metastasis in extremity soft tissue sarcomas: risk factors and survival analysis using the SEER registry. Musculoskelet Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 16. Keung E, Chiang Y, Voss R, et al. Defining the incidence and clinical significance of lymph node metastasis in soft tissue sarcoma. Eur J Surg Oncol. 2018;44(1):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johannesmeyer D, Smith V, Cole D, Esnaola N, Camp E. The impact of lymph node disease in extremity soft-tissue sarcomas: a population-based analysis. Am J Surg. 2013;206(3):289–295. [DOI] [PubMed] [Google Scholar]
- 18. Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113(3):573–581. [DOI] [PubMed] [Google Scholar]
- 19. Ferguson P, Deheshi B, Chung P, et al. Soft tissue sarcoma presenting with metastatic disease: outcome with primary surgical resection. Cancer 2011;117(2):372–379. [DOI] [PubMed] [Google Scholar]
- 20. DePinho R. The age of cancer. Nature. 2000;408(6809):248–254. [DOI] [PubMed] [Google Scholar]
- 21. Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31(13):1649–1655. [DOI] [PubMed] [Google Scholar]
- 22. Kattan M, Leung D, Brennan M. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20(3):791–796. [DOI] [PubMed] [Google Scholar]
- 23. Mariani L, Miceli R, Kattan M, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer 2005;103(2):402–408. [DOI] [PubMed] [Google Scholar]
- 24. Callegaro D, Miceli R, Bonvalot S, et al. Development and external validation of two nomograms to predict overall survival and occurrence of distant metastases in adults after surgical resection of localised soft-tissue sarcomas of the extremities: a retrospective analysis. Lancet Oncol. 2016;17(5):671–680. [DOI] [PubMed] [Google Scholar]
- 25. Fisher S, Chiang Y, Feig B, et al. Comparative performance of the 7th and 8th editions of the American Joint Committee on cancer staging systems for soft tissue sarcoma of the trunk and extremities. Ann Surg Oncol. 2018;25(5):1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyer M, Seetharam M. First-line therapy for metastatic soft tissue sarcoma. Curr Treat Options Oncol. 2019;20(1):6. [DOI] [PubMed] [Google Scholar]
- 27. Zhang S, Wang Z, Wang W, Wang X, Zhou Y. Novel nomograms individually predict the survival of patients with soft tissue sarcomas after surgery. Cancer Manag Res. 2019;11:3215–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rehders A, Peiper M, Stoecklein N, et al. Hepatic metastasectomy for soft-tissue sarcomas: is it justified? World J Surg. 2009;33(1):111–117. [DOI] [PubMed] [Google Scholar]
- 29. Grimme F, Seesing M, van Hillegersberg R, et al. Liver resection for hepatic metastases from soft tissue sarcoma: a nationwide study. Dig Surg. 2019;36(6):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Predina J, Puc M, Bergey M, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol. 2011;6(5):913–919. [DOI] [PubMed] [Google Scholar]
- 31. Keung E, Fairweather M, Raut C. Surgical management of metastatic disease. Surg Clin North Am. 2016;96(5):1175–1192. [DOI] [PubMed] [Google Scholar]
- 32. Nussbaum D, Rushing C, Lane W, Cardona D, Kirsch D, Peterson B, Blazer D. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17(7):966–975. [DOI] [PubMed] [Google Scholar]
- 33. Gutierrez J, Perez E, Franceschi D, Moffat F, Livingstone A, Koniaris L. Outcomes for soft-tissue sarcoma in 8249 cases from a large state cancer registry. J Surg Res. 2007;141(1):105–114. [DOI] [PubMed] [Google Scholar]
- 34. Frezza A, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med. 2017;15(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frustaci S, Gherlinzoni F, De Paoli A, et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19(5):1238–1247. [DOI] [PubMed] [Google Scholar]
- 36. Grobmyer S, Maki R, Demetri G, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004, 15(11):1667–1672. [DOI] [PubMed] [Google Scholar]
- 37. Blay J, van Glabbeke M, Verweij J, et al. Advanced soft-tissue sarcoma: a disease that is potentially curable for a subset of patients treated with chemotherapy. Eur J Cancer. 2003;39(1):64–69. [DOI] [PubMed] [Google Scholar]