Abstract
Background:
It was controversial that whether LUAD patients with brain metastases (BMs) and EGFR sensitive mutations should be conducted using brain radiotherapy when treated with first-generation EGFR-TKI. Herein, a retrospective study was designed to compare the efficacy of first-generation EGFR-TKI combined with brain radiotherapy and EGFR-TKI alone as first-line treatment for these LUAD patients.
Patients and Methods:
We retrospectively analyzed the status of patients with advanced LUAD carrying EGFR sensitive mutations who received first-generation EGFR-TKI treatment in our center. iPFS was the first time of intracranial progression or death from the diagnosis of BMs, PFS was the time of progression of any site or death from the diagnosis of BMs, and OS was the time of confirmed BMs to death or the last follow-up time. Differences in characteristics between groups were compared using the Chi-square test. The Kaplan-Meier method was used to calculate the iPFS, PFS, and OS. Univariate analysis, multivariate analysis, and subgroup analysis were conducted by Cox regression model.
Results:
There were 77 patients (77/134, 57.5%) in the TKI + RT group and 57 patients (57/134, 42.5%) in the TKI group. TKI + RT group had a significant higher intracranial ORR and DCR, and the combination therapy was independently significantly associated with a longer iPFS (18.9 vs. 10.5 months, P = 0.0009), systematic PFS (12.5 vs. 8.4 months, P = 0.0071) and OS (30.8 vs. 22.7 months, P = 0.0183). Females, non-smokers, and younger patients benefited more from the combination therapy. Subgroup analysis demonstrated that the combination therapy could improve the iPFS in patients with more than 3 BMs (P = 0.005); however, it couldn’t improve the OS for these patients.
Conclusion:
Our study confirmed the effect of the combination of EGFR-TKI and brain radiotherapy as first-line treatment for LUAD patients with BMs and EGFR sensitive mutations.
Keywords: LUAD, EGFR, TKI, brain metastases, radiotherapy
Introduction
Lung cancer is the first leading cause of cancer-related death, and its 5-year survival rate is only 19%.1 And non-small cell lung cancer (NSCLC) is the most routine type, accounting for about 85% of all lung cancer patients.2 For NSCLC, brain metastases (BMs) are one of the most common metastatic sites.3 According to reports, when NSCLC occurred, 20%-40% of the patients would suffer from BMs.4 Once the BMs occurred, the patients’ quality of life would decline and the median overall survival time (OS) was only 6 months.5 The epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor on the cell surface. Mutations of EGFR make it continuously activated, causing unlimited growth of cells, and then tumors formed.6 Sensitizing EGFR mutations are the most common actionable driver mutations found in patients with NSCLC, which was considered predictive of sensitivity to EGFR- tyrosine kinase inhibitor (TKI) therapy.7 For EGFR sensitive mutant patients, the rate of BMs was even higher, almost 31.4% to 64.7% of NSCLC patients with EGFR sensitive mutations developed in BMs while it was accounted for 19.7% to 35.3% for EGFR wild type patients.4,8,9 It was hard for traditional macromolecular drugs and antibodies to cross the blood-brain barrier (BBB) to locally form a higher drug concentration, which increases the difficulty of treatment for patients with BMs.10
It is recognized that the excellent curative effect of EGFR-TKI makes it the first choice for patients with advanced NSCLC with EGFR sensitive mutations.11-13 However, to date, there is no standard treatment strategy for patients with EGFR mutations and BMs. In the past, brain radiotherapy, including whole brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS), was the most important strategy for BMs.14,15 However, in the era of targeted therapy, the necessity and intervetion timing of local therapy have been questioned.16 The study named BRAIN compared the efficacy of icotinib and WBRT +/- chemotherapy for patients with EGFR mutations and BMs. The results revealed that icotinib significantly prolonged the intracranial progress-free survival (iPFS), and EGFR-TKI was recommended as the first-line treatment for patients with EGFR-mutation and BMs.17 However, the patients in this study had a limited burden of tumors, and only a small part of them had brain symptoms. In the real world, it was still controversial whether the EGFR-TKI alone could achieve the best effect. Clinicians had different opinions on this issue. Some of them believe that brain radiotherapy should be intervened after the progression of intracranial disease, and premature radiotherapy is considered overtreatment. However, another recommendation is to intervene in brain radiotherapy as soon as possible.16 This study aims to compare the clinical efficacy of first-generation EGFR-TKI combined brain radiotherapy with EGFR-TKI alone for lung adenocarcinoma (LUAD) patients with EGFR sensitive mutations and BMs.
Materials and Methods
Patients
We retrospectively reviewed the patients of newly diagnosed or recurrent after radical resection of NSCLC between Sep 2015 to May 2019 in the Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China). The patients who met the following inclusion criteria were included: (1) pathological confirmed, (2) computed tomography (CT) or magnetic resonance imaging (MRI) examination of the brain to confirm the presence of BMs before treatment, (3) carrying EGFR sensitive mutations, (4) receiving first-generation EGFR-TKI as first-line treatment. Patients who met the following exclusion criteria were excluded: (1) with other malignant tumors or chronic inflammatory diseases at the same time or before, (2) with insertion mutations in exon 20, (3) with the history of EGFR-TKI or brain radiotherapy, (4) the follow-up time not more than 6 months.
The study was conducted in compliance with the Helsinki Declaration of 1975 and was approved by the Institutional Review Board of Tongji Medical College of Huazhong University of Science and Technology.
Treatment Method
All patients received first-line EGFR-TKI treatment: icotinib po 125 mg tid; gefitinib 250 mg po qd; erlotinib 150 mg po qd. Adjusting the dose according to the doctor’s advice when adverse reactions and drug resistance occurred. On the other hand, for the patients who received brain radiotherapy, the individualized radiotherapy regimen was chosen according to the patient’s condition, including WBRT (2-3.6Gy/F, 10-15F), WBRT combined with SRS (6-13Gy/F, 1-3F) / IMRT (2-3Gy/F, 7-15F), or SRS (8-15Gy/F, 1-3F) alone.
Data Collection
Patient characteristics including gender, age, smoking history, brain metastasis status, Karnofsky Performance Status (KPS) score, EGFR mutation status, and treatment status were obtained from the electronic medical record system of the Cancer center, Union Hospital of Tongji Medical College, Huazhong university of Science and Technology. The last follow-up time was Jan 20, 2020. The efficacy evaluation was conducted using Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST 1.1) standard: complete response (CR) was defined as disappearance of all target lesions, partial response (PR) was defined as at least a 30% decrease in the sum of the diameters of target lesions, progressive disease (PD) was defined as at least a 20% increase in the sum of diameters of target lesions or appearance of one or more new lesions, and stable disease (SD) was between PR and PD.18 iPFS was defined as the first time of intracranial progression or death from the diagnosis of brain metastases, PFS was defined as the time of progression of any site or death from the diagnosis of brain metastasis, and overall survival (OS) was defined as the time of confirmed brain metastases to death or the last follow-up time. It was defined as censored data if the event had not occurred by the last follow-up time.
Statistical Analysis
Differences between groups were compared using the Chi-square test. Survival probabilities were estimated using the Kaplan-Meier method and a log-rank test to compare the differences between groups. Univariate analysis, multivariate analysis, and subgroup analysis were conducted by Cox regression model. In the multivariate Cox regression model including variables with P < 0.01 in the univariate Cox regression, but the variable of symptoms was excluded because of the relevance to brain radiotherapy. All statistical analyses were calculated using SPSS 24.0. Drawings were performed with GraphPad Prism 7.0 or Microsoft Excel for Mac 16.25. A 2-sided P value <0.05 was considered statistically significant.
Results
Patient Characteristics
The baseline characteristics and treatment information for the patients are listed in Table 1 . 134 LUAD patients were enrolled for the analyses totally, 77 (57.5%) of them were treated with first-generation EGFR-TKI combined with brain radiotherapy (TKI + RT group) while 57 (42.5%) patients were treated with first-generation EGFR-TKI alone (TKI group). The median patient age was 56 (range 30-83) years, most of them were female (63.4%) and non-smokers (70.1%).
Table 1.
Baseline Demographics and Characteristics of All Patients.
| Characteristics | TKI + RT group | TKI group | P value |
|---|---|---|---|
| (n = 77) | (n = 57) | ||
| Age | |||
| ≤60 | 50 (64.9) | 41 (71.9) | 0.391 |
| >60 | 27 (35.1) | 16 (28.1) | |
| Gender | |||
| Male | 30 (39.0) | 19 (33.3) | 0.504 |
| Female | 47 (61.0) | 38 (66.7) | |
| Smoking | |||
| Never | 55 (71.4) | 39 (68.4) | 0.707 |
| Current / ex-smoking | 22 (28.6) | 18 (31.6) | |
| KPS score | |||
| ≤80 | 39 (50.6) | 28 (49.1) | 0.861 |
| >80 | 38 (49.4) | 29 (50.9) | |
| T stage | |||
| 1 | 16 (20.8) | 14 (24.6) | 0.072 |
| 2 | 25 (32.5) | 21 (36.8) | |
| 3 | 10 (13.0) | 2 (3.5) | |
| 4 | 22 (28.5) | 11 (19.3) | |
| X | 4 (5.2) | 9 (15.8) | |
| N stage | |||
| 0 | 6 (7.8) | 3 (5.3) | 0.192 |
| 1 | 19 (24.7) | 10 (17.5) | |
| 2 | 18 (23.4) | 9 (15.8) | |
| 3 | 32 (41.5) | 29 (50.9) | |
| X | 2 (2.6) | 6 (10.5) | |
| Number of brain metastases | |||
| ≤3 | 28 (36.4) | 32 (56.1) | 0.023* |
| >3 | 49 (63.6) | 25 (43.9) | |
| Symptoms | |||
| No | 38 (49.4) | 48 (84.2) | 0.000* |
| Yes | 39 (50.6) | 9 (15.8) | |
| Extracranial distant metastases | |||
| No | 31 (40.3) | 18 (31.6) | 0.302 |
| Yes | 46 (59.7) | 39 (68.4) | |
| EGFR mutation | |||
| Del-19 | 37 (48.0) | 27 (47.4) | 0.561 |
| 21-L858R | 36 (46.8) | 29 (50.9) | |
| Other | 4 (5.2) | 1 (1.7) | |
| EGFR TKI | |||
| Erlotinib | 14 (18.2) | 5 (8.8) | 0.19 |
| Icotinib | 39 (50.6) | 37 (64.9) | |
| Gefitinib | 22 (28.6) | 15 (26.3) | |
| Other | 2 (2.6) | 0 (0.0) | |
| Combination of CT | |||
| No | 49 (63.6) | 34 (59.6) | 0.638 |
| Yes | 28 (36.4) | 23 (40.4) | |
| Combination of ADD | |||
| No | 63 (81.8) | 52 (91.2) | 0.123 |
| Yes | 14 (18.2) | 5 (8.8) |
TKI, tyrosine kinase inhibitor; RT, radiotherapy; KPS, Karnofsky Performance Status; EGFR, epidermal growth factor receptor; CT, Chemotherapy; AAD, anti-angiogenic drugs; *P < 0.05 indicated statistical significance.
All patients were carrying EGFR sensitive mutations and received first-generation EGFR-TKI as first-line treatment. The patient demographics and characteristics were well-balanced in most variables such as gender, age, KPS, smoking history, and so on. However, the TKI + RT group had more brain metastases sites (more than 3 brain metastases sites: 63.6% vs. 43.9%, P = 0.023) and apparently symptoms (50.6% vs. 19.8%, P < 0.001) of intracranial hypertension.
The Best Response Before Progression
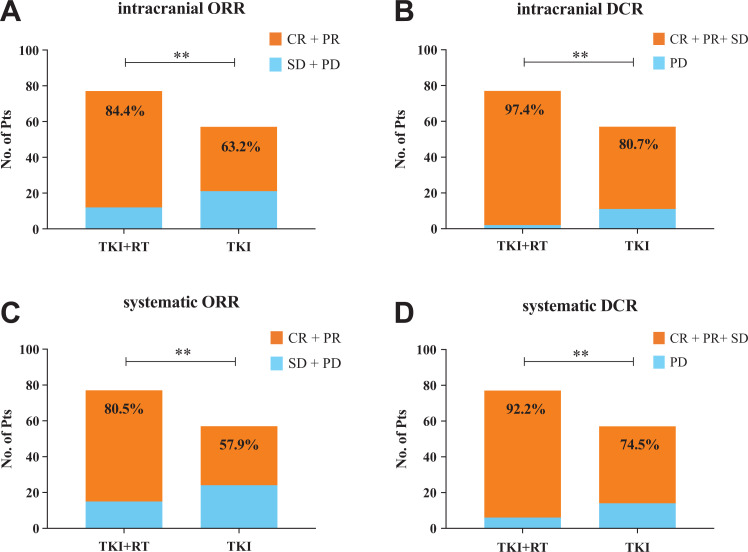
The best response before progression was judged according to RECIST 1.1 (Table S1). Then we compared the objective response rate (ORR) and disease control rate (DCR) of intracranial and systematic between groups. As shown in Figure 1 , TKI + RT group had a significant higher intracranial ORR (84.4% vs. 63.2%, P < 0.01) and DCR (97.4% vs. 80.7%, P < 0.01) than TKI group. At the same time, the combination of first-generation EGFR-TKI and brain radiotherapy has brought benefits for systematic disease control.
Figure 1.
The objective efficacy of 2 groups. The best response of intracranial and systematic was judged according to RECIST 1.1. The chi-square test was used to compare the ORR and DCR between the 2 groups. A-D showed the differences of intracranial ORR, intracranial DCR, systematic ORR and systematic DCR respectively between groups. No. of Pts, number of patients; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate. **P < 0.01.
The Differences in Survival Probability Between TKI + RT Group and TKI Group
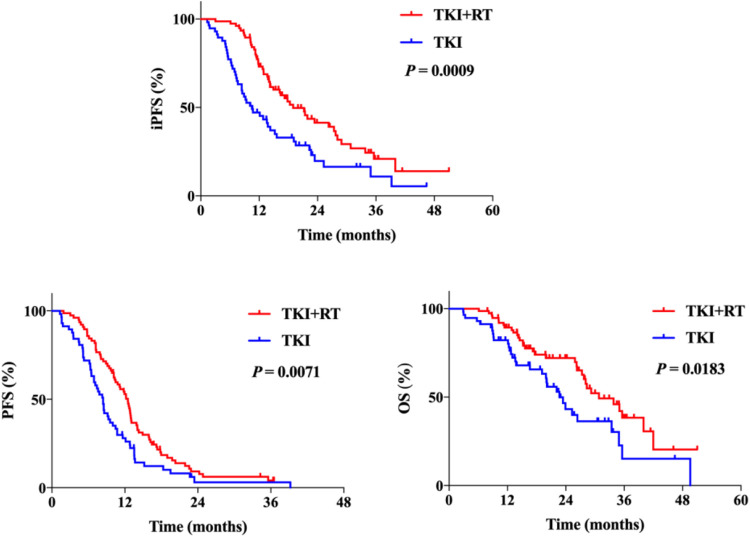
With the median follow-up time of 27.9 months (95%CI 22.0-33.8 months), there were 93 patients (69.4%) had intracranial progression, 124 patients (92.5%) had systematic progression and 65 (48.5%) of them died. The median iPFS, PFS, and OS were 18.9 months, 12.5 months, and 30.8 months in group TKI + RT, while in TKI group hey were 10.5 months, 8.4 months, and 22.7months, respectively. Kaplan-Meier survival curves were plotted for iPFS, PFS, and OS ( Figure 2 ), showing the combination of EGFR-TKI and brain radiotherapy was significantly associated with longer iPFS (P = 0.0009), systematic PFS (P = 0.0071) and OS (P = 0.0183). The HRs were 0.510 (95% CI 0.330-0.787) for iPFS, 0.622 (95%CI 0.427-0.906) for PFS and 0.564 (95%CI 0.340-0.938) for OS.
Figure 2.
The Kaplan-Meier survival curves of iPFS, PFS and OS. iPFS, intracranial progression-free survival; PFS, progression-free survival; OS, overall survival.
Key Subgroups Analyses
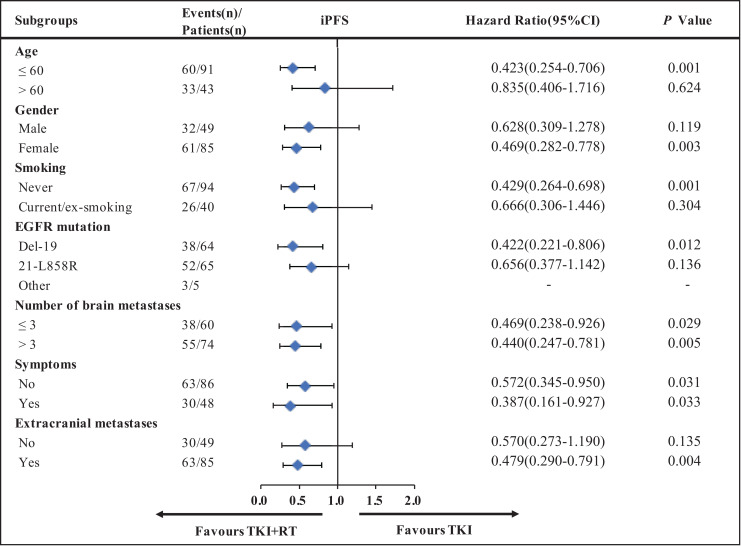
To explore the beneficial populations for combination therapy, HRs and associated 95% CIs were assessed using the Cox proportional hazards model for subgroup analyses. Variables of age (≤60 years or >60 years), gender (male or female), smoking status (never or current/ex-smoking), EGFR mutation status (Del-19, 21-L858 R), number of brain metastases (≤3 or >3), symptoms (yes or no) and extracranial metastases (yes or no) have been taken into consideration. TKI + RT was better than TKI concerned to iPFS ( Figure 3 ) in most of the subgroups examined. Especially for females, non-smokers, and younger patients, TKI + RT prolonged iPFS, PFS, and OS ( Figure 3 , Figure S1). However, the combination of EGFR-TKI and brain radiotherapy could only prolong the iPFS and PFS for patients with more brain metastases, but it couldn’t improve the overall survival.
Figure 3.
Key subgroup analyses for iPFS. iPFS, intracranial progression-free survival; CI, confidence interval.
Univariate and Multivariate Analysis of Survival Probabilities for Patients Treated with First-Generation of EGFR-TKI
Univariate analysis identified EGFR Del-19, brain radiotherapy, erlotinib, and a combination of chemotherapy or antiangiogenic drugs as being significantly associated with better iPFS. Multivariate analysis revealed brain radiotherapy as an independent predictive factor for better iPFS (HR 0.517, 95%CI 0.339-0.789, P = 0.002), as well as EGFR Del-19, erlotinib and a combination of chemotherapy or antiangiogenic drugs ( Table 2 ).
Table 2.
Univariate Analysis and Multivariate Analysis of iPFS.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | ||||||
| ≤60 | 1 | |||||
| >60 | 1.135 | 0.741-1.741 | 0.56 | |||
| Gender | ||||||
| Male | 1 | |||||
| Female | 1.399 | 0.905-2.161 | 0.13 | |||
| Smoking | ||||||
| Never | 1 | |||||
| Current/ex-smoking | 0.718 | 0.455-1.133 | 0.155 | |||
| KPS | ||||||
| ≤80 | 1 | |||||
| >80 | 0.771 | 0.512-1.160 | 0.212 | |||
| Number of brain metastases | ||||||
| ≤3 | 1 | |||||
| >3 | 1.249 | 0.824-1.894 | 0.295 | |||
| Symptoms | ||||||
| No | 1 | |||||
| Yes | 0.72 | 0.465-1.113 | 0.139 | |||
| Extracranial metastases | ||||||
| No | 1 | |||||
| Yes | 1.3 | 0.841-2.010 | 0.238 | |||
| EGFR mutation | ||||||
| Del-19 | 1 | 1 | ||||
| 21-L858R | 1.766 | 1.158-2.693 | 0.008 | 1.875 | 1.212-2.899 | 0.005 |
| Other | 1.114 | 0.339-3.664 | 0.859 | 2.169 | 0.615-7.656 | 0.229 |
| Brain radiotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.506 | 0.336-0.762 | 0.001 | 0.517 | 0.339-0.789 | 0.002 |
| EGFR TKI | ||||||
| Erlotinib | 1 | 1 | ||||
| Icotinib | 2.341 | 1.202-4.562 | 0.012 | 2.905 | 1.418-5.952 | 0.004 |
| Gefitinib | 2.005 | 0.983-4.089 | 0.056 | 2.536 | 1.179-5.458 | 0.017 |
| Other | 1.016 | 0.223-4.630 | 0.983 | 2.337 | 0.482-11.323 | 0.292 |
| Combination of CT or AAD | ||||||
| No | 1 | 1 | ||||
| Yes | 0.627 | 0.413-0.951 | 0.028 | 0.528 | 0.343-0.812 | 0.004 |
HR, hazard ratio; CI, confidence interval; KPS, Karnofsky Performance Status; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; CT, Chemotherapy; AAD, anti-angiogenic drugs.
For systematic PFS and OS, multivariate analysis showed that brain radiotherapy, erlotinib, and combination of chemotherapy or antiangiogenic drugs were independent predictive factors. What’s more, EGFR Del-19 was also associated with better OS. (Table S2, Table S3).
Discussion
EGFR-TKI is the first choice for NSCLC patients with EGFR sensitive mutations,19 but drug resistance is an inevitable challenge. Almost 30% of patients who can’t receive back-line treatment after first-line treatment of EGFR-TKI as a study reported.20 It suggests that the importance of extending the resistance time of first-line EGFR-TKI. Several studies have demonstrated the efficacy of radiotherapy combined with EGFR-TKI.21,22 However, it’s still controversy about the use of EGFR-TKI combined brain radiotherapy, especially in first-line treatment. A multi-institution retrospective study suggested that EGFR-TKI combined with brain radiotherapy (whether SRS or WBRT) could significantly prolong the overall survival of patients (46 vs. 30 vs. 25 months, P < 0.001).23 While another study which compared the efficacy of EGFR-TKI + WBRT with EGFR-TKI alone, and found that a combination of WBRT did not improve the intracranial local control rate of patients with EGFR sensitive mutations, nor did it bring long-term survival benefits.24 Our study proved the efficacy of first-generation EGFR-TKI combined with brain radiotherapy as a first-line treatment for LUAD patients with EGFR mutations and BMs. BBB is the main reason that affects the concentration of drug in the cerebrospinal fluid. Studies have found that brain radiotherapy can destroy BBB and increase the concentration of TKIs.25,26 Besides, EGFR mutant is a radiotherapy sensitive gene, and EGFR-TKI plays a role in radio sensitization.27 This may be the mechanism by which EGFR-TKI combined with brain radiotherapy can improve the local control rate.
WBRT used to be the main treatment for brain metastases from non-small cell lung cancer, which can prolong survival to a certain extent.14 However, the role of WBRT is gradually weakened because of the serious side effects. In our study, we didn’t observe adverse reactions above grade 3. Unfortunately, we were unable to understand the long-term cognitive function differences between the 2 groups because we were unable to fill in relevant scales in the retrospective study. But it was reported that the side effects were under control in previous researches.28,29 And the hippocampus protection technology and neuroprotective drugs can also reduce the neurological damage caused by radiotherapy.30,31
We have a positive attitude towards combination therapy. As Table 1 showen, patients with symptoms and more brain lesions were more likely to be considered for early brain radiotherapy. However, the outcome suggested that EGFR-TKI combined with brain radiotherapy could improve iPFS regardless of symptoms or number of brain metastases. In addition, chemotherapy and antiangiogenic drugs could also bring benefits to LUAD patients with EGFR-mutation and BMs. A recent meta-analysis compared the anti-VEGF plus erlotinib vs erlotinib alone as first-line therapy for advanced NSCLC harboring an EGFR mutation, and it revealed that the combination therapy was significantly associated with prolonged PFS.32 In addition, chemotherapy combined with TKI therapy could also increase efficacy.33 More evidence is needed to explore which combination therapy is more effective for patients with BMs.
However, there are some limitations in our study. First of all, it was a retrospective study, the selection bias and recall bias could not be avoided. Besides, the side effects of combination therapy such as cognitive dysfunction couldn’t be assessed for we unable to fill in the relevant scale. What’s more, the order of TKI and radiotherapy is still an important question to solve. We are looking forward to more evidence to support our conclusions.
Conclusion
In conclusion, this study showed the combination of first-generation EGFR-TKI with brain radiotherapy as first-line treatment may bring benefits to LUAD patients harboring EGFR sensitive mutations and BMs.
Supplemental Material
Supplemental Material, sj-pdf-1-tct-10.1177_1533033821997819 for The Efficacy of First-Generation EGFR-TKI Combined With Brain Radiotherapy as the First-Line Treatment for Lung Adenocarcinoma Patients With Brain Metastases and EGFR Sensitive Mutations: A Retrospective study by Yuting Liu, Juanjuan Wang, Jingjing Wu, Qifan Yang, Yulan Zeng, Di Wu, Chen Tian, Yue Hu, Feifei Gu, Chang Li, Kai Zhang and Li Liu in Technology in Cancer Research & Treatment
Footnotes
Authors’ Note: The study was conducted in compliance with the Helsinki Declaration of 1975 and was approved by the Institutional Review Board of Tongji Medical College of Huazhong University of Science and Technology [S224]. All of the patients had written/verbal consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the National Key Research and Development Program of China (2016YFC1303800) and National Natural Science Foundation of China (81773056).
ORCID iD: Yuting Liu, MD  https://orcid.org/0000-0001-9175-514X
https://orcid.org/0000-0001-9175-514X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Travis WD, Brambilla E, Nicholson AG. et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. [DOI] [PubMed] [Google Scholar]
- 3. D’antonio C, Passaro A, Gori B. et al. Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol. 2014;(3):101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shin DY, Kim CH, Park S. et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9(2):195–199. [DOI] [PubMed] [Google Scholar]
- 5. Waqar SN, Samson PP, Robinson CG. et al. Non-small-cell lung cancer with brain metastasis at presentation. Clin Lung Cancer. 2018;19(4):e373–e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12(18):5268–5272. [DOI] [PubMed] [Google Scholar]
- 7. Castellanos E, Feld E, Horn L. Driven by mutations: the predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12(4):612–623. [DOI] [PubMed] [Google Scholar]
- 8. Iuchi T, Shingyoji M, Itakura M. et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol. 2015;20(4):674–679. [DOI] [PubMed] [Google Scholar]
- 9. Pechoux CL, Dhermain F, Besse B. Whole brain radiotherapy in patients with NSCLC and brain metastases. Lancet. 2016;388(10055):1960–1962. [DOI] [PubMed] [Google Scholar]
- 10. Addeo R, De Rosa C, Faiola V. et al. Phase 2 trial of temozolomide using protracted low-dose and whole-brain radiotherapy for nonsmall cell lung cancer and breast cancer patients with brain metastases. Cancer. 2008;113(9):2524–2531. [DOI] [PubMed] [Google Scholar]
- 11. Zhou C, Wu Y-L, Chen G. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. [DOI] [PubMed] [Google Scholar]
- 12. Soria JC, Wu YL, Nakagawa K. et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990–998. [DOI] [PubMed] [Google Scholar]
- 13. Shi YK, Wang L, Han BH. et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443–2450. [DOI] [PubMed] [Google Scholar]
- 14. Li J, Bentzen SM, Renschler M. et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260–1266. [DOI] [PubMed] [Google Scholar]
- 15. Zindler JD, Slotman BJ, Lagerwaard FJ. Patterns of distant brain recurrences after radiosurgery alone for newly diagnosed brain metastases: implications for salvage therapy. Radiother Oncol. 2014;112(2):212–216. [DOI] [PubMed] [Google Scholar]
- 16. Zhuang H, Shi S, Chang JY. Treatment modes for EGFR mutations in patients with brain metastases from non-small cell lung cancer: controversy, causes, and solutions. Transl Lung Cancer Res. 2019;8(4):524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Zhou C, Huang Y. et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med. 2017;5(9):707–716. [DOI] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 19. Gao G, Ren S, Li A. et al. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: a meta-analysis from six phase III randomized controlled trials. Int J Cancer. 2012;131(5): E822–E829. [DOI] [PubMed] [Google Scholar]
- 20. Planchard D, Boyer MJ, Lee J-S. et al. Postprogression outcomes for osimertinib versus standard-of-care EGFR-TKI in patients with previously untreated EGFR-mutated advanced non-small cell lung cancer. Clin Cancer Res. 2019;25(7):2058–2063. [DOI] [PubMed] [Google Scholar]
- 21. Weickhardt AJ, Scheier B, Burke JM. et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Q, Zhou F, Liu H. et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol. 2018;13(9):1383–1392. [DOI] [PubMed] [Google Scholar]
- 23. Magnuson WJ, Lester-Coll NH, Wu AJ. et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35(10):1070–1077. [DOI] [PubMed] [Google Scholar]
- 24. Jiang T, Su C, Li X. et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol. 2016;11(10):1718–1728. [DOI] [PubMed] [Google Scholar]
- 25. Khalifa J, Amini A, Popat S. et al. International association for the study of lung cancer advanced radiation technology committee. Brain metastases from NSCLC: radiation therapy in the era of targeted therapies. J Thorac Oncol. 2016;11(10):1627–1643. [DOI] [PubMed] [Google Scholar]
- 26. Zhang J, Yu J, Sun X. et al. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of central nerve system metastases from non-small cell lung cancer. Cancer Lett. 2014;351(1):6–12. [DOI] [PubMed] [Google Scholar]
- 27. Johung KL, Yao X, Li F. et al. A clinical model for identifying radiosensitive tumor genotypes in non-small cell lung cancer. Clin Cancer Res. 2013;19(19):5523–5532. [DOI] [PubMed] [Google Scholar]
- 28. Welsh JW, Komaki R, Amini A. et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhuang H, Yuan Z, Wang J. et al. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Des Devel Ther. 2013;7:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gondi V, Pugh SL, Tome WA. et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rapp SR, Case LD, Peiffer A. et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33(15):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Landre T, Des Guetz G, Chouahnia K. et al. First-line angiogenesis inhibitor plus erlotinib versus erlotinib alone for advanced non-small-cell lung cancer harboring an EGFR mutation. J Cancer Res Clin Oncol. 2020;146:3333–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noronha V, Patil VM, Joshi A. et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124–136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-tct-10.1177_1533033821997819 for The Efficacy of First-Generation EGFR-TKI Combined With Brain Radiotherapy as the First-Line Treatment for Lung Adenocarcinoma Patients With Brain Metastases and EGFR Sensitive Mutations: A Retrospective study by Yuting Liu, Juanjuan Wang, Jingjing Wu, Qifan Yang, Yulan Zeng, Di Wu, Chen Tian, Yue Hu, Feifei Gu, Chang Li, Kai Zhang and Li Liu in Technology in Cancer Research & Treatment