Significance
Genetics, notably pathogenic genetic variants in sarcomere protein genes, play a major role in development of hypertrophic cardiomyopathy (HCM). However, degree of contribution from epigenetic and environmental factors in clinical presentation of HCM is currently unclear. We investigated phenotypic differences between identical twins with pathogenic sarcomere protein gene variants and demonstrated their discordant HCM presentation despite having virtually identical genomes. Our study underscores the important contribution of epigenetics and environment in disease progression in genetically diagnosed HCM patients.
Keywords: hypertrophic cardiomyopathy, identical twins, genetics
Abstract
Hypertrophic cardiomyopathy (HCM) is a disease of heart muscle, which affects ∼1 in 500 individuals and is characterized by increased left ventricular wall thickness. While HCM is caused by pathogenic variants in any one of eight sarcomere protein genes, clinical expression varies considerably, even among patients with the same pathogenic variant. To determine whether background genetic variation or environmental factors drive these differences, we studied disease progression in 11 pairs of monozygotic HCM twins. The twin pairs were followed for 5 to 14 y, and left ventricular wall thickness, left atrial diameter, and left ventricular ejection fraction were collected from echocardiograms at various time points. All nine twin pairs with sarcomere protein gene variants and two with unknown disease etiologies had discordant morphologic features of the heart, demonstrating the influence of nonhereditable factors on clinical expression of HCM. Whole genome sequencing analysis of the six monozygotic twins with discordant HCM phenotypes did not reveal notable somatic genetic variants that might explain their clinical differences. Discordant cardiac morphology of identical twins highlights a significant role for epigenetics and environment in HCM disease progression.
Hypertrophic cardiomyopathy (HCM) is characterized by left ventricular (LV) hypertrophy accompanied by nondilated ventricular chambers in the absence of other cardiac or systemic disease that would cause similar cardiac morphology (1). HCM is clinically recognized in ∼1 in 500 individuals, and >70% of familial HCM patients carry a pathogenic or likely pathogenic variant in genes encoding cardiac sarcomere proteins (2, 3) (denoted as HCM variants). Most HCM variants alter thick filament proteins myosin heavy chain 7 (MYH7) and myosin binding protein C (MYBPC3). Other HCM variants in genes encoding thin filament proteins, such as troponin T (TNNT2), account for a minority of cases (4, 5).
HCM variants generally have high penetrance but produce variable expression of clinical manifestations (6–13). Some variant carriers manifest LV hypertrophy early in childhood, while others have normal LV wall thickness (LVWT) until the sixth or seventh decade of life. Hypertrophy can range from the upper limit of normal (LVWT = 12 mm) to massive (>30 mm) and can occur with minimal symptoms, advanced heart failure, or fatal arrhythmias (3, 10). Approximately 25% of patients die from HCM-related adverse events (14), including sudden cardiac death, thromboembolic stroke, and heart failure. Although patients with HCM variants have more adverse outcomes than patients with unknown causes for HCM (15, 16), the mechanisms accounting for diverse responses to variants within the same or different HCM genes are unknown.
A classic approach to defining the contribution of genetic and environmental factors to variable progression of human disorders is to compare clinical phenotypes in monozygotic (MZ) twins, who have identical genome sequences. Few case reports have evaluated MZ twins with HCM (17–22). Wang et al. (19) showed that a twin pair with a pathogenic sarcomere gene mutation (MYH7 G768R) had similar LVWT but different amounts of fibrosis, measured by late gadolinium enhanced magnetic resonance imaging. Jansweijer et al. (22) examined interventricular septum thickness (IVSd) from 11 MZ twin pairs with HCM, including 5 pairs with sarcomeric variants, and found no significant heritability for IVSd in HCM. In contrast, a recent study demonstrated concordant morphologic findings and clinical course of identical twins with HCM, suggesting little environmental influence on clinical expression of HCM (20). Whether MZ twins with HCM variants have similar or different clinical courses remains uncertain. To more fully identify factors influencing HCM progression, we characterized longitudinal disease progression in 11 MZ twin pairs, including 9 with pathogenic sarcomere variants over 5 to 14 y.
Results
Among 11 twin pairs, monozygosity was confirmed by microsatellite analysis (1 pair), whole genome sequencing (WGS; 6 pairs), placental pathology (1 pair), and medical record (3 pairs) (Table 1). Genetic analyses for HCM were prompted by clinical manifestations in nine twin pairs and by familial cascade testing in two twin pairs. We compared LVWT, left atrial (LA) size, and LV ejection fraction (LVEF), standard measures of clinical expression and severity of HCM (1), by retrospective analyses of echocardiograms and clinical cardiac records. Twins were considered discordant if maximal LVWT or LA size differed by ≥20% (23, 24), or if absolute difference in LVEF ≥ 10% was noted between the two twins in at least two echocardiographic studies a year or more apart (25–27).
Table 1.
Clinical characteristics of MZ twins
| ID | Sex | Variant | Dx | Age of Dx | Monozygosity confirmation | Comorbidities† | ICD‡ | AF | CHF | NYHA | LVOT obstruction | Interventions‡ | F/U (y) | BMI | |
| 00554 | M | MYBPC3: c.2373insG (p.Trp792Valfs*41) | Preclinical | Placental pathology | ASD | N | N | N | I | N | N | 5 | 14.1 | ||
| 00555 | M | HCM | 4.5 | — | Y (12) | N | N | I | N | N | 5 | 14.6 | |||
| 00168 | F | MYBPC3: c.1892delT (p.Phe631Serfs*32) | Preclinical | Medical record | — | N | N | N | I | N | N | 6 | 25.8 | ||
| 00940 | F | Preclinical | — | N | N | N | I | N | N | 6 | 28.5 | ||||
| PC13 | F | MYBPC: c.927-9G > A | HCM | 51 | WGS | Hypothyroidism | N | N | N | II | Y | Myectomy (62) | 14 | 24.4 | |
| Myxomatous MV | |||||||||||||||
| PC14 | F | HCM | 52 | — | N | N | N | I | N | N | 14 | 26.5 | |||
| GH1 | F | MYBPC3: c.655G > C (p.Val219Leu) | HCM | 21 | WGS | — | N | N | N | I | Y | N | 9 | ||
| GH2 | F | HCM | 21 | — | N | N | N | I | N | N | 7 | ||||
| OU3 | F | MYBPC3: c.2735delG (p.Gly912Alafs*12) | HCM | 46 | WGS | HTN | Y (51) | N | N | I | N | N | 6 | 26.2 | |
| OU4 | F | HCM | 48 | HTN | N | N | N | I | N | N | 7 | ||||
| ALH1 | F | MYBPC3: c.772G > A (p.Glu258Lys) | HCM | 40 | WGS | HTN | N | N | N | II | N | N | 6 | ||
| ALH2 | F | HCM | 44 | — | N | N | N | I | N | N | 4 | ||||
| NF111 | F | MYH7: c.1012G > A (p.Val338Met) | HCM | 14 | WGS | — | Y (18) | N | N | I | Y | N | 7 | 26.6 | |
| NF112 | F | HCM | 14 | — | Y (18) | N | N | I | N | N | 7 | 23.4 | |||
| 1212 | F | MYH7: c.1988G > A (p.Arg663His) | HCM | 39 | Microsatellites | — | Y (51) | Y | N | I | Y | AV node ablation (51) | 7 | 24.8 | |
| 1213 | F | HCM | 39 | MR/TR | N | Y | Y (66) EF = 40 | IV | N | Pacemaker (63) | 7 | 26.7 | |||
| HTN | MV and TV annuloplasty (68) | ||||||||||||||
| HLD | LA appendage ligation (68) | ||||||||||||||
| DM | AV node ablation (68) | ||||||||||||||
| CKD | |||||||||||||||
| ZN1 | F | TNNT2: c.275G > A (p.Arg92Gln) | HCM | 14 | WGS | — | Y (28) | N | N | II | N | N | 6 | ||
| ZN2 | F | HCM | 14 | — | Y (28) | N | N | II | N | N | 6 | ||||
| 00872 | F | Unknown | HCM | 3 | Medical record | — | Y (21) | N | Y | III | N | N | 5 | ||
| 00925 | F | HCM | 17 | NSVT | Y (21) | N | Y | II | N | N | 16 | ||||
| 00141 | M | Unknown | HCM | 15 | Medical record | HTN | Y (19) | N | N | I | Y | N | 5 | ||
| 00142 | M | HCM | 14 | — | Y (19) | N | N | I | Y | N | 6 |
Abbreviations: ASD, atrial septal defect; AV, atrioventricular; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; Dx, diagnosis; EF, ejection fraction; F/U, follow-up; HLD, hyperlipidemia; HTN, hypertension; ICD, implantable cardioverter, defibrillator; LVOT, left ventricular outflow tract; MR, mitral regurgitation; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association functional classification; TR, tricuspid regurgitation.
No patient had a history of stroke, coronary artery disease, cardiac arrest, or inappropriate ICD firing.
Number in parenthesis indicates age at time of procedure.
Nine twin pairs had HCM-causing variants in MYBPC3 (n = 6), MYH7 (n = 2), and TNNT2 (n = 1) genes. The causes of HCM in two twin pairs, one of which tested negative for the sarcomeric gene panel and the other declined genetic testing, remain unknown. Seven individuals had cardiovascular comorbidities, including hypertension, hyperlipidemia, secundum atrial septal defect (status post percutaneous closure), and myxomatous mitral valve. These comorbidities, except for hypertension, were discordant between some twin pairs. Eleven individuals, inclusive of four twin pairs, had an implantable cardioverter defibrillator for primary prevention or a pacemaker. Individual PC13 underwent myectomy at age 62 y to ameliorate symptoms of chest pain, exertional dyspnea, limited exercise capacity, tachycardia, and presyncopal events.
To determine if LVWT differences observed between identical twins reflected technical variation between different observers, we analyzed 2,317 HCM patients with HCM-causing genetic variants enrolled in the Sarcomeric Human Cardiomyopathy Registry (SHaRe), who have had one or more echocardiograms in a single year. The mean difference between the two echocardiograms performed in the same year was 1.11 ± 1.2 mm on the same individual (n = 17,137). In contrast, 54 paired LVWT measurements from 2 identical twins within 1 y differed by 4.75 ± 0.7 mm, which is significantly different (P < 1e-16) from the mean LVWT observed between measurements made in the same SHaRe subject.
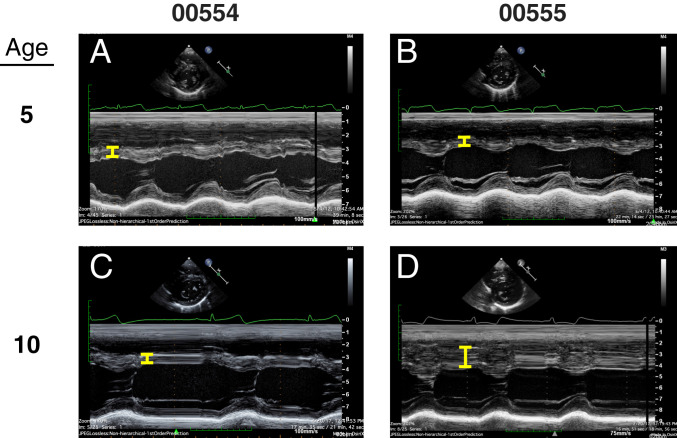
All twins, with or without an HCM variant, shared classic asymmetry of the interventricular septum and had discordant LVWT over extended periods during this study (Fig. 1 and SI Appendix, Fig. S1 and Table S1). LVWT differences were typically evident early and maintained throughout longitudinal follow-up (5 to 14 y). Twin pair 00554/00555, with pediatric onset of HCM, had comparable LVWT at age 5 y, but over the ensuing 7 y the degree of hypertrophy markedly diverged (Figs. 1 and 2). As previously found in HCM cohorts (28, 29), LVWT of twin pairs 00554/00555 and 00168/00940 did not correlate with body mass index (BMI) (SI Appendix, Fig. S2) (P = NS, not significant).
Fig. 1.
LVWT of MZ twins with pathogenic/likely pathogenic sarcomere variants. Asterisks (*) represent at least 20% difference in LVWT between echocardiogram readings of each twin pair at each time point. First, echocardiograms of twin pairs taken in the same year were considered. If a subject’s twin did not have an echocardiogram taken in the same year, then echocardiograms taken within 3 y were considered. Only LVWT measurements taken in the same region of the ventricle (septum) were considered. PC13 obtained myectomy at the age of 62, indicated by blue arrow. Dotted lines represent normal range of LVWT (43).
Fig. 2.
Representative images of echocardiograms from twin pair 00554 and 00555 at age 5 y (A and B) and 10 y (C and D). Yellow bracket indicates LVWT measured in M-mode.
Approximately 20% of HCM patients develop LA enlargement, a morphologic abnormality that conveys increased risk for atrial fibrillation (AF), embolic stroke, and reduced survival (30–33). Although LVWT differed between the all MZ twin pairs, LA diameters were discordant in only six of the nine twin pairs (ALH1/ALH2, OU3/OU4, PC13/PC14, ZN1/ZN2, 00168/00940, 00554/00555) (Fig. 3 and SI Appendix, Table S1). Only one twin pair (1212/1213) developed AF, perhaps due to the overall young age of the twin cohort.
Fig. 3.
LA diameter of MZ twins with pathogenic/likely pathogenic sarcomere variants. Asterisks (*) represent at least 20% difference in LA size between echocardiogram readings of each twin pair at each time point. First, echocardiograms of twin pairs taken in the same year were considered. If a subject’s twin did not have an echocardiogram taken in the same year, then echocardiograms taken within 3 y of that echocardiogram were considered. Normal LA size (AP dimension) for females is 27 to 38 mm, and for males is 30 to 40 mm (43). Discordant twins are denoted by solid lines, concordant twins by dashed lines.
LVEF is typically normal or supranormal in HCM patients, and reduced LVEF can herald the onset of progressive dysfunction that results in heart failure. Four of seven twin pairs demonstrated discordance in LVEF (ALH1/ALH2, PC13/PC14, 00168/00940, 00554/00555) (SI Appendix, Fig. S3).
To investigate whether somatic genetic variants might account for these discordant phenotypes in MZ twins, we performed WGS analysis of six MZ twin pairs (ALH1/ALH2, GH1/GH2, NF111/NF112, OU3/OU4, PC13/PC14, ZN1/ZN2) whose DNA was available for sequencing analysis. We did not identify any single nucleotide variants (SNV) or structural variants in HCM genes (ACTC1, FHL1, GLA, MYBPC3, MYH7, MYL2, MYL3, PRKAG2, TNNI3, TNNT2, TPM1) (5) or dilated cardiomyopathy genes (ACTC1, BAG3, DSP, LMNA, MYH7, NEXN, PLN, TNNC1, TNNT2, TPM1, TTN, VCL) (34) that differed between each twin pair. Furthermore, although our analysis identified a number of somatic variants in twin pairs, none of these somatic variants (coding, noncoding, or structural variants) were shared between any two or more twin pairs.
Discussion
Our analyses of cardiac morphology and function in 11 MZ HCM twin pairs, who were followed over 5 to 14 y, demonstrated LVWT discordance in all twin pairs. The discordant LVWT in all of the MZ twin pairs suggest that epigenetic and environmental factors, rather than background genetic variation, play a major role in hypertrophic remodeling.
In contrast to LVWT, LA dimensions were concordant in three of nine MZ twin pairs with HCM-causing variants. An appealing hypothesis to explain this greater degree of concordance is that LA size reflects impaired ventricular relaxation, which results directly from sarcomere dysfunction rather than environmentally controlled hemodynamic function. Four of seven MZ twin pairs with available LVEF data demonstrated discordance, suggestive of environmental factors influencing ventricular function in HCM in addition to its structural traits, such as LVWT. However, as LVEF is usually preserved or somewhat enhanced in HCM until late stages of the disease and only one twin pair was over 60 y of age, longer follow-up is needed to better assess the influence of genetic and nongenetic factors on ventricular function.
Our finding that MZ twins with sarcomeric variants known to perturb structural traits in HCM have discordant phenotypes is consistent with the conclusions drawn from Jansweijer et al. (22), where the authors compared the first available measurement of IVSd of 11 MZ HCM twins. We followed the twin pairs for 5 to 14 y rather than a cross-sectional analysis to minimize sampling bias from analyzing one-time measurements and to study their clinical course over long periods of time. Nine of 11 twin pairs in the present study had pathogenic HCM variants identified, compared to less than 50% of twin pairs with a known genetic mutation in Jansweijer et al. Identification of pathogenic sarcomeric variants ensures that subjects in the present study indeed have HCM rather than cardiomyopathy that mimics HCM, such as hypertensive cardiomyopathy or infiltrative cardiomyopathy. Furthermore, WGS analyses demonstrate that somatic mutation is unlikely to explain discordant phenotypes of the HCM MZ twins. The clinical course of HCM patients with pathogenic mutations affecting sarcomere genes is clearly impacted by epigenetic and environmental factors that might include microbial infection, diet, or exercise.
Limitations of our study include the retrospective nature of the analysis and possible interobserver variabilities of echocardiographic measurements. To minimize confounding from interobserver variation, we defined phenotypes to be discordant if maximal LVWT or LA size differed by ≥20% or if absolute LVEF differed by ≥10% based on published measurement variabilities (23–27). Furthermore, as noted in the results, echocardiographic measurements in discordant HCM twins were significantly greater than technical variation of the measurements in a single year from 2,317 HCM patients enrolled in SHaRe.
Our data infer a critical role for environmental factors in determining cardiac morphologic progression in HCM patients. Presumably, the consequences of different environmental factors are mediated through changes in the epigenome. Some of these epigenetic modulations will likely be identified through studies of small and large animal HCM models (35, 36). However, epidemiologic studies of HCM patients will be required to identify those environmental factors that mediate human HCM progression; information that will benefit future HCM patients.
Materials and Methods
MZ Twin Cohort.
Data from 8 of the 11 twin pairs were acquired from SHaRe. Three additional twin pairs were subjects in studies approved by and carried out according to the Brigham and Women’s Hospital (BWH) Human Subjects Committee. Clinical measures were obtained by review of each subjects’ medical record as recorded in the SHaRe database or in medical records of BWH. All subjects gave informed consent to participate in research.
Next-Generation Sequencing and Variant Analysis.
Genomic DNA extracted from peripheral blood sample was sequenced using Illumina HiSeq instruments. All sequencing reads were aligned to hg38 (GRCh38) using BWA-MEM with the -Y option (BWA v0.7.15). SNVs and small indels were identified using the Genome Analysis Tool Kit (GATK; v4.1) Haplotype Caller tool (37). Structural variants were analyzed using Manta, Wham, and MELT as part of the GATK-SV pipeline (38). The SNVs were annotated using vcfanno (v0.3.2), dbSNP (build 151), the 1000 Genomes Project (phase 3), gnomAD (v3.0), SnpEff (v4.3t, annotation database GRCh38.86) (39), and dbNSFP (v3.5a). High-quality variants [pass GATK Variant Score Quality Recalibration (VSQR) truth sensitivity threshold 99.5 for SNVs, 99.0 for indels), a minimum depth of 10, genotype quality ≥ 20, and Phred-scaled quality score (QUAL) ≥ 30] were filtered for rare heterozygous variants [defined as minor allele frequency (MAF) < 1.00e-04] in gnomAD (40) and the 1000 Genomes Project (41). Loss-of-function variants (labeled by SnpEff as nonsense, canonical splice-site, frameshift indels, start loss, stop lost, and stop gained) and damaging missense variants [deleterious by MetaSVM (42)] were considered potentially pathogenic to the disease.
Supplementary Material
Acknowledgments
We thank the patients and investigators, who participated in enrollment. This study was supported by the following funding: Department of Health and Human Services, NIH, National Heart, Lung, and Blood Institute (NHBLI) Grants 5R01HL084553, 5R011HL080494, and X01HL143310 (to C.E.S. and J.G.S.); Fondation Leducq 16-CVD 03 (to C.E.S. and J.G.S.); a John S. Ladue Fellowship at Harvard Medical School (to Y.K.); NIH, NHLBI 5T32HL007208 (to Y.K.); and a Sarnoff Cardiovascular Research Foundation Fellowship (to G.G.R.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021717118/-/DCSupplemental.
Data Availability
All study data are included in the article and supporting information.
References
- 1.Gersh B. J.et al.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons , 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Thorac. Cardiovasc. Surg. 142, 1303–1338 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Maron B. J., et al., Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary Artery Risk Development in (Young) Adults. Circulation 92, 785–789 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Semsarian C., Ingles J., Maron M. S., Maron B. J., New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 65, 1249–1254 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Thomson K. L., et al., Analysis of 51 proposed hypertrophic cardiomyopathy genes from genome sequencing data in sarcomere negative cases has negligible diagnostic yield. Genet. Med. 21, 1576–1584 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh R.et al.; Exome Aggregation Consortium , Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet. Med. 19, 192–203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen M. K., et al., Penetrance of hypertrophic cardiomyopathy in children and adolescents: A 12-year follow-up study of clinical screening and predictive genetic testing. Circulation 127, 48–54 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Pasquale F., et al., Long-term outcomes in hypertrophic cardiomyopathy caused by mutations in the cardiac troponin T gene. Circ. Cardiovasc. Genet. 5, 10–17 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Maron B. J., Spirito P., Wesley Y., Arce J., Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. N. Engl. J. Med. 315, 610–614 (1986). [DOI] [PubMed] [Google Scholar]
- 9.Gray B., Ingles J., Semsarian C., Natural history of genotype positive-phenotype negative patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 152, 258–259 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Vermeer A. M. C., Clur S. B., Blom N. A., Wilde A. A. M., Christiaans I., Penetrance of hypertrophic cardiomyopathy in children who are mutation positive. J. Pediatr. 188, 91–95 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Andersen P. S., et al., Genetic and phenotypic characterization of mutations in myosin-binding protein C (MYBPC3) in 81 families with familial hypertrophic cardiomyopathy: Total or partial haploinsufficiency. Eur. J. Hum. Genet. 12, 673–677 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Walsh R., et al., Defining the genetic architecture of hypertrophic cardiomyopathy: Re-evaluating the role of non-sarcomeric genes. Eur. Heart J. 38, 3461–3468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurizi N., et al., Clinical course and significance of hypertrophic cardiomyopathy without left ventricular hypertrophy. Circulation 139, 830–833 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Maron B. J., Rowin E. J., Casey S. A., Garberich R. F., Maron M. S., What do patients with hypertrophic cardiomyopathy die from? Am. J. Cardiol. 117, 434–435 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Marian A. J., On genetic and phenotypic variability of hypertrophic cardiomyopathy: Nature versus nurture. J. Am. Coll. Cardiol. 38, 331–334 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho C. Y., et al., Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: Insights f+rom the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 138, 1387–1398 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovács A., et al., Hypertrophic cardiomyopathy in a monozygotic twin pair: Similarly different. Circ. Cardiovasc. Imaging 9, e004794 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Palka P., Lange A., Burstow D. J., Different presentation of hypertrophic cardiomyopathy in monozygotic twins. Heart 89, 751 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Li W., Han Y., Chen Y., Different clinical presentation and tissue characterization in a monozygotic twin pair with MYH7 mutation-related hypertrophic cardiomyopathy. Int. Heart J. 60, 477–481 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Maron B. J., et al., Adult monozygotic twins with hypertrophic cardiomyopathy and identical disease expression and clinical course. Am. J. Cardiol. 127, 135–138 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Reid J. M., Houston A. B., Lundmark E., Hypertrophic cardiomyopathy in identical twins. Br. Heart J. 62, 384–388 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansweijer J. A., et al., Heritability in genetic heart disease: The role of genetic background. Open Heart 6, e000929 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallerson D. C., Devereux R. B., Reproducibility of echocardiographic left ventricular measurements. Hypertension 9, 6–18 (1987). [DOI] [PubMed] [Google Scholar]
- 24.Schoenmaker N. J., et al., Low agreement between cardiologists diagnosing left ventricular hypertrophy in children with end-stage renal disease. BMC Nephrol. 14, 170 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johri A. M., et al., Can a teaching intervention reduce interobserver variability in LVEF assessment: A quality control exercise in the echocardiography lab. JACC Cardiovasc. Imaging 4, 821–829 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Frikha Z., et al., Reproducibility in echocardiographic assessment of diastolic function in a population based study (the STANISLAS Cohort study). PLoS One 10, e0122336 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otterstad J. E., Froeland G., St John Sutton M., Holme I., Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur. Heart J. 18, 507–513 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Canepa M., et al., Comparison of clinical presentation, left ventricular morphology, hemodynamics, and exercise tolerance in obese versus nonobese patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 112, 1182–1189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balaji S., et al., Impact of obesity on left ventricular thickness in children with hypertrophic cardiomyopathy. Pediatr. Cardiol. 40, 1253–1257 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Maron B. J., Maron M. S., Hypertrophic cardiomyopathy. Lancet 381, 242–255 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Olivotto I., et al., Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 104, 2517–2524 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Kim K.-J., et al., Left atrial mechanical function and global strain in hypertrophic cardiomyopathy. PLoS One 11, e0157433 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siontis K. C., et al., Atrial fibrillation in hypertrophic cardiomyopathy: Prevalence, clinical correlations, and mortality in a large high-risk population. J. Am. Heart Assoc. 3, e001002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzarotto F., et al., Reevaluating the genetic contribution of monogenic dilated cardiomyopathy. Circulation 141, 387–398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson R. L., et al., Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc. Natl. Acad. Sci. U.S.A. 115, E8143–E8152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geisterfer-Lowrance A. A., et al., A mouse model of familial hypertrophic cardiomyopathy. Science 272, 731–734 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Van der Auwera G. A.et al., From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43, 11.10.1-11.10.33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins R. L.et al.; Genome Aggregation Database Production Team; Genome Aggregation Database Consortium , A structural variation reference for medical and population genetics. Nature 581, 444–451 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cingolani P., et al., A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6, 80–92 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lek M.et al.; Exome Aggregation Consortium , Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auton A.et al.; 1000 Genomes Project Consortium , A global reference for human genetic variation. Nature 526, 68–74 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong C., et al., Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 24, 2125–2137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang R. M., et al., Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1–39.e14 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.