Abstract
The process of evaluating the efficacy and toxicity of drugs is important in the production of new drugs to treat diseases. Testing in humans is the most accurate method, but there are technical and ethical limitations. To overcome these limitations, various models have been developed in which responses to various external stimuli can be observed to help guide future trials. In particular, three-dimensional (3D) cell culture has a great advantage in simulating the physical and biological functions of tissues in the human body. This article reviews the biomaterials currently used to improve cellular functions in 3D culture and the contributions of 3D culture to cancer research, stem cell culture and drug and toxicity screening.
Keywords: 3D cell culture, biomaterials, drug screening, alternative model
1. Introduction
The human body consists of highly sophisticated biological systems [1]. Cells form tissues in various combinations and patterns, tissues form organs with different types of tissues, and organs are organically connected to maintain the human body [2]. For a long time, two-dimensional (2D) cell culturing has been carried out on widely available flat plastic dishes to mimic the complex human body [3,4]. However, in a 2D culture system, the cells spread on the flat and hard surfaces and proliferate unnaturally. There is a difference in the cellular morphology, functions, and overall behavior compared to those in the natural environment [4]. In addition, the phenotype of the cell is not accurately reproduced in 2D culture. Indeed, chondrocytes grown in vitro to a large number gradually lose their differentiated phenotype, which is indicated by the loss of synthesis of type II collagen during 2D cell culture [5,6,7]. Similarly, 2D cultured primary human hepatocytes (PHHs) show rapid declines in critical phenotypic functions, such as cytochrome P-450 (CYP450) enzyme activities, insulin responsiveness, and expression of the master liver transcription factor hepatocyte nuclear factor 4α within hours to days [8]. Moreover, the transwell culture system, a kind of layered 2D culture system, was introduced as a co-culture system to simulate the in vivo environment, but this method has limitations in maintaining or improving cellular function over a long time [9,10]. Thus, cell culturing should be adapted to better reflect the natural environment.
Cells in the natural environment are embedded in the extracellular matrix (ECM), forming a complex three-dimensional (3D) structure [11]. The ECM plays the role of regulating cell-to-cell interactions, cell adhesion, differentiation, and growth [12,13,14]. Therefore, an understanding of ECM composition and structure is critical for the development of novel 3D cultures for predicting biological mechanisms and therapeutic effects. Mounting evidence has shown that physiologically more relevant factors can be revealed by imitation of the components and structure of the ECM in the natural environment [13,15,16]. In particular, cells cultured in a 3D microenvironment with ECM components showed realistic morphology and expressed several genes that failed to be expressed in a 2D culture [5,6,7]. Moreover, these cells synthesized ECM as they do in vivo for regeneration [11,12,13,14]. Thus, 3D cell culturing requires the use of biomaterials with a high level of similarity with the ECM for the enhancement of cellular functions.
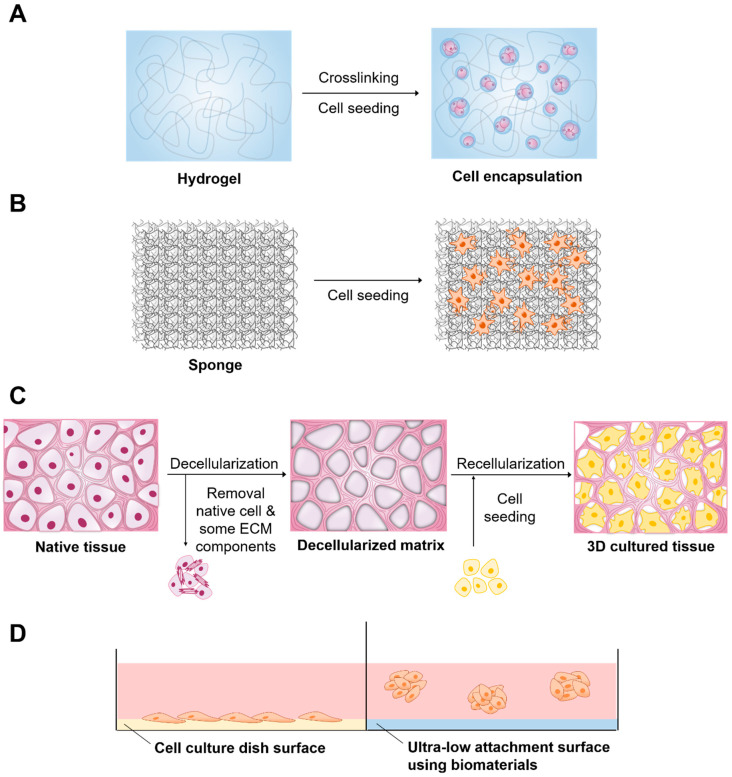
A number of 3D culture systems are already available (Table 1). Biomaterials are available for 3D cell culture to improve the efficiency of culture and cell functions in various forms, including hydrogels, solid scaffolds, decellularized native tissue, and ultra-low attachment (ULA) surface (Figure 1). Knowledge of 3D culture methods has significantly increased, which has resulted in the development of numerous applications. Thus, this review addresses the applications of biomaterials in 3D cell culture and the contribution of 3D cell culture to cancer research, stem cell research, and drug and toxicity screening.
Table 1.
Types of biomaterials used in three-dimensional (3D) cell culture and their advantages and disadvantages.
| Type | Advantage | Disadvantage | References |
|---|---|---|---|
| Hydrogel | Tissue like flexibility Easily supplies water-soluble factors to cells |
Low mechanical resistance | [5,11,13,17,18] |
| Solid scaffold | Various materials can be used Physical strength is easily adjusted |
Difficulty in homogeneous dispersion of cells | [15,16,19,20,21] |
| Decellularized native tissue | Provides complex biochemistry, biomechanics and 3D tissues of tissue-specific extracellular matrix (ECM) | Decrease of mechanical properties (roughness, elasticity, and tension strength) of the tissues as compared to the native group | [22,23,24,25,26] |
| Ultra-low attachment surface | Provides an environment similar to in vivo conditions | Difficulty in mass production Lack of uniformity between spheroids |
[27,28,29,30,31] |
Figure 1.
Biomaterials and related method of three-dimensional (3D) cell culture preparation. (A) Hydrogel, (B) Solid scaffold, (C) Decellularized native tissue (D) Ultra-low attachment surface.
2. Applications of Biomaterials in 3D Cell Culture
2.1. Hydrogels
Hydrogels have 3D structure, hydrophilic property, and polymeric networks capable of absorbing large amounts of water or biological fluids [32]. These hydrogels can mimic soft and wet environments similar to ECM of native tissues and promote the transportation of O2, nutrients, waste and soluble factors [33]. Therefore, they have received much attention in 3D cell culture [6,14]. Hydrogels are categorized as either synthetic or natural (Table 2). As the name suggests, synthetic hydrogels are composed of unnatural molecules such as poly vinyl alcohol (PVA), poly-2-hydroxyethyl methacrylate (pHEMA), and poly ethylene glycol (PEG). These materials can provide mechanical support for various types of cells [14]. However, they are biologically inert. In addition, they lack endogenous factors essential for cell behavior and act mainly as a template to permit cell function. Thus, synthetic hydrogels need modification with suitable biological components to promote signals of cellular function. On the other hand, synthetic hydrogels such as PEG represent a very good candidate for the encapsulation of various bioactive factors, drugs, and chemicals to avoid complicating systemic factors derived from hydrogels for a more controlled comparison of encapsulated materials [34]. Recently, it has been reported that the combination of arginine-glycine-aspartic acid (RGD) groups or alginate-PEG hydrogel improved the spread and proliferation of fibroblasts and enhanced the osteogenic differentiation of mesenchymal stem cells (MSCs) for 3D cell culture [35]. Similarly, PEG hydrogels were used to culture and expand a variety of neural and glial cell types simply by altering the material properties of the hydrogel [36].
Table 2.
Synthetic and natural hydrogels for 3D cell culture.
| Properties | Materials | Cells | Applications | |
|---|---|---|---|---|
| Synthetic | Provide structural support to various cell types | PVA | Mouse 129 teratocarcinoma AT805 derived cells (ATDC5) [37], Human iPS cells (HPS0077) [38] | Repair cartilage [37], promote differentiation [38] |
| pHEMA | Bovine ear chondrocytes [39] | Proliferate chondrocytes [39] | ||
| PEG | Ovarian Follicle cell [40], human mesenchymal stem cells (hMSCs) [41] | Promote cell survival, growth [40], and viability by encapsulation [41] | ||
| Natural | Support cellular activities and are biocompatible and biodegradable | Collagen | Human umbilical vein endothelial cells (HUVECs) [42] | Form stable EC networks [42] |
| Alginate | Human adipose-derived stem cells (hASCs) [43], rat astroglioma (LRM55) [44] | Maintain their ability to secrete therapeutic factors [43], maintain the viability and function [44] | ||
| Hyaluronic acid | Human induced pluripotent stem cell-derived neural progenitor cells (hiPSC-NPCs) [45], human breast cancer MCF-7 cells [46] | Promote neural differentiation [45], higher tumorigenic capability of MCF-7 cells [46] |
Natural hydrogels are made up of natural substances such as collagen, alginate, hyaluronic acid and many more that promote several cellular functions with a range of endogenous factors present, which can benefit the viability, proliferation, and differentiation of many cell types [47,48,49,50,51,52,53]. However, due to the complexity and undefined nature of these hydrogels, it is difficult to very accurately determine which signals promote cellular function. Collagen is one of the abundantly present proteins in the ECM [54]. These compounds possess native tissue-like properties and characteristics. Thus, collagen can be used to create gels for 3D cell culture [48,55]. Collagen can be used in culturing various cell types to improve cell growth, adhesion, and differentiation. Cells can proliferate and form tissue-like structures within the collagen matrix [56]. Collagen plays an important role in maintaining the chondrocyte phenotype and supporting chondrogenesis, both in vitro and in vivo [57,58]. Previous studies have shown that type I collagen promotes the proliferation of chondrocytes and that type II collagen supports the chondrogenic differentiation of MSCs [59,60]. The results from this study suggest that there is clinical value in the cartilage repair capabilities of Col I/II hydrogel with encapsulated MSCs [61,62]. Nonetheless, the long-term performance of pure type I collagen may be compromised by significant shrinkage and weak mechanical properties [63,64]. To control these problems in collagen hydrogels, one possibility is to introduce additional molecular bonds between the collagen fibrils via different chemical cross-linkers. Lotz et al. aimed to improve the long-term stability and mechanical properties of collagen hydrogels by using the nontoxic chemical cross-linker four-armed succinimidyl glutarate polyethylene glycol (PEG-SG) to obviate negative impacts on cell viability. The hydrogels showed increased mechanical stability and compression E-modulus compared with pure collagen. This could indicate a more sterically rigid molecular network, rendering human dermal fibroblasts, and human epidermal keratinocytes unable to contract the hydrogel. This leads to a reproducible generation of full-thickness skin equivalents for in vitro testing or clinical application [65]. Incorporation of other materials into polymeric hydrogels can also be a suitable option to overcome these problems and improve the biological performance of the hydrogels. Sun et al. observed that collagen–chitosan could promote axonal regeneration and neurological recovery compared with collagen–chitosan hydrogels fabricated by traditional technology. In addition, it was demonstrated that 3D printing of collagen–chitosan decreased the formation of scars and cavities, and improved the regeneration of nerve fibers as well as functional recovery in rats [66,67]. Ying et al. fabricated a porous structure of this collagen–hyaluronic acid (HA) hydrogel that contributed to water retention, gas exchange, nutrition penetration, and cell dwelling [68]. In addition, these materials are suitable to study tissue reconstruction by seeding co-cultures of fibroblasts and endothelial cells within the collagen matrix [69]. In this system, fibroblasts form connective tissue, and endothelial cells produce angiogenic growth factors and vasculature.
Hyaluronic acid (HA) is distributed in many tissues, such as skin and cartilage [70]. HA can be obtained not only from animal tissues, but also via microbial fermentation in Escherichia coli to produce animal-free HA [71]. The role of HA in tissue is to promote cellular survival, migration, angiogenesis, and differentiation by transduction of intracellular signals [72,73,74]. In addition, the higher content of HA present in the cancer microenvironment promotes tumor progression and resistance to anticancer drugs [75,76]. Tumor cells showed decreased adhesion to the surface of HA. These properties promote the production of tumor spheroids and mimic cell HA signaling in the tumor microenvironment for anticancer drug screening purposes. Ahrens et al. reported that HA promotes the growth rate of melanoma cells by enhancing the secretion of basic fibroblast growth factor (bFGF) [77]. Other researchers have reported that when cells are 3D cultured in the presence of HA, the activity of multidrug resistance proteins is enhanced and therapeutic effectiveness is reduced compared to the 2D cultured cells [78]. Another interesting application of HA hydrogel is to improve the efficacy of 3D cell culture by mixing these materials with various substances. Lou et al. reported that HA-collagen hydrogels promoted cell spreading, fiber remodeling, and focal adhesion in 3D cell culture [49]. Häckel and coworkers demonstrated that human nucleus pulposus cells cultured in fibrin-HA hydrogels showed an increase in collagen type II and carbonic anhydrase XII gene expression [79]. Furthermore, Lee et al. also reported that chitosan/HA blend hydrogels exhibited enhanced physical stability, mechanical properties, cell binding affinity, and tissue compatibility [80]. Recently, HA combined with alginate and fibrin has been used as a bioink for 3D bioprinting of peripheral nerve tissue regeneration [81]. Finally, acetylated HA (AcHA) was used to enhance the mechanical strength of the thermogel via simple blending of modified glycol chitosan. The blended gel showed not only good cell binding affinity in vitro and biocompatibility in vivo, but also more effective cartilage formation than that of the original hydrogel [80].
Alginate is derived from the cells of brown algae, and its monomers have the ability to cross-link to form hydrogels [50]. Normally, alginate does not interact directly with mammalian cells and is not degradable [82]. Thus, when hydrogels exhibiting minimal degradation are desired, alginate is selected for these studies. In addition, cell adhesion can be improved via covalent coupling of the RGD cell adhesion peptide to the alginate chains [83]. A previous study reported a material approach to tune the rate of stress relaxation of hydrogels for 3D culture, independent of the hydrogel’s initial elastic modulus, cell adhesion ligand density, and degradation. The influence of substrate stress relaxation on cell spreading and proliferation was enhanced when RGD cell adhesion and ligand density was increased in gels with faster relaxation [84]. Another study reported that stem cells encapsulated in ionically crosslinked alginate hydrogels undergo predominantly adipogenic differentiation at initial moduli of 1–10 kPa and predominantly osteogenic differentiation at initial moduli of 11–30 kPa [85]. Recently, alginate hydrogels have been extensively used as bioinks to provide 3D cell growth because of their relatively higher viscosity and rapid crosslinking process after printing [86,87]. In addition, oxidized alginates showed great potential as ink for bioprinting [88]. Finally, alginate hydrogels encapsulating stem cells have been investigated for the prevention of immune rejection of transplanted cells [89,90]. Stock and coworkers reported that alginate capsules prevented infiltration of immune cells while allowing smaller molecules, such as oxygen, nutrients, glucose, and insulin to diffuse freely through the capsule [91].
2.2. Porous and Fibrous Scaffolds
Solid scaffold-based cell culturing is one of the older techniques used in the field of 3D cell culture [92]. In this system, scaffolds may facilitate proliferation, cell adhesion, and signaling activities between the cells. These efficacies of a scaffold are affected by the materials that make up the scaffold and its physical structures, such as exposed surface, pore size, pore distribution, and interconnectivity (Table 3). These solid scaffolds are mainly porous foams or fibrous meshes fabricated from synthetic polymers, such as poly(glycolic acid) (PGA), poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), and polycaprolactone (PCL), and naturally derived polymers, such as collagen, hyaluronic acid, fibrin, alginate, gelatine, silk, and chitosan [93,94,95,96,97,98,99].
Table 3.
Fabrication of porous scaffolds: advantages and disadvantages.
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Particulate Leaching | Modulate pore size and porosity | Limited pore shape and size | [15] |
| Solvent Casting | Modulate pore size and porosity Easy incorporation of drugs within the scaffold |
Low pore interconnectivity | [100,101] |
| Emulsion Templating | Modulate particle size, high porosity, interconnectivity | Difficulty in obtaining emulsions with sufficient monodispersity for crystallization | [16,102,103] |
| Gas Foaming | Modulate pore size and porosity Free of toxic organic solvents |
Unexpected pore interconnectivity | [104,105,106] |
| Melt Molding | Modulate pore size and porosity | High temperature required when molding | [107] |
Porous foam-solid scaffolds have high porosity and a uniform interconnected structure. Many attempts have been made to fabricate porous foam-solid scaffolds [108,109]. Particulate leaching is a physical process that involves casting polymers around soluble beads known as porogens [100]. Solvent casting uses a polymer dissolved in an organic solvent. This solution is mixed with ceramic particles and poured into a predefined 3D mold, which is left to set. The solvent casting and particulate leaching (SCPL) method, which combines the particulate leaching method and solvent casting method, has been used to produce scaffolds for the culture of osteoblasts and osteogenic differentiation of stem cells [110]. Mouse embryonic osteoblast cells (MC3T3-E1) cultured on PLGA scaffolds made with the SCPL method showed increased alkaline phosphatase activity and expression of type I collagen [111]. Emulsion templating is one of the common methods for the fabrication of porous scaffolds [16]. Porous polymers can be generated within high internal phase emulsions. It has been reported that metabolic activity is improved when hepatocytes are cultured on PCL scaffolds containing various factors by emulsion templating [112,113]. The gas forming technique is performed by agitating the polymer and creating foam [104]. High-pressure gases such as CO2 can be used as the gas foaming agent, and the porosity of the scaffold can be controlled by the amount of gas dissolved in the polymer. The melt molding method uses both polymer and porogen, which are poured into a mold and heated above the polymer glass transition temperature [107,114]. Various types of cells have been successfully cultured in 3D on porous solid scaffolds [115,116,117,118]. Vascular smooth muscle cells adhered to and proliferated in engineered smooth muscle tissue on highly porous and elastic tubular scaffolds [119]. Human hepatoma cells showed higher cell infiltration in PLGA scaffold fabricated by the gas foaming method. The porous PLGA scaffold fabricated by the particulate leaching method supported cell adhesion and growth. After implantation, there was better bone and cartilage formation inside the scaffold [120,121].
Fibrous scaffolds provide a large surface area for cell growth in 3D cell culture (Table 4). These structures allow appropriate space for gas and nutrition exchange and cell infiltration. In addition, fibrous scaffolds can imitate oriented and aligned tissues, which include skeletal muscles, the central nervous system, and cardiac tissues [122,123,124,125,126]. Accordingly, the aligned fibers help control stem cell differentiation into the desired cell type [127,128,129,130]. Several natural and synthetic polymers, including collagen, gelatine, hyaluronic acid, alginate, chitosan, silk, PLA, PLGA, and others, have been used for the fabrication of fibrous scaffolds [124,129,131,132]. The fiber mesh is either knitted or woven into 3D patterns of different pore sizes [133]. However, these materials do not have sufficient mechanical and structural stability. Fiber bonding was thus developed to overcome the drawbacks of fiber mesh. Enhanced mechanical strength is provided by binding the fibers at the joints or intersections by raising temperatures above the polymer melting points or by using special solvents [134]. The electrospinning method uses an electric field generated using two electrodes (one each placed in the polymer and collector solutions) having electric charges of opposite polarity for the production of continuous fibers ranging from submicron to nanomicron diameters. This system allows cells to adhere and elongate along the fibers, which induces cell alignment and directionality in the cultures [135]. Lee et al. fabricated PLLA fibrous scaffolds using the electrospinning method, and their morphologies were controlled by the fiber collection speed. Therefore, the morphology of the designed fibrous scaffolds in this work has successfully controlled cell alignment as well as the direction of calcification [136]. For phase separations, two phases that are polymer rich and polymer poor are formed upon the addition of water, inducing phase separation [137,138]. Upon cooling below the solvent melting point followed by vacuum drying, the scaffold is obtained. This method can easily be combined with other fabrication technologies, such as particulate leaching, to design 3D structures with the desired pore morphology. Finally, nanofibers can be generated by the self-assembly of synthetic or natural molecules [139,140]. These scaffolds fabricated by self-assembly facilitated attachment and migration of hepatocytes, stem cells, and endothelial cells [141,142].
Table 4.
Fabrication of fibrous scaffolds: advantages and disadvantages.
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Fiber Mesh | High surface area for cell attachment | Low structural stability | [21] |
| Fiber Bonding | High surface to volume ratio, high porosity | Limited applications to other polymers | [132] |
| Electrospinning | Induces cell alignment and directionality | Limited by cell seeding | [143,144,145] |
| Phase Separation | No reduction in the activity of molecules | Difficult to control the scaffold morphology | [138,146] |
| Self-Assembly | Form extremely stable scaffolds, less use of organic solvent | Expensive material, complicated and elaborate process | [147,148] |
Another way to fabricate a porous scaffold is 3D printing. This method allows for easier and more detailed manufacturing than the above two methods and allows various architectures and control of mechanical stability [149]. The method can be applied to bone tissue engineering by taking advantage of its strong mechanical strength [150]. Chitosan material, which has been used as a soft scaffold in the form of a conventional hydrogel, can also be used for bone tissue engineering after reinforcement of its mechanical strength by applying this method. This can be done by incorporating a chitosan thermogel into a porous PCL scaffold [151].
2.3. Decellularized Native Tissue
One of the ideal scaffolds is a decellularized matrix that provides natural geometric morphology, flexibility, and mechanical strength, which is difficult to mimic perfectly with synthetic scaffolds. Recently, various approaches have been introduced to fabricate decellularized scaffolds, including perfusion of the whole organ (recommended for dense organs/tissues), application of a pressure gradient (employed for hollow tissues), use of supercritical fluid (appropriate for long-standing storage of decellularized scaffolds), and immersion and agitation (suitable for thin tissues) [152,153,154,155,156,157]. During the decellularization process, the cells are eliminated to inhibit inflammatory reactions or immediate rejection after implantation. The ECM derived from decellularized matrix provides an endogenous environment, from a biochemical and anatomical point of view, for regeneration of target organs. Recellularization is performed by direct injection of cells into the vein because of its proper vascular diameter and accessibility. This method is broadly used for blood vessel recellularization of different organs, such as the heart, lungs, and liver [158,159,160]. Another approach for recellularization is cell inoculation into mass media by allowing cells to recover through the circuit to seed scaffolds, but the efficiency of this method is lower than that of direct injection using veins [161]. In various studies, researchers investigated the effect of decellularized scaffolds on cell proliferation and construction of organs, including the liver, heart, lung, kidney, and pancreas [162,163,164,165,166,167].
2.4. Ultra-Low Attachment Surface
Cell culture plates can be covered with biomaterials with low cell-binding properties to prevent the cells from adhering to the surface. This method is one of the older techniques to generate self-assembled cellular structures in media for 3D cell culture. This system inhibits the attachment of cells to the surface of the culture plate, resulting in force floating of cells. Force floating improves cell-to-cell interactions, enabling multicellular aggregation. To provide attachment-resistant cell surfaces, cell culture plates or surfaces are coated with polymers that possess low cell-binding properties, such as 2-hydroxyethyl methacrylate (poly-HEMA), polyethylene glycol, chitosan, agar, and agarose [168,169,170,171,172]. These polymers allow greater cell-cell interactions rather than cell-substrate interactions, which enables spontaneous spheroid formation [173]. Cell culture plates could have flat or round surfaces. The flat surface causes the formation of irregularly sized spheroids. However, round surfaces are capable of generating single spheroids. Various types of cells have successfully formed spheroids on plates or round surfaces with low cell-binding properties [174,175,176,177,178]. In this system, cells accumulate as clusters and synthesize their own ECM. In addition, the signaling and communication between cells in spheroids were enhanced, and gap junctions were created that can facilitate the exchange of ions, small molecules and electrical currents. This method can be used for high-throughput screening [179]. Recently, Cho et al. fabricated new polymers with low cell-binding properties for spheroid generation [180]. Spontaneous spheroid formation was completed within a few days, and the size of the spheroids varied with the cell density.
3. Applications of Three-Dimensional Cell Culture
3.1. Cancer Research and Drug Screening
Three-dimensional (3D) cell culture has several applications in the field of biological science. These applications can be divided into three categories: cancer research, stem cell culture, and drug toxicity and screening. With the growing demand for effective validation in the field of oncology, there is a demand for research tools that provide clinically significant results for safety and efficacy testing of anticancer drugs. Moreover, 3D culture techniques have been able to produce 3D models, such as oncospheres, spheroids, and other tumor models that closely resemble the natural tumor microenvironment due to the proper supply of nutrients, oxygen, and intercellular interactions [181,182]. Increasing the scope of this technology could reduce the number of animals sacrificed for research. In addition, various meaningful results that could not be solved with animal models can be obtained.
Animal models and 2D culture systems have been a standard to determine anticancer drug effects on growth inhibition and apoptosis. However, the results produced from these systems showed relatively low similarity in clinical outcomes [183,184]. Owing to their close resemblance to tumor microenvironments, 3D culture systems are gradually being used to replace animal and 2D culture models for screening anticancer drugs that are in their initial stages of development. The spheroids contain proliferating cells on the surface and quiescent cells in the core due to limited penetration of nutrients and oxygen, and necrotic areas may be included in larger spheroids that resemble native solid tumors [185,186]. Several studies have shown differences in viability in 2D- and 3D-cultured cancer cells [187,188]. The cells were treated with the same concentration of tirapazamine (TPZ). Cells cultured in 2D were more resistant to treatment with TPZ and showed 72% viability. In contrast, cells cultured in 3D were more responsive to treatment with TPZ, with 40% viability. The difference was correlated with the fact that TPZ is a hypoxic activated cytotoxin, which works more effectively on cells cultured in 3D because the core of the spheroid is hypoxic due to limited oxygen diffusion. Some studies have reported that cancer cells in 3D models show increased drug resistance compared to those in 2D culture [189]. The variation in response to drugs can be explained by limitations on the mass transfer of the drugs in 3D culture systems compared to 2D cultured cells [190,191].
Cancer cells grown and maintained in 3D models show phenotypic heterogeneity [192]. This is important because cells of the same tumor group also change morphologically and functionally as gene expression, differentiation and proliferation rates change. Due to the diversity of cancer cells, it is difficult to develop drugs that accurately target tumor cells. In this regard, appropriate genetic modifications of ovarian epithelial cancer in a 3D environment have been investigated during development and progression [193,194,195]. Therefore, 3D models are used to study changes in gene expression-related tumor microenvironments. Moreover, 3D cell culture was used to confirm the presence of cancer stem cells [196]. Numerous studies have demonstrated that cancer stem cells can generate tumor-spheres by a suspension of single cells in serum-free conditions. Thus, scaffold-free methods are being used for 3D culture of cancer stem cells. In addition, 3D coculture of cancer cells and normal cells was used to confirm the specificity of drugs for cancer cells [197,198]. These studies suggest the possibility of 3D models in anticancer drug screening to more accurately represent tumor models.
3.2. Stem Cell Research and Drug Screening
Recently, stem cells have become an important research tool in biology, medicine and toxicology, leading to a rapidly developing new scientific field. The importance of stem cells is evidenced by the increasing number of articles published each year. This rapid development requires new means of providing high-quality, well-defined and scalable techniques for the generation of stem cells. It is essential to proliferate stem cells to be used as a tool in scientific disciplines. Embryoid bodies (EBs) are 3D aggregates generated in suspension by pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) [199]. EB differentiation is a common tool to generate specific cell lineages from PSCs [200,201]. Many 3D culture methods of PSCs in the form of EBs have been developed. Conventional methods for forming EBs include scaffold-free methods such as the ultra-low attachment (ULA) method, hanging drop methods, and suspension culture methods. In particular, EB formation can be more accurately controlled by the inoculation of known cell numbers within single drops suspended from the lid of a Petri dish [199,202]. While this method enables control of EB size by altering cell density per drop, the formation of hanging drops is labor intensive and does not facilitate scalable cultures. Additionally, the media cannot be readily exchanged without disturbing the EBs every 2–3 days. Recently, new methods have been developed to enable media exchange using a modified hanging drop plate [203]. In addition, these methods have also been designed to readily separate EBs within individual wells on adhesive substrates for the analysis of EBs [204,205]
One of the major efforts in the stem cell field is to develop therapeutic applications for the regeneration of diseased, dysfunctional or complex injured tissues. To that end, it is essential to secure technology for stem cell proliferation with increased cell viability and differentiation potential [206]. Various studies have been conducted to prove the convenience of direct transplantation, improved graft adherence, and increased cellular loading densities through 3D cell culture [207]. Studies have reported hydrogel encapsulation in 3D cell culture for regeneration of cartilage, bone, liver, cardiac, cortical, brain, and skin tissues [208,209,210,211,212,213,214]. This method facilitates cell growth, differentiation and migration of many cell types, such as neural stem cells, mesenchymal stem cells, adipose-derived stem cells, and PSCs. In addition, hydrogels with stem cells have demonstrated therapeutic effects in xenograft models [215].
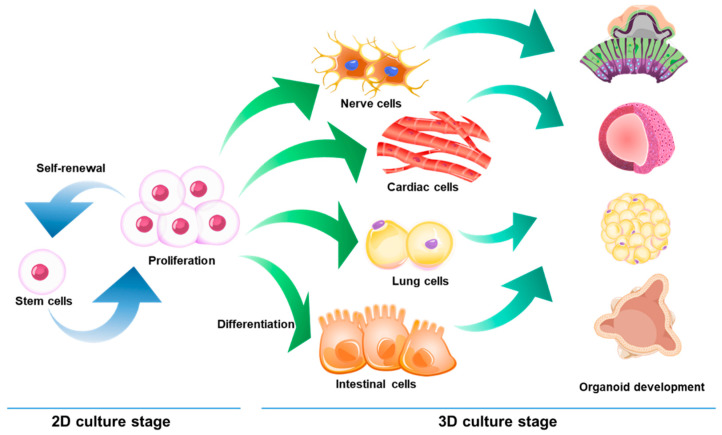
Three-dimensional (3D) cell culture has emerged as a promising method for the generation of organoids that could serve as a model to study various disease mechanisms or the toxicity of new drugs (Figure 2). Organoids are highly heterogeneous 3D structures that exhibit typical tissue architecture. Thus, organoids can serve as a valuable developmental model to explore new drugs [216,217]. To date, several protocols have been developed for the in vitro generation of organoids for the brain, gut, retina, liver, skin, and kidney [218,219,220,221,222]. Organoid formation generally requires culturing stem cells or progenitor cells in a 3D environment [223,224]. Hydrogels such as Matrigel or collagen gel are used to provide a 3D environment. When stem cells are used for the creation of the organoid, cells are allowed to form embryoid bodies. These embryoid bodies are an example of organoids. Cardiac organoids generated from cardiomyocytes have the ability to spontaneously contract [225]. These can produce electrophysiological measurements when plated on multielectrode arrays. Hairy human skin can be generated using a unique approach of organoid formation. PSCs can be differentiated into epidermis, dermis, fat, nerves and hair buds that produce hair [226]. Several studies have shown that more realistic outcomes can be achieved to study disease mechanisms by organoids and how cells respond to drug treatments [227,228]. However, standard protocols of organoid generation have yet to be defined. Therefore, the development of organoid culture platforms for large-scale production, organoid-based high-content screening platforms, and finely controlled systems should be continued. Finally, developing organoids generated from PSCs in an organ-on-chip system is also an area to be explored. The function of various organs or tissues, such as the lung, liver, kidney, heart, and gut, can be realized by using microfluidic device technology in an organ-on-chip system [229,230]. The successful development of organ-on-chip system will be able to mimic the systemic circulation of humans and animals under in vitro conditions and will provide us with vast benefits as an alternative model for drug screening and toxicology tests.
Figure 2.
Stem cell-derived organoids.
4. Conclusions and Future Perspectives
Herein, we introduced 3D cell culture methods and summarized the trends in the application of this technology. The 3D cell culture technologies have already emerged as an important tool that can facilitate research in the biomedical field with more realistic results. However, 3D culture methods have many problems to overcome. First, there are no standardized culture methods. The 3D cell culture method is more difficult and more complicated than that of 2D cell culture. Thus, it has low reproducibility and requires a high level of skill. The second problem is that 3D cell culture is still inappropriate for completely simulating the intrinsic properties of cells. One example is a vascular problem. In the body, tissues are constantly supplied with nutrients and oxygen from microvessels in the tissues. Cells cannot survive beyond 150 μm from a microvessel. Thus, angiogenesis is an important consideration for successful 3D cell culture. When tissue is regenerated in vitro, the 3D tissue should be supplied with nutrients and oxygen around it until blood vessels grow into the tissue. In this case, growth factors as well as vascular endothelial cells and stem cells may be used to promote angiogenesis. However, the problem is that necrosis can occur before blood vessels are formed due to the insufficient supply of nutrients and oxygen within the 3D tissue. Finally, even if this simulation is correctly achieved, there is still a lack of research on analytical methods for verification. Most analyses now depend on visual images. Therefore, it can be difficult to clearly judge the standards and grasp the characteristics inside 3D structures. For this reason, standardization of the analysis of 3D cell culture is still difficult. Although there are still many problems to overcome in 3D cell culture, the introduction of advanced 3D cell culture technologies has attracted several researchers to shift their attention from 2D to 3D cell culture systems. Thus, 3D cell culture technology is expected to contribute greatly to future research with the potential to create excellent outputs.
Acknowledgments
We thank Hye-Eun Shim (Research Group for Biomimetic Advanced Technology, KIT) for technical support in illustration of Figure 2.
Abbreviations
| 3D | Three-dimensional |
| 2D | Two-dimensional |
| PHHs | Primary human hepatocytes |
| CYP450 | cytochrome P-450 |
| ECM | Extracellular matrix |
| ULA | Ultra-low attachment |
| PVA | Poly (vinyl alcohol) |
| PLGA | Poly(lactic-co-glycolic acid) |
| pHEMA | Poly-2-hydroxyethyl methacrylate |
| PEG | Poly ethylene glycol |
| RGD | Arginine-glycine-aspartic acid |
| MSCs | Mesenchymal stem cells |
| PEG-SG | Four-arm succinimidyl glutarate polyethylene glycol |
| hMSC | Human mesenchymal stem cell |
| HUVEC | Human umbilical vein endothelial cell |
| hASC | Human adipose-derived stem cell |
| hiPSC-NPC | Human induced pluripotent stem cell-derived neural progenitor cell |
| HA | Hyaluronic acid |
| bFGF | Basic fibroblast growth factor |
| AcHA | Acetylated HA |
| PGA | Poly (glycolic acid) |
| PLA | Poly (lactic acid) |
| PCL | Polycarprolactone |
| SCPL | Solvent casting & particulate leaching |
| TPZ | Tirapazamine |
| EB | Embryoid body |
| PSC | Pluripotent stem cell |
| ESC | Embryonic stem cell |
| iPSC | Induced pluripotent stem cell |
Author Contributions
All authors have contributed to the conceptualization, writing and approved the final version of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Bio&Medical Technology Development Program (NRF-2016M3A9B4919655 and NRF-2019M3A9H1103331) and Basic Science Research Program (NRF-2020R1A2C2100794) of the National Research Foundation funded by the Korean government.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Acosta D., Anuforo D.C., Smith R.V. Cytotoxicity of acetaminophen and papaverine in primary cultures of rat hepatocytes. Toxicol. Appl. Pharmacol. 1980;53:306–314. doi: 10.1016/0041-008X(80)90431-7. [DOI] [PubMed] [Google Scholar]
- 2.Baharvand H., Hashemi S.M., Ashtiani S.K., Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int. J. Dev. Biol. 2006;50:645–652. doi: 10.1387/ijdb.052072hb. [DOI] [PubMed] [Google Scholar]
- 3.Knight E., Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015;227:746–756. doi: 10.1111/joa.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu K.-P., Li X.-F., Yu F.-S.X. Corneal Organ Culture Model for Assessing Epithelial Responses to Surfactants. Toxicol. Sci. 2000;58:306–314. doi: 10.1093/toxsci/58.2.306. [DOI] [PubMed] [Google Scholar]
- 5.Caliari S.R., Burdick J.A. A practical guide to hydrogels for cell culture. Nat. Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sepantafar M., Maheronnaghsh R., Mohammadi H., Radmanesh F., Hasani-Sadrabadi M.M., Ebrahimi M., Baharvand H. Engineered Hydrogels in Cancer Therapy and Diagnosis. Trends Biotechnol. 2017;35:1074–1087. doi: 10.1016/j.tibtech.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Debnath T., Ghosh S., Potlapuvu U.S., Kona L., Kamaraju S.R., Sarkar S., Gaddam S., Chelluri L.K. Proliferation and differentiation potential of human adipose-derived stem cells grown on chitosan hydrogel. PLoS ONE. 2015;10:e0120803. doi: 10.1371/journal.pone.0120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ware B.R., Durham M.J., Monckton C.P., Khetani S.R. A Cell Culture Platform to Maintain Long-term Phenotype of Primary Human Hepatocytes and Endothelial Cells. Cell. Mol. Gastroenterol. Hepatol. 2018;5:187–207. doi: 10.1016/j.jcmgh.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hira V.V.V., Breznik B., van Noorden C.J.F., Lah T., Molenaar R.J. 2D and 3D in vitro assays to quantify the invasive behavior of glioblastoma stem cells in response to SDF-1α. BioTechniques. 2020;69:339–346. doi: 10.2144/btn-2020-0046. [DOI] [PubMed] [Google Scholar]
- 10.Noonan J., Grassia G., MacRitchie N., Garside P., Guzik T.J., Bradshaw A.C., Maffia P. A Novel Triple-Cell Two-Dimensional Model to Study Immune-Vascular Interplay in Atherosclerosis. Front. Immunol. 2019;10:849. doi: 10.3389/fimmu.2019.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benoit D.S., Schwartz M.P., Durney A.R., Anseth K.S. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat. Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tibbitt M.W., Anseth K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dikovsky D., Bianco-Peled H., Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials. 2006;27:1496–1506. doi: 10.1016/j.biomaterials.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Wu L., Jing D., Ding J. A “room-temperature” injection molding/particulate leaching approach for fabrication of biodegradable three-dimensional porous scaffolds. Biomaterials. 2006;27:185–191. doi: 10.1016/j.biomaterials.2005.05.105. [DOI] [PubMed] [Google Scholar]
- 16.Dikici B.A., Claeyssens F. Basic Principles of Emulsion Templating and Its Use as an Emerging Manufacturing Method of Tissue Engineering Scaffolds. Front. Bioeng. Biotechnol. 2020;8:875. doi: 10.3389/fbioe.2020.00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt N.C., Hallam D., Karimi A., Mellough C.B., Chen J., Steel D.H.W., Lako M. 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomater. 2017;49:329–343. doi: 10.1016/j.actbio.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 18.King W.J., Jongpaiboonkit L., Murphy W.L. Influence of FGF2 and PEG hydrogel matrix properties on hMSC viability and spreading. J. Biomed. Mater. Res. A. 2010;93:1110–1123. doi: 10.1002/jbm.a.32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maximova N., Österberg M., Koljonen K., Stenius P. Lignin adsorption on cellulose fibre surfaces: Effect on surface chemistry, surface morphology and paper strength. Cellulose. 2001;8:113–125. doi: 10.1023/A:1016721822763. [DOI] [Google Scholar]
- 20.Hochleitner G., Chen F., Blum C., Dalton P.D., Amsden B., Groll J. Melt electrowriting below the critical translation speed to fabricate crimped elastomer scaffolds with non-linear extension behaviour mimicking that of ligaments and tendons. Acta Biomater. 2018;72:110–120. doi: 10.1016/j.actbio.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Gomes M.E., Azevedo H.S., Moreira A.R., Ellä V., Kellomäki M., Reis R.L. Starch–poly(ε-caprolactone) and starch–poly(lactic acid) fibre-mesh scaffolds for bone tissue engineering applications: Structure, mechanical properties and degradation behaviour. J. Tissue Eng. Regen. Med. 2008;2:243–252. doi: 10.1002/term.89. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C., Li Y., Peng G., Lei X., Zhang G., Gao Y. Decellularized liver matrix-modified chitosan fibrous scaffold as a substrate for C3A hepatocyte culture. J. Biomater. Sci. Polym. Ed. 2020;31:1041–1056. doi: 10.1080/09205063.2020.1738690. [DOI] [PubMed] [Google Scholar]
- 23.Xie M., Wang Z., Wan X., Weng J., Tu M., Mei J., Wang Z., Du X., Wang L., Chen C. Crosslinking effects of branched PEG on decellularized lungs of rats for tissue engineering. J. Biomater. Appl. 2019;34:965–974. doi: 10.1177/0885328219885068. [DOI] [PubMed] [Google Scholar]
- 24.Taylor D.A., Lee P.-F., Barac Y., Hochman-Mendez C., Sampaio L.C. Decellularization of whole hearts for cardiac regeneration. Emerg. Technol. Heart Dis. 2020;1:291–310. [Google Scholar]
- 25.DeQuach J.A., Yuan S.H., Goldstein L.S.B., Christman K.L. Decellularized Porcine Brain Matrix for Cell Culture and Tissue Engineering Scaffolds. Tissue Eng. Part A. 2011;17:2583–2592. doi: 10.1089/ten.tea.2010.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo Y., Jung Y., Kim S.H. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater. 2018;67:270–281. doi: 10.1016/j.actbio.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Tomomi G., Otsuji J., Bin A., Yoshimura M., Tomura D., Tateyama I., Minami Y., Yoshikawa K., Aiba J.E., Heuser T., et al. A 3D Sphere Culture System Containing Functional Polymers for Large-Scale Human Pluripotent Stem Cell Production. Stem Cell Rep. 2014;2:734–745. doi: 10.1016/j.stemcr.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howes L., Richardson R.D., Finlay D., Vuori K. 3-Dimensional culture systems for anti-cancer compound profiling and high-throughput screening reveal increases in EGFR inhibitor-mediated cytotoxicity compared to monolayer culture systems. PLoS ONE. 2014;9:e108283. doi: 10.1371/journal.pone.0108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cesarz Z., Tamama K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:9176357. doi: 10.1155/2016/9176357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong C.S., Zhou X., Han J., Huang C.Y., Nashed A., Khatri S., Mattson G., Fukunishi T., Zhang H., Hibino N. In vivo therapeutic applications of cell spheroids. Biotechnol. Adv. 2018;36:494–505. doi: 10.1016/j.biotechadv.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Daly C., Davidson M.D., Burdick J.A. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 2021;12:753. doi: 10.1038/s41467-021-21029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caló E., Khutoryanskiy V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015;65:252–267. doi: 10.1016/j.eurpolymj.2014.11.024. [DOI] [Google Scholar]
- 33.Schmidt J.J., Rowley J., Kong H.J. Hydrogels used for cell-based drug delivery. J. Biomed. Mater. Res. A. 2008;87:1113–1122. doi: 10.1002/jbm.a.32287. [DOI] [PubMed] [Google Scholar]
- 34.Senol S., Akyol E. Preparation and characterization of pH-sensitive hydrogels from photo-crosslinked poly(ethylene glycol) diacrylate incorporating titanium dioxide. Mater. Sci. Pol. 2020;38:443–449. [Google Scholar]
- 35.Nam S., Stowers R., Lou J., Xia Y., Chaudhuri O. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials. 2019;200:15–24. doi: 10.1016/j.biomaterials.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampe K.J., Mooney R.G., Bjugstad K.B., Mahoney M.J. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. J. Biomed. Mater. Res. Part A. 2010;94A:1162–1171. doi: 10.1002/jbm.a.32787. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y., Li M., Liu B., Xiang J., Cui Z., Qu X., Qiu D., Tian Y., Yang Z. Ultra-tough injectable cytocompatible hydrogel for 3D cell culture and cartilage repair. J. Mater. Chem. B. 2018;6:1351–1358. doi: 10.1039/C7TB03177G. [DOI] [PubMed] [Google Scholar]
- 38.Muduli S., Chen L.-H., Li M.-P., Heish Z.-w., Liu C.-H., Kumar S., Alarfaj A.A., Munusamy M.A., Benelli G., Murugan K., et al. Stem cell culture on polyvinyl alcohol hydrogels having different elasticity and immobilized with ECM-derived oligopeptides. J. Polym. Eng. 2017;37:647–660. doi: 10.1515/polyeng-2016-0193. [DOI] [Google Scholar]
- 39.Passos M.F., Carvalho N.M.S., Rodrigues A.A., Bavaresco V.P., Jardini A.L., Maciel M.R.W., Filho R.M. PHEMA Hydrogels Obtained by Infrared Radiation for Cartilage Tissue Engineering. Int. J. Chem. Eng. 2019;2019:4249581. doi: 10.1155/2019/4249581. [DOI] [Google Scholar]
- 40.Mendez U., Zhou H., Shikanov A. Synthetic PEG Hydrogel for Engineering the Environment of Ovarian Follicles. Methods Mol. Biol. 2018;1758:115–128. doi: 10.1007/978-1-4939-7741-3_9. [DOI] [PubMed] [Google Scholar]
- 41.Wang D., Yang X., Liu Q., Yu L., Ding J. Enzymatically cross-linked hydrogels based on a linear poly(ethylene glycol) analogue for controlled protein release and 3D cell culture. J. Mater. Chem. B. 2018;6:6067–6079. doi: 10.1039/C8TB01949E. [DOI] [PubMed] [Google Scholar]
- 42.Andrée B., Ichanti H., Kalies S., Heisterkamp A., Strauß S., Vogt P.-M., Haverich A., Hilfiker A. Formation of three-dimensional tubular endothelial cell networks under defined serum-free cell culture conditions in human collagen hydrogels. Sci. Rep. 2019;9:5437. doi: 10.1038/s41598-019-41985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hached F., Vinatier C., Pinta P.-G., Hulin P., le Visage C., Weiss P., Guicheux J., Billon-Chabaud A., Grimandi G. Polysaccharide Hydrogels Support the Long-Term Viability of Encapsulated Human Mesenchymal Stem Cells and Their Ability to Secrete Immunomodulatory Factors. Stem Cells Int. 2017;2017:9303598. doi: 10.1155/2017/9303598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frampton J.P., Hynd M.R., Shuler M.L., Shain W. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed. Mater. 2011;6:015002. doi: 10.1088/1748-6041/6/1/015002. [DOI] [PubMed] [Google Scholar]
- 45.Wu S., Xu R., Duan B., Jiang P. Three-dimensional hyaluronic acid hydrogel-based models for in vitro human iPSC-derived NPC culture and differentiation. J. Mater. Chem. B. 2017;5:3870–3878. doi: 10.1039/C7TB00721C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suo A., Xu W., Wang Y., Sun T., Ji L., Qian J. Dual-degradable and injectable hyaluronic acid hydrogel mimicking extracellular matrix for 3D culture of breast cancer MCF-7 cells. Carbohydr. Polym. 2019;211:336–348. doi: 10.1016/j.carbpol.2019.01.115. [DOI] [PubMed] [Google Scholar]
- 47.Bao Z., Xian C., Yuan Q., Liu G., Wu J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019;8:1900670. doi: 10.1002/adhm.201900670. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y.-B., Polio S., Lee W., Dai G., Menon L., Carroll R.S., Yoo S.-S. Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp. Neurol. 2010;223:645–652. doi: 10.1016/j.expneurol.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Lou J., Stowers R., Nam S., Xia Y., Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials. 2018;154:213–222. doi: 10.1016/j.biomaterials.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Park S.H., Kim K., Lim J.H., Lee S.J. Selective lithium and magnesium adsorption by phosphonate metal-organic framework-incorporated alginate hydrogel inspired from lithium adsorption characteristics of brown algae. Sep. Purif. Technol. 2019;212:611–618. doi: 10.1016/j.seppur.2018.11.067. [DOI] [Google Scholar]
- 51.Ullah F., Javed F., Zakaria M.R., Jamila D.N., Khattak R., Khan A., Akil H.M. Determining the Molecular-weight and interfacial properties of chitosan built nanohydrogel for controlled drug delivery applications. Biointerface Res. Appl. Chem. 2019;9:4452–4457. [Google Scholar]
- 52.Ullah F., Javed F., Khan A., Kudus M., Jamila N., Minhaz A., Akil H. Synthesis and surface modification of chitosan built nanohydrogel with antiviral and antimicrobial agent for controlled drug delivery. Biointerface Res. Appl. Chem. 2019;9:4439–4445. [Google Scholar]
- 53.Kim J.-S., Kim T.H., Kang D.L., Baek S.Y., Lee Y., Koh Y.-G., Kim Y.I. Chondrogenic differentiation of human ASCs by stiffness control in 3D fibrin hydrogel. Biochem. Biophys. Res. Commun. 2020;522:213–219. doi: 10.1016/j.bbrc.2019.11.049. [DOI] [PubMed] [Google Scholar]
- 54.Faraj K.A., van Kuppevelt T.H., Daamen W.F. Construction of Collagen Scaffolds That Mimic the Three-Dimensional Architecture of Specific Tissues. Tissue Eng. 2007;13:2387–2394. doi: 10.1089/ten.2006.0320. [DOI] [PubMed] [Google Scholar]
- 55.Cui Z.-K., Li S.-Y., Liao K., Wang Z.-J., Guo Y.-L., Tang L.-S., Tang S.-B., Ma J., Chen J.-S. Characteristics of neural growth and cryopreservation of the dorsal root ganglion using three-dimensional collagen hydrogel culture vs. conventional culture. Neural Regen. Res. 2021;16:1856–1864. doi: 10.4103/1673-5374.306097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smeriglio P., Dhulipala L., Lai J.H., Goodman S.B., Dragoo J.L., Smith R.L., Maloney W.J., Yang F., Bhutani N. Collagen VI Enhances Cartilage Tissue Generation by Stimulating Chondrocyte Proliferation. Tissue Eng. Part A. 2014;21:840–849. doi: 10.1089/ten.tea.2014.0375. [DOI] [PubMed] [Google Scholar]
- 57.Pachence J.M. Collagen-based devices for soft tissue repair. J. Biomed. Mater. Res. 1996;33:35–40. doi: 10.1002/(SICI)1097-4636(199621)33:1<35::AID-JBM6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 58.Benea H., Tomoaia G., Soritau O., Pasca R.D. A Review on the Reconstruction of Articular Cartilage Using Collagen Scaffolds. Rom. Biotechnol. Lett. 2016;21:11735–11743. [Google Scholar]
- 59.Jin G.-Z., Kim H.-W. Effects of Type I Collagen Concentration in Hydrogel on the Growth and Phenotypic Expression of Rat Chondrocytes. Tissue Eng. Regen. Med. 2017;14:383–391. doi: 10.1007/s13770-017-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamaddon M., Burrows M., Ferreira S.A., Dazzi F., Apperley J.F., Bradshaw A., Brand D.D., Czernuszka J., Gentleman E. Monomeric, porous type II collagen scaffolds promote chondrogenic differentiation of human bone marrow mesenchymal stem cells in vitro. Sci. Rep. 2017;7:43519. doi: 10.1038/srep43519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kilmer C.E., Battistoni C.M., Cox A., Breur G.J., Panitch A., Liu J.C. Collagen Type I and II Blend Hydrogel with Autologous Mesenchymal Stem Cells as a Scaffold for Articular Cartilage Defect Repair. Acs Biomater. Sci. Eng. 2020;6:3464–3476. doi: 10.1021/acsbiomaterials.9b01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irawan V., Sung T.-C., Higuchi A., Ikoma T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018;15:673–697. doi: 10.1007/s13770-018-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez-Puyana V., Romero A., Guerrero A. Influence of collagen concentration and glutaraldehyde on collagen-based scaffold properties. J. Biomed. Mater. Res. Part A. 2016;104:1462–1468. doi: 10.1002/jbm.a.35671. [DOI] [PubMed] [Google Scholar]
- 64.Nong L.-M., Zhou D., Zheng D., Jiang Y.-Q., Xu N.-W., Zhao G.-Y., Wei H., Zhou S.-Y., Han H., Han L. The effect of different cross-linking conditions of EDC/NHS on type II collagen scaffolds: An in vitro evaluation. Cell Tissue Bank. 2019;20:557–568. doi: 10.1007/s10561-019-09790-7. [DOI] [PubMed] [Google Scholar]
- 65.Lotz C., Schmid F.F., Oechsle E., Monaghan M.G., Walles H., Groeber-Becker F. Cross-linked Collagen Hydrogel Matrix Resisting Contraction To Facilitate Full-Thickness Skin Equivalents. Acs Appl. Mater. Interfaces. 2017;9:20417–20425. doi: 10.1021/acsami.7b04017. [DOI] [PubMed] [Google Scholar]
- 66.Li H., Koenig A.M., Sloan P., Leipzig N.D. In vivo assessment of guided neural stem cell differentiation in growth factor immobilized chitosan-based hydrogel scaffolds. Biomaterials. 2014;35:9049–9057. doi: 10.1016/j.biomaterials.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 67.Sun Y., Yang C., Zhu X., Wang J.-J., Liu X.-Y., Yang X.-P., An X.-W., Liang J., Dong H.-J., Jiang W., et al. 3D printing collagen/chitosan scaffold ameliorated axon regeneration and neurological recovery after spinal cord injury. J. Biomed. Mater. Res. Part A. 2019;107:1898–1908. doi: 10.1002/jbm.a.36675. [DOI] [PubMed] [Google Scholar]
- 68.Ying H., Zhou J., Wang M., Su D., Ma Q., Lv G., Chen J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C. 2019;101:487–498. doi: 10.1016/j.msec.2019.03.093. [DOI] [PubMed] [Google Scholar]
- 69.Kolodney M.S., Wysolmerski R.B. Isometric contraction by fibroblasts and endothelial cells in tissue culture: A quantitative study. J. Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Price R.D., Berry M.G., Navsaria H.A. Hyaluronic acid: The scientific and clinical evidence. J. Plast. Reconstr. Aesthetic Surg. 2007;60:1110–1119. doi: 10.1016/j.bjps.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Liu L., Liu Y., Li J., Du G., Chen J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Factories. 2011;10:99. doi: 10.1186/1475-2859-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lei Y., Gojgini S., Lam J., Segura T. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials. 2011;32:39–47. doi: 10.1016/j.biomaterials.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seidlits S.K., Khaing Z.Z., Petersen R.R., Nickels J.D., Vanscoy J.E., Shear J.B., Schmidt C.E. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–3940. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 74.Inoue M., Katakami C. The effect of hyaluronic acid on corneal epithelial cell proliferation. Investig. Ophthalmol. Vis. Sci. 1993;34:2313–2315. [PubMed] [Google Scholar]
- 75.Chen J.-W.E., Pedron S., Shyu P., Hu Y., Sarkaria J.N., Harley B.A.C. Influence of Hyaluronic Acid Transitions in Tumor Microenvironment on Glioblastoma Malignancy and Invasive Behavior. Front. Mater. 2018;5:39. doi: 10.3389/fmats.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boregowda R.K., Appaiah H.N., Siddaiah M., Kumarswamy S.B., Sunila S., Thimmaiah K.N., Mortha K., Toole B., Banerjee S.D. Expression of hyaluronan in human tumor progression. J. Carcinog. 2006;5:2. doi: 10.1186/1477-3163-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Assmann V., Fieber C., Herrlich P., Hofmann M., Termeer C.C., Ahrens T., Simon J.C. CD44 is the Principal Mediator of Hyaluronic-Acid-Induced Melanoma Cell Proliferation. J. Investig. Dermatol. 2001;116:93–101. doi: 10.1046/j.1523-1747.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 78.Bucatariu S.-M., Constantin M., Varganici C.-D., Rusu D., Nicolescu A., Prisacaru I., Carnuta M., Anghelache M., Calin M., Ascenzi P., et al. A new sponge-type hydrogel based on hyaluronic acid and poly(methylvinylether-alt-maleic acid) as a 3D platform for tumor cell growth. Int. J. Biol. Macromol. 2020;165:2528–2540. doi: 10.1016/j.ijbiomac.2020.10.095. [DOI] [PubMed] [Google Scholar]
- 79.Häckel S., Zolfaghar M., Du J., Hoppe S., Benneker L.M., Garstka N., Peroglio M., Alini M., Grad S., Yayon A., et al. Fibrin-Hyaluronic Acid Hydrogel (RegenoGel) with Fibroblast Growth Factor-18 for In Vitro 3D Culture of Human and Bovine Nucleus Pulposus Cells. Int. J. Mol. Sci. 2019;20:5036. doi: 10.3390/ijms20205036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee E.J., Kang E., Kang S.-W., Huh K.M. Thermo-irreversible glycol chitosan/hyaluronic acid blend hydrogel for injectable tissue engineering. Carbohydr. Polym. 2020;244:116432. doi: 10.1016/j.carbpol.2020.116432. [DOI] [PubMed] [Google Scholar]
- 81.Ning L., Zhu N., Mohabatpour F., Sarker M.D., Schreyer D.J., Chen X. Bioprinting Schwann cell-laden scaffolds from low-viscosity hydrogel compositions. J. Mater. Chem. B. 2019;7:4538–4551. doi: 10.1039/C9TB00669A. [DOI] [Google Scholar]
- 82.Lee K.Y., Mooney D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee K.Y., Mooney D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001;101:1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 84.Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S.A., Weaver J.C., Huebsch N., Lee H.-P., Lippens E., Duda G.N., et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huebsch N., Arany P.R., Mao A.S., Shvartsman D., Ali O.A., Bencherif S.A., Rivera-Feliciano J., Mooney D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nakamura M., Iwanaga S., Henmi C., Arai K., Nishiyama Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication. 2010;2:014110. doi: 10.1088/1758-5082/2/1/014110. [DOI] [PubMed] [Google Scholar]
- 87.Gao T., Gillispie G.J., Copus J.S., Pr A.K., Seol Y.-J., Atala A., Yoo J.J., Lee S.J. Optimization of gelatin–alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication. 2018;10:034106. doi: 10.1088/1758-5090/aacdc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jia J., Richards D.J., Pollard S., Tan Y., Rodriguez J., Visconti R.P., Trusk T.C., Yost M.J., Yao H., Markwald R.R., et al. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014;10:4323–4331. doi: 10.1016/j.actbio.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alagpulinsa D.A., Cao J.J.L., Driscoll R.K., Sîrbulescu R.F., Penson M.F.E., Sremac M., Engquist E.N., Brauns T.A., Markmann J.F., Melton D.A., et al. Alginate-microencapsulation of human stem cell–derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am. J. Transplant. 2019;19:1930–1940. doi: 10.1111/ajt.15308. [DOI] [PubMed] [Google Scholar]
- 90.De Vos P., Faas M.M., Strand B., Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603–5617. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 91.Stock A., Manzoli V., de Toni T., Abreu M.M., Poh Y.-C., Ye L., Roose A., Pagliuca F.W., Thanos C., Ricordi C., et al. Conformal Coating of Stem Cell-Derived Islets for β Cell Replacement in Type 1 Diabetes. Stem Cell Rep. 2020;14:91–104. doi: 10.1016/j.stemcr.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng K., Kisaalita W.S. Exploring cellular adhesion and differentiation in a micro-/nano-hybrid polymer scaffold. Biotechnol. Prog. 2010;26:838–846. doi: 10.1002/btpr.391. [DOI] [PubMed] [Google Scholar]
- 93.Weigel T., Schinkel G., Lendlein A. Design and preparation of polymeric scaffolds for tissue engineering. Expert Rev. Med Devices. 2006;3:835–851. doi: 10.1586/17434440.3.6.835. [DOI] [PubMed] [Google Scholar]
- 94.Mondal S., Nguyen T.P., Pham V.H., Hoang G., Manivasagan P., Kim M.H., Nam S.Y., Oh J. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram. Int. 2020;46:3443–3455. doi: 10.1016/j.ceramint.2019.10.057. [DOI] [Google Scholar]
- 95.Eslami H., Lisar H.A., Kashi T.S.J., Tahriri M., Ansari M., Rafiei T., Bastami F., Shahin-Shamsabadi A., Abbas F.M., Tayebi L. Poly(lactic-co-glycolic acid)(PLGA)/TiO2 nanotube bioactive composite as a novel scaffold for bone tissue engineering: In vitro and in vivo studies. Biologicals. 2018;53:51–62. doi: 10.1016/j.biologicals.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Le C.M.T., Monajjemi M., Mollaamin F., Dang C. Simulation & modelling of dilute solutions in drop-on-demand inkjet printing: A review. Biointerface Res. Appl. Chem. 2019;9:4474–4484. [Google Scholar]
- 97.Trakoolwannachai V., Kheolamai P., Ummartyotin S. Characterization of hydroxyapatite from eggshell waste and polycaprolactone (PCL) composite for scaffold material. Compos. Part B: Eng. 2019;173:106974. doi: 10.1016/j.compositesb.2019.106974. [DOI] [Google Scholar]
- 98.Bagheri-Hosseinabadi Z., Mesbah-Namin S.A., Salehinejad P., Seyedi F. Fibrin scaffold could promote survival of the human adipose-derived stem cells during differentiation into cardiomyocyte-like cells. Cell Tissue Res. 2018;372:571–589. doi: 10.1007/s00441-018-2799-9. [DOI] [PubMed] [Google Scholar]
- 99.Yan R., Chen Y., Gu Y., Tang C., Huang J., Hu Y., Zheng Z., Ran J., Heng B., Chen X., et al. A collagen-coated sponge silk scaffold for functional meniscus regeneration. J. Tissue Eng. Regen. Med. 2019;13:156–173. doi: 10.1002/term.2777. [DOI] [PubMed] [Google Scholar]
- 100.Thadavirul N., Pavasant P., Supaphol P. Development of polycaprolactone porous scaffolds by combining solvent casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. J. Biomed. Mater. Res. Part A. 2014;102:3379–3392. doi: 10.1002/jbm.a.35010. [DOI] [PubMed] [Google Scholar]
- 101.Sin D., Miao X., Liu G., Wei F., Chadwick G., Yan C., Friis T. Polyurethane (PU) scaffolds prepared by solvent casting/particulate leaching (SCPL) combined with centrifugation. Mater. Sci. Eng. 2010;30:78–85. doi: 10.1016/j.msec.2009.09.002. [DOI] [Google Scholar]
- 102.Zhou S., Bismarck A., Steinke J.H.G. Ion-responsive alginate based macroporous injectable hydrogel scaffolds prepared by emulsion templating. J. Mater. Chem. B. 2013;1:4736–4745. doi: 10.1039/c3tb20888e. [DOI] [PubMed] [Google Scholar]
- 103.Yadav A., Pal J., Nandan B., Srivastava R.K. Macroporous scaffolds of cross-linked Poly(ɛ-caprolactone) via high internal phase emulsion templating. Polymer. 2019;176:66–73. doi: 10.1016/j.polymer.2019.05.034. [DOI] [Google Scholar]
- 104.Salerno A., Oliviero M., Di Maio E., Iannace S., Netti P.A. Design of porous polymeric scaffolds by gas foaming of heterogeneous blends. J. Mater. Sci. 2009;20:2043–2051. doi: 10.1007/s10856-009-3767-4. [DOI] [PubMed] [Google Scholar]
- 105.Bak T.-Y., Kook M.-S., Jung S.-C., Kim B.-H. Biological Effect of Gas Plasma Treatment on CO2 Gas Foaming/Salt Leaching Fabricated Porous Polycaprolactone Scaffolds in Bone Tissue Engineering. J. Nanomater. 2014;2014:657542. doi: 10.1155/2014/657542. [DOI] [Google Scholar]
- 106.Ji C., Annabi N., Khademhosseini A., Dehghani F. Fabrication of porous chitosan scaffolds for soft tissue engineering using dense gas CO2. Acta Biomater. 2011;7:1653–1664. doi: 10.1016/j.actbio.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 107.Gomes M.E., Ribeiro A.S., Malafaya P.B., Reis R.L., Cunha A.M. A new approach based on injection moulding to produce biodegradable starch-based polymeric scaffolds: Morphology, mechanical and degradation behaviour. Biomaterials. 2001;22:883–889. doi: 10.1016/S0142-9612(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 108.El-Ayoubi R., Eliopoulos N., Diraddo R., Galipeau J., Yousefi A.-M. Design and Fabrication of 3D Porous Scaffolds to Facilitate Cell-Based Gene Therapy. Tissue Eng. Part A. 2008;14:1037–1048. doi: 10.1089/ten.tea.2006.0418. [DOI] [PubMed] [Google Scholar]
- 109.Bonfield W. Designing porous scaffolds for tissue engineering. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006;364:227–232. doi: 10.1098/rsta.2005.1692. [DOI] [PubMed] [Google Scholar]
- 110.Prasad M., Sankar R., Katiyar V. State of Art on Solvent Casting Particulate Leaching Method for Orthopedic ScaffoldsFabrication. Mater. Today. 2017;4:898–907. doi: 10.1016/j.matpr.2017.01.101. [DOI] [Google Scholar]
- 111.Deng Y., Zhang M., Chen X., Pu X., Liao X., Huang Z., Yin G. A novel akermanite/poly (lactic-co-glycolic acid) porous composite scaffold fabricated via a solvent casting-particulate leaching method improved by solvent self-proliferating process. Regen. Biomater. 2017;4:233–242. doi: 10.1093/rb/rbx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiao Y., Zhou M., Zhang M., Liu W., Zhou Y., Lang M. Hepatocyte culture on 3D porous scaffolds of PCL/PMCL. Colloids Surf. B. 2019;173:185–193. doi: 10.1016/j.colsurfb.2018.09.064. [DOI] [PubMed] [Google Scholar]
- 113.Shim J.-H., Kim A.J., Park J.Y., Yi N., Kang I., Park J., Rhie J.-W., Cho D.-W. Effect of solid freeform fabrication-based polycaprolactone/poly(lactic-co-glycolic acid)/collagen scaffolds on cellular activities of human adipose-derived stem cells and rat primary hepatocytes. J. Mater. Sci. 2013;24:1053–1065. doi: 10.1007/s10856-013-4867-8. [DOI] [PubMed] [Google Scholar]
- 114.Riesco R., Boyer L., Blosse S., Lefebvre P.M., Assemat P., Leichle T., Accardo A., Malaquin L. Water-in-PDMS Emulsion Templating of Highly Interconnected Porous Architectures for 3D Cell Culture. Acs Appl. Mater. Interfaces. 2019;11:28631–28640. doi: 10.1021/acsami.9b07564. [DOI] [PubMed] [Google Scholar]
- 115.Wu S., Liu X., Yeung K.W.K., Liu C., Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. 2014;80:1–36. doi: 10.1016/j.mser.2014.04.001. [DOI] [Google Scholar]
- 116.Lemon G., Waters S.L., Rose F.R.A.J., King J.R. Mathematical modelling of human mesenchymal stem cell proliferation and differentiation inside artificial porous scaffolds. J. Theor. Biol. 2007;249:543–553. doi: 10.1016/j.jtbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 117.Zhang M., Boughton P., Rose B., Lee C.S., Hong A.M. The Use of Porous Scaffold as a Tumor Model. Int. J. Biomater. 2013;2013:396056. doi: 10.1155/2013/396056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dar A., Shachar M., Leor J., Cohen S. Optimization of cardiac cell seeding and distribution in 3D porous alginate scaffolds. Biotechnol. Bioeng. 2002;80:305–312. doi: 10.1002/bit.10372. [DOI] [PubMed] [Google Scholar]
- 119.Ardila D.C., Tamimi E., Doetschman T., Wagner W.R., Geest J.P.V. Modulating smooth muscle cell response by the release of TGFβ2 from tubular scaffolds for vascular tissue engineering. J. Control. Release. 2019;299:44–52. doi: 10.1016/j.jconrel.2019.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ren T., Ren J., Jia X., Pan K. The bone formation in vitro and mandibular defect repair using PLGA porous scaffolds. J. Biomed. Mater. Res. Part A. 2005;74A:562–569. doi: 10.1002/jbm.a.30324. [DOI] [PubMed] [Google Scholar]
- 121.Chang N.J., Lam C.F., Lin C.C., Chen W.L., Li C.F., Lin Y.T., Yeh M.L. Transplantation of autologous endothelial progenitor cells in porous PLGA scaffolds create a microenvironment for the regeneration of hyaline cartilage in rabbits. Osteoarthr. Cartil. 2013;21:1613–1622. doi: 10.1016/j.joca.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 122.Bloise N., Berardi E., Gualandi C., Zaghi E., Gigli M., Duelen R., Ceccarelli G., Cortesi E.E., Costamagna D., Bruni G., et al. Ether-Oxygen Containing Electrospun Microfibrous and Sub-Microfibrous Scaffolds Based on Poly(butylene 1,4-cyclohexanedicarboxylate) for Skeletal Muscle Tissue Engineering. Int. J. Mol. Sci. 2018;19:3212. doi: 10.3390/ijms19103212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.MacQueen L.A., Alver C.G., Chantre C.O., Ahn S., Cera L., Gonzalez G.M., O’Connor B.B., Drennan D.J., Peters M.M., Motta S.E., et al. Muscle tissue engineering in fibrous gelatin: Implications for meat analogs. NPJ Sci. Food. 2019;3:20. doi: 10.1038/s41538-019-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jang S.R., Kim J.I., Park C.H., Kim C.S. The controlled design of electrospun PCL/silk/quercetin fibrous tubular scaffold using a modified wound coil collector and L-shaped ground design for neural repair. Mater. Sci. Eng. C. 2020;111:110776. doi: 10.1016/j.msec.2020.110776. [DOI] [PubMed] [Google Scholar]
- 125.Liu H., Wang Y., Yang Y., Wang A., Huang C., Zhao Z., Li P., Liu M., Fan Y. Aligned graphene/silk fibroin conductive fibrous scaffolds for guiding neurite outgrowth in rat spinal cord neurons. J. Biomed. Mater. Res. Part A. 2020 doi: 10.1002/jbm.a.37031. [DOI] [PubMed] [Google Scholar]
- 126.Joshi J., Brennan D., Beachley V., Kothapalli C.R. Cardiomyogenic differentiation of human bone marrow-derived mesenchymal stem cell spheroids within electrospun collagen nanofiber mats. J. Biomed. Mater. Res. Part A. 2018;106:3303–3312. doi: 10.1002/jbm.a.36530. [DOI] [PubMed] [Google Scholar]
- 127.Damaraju S.M., Shen Y., Elele E., Khusid B., Eshghinejad A., Li J., Jaffe M., Arinzeh T.L. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials. 2017;149:51–62. doi: 10.1016/j.biomaterials.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 128.Rahmani A., Nadri S., Kazemi H.S., Mortazavi Y., Sojoodi M. Conductive electrospun scaffolds with electrical stimulation for neural differentiation of conjunctiva mesenchymal stem cells. Artif. Organs. 2019;43:780–790. doi: 10.1111/aor.13425. [DOI] [PubMed] [Google Scholar]
- 129.Brennan M., Eichholz K.F., Hoey D.A. The effect of pore size within fibrous scaffolds fabricated using melt electrowriting on human bone marrow stem cell osteogenesis. Biomed. Mater. 2019;14:065016. doi: 10.1088/1748-605X/ab49f2. [DOI] [PubMed] [Google Scholar]
- 130.Birhanu G., Javar H.A., Seyedjafari E., Zandi-Karimi A., Telgerd M.D. An improved surface for enhanced stem cell proliferation and osteogenic differentiation using electrospun composite PLLA/P123 scaffold. Artif. CellsNanomed. Biotechnol. 2018;46:1274–1281. doi: 10.1080/21691401.2017.1367928. [DOI] [PubMed] [Google Scholar]
- 131.Boland E.D., Wnek G.E., Simpson D.G., Pawlowski K.J., Bowlin G.L. Tailoring tissue engineering scaffolds using electrostatic processing techniques: A study of poly(glycolic acid) electrospinning. J. Macromol. Sci. Part A. 2001;38:1231–1243. doi: 10.1081/MA-100108380. [DOI] [Google Scholar]
- 132.Eberli D., Filho L.F., Atala A., Yoo J.J. Composite scaffolds for the engineering of hollow organs and tissues. Methods. 2009;47:109–115. doi: 10.1016/j.ymeth.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 133.Sartuqui J., D’Elía N.L., Ercoli D., de Alcazar D.S., Cortajarena A.L., Messina P.V. Mechanical performance of gelatin fiber mesh scaffolds reinforced with nano-hydroxyapatite under bone damage mechanisms. Mater. Today Commun. 2019;19:140–147. doi: 10.1016/j.mtcomm.2019.01.004. [DOI] [Google Scholar]
- 134.Lee S.J., Oh S.H., Liu J., Soker S., Atala A., Yoo J.J. The use of thermal treatments to enhance the mechanical properties of electrospun poly(ɛ-caprolactone) scaffolds. Biomaterials. 2008;29:1422–1430. doi: 10.1016/j.biomaterials.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 135.Hosseini V., Evrova O., Hoerstrup S.P., Vogel V. A Simple Modification Method to Obtain Anisotropic and Porous 3D Microfibrillar Scaffolds for Surgical and Biomedical Applications. Small. 2018;14:1702650. doi: 10.1002/smll.201702650. [DOI] [PubMed] [Google Scholar]
- 136.Lee S., Nagata F., Kato K., Nakano T. Bone apatite anisotropic structure control via designing fibrous scaffolds. RSC Adv. 2020;10:13500–13506. doi: 10.1039/D0RA01295E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nam Y.S., Park T.G. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J. Biomed. Mater. Res. 1999;47:8–17. doi: 10.1002/(SICI)1097-4636(199910)47:1<8::AID-JBM2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 138.Tu C., Cai Q., Yang J., Wan Y., Bei J., Wang S. The fabrication and characterization of poly(lactic acid) scaffolds for tissue engineering by improved solid–liquid phase separation. Polym. Adv. Technol. 2003;14:565–573. doi: 10.1002/pat.370. [DOI] [Google Scholar]
- 139.Zhang S., Gelain F., Zhao X. Designer self-assembling peptide nanofiber scaffolds for 3D tissue cell cultures. Semin. Cancer Biol. 2005;15:413–420. doi: 10.1016/j.semcancer.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 140.Chen W., Ma J., Zhu L., Morsi Y., Ei-Hamshary H., Al-Deyab S.S., Mo X. Superelastic, superabsorbent and 3D nanofiber-assembled scaffold for tissue engineering. Colloids Surf. B. 2016;142:165–172. doi: 10.1016/j.colsurfb.2016.02.050. [DOI] [PubMed] [Google Scholar]
- 141.Wang S., Nagrath D., Chen P.C., Berthiaume F., Yarmush M.L. Three-Dimensional Primary Hepatocyte Culture in Synthetic Self-Assembling Peptide Hydrogel. Tissue Eng. Part A. 2008;14:227–236. doi: 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- 142.Galler K.M., Cavender A., Yuwono V., Dong H., Shi S., Schmalz G., Hartgerink J.D., D’Souza R.N. Self-Assembling Peptide Amphiphile Nanofibers as a Scaffold for Dental Stem Cells. Tissue Eng. Part A. 2008;14:2051–2058. doi: 10.1089/ten.tea.2007.0413. [DOI] [PubMed] [Google Scholar]
- 143.Thorvaldsson A., Stenhamre H., Gatenholm P., Walkenström P. Electrospinning of Highly Porous Scaffolds for Cartilage Regeneration. Biomacromolecules. 2008;9:1044–1049. doi: 10.1021/bm701225a. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Y., Ouyang H., Lim C.T., Ramakrishna S., Huang Z.-M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. Part B. 2005;72B:156–165. doi: 10.1002/jbm.b.30128. [DOI] [PubMed] [Google Scholar]
- 145.Lannutti J., Reneker D., Ma T., Tomasko D., Farson D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C. 2007;27:504–509. doi: 10.1016/j.msec.2006.05.019. [DOI] [Google Scholar]
- 146.Cai Q., Yang J., Bei J., Wang S. A novel porous cells scaffold made of polylactide–dextran blend by combining phase-separation and particle-leaching techniques. Biomaterials. 2002;23:4483–4492. doi: 10.1016/S0142-9612(02)00168-0. [DOI] [PubMed] [Google Scholar]
- 147.Blum S., Soto C.M., Wilson C.D., Brower T.L., Pollack S.K., Schull T.L., Chatterji A., Lin T., Johnson J.E., Amsinck C., et al. An Engineered Virus as a Scaffold for Three-Dimensional Self-Assembly on the Nanoscale. Small. 2005;1:702–706. doi: 10.1002/smll.200500021. [DOI] [PubMed] [Google Scholar]
- 148.Liu Y., Wang S., Lee J.W., Kotov N.A. A Floating Self-Assembly Route to Colloidal Crystal Templates for 3D Cell Scaffolds. Chem. Mater. 2005;17:4918–4924. doi: 10.1021/cm048050g. [DOI] [Google Scholar]
- 149.Zhang X., Morits M., Jonkergouw C., Ora A., Valle-Delgado J.J., Farooq M., Ajdary R., Huan S., Linder M., Rojas O., et al. Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel. Biomacromolecules. 2020;21:1875–1885. doi: 10.1021/acs.biomac.9b01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sasayama S., Hara T., Tanaka T., Honda Y., Baba S. Osteogenesis of Multipotent Progenitor Cells using the Epigallocatechin Gallate-Modified Gelatin Sponge Scaffold in the Rat Congenital Cleft-Jaw Model. Int. J. Mol. Sci. 2018;19:3803. doi: 10.3390/ijms19123803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dong L., Wang S.J., Zhao X.R., Zhu Y.F., Yu J.K. 3D- Printed Poly(epsilon-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017;7:13412. doi: 10.1038/s41598-017-13838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guyette J.P., Gilpin S.E., Charest J.M., Tapias L.F., Ren X., Ott H.C. Perfusion decellularization of whole organs. Nat. Protoc. 2014;9:1451–1468. doi: 10.1038/nprot.2014.097. [DOI] [PubMed] [Google Scholar]
- 153.Daryabari S.S., Kajbafzadeh A.-M., Fendereski K., Ghorbani F., Dehnavi M., Rostami M., Garajegayeh B.A., Tavangar S.M. Development of an efficient perfusion-based protocol for whole-organ decellularization of the ovine uterus as a human-sized model and in vivo application of the bioscaffolds. J. Assist. Reprod. Genet. 2019;36:1211–1223. doi: 10.1007/s10815-019-01463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krishtul S., Baruch L., Machluf M. Processed Tissue–Derived Extracellular Matrices: Tailored Platforms Empowering Diverse Therapeutic Applications. Adv. Funct. Mater. 2020;30:1900386. doi: 10.1002/adfm.201900386. [DOI] [Google Scholar]
- 155.Sierad L.N., Shaw E.L., Bina A., Brazile B., Rierson N., Patnaik S.S., Kennamer A., Odum R., Cotoi O., Terezia P., et al. Functional Heart Valve Scaffolds Obtained by Complete Decellularization of Porcine Aortic Roots in a Novel Differential Pressure Gradient Perfusion System. Tissue Eng. Part C. 2015;21:1284–1296. doi: 10.1089/ten.tec.2015.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Syed O., Walters N.J., Day R.M., Kim H.-W., Knowles J.C. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 2014;10:5043–5054. doi: 10.1016/j.actbio.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 157.Casali D.M., Handleton R.M., Shazly T., Matthews M.A. A novel supercritical CO2-based decellularization method for maintaining scaffold hydration and mechanical properties. J. Supercrit. Fluids. 2018;131:72–81. doi: 10.1016/j.supflu.2017.07.021. [DOI] [Google Scholar]
- 158.David M.S., Saubaméa B., Susen S., Kindo M., Bruneval P., van Belle E., Jansen P., Roussel J.-C., Latrémouille C., Carpentier A. Bioprosthetic Total Artificial Heart Induces a Profile of Acquired Hemocompatibility with Membranes Recellularization. J. Am. Coll. Cardiol. 2017;70:404–406. doi: 10.1016/j.jacc.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 159.Tsuchiya T., Sivarapatna A., Rocco K., Nanashima A., Nagayasu T., Niklason L.E. Future prospects for tissue engineered lung transplantation. Organogenesis. 2014;10:196–207. doi: 10.4161/org.27846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Caires-Júnior L.C., Goulart E., Telles-Silva K.A., Araujo B.H.S., Musso C.M., Kobayashi G., Oliveira D., Assoni A., Carvalho V.M., Ribeiro A.F., Jr., et al. Pre-coating decellularized liver with HepG2-conditioned medium improves hepatic recellularization. Mater. Sci. Eng. C. 2021;121:111862. doi: 10.1016/j.msec.2020.111862. [DOI] [PubMed] [Google Scholar]
- 161.Soto-Gutierrez A., Zhang L., Medberry C., Fukumitsu K., Faulk D., Jiang H., Reing J., Gramignoli R., Komori J., Ross M., et al. A Whole-Organ Regenerative Medicine Approach for Liver Replacement. Tissue Eng. Part C. 2011;17:677–686. doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hillebrandt K.H., Everwien H., Haep N., Keshi E., Pratschke J., Sauer I.M. Strategies based on organ decellularization and recellularization. Transpl. Int. 2019;32:571–585. doi: 10.1111/tri.13462. [DOI] [PubMed] [Google Scholar]
- 163.Shimoda H., Yagi H., Higashi H., Tajima K., Kuroda K., Abe Y., Kitago M., Shinoda M., Kitagawa Y. Decellularized liver scaffolds promote liver regeneration after partial hepatectomy. Sci. Rep. 2019;9:12543. doi: 10.1038/s41598-019-48948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hodgson M.J., Knutson C.C., Momtahan N., Cook A.D. Decellularized Scaffolds and Organogenesis. Humana Press; New York, NY, USA: 2017. Extracellular Matrix from Whole Porcine Heart Decellularization for Cardiac Tissue Engineering; pp. 95–102. [DOI] [PubMed] [Google Scholar]
- 165.Ohata K., Ott H.C. Human-scale lung regeneration based on decellularized matrix scaffolds as a biologic platform. Surg. Today. 2020;50:633–643. doi: 10.1007/s00595-020-02000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xue A., Niu G., Chen Y., Li K., Xiao Z., Luan Y., Sun C., Xie X., Zhang D., Du X., et al. Recellularization of well-preserved decellularized kidney scaffold using adipose tissue-derived stem cells. J. Biomed. Mater. Res. Part A. 2018;106:805–814. doi: 10.1002/jbm.a.36279. [DOI] [PubMed] [Google Scholar]
- 167.Damodaran R.G., Vermette P. Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture. J. Tissue Eng. Regen. Med. 2018;12:1230–1237. doi: 10.1002/term.2655. [DOI] [PubMed] [Google Scholar]
- 168.Acikgöz A., Giri S., Cho M.G., Bader A. Morphological and Functional Analysis of Hepatocyte Spheroids Generated on Poly-HEMA-Treated Surfaces under the Influence of Fetal Calf Serum and Nonparenchymal Cells. Biomolecules. 2013;3:242–269. doi: 10.3390/biom3010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Imaninezhad M., Hill L., Kolar G., Vogt K., Zustiak S.P. Templated Macroporous Polyethylene Glycol Hydrogels for Spheroid and Aggregate Cell Culture. Bioconjugate Chem. 2019;30:34–46. doi: 10.1021/acs.bioconjchem.8b00596. [DOI] [PubMed] [Google Scholar]
- 170.Chen Y.-H., Wang I.J., Young T.-H. Formation of Keratocyte Spheroids on Chitosan-Coated Surface Can Maintain Keratocyte Phenotypes. Tissue Eng. Part A. 2009;15:2001–2013. doi: 10.1089/ten.tea.2008.0251. [DOI] [PubMed] [Google Scholar]
- 171.Vinci M., Gowan S., Boxall F., Patterson L., Zimmermann M., Court W., Lomas C., Mendiola M., Hardisson D., Eccles S.A. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10:29. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hasebe Y., Okumura N., Koh T., Kazama H., Watanabe G., Seki T., Ariga T. Formation of rat hepatocyte spheroids on agarose. Hepatol. Res. 2005;32:89–95. doi: 10.1016/j.hepres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 173.Nie Y., Xu X., Wang W., Ma N., Lendlein A. Spheroid formation of human keratinocyte: Balancing between cell-substrate and cell-cell interaction. Clin. Hemorheol. Microcirc. 2020;76:329–340. doi: 10.3233/CH-209217. [DOI] [PubMed] [Google Scholar]
- 174.Goričan L., Gole B., Potočnik U. Head and Neck Cancer Stem Cell-Enriched Spheroid Model for Anticancer Compound Screening. Cells. 2020;9:1707. doi: 10.3390/cells9071707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Baze A., Parmentier C., Hendriks D.F.G., Hurrell T., Heyd B., Bachellier P., Schuster C., Ingelman-Sundberg M., Richert L. Three-Dimensional Spheroid Primary Human Hepatocytes in Monoculture and Coculture with Nonparenchymal Cells. Tissue Eng. Part C. 2018;24:534–545. doi: 10.1089/ten.tec.2018.0134. [DOI] [PubMed] [Google Scholar]
- 176.Hagemann J.A.N., Jacobi C., Hahn M., Schmid V., Welz C., Schwenk-Zieger S., Stauber R., Baumeister P., Becker S. Spheroid-based 3D Cell Cultures Enable Personalized Therapy Testing and Drug Discovery in Head and Neck Cancer. Anticancer Res. 2017;37:2201. doi: 10.21873/anticanres.11555. [DOI] [PubMed] [Google Scholar]
- 177.Ramaiahgari S.C., Waidyanatha S., Dixon D., DeVito M.J., Paules R.S., Ferguson S.S. From the Cover: Three-Dimensional (3D) HepaRG Spheroid Model with Physiologically Relevant Xenobiotic Metabolism Competence and Hepatocyte Functionality for Liver Toxicity Screening. Toxicol. Sci. 2017;159:124–136. doi: 10.1093/toxsci/kfx122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ducker M., Millar V., Ebner D., Szele F.G. A Semi-automated and Scalable 3D Spheroid Assay to Study Neuroblast Migration. Stem Cell Rep. 2020;15:789–802. doi: 10.1016/j.stemcr.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kessel S., Cribbes S., Déry O., Kuksin D., Sincoff E., Qiu J., Chan L.L.-Y. High-Throughput 3D Tumor Spheroid Screening Method for Cancer Drug Discovery Using Celigo Image Cytometry. Slas Technol. 2016;22:454–465. doi: 10.1177/2211068216652846. [DOI] [PubMed] [Google Scholar]
- 180.Cho M.O., Li Z., Shim H.-E., Cho I.-S., Nurunnabi M., Park H., Lee K.Y., Moon S.-H., Kim K.-S., Kang S.-W., et al. Bioinspired tuning of glycol chitosan for 3D cell culture. NPG Asia Mater. 2016;8:e309. doi: 10.1038/am.2016.130. [DOI] [Google Scholar]
- 181.Loessner D., Rockstroh A., Shokoohmand A., Holzapfel B.M., Wagner F., Baldwin J., Boxberg M., Schmalfeldt B., Lengyel E., Clements J.A., et al. A 3D tumor microenvironment regulates cell proliferation, peritoneal growth and expression patterns. Biomaterials. 2019;190–191:63–75. doi: 10.1016/j.biomaterials.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 182.Shang M., Soon R.H., Lim C.T., Khoo B.L., Han J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip. 2019;19:369–386. doi: 10.1039/C8LC00970H. [DOI] [PubMed] [Google Scholar]
- 183.Hoarau-Véchot J., Rafii A., Touboul C., Pasquier J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018;19:181. doi: 10.3390/ijms19010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Thippabhotla S., Zhong C., He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci. Rep. 2019;9:13012. doi: 10.1038/s41598-019-49671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Groebe K., Mueller-Klieser W. On the relation between size of necrosis and diameter of tumor spheroids. Int. J. Radiat. Oncol. Biol. Phys. 1996;34:395–400. doi: 10.1016/0360-3016(95)02065-9. [DOI] [PubMed] [Google Scholar]
- 186.Anada T., Fukuda J., Sai Y., Suzuki O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials. 2012;33:8430–8441. doi: 10.1016/j.biomaterials.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 187.Barros S., Costa E.C., Nunes A.S., de Melo-Diogo D., Correia I.J. Comparative study of the therapeutic effect of Doxorubicin and Resveratrol combination on 2D and 3D (spheroids) cell culture models. Int. J. Pharm. 2018;551:76–83. doi: 10.1016/j.ijpharm.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 188.Paškevičiūtė M., Petrikaitė V. Differences of statin activity in 2D and 3D pancreatic cancer cell cultures. Drug Des. Dev. Ther. 2017;11:3273–3280. doi: 10.2147/DDDT.S149411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aline G.S., Beatriz B.S.I., Esther C.-F., Leticia S.B., Brito S.J., Karina M., Luiz R.G., Vivian A.-G. Comparative Assay of 2D and 3D Cell Culture Models: Proliferation, Gene Expression and Anticancer Drug Response. Curr. Pharm. Des. 2018;24:1689–1694. doi: 10.2174/1381612824666180404152304. [DOI] [PubMed] [Google Scholar]
- 190.Riedl A., Schlederer M., Pudelko K., Stadler M., Walter S., Unterleuthner D., Unger C., Kramer N., Hengstschläger M., Kenner L., et al. Comparison of cancer cells in 2D vs. 3D culture reveals differences in AKT–mTOR–S6K signaling and drug responses. J. Cell Sci. 2017;130:203. doi: 10.1242/jcs.188102. [DOI] [PubMed] [Google Scholar]
- 191.Luca C., Mersch S., Deenen R., Schmidt S., Messner I., Schäfer K.-L., Baldus S.E., Huckenbeck W., Piekorz R.P., Knoefel W.T., et al. Impact of the 3D Microenvironment on Phenotype, Gene Expression, and EGFR Inhibition of Colorectal Cancer Cell Lines. PLoS ONE. 2013;8:e59689. doi: 10.1371/journal.pone.0059689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kimlin L.C., Casagrande G., Virador V.M. In vitro three-dimensional (3D) models in cancer research: An update. Mol. Carcinog. 2013;52:167–182. doi: 10.1002/mc.21844. [DOI] [PubMed] [Google Scholar]
- 193.Zietarska M., Maugard C.M., Filali-Mouhim A., Alam-Fahmy M., Tonin P.N., Provencher D.M., Mes-Masson A.-M. Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC) Mol. Carcinog. 2007;46:872–885. doi: 10.1002/mc.20315. [DOI] [PubMed] [Google Scholar]
- 194.Loessner D., Stok K.S., Lutolf M.P., Hutmacher D.W., Clements J.A., Rizzi S.C. Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494–8506. doi: 10.1016/j.biomaterials.2010.07.064. [DOI] [PubMed] [Google Scholar]
- 195.Lee J.M., Mhawech-Fauceglia P., Lee N., Parsanian L.C., Lin Y.G., Gayther S.A., Lawrenson K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013;93:528–542. doi: 10.1038/labinvest.2013.41. [DOI] [PubMed] [Google Scholar]
- 196.Bielecka Z.F., Maliszewska-Olejniczak K., Safir I.J., Szczylik C., Czarnecka A.M. Three-dimensional cell culture model utilization in cancer stem cell research. Biol. Rev. 2017;92:1505–1520. doi: 10.1111/brv.12293. [DOI] [PubMed] [Google Scholar]
- 197.Chiew G.G.Y., Wei N., Sultania S., Lim S., Luo K.Q. Bioengineered three-dimensional co-culture of cancer cells and endothelial cells: A model system for dual analysis of tumor growth and angiogenesis. Biotechnol. Bioeng. 2017;114:1865–1877. doi: 10.1002/bit.26297. [DOI] [PubMed] [Google Scholar]
- 198.Kuen J., Darowski D., Kluge T., Majety M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS ONE. 2017;12:e0182039. doi: 10.1371/journal.pone.0182039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kurosawa H. Methods for inducing embryoid body formation: In vitro differentiation system of embryonic stem cells. J. Biosci. Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 200.Giobbe G.G., Zagallo M., Riello M., Serena E., Masi G., Barzon L., di Camillo B., Elvassore N. Confined 3D microenvironment regulates early differentiation in human pluripotent stem cells. Biotechnol. Bioeng. 2012;109:3119–3132. doi: 10.1002/bit.24571. [DOI] [PubMed] [Google Scholar]
- 201.Pettinato G., Wen X., Zhang N. Engineering Strategies for the Formation of Embryoid Bodies from Human Pluripotent Stem Cells. Stem Cells Dev. 2015;24:1595–1609. doi: 10.1089/scd.2014.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ludwig T.E., Levenstein M.E., Jones J.M., Berggren W.T., Mitchen E.R., Frane J.L., Crandall L.J., Daigh C.A., Conard K.R., Piekarczyk M.S., et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 203.Yoon S., Yoo S.J., Lee J.E., You S., Lee H.T., Yoon H.S. Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation. 2006;74:149–159. doi: 10.1111/j.1432-0436.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- 204.Mohr J.C., de Pablo J.J., Palecek S.P. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27:6032–6042. doi: 10.1016/j.biomaterials.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 205.Ungrin M.D., Joshi C., Nica A., Bauwens C., Zandstra P.W. Reproducible, Ultra High-Throughput Formation of Multicellular Organization from Single Cell Suspension-Derived Human Embryonic Stem Cell Aggregates. PLoS ONE. 2008;3:e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Meng X., Leslie P., Zhang Y., Dong J. Stem cells in a three-dimensional scaffold environment. SpringerPlus. 2014;3:80. doi: 10.1186/2193-1801-3-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Regmi S., Pathak S., Thanh T.P., Nguyen T.T., Sung J.-H., Yook S., Kim J.O., Yong C.S., Choi I., Doh K.-O., et al. Intraportally delivered stem cell spheroids localize in the liver and protect hepatocytes against GalN/LPS-induced fulminant hepatic toxicity. Stem Cell Res. Ther. 2019;10:230. doi: 10.1186/s13287-019-1337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sasai Y. Next-Generation Regenerative Medicine: Organogenesis from Stem Cells in 3D Culture. Cell Stem Cell. 2013;12:520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 209.Liu Q., Wang J., Chen Y., Zhang Z., Saunders L., Schipani E., Chen Q., Ma P.X. Suppressing mesenchymal stem cell hypertrophy and endochondral ossification in 3D cartilage regeneration with nanofibrous poly(l-lactic acid) scaffold and matrilin-3. Acta Biomater. 2018;76:29–38. doi: 10.1016/j.actbio.2018.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yu L., Wu Y., Liu J., Li B., Ma B., Li Y., Huang Z., He Y., Wang H., Wu Z., et al. 3D Culture of Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs) Could Improve Bone Regeneration in 3D-Printed Porous Ti6Al4V Scaffolds. Stem Cells Int. 2018;2018:2074021. doi: 10.1155/2018/2074021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xu L., Wang S., Sui X., Wang Y., Su Y., Huang L., Zhang Y., Chen Z., Chen Q., Du H., et al. Mesenchymal Stem Cell-Seeded Regenerated Silk Fibroin Complex Matrices for Liver Regeneration in an Animal Model of Acute Liver Failure. Acs Appl. Mater. Interfaces. 2017;9:14716–14723. doi: 10.1021/acsami.7b02805. [DOI] [PubMed] [Google Scholar]
- 212.Wang K.-L., Xue Q., Xu X.-H., Hu F., Shao H. Recent progress in induced pluripotent stem cell-derived 3D cultures for cardiac regeneration. Cell Tissue Res. 2021;164:1–10. doi: 10.1007/s00441-021-03414-x. [DOI] [PubMed] [Google Scholar]
- 213.Song L., Yuan X., Jones Z., Griffin K., Zhou Y., Ma T., Li Y. Assembly of Human Stem Cell-Derived Cortical Spheroids and Vascular Spheroids to Model 3-D Brain-like Tissues. Sci. Rep. 2019;9:5977. doi: 10.1038/s41598-019-42439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gizaw M., Faglie A., Pieper M., Poudel S., Chou S.F. The Role of Electrospun Fiber Scaffolds in Stem Cell Therapy for Skin Tissue Regeneration. Med. ONE. 2019;4:e190002. doi: 10.20900/mo.20190002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jeena K., Manju C.A., Sajesh K.M., Gowd G.S., Sivanarayanan T.B., Mol C.D., Manohar M., Nambiar A., Nair S.V., Koyakutty M. Brain-Tumor-Regenerating 3D Scaffold-Based Primary Xenograft Models for Glioma Stem Cell Targeted Drug Screening. ACS Biomater. Sci. Eng. 2019;5:139–148. doi: 10.1021/acsbiomaterials.8b00249. [DOI] [PubMed] [Google Scholar]
- 216.Saengwimol D., Rojanaporn D., Chaitankar V., Chittavanich P., Aroonroch R., Boontawon T., Thammachote W., Jinawath N., Hongeng S., Kaewkhaw R. A three-dimensional organoid model recapitulates tumorigenic aspects and drug responses of advanced human retinoblastoma. Sci. Rep. 2018;8:15664. doi: 10.1038/s41598-018-34037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ranga A., Gjorevski N., Lutolf M.P. Drug discovery through stem cell-based organoid models. Adv. Drug Deliv. Rev. 2014;69–70:19–28. doi: 10.1016/j.addr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 218.Baptista P.M., Siddiqui M.M., Lozier G., Rodriguez S.R., Atala A., Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- 219.Kim Y., Ju J.H. Generation of 3D Skin Organoid from Cord Blood-derived Induced Pluripotent Stem Cells. JoVE. 2019;146:e59297. doi: 10.3791/59297. [DOI] [PubMed] [Google Scholar]
- 220.Wu H., Uchimura K., Donnelly E.L., Kirita Y., Morris S.A., Humphreys B.D. Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics. Cell Stem Cell. 2018;23:869–881.e868. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Völkner M., Zschätzsch M., Rostovskaya M., Overall R.W., Busskamp V., Anastassiadis K., Karl M.O. Retinal Organoids from Pluripotent Stem Cells Efficiently Recapitulate Retinogenesis. Stem Cell Rep. 2016;6:525–538. doi: 10.1016/j.stemcr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kelava I., Lancaster M.A. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev. Biol. 2016;420:199–209. doi: 10.1016/j.ydbio.2016.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dutta D., Heo I., Clevers H. Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol. Med. 2017;23:393–410. doi: 10.1016/j.molmed.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 224.Boonekamp E., Kretzschmar K., Wiener D.J., Asra P., Derakhshan S., Puschhof J., López-Iglesias C., Peters P.J., Basak O., Clevers H. Long-term expansion and differentiation of adult murine epidermal stem cells in 3D organoid cultures. Proc. Natl. Acad. Sci. USA. 2019;116:14630. doi: 10.1073/pnas.1715272116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hoang P., Wang J., Conklin B.R., Healy K.E., Ma Z. Generation of spatial-patterned early-developing cardiac organoids using human pluripotent stem cells. Nat. Protoc. 2018;13:723–737. doi: 10.1038/nprot.2018.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tchieu J., Zimmer B., Fattahi F., Amin S., Zeltner N., Chen S., Studer L. A Modular Platform for Differentiation of Human PSCs into All Major Ectodermal Lineages. Cell Stem Cell. 2017;21:399–410.e397. doi: 10.1016/j.stem.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wilson W.R., Hay M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 228.Minchinton I., Tannock I.F. Drug penetration in solid tumours. Nat. Rev. Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 229.Geraili A., Jafari P., Hassani M.S., Araghi B.H., Mohammadi M.H., Ghafari A.M., Tamrin S.H., Modarres H.P., Kolahchi A.R., Ahadian S., et al. Controlling Differentiation of Stem Cells for Developing Personalized Organ-on-Chip Platforms. Adv. Healthc. Mater. 2018;7:1700426. doi: 10.1002/adhm.201700426. [DOI] [PubMed] [Google Scholar]
- 230.Cochrane A.A., Albers H.J., Passier R., Mummery C.L., van den Berg A., Orlova V.V., van der Meer A.D. Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Adv. Drug Deliv. Rev. 2019;140:68–77. doi: 10.1016/j.addr.2018.06.007. [DOI] [PubMed] [Google Scholar]