Abstract
Purpose:
Cancer-related cognitive impairment (CRCI) is a common neurotoxicity among patients with breast and other cancers. Neuroimaging studies have demonstrated measurable biomarkers of CRCI but have largely neglected the potential heterogeneity of the syndrome.
Methods:
We used retrospective functional MRI data from 80 chemotherapy-treated breast cancer survivors to examine neurophysiologic subtypes or “biotypes” of CRCI. The breast cancer group consisted of training (N = 57) and validation (N = 23) samples.
Results:
An unsupervised clustering approach using connectomes from the training sample identified three distinct biotypes. Cognitive performance (p < 0.05, corrected) and regional connectome organization (p < 0.001, corrected) differed significantly between the biotypes and also from 103 healthy female controls. We then built a random forest classifier using connectome features to distinguish between the biotypes (accuracy = 91%) and applied this to the validation sample to predict biotype assignment. Cognitive performance (p < 0.05, corrected) and regional connectome organization (p < 0.005, corrected) differed significantly between the predicted biotypes and healthy controls. Biotypes were also characterized by divergent clinical and demographic factors as well as patient reported outcomes.
Conclusions:
Neurophysiologic biotypes may help characterize the heterogeneity associated with CRCI in a data driven manner based on neuroimaging biomarkers.
Implications for Cancer Survivors:
Our novel findings provide a foundation for detecting potential risk and resilience factors that warrant further study. With further investigation, biotypes might be used to personalize assessments of and interventions for CRCI.
Introduction
Cancer-related cognitive impairment (CRCI) is common among breast cancer survivors, affecting up to 75% or more of these women [1]. The etiology of CRCI remains largely unclear but likely involves cancer pathogenesis, treatment-related neurotoxicity, and patient-related factors, among others [1]. CRCI has been shown to persist decades into remission in many women and even worsens over time in some [2, 3]. CRCI is frequently assessed using neuropsychological testing. However, a clear definition of cognitive impairment and/or an appropriate statistical threshold to distinguish impaired versus non-impaired remains lacking [4, 5]. The use of outdated/insensitive measures and/or inappropriate normative samples is common in the literature and creates discrepancies across findings [4, 6].
The National Institute of Mental Health Research Domain Criteria (RDoC) project asserts that psychiatric conditions are brain-based and should be viewed dimensionally rather than dichotomously [7]. Thus, RDoC emphasizes elucidation of the neural signatures of psychiatric conditions and seeks to identify relevant disease subtypes towards precision clinical management [8, 7]. CRCI has been shown by numerous neuroimaging studies to be a brain-based disorder characterized by widespread disruption of large-scale neural networks [9–14]. However, the heterogeneity of CRCI has not been adequately investigated as most studies have examined it dichotomously (impaired vs. not impaired).
Neuroimaging can be used in combination with machine learning (a type of artificial intelligence) to identify neurobiologically distinct subtypes, or “biotypes” of complex syndromes. This may provide a means of biologically diagnosing brain-based syndromes based on a continuum of neurologic alteration. For example, using resting state fMRI (rsfMRI) in combination with machine learning methods, Drysdale et al. (2017) distinguished four different biotypes of depression that had unique clinical characteristics [15]. They examined the association of whole-brain functional connectivity (i.e. connectome) data with depressive symptom data to determine neurophysiological subtypes of depression. The connectome, or brain network, incorporates both biologic and environmental processes including effects of age, education, socioeconomic status, gender, and disease susceptibility, among others [16–21] making it a unique and parsimonious biomarker. We have shown that altered connectome organization is consistently associated with CRCI in both patients and animals [22–31]. In the present study, we used functional connectomes and unsupervised (data-driven) machine learning techniques to determine if there are distinct neural signatures in breast cancer survivors at risk for CRCI. We hypothesized that there would be multiple biotypes suggesting that CRCI is not a binary impairment.
Methods
Participants
We examined retrospective rsfMRI, cognitive, clinical and demographic data from 80 chemotherapy-treated breast cancer survivors age 35–73 years who had completed all primary treatments (surgery, radiation, chemotherapy) excluding hormone blockade at least 6 months before study enrollment. Datasets were used from two different studies. Study 1 (2008–2013) included 57 survivors and focused on determining neuroimaging correlates of CRCI. Study 2 (2010–2011) involved 23 survivors and focused on examining genetic markers in addition to neuroimaging correlates of CRCI. T-tests indicated that were no differences between the two samples in terms of demographic or medical variables, cognitive or psychological function (p > 0.17).
We also utilized cognitive and demographic data collected during 2008–2013 from 103 healthy female controls (Table 1). A subset of controls had available rsfMRI data (N = 82) that was used for comparison. Those without did not differ from those with rsfMRI data in terms of demographics, cognitive or psychological function via t-test (p > 0.34). Survivors and controls participated in prior studies from our laboratory focused on neuroimaging correlates of cognitive dysfunction. Breast cancer survivors were free from disease and had no history of relapse or recurrence at the time of evaluation. Participants were excluded for neurologic, psychiatric or medical conditions known to affect cognitive function. This study was approved by the Stanford University Institutional Review Board.
Table 1.
Demographic and Clinical Data for Training Sample. Data are shown as mean (standard deviation) unless otherwise indicated.
| Biotype 1 N = 24 | Biotype 2 N = 16 | Biotype 3 N = 17 | Controls N = 103 | |
|---|---|---|---|---|
| Age (years) | 50 (9.7) | 53 (7.9) | 52 (8.7) | 49 (13) |
| Education (years) | 16.5 (2.7) | 17.6 (3.2) | 16.5 (2.2) | 16.9 (2.5) |
| Postmenopausal | 54% | 69% | 59% | 64% |
| Radiotherapy1 | 50% | 94% | 65% | NA |
| Hormone Blockade | 54% | 63% | 65% | NA |
| Stage at Diagnosis (I,II,III)2 | 21%, 67%, 13% | 6%,50%,44% | 18%,65%,18% | NA |
| Anthracycline Chemotherapy | 71% | 63% | 71% | NA |
| Time Off-Therapy (months)3 | 24 (22) | 43 (39) | 64 (82) | NA |
| Minority4 | 33% | 19% | 12% | 16% |
Significantly different for Biotype 2 vs. 1, p = 0.002, d = 1.0 and vs. 3, p = 0.02, d = 0.67
Significantly different for Biotype 2 vs. 1, p = 0.02, d = 0.69 and vs. 3, p = 0.04, d = 0.57
Significantly different for Biotype 1 vs. 3, p = 0.02, d = 0.67
Significantly different for Biotype 1 vs. 3, p = 0.05, d = 0.50
Connectome Construction
Functional magnetic resonance imaging (fMRI) data were obtained while participants rested with eyes closed using a T2* weighted [32] gradient echo spiral pulse sequence: TR = 2000msec, TE = 30msec, flip angle = 80° and 1 interleave, FOV = 22cm, matrix = 64×64, in-plane resolution = 3.4375, number of volumes = 216 with a 3T GE Signa HDx whole body scanner (GE Medical Systems, Milwaukee, WI). A high-order shimming method was employed to reduce field heterogeneity. A high-resolution, 3D IR prepared FSPGR scan was also acquired and used for spatial normalization of fMRI: TR: 8.5, TE: minimum, flip: 15 degrees, TI: 400 ms, BW: +/− 31.25 kHz, FOV: 22cm, Phase FOV: 0.75, slice thickness: 1.5mm, 124 slices, 256×256 @ 1 NEX, scan time: 4:33.
Functional connectivity preprocessing was performed with Statistical Parametric Mapping 8 and CONN Toolboxes [33, 34] implemented in Matlab v2019b (Mathworks, Inc, Natick, MA). Briefly, this involved realignment, coregistration with the segmented anatomic volume, spatial normalization, artifact detection and smoothing (FWHM = 8mm) followed by band-pass filtering (0.008–0.09 Hz). The CompCor correction method was used to reduce physiological and other non-neuronal noise artifacts [35]. This method involves extracting signal from white matter and cerebrospinal fluid regions using principal component analysis and then regressing these signals out of the total fMRI signal. Motion parameters from realignment were included as regressors and images identified as motion or signal outliers were excluded. Temporal correlations between all possible pairs of 268 regions [36] were computed based on the corrected fMRI signal. This resulted in a 268×268 connectivity matrix for each participant. The 268 connectome nodes (regions) were ordered by their membership in 1 of 8 previously defined functional networks: medial frontal, frontal-parietal, default mode, subcortical/cerebellar, motor, visual 1, visual 2 and visual association [36].
Cognitive Performance
Participants underwent standardized neuropsychological testing to assess attention, processing speed and cognitive flexibility with the CTMT - Comprehensive Trail Making Test Trails 1 and 5 [37], verbal fluency with the Delis-Kaplan Executive Function System Letter Fluency test [38] and verbal learning and retention with the RAVLT - Rey Auditory Verbal Learning Test [39]. Only CTMT 1 and 5 were examined as these are similar to Trails A and B, respectively from the traditional Trail Making Test. Test scores were converted to T scores based on published normative data for each test and then averaged to create a composite cognitive score.
Patient Reported Outcomes
Self-ratings of psychological function were obtained using the Total Score of the Clinical Assessment of Depression (CAD), which measures, depression, anxiety and fatigue [40]. Subjective executive function was measured with the Global Executive Score of the Behavioral Rating Inventory of Executive Function - Adult Version (BRIEF-A) [41]. Scores were converted to T scores based on the published normative data and reversed to be consistent with cognitive testing such that lower scores correspond to poorer function.
Identifying Biotypes
Figure 1 provides a graphical overview of the methods for identifying biotypes. Breast cancer participants from Study 1 were assigned to a training sample (N = 57) and those from Study 2 to a validation sample (N = 23). Using only the training sample, we identified the most relevant features of the 268×268 connectome by correlating the low dimensional, cognitive composite score with each edge (connection) of the connectome. Significantly correlated (r >0, p < 0.05) edges within each of the 8 networks described above were summed resulting in 8 connectome features for each training sample participant. This method of feature selection has been previously used for neurophysiologic biotyping [15] and connectome-based predictive modeling of behavioral outcomes [42]. Although cognitive performance is used both to select connectome features and evaluate cognition between resultant biotypes, feature selection utilized the low dimensional composite score and biotype comparisons involved the high dimensional, individual cognitive test scores. More importantly, feature selection was conducted only in the training sample and thus cognitive differences in the validation sample were not biased by feature selection.
Figure 1.
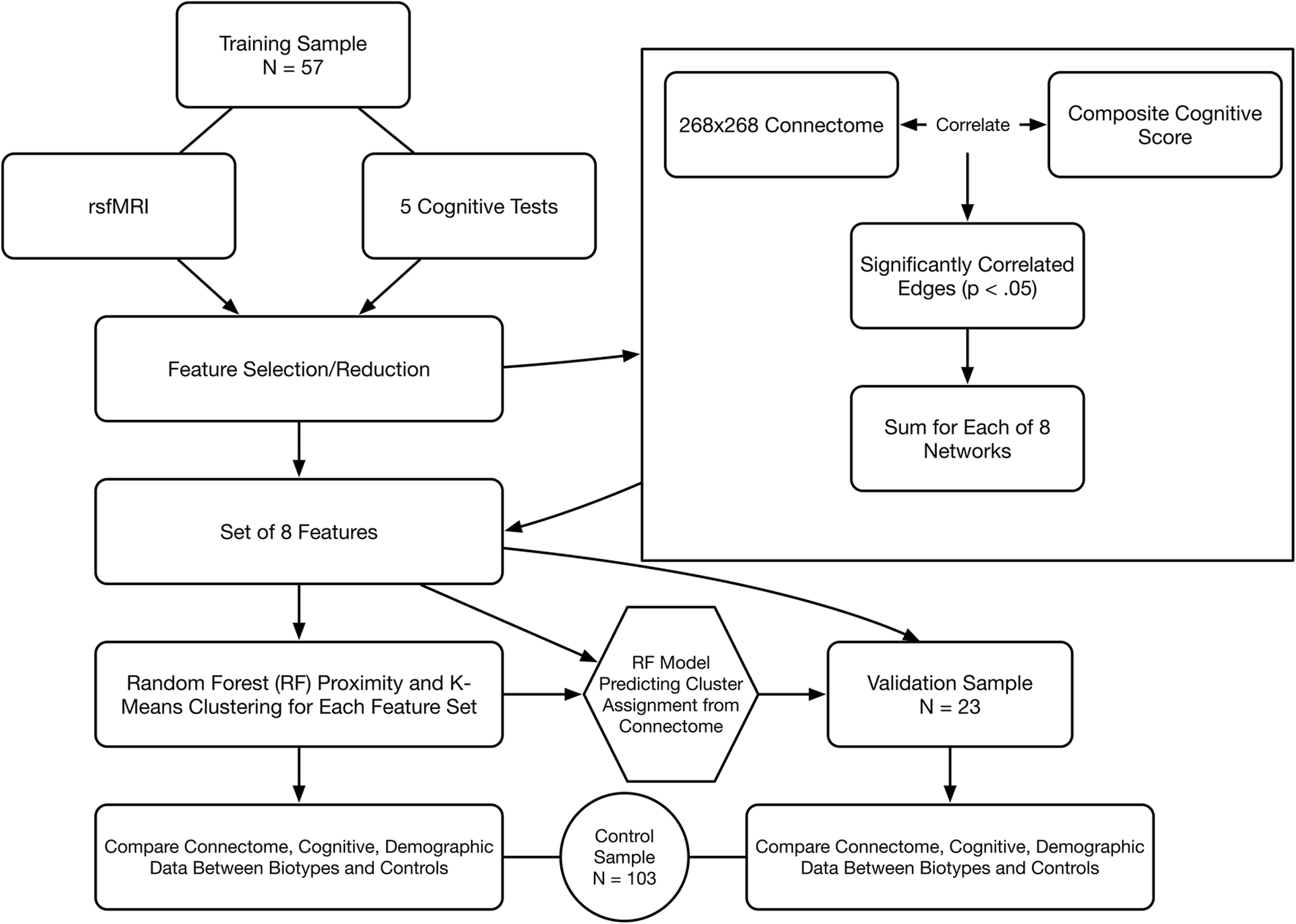
Analysis Overview. The breast cancer sample was divided into training and validation samples. Resting state fMRI (rsfMRI) was used to construct 268×268 functional connectomes for each participant. Connectome edge (connection) weights that significantly correlated with the low dimensional, composite cognitive score were summed according to their membership within each of 8 functional networks. These 8 features were entered into an unsupervised random forest model to calculate a proximity matrix followed by K-means clustering to determine cluster (biotype) assignments. High dimensional regional connectome organization and individual cognitive domain performance was compared between biotypes and controls with correction for multiple comparisons. A random forest classification model was built using the 8 network features and applied to the validation sample to predict biotype. Connectome organization and cognitive performance were then compared between predicted biotypes and controls.
Next, an unsupervised random forest algorithm (trees = 500, mtry = 3) was used to generate a proximity matrix [43, 44] for the reduced feature set. The proximity matrix was then input to a K-means clustering algorithm that included a method to evaluate and compare 30 different indices for determining the optimal number of clusters [45]. This resulted in a data-driven cluster assignment for each participant in the training sample.
Characterizing Biotypes in the Training Sample
Cluster assignments were considered as the participant’s biotype. Performance on the high dimensional, individual cognitive tests including RAVLT Immediate Recall, RAVLT Delayed Recall, CTMT 1, CTMT 5 and Letter Fluency were compared between biotypes and that of healthy controls using a pairwise Wilcoxon rank sum test corrected for multiple comparisons using false discovery rate (FDR) [46].
Differences in regional connectome organization between biotypes and healthy controls were assessed using the Network-Based Statistic (NBS) [47] for the entire, high dimensional, 268×268 connectivity matrix (t > 3.5, p < 0.05, 5000 permutations) controlling for multiple comparisons using family-wise error rate. This method identifies connected substructures, or components, within the larger network without a priori assumptions regarding region membership in the components.
We also explored differences in demographic (age, education, minority status, menopausal status) and patient reported outcomes (BRIEF-A and CAD) between the biotypes and controls. We compared clinical data including history of radiotherapy, history of hormone blockade therapy, time since primary treatment completion and stage at diagnosis between the biotypes. Pairwise Wilcoxon rank sum or Chi square tests were used as appropriate. Because these were exploratory analyses for hypothesis generation, multiple comparisons correction was not applied.
Predicting and Characterizing Biotypes in the Validation Sample
The significantly correlated edge features from the training sample were entered into a supervised random forest algorithm to classify the biotypes (trees = 500, mtry = 3). The model distinguished between the three biotypes with 91% accuracy (p < 0.0001). The random forest model was then applied to the validation sample data using the sums from the same network edges identified during feature selection within the training sample. This resulted in a predicted biotype for each participant in the validation sample. Cognitive function, connectome organization, patient reported outcomes, demographic and clinical data were compared between the predicted biotypes in the validation sample and controls using the same methods as described above for the training sample.
Network-based statistic analysis was conducted using the Network-Based Statistic Toolbox [47] implemented in Matlab v2019b (Mathworks, Inc., Natick, MA). All other analyses were conducted in the R Statistical Package v3.6.2 (R Foundation, Vienna, Austria) including the “randomForest” [48] and “NbClust” [45] libraries. Effect sizes were calculated using Cohen’s d [49].
Results
Training Sample
Biotypes:
Three biotypes were identified from connectome edge features. Biotype 1 included 24 participants, biotype 2 = 16 and biotype 3 = 17.
Cognitive Function:
As shown in Figure 2A, biotype 1 showed lower performance across cognitive tests compared to controls (p < 0.0001, corrected, d = 1.1–2.3) while biotype 3 showed lower performance on 4 out of 5 tests (p < 0.02, corrected, d = 0.45–1.4). Biotype 2 showed lower performance only on Letter Fluency (p = 0.01, corrected) with effect sizes across cognitive tests ranging from d = 0.14 to 0.85.
Figure 2.

Cognitive Differences Between Biotypes and Controls (N = 103). Boxplots and FDR corrected pairwise comparison p values/Cohen’s d are shown for the training sample (A) and the validation sample (B).
Connectome Organization:
Regional connectome organization differed significantly (p < 0.001, corrected) between each biotype and controls with effect sizes ranging from d = 0.70 to 1.7. Given the large number of significant connectome edges identified by NBS, we calculated the percent of significantly hypoconnected (lower connectivity compared to controls) and hyperconnected (higher connectivity compared to controls) connectome edges within each of the 9 predefined functional networks to help simplify the interpretation of the NBS findings (Figure 3). Based on these percentages, qualitatively, biotype 1 demonstrated relatively higher medial frontal network disruption and relatively lower default mode network dysconnectivity. Although hypoconnectivity (lower connectivity compared to controls) was most typical across biotypes, biotype 2 showed relatively more hyperconnectivity (higher connectivity compared to controls) affecting 7 out of 8 networks, particularly the subcortical/cerebellar and motor networks. Biotype 3 showed relatively greater disruption of default mode network and the least alteration of subcortical/cerebellar networks. Medial frontal and subcortical/cerebellar networks were the most commonly disrupted across the biotypes compared to controls.
Figure 3.

Connectome Differences Between Biotypes and Controls (N = 82). Network-based statistic analysis (NBS) demonstrated that regional connectome organization differed significantly (p < 0.005, corrected) between each biotype and controls (d = Cohen’s effect sizes for NBS). For illustration purposes, bar graphs show the percent of significantly hypoconnected (lower connectivity compared to controls) and hyperconnected (higher connectivity compared to controls) connectome edges from NBS tabulated within each of 8 predefined functional networks. Training sample biotypes (TB) are shown in the top row and validation biotypes (VB) are in the bottom row. MF = medial-frontal, FP = frontal-parietal, DM = default mode, SC = subcortical-cerebellar, MT = motor, V1 = visual 1, V2 = visual 2, VA = visual association networks.
Clinical and Demographic Variables:
There were no significant differences in age, education, history of hormone blockade therapy, or menopausal status between the biotypes and also no differences between biotypes and controls in age, education or menopausal status (Table 1). However, biotype 1 had shorter time off therapy (p = 0.02, d = 0.67) and more minority participants (p = 0.05, d = 0.50) compared to biotype 3. Biotype 2 was characterized by higher disease stage with significantly more stage III survivors compared to Biotypes 1 (p = 0.02, d = 0.69) and 3 (p = 0.04, d = 0.57). Biotype 2 also had more participants with a history of radiotherapy compared to biotypes 1 (p = 0.002, d = 1.0) and 3 (p = 0.02, d = 0.67).
Patient Reported Outcomes:
Biotypes 1 and 2 showed lower BRIEF-A scores compared to controls (p < 0.001, d = 1.1–1.2) indicating lower perceived executive function. Biotype 1 demonstrated lower CAD scores compared to biotype 3 (p = 0.03, d = 0.68) and controls (p = 0.01, d = 0.55, Figure 4) indicating lower psychological functioning.
Figure 4.

Patient Reported Outcomes for Biotypes and Controls (N = 103). Boxplots and pairwise comparison p values/Cohen’s d are shown for the training sample in the top row and the validation sample in the bottom row. BRIEF-A = Behavioral Rating Inventory of Executive Function Adult Version; CAD = Clinical Assessment of Depression.
Validation Sample
Biotypes:
The random forest classifier derived from the training sample predicted that 12 participants were biotype 1 and 11 were biotype 2. No participants were predicted to be biotype 3.
Cognitive Function:
As shown in Figure 2B, biotype 1 demonstrated significantly lower performance compared to controls across cognitive tests (p < 0.01, corrected, d = 0.50–1.7) and biotype 2 showed significantly lower Letter Fluency performance (p = 0.01, corrected, d = 1.3), consistent with the training sample.
Connectome Organization:
Regional connectivity differed significantly between each of the predicted biotypes and controls (p < 0.005, corrected, d = 0.97–2.4, Figure 3).
Clinical and Demographic Variables:
Consistent with the training sample, biotype 1 showed higher ratio of minority participants compared to biotype 2 (p = 0.008, d = 1.3) and controls (p = 0.01, d = 0.42). Biotype 1 also demonstrated significantly shorter time since treatment completion (p < 0.001, d = 2.9) compared to biotype 2. However, history of radiotherapy and disease stage were not significantly different (Table 2).
Table 2.
Demographic and Clinical Data for Validation Sample. Data are shown as mean (standard deviation) unless otherwise indicated
| Biotype 1 N = 12 | Biotype 2 N = 11 | Controls N = 103 | |
|---|---|---|---|
| Age (years) | 48 (9.6) | 52 (7.9) | 49 (13) |
| Education (years) | 16.1 (2.0) | 16.3 (.89 | 16.9 (2.5) |
| Postmenopausal | 75% | 75% | 64% |
| Radiotherapy | 100% | 100% | NA |
| Hormone Blockade | 75% | 100% | NA |
| Stage at Diagnosis (I,II,III) | 33%,50%,17% | 50%,25%,25% | NA |
| Anthracycline Chemotherapy | 75% | 100% | NA |
| Time Off-Therapy (months)1 | 31 (9.9) | 64 (12.7) | NA |
| Minority2 | 42% | 0% | 16% |
Significantly different for Biotype 1 vs. 2, p < 0.0001, d = 2.9
Significantly different for Biotype 1 vs 2, p = 0.008, d = 1.3 and vs controls, p = 0.01, d = 0.42
Patient Reported Outcomes:
Biotype 1 had lower BRIEF-A scores compared to controls (p = 0.01, d = 0.78) but CAD scores were not different between the groups (Figure 4).
Discussion
Cognitive impairment is a well-known adverse effect of cancer and its treatments but remains poorly understood due in part to a lack of standardized definition or diagnosis. Previous studies have examined cognitive impairment as a binary syndrome wherein individuals are simply impaired or not impaired, which is unlikely to be accurate given the complexity of cognitive function. Using data driven methods, we identified 3 potential biotypes among a sample of breast cancer survivors with a history of chemotherapy treatment. We then implemented machine learning classification to predict biotype assignment in a separate sample of chemotherapy-treated breast cancer survivors. We compared connectome, cognitive, patient reported, demographic and clinical characteristics of the biotypes with those of healthy female controls.
Differences in regional connectome organization for each biotype compared to controls were very robust in both training and validation samples with patterns of connectome difference being distinct for each biotype. As in previous studies [9, 50], these differences included both hyper- and hypo-connectivity. Hyperconnectivity may reflect recovery-related remodeling of network organization [51] and could thus help explain the higher cognitive function of biotype 2. Ideally, the training and validation biotypes would show similar patterns of connectome difference from controls. While there was overlap, differences were more apparent. This likely stems from the small sample size and also the fact that the random forest classifier was not perfect (91% accuracy). In addition to larger samples, future CRCI biotype research should also include investigations of effective connectivity which could aid in further narrowing down target brain regions and connections. To date there have been no effective connectivity studies in CRCI. With further research and validation, biotyping could help inform precision assessment of and intervention for CRCI. For example, test development could focus on examining cognitive processes known to be supported by the particular networks that distinguish each CRCI biotype.
Biotypes demonstrated significantly different cognitive function from controls and each other. Biotype 1 showed the lowest performance, differing from controls on all cognitive tests in both the training and validation samples. Next was biotype 3 which differed from controls on all cognitive tests but one. Biotype 2 showed the highest performance in both the training and validation samples, differing from controls consistently only on Letter Fluency. Qualitatively, compared to controls, biotype 1 tended to show a range of impaired to average performance, biotype 3 was average and biotype 2 showed average to above average performance. Thus, there may be additional biotypes that we did not have power to detect in this sample. Reliance on imprecise measures (i.e. neuropsychological tests) that are not designed for this syndrome [6] likely also contributes to our results.
In both the training and validation samples, biotype 1, which had the lowest cognitive function, was also characterized by shortest time off-therapy and lowest BRIEF-A scores, indicating poorest perceived executive function. Biotype 1 also showed lower CAD scores indicating increased depression, anxiety and/or fatigue. The range of CAD scores for this group in both the training and validation samples extended much lower than the other biotypes, into the impaired category, based on clinical cutoffs established for this test [40]. Biotype 1 also had a higher number of minority women raising the issue of health disparities in CRCI. Reduced quality of life, including mental health, has been previously demonstrated in racial/ethnic minority breast cancer survivors [52] but few if any studies have been conducted regarding CRCI disparities. Thus biotype 1 appeared to capture several important risk factors for CRCI suggesting that this may be a disadvantaged group that should be prioritized for early intervention and supportive care.
In the training sample, biotype 2 had significantly higher disease stage with more than double the number of patients with stage III disease and also more patients were treated with radiotherapy than the other biotypes. These findings are surprising given that this biotype had the highest cognitive function yet disease severity and radiotherapy have been associated with worse cognitive functioning in past studies [53, 54]. Of course, prior studies examined these variables on average across all participants. Thus, biotype 2 could represent an advantaged subgroup via increased reserve or resilience to cognitive decline irrespective of disease and treatment risk factors. There was no difference between biotypes in education level, which is a proxy of cognitive reserve/resilience, but the range was restricted as most participants were highly educated. There could be some differences in SES, physical function, or some other variable(s) for which we currently have no data that warrant further investigation. However, this finding was not observed in the validation sample and thus requires replication.
Biotype 3 demonstrated cognitive performance that was in between biotype 1 (lowest) and biotype 2 (highest). It is possible, given the longer time off-therapy for this group, that these women had more time to recover compared to biotype 1 but simultaneously did not have the potential neural reserve of biotype 2. Increased resilience could manifest as lower impairment despite greater insult from disease and treatments and/or capacity for earlier neural recovery [55]. There were no biotype 3’s present in our validation sample. It is likely that not all identified biotypes will be represented in a particular sample, unless perhaps the sample is very large. The purpose of the validation sample was to demonstrate that whatever biotypes were present showed significantly different cognitive, connectome and other differences from controls similar to the training sample, which was the case here. Further research is needed with a larger independent sample.
A previous study by Smith et al. demonstrated biotypes within healthy adults that were characterized by different profiles of lifestyle, demographics and cognitive abilities [56]. Thus, it is likely that distinct biotypes exist within our healthy control group that may show more or less difference in cognitive function and other characteristics compared to one or more of the breast cancer biotypes. This may help explain why some studies do not detect cognitive differences in breast cancer patients and survivors compared to healthy controls [57]. Further studies are required with much larger samples in order to compare breast cancer and healthy control biotypes.
In addition to the small sample, these results were influenced by our choice of connectome parcellation scheme, feature reduction strategy, machine learning algorithm and cognitive tests. We employed the same parcellation scheme and feature reduction strategy shown by previous studies to predict individual differences in behavioral outcomes [58, 59, 42] but there is not a standard connectome parcellation. We used the mean across cognitive tests in order to maintain simplicity and reduce bias of feature reduction. However, alternate composite score methods such as principal components analysis could be considered in future studies with larger samples. Random forest is consistently a flexible, high performance algorithm across disciplines but future studies involving larger samples should compare different algorithms. Cognitive testing, clinical data and patient reported outcomes were limited due to the retrospective nature of the study.
In conclusion, CRCI is likely not a binary syndrome. Although CRCI research remains critically in need of “deep” studies involving longitudinal designs to track individual cognitive trajectories, “broad” studies involving large cross-sectional samples are extremely useful for determining the differences between individuals [60]. These between-group differences provide important insights regarding syndrome dimensions and phenotypes that help refine our understanding of the syndrome. Our findings strongly suggest that further “broad” work is required to define biotypes in parallel with “deep” studies to investigate questions such as whether biotypes remain stable over time and what are the risks/protective factors associated with these biotypes. Importantly, our results support the need to better characterize the heterogeneity of CRCI. This line of research may help us to biologically diagnosis CRCI in the future and identify syndrome-specific neural mechanisms to guide development of precision assessments and interventions.
Acknowledgements
The authors wish to thank the faculty and staff of the Richard M. Lucas Center for Imaging. This research was funded by the National Institutes of Health (1DP2OD004445 to SRK) and the Stanford Cancer Center (Developmental Cancer Research Award to SRK).
Funding: This study was funded by the National Institutes of Health (1DP2OD004445 to SRK) and the Stanford Cancer Center (Developmental Cancer Research Award to SRK).
Footnotes
Conflict of Interest: Each author declares that he/she has no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26(1):102–13. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesler SR, Rao A, Blayney DW, Oakley-Girvan IA, Karuturi M, Palesh O. Predicting Long-Term Cognitive Outcome Following Breast Cancer with Pre-Treatment Resting State fMRI and Random Forest Machine Learning. Front Hum Neurosci. 2017;11:555. doi: 10.3389/fnhum.2017.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Willik KD, Koppelmans V, Hauptmann M, Compter A, Ikram MA, Schagen SB. Inflammation markers and cognitive performance in breast cancer survivors 20 years after completion of chemotherapy: a cohort study. Breast Cancer Res. 2018;20(1):135. doi: 10.1186/s13058-018-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein LJ, McCreath GA, Komeylian Z, Rich JB. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: A multilevel meta-analysis. Neurosci Biobehav Rev. 2017;83:417–28. doi: 10.1016/j.neubiorev.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Shilling V, Jenkins V, Trapala IS. The (mis)classification of chemo-fog--methodological inconsistencies in the investigation of cognitive impairment after chemotherapy. Breast Cancer Res Treat. 2006;95(2):125–9. doi: 10.1007/s10549-005-9055-1. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz TS, Suls J, Treviño M. A Call for a Neuroscience Approach to Cancer-Related Cognitive Impairment. Trends in Neurosciences. 2018;41(8):493–6. doi: 10.1016/j.tins.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 8.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen CY, Chen VC, Yeh DC, Huang SL, Zhang XR, Chai JW et al. Association of functional dorsal attention network alterations with breast cancer and chemotherapy. Scientific reports. 2019;9(1):104. doi: 10.1038/s41598-018-36380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, Zhang XD, Zheng G, Zhang LJ. Chemotherapy-induced brain changes in breast cancer survivors: evaluation with multimodality magnetic resonance imaging. Brain Imaging Behav. 2019. doi: 10.1007/s11682-019-00074-y. [DOI] [PubMed] [Google Scholar]
- 11.Chen BT, Jin T, Patel SK, Ye N, Ma H, Wong CW et al. Intrinsic brain activity changes associated with adjuvant chemotherapy in older women with breast cancer: a pilot longitudinal study. Breast Cancer Res Treat. 2019. doi: 10.1007/s10549-019-05230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apple AC, Schroeder MP, Ryals AJ, Wagner LI, Cella D, Shih PA et al. Hippocampal functional connectivity is related to self-reported cognitive concerns in breast cancer patients undergoing adjuvant therapy. NeuroImage Clinical. 2018;20:110–8. doi: 10.1016/j.nicl.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mo C, Lin H, Fu F, Lin L, Zhang J, Huang M et al. Chemotherapy-induced changes of cerebral activity in resting-state functional magnetic resonance imaging and cerebral white matter in diffusion tensor imaging. Oncotarget. 2017;8(46):81273–84. doi: 10.18632/oncotarget.18111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao H, Chen X, Yan Y, He X, Hu S, Kong J et al. Functional connectivity change of brain default mode network in breast cancer patients after chemotherapy. Neuroradiology. 2016. doi: 10.1007/s00234-016-1708-8. [DOI] [PubMed] [Google Scholar]
- 15.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marques P, Soares JM, Magalhaes R, Santos NC, Sousa N. The Bounds Of Education In The Human Brain Connectome. Scientific reports. 2015;5:12812. doi: 10.1038/srep12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K et al. Sex differences in the structural connectome of the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(2):823–8. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun J, Tong S, Yang GY. Reorganization of Brain Networks in Aging and Age-related Diseases. Aging Dis. 2012;3(2):181–93. [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnadas R, Kim J, McLean J, Batty GD, McLean JS, Millar K et al. The envirome and the connectome: exploring the structural noise in the human brain associated with socioeconomic deprivation. Front Hum Neurosci. 2013;7:722. doi: 10.3389/fnhum.2013.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Allen G, Zhu H, Dunson D. Relationships between Human Brain Structural Connectomes and Traits. bioRxiv. 2018. doi: 10.1101/256933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuladhar AM, van Uden IW, Rutten-Jacobs LC, Lawrence A, van der Holst H, van Norden A et al. Structural network efficiency predicts conversion to dementia. Neurology. 2016;86(12):1112–9. doi: 10.1212/WNL.0000000000002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amidi A, Leemans A, Kesler SR, Agerbæk M, Wu LM, Zachariae R. Changes in Brain Structural Networks and Cognitive Functions in Testicular Cancer Patients Receiving Cisplatin-Based Chemotherapy. JNCI: Journal of the National Cancer Institute. 2017;109(12):djx085–djx. doi: 10.1093/jnci/djx085. [DOI] [PubMed] [Google Scholar]
- 23.Bruno J, Hosseini SM, Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol Dis. 2012;48(3):329–38. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu GS, Boukelmoune N, Chiang ACA, Peng B, Rao V, Kingsley C et al. Nasal administration of mesenchymal stem cells restores cisplatin-induced cognitive impairment and brain damage in mice. Oncotarget. 2018;9(85):35581–97. doi: 10.18632/oncotarget.26272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosseini SM, Koovakkattu D, Kesler SR. Altered small-world properties of gray matter networks in breast cancer. BMC Neurol. 2012;12:28. doi: 10.1186/1471-2377-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesler SR, Blayney DW. Neurotoxic Effects of Anthracycline- vs Nonanthracycline-Based Chemotherapy on Cognition in Breast Cancer Survivors. JAMA Oncol. 2016;2(2):185–92. doi: 10.1001/jamaoncol.2015.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kesler SR, Gugel M, Huston-Warren E, Watson C. Atypical Structural Connectome Organization and Cognitive Impairment in Young Survivors of Acute Lymphoblastic Leukemia. Brain connectivity. 2016;6(4):273–82. doi: 10.1089/brain.2015.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kesler SR, Gugel M, Pritchard-Berman M, Lee C, Kutner E, Hosseini SM et al. Altered resting state functional connectivity in young survivors of acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61(7):1295–9. doi: 10.1002/pbc.25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesler SR, Noll K, Cahill DP, Rao G, Wefel JS. The effect of IDH1 mutation on the structural connectome in malignant astrocytoma. J Neurooncol. 2017;131(3):565–74. doi: 10.1007/s11060-016-2328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kesler SR, Ogg R, Reddick WE, Phillips N, Scoggins M, Glass JO et al. Brain Network Connectivity and Executive Function in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Brain connectivity. 2018;8(6):333–42. doi: 10.1089/brain.2017.0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kesler SR, Watson CL, Blayney DW. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol Aging. 2015;36(8):2429–42. doi: 10.1016/j.neurobiolaging.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med. 1998;39(3):361–8. [DOI] [PubMed] [Google Scholar]
- 33.Ashburner J SPM: a history. NeuroImage. 2012;62(2):791–800. doi: 10.1016/j.neuroimage.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity. 2012;2(3):125–41. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 35.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage. 2013;82:403–15. doi: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith SR, Servesco AM, Edwards JW, Rahban R, Barazani S, Nowinski LA et al. Exploring the validity of the comprehensive trail making test. The Clinical Neuropsychologist. 2008;22(3):507–18. [DOI] [PubMed] [Google Scholar]
- 38.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corp; 2008. [Google Scholar]
- 39.de Sousa Magalhães S, Malloy-Diniz LF, Hamdan AC. Validity convergent and reliability test-retest of the Rey Auditory Verbal Learning Test. Age (years). 2012;20(4.5):19.1. [Google Scholar]
- 40.Aghakhani A, Chan EK. Test Reviews: Bracken, B. A., & Howell, K. (2004). Clinical Assessment of Depression. Odessa, FL: Psychological Assessment Resources. Journal of Psychoeducational Assessment. 2007;25(4):416–22. doi: 10.1177/0734282907300383. [DOI] [Google Scholar]
- 41.Roth RM, Lance CE, Isquith PK, Fischer AS, Giancola PR. Confirmatory factor analysis of the behavior rating inventory of executive function-adult version in healthy adults and application to attention-deficit/hyperactivity disorder. Archives of clinical neuropsychology. 2013;28(5):425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen X, Finn ES, Scheinost D, Rosenberg MD, Chun MM, Papademetris X et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc. 2017;12(3):506–18. doi: 10.1038/nprot.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi T, Horvath S. Unsupervised Learning With Random Forest Predictors. Journal of Computational and Graphical Statistics. 2006;15(1):118–38. doi: 10.1198/106186006X94072. [DOI] [Google Scholar]
- 44.Breiman L Random forests. Machine Learning. 2001;45(1):5–32. doi:Doi 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 45.Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. 2014. 2014;61(6):36. doi: 10.18637/jss.v061.i06. [DOI] [Google Scholar]
- 46.Benjamini Y, Yekutieli D. The Control of the False Discovery Rate in Multiple Testing under Dependency. The Annals of Statistics. 2001;29(4):1165–88. [Google Scholar]
- 47.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53(4):1197–207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 48.Liaw A, Wiener M. Classification and Regression by randomForest. R News. 2002;2(3):18–22. doi:citeulike-article-id:1121494. [Google Scholar]
- 49.Cohen J Statistical power analysis for the behavioral sciences. Routledge; 2013. [Google Scholar]
- 50.Hosseini SM, Kesler SR. Multivariate pattern analysis of FMRI in breast cancer survivors and healthy women. J Int Neuropsychol Soc. 2014;20(4):391–401. doi: 10.1017/S1355617713001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sahnoune I, Inoue T, Kesler SR, Rodgers SP, Sabek OM, Pedersen SE et al. Exercise ameliorates neurocognitive impairments in a translational model of pediatric radiotherapy. Neuro Oncol. 2018;20(5):695–704. doi: 10.1093/neuonc/nox197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seiler A, Murdock KW, Garcini LM, Chirinos DA, Ramirez J, Jackson B et al. Racial/Ethnic Disparities in Breast Cancer Incidence, Risk Factors, Health Care Utilization, and Outcomes in the USA. Current Breast Cancer Reports. 2017;9(2):91–9. doi: 10.1007/s12609-017-0247-6. [DOI] [Google Scholar]
- 53.Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, Boogerd W, Reneman L, Schagen SB. Neurotoxicity in breast cancer survivors >/=10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015;9(2):275–84. doi: 10.1007/s11682-014-9305-0. [DOI] [PubMed] [Google Scholar]
- 54.Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110(1):143–52. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stern Y An approach to studying the neural correlates of reserve. Brain Imaging Behav. 2017;11(2):410–6. doi: 10.1007/s11682-016-9566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18(11):1565–7. doi: 10.1038/nn.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li M, Caeyenberghs K. Longitudinal assessment of chemotherapy-induced changes in brain and cognitive functioning: A systematic review. Neurosci Biobehav Rev. 2018;92:304–17. doi: 10.1016/j.neubiorev.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Finn ES, Scheinost D, Finn DM, Shen X, Papademetris X, Constable RT. Can brain state be manipulated to emphasize individual differences in functional connectivity? NeuroImage. 2017;160:140–51. doi: 10.1016/j.neuroimage.2017.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664–71. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cornblath EJ, Lydon-Staley DM, Bassett DS. Harnessing networks and machine learning in neuropsychiatric care. Current opinion in neurobiology. 2019;55:32–9. doi: 10.1016/j.conb.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


