Significance
This study reveals that the tryptophan-degrading reaction catalyzed by indoleamine 2,3-dioxygenase 1 (IDO1) is linked to reactive oxygen species (ROS) scavenging in Gr-1+CD11b+ myeloid cells. The IDO1-mediated ROS scavenging promotes myeloid-derived suppressor cell characteristics in Gr-1+CD11b+ cells, suppressing their differentiation into proinflammatory neutrophils. These results could explain the increased lethality in graft-versus-host disease as well as the enhanced proinflammatory and reduced regulatory T cell responses after transplantation of IDO1-deficient bone marrow cells. Our findings provide a mechanistic insight into the immune-modulatory roles of IDO1.
Keywords: IDO, Gr-1+CD11b+ cell, myeloid-derived suppressor cell, ROS, GVHD
Abstract
Tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenase 1 (IDO1) also has an immunological function to suppress T cell activation in inflammatory circumstances, including graft-versus-host disease (GVHD), a fatal complication after allogeneic bone marrow transplantation (allo-BMT). Although the mononuclear cell expression of IDO1 has been associated with improved outcomes in GVHD, the underlying mechanisms remain unclear. Herein, we used IDO-deficient (Ido1−/−) BMT to understand why myeloid IDO limits the severity of GVHD. Hosts with Ido1−/− BM exhibited increased lethality, with enhanced proinflammatory and reduced regulatory T cell responses compared with wild type (WT) allo-BMT controls. Despite the comparable expression of the myeloid-derived suppressor cell (MDSC) mediators, arginase-1, inducible nitric oxide synthase, and interleukin 10, Ido1−/− Gr-1+CD11b+ cells from allo-BMT or in vitro BM culture showed compromised immune-suppressive functions and were skewed toward the Ly6ClowLy6Ghi subset, compared with the WT counterparts. Importantly, Ido1−/−Gr-1+CD11b+ cells exhibited elevated levels of reactive oxygen species (ROS) and neutrophil numbers. These characteristics were rescued by human IDO1 with intact heme-binding and catalytic activities and were recapitulated by the treatment of WT cells with the IDO1 inhibitor L1-methyl tryptophan. ROS scavenging by N-acetylcysteine reverted the Ido1−/−Gr-1+CD11b+ composition and function to an MDSC state, as well as improved the survival of GVHD hosts with Ido1−/− BM. In summary, myeloid-derived IDO1 enhances GVHD survival by regulating ROS levels and limiting the ability of Gr-1+CD11b+ MDSCs to differentiate into proinflammatory neutrophils. Our findings provide a mechanistic insight into the immune-regulatory roles of the metabolic enzyme IDO1.
Indoleamine 2,3-dioxygenase 1 (IDO1) is a heme-binding metabolic enzyme that catalyzes the conversion of tryptophan (Trp) into kynurenine (Kyn). In addition to Trp catabolism, IDO1 has long been recognized to have immune-regulatory roles, preventing excessive inflammation (1). IDO1 is up-regulated in response to inflammatory stimuli, including Toll-like receptor (TLR) and type I/II interferon (IFN) signaling (1, 2). The induction of IDO1 after TLR9 stimulation has been demonstrated to mitigate experimental colitis (3). Catalytic function blockade in mice by pharmacological inhibition or genetic ablation of IDO1 (Ido1−/−) enhanced inflammation and aggravated autoimmune diseases, including experimental autoimmune encephalomyelitis (EAE) (4). The enhanced immune responses induced by IDO1 deficiency were associated with increased T helper (Th)1/Th17 responses; in contrast, regulatory T cell (Treg) responses were repressed (4–6). Consistently, IDO1 inhibition enhanced antitumor immune responses (7–9). The immune-regulatory effects of IDO1 have been ascribed to the depletion of Trp (10, 11) and the production of toxic catabolites along the Kyn pathway (4, 12–14). However, it remains unclear whether additional mechanisms are involved in IDO1-mediated immune suppression.
Graft-versus-host disease (GVHD) is a severe inflammatory disease for which IDO1 has been shown to play a protective role (2, 14, 15). GVHD often develops as an adverse systemic complication following allogeneic hematopoietic stem cell transplantation (allo-HSCT) and is induced by activation of donor T cells reactive to the recipient’s major histocompatibility complexes (MHCs) and/or minor histocompatibility antigens (MiHAs) (16). Allo-reactivity of the activated donor T cells promotes tissue inflammation in the host, leading to morbidity and mortality. IDO1 deficiency in the bone marrow (BM) of the donor or the recipient has been linked to increased lethality (2, 14, 15), indicating a crucial role of IDO1 expression in the parenchymal and hematopoietic compartments in preventing GVHD. Kyn produced in IDO1-expressing lung epithelial cells and tissue macrophages suppressed T cell activation by binding to and activating immunomodulatory aryl hydrocarbon receptors (AhRs), which could explain the GVHD aggravation in Ido1−/− recipients (14). Nevertheless, the mechanisms behind GVHD exacerbation by Ido1−/− BM transfer remain obscure. Wild-type (WT) donor antigen-presenting cells prolonged survival in GVHD regardless of epithelial cell expression of IDO1, and IDO1 up-regulation after treatment of donor BM with TLR ligands reduced GVHD severity (2). These findings suggest an important role of IDO1 expressed by donor-derived myeloid cells in preventing severe GVHD. However, the immune-regulatory roles of IDO1 expressed in myeloid cells (termed myeloid IDO1 hereafter) remain elusive.
Myeloid-derived suppressor cells (MDSCs) are innate cells that have immune-suppressive functions (17). Conventionally, MDSCs are identified as Gr-1+CD11b+ cells and can be further classified into Ly6ChiLy6Glow monocytic (M) or Ly6ClowLy6Ghi polymorphonuclear (PMN) subsets. MDSCs produce various immune-suppressive mediators, including arginase-1 (Arg-1), inducible nitric oxide synthase (iNOS), and interleukin 10 (IL-10) (17, 18). Their ability to enhance Treg responses has also been reported (19, 20). As immature cells, MDSCs maintain the ability to differentiate into dendritic cells (DCs), macrophages, or neutrophils (21, 22). In GVHD, MDSCs derived from donor BM are the major population of myeloid cells expanding in the host (23), and along with Tregs they suppress GVHD (24–26). We previously reported that transplantation of MyD88-deficient (Myd88−/−) BM suppressed Gr-1+CD11b+ cell expansion and polarized the differentiation of Gr-1+CD11b+ cells into DCs, aggravating GVHD (27, 28). These findings indicate that increasing the number of undifferentiated Gr-1+CD11b+ cells is essential for MDSC-mediated immune suppression in GVHD. Additionally, the finding that IDO1 expression in mononuclear cells, rather than in parenchymal cells, correlated positively with the survival of GVHD patients (29) suggested that IDOl expression in myeloid cells might be involved in the MDSC-mediated suppression of GVHD. Understanding the role of IDO1 in the function of MDSCs derived from the donor BM could lead to novel therapeutic strategies for the treatment of GVHD.
In this study, we investigated the mechanisms underlying GVHD aggravation in hosts transplanted with IDO1-deficient BM. We found that IDO1 deficiency in donor BM did not affect the expansion of Gr-1+CD11b+ cells in GVHD hosts but polarized them toward a Ly6ClowLy6Ghi phenotype, reducing their immune-regulatory potential. This phenomenon was ascribed to increased reactive oxygen species (ROS) generation in the Ido1−/− Gr-1+CD11b+ cells and their skewing to neutrophil differentiation. Treatment of ROS-scavenging chemical reversed this phenomenon. Our findings suggest that the immune-regulatory roles of IDO1 are mediated by ROS scavenging and suppression of the differentiation of Gr-1+CD11b+ cells.
Results
Systemic Enhancement of Inflammatory T Cell Immunity in GVHD Hosts with IDO-KO BM Transplantation.
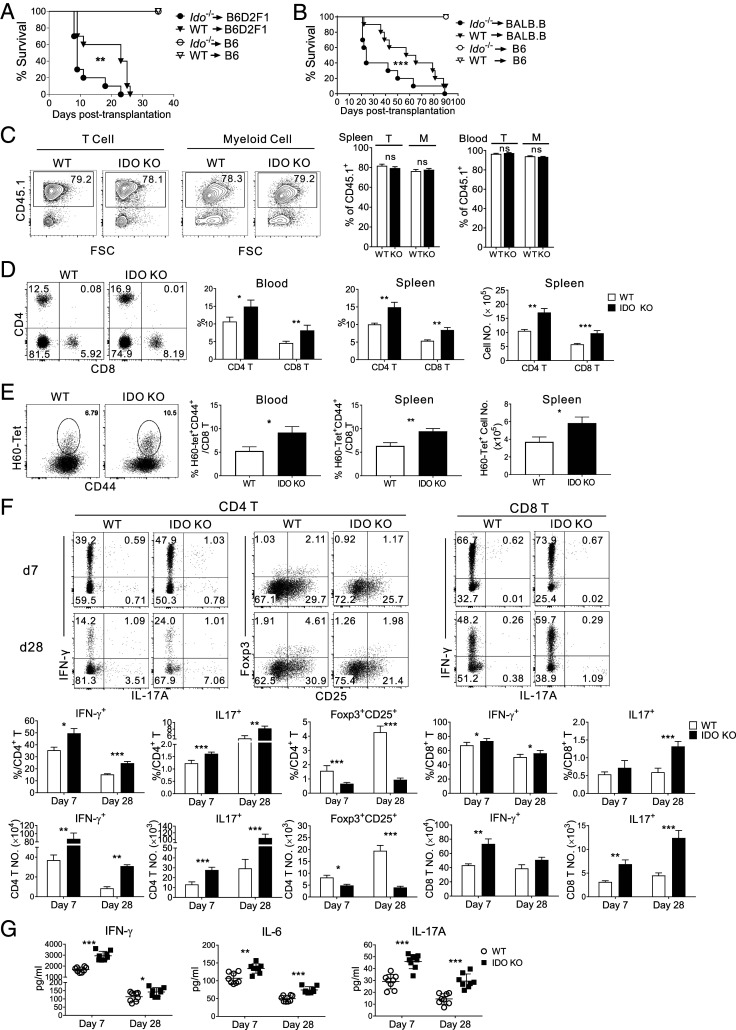
The importance of donor-derived myeloid IDO1 in GVHD attenuation was confirmed by GVHD aggravation after transplantation of Ido1−/− BM. BM cells isolated from Ido1−/− CD45.1+ C57BL/6 (B6) mice, together with WT CD45.1+B6 T cells, were transplanted into haploidentical B6D2F1 (Ido1−/− BM + WT T → B6D2F1) or MHC-matched/MiHA-mismatched BALB.B (Ido1−/− BM + WT T → BALB.B) allogeneic recipients. In both cases, the allogeneic recipients succumbed to severe GVHD, showing significantly reduced mean survival times (MSTs; 9 and 24 d, respectively) compared with the 23- and 61-d MSTs of the WT counterparts that received WT BM and T cells (WT BM + WT T → B6D2F1 and BALB.B, respectively; Fig. 1 A and B). All syngeneic B6 recipients of either WT or Ido1−/− BM showed long-term disease-free survival.
Fig. 1.
GVHD aggravation in IDO-KO BM recipients with systemic enhancement of proinflammatory T cell alloimmunity. (A and B) GVHD induction by transplantation of CD45.1+ IDO-KO or WT BM together with CD45.1+B6 WT T cells into (A) haploidentical recipient B6D2F1 or (B) MHC-matched but MiHA-mismatched BALB.B mice. Survival rates are also shown. Data are representative of two independent experiments (n = 6 to 10 per group per experiment). **P < 0.01, ***P < 0.001 as determined by log-rank (Mantel–Cox) test. (C–G) Analyses were performed using BALB.B hosts. (C) T and myeloid cell chimerism in the spleen and peripheral blood of IDO-KO-BM or WT-BM hosts on day 7 posttransplantation. Representative CD45.1/FSC profiles of T (CD3+) and myeloid cells (CD3-CD19−) in the spleens are shown. Proportions of CD45.1+ cells in T and myeloid (M) cells are plotted. (D and E) Flow cytometric analyses of (D) CD4+ and CD8+ T cells and (E) H60-tetramer-binding CD8+ T cells in the blood and spleen on day 7 posttransplantation. Representative flow cytometric data of splenocytes gated on CD45.1+ cells are shown. Percentages and numbers of total CD45.1+ CD4+ and CD8+ T cells, and H60-tetramer-binding CD8+ T cells are plotted. (F) Flow cytometric analysis of IFN-γ– and IL-17A–producing CD45.1+ CD4+ and CD8+ T cells, as well as of CD45.1+Foxp3+CD25+ Tregs in the spleens. Percentages and numbers of the corresponding cells are shown. (G) Serum IFN-γ, IL-6, and IL-17 levels. Representative data from at least three (C–G) independent experiments (C–F, n = 3 per group per experiment; G, n = 8 per group per experiment) are presented as means ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001 as determined by Student’s t test). ns, not statistically significant.
To maintain the viability of the recipients for several weeks after BM transplantation (BMT), we utilized the MiHA-mismatched model (CD45.1+ Ido1−/− or WT BM + WT T → CD45.2+ BALB.B; hereafter termed IDO-KO-BM host or WT-BM host, respectively) for ensuing mechanistic studies. Both the IDO-KO-BM and WT-BM hosts showed >75% (80% on average) donor chimerism in T and myeloid cells in the spleen and >95% in peripheral blood on day 7 posttransplantation (Fig. 1C). Next, T and myeloid-cell analyses were performed with gating on CD45.1+ or CD45.2- cells. T cell (CD45.1+) profiling on day 7 posttransplantation indicated significantly higher expression of CD4+ and CD8+ T cells in the peripheral blood leukocytes (PBLs) of IDO-KO-BM versus WT-BM hosts (Fig. 1D). Tetramer staining for H60, a dominant MiHA in the B6 → BALB.B GVHD setting (23), confirmed that the frequencies of alloantigen H60-specific CD8+ T cells (H60-tetramer+CD44+CD8+ T cells) were higher in PBLs of the IDO-KO-BM host compared with the WT-BM host (Fig. 1E). The fractions and numbers of total CD4+ and CD8+ T cells, as well as H60-specific CD8+ T cells, were also significantly higher in the spleens of IDO-KO-BM hosts compared with their WT counterparts (Fig. 1 D and E). Additionally, the proportions and numbers of CD4+ and CD8+ T cells producing IFN-γ and IL-17 were significantly higher, while those of Foxp3+CD25+CD4+ Treg cells were significantly lower, in the spleens of IDO-KO-BM hosts relative to the WT-BM hosts on days 7 and 28 posttransplantation (Fig. 1F), as were T cells infiltrating the mesenteric lymph nodes, liver, and lungs (SI Appendix, Fig. S1). Moreover, the proinflammatory cytokines IFN-γ, IL-6, and IL-17A were detected at significantly higher levels in the serum of IDO-KO-BM hosts compared with their WT counterparts (Fig. 1G). These results demonstrated that IDO1 deficiency in donor BM reduced Tregs and increased allo-responsive Th1 and Th17 cells, suggesting a systemic enhancement of proinflammatory T cell alloimmunity and repression of Treg responses in the IDO-KO-BM hosts. This systemic enhancement of proinflammatory T cell responses in GVHD hosts with IDO-KO BM is consistent with previous findings in tumor and EAE models lacking IDO1 function or expression (4, 8).
Compositional Changes and Reduced Suppressive Function of Gr-1+CD11b+ Cells Generated in IDO-KO Hosts.
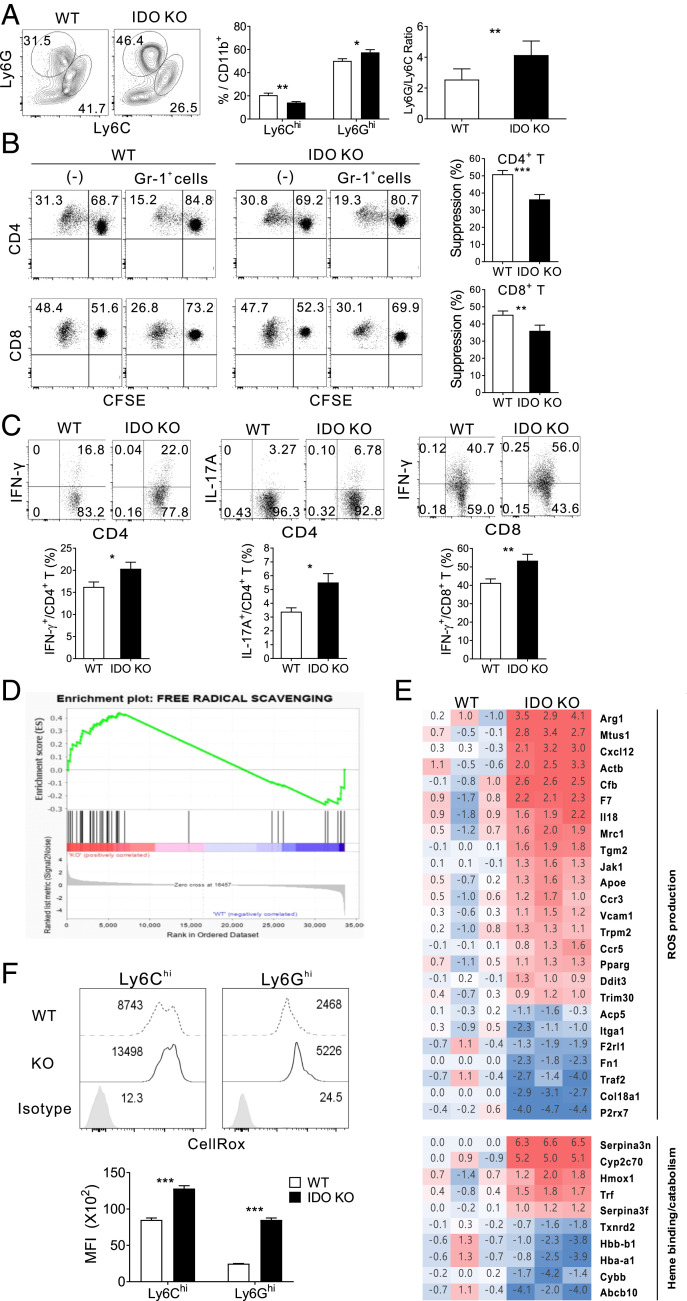
Given the importance of suppressive Gr-1+CD11b+ cells generated en masse in preventing severe GVHD (27, 28, 30), we characterized the subsets of Gr-1+CD11b+ cells that develop in IDO-KO hosts. The composition of BM cells before transplantation was similar between IDO-KO and WT (day 0; SI Appendix, Fig. S2A). On day 7 posttransplantation, the overall proportions and numbers of donor-derived (CD45.2-) CD11c−CD11b+ non-DC myeloid cells and Gr-1+CD11b+ cells in the spleens of IDO-KO-BM hosts were in the normal range compared with the WT-BM hosts (SI Appendix, Fig. S2 B and C). However, the compositions of Gr-1+CD11b+ cells in IDO-KO hosts were notable in that the fraction of Ly6ClowLy6Ghi cells (hereafter termed Ly6Ghi) was significantly higher, while that of Ly6ChiLy6Glow cells (hereafter termed Ly6Chi) was significantly lower, compared with their WT counterparts (Fig. 2A). No such significant compositional difference was observed between host residual (CD45.2+) Gr-1+CD11b+ cells of the two BM hosts (SI Appendix, Fig. S2D). Consistent with a previous report of normal development and function of DCs in IDO-KO mice (31), the profiles of donor-derived CD11c+ DCs, including the CD8α+ conventional DC1 (cDC1), CD11b+ cDC2, and B220+ plasmacytoid DC subsets, did not differ significantly between IDO-KO-BM and WT-BM hosts (SI Appendix, Fig. S2E). When coincubated with activated T cells, however, splenic Gr-1+ cells (all CD11b+) from IDO-KO-BM hosts exhibited less suppressive activity, allowing enhanced proliferation and increased cytokine production of CD4+ or CD8+ T cells with anti-CD3 stimulation (Fig. 2 B and C). The reduced suppression activity of Gr-1+ cells from IDO-KO-BM hosts was not associated with apoptosis, because Gr-1+ cells isolated ex vivo, as well as the Gr-1+ and activated T cells in the suppression assay, showed similar degrees of Annexin-V staining compared with their WT counterparts (SI Appendix, Fig. S3A). Unexpectedly, both the Ly6Chi and Ly6Ghi subsets of Gr-1+CD11b+ cells from IDO-KO-BM hosts expressed typical functional mediators of MDSC, such as iNOS, Arg-1, and IL-10, at levels similar to those of their WT counterparts (SI Appendix, Fig. S3B). Thus, Gr-1+CD11b+ progenies of IDO-KO BM in GVHD hosts were skewed toward the Ly6Ghi subset and exhibited less suppressive activity compared with their WT counterparts, despite the nondisrupted expression of typical functional mediators of MDSCs.
Fig. 2.
Reduced immune-suppressive activity and elevated ROS generation in Gr-1+CD11b+ cells from IDO-KO hosts. (A) Flow cytometric analysis of CD11c−CD11b+ cell subsets in the spleens of BALB.B GVHD hosts on day 7 posttransplantation. Representative Ly6G/Ly6C profiles of CD45.2-CD11c−CD11b+ cells are shown. Percentages and numbers of Ly6Chi and Ly6Ghi cells are plotted. (B) Immune suppression assessed by CFSE dilution of activated CD4 and CD8 T cells. Representative CD4/CD8 FACS profiles were obtained after 72 h coincubation with Gr-1+ (Ly6G+) cells from WT or IDO-KO hosts on day 7 posttransplantation. Suppression is expressed as the percentage of undivided CFSE (Gr-1+ cells) compared with that of the control (−). (C) Representative IFN-γ/CD4, IL-17A/CD4, and IFN-γ/CD8 FACS profiles of the cocultured T cells. Percentages of cytokine-producing cells are plotted. (D and E) Transcriptome analysis of splenic Gr-1+ cells MACS-purified from WT-BM and IDO-KO-BM hosts on day 7 posttransplantation. Two independent transcriptome analyses using pooled RNAs from three mice per group were performed. (D) GSEA enrichment plots for classifying significant genes of IDO-KO GVHD hosts in terms of free radical scavenging function. (E) Heat maps of the expression of ROS and heme metabolism genes. Numbers represent normalized fold changes of KO and WT to syngeneic (WT BM + T → B6) control. (F) Flow cytometric detection of ROS in Ly6Chi and Ly6Ghi cells of IDO-KO-BM and WT-BM hosts on day 7 posttransplantation. Representative CellROX-staining FACS data gated on CD45.2- Gr-1+CD11b+ cells are shown. Geometric mean fluorescence intensity (MFI) values are plotted. The data in A–C and F are representative of at least three independent experiments (n = 3 per group per experiment) and are presented as means ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001 as determined by Student’s t test).
Enhanced ROS Generation in CD11b+Gr-1+ Cells of IDO-KO Hosts.
To investigate the molecular pathways regulated by myeloid IDO1, we compared the transcriptomic profiles of splenic Gr-1+ cells isolated by magnetic-activated cell sorting (MACS) from IDO-KO-BM hosts and WT-BM hosts on day 7 posttransplantation. Gr-1+ cells from syngeneic non-GVHD hosts (WT BM + T → B6) were included as controls. Differentially expressed genes (DEGs) between the IDO-KO and WT Gr-1+ cells were identified after normalization against the non-GVHD Gr-1+ control (SI Appendix, Fig. S4 A and B). Many of the DEGs in this IDO-KO versus WT Gr-1+ set overlapped with those previously identified in the MyD88-KO versus WT Gr-1+ set under GVHD conditions (28). However, a number of DEGs in the Free Radical Scavenging category were unique to the IDO-KO versus WT Gr-1+ pair, and gene set enrichment analysis (GSEA) revealed that this category was significantly enriched in the IDO-KO DEGs (false discovery rate <0.05; Fig. 2D). This finding was confirmed by Gene Ontology (GO) analysis, in which the synthesis of ROS was identified as a significant gene ontology (SI Appendix, Fig. S4C). Moreover, IDO-KO Gr-1+ cells up-regulated genes encoding enzymes that regulate ROS production (Arg1, Tgm2, Jak1, F7, and Cfb), chemokine receptors and recognition molecules (Ccr3, Ccr5, Vcam1, and Mrc1), cytokines (Cxcl12 and Il18), and heme and iron catabolism proteins (Serpina3n, Hmox1, Cyp2c70, and Trf), compared with their WT counterparts (Fig. 2E and SI Appendix, Fig. S4C). Conversely, genes encoding heme-binding proteins (Hba1 and Hbb) and genes related to ROS scavenging (Abcb10, Cybb, and Txnrd2) were down-regulated in IDO-KO Gr-1+ cells. Finally, in flow cytometric analysis, ROS levels, detected by CellRox staining, were significantly higher in the Ly6Chi and Ly6Ghi subsets of donor-derived Gr-1+ cells from IDO-KO-BM hosts compared with their WT counterparts (Fig. 2F). Host (CD45.2+) Gr-1+ cells in the IDO-KO-BM hosts, which accounted for 10 to 15% of splenic Gr-1+ cells, also showed a higher level of ROS than their WT counterparts (SI Appendix, Fig. S4D), suggesting that IDO-KO donor-derived Gr-1+ cells indirectly increase the ROS level in host residual Gr-1+ cells. Of note, this ROS elevation in host Gr-1+ cells was accompanied by Arg-1 elevation, possibly explaining inclusion of Arg1 in the DEG list. Collectively, these results suggested that myeloid IDO1 limits ROS generation in the Ly6Chi and Ly6Ghi subsets of Gr-1+CD11b+ myeloid cells.
Enhanced Ly6Ghi Cell and ROS Generation In Vitro by Gr-1+CD11b+ Progeny of IDO-KO BM.
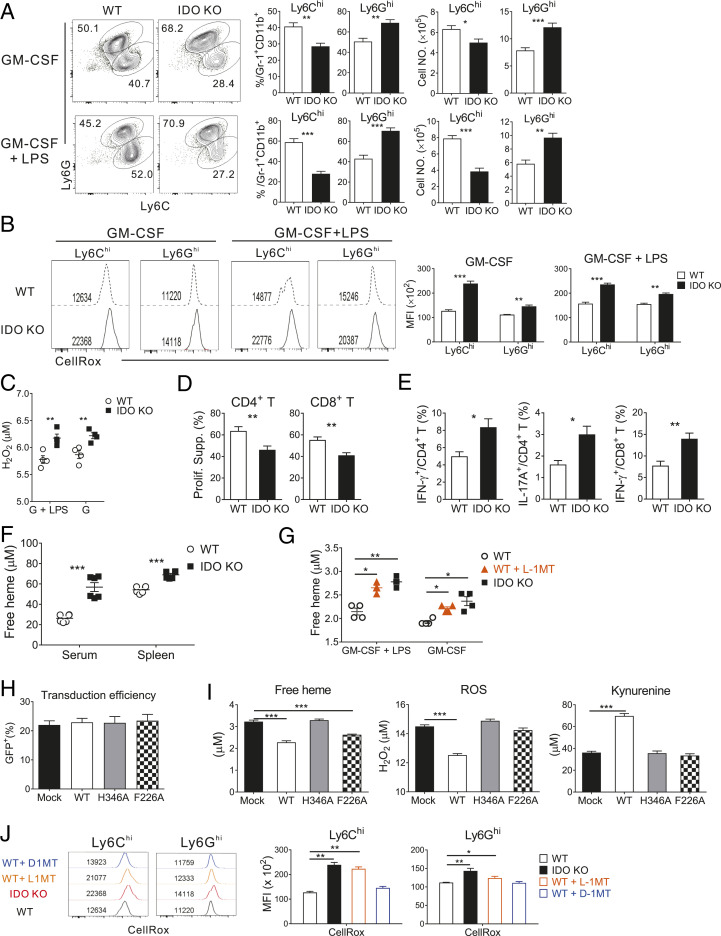
To determine whether the elevation of ROS was maintained in in vitro myelopoiesis of IDO-KO BM, BM cells isolated from naïve Ido1−/− or WT mice were evaluated after culture in the presence of granulocyte macrophage-colony stimulating factor (GM-CSF) for 4 d with or without lipopolysaccharide (LPS) supplementation (28). Flow cytometric analysis of Gr-1+CD11b+ cells from WT and IDO-KO BM cell culture revealed that greater numbers and frequencies of Ly6Ghi cells were generated in IDO-KO BM culture compared with the WT control, while Ly6Chi cell generation was reduced (Fig. 3A). Cellular ROS production was also elevated in both the Ly6Ghi and Ly6Chi cell subsets of IDO-KO BM cell culture (Fig. 3B). Moreover, hydrogen peroxide (H2O2) was detected at higher concentrations in the supernatant of IDO-KO BM cell culture compared with the WT counterpart (Fig. 3C). LPS supplementation did not affect this trend. Furthermore, the in vitro IDO-KO Gr-1+ cells exhibited reduced suppressive activity, as higher proliferation and cytokine production were detected in coincubated T cells compared with the WT counterparts (Fig. 3 D and E). The expression of iNOS, Arg-1, and IL-10 was comparable between IDO-KO and WT progenies (SI Appendix, Fig. S3C). Thus, the ex vivo findings obtained with the Gr-1+CD11b+ cells from IDO-KO-BM hosts were reproduced in vitro with their counterparts generated from IDO-KO BM. This confirmed that ROS elevation and Ly6Ghi subset skewing were intrinsic properties of IDO-KO BM, indicating that IDO1 reduces the generation of ROS and the Ly6Ghi cell subset in Gr-1+CD11b+ cells.
Fig. 3.
The catalytic activity of IDO1 regulates ROS levels. (A–E, G, and J) IDO-KO and WT BM were cultured in the presence of GM-CSF (40 ng/mL) alone or together with LPS (1 μg/mL) and analyzed on day 4. (A and B) Flow cytometric analysis of in vitro-generated Gr-1+CD11b+ cells (n = 3 per group per experiment). (A) Ly6C versus Ly6G profiles. Percentages and numbers of Ly6Chi and Ly6Ghi cells are plotted. (B) Representative histograms of CellRox staining of Ly6Chi and Ly6Ghi cells are shown along with MFI values. (C) Quantification of extracellular H2O2 in the supernatants of in vitro BM cell cultures (n = 4 per group per experiment). Concentrations of H2O2 measured by Amplex Red assay are plotted. (D and E) Immunosuppression assay using in vitro-generated Ly6G+ cells (n = 3 per group per experiment). (D) Percentages of suppression of T cell proliferation and (E) cytokine production in activated T cells are plotted. (F and G) Quantification of free heme by QuantiChrom heme assay. (F) Serum and interstitial fluid of the spleen from BALB.B GVHD hosts on day 7 posttransplantation Each symbol represents a sample pooled from two mice (n = 6 per group per experiment). (G) Supernatants from BM cell cultures were analyzed. l-1MT (100 μM) was added to naïve WT BM cell cultures. Free heme concentrations are plotted (n = 4 per group per experiment). (H and I) WT, heme-binding mutant (H346A), and catalytic mutant (F226A) hu-IDO1 genes were transduced into IDO-KO BM cells cultured in the presence of GM-CSF (40 ng/mL), SCF (20 ng/mL), IL-3 (20 ng/mL), and IL-6 (50 ng/mL). n = 3 per group per experiment. (H) Transduction efficiency was determined by flow cytometric measurement of the percentages of green fluorescent protein (GFP)-expressing cells 1 d after the last transduction, and assays were performed when efficiency was similar among the samples (∼20%). (I) Concentrations of free heme, extracellular H2O2 (ROS), and Kyn in culture supernatants of transduced IDO-KO BM are plotted. (J) Detection of intracellular ROS in Ly6Chi and Ly6Ghi cells generated from BM cells in the presence of GM-CSF. WT BM samples were additionally treated with l-1MT or d-1MT. CellRox-stained MFI values are plotted (n = 3 per group per experiment). Data are representative of two (H and I) and at least three (A–G and J) independent experiments and are presented as means ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001 as determined by Student’s t test).
ROS Regulation by IDO1.
Heme binding is required for the catalytic function of IDO1 (32). Free heme and heme-derived iron show prooxidant and cytotoxic activity and up-regulate genes for heme metabolism such as heme oxygenase Hmox1 and transferrin Trf (33, 34). As the transcriptome data of IDO-KO Gr-1+CD11b+ cells showed up-regulation of genes involved in heme and iron catabolism (Fig. 2E), we hypothesized that enhanced ROS generation in the absence of IDO1 was caused by a failure to sequester free heme. In support of this hypothesis, the free heme concentration in the serum and spleen interstitial fluid from GVHD hosts was significantly higher in IDO-KO than in WT samples (Fig. 3F). Levo-1-methyl tryptophan (l-1MT) is a Trp analog that blocks the catalytic activity of IDO1 (35). l-1MT treatment in WT BM cell culture increased the free heme to a level similar to that of IDO-KO control supernatants, irrespective of LPS supplementation (Fig. 3G), indicating that the catalytic activity of IDO1 was associated with free heme sequestration.
Having found a positive association between free heme sequestration and the catalytic function of IDO1, we evaluated the enzymology of IDO1. IDO-KO BM cells were transduced in vitro so that myeloid progeny would express a mutant type of human-IDO1 (hu-IDO), F226A, which retains heme binding but has defective catalytic function owing to alanine substitution at position (p) 226 (36). WT IDO1 and the H346A mutant (alanine substitution at p346 of the heme-binding pocket), defective in both heme-binding and catalytic activities (37), were also transduced as controls. The free heme concentration in the cell culture supernatant was measured under conditions in which the different hu-IDO proteins showed similar expression (∼25% of cells) (Fig. 3H). As expected, the expression of F226A or WT hu-IDO reduced free heme levels compared with the mock and F346A samples (Fig. 3I). However, despite sequestering free heme, F226A did not lower ROS production, as determined by the failure of F226A to reduce H2O2 compared with the mock and H346A samples. Only WT hu-IDO lowered the H2O2 level and, uniquely, produced a considerable amount of the Trp catabolite, Kyn (Fig. 3I). Also, the catalytic inhibitor l-1MT, but not the pseudoinhibitor dextro (d)-1MT, consistently increased the ROS level in Ly6Chi and Ly6Ghi subsets of WT Gr-1+CD11b+ progeny (Fig. 3J). Taken together, the results suggest that the catabolic activity of IDO1 limits the production of ROS in Gr-1+CD11b+ BM cells.
Increased Neutrophil Generation from IDO-KO BM.
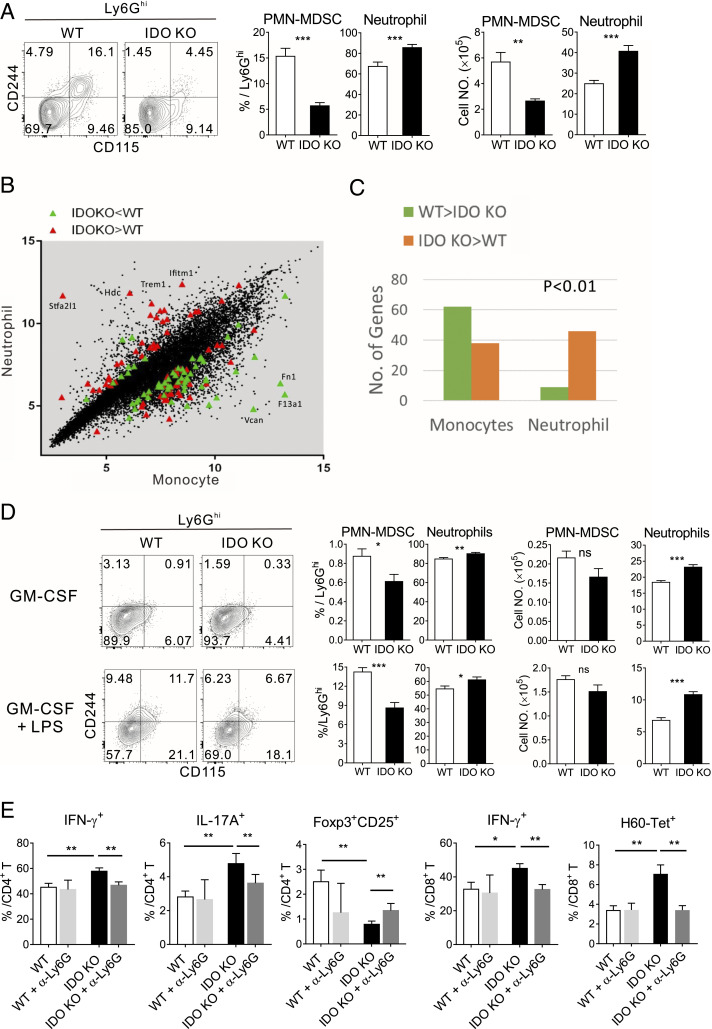
Neutrophils express Ly6G and are strong candidates for ROS production (22, 38). Hence, we tested the possibility that elevated ROS in IDO-KO Ly6Ghi cells in GVHD is caused by neutrophil differentiation rather than immunosuppressive PMN-MDSCs. Using CD244 and CD115 surface markers to distinguish neutrophils (CD244−CD115−) and PMN-MDSCs (CD244+ and CD115+) in Ly6Ghi cells (22, 28), we found that the proportion of neutrophils was significantly higher in Ly6Ghi cells from IDO-KO hosts, while that of PMN-MDSC was lower, compared with their WT counterparts (Fig. 4A). Consistently, genes involved in neutrophil development and activity, such as Stfa2l1 (39), Hdc (40), and Ifitm1 (41), were up-regulated; in contrast, monocyte-related genes were down-regulated in IDO-KO Gr-1+ cells (Fig. 4B). Moreover, comparison of transcriptome profiles with those of classical neutrophils and monocytes retrieved from the ImmGen Project indicated that the up-regulated genes in the IDO-KO transcriptomes were enriched in the classical neutrophil profiles (Fig. 4C). Neutrophil enrichment was also observed in the flow cytometric analysis of Ly6Ghi pool generated in vitro from IDO-KO BM (Fig. 4D). Furthermore, administration of a neutrophil-depleting anti-Ly6G antibody (Ab) into IDO-KO-BM hosts on days 4 and 8 posttransplantation resulted in a significant reduction in IFN-γ+ or IL-17+ cells in CD4/CD8 T cells and H60-tetrmer+ CD8 T cells but an increase in Foxp3+CD25+ Treg cells in the spleen, compared to isotype Ab-treated control hosts on day 14 posttransplantation (Fig. 4E and SI Appendix, Fig. S4E). No such depletion effect was detected in WT-BM hosts. Taken together, these results demonstrated that IDO-KO Gr-1+CD11b+ progeny generated in GVHD hosts in vivo and in vitro are skewed toward neutrophils, and removal of the neutrophils attenuates proinflammatory T cell alloimmunity in vivo, potentially explaining why the IDO-KO Gr-1+ cells were less immunosuppressive than their WT counterparts (Figs. 2 and 3) and IDO-KO-BM hosts showed heightened T cell alloimmunity (Fig. 1).
Fig. 4.
Enhanced neutrophil generation by IDO-KO BM cells in GVHD hosts in vivo and in vitro. (A) Detection of neutrophils in the Ly6Ghi cells generated in GVHD hosts on day 7 posttransplantation. Representative CD115 and CD244 FACS profiles of Ly6Ghi (CD45.2-) cells are shown. Percentages of PMN-MDSCs and neutrophils in Ly6Ghi cells and the numbers are plotted. (B and C) Comparison of the transcriptome of the IDO-KO and WT Gr-1+ cells with those of mouse neutrophils and monocytes retrieved from ImmGen (Immunological Genome Project; https://www.immgen.org/). (B) The DEGs up-regulated in IDO-KO (labeled as IDO KO > WT; red) and down-regulated in IDO-KO (labeled as IDO-KO < WT; green) were plotted (monocyte versus neutrophil scatter plot). Some representative genes are marked with their names. (C) Counts of up- and down-regulated genes in IDO-KO for monocyte and neutrophil areas (below and above, respectively, the diagonal of the scatter plot in B) were tested for significance by Fisher’s exact test. (D) Fractions and numbers of PMN-MDSCs and neutrophils among Ly6Ghi cells generated in vitro from the TCD-BM of IDO-KO and WT mice. Representative CD115 and CD244 FACS profiles of Ly6Ghi cells are shown. (E) T cell phenotype of BALB.B GHVD hosts treated with a neutrophil-depleting or control Ab. Splenic T cells were analyzed on day 14 posttransplantation. Frequencies of IFN-γ+ or IL-17+ cells in CD4+ and CD8+ T cells, Foxp3+CD25+ Tregs, and H60-tetramer+ CD8 T cells are plotted. Data (A, D, and E) represent three independent experiments (n = 3 per group per experiment) and are presented as means ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001 as determined by Student’s t test). ns, not statistically significant.
ROS Scavenging Reverses the Aberrations of IDO1-Deficient Myeloid Cells In Vitro and in GVHD.
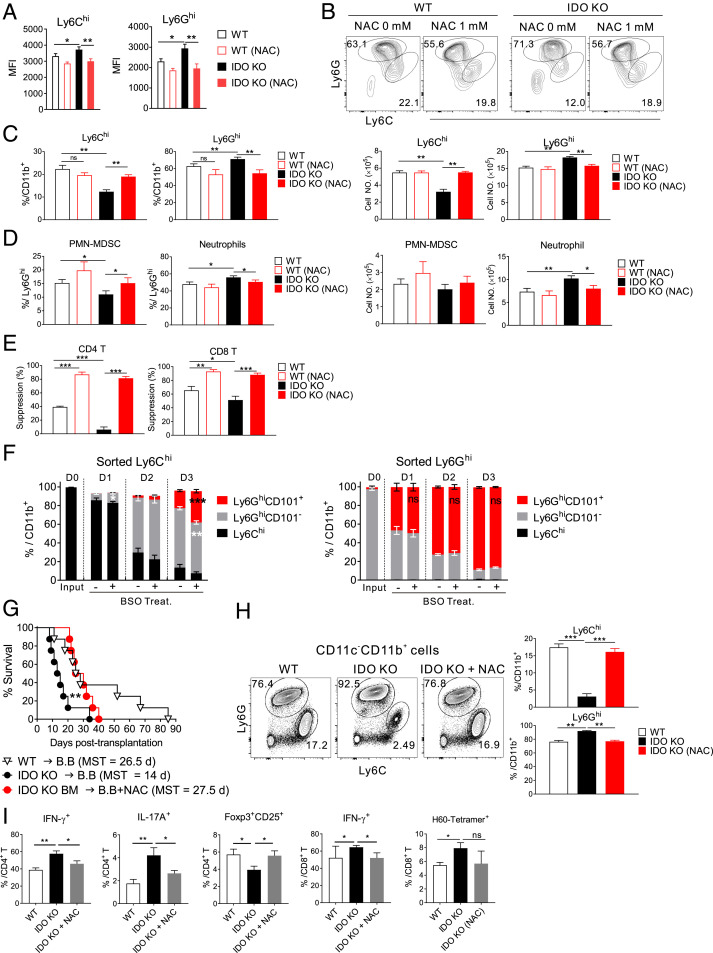
To clarify the consequences of elevated ROS levels in IDO-KO Gr-1+CD11b+ cells, N-acetylcysteine (NAC), a ROS-scavenging synthetic glutathione (GSH) precursor (42), was added to IDO-KO BM cell culture. NAC treatment not only reduced the ROS level in the Ly6Chi and Ly6Ghi subsets (Fig. 5A) but also increased the percentage of Ly6Chi cell subset significantly, with a concomitant reduction in the percentage of Ly6Ghi cell subset in IDO-KO BM cultures compared with the untreated controls (Fig. 5 B and C). The fraction of Ly6Ghi PMN-MDSC was increased in the NAC-treated IDO-KO subset, while the neutrophil fraction was reduced (Fig. 5D). Thus, ROS scavenging by NAC reversed the tendency of IDO-KO Gr-1+CD11b+ cells to increase neutrophil levels, converting their composition to the WT Gr-1+CD11b+ cellular composition. Accordingly, Gr-1+ cells from the NAC-treated IDO-KO Gr-1+CD11b+ progenies showed significantly enhanced immunosuppressive activities compared with the untreated IDO-KO controls (Fig. 5E). By contrast, ROS elevation promoted neutrophil generation from WT cells. In progeny-tracing analysis, fluorescence-activated cell sorter (FACS)-sorted WT Ly6Chi cells generated CD244−CD115− neutrophils, including those expressing CD101 neutrophil maturation marker (43), when cultured in the presence of GM-CSF (SI Appendix, Fig. S5A). Tracing with CD101 marker demonstrated that the proportions of Ly6Chi (CD101−) cells gradually decreased, whereas those of Ly6Ghi cells, both the CD101− (PMN-MDSC + immature neutrophils) and CD101+ (mature neutrophils) subsets, gradually increased (Fig. 5F and SI Appendix, Fig. S5B). Therefore, Ly6Chi cells differentiate into Ly6Ghi mature neutrophils via intermediate immature stages. When ROS-elevating l-buthionine-S, R-sulfoximine (BSO), a GSH-depleting agent (44), was added, neutrophil differentiation from Ly6Chi cells into mature neutrophils was significantly increased compared with untreated controls. In case of sorted Ly6Ghi (CD101−) cells, GM-CSF drove their differentiation to CD101+ mature neutrophils also. However, BSO-induced significant enhancement was not observed for Ly6Ghi cell differentiation, implying a preferential effect of ROS on Ly6Chi cell differentiation. Thus, differentiation of Ly6Chi cells into Ly6Ghi neutrophils is affected by ROS elevation, explaining the reduced Ly6Chi fraction and neutrophil skewing in IDO-KO Gr-1+CD11b+ cells.
Fig. 5.
Regulation of ROS and neutrophil generation by IDO1 for GVHD protection. (A–E) IDO-KO or WT BM cells were cultured in the presence of GM-CSF and LPS with or without NAC (1 mM) for 4 d. (A) Cellular ROS levels in Ly6Chi and Ly6Ghi cells generated in vitro. MFI values of CellRox staining of the in vitro-generated cells are plotted. (B) Representative Ly6C and Ly6G FACS profiles for the in vitro-generated CD11b+ cells are shown. (C and D) Percentages and numbers of (C) Ly6Chi and Ly6Ghi cells in CD11b+ cells and (D) neutrophils and PMN-MDSCs in Ly6Ghi cells are plotted. (E) Immunosuppressive activity of Gr-1+ cells generated from IDO-KO and WT BM in the presence or absence of the ROS scavenger NAC. Percent suppressions of T cell proliferation are plotted. (F) Effect of ROS elevation on the differentiation of WT Ly6Chi and Ly6Ghi cells in vitro. Ly6Chi and Ly6Ghi cells FACS-sorted from naïve WT BM (day 0, D0) were cultured in the presence of GM-CSF alone or together with BSO (1 mM). Cultured cells were analyzed daily (days 1 to 3, D1–D3) by flow cytometry. Fractions of Ly6Chi, Ly6GhiCD101-, and Ly6GhiCD101+ cells in CD11b+ cells are plotted. (G) Enhanced GVHD survival of IDO-KO-BM hosts (BALB.B) with NAC treatment. NAC or PBS (control) treatment began at day 4 posttransplantation. NACs (1 mg/mouse) were injected intraperitoneally five times a week for 3 wk. The survival rates of GVHD hosts are plotted. Data are representative of two independent experiments (n = 8 per group per experiment). **P < 0.01 as determined by log-rank (Mantel–Cox) test. (H) Representative Ly6G/Ly6C profiles of PBLs on day 10 posttransplantation are shown with gating on CD45.2-CD11c−CD11b+ cells. Percentages of Ly6Chi or Ly6Ghi cells in CD11b+ cells are plotted. (I) T-cell phenotype of NAC-treated IDO-KO-BM hosts. GVHD hosts were treated daily with NAC or PBS from day 4 posttransplantation and analyzed for the frequencies of proinflammatory CD4/CD8 T cells, H60-tetramer+ CD8 T cells, and Treg cells in the spleens on day 7 posttransplantation. Data are representative of two (H; n = 3 per group per experiment) or three (A–F and I); n = 3 per group per experiment) independent experiments and are presented as means ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001 as determined by Student’s t test). ns, not statistically significant.
Next, we extended the in vitro effect of ROS scavenging to the in vivo GVHD model. NAC treatment significantly increased the survival rate of IDO-KO-BM hosts, compared with untreated IDO-KO-BM hosts, to a level comparable to that of WT-BM hosts (Fig. 5G). The fractions of Ly6Chi Gr-1+CD11b+ cells in IDO-KO-BM hosts were significantly increased, while the percentages of the Ly6Ghi subset were reduced in the NAC-treated hosts compared with untreated controls; the values for NAC-treated IDO-KO hosts were similar to those of the WT host counterparts (Fig. 5H). In the spleens of NAC-treated IDO-KO-BM hosts, fractions of proinflammatory T cell (IFN-γ+ or IL-17+ CD4/CD8 T cells) and H60-tetramer+ CD8 T cell fractions were reduced, while Treg cell proportions were increased, compared with untreated controls (Fig. 5I). This indicated that NAC-induced GVHD alleviation was accompanied by attenuation of proinflammatory T cell alloimmunity. The enhanced immunosuppression is likely irrelevant with Kyn, an inhibitory metabolite of IDO-family enzymes, because the Kyn level was not increased in the supernatants of NAC-treated IDO-KO BM cultures or in the serum of NAC-treated IDO-KO-BM hosts (SI Appendix, Fig. S5C).
Finally, we evaluated whether the findings obtained with mouse models could be recapitulated in humans by bioinformatics analysis of published transcriptome data in patients with allo-HSCT (45). Analysis of monocytic (M; CD14+HLA-DR−) and granulocytic (G; CD33+CD14− HLA-DR−) MDSC demonstrated that IDO1 transcript level was higher in M-MDSC than in G(PMN)-MDSC (SI Appendix, Fig. S6), consistent with publications indicating M-MDSC as a main source of IDO expression in murine tumor models (46, 47). Furthermore, IDO1 expression was strongly associated with the expression of genes in the Regulation of ROS category, notably showing a positive correlation with the expression of SIRT1 and HIF1, cellular redox and oxygen sensors (48) and a negative correlation with ROS regulators (e.g., SOD1, GSTP1, CAT, NOXA1, and MPO) (49). These results are in line with the aforementioned findings in mice, which implies the functional significance of IDO in ROS regulation in Ly6Chi M-MDSCs. Altogether, we conclude that an important immunoregulatory role of myeloid IDO1 is the prevention of ROS elevation in Gr-1+CD11b+ cells via its catalytic domain. This limits Gr-1+CD11b+ cell differentiation into proinflammatory neutrophils, while sparing MDSCs that suppress alloimmunity and alleviate GVHD.
Discussion
Gr-1+CD11b+ MDSCs suppress T cell response and contribute to the prevention of severe GVHD development after allo-HSCT. These myeloid cells employ several molecules, including IDO1, as functional mediators. In this study, using a GVHD model in which BM-derived myeloid cells are devoid of IDO1 expression, we elucidated that the catabolic activity of IDO1 regulates ROS generation and maintenance of Gr-1+CD11b+ cells in the MDSC state.
The main characteristics of IDO1-deficient Gr-1+CD11b+ cells were ROS elevation and neutrophil skewing, providing insight into the roles of IDO1 in ROS regulation and maintenance of MDSCs. The primary role for IDO1 is ROS scavenging through its oxygenase activity in the Trp-degrading redox chemical reaction, as evidenced by the fact that ROS scavenging induced conversion of the IDO-KO Gr-1+CD11b+ cells, in terms of both their composition and suppressive activity, into their MDSC-prone WT counterparts. From an immunological point of view, however, the maintenance of Gr-1+CD11b+ cells in the MDSC state has significant implications. MDSCs represent a major population responsible for immune suppression under various pathological conditions (17, 18). Furthermore, MDSCs promote Treg differentiation and activation (19, 20). Therefore, maintaining MDSC would be helpful to establish and support an environment conducive to T cell suppression under pathological conditions. Thus, we suggest that the contribution to the increased MDSC expression in the myeloid compartment via the catalytic activity-associated ROS control represents a fundamental mechanism underlying the immune-regulatory function of IDO1.
Gr-1+CD11b+ cells are a major myeloid population that shows expansion in GVHD hosts (23, 27). We have previously emphasized the importance of increased Gr-1+CD11b+ cell numbers, including MDSCs, in preventing GVHD, as well as identified MyD88 as a key molecule responsible for this increase (27, 30). The current study revealed that IDO1 is not directly involved in increasing the number of Gr-1+CD11b+ cells but suppresses their differentiation to neutrophils instead, while sparing MDSCs. In this respect, IDO1, together with MyD88, is critical for endowing donor BM-derived myeloid cells with the ability to protect the host from severe GVHD.
Our results demonstrate that ROS scavenging enhances the ability of Gr-1+CD11b+ cells to function as MDSCs, attenuating GVHD. More specifically, ROS scavenging reduces neutrophil numbers, resulting in an increased proportion of MDSCs in IDO-KO Gr-1+CD11b+ cells and survival of IDO-KO-BM hosts. This ROS scavenging by NAC and subsequent prevention of neutrophil enrichment contribute to alleviation of proinflammatory T cell alloimmunity in IDO-KO-BM hosts. In contrast, forced ROS elevation by BSO promotes neutrophil generation from WT Gr-1+CD11b+ cells, more specifically from Ly6Chi cells. This is consistent with previous evidence that ROS dysregulation is associated with GVHD and that neutrophils increase GVHD severity (50, 51). However, studies in tumor models have suggested that ROS elevation is required for the suppressive activity of MDSCs (21, 52–54). In the suppressive MDSCs, hydrogen peroxide, not the superoxide anion radical, was identified as the major part of the elevated ROS (21). H2O2 scavenging by catalase or the absence of the ROS-producing Nox2 (NADPH oxidase 2) led to loss of the suppressive function of MDSCs and promoted MDSC differentiation into tumor-associated macrophages and DCs, thereby enhancing antitumor immunity (21, 52). Loss of MDSC due to differentiation into such lineages may explain the functional loss. In GVHD hosts, various factors, such as irradiation, microbial exposure, and T cell-mediated tissue damage, can lead to ROS generation and neutrophil activation (51, 55–57). As an inflammatory disease, GVHD severity is linked to ROS production by hematopoietic cells. For example, in a previous study on Nox2-deficient BM transplantation, Nox2 deficiency in donor BM cells did not alleviate GVHD, probably because of the presence of Nox2 in host hematopoietic cells (51). However, Nox2 deficiency in both donor and host hematopoietic cells reduced the GVHD lethality (51). By contrast, ROS elevation by IDO-KO BM transplantation, even in IDO-sufficient host, is enough to reduce survival. IDO1 is unique in that it utilizes superoxide anion radicals as both a cofactor and a substrate (32) and is also reported to have peroxygenase activity, thereby consuming peroxide (58). IDO-1 is up-regulated in Ly6Chi M-MDSCs (46, 47) (SI Appendix, Fig. S6), which are susceptible to ROS levels and show expedited differentiation into neutrophils in the presence of ROS elevation (Fig. 5F). Thus, IDO1 deficiency may lead to hyper-ROS elevation beyond the levels required for suppression by MDSCs, thereby promoting neutrophil generation and activation in GVHD hosts. To resolve these seemingly equivocal findings, we propose that MDSCs require ROS elevation within a certain range to function as suppressor cells, whereas functionality is lost when they further differentiate into a specific lineage under circumstances where ROS levels are below or above the critical range.
IDO1 is the initial and rate-liming enzyme for Trp degradation in the Kyn pathway. It oxidizes the pyrrole rings of l-Trp and other molecules, such as serotonin and tryptamine, undergoing conformational changes from the ferric (Fe3+) to ferrous (Fe2+) state for reductive activation (32, 59). During the catalytic reaction, IDO1 can utilize superoxide anion radicals and peroxides (32, 58). These redox activities scavenge ROS, supporting our results showing a marginal ROS scavenging effect by the heme-binding activity alone (F226A mutant of Hu-IDO), as opposed to the profound effect of the catalytically active WT hu-IDO. Thus, Trp degradation along the Kyn pathway is an anti-ROS redox reaction. This is consistent with a report on the radical scavenging and antioxidant properties of the Trp catabolites, 3-hydroxyanthranilic acid and 3-hydroxykynurenine (60). Furthermore, IDO1 itself is intimately linked to the ROS level, as elevation and scavenging of ROS was reported to induce up- or down-regulation of IDO1 expression in DCs (61). These findings are in line with a previous hypothesis emphasizing the importance of the production of Trp catabolite in inducing T cell death. As shown previously (2), depletion of Trp and activation of GCN-kinase appears to be irrelevant, at least as a mechanism of IDO1-mediated GVHD suppression. Binding to and then activation of AhR was suggested as a mechanism underlying the Trp catabolite-associated suppressive effects (12, 13) and was relevant to the IDO1 expressed by parenchymal compartment of GVHD hosts (14). Here, we demonstrated that IDO1 catalytic activity-based ROS scavenging maintains MDSCs, representing a critical immunosuppressive mechanism of myeloid IDO1. Therefore, whether ROS control by IDO1 is associated with the suppressive effects of parenchymal IDO1 merits further investigation.
The immunological attributes of myeloid IDO1 reported here have parallels with those seen in other disease models (4, 7, 8). Blockade of Treg to Th17 conversion has been suggested as a function of IDO1 in previous tumor and EAE studies (4–6, 8, 9). However, the underlying mechanism has remained elusive. Based on our findings, we propose that the role of IDO1 in the blockade of Treg to Th17 conversion is mediated by the increase of Gr-1+CD11b+ MDSCs, suggesting that the regulation of ROS levels and increase of MDSC numbers constitute the fundamental immune-regulatory mechanism of IDO1, applicable to other pathological conditions in addition to GVHD.
In summary, this study identified an immune-regulatory mechanism of myeloid IDO1, thereby providing insight into the association between metabolic and immune regulatory functions and potentially facilitating the development of preventative and therapeutic strategies for GVHD.
Materials and Methods
Mice.
C.B10-H2b/LiMcdJ (BALB.B), C57BL/6 (B6), B6.129-Ido1tm1Alm/J (IDO-KO), B6.SJL-PtprcaPep3b/BoyJ (CD45.1+), and B6D2F1 mice were obtained from The Jackson Laboratory. Mice were housed under specific pathogen-free conditions at the Biomedical Center for Animal Resource Development of Seoul National University College of Medicine (Seoul, Korea). All experiments were performed using mice aged between 8 and 12 wk, with approval from The Institutional Animal Care and Use Committee of Seoul National University.
GVHD Induction.
IDO-KO or WT mice on CD45.1+B6 background were used as BM and T cell donors to induce GVHD (SI Appendix, Supplemental Methods).
Cell Staining and Flow Cytometry.
Cells stained with antibodies (SI Appendix, Supplemental Methods) were analyzed using a FACS LSRII instrument (BD Biosciences) and FlowJo software (Tree Star).
Serum Enzyme-Linked Immunosorbent Assay.
The serum concentrations of IL-6, IL-17, and IFN-γ were measured using a commercial enzyme-linked immunosorbent assay kit (BioLegend).
BM Cell Culture and Immunosuppression Assay.
Details are described in SI Appendix, Supplemental Methods.
Bioinformatics Analysis of Gene Expression Profiles.
Gr-1+ cells were MACS-purified from splenocytes on day 7 posttransplantation for transcriptome and bioinformatics analyses (SI Appendix, Supplemental Methods).
Measurement of ROS Levels.
Details are in SI Appendix, Supplemental Methods.
Retroviral Plasmid DNA Construction and Transduction.
Details are in SI Appendix, Supplemental Methods.
Quantification of Heme and Kyn.
Details are in SI Appendix, Supplemental Methods.
Statistical Analysis.
All statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc.).
Supplementary Material
Acknowledgments
We thank Dr. Derry Roopenian (The Jackson Laboratory, Bar Harbor, ME) for critically reading the manuscript. This work was supported by grants from the National Research Foundation (Basic Research Laboratory 2017R1A4A1015745; Basic Research Program 2019R1A2C3004336) and the Healthcare Technology R&D Project, Ministry of Health and Welfare (HI16C0047), Republic of Korea, and the Seoul National University Hospital (03-2015-0090).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.R.M.v.d.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2011170118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Munn D. H., Mellor A. L., Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 34, 137–143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jasperson L. K., et al., Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood 114, 5062–5070 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciorba M. A., et al., Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J. Immunol. 184, 3907–3916 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan Y., et al., IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 185, 5953–5961 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baban B., et al., IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J. Immunol. 183, 2475–2483 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H., Chi H., Metabolic control of Treg cell stability, plasticity, and tissue-specific heterogeneity. Front. Immunol. 10, 2716 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma M. D., et al., Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Invest. 117, 2570–2582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma M. D., et al., Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood 113, 6102–6111 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson T. S., Mcgaha T., Munn D. H., Chemo-immunotherapy: Role of indoleamine 2,3-dioxygenase in defining immunogenic versus tolerogenic cell death in the tumor microenvironment. Adv. Exp. Med. Biol. 1036, 91–104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn D. H., et al., GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 22, 633–642 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Ravishankar B., et al., The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc. Natl. Acad. Sci. U.S.A. 112, 10774–10779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opitz C. A., et al., An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Bessede A., et al., Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511, 184–190 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S. M., et al., Inhibition of acute lethal pulmonary inflammation by the IDO-AhR pathway. Proc. Natl. Acad. Sci. U.S.A. 114, E5881–E5890 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jasperson L. K., et al., Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood 111, 3257–3265 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrara J. L., Cooke K. R., Teshima T., The pathophysiology of acute graft-versus-host disease. Int. J. Hematol. 78, 181–187 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Gabrilovich D. I., Nagaraj S., Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veglia F., Perego M., Gabrilovich D., Myeloid-derived suppressor cells coming of age. Nat. Immunol. 19, 108–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindau D., Gielen P., Kroesen M., Wesseling P., Adema G. J., The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138, 105–115 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen L., et al., Interplay between myeloid-derived suppressor cells (MDSCs) and Th17 cells: Foe or friend? Oncotarget 7, 35490–35496 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusmartsev S., Gabrilovich D. I., Inhibition of myeloid cell differentiation in cancer: The role of reactive oxygen species. J. Leukoc. Biol. 74, 186–196 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Youn J. I., Nagaraj S., Collazo M., Gabrilovich D. I., Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi E. Y., et al., Real-time T-cell profiling identifies H60 as a major minor histocompatibility antigen in murine graft-versus-host disease. Blood 100, 4259–4265 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Di Ianni M., et al., Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Koehn B. H., Blazar B. R., Role of myeloid-derived suppressor cells in allogeneic hematopoietic cell transplantation. J. Leukoc. Biol. 102, 335–341 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blazar B. R., MacDonald K. P. A., Hill G. R., Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood 131, 2651–2660 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim J. Y., et al., MyD88 in donor bone marrow cells is critical for protection from acute intestinal graft-vs.-host disease. Mucosal Immunol. 9, 730–743 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Lee Y. K., et al., Skewed dendritic cell differentiation of MyD88-deficient donor bone marrow cells, instead of massive expansion as myeloid-derived suppressor cells, aggravates GVHD. Immune Netw. 18, e44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park G., et al., A paradoxical pattern of indoleamine 2,3-dioxygenase expression in the colon tissues of patients with acute graft-versus-host disease. Exp. Hematol. 42, 734–740 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Lee Y. K., Kang M., Choi E. Y., TLR/MyD88-mediated innate immunity in intestinal graft-versus-host disease. Immune Netw. 17, 144–151 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Faudeur G., de Trez C., Muraille E., Leo O., Normal development and function of dendritic cells in mice lacking IDO-1 expression. Immunol. Lett. 118, 21–29 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Thomas S. R., Stocker R., Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 4, 199–220 (1999). [DOI] [PubMed] [Google Scholar]
- 33.Jeney V., et al., Pro-oxidant and cytotoxic effects of circulating heme. Blood 100, 879–887 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Dutra F. F., Bozza M. T., Heme on innate immunity and inflammation. Front. Pharmacol. 5, 115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Löb S., et al., IDO1 and IDO2 are expressed in human tumors: Levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol. Immunother. 58, 153–157 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugimoto H., et al., Crystal structure of human indoleamine 2,3-dioxygenase: Catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc. Natl. Acad. Sci. U.S.A. 103, 2611–2616 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Littlejohn T. K., Takikawa O., Truscott R. J., Walker M. J., Asp274 and his346 are essential for heme binding and catalytic function of human indoleamine 2,3-dioxygenase. J. Biol. Chem. 278, 29525–29531 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Yang W., et al., Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 10, 1076 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ericson J. A.et al.; ImmGen Consortium , Gene expression during the generation and activation of mouse neutrophils: Implication of novel functional and regulatory pathways. PLoS One 9, e108553 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka S., et al., Expression of L-histidine decarboxylase in granules of elicited mouse polymorphonuclear leukocytes. Eur. J. Immunol. 34, 1472–1482 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Hofman V. J., et al., Gene expression profiling in human gastric mucosa infected with Helicobacter pylori. Mod. Pathol. 20, 974–989 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Yim C. Y., Hibbs J. B. Jr, McGregor J. R., Galinsky R. E., Samlowski W. E., Use of N-acetyl cysteine to increase intracellular glutathione during the induction of antitumor responses by IL-2. J. Immunol. 152, 5796–5805 (1994). [PubMed] [Google Scholar]
- 43.Evrard M., et al., Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity 48, 364–379.e8 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Bailey H. H., L-S,R-buthionine sulfoximine: Historical development and clinical issues. Chem. Biol. Interact. 111-112, 239–254 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Lee S. E., et al., Matrix metalloproteinase-9 in monocytic myeloid-derived suppressor cells correlate with early infections and clinical outcomes in allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 24, 32–42 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Meireson A., Devos M., Brochez L., IDO expression in cancer: Different compartment, different functionality? Front. Immunol. 11, 531491 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li A., et al., Indoleamine 2,3-dioxygenase 1 inhibition targets anti-PD1-resistant lung tumors by blocking myeloid-derived suppressor cells. Cancer Lett. 431, 54–63 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim J. H., et al., Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol. Cell 38, 864–878 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Zhang L., et al., Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 26, 101284 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paczesny S., et al., A biomarker panel for acute graft-versus-host disease. Blood 113, 273–278 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwab L., et al., Neutrophil granulocytes recruited upon translocation of intestinal bacteria enhance graft-versus-host disease via tissue damage. Nat. Med. 20, 648–654 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Corzo C. A., et al., Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohl K., Tenbrock K., Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front. Immunol. 9, 2499 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayakumar A., Bothwell A. L. M., Functional diversity of myeloid-derived suppressor cells: The multitasking hydra of cancer. J. Immunol. 203, 1095–1103 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akira S., Takeda K., Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Amer J., et al., The oxidative status of blood cells in a murine model of graft-versus-host disease. Ann. Hematol. 86, 753–758 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Themeli M., et al., Alloreactive microenvironment after human hematopoietic cell transplantation induces genomic alterations in epithelium through an ROS-mediated mechanism: In vivo and in vitro study and implications to secondary neoplasia. Leukemia 24, 536–543 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Kuo H. H., Mauk A. G., Indole peroxygenase activity of indoleamine 2,3-dioxygenase. Proc. Natl. Acad. Sci. U.S.A. 109, 13966–13971 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y., Indoleamine 2,3-dioxygenase–A new antioxidant enzyme. Mater. Med. Pol. 21, 244–250 (1989). [PubMed] [Google Scholar]
- 60.Christen S., Peterhans E., Stocker R., Antioxidant activities of some tryptophan metabolites: Possible implication for inflammatory diseases. Proc. Natl. Acad. Sci. U.S.A. 87, 2506–2510 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogasawara N., et al., Hemoglobin induces the expression of indoleamine 2,3-dioxygenase in dendritic cells through the activation of PI3K, PKC, and NF-kappaB and the generation of reactive oxygen species. J. Cell. Biochem. 108, 716–725 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.