Abstract
Background
An important aspect of end-of-life care is the place of death. A majority of cancer patients prefer home death to hospital death. At the same time, the actual location of death is often against patient’s last-known wish. The aim of this study was to analyze whether socioeconomic factors influence if Swedish palliative cancer patients die at home or at a hospital. There is no previous study on location of death encompassing several years in Swedish cancer patients.
Methods
Data was collected from the Swedish Register of Palliative Care for patients diagnosed with brain tumor, lung, colorectal, prostate or breast cancer recorded between 2011 and 2014. The data was linked to the Swedish Cancer Register, the Cause of Death Register and the Longitudinal Integration Database for health-insurance and labor-market studies. A total of 8990 patients were included.
Results
We found that marital status was the factor that seemed to affect the place of death. Lack of a partner, compared to being married, was associated with a higher likelihood of dying at a hospital.
Conclusion
Our findings are in line with similar earlier studies encompassing only 1 year and based on patients in other countries. Whether inequalities at least partly explain the differences remains to be investigated. Patients dying of cancer in Sweden, who do not have a life partner, may not have the option of dying at home due to lack of informal support. Perhaps the need of extensive community support services to enable home death have to improve, and further studies are warranted to answer this question.
Keywords: Location of death, Palliative care, Cancer patients, Register study, Socioeconomy
Background
An important aspect of end-of-life care is the place of death [1]. A majority of cancer patients across all socioeconomic groups prefer home death to hospital death [2, 3]. However, most deaths in developed countries occur in a hospital, and previous studies report that the actual location of death is often against patients last-known wish [4, 5]. As meeting patients’ choice of death place has been seen as a robust indicator of quality in end-of-life care, it is important to explore the issue and plan supportive care accordingly [6]. The likelihood of home death in comparison to hospital death seems to be greater among palliative cancer patients living in rural areas than those living in urban areas in Sweden. This geographical discrepancy can be explained, at least partly, by closer proximity to hospital emergency departments in larger cities than in the countryside. This explanation is in particular attributable to patients with acute conditions or refractory symptoms, which are common in specific cancer types such as lung cancer [7, 8]. Also, patients living in rural areas could be more likely to prefer home death since they would otherwise have to receive inpatient care far away from their families. Moreover, one previous Swedish study, focusing on 1 year only, has shown associations between socioeconomic factors (such as educational level and marital status) and place of death, indicating that socioeconomic inequalities influence end-of-life care [6].
The aim of the current study, which encompasses several years, is to analyze whether socioeconomic factors such as educational level, income and marital status influence if Swedish palliative patients die at home or at a hospital.
Methods
Data collection
As part of a doctoral project, data was obtained from the Swedish Register of Palliative Care (SRPC), which is a National quality register developed in 2005 and contains data that is collected retrospectively post mortem through a structured questionnaire that is filled out by responsible health care professionals, and includes thirty questions of interest in palliative care regarding the last week of life of the deceased. Data was collected for patients recorded from January 2011 through December 2014. Patients who were diagnosed and died due to cancer within the defined period were included. Record linkage was made between SRPC and the Swedish Cancer Register (SCR) by using the personal identity numbers assigned to all Swedish permanent citizens at birth [9]. Data was collected from patients with lung cancer (ICD, International Classification of disease, C34), high grade brain tumors (C71), prostate cancer (C61), breast cancer (C50) and colorectal cancer (C18, C19 & C20). Information on underlying cause of death was obtained from the Cause of Death Register (CDR) [10], coded according to ICD system. The coverage of reported events in CDR is comprehensive, estimated at more than 99% of all deaths [11]. Additional individual information regarding socioeconomic data was retrieved from the Longitudinal Integration Database for health-insurance and labor-market studies (LISA in the Swedish acronym) managed by Statistics Sweden, including data on income, education, employment, country of birth etc. [12]. Since there are only a few Hospices in Sweden, we have chosen not to involve them in the study.
Definitions of socioeconomic data
Marital status was defined as married, unmarried, widow/widower or divorced at the year of death. Education level was stratified as low, middle or high. Low education level was defined as 9 years or less in compulsory school. Middle education level was defined as having attended secondary level school (Sw: gymnasium). High education level was defined as university studies. Income was defined as the disposable income (individualized by family) of the deceased at the year of death. Numbers are presented in thousands of Swedish crowns (SEK).
Statistical analysis
Patients’ characteristics at baseline were presented with standard descriptive statistics. We used multiple logistic regressions to assess the relationship between socioeconomic factors and if patients died at home versus at a hospital. Our dependent binary variable equaled 1 if the individual had died at home and 0 if he or she died at a hospital. Our primary independent variables of interest were the patients’ marital status, education level, and disposable income (individualized by family). We also included two possible confounder variables: age and sex. Age and disposable income were treated as continuous variables and fitted by restricted cubic splines, as they tended to be non-linear. Adjusted odds ratios (aOR) and corresponding 95% confidence intervals (CI) were used as inference for all variables in the models. Confidence intervals that don’t overlap the null value indicate statistical significance. Data processing and statistical analysis were performed with the statistical software R.
Results
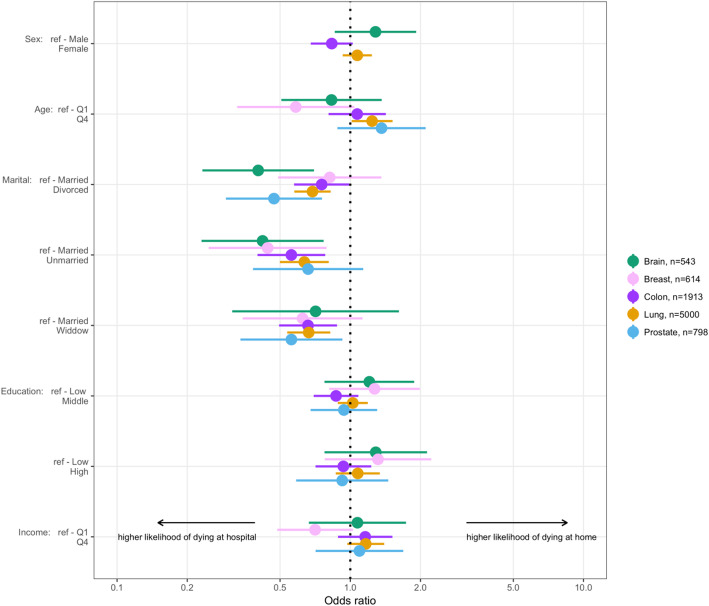
A total of 8990 patients were included in the study. Of these, 561 had a brain tumor, 621 had breast cancer, 1935 had colorectal cancer, 5062 had lung cancer and 811 had prostate cancer. For full demographic characteristics of the study population, see Table 1. For patients with lung cancer, the likelihood of dying at a hospital compared to dying at home was significantly higher for divorced (OR 0,69; 95% CI: 0.58–0.82), unmarried (OR 0,64; 95% CI: 0.50–0.81) and widowed (OR 0,66; 95% CI: 0.54–0.82) patients compared to married patients, holding all other predictors constant. For colorectal cancer, too, the likelihood of dying at a hospital compared to dying at home was significantly higher for divorced (OR 0,75; 95% CI: 0.57–0.99), unmarried (OR 0,56; 95% CI: 0.40–0.78) and widowed (OR 0,66; 95% CI: 0.49–0.88) patients compared to married patients. The same tendency was also seen for the other tumor types, although for brain tumors, the association with place of death was statistically significant only for divorced (OR 0,40; 95% CI: 0.23–0.70) and unmarried (OR 0,42; 95% CI: 0.23–0.77), for breast cancer only for unmarried (OR 0,44; 95% CI: 0.25–0.79), and for prostate cancer only for divorced (OR 0,47; 95% CI: 0.29–0.76) and widowed (OR 0,56; 95% CI: 0.34–0.92) when compared to married patients. There was a trend for higher likelihood of dying at home for patients with breast cancer and brain tumors with a high or middle education level compared to patients with a low education level. The differences were, not statistically significant, however, as the confidence intervals overlapped the null value. There was also a non-isolated trend for higher likelihood of dying at a hospital for patients with breast cancer with a high family income (fourth quartile) as compared to patients with a low family income (first quartile). For odds ratios for dying at home vs. dying at hospital for different tumor types, see Table 2. The findings are visualized in Fig. 1.
Table 1.
Demographic characteristics of study population stratified by place of death
| Total, n (%) | Home, n (%) | Hospital, n (%) | |||
|---|---|---|---|---|---|
| Brain | Sex | Female | 207 (100%) | 92 (44.4%) | 115 (55.6%) |
| Male | 354 (100%) | 147 (41.5%) | 207 (58.5%) | ||
| NA | 0 | 0 | 0 | ||
| Age | Median (Q1-Q4) min-max | 65 (53–74) 0–89 | 65 (52–74) 5–89 | 65 (54–74) 0–89 | |
| NA | 0 | 0 | 0 | ||
| Marital | Divorced | 78 (100%) | 21 (26.9%) | 57 (73.1%) | |
| Married | 365 (100%) | 176 (48.2%) | 189 (51.8%) | ||
| Unmarried | 86 (100%) | 29 (33.7%) | 57 (66.3%) | ||
| Widow | 32 (100%) | 13 (40.6%) | 19 (59.4%) | ||
| NA | 0 | 0 | 0 | ||
| Education | Low | 151 (100%) | 56 (37.1%) | 95 (62.9%) | |
| Middle | 224 (100%) | 100 (44.6%) | 124 (55.4%) | ||
| High | 168 (100%) | 73 (43.5%) | 95 (56.5%) | ||
| NA | 18 | 10 | 8 | ||
| Income | Median (Q1-Q4) min-max | 193.2 (126.76–312.32) -527.5-4372.9 | 192 (125.46–320.54) -527.5-1339.3 | 195.1 (130.34–307.34) -333.6-4372.9 | |
| NA | 12 | 7 | 5 | ||
| Breast | Sex | Female | 619 (100%) | 174 (28.1%) | 445 (71.9%) |
| Male | 2 (100%) | 0 (0%) | 2 (100%) | ||
| NA | 0 | 0 | 0 | ||
| Age | Median (Q1-Q4) min-max | 69 (56–82) 27–100 | 66 (54–81) 33–94 | 69 (56–82.8) 27–100 | |
| NA | 0 | 0 | 0 | ||
| Marital | Divorced | 108 (100%) | 31 (28.7%) | 77 (71.3%) | |
| Married | 275 (100%) | 93 (33.8%) | 182 (66.2%) | ||
| Unmarried | 98 (100%) | 19 (19.4%) | 79 (80.6%) | ||
| Widow | 140 (100%) | 31 (22.1%) | 109 (77.9%) | ||
| NA | 0 | 0 | 0 | ||
| Education | Low | 213 (100%) | 53 (24.9%) | 160 (75.1%) | |
| Middle | 245 (100%) | 74 (30.2%) | 171 (69.8%) | ||
| High | 156 (100%) | 44 (28.2%) | 112 (71.8%) | ||
| NA | 7 | 3 | 4 | ||
| Income | Median (Q1-Q4) min-max | 141.7 (103–214.8) -44.7-6627.6 | 135.3 (96.18–196.04) 0–590 | 143.4 (104.76–222.54) -44.7-6627.6 | |
| NA | 0 | 0 | 0 | ||
| Colorectal | Sex | Female | 927 (100%) | 307 (33.1%) | 620 (66.9%) |
| Male | 1008 (100%) | 395 (39.2%) | 613 (60.8%) | ||
| NA | 0 | 0 | 0 | ||
| Age | Median (Q1-Q4) min-max | 74 (64–83) 15–99 | 73 (63–82) 22–97 | 74 (64–83) 15–99 | |
| NA | 0 | 0 | 0 | ||
| Marital | Divorced | 316 (100%) | 106 (33.5%) | 210 (66.5%) | |
| Married | 999 (100%) | 415 (41.5%) | 584 (58.5%) | ||
| Unmarried | 212 (100%) | 62 (29.2%) | 150 (70.8%) | ||
| Widow | 408 (100%) | 119 (29.2%) | 289 (70.8%) | ||
| NA | 0 | 0 | 0 | ||
| Education | Low | 722 (100%) | 263 (36.4%) | 459 (63.6%) | |
| Middle | 758 (100%) | 265 (35%) | 493 (65%) | ||
| High | 433 (100%) | 165 (38.1%) | 268 (61.9%) | ||
| NA | 22 | 9 | 13 | ||
| Income | Median (Q1-Q4) min-max | 160.85 (118.16–249.22) -96-3319.7 | 166 (119.36–265.18) -96-1646.4 | 158.3 (117.7–241.74) 0.5–3319.7 | |
| NA | 1 | 0 | 1 | ||
| Lung | Sex | Female | 2391 (100%) | 517 (21.6%) | 1874 (78.4%) |
| Male | 2671 (100%) | 595 (22.3%) | 2076 (77.7%) | ||
| NA | 0 | 0 | 0 | ||
| Age | Median (Q1-Q4) min-max | 71 (63.2–79) 14–97 | 72 (64–80) 14–95 | 71 (63–79) 23–97 | |
| NA | 0 | 0 | 0 | ||
| Marital | Divorced | 1171 (100%) | 216 (18.4%) | 955 (81.6%) | |
| Married | 2472 (100%) | 627 (25.4%) | 1845 (74.6%) | ||
| Unmarried | 589 (100%) | 101 (17.1%) | 488 (82.9%) | ||
| Widow | 829 (100%) | 168 (20.3%) | 661 (79.7%) | ||
| NA | 1 | 0 | 1 | ||
| Education | Low | 2142 (100%) | 465 (21.7%) | 1677 (78.3%) | |
| Middle | 2117 (100%) | 462 (21.8%) | 1655 (78.2%) | ||
| High | 742 (100%) | 178 (24%) | 564 (76%) | ||
| NA | 61 | 7 | 54 | ||
| Income | Median (Q1-Q4) min-max | 157.3 (116.5–238.7) -369.8-4260 | 161.75 (118.38–255.1) -60.5-1901.1 | 156.4 (116.4–233.96) -369.8-4260 | |
| NA | 5 | 2 | 3 | ||
| Prostate | Sex | Male | 811 (100%) | 295 (36.4%) | 516 (63.6%) |
| NA | 0 | 0 | 0 | ||
| Age | Median (Q1-Q4) min-max | 76 (68–84) 48–97 | 77 (68–84) 48–95 | 76 (68–84) 49–97 | |
| NA | 0 | 0 | 0 | ||
| Marital | Divorced | 116 (100%) | 28 (24.1%) | 88 (75.9%) | |
| Married | 517 (100%) | 214 (41.4%) | 303 (58.6%) | ||
| Unmarried | 79 (100%) | 24 (30.4%) | 55 (69.6%) | ||
| Widow | 99 (100%) | 29 (29.3%) | 70 (70.7%) | ||
| NA | 0 | 0 | 0 | ||
| Education | Low | 370 (100%) | 140 (37.8%) | 230 (62.2%) | |
| Middle | 287 (100%) | 100 (34.8%) | 187 (65.2%) | ||
| High | 141 (100%) | 51 (36.2%) | 90 (63.8%) | ||
| NA | 13 | 4 | 9 | ||
| Income | Median (Q1-Q4) min-max | 173.6 (134.5–255.7) -0.6-6657.2 | 176.2 (136.04–252.72) -0.6-954.6 | 170.85 (134.4–257.7) 0–6657.2 | |
| NA | 0 | 0 | 0 | ||
Table 2.
Odds Ratios for dying at home vs dying at hospital for different tumor types
| aOR (95% CI) | ||
|---|---|---|
| Brain, n = 543 | ||
| Sex | Male | Ref |
| Female | 1.28 (0.86–1.92) | |
| Age | Q1 | Ref |
| Q4 | 0.83 (0.5–1.36) | |
| Marital | Married | Ref |
| Divorced | 0.4 (0.23–0.7)a | |
| Unmarried | 0.42 (0.23–0.76)a | |
| Widow | 0.71 (0.31–1.62) | |
| Education | Low | Ref |
| Middle | 1.24 (0.79–1.95) | |
| High | 1.22 (0.75–1.99) | |
| Income | Q1 | Ref |
| Q4 | 1.08 (0.67–1.75) | |
| Breast, n = 614 | ||
| Age | Q1 | Ref |
| Q4 | 0.58 (0.33–1.04) | |
| Marital | Married | Ref |
| Divorced | 0.81 (0.49–1.35) | |
| Unmarried | 0.44 (0.25–0.79)a | |
| Widow | 0.62 (0.34–1.12) | |
| Education | Low | Ref |
| Middle | 1.28 (0.82–2.01) | |
| High | 1.29 (0.76–2.17) | |
| Income | Q1 | Ref |
| Q4 | 0.71 (0.49–1.03) | |
| Colorectal, n = 1913 | ||
| Sex | Male | Ref |
| Female | 0.83 (0.68–1.02) | |
| Age | Q1 | Ref |
| Q4 | 1.07 (0.81–1.42) | |
| Marital | Married | Ref |
| Divorced | 0.75 (0.57–0.99)a | |
| Unmarried | 0.56 (0.4–0.78)a | |
| Widow | 0.66 (0.49–0.88)a | |
| Education | Low | Ref |
| Middle | 0.87 (0.7–1.09) | |
| High | 0.92 (0.71–1.21) | |
| Income | Q1 | Ref |
| Q4 | 1.16 (0.89–1.52) | |
| Lung, n = 5000 | ||
| Sex | Male | Ref |
| Female | 1.07 (0.93–1.24) | |
| Age | Q1 | Ref |
| Q4 | 1.24 (1.01–1.52) | |
| Marital | Married | Ref |
| Divorced | 0.69 (0.58–0.82)a | |
| Unmarried | 0.63 (0.5–0.81)a | |
| Widow | 0.66 (0.54–0.82)a | |
| Education | Low | Ref |
| Middle | 1.02 (0.88–1.19) | |
| High | 1.07 (0.87–1.32) | |
| Income | Q1 | Ref |
| Q4 | 1.16 (0.97–1.4) | |
| Prostate, n = 798 | ||
| Age | Q1 | Ref |
| Q4 | 1.36 (0.88–2.11) | |
| Marital | Married | Ref |
| Divorced | 0.47 (0.29–0.76)a | |
| Unmarried | 0.66 (0.38–1.14) | |
| Widow | 0.56 (0.34–0.92)a | |
| Education | Low | Ref |
| Middle | 0.94 (0.67–1.31) | |
| High | 0.92 (0.6–1.42) | |
| Income | Q1 | Ref |
| Q4 | 1.1 (0.71–1.69) | |
astatistically significant
An Odds ratio greater than 1 implies a higher likelihood of home death and one less than 1 implies a higher likelihood of in hospital deaths
Fig. 1.
Odds ratios for dying at home vs dying at hospital for different tumor types
Discussion
In this register-based study we found that socioeconomic factors seem to have an impact on the place of death for all patient groups included, i.e. with brain tumors, breast, colorectal, lung and prostate cancer. Marital status was found to be the most important factor predicting place of death where the lack of a partner (i.e. being divorced, unmarried or widowed) was associated with a higher likelihood of dying at a hospital, compared to married patients. For income and education level the associations were not statistically significant, although patients with breast cancer and a high income tended to die at a hospital compared to those with low income.
The main strength of the present study is the large number of patients who have been identified through the SRPC, which covers a majority of patients who have died from a cancer diagnosis in Sweden. The SRPC has high validity but, nevertheless, a concern with all register-based studies is the quality of the register data. There are previous studies on cancer patients in other countries and one Swedish study which included patients from one single year. There is however no earlier study on Swedish patients encompassing several years. One remark about the data from the present study is the high representation of patients with lung cancer and the relatively low representation of patients with breast and prostate cancer, which are the two most common cancer types in Sweden. A possible explanation is that since only patients that were both diagnosed and died due to cancer during 2011 through 2014 were included, patients who survived more than 4 years after diagnosis, or were diagnosed prior to 2011 were not included. The relative 5-year survival of breast and prostate cancer is about 80–90%, whereas the relative 5-year survival rate for lung cancer is around 40%, and this difference could explain the relatively low percentage of breast and prostate cancer patients.
The impact of marital status on place of death in cancer patients has been studied previously with similar results as in the present study. In a study by Öhlén et al., associations between place of death and cancer types, and individual, socioeconomic and environmental characteristics were investigated for all cancer deaths in 2012 in Sweden [6]. Being married was associated with a higher likelihood of dying at home when compared to being unmarried, whereas education level did not seem to affect this likelihood. Cohen et al. have reported similar results on place of death in two studies with cross-sectional data from death certificates from various countries during 2002–2003 and 2008 [13, 14]. In both studies it was found that being married consistently increased the chances of dying at home in all countries. A higher education level was associated with a higher likelihood of dying at home in some of the countries, whereas in other countries the opposite was seen. In a study of factors affecting place of cancer death in London and New York City in the years 1995 through 1998 it was found that being in the lowest tercile of socioeconomic status as compared to the highest lowered the odds of death at home by 22% in London and 39% in New York City [15]. We did not find similar associations in our study. Perhaps the differences are at least partly explained by various socioeconomic conditions in different countries. Other studies have also found a significant association between having a partner and a higher likelihood of dying at home, whereas the relationship with income, occupation and education is less clear [16, 17].
In Sweden the bulk of health and medical costs is funded by regional and municipal taxes [18]. Thus the nominal cost for the individual patient is probably not an important factor in end of life care. In urban areas most of the home care is carried out by the regions, and in rural areas mainly as a cooperation between the regions/specialized palliative home care teams and nurses from the municipalities [19]. Nevertheless, home care is in principal generally available to Swedish cancer patients.
Having a partner might facilitate the home caregiving of the patient. Moreover, married people are probably more likely to have children, which further enhances the opportunities for informal support in end of life care. However, we don’t know the support-giving capabilities of family members, and we have not included if the patient’s need of external support such as home service and nursing visits was met. In addition, patients would probably not want to leave their loved ones and be cared for in a hospital. Further studies with interviews at the individual level should be conducted to answer these questions.
Conclusion
We have conducted the first study encompassing several years on location of death in Swedish palliative cancer patients. Marital status was found to be the most important factor associated with place of death where the lack of a partner (i.e. being divorced, unmarried or widowed) was associated with a higher likelihood of dying at a hospital. Patients dying of cancer in Sweden, who do not have a life partner, may not have the option of dying at home due to lack of informal support. Perhaps community support services to enable home death have to improve, and further studies are warranted to answer this question.
Acknowledgments
Not applicable.
Authors’ contributions
JN, TC, MB and SB have contributed to study design and data withdrawal. TC has drafted statistical processing. JN, TC, GH, GU, MB, SB, MH and BA have made substantial interpretations of the results. GH, GU and JN have contributed with manuscript processing. JN, TC, GH, GU, MB, SB, MH and BA have substantively revised the manuscript. The authors read and approved the final manuscript.
Funding
Gävle Cancerfond to enable the statistical analyses. Open Access funding provided by Umea University.
Availability of data and materials
The dataset will not be published, since there are multiple variables that together could be used to identify individual patients. However data are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Approved by the Local Ethics Committee (Sw: Etikprövningsmyndigheten) Uppsala, DNR 2015/131.
Permission to access raw data from the SRPC was granted by the management team of SRPC on 4 Sept. 2015.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Clayton JM, Butow PN, Arnold RM, Tattersall MH. Discussing end-of-life issues with terminally ill cancer patients and their carers: a qualitative study. Support Care Cancer. 2005;13(8):589–599. doi: 10.1007/s00520-004-0759-2. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Gomes B, Higginson IJ. End-of-life care--what do cancer patients want? Nat Rev Clin Oncol. 2014;11(2):100–108. doi: 10.1038/nrclinonc.2013.217. [DOI] [PubMed] [Google Scholar]
- 3.Townsend J. Terminal cancer care and patients’ preference for place of death: a prospective study. Br Med J. 1990;301(6749):415–417. doi: 10.1136/bmj.301.6749.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomes B. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care. 2013;12:7. doi: 10.1186/1472-684X-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson J, Blomberg C, Holgersson G, Carlsson T, Bergqvist M, Bergstrom S. End-of-life care: where do cancer patients want to die? A systematic review. Asia-Pacific J Clin Oncol. 2017;13(6):356. doi: 10.1111/ajco.12678. [DOI] [PubMed] [Google Scholar]
- 6.Ohlen J, Cohen J, Hakanson C. Determinants in the place of death for people with different cancer types: a national population-based study. Acta oncologica (Stockholm, Sweden) 2017;56(3):455–461. doi: 10.1080/0284186X.2016.1250946. [DOI] [PubMed] [Google Scholar]
- 7.Mayer DK, Travers D, Wyss A, Leak A, Waller A. Why do patients with cancer visit emergency departments? Results of a 2008 population study in North Carolina. J Clin Oncol. 2011;29(19):2683–2688. doi: 10.1200/JCO.2010.34.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westfall K, Moore D, Meeneghan M, Jarr S, Valgus J, Bernard S. The Impact on Resource Utilization of Supportive Care Consults on Patients at the University of North Carolina Hospital, 2010-2012. J Palliat Med. 2017;21(2):216. doi: 10.1089/jpm.2016.0482. [DOI] [PubMed] [Google Scholar]
- 9.Swedish Cancer Registry [https://www.socialstyrelsen.se/register/halsodataregister/cancerregistret/inenglish].
- 10.Dödsorsaksregistret [http://www.socialstyrelsen.se/register/dodsorsaksregistret].
- 11.Statistik om Dödsorsaker [http://www.socialstyrelsen.se/SiteCollectionDocuments/2016-8-3-beskrivning-av-statistiken.pdf].
- 12.Longitudinal integration database for health insurance and labour market studies (LISA by swedish acronym) [http://www.scb.se/lisa-en]. [DOI] [PMC free article] [PubMed]
- 13.Cohen J, Houttekier D, Onwuteaka-Philipsen B, Miccinesi G, Addington-Hall J, Kaasa S, Bilsen J, Deliens L. Which patients with cancer die at home? A study of six European countries using death certificate data. J Clin Oncol. 2010;28(13):2267–2273. doi: 10.1200/JCO.2009.23.2850. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J, Pivodic L, Miccinesi G, Onwuteaka-Philipsen BD, Naylor WA, Wilson DM, Loucka M, Csikos A, Pardon K, Van den Block L, et al. International study of the place of death of people with cancer: a population-level comparison of 14 countries across 4 continents using death certificate data. Br J Cancer. 2015;113(9):1397–1404. doi: 10.1038/bjc.2015.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decker SL, Higginson IJ. A tale of two cities: factors affecting place of cancer death in London and New York. Eur J Pub Health. 2007;17(3):285–290. doi: 10.1093/eurpub/ckl243. [DOI] [PubMed] [Google Scholar]
- 16.Hunt RW, D'Onise K, Nguyen AT, Venugopal K. Where patients with Cancer die: a population-based study, 1990 to 2012. J Palliat Care. 2018;34(4):224. doi: 10.1177/0825859718814813. [DOI] [PubMed] [Google Scholar]
- 17.Johnson MJ, Allgar V, Chen H, Dunn L, Macleod U, Currow DC. The complex relationship between household income of family caregivers, access to palliative care services and place of death: a national household population survey. Palliat Med. 2018;32(2):357–365. doi: 10.1177/0269216317711825. [DOI] [PubMed] [Google Scholar]
- 18.Healthcare in Sweden [https://sweden.se/society/health-care-in-sweden/#].
- 19.Home care - a map of Reviews [https://www.sbu.se/contentassets/e6c06e9b0d1545aaaeb3d2ef44918531/hemvard_2014-12-22.pdf].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset will not be published, since there are multiple variables that together could be used to identify individual patients. However data are available from the corresponding author on reasonable request.